Abstract
In recent years, the continuous occurrence of multi-drug resistance in the clinic has made people pay more attention to the transporter. Changes in the expression and activity of transporters can cause corresponding changes in drug pharmacokinetics and pharmacodynamics. The drug-drug interactions (DDI) caused by transporters can seriously affect drug effectiveness and toxicity. In the development of pharmaceutical preparations, people have increasingly concerned about the effects and regulation of transporters in drug effects. To improve the targeting and physicochemical properties of drugs, the development of targeted agents is very rapid. Among them, novel nano-formulations are the best. With the continuous innovation and development of nano-formulation, its application has become more and more extensive. Nano-formulation has exerted certain advantages in the drug development based on transporters, and is also involved in the combination of targeted transporters. This review focuses on the application of novel nano-agents targeting transporters and the introduction of drug-transporter-based nano-formulations.
Keywords: Drug transporter, Nano-formulation, Drug delivery system, Bioavailability, P-gp
Graphical abstract
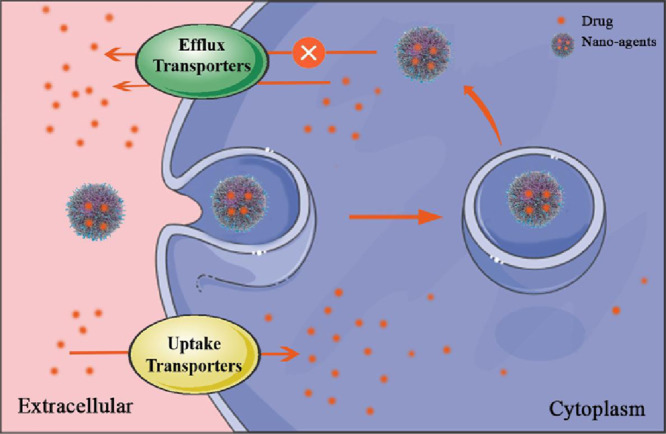
Some medicines enter or exit target cells through transporters. Nano-agents targeting transporters can enhance the targeted uptake of the drug while prevent the efflux of it.
1. Introduction
Drug needs to undergo absorption, distribution, metabolism and excretion processes to work in the body. It must cross multiple layers of biofilm after numerous transfers. This process is called drug transmembrane transport. Most drugs can be transported across the membrane by passive transport [1], such as simple diffusion and dissimilation. Some drugs require active transport to complete the transmembrane transport process. Among them, dissimilation diffusion and active transport require the participation of corresponding transporters. Besides, eukaryotic cells typically undergo transmembrane transport of macromolecules and particulate matter through endocytosis and exocytosis, such as receptor-mediated endocytosis and non-specific endocytosis.
Receptor-mediated endocytosis is an essential process in which viruses and biological particles enter cells. Studies have shown that abnormal regulation of specific receptors can cause related disorders. In the process of crossing the blood-brain barrier, drugs can also be transported by adsorption-mediated endocytosis. For example, studies by Junichi Matsumoto [2] found that red blood cell-derived extracellular vesicles containing α-synuclein pass the blood-brain barrier (BBB) in this way, which can be applied to Parkinson's treatment. In general, transmembrane transport processes such as active transport, dissimilation, and efflux secretion of drugs are carried out in the presence of transporters. The relevant influx and efflux transporters determine the plasma and tissue concentrations of the drug to some extent. According to the biopharmaceutics classification system (BCS) [3], high-through and high-dissolved drugs are not affected by transporters; low-soluble, high-permeability drugs are greatly affected by efflux transporters; high-dissolved, low-permeability drugs are affected by ingestion transporters. For low-solubility, low-permeability drugs, both efflux transporters and uptake transporters affect them. Therefore, carrier-mediated transmembrane transport has received extensive attention. Drug transporters are recognized as a decisive factor for drug delivery and drug interaction. The research on the mechanism of uptake and efflux transporters lays a foundation for the development and improvement of drugs.
In order to avoid the situation mentioned above, we hope to innovate the traditional preparations and break through some limitations of the conventional drug delivery system. The targeted therapy has emerged, which can be further divided into active targeting agents, passive targeting agents, and physical targeting agents according to their methods of action. Among them, the passive targeted preparation refers to the drug-using microspheres, liposomes, polymer micelles and other microparticles as carriers, through a normal physiological process, enrichment in specific targets in the body. This agent achieves tumor targeting based on the enhanced permeability and retention effect of the peripheral vascular system. The actively targeted formulation refers to the modification of the loaded drug microparticles and the delivery to a specific target organ in combination with the target cell receptor to achieve targeted therapy. With the development of nanotechnology, targeted drug delivery based on nanoparticles has attracted more and more attention and has gradually become the focus of targeted therapy. Currently, active nanocarriers have not received food and drug administration (FDA) approval, and only a few clinical trials are underway [4]. Herein, we focus on the application of novel nano-agents targeting transporters and the introduction of drug-transporter-based nano-formulations.
2. The role of transporters and efflux systems in drug delivery
2.1. Transporter
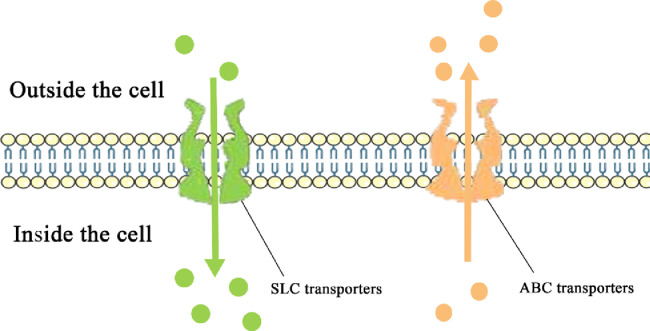
Drug transporters can be divided into two families: solute carrier transporters (SLC transporters) and ATP-binding cassette transporters (ABC transporters) (Fig. 1). They have different expression profiles in human tissues, and transporters of different sites correspond to different treatments for drugs or endogenous compounds. Transporters widely distributed in the gut, liver, and kidney are closely related to the elimination of drugs. Drugs and their metabolites usually need to be excreted through the urine and bile. Transporters expressed in the BBB protect sensitive tissues from the potential toxicity of the drug.
Fig. 1.
Drug Transporter family.
The expression of transporters in different tissues is different, which suggests that we can use drug transporters to enhance the ability of drugs to concentrate at the target site, and develop related targeted agents, thereby enhancing drug targeting, improving drug efficacy and reducing toxic side effects. Therefore, studies on the selectivity of transporter expression and the mechanism of transport are critical. To date, over 400 membrane transporters have been identified and characterized in human cells at both the molecule level and functional level. The distribution of various uptake transporters and efflux transporters in tissues is shown in Table 1 according to the figures adapted from the previously published studies [5], [6], [7], [8], (Table 2).
Table 1.
Distribution of transporters in tissues.
| Position | Influx transporter type | Efflux transporter type |
|---|---|---|
| Intestine | PEPT; OATPs; OCTNs; OCT1; OCT2 | MDR1; MRP1/2/3/4/5; BCRP |
| Liver | OATP1B1; OATP1B3; OATP2B1; OCT1; OAT2 | MDR1/2/3; MRP2/3/4; BCRP; BSEP |
| Kidney | OCT2; OAT1; OAT3; OAT4; PEPT2; GLUTs | MDR1; MRP2/4; OCTN1/2; MATE1/2; OCT4; OATP1A2 |
| Blood-brain barrier | GLUT1; OAT3; OATP2B1; OCTN2; OCT1/2/3 | MDR1; MRP1/2/3/4/5; BCRP |
Table 2.
Preparation materials for several common nano preparations.
| Nano preparation | Common materials |
|---|---|
| Microspheres | Natural polysaccharide; Gelatin; Poly(lactic-co-glycolic acid); Polystyrene; Polyacrylic acid; Resin; Alginate |
| Nanoparticles | Poly(lactic-co-glycolic acid); Inorganic metal; Nano-powder; Nanofibers; Nanofilm; Nano-block |
| Liposomes | Phosphatidylethanolamine; Cholesterol; Soy sterol; Phosphatidylcholine; Sphingomyelin; Stearic acid amide |
| Dendrimers | PAMAM; PLLD; Propylene imine; Polymethyl methacrylate; Polystyrene; Polyacetylene |
| Polumeric micelles | Polyester; Polyether; Polyamino acid; Polyethylene glycol; Polyethylene oxide |
2.1.1. Influx transporter
Organic anion transfer peptide (the OATP family): The OATP family belongs to the SLC21 subfamily and the human OATP family consists of 11 members [9]: OATP1A2, OATP1B1, OATP1B3, OATP1C1, OATP2A1, OATP2B1, OATP3A1, OATP4A1, OATP4C1, OATP5A1, OATP6A1. OATP1A2 expressed in cerebral capillaries mainly mediates metabolites (such as glutathione) and uptake of drugs (such as opioid peptides). OATP1B1 and OATP1B3 are expressed only in the liver; whereas OATP2B1, OATP3A1, OATP4A1 are expressed in many tissues, such as the small intestine and liver.
The OATP family has a wide range of substrates, which can not only transport organic anions and anionic complexes, but also identify some neutral and zwitterionic compounds, to be the key determinant in the cellular uptake of many endogenous and exogenous chemicals. There are substrate overlaps between different OATPs, but the affinity is different. Several clinically used drugs have been identified as substrates for OATP transporters, such as statins, methotrexate, rifampicin, cardiac glycosides, etc. [10]. Since a large number of drugs need to be taken up by the OATP1B1 and OATP1B3 transporters on the liver, the substrate range of these two transporters are extensive. A large number of studies have been conducted on OATP-mediated drug transport and drug interactions. Kalliokoski et al. [9] summarized the genetic effects of OATP1B1, OATP1A2, OATP1B3, OATP2B1 and their effects on drug interactions, indicating that OATP families play an important role in drug pharmacokinetics and DDI profiles. Studies have shown that OATP2B1 in endothelial cells of human brain capillaries can mediate brain transport of morphine and its metabolite morphine-6-glucuronide (M6G), and its expression regulation is associated with morphine tolerance [11]. The mRNA level is significantly reduced, and lower levels of OATP3A1 may additionally result in lower estrone-sulfate (E1-S) uptake and further limit its conversion to a biologically active form, revealing a novel mechanism by which OATP3A1 may be involved in colorectal cancer regulation. In addition, OATP4A1 has potential in the assessment of colorectal cancer as a biomarker, helping to provide personalized treatment for patients [12].
Organic anion transporter (the oat family): Organic anion transporter belongs to the SLC22A superfamily. The OAT family in humans includes OAT1, OAT2, OAT3, OAT4, OAT5, OAT7, OAT10, and URAT1 [13], which are widely distributed in the body. In the kidney, OAT1 and OAT3 use a tertiary transport mechanism to move the organic anion/drug through the basolateral membrane into the proximal tubule cells [14]. Then the organic anion/drug enters the urine through the apical membrane to complete the elimination. The human liver contains OAT2, OAT5 and OAT7, which plays a role in bile secretion and drug excretion through bile. The substrate of the Organic cationic organic cation transporter (OCT) are hydrophilic organic anions, such as steroid hormone conjugates, biogenic amines, and various drug which is different from the OATP transporter in transporting larger hydrophobic organic anions. OATs can mediate the secretion and reabsorption of organic anions, like some neutral particles and organic cations, and the Na+ concentration gradient is required for the transport process. OAT1 and OAT3 transporters are widely distributed in the kidney. In view of the extensive nature of OATs substrate, the change of OATs activity is a key factor in many clinical conditions. For most drugs, interaction was demonstrated in vitro by inhibition of OAT-mediated transport of model substrates; for some drugs, transport by OATs was directly proven. Burckhardt [15] hold that drug–drug interactions at OAT1 and OAT3 may retard renal drug secretion and cause untoward effects, since OAT1 and OAT3 show comparable affinities for diuretics, cephalosporins, and nonsteroidal anti-inflammatory drugs whereas OAT2 has a lower affinity to most of these compounds. Nieskens Tom T. G's data [16] demonstrate that functional OAT1 was directly responsible for cytotoxicity of adefovir, cidofovir, and tenofovir, while a drug interaction with zidovudine was not associated with decreased cell viability. Some results also suggested that interactions of the human organic anion with its substrates may significantly impact the dosing, efficacy and toxicity of related drugs (methotrexate, nonsteroidal anti-inflammatory drugs, organic anion transport inhibitors).
Organic cationic organic cation transporter (The OCT family): Organic cation transporter, as a member of the SLC superfamily, is widely distributed in the human body. It is an important factor that affects the absorption, distribution and metabolism of organic cations. The OCT family mainly includes OCT1, OCT2, and OCT3, as well as several subtypes of OCTN1, OCTN2, and OCT6. OCT1 is mainly distributed in the liver, which is the first step of biotransformation of exogenous substances; OCT2 is mainly distributed in the kidney. There is also a distribution of OCT3 in brain tissue, which can mediate the transport of endogenous substances to the central nervous system [17].
Substrates for OCT proteins are endogenously cationic substances and several cationic drugs. Endogenous compounds transported by or inhibiting the transport by all three OCT proteins include neurotransmitters. OCT1-3 translocate a variety of organic cations with widely differing molecular structures, and are inhibited by a large number of additional compounds that are not transported [18]. Several endogenous compounds that are transported by human OCTs have been identified, Koepsell summarize the specific compounds transport by human OCTs (hOCT1, hOCT2, hOCT3) in his article [19]. As for the drugs, like metformin [20], an anti-diabetic drug, is distributed to the liver via hOCT1-mediated transport, and mainly excreted into urine via hOCT2 in humans without metabolism. Drug–drug interactions at OCTs may change pharmacokinetics, pharmacodynamics and drug toxicity. All these results suggest that we can rationally utilize drug interactions based on the regulatory mechanisms of OCTs to overcome the changes in transporters.
In addition to the several SLC family members mentioned above, oligopeptide transporter (PEPT) is also widely distributed in humans. It is a crucial uptake protein in the intestine, which mainly mediates the transport of peptides to cells, including dipeptides, tripeptides and peptidomimetics. PEPT are mainly divided into two types: PEPT1 and PEPT2, which a uses electrochemical proton gradient as a driving force and has a strong dependence on extracellular pH and membrane potential. It can transport β-lactam antibiotics, angiotensin-converting enzyme inhibitors, aminopeptidase inhibitors and other drugs [21].
2.1.2. Efflux transporter
ABC transporter: Multidrug resistance protein P-glycoprotein (P-gp) encoded by the ABCB1 gene causes multidrug resistance (MDR) in tumor cells by mediating drug efflux. P-gp in humans includes MDR1 and MDR3. MDR1 is widely distributed in humans, especially in the liver, intestine, blood-brain barrier, blood-testis barrier, placenta and kidney [22]. It is abundantly expressed in tumor cells and also distributed in normal tissue cells. The substrate of P-gp has a massive difference in structure and function [23]. It is difficult to define canonical properties of P-gp substrates, including small molecules, polysaccharides and proteins. Some involve aromatic heterocycles, some have positively charged nitrogen residues, but most are hydrophobic compounds. At present, many studies aim to classify and predict the substrate of P-gp by using quantitative structure-activity relationship, homology modeling, molecular docking and molecular dynamics simulation.
Since P-gp can be inhibited or induced, it has a significant influence on the pharmacokinetics of drugs. To reverse the MDR of tumor cells, the regulation mechanism of P-gp expression research is always going on. On the one hand, single nucleotide polymorphism (SNPs) is an important factor in the variation of P-gp protein, which affects the activity and function of P-gp protein. Among the three SNPs of the MDR1 gene coding region (C3435T, C1236T and G2677T/A), C3435T is the SNP most widely studied [24]. On the other hand, many medications can also regulate the expression of P-gp through physiological pathways. In order to solve the MDR caused by P-gp, the use of P-gp inhibitor is an effective solution. However, due to the diversity of binding sites on P-gp structure and the low resolution of protein crystal structure, the research progress is limited [25]. The existing P-gp inhibitors are classified into two types, synthetic and natural sources. Recently, the synthetic flavonoid dimer FD18 represents a new generation of low-toxic and high-affinity P-gp inhibitors [26]. Studies have shown that Janus kinase 2 (JAK2) inhibitors, such as XL019, CEP-33,779, can inhibit P-gp in drug-resistant cancer cells, and XL019 has higher specificity than verapamil [27]. Certain naturally derived drugs (glycosides, terpenes, alkaloids, flavonoids, and phenols) can also modulate the activity of drug-metabolizing enzymes and ABC transporters, and have an inhibitory effect on P-gp [28]. These naturally-derived compounds are less toxic in inhibiting P-gp expression, but they have limited physical and chemical properties. The emergence of new microparticle delivery systems has improved the physicochemical properties of natural medicines to some extent, which has contributed to the development of P-gp inhibitors of natural origin.
Multidrug resistance-associated proteins (MRPs): In addition to the up-regulation of MDR protein expression, increased expression of MRPs can also cause tumor resistance. MRPs belong to the ABCC family and contain 13 members, of which MRP1 to MRP9 are the major transporters indicated to cause MDR in tumor cells by extruding anticancer drugs out of the cell [29]. Different MRPs are expressed differentially in the liver, kidney, intestine and BBB. Anne T. Nies found that in hepatocellular carcinoma cell, the expression levels of MRP3 and MRP2 are higher, which possibly contributes to the intrinsic MDR phenotype of hepatocellular carcinoma [30]. In the normal liver cell, it may function as a compensatory mechanism to prevent the accumulation of anionic substrates in hepatocytes. The abnormal expression of transporters in these different sites, leads to the change of the pharmacokinetics of related drugs, which leads to the phenomenon of multi-drug resistance.
The human MRP/ABCC transporters except MRP9/ABCC12 are all able to transport organic anions, such as drugs conjugated to glutathione, sulfate or glucuronate [12]. The substrate specificity of MRP1 initially seemed to be similar to that of P-gp; MRP2 have a similar substrate specificity to MRP1, but it can transport cisplatin (CDDP) and some carcinogens [31]; MRP3 can transport organic compounds conjugated to glutathione, sulfate, or glucuronate [32]; MRP4 and MRP5 have attracted attention by their ability to transport cyclic nucleotides and many nucleotide analogs [33]; both the physiologic function and the potential involvement of MRP6 in drug resistance are still unclear. According to the substrate-binding transport mechanism of MRPs, many drugs can act as inhibitors to reverse MRPs-mediated MDR, such as cyclosporin A, rapamycin, rapalogs, deforolimus, everolimus and temsirolimus [34]. Inhibition of MRPs can alter the disposition of endogenous substrates, which may have toxicological implications including hyperbilirubinemia and cholestatic drug induced live injury (DILI). Therefore, it is necessary to carry out a simulation evaluation to avoid drug-induced liver damage caused by effective inhibitors.
Breast cancer resistance protein (BCRP): BCRP is another ATP-binding cassette (ABC) transporter capable of causing cancer drug resistance, formally designated as ABCG2. It was found in one such cell line (MCF-7/AdrVp) as a novel member of the G subfamily of ABC transporters. The greatest expression was seen in placental tissue. As a critical factor in preventing the absorption of toxic compounds, high ABCG2 expression has been found primarily in the placenta and intestine, but also in the brain endothelium, prostate, testes, ovaries, liver, adrenal gland, uterus, and central nervous system, which can effectively protect the maternal-fetal barrier, BBB and blood-testis barrier, just like P-gp, MRPs. In addition to the low levels of expression in normal tissue cells, BCRP is overexpressed in acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and solid tumor cells, in which the BCRP increase the course of chemotherapy drug efflux [35]. BCRP expression and activity have important effects on the disposal, clearance, accumulation of the drug [36].
BCRP can identify a wide range of substrates which is similar to P-gp and MRPs, including anthracenes (mitoxantrone), camptothecin (CPT) derivates (topotecan), polyglutamate (such as methotrexate), and nucleoside analogs (AZT), tyrosine kinase inhibitor (imatinib), conjugated organic anion, 3‑hydroxy-3-methylglutaryl-coenzyme A, reductase inhibitor (pitavastatin), carcinogen (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine) and flavonoids (xylogen) according to the review written by Mao [37]. Among these drugs, the anticancer drugs passed out by BCRP occupied major part one. Since BCRP plays a protective role in normal tissues, we need to pay attention to the possible adverse effects of inhibitors on normal cells during the development of BCRP inhibitors [38]. When using BCRP inhibitors in combination with other drugs, we should know the effects of BCRP-mediated drug interactions on clinical use.
In addition to the ABC transporters, the efflux transporter also comprises multidrug and toxin efflux transporters (MATEs). MATEs are mainly distributed in the small intestine, kidney and liver, and play an essential role in the transport of organic cations in vivo. Omote et al. [39] introduced the discovery, structure and function of MATEs.
2.2. Effects of transporters on drug transport and DDI
Both influx and efflux transporters are contained in various tissues in the human body, therefore, when we use drugs, we may face the following problems: (1). The drug is the substrate for uptake transporters and efflux transporters, the plasma concentration of the drug may be lowered due to an increase in drug efflux. (2). Two or more drugs are used at the same time, the crossover of the transporter substrate may cause drug interactions, which may impact on the efficacy of the drug, and may even increase the cytotoxicity of the drug.
Results suggest that coadministration of the human peptide transporter 1 (hPEPT1) substrates valacyclovir and cephalexin minimally reduced the acyclovir AUC, which contributes to significant inter-individual variability of aciclovir pharmacokinetics [40]. Besides, Masanori Nakakariya et al. [41] did a series of research on appropriate risk criteria for OATP inhibition based on the clinical relevancy between OATP inhibitors and drug-induced adverse effect. On the basis of pharmacogenetic and clinical DDI results [42], a total of 12 drugs (e.g. atenolol, celiprolol, and fexofenadine) were identified as possible clinical substrates of OATP2B1 and OATP1A2. Due to the substrate overlap in OATP and MRP2 in the basal side of the liver, OATP-mediated hepatic uptake and MRP2-mediated bile efflux synergy to achieve hepatobiliary transport. Wang et al. [43] revealed that antivirals, antibiotics, neuroprotective agents, and proton pump inhibitors all showed varying degrees of inhibition of OAT3-mediated uptake of dicaffeoylquinic acids (DCQAs) in vitro. Besides, Huo et al. [44] indicated that imipenem and cilastatin were substrates of hOAT1 and hOAT3, and cilastatin inhibited hOAT1/3-mediated transport of imipenem. Moreover, imipenem exhibited hOAT1/3-dependent cytotoxicity. Pitavastatin [45] and rosuvastatin are the substrates of OATP1B1 and BCRP, which remind us pay attention to the OATP1B1 or BCRP‐mediated DDI of pitavastatin or rosuvastatin in coadministration with other drugs. Concomitant drugs based on transporters have made breakthroughs in cancer treatment, Liu et al. [46] demonstrated that epigenetic activation of OCT2 by decitabine sensitizes RCC cells to oxaliplatin both in vitro and in xenografts. As a classic substrate of P-gp, paclitaxel can be out of cells and induces drug resistance, which affects dosing schedule and drug efficacy. Besides, Jörg et al. [47] summarized the transporters and DDI, in particular clinically observed DDI attributable to inhibition or induction of P-gp, BCRP, OATPs, OATs and OCT2. Based on the above analysis, the DDI potential of novel drugs was investigated in both nonclinical and clinical studies. Otherwise, the choice of drug dosage requires full consideration of the transporter's expression and activity when we choose the drug dose.
To summarize, defining the drug and related transporter transport mechanism, it has guiding significance for clinical combination, avoiding drug-drug and -food interactions. Beyond all doubt, it's helpful to reduce the toxicity of the drug and enhance its targeting and stability of the drug continuously. Research shows the DDI and -food (DFI) interactions on these transporters are considered to affect bioavailability of their substrate drugs. In the process of using multi-drug combination therapy, we need to carefully analyze the interactions between drugs based on drug transporters to avoid adverse reactions. The emergence of new nanotechnology has dramatically improved this phenomenon. The nanomaterials encapsulated drugs to achieve targeted drug delivery, enhance the stability of the drug in the body, and enhance the efficacy while avoiding toxicity. Compared with the combined use of traditional preparations, nanomaterials based on drug interaction have better pharmacokinetics and enhanced targeting. Therefore, nanomaterials loaded with multiple drugs are under constant development in the combined use.
3. Development of new drug delivery systems
A nano-targeted preparation, also known as a targeted nano-drug delivery system, refers to a drug delivery system that concentrates explicitly a drug on a specific tissue or organ by using a particular drug carrier or drug delivery technique. It has the characteristics of specificity, targeting, long duration of drug action, small side effects, and wide drug loading range. It also provides some benefits over conventional preparations, including increasing the solubility of hydrophobic drugs, enhancing the stability of the drug in vivo, and improving its epithelial permeability [48]. Compared with traditional preparations, the most prominent characteristic of targeted preparations is that drugs can be delivered to the target site to the maximum extent, the bioavailability of drugs can be improved, and the therapeutic effect can be maximized. In the continuous development of nanotechnology, the targeted preparations of nanoparticles, liposomes, polymer micelles, dendrimers and microspheres have become more and more prominent in cancer treatment. It has received extensive attention and research.
3.1. Microspheres
Microsphere refers to a microparticle system prepared by encapsulating or dispersing a drug in a polymer material, and its particle diameter is generally between 1 and 250 µm. The skeleton material of the microsphere mainly includes gelatin, alginate, chitosan and polylactic acid-co-glycolic acid (PLGA) [49]. Due to the protective effect of the carrier, the microsphere preparation can effectively improve the stability of the drug, and at the same time reduce the toxicity of the drug to the organ. Compared with traditional preparations, microspheres have a high surface area and volume ratio, which can be in close contact with the mucosal layer at the absorption site and prolong the retention time [50]. Therefore, it has the potential of targeted and controlled release drugs. Zhang et al. [51] encapsulated curcumin in microspheres to prepare curcumin-carrier chitosan microspheres (Cur-PS-CMs). The results showed that the microsphere preparation improved the water solubility and stability of curcumin. Besides, Cheng et al. [52] constructed curcumin-PLGA microspheres and studied its release in vitro. When microspheres are combined with an adhesive polymer (insulin, gentamicin, vasopressin, testosterone and its esters, and dopamine) to form mucoadhesive microspheres, it enhances drug absorption and bioavailability [53]. As we all known, the use of non-steroidal anti-inflammatory drug aspirin is limited by its sudden release. PLGA is selected as the drug carrier for the most developed microsphere preparations. However, PLGA microspheres also have many problems to be solved, such as severe drug release, incomplete drug release, low repetition rate and low yield. At present, for the preparation of microspheres, more attention is paid to the use of natural polymers. By using biodegradable materials, controlled release and sustained release can be realized and the safety of the preparation can be improved. Developmental applications in vaccination, by controlled release of embedded antigens, simulate repeated injections of conventional vaccines [54]. As for this problem, Sharma et al. [55] summarized the preparation methods and recent developments of natural polymer microspheres.
3.2. Nanoparticles
Nanoparticles are solid colloids with a particle size of 1–100 nm [56]. They can be divided into nanospheres and nanocapsules according to their skeleton morphology. As a representative of new drug carriers, nanoparticles have always been a research hotspot in particle delivery systems. Several anticancer drugs have successfully used nanomaterials to formulate corresponding nano-formulations, including paclitaxel, doxorubicin (DOX), dexamethasone and 5-fluorouracil (5-FU).
In order to improve the performance of nanoparticles, it is often necessary to modify the nanoparticles or use high-quality synthetic materials. Monsalve et al. [57] found that the interaction between cationic chitosan and the negative charge of brain endothelial cells makes anti-transferrin receptor antibody (OX26)-conjugated nanoparticles have much higher brain uptake than unmodified. It has the potential to be a drug that targets the brain. Polyethyl glycol 1000 (PEG1000) vitamin E succinate (D – alpha-tocopheryl polyethylene glycol 1000 succinate, TPGS) is a safe, nontoxic material, and it has an excellent effect on inhibiting P-gp [58]. Yang et al. [11] used dendrigraft poly-l-lysine-Polyethylene glycol/picrin-encapsulated siRNA (OATP2B1) nanoparticles which can deliver siRNA to the mouse brain to study the role of OATP2B1 in morphine brain transport and drug tolerance. As the study above, the novel nanoparticle successfully passed through the blood-brain barrier, and the targeting and gene silencing effects were better.
In the development of nanoparticles, inorganic nanoparticles have attracted attention due to their simple preparation process, the scalability of synthesis, and the controllability of shape and size. Inorganic nanoparticles refer to nanoparticles made of inorganic materials, including metal nanoparticles, carbon nanoparticles, silica nanoparticles, calcium carbonate nanoparticles, and layered double hydroxide nanoparticles for transporting siRNA. These inorganic nanoparticles are mainly used for imaging and diagnosis. The carbon nanotubes [59] are formed by crimping a single layer or a plurality of carbon atoms, and the tubular structure is a basic unit of a regular hexagon. The tube wall and the diameter can be divided into two types: the single-walled carbon nanotubes (SWCNT) and multi-walled carbon nanotubes (MWCNT). The comparative results confirmed that SWCNT modified electrodes can be exploited for better amplification signal as compared to MWCNT [60]. Modification of carbon nanotubes can reduce their toxicity and enhance the targeting of delivery, which improve the absorption capacity of drugs. For example, oxidized MWCNT loaded with DOX, conjugated to PEG and angiopep-2 can penetrate the BBB [61], which is an ideal dual targeting according to delivery system intracellular tracking (in vitro) and in vivo fluorescence imaging. Nowadays, many hollow inorganic nanoparticles are used for siRNA delivery. Varshosaz [62] introduced the application and development of inorganic nanoparticles in siRNA delivery.
A major limitation of nanoparticles is that they are easily conditioned by plasma proteins and cleared by the reticuloendothelial system, which results in ineffective tumor targeting and is one of the most challenging tasks in nanomedicine [63]. Thus, electrically neutral hydrophilic surface coatings covering nanoparticles can facilitate the development of nanoparticles for whole-body applications. PEG is the most commonly used hidden layer material, PEG-copolymer-coated iron oxide nanoparticles to avoid the reticuloendothelial system [64]. However, the ability of PEG-modified nanoparticles to absorb and escape in vivo is limited in the process of use. Now, more materials have been developed to replace PEG, such as chitosan, dextran, hyaluronic acid. Besides, Talkar et al. [65] focuses on the mechanism of nanoparticle's toxicity and the various toxicity issues associated with nanoparticles through mucosal routes. At present, research on nanoparticles has focused on surface modification of nanoparticles to improve drug targeting, stability, drug loading, and controlled release. At the same time, the nano-drug synthesis technology and processing technology are continuously optimized and industrialized.
3.3. Liposomes
Its unique phospholipid bilayer structure (similar to physiological membrane) had made it more compatible with the lipoidal layer of BBB and helps the drug to enter the brain. Agrawal et al. highlighted such modification made to the surface of the liposomes to increase the bioavailability of drug in brain region [66].
Liposomes are microscopic spheres formed by a lipid bilayer enveloping an aqueous phase medium, about 80–200 nm in size, similar in structure to biofilms. The formation of liposomes enables them to carry hydrophobic, hydrophilic and amphiphilic substance. Hydrophobic drugs can be directly inserted into liposomes, such as paclitaxel and amphotericin B. Water-soluble drugs, such as DOX, mitoxantrone, irinotecan, vincristine, need to be added to the buffer to be dissolved during liposome formation [67]. A number of nebulizable liposome formulations have reached clinical trials. For example, Arikace® (liposomal amikacin) and Pulmaquin® (liposomal ciprofloxacin) are antibacterial formulations currently in advanced stages of development [68]. Thermosensitive liposomes, pH-sensitive liposomes, cationic liposomes, ligand targeting liposomes, long-circulating liposomes are prepared by modifying the surface of conventional liposomes [69]. Thermosensitive liposomes achieve drug release by changing the phase transition temperature of synthetic liposome materials, but thermosensitive liposomes are still lacking in targeting, stability and encapsulation efficiency. The pH-sensitive liposomes are synthesized from liposomes modified with pH-sensitive groups, like pH-sensitive liposomes based on phosphatidylethanolamine. Regarding the mechanism of pH sensitivity, Yang et al. [70] demonstrated that the pH-related modifications in the surface microstructure of liposomes are related to the controlled release of tumor-targeted drug delivery systems. In order to prevent the rapid elimination of the mononuclear phagocytic system during liposome cycling, liposomes are usually linked to PEG to make long-circulating liposomes. The cationic liposome is composed of positively charged lipid vesicles, which is suitable for encapsulating proteins, nucleic acid drugs and gene therapy. Halevas et al. [71] synthesized a novel magnetic cationic liposome nanoformulation for encapsulation of a ternary V(IV)-curcumin-bipyridine (VCur) complex. Although cationic liposomes are often used for gene transfection in vitro, they have shortcomings such as low gene silencing activity and high systemic toxicity, which limit their clinical application. Ligand-targeted liposomes [72] are specifically ligand-modified (such as proteins, peptides, monoclonal antibodies, etc.) on the surface of liposomes, allowing the liposome preparation to be targeted after attaching to the drug. It increases the local concentration of the drug and prolongs the half-life of the drug. In addition to the lipids mentioned above, magnetic liposomes also have broad application prospects. Magnetic liposome encapsulates nano-magnetic particles into the liposome, which improves the controllability of the drug based on improved targeting. Like magnetic-immuno-loop-mediated isothermal amplification [73] as an alternative approach to be used in the ultrasensitive detection of P-gp.
At present, the research pays more attention to the combined application of several liposome modifications. Huang et al. [74] synthesized paclitaxel and docetaxel (DTX) prodrugs to prepare ephrin A2 targeted immunoliposomes, which have pH-sensitive properties at the same time. The active drug is continuously delivered to the solid tumor. Also, Pradhan et al. [75] synthesized a multifunctional DOX-loaded temperature-responsive magnetic liposome and surface-modified with folic acid (ligand) to target magnetic substances and biopharmaceuticals, which achieves targeted drug release in hyperthermia. Experiments have been carried out to connect pH-sensitive liposomes to polyethylene glycol to synthesize long-circulating pH-sensitive liposomes. This method not only retains the pH-sensitive characteristics, but also avoids the short-term residence time of pH-sensitive liposomes. The above various modified liposomes achieve better drug delivery targeting and delivery efficiency.
3.4. Dendrimers
Dendrimers are highly branched, monodisperse three-dimensional spherical polymers. Their structure can be controlled by different synthetic processes, including the shape, size, charge and solubility characteristics. The application of three common materials in delivery systems are PAMAM, poly(L-lysine) dendron (PLLD) and polypropylenimine (PPI). Dendrimers are easily modified on the surface and can be conjugated to drugs or genes. They have been widely used in biomedical fields [76], such as drug carriers, gene carriers, nuclear magnetic resonance contrast agents. The carrier system constructed from dendrimers has unique drug loading characteristics. A large number of functional groups on the surface can be covalently linked to the drug, and the hollow hydrophobic cavity inside the sphere can also embed hydrophobic small molecule drugs through hydrogen bonding, electrostatic interaction, and van der Waals force.
When designing the drug carrier, other groups can be added for surface modification. Surface functionalization of dendrimers can be carried out to renders excessive specificity to dendrimer and improve therapeutic efficiency. It has been reported [77] in the literature that dendrimers were pre-modified with arginine-glycine-aspartic (RGD) peptides, poly(ethylene glycol) chains, and acetyl groups to be endowed with cancer cell specificity and biocompatibility. Besides, studies have shown that lecithin modification on polyamidoamine (PAMAM) molecules can enhance the drug loading of PAMAM for DOX, and PEG, plectin-1 peptide, graphene oxide, hyaluronic acid can modify PAMAM, and then improve carrier targeting [78]. Besides, Li et al. [79] prepared a selenium-platinum polylysine dendrimer with anticancer activity by coordinating platinum and selenium-containing dendrimers to improve the drugs’ stability and monodispersity.
Compared with other nano-agents, dendrimers have higher transfection efficiency and stability than viral and liposome carriers, which can prolong the survival time of gene drugs in vivo. For example, the delivery of active siRNA by dendrimers increases the stability of siRNA in plasma and prolongs the half-life of such drugs. For the use of dendrimers in siRNA delivery and gene silencing, Tambea et al. [80] have been described in detail in the relevant literature. Also, Palmerston et al. [76] made a detailed review of the recent status of dendrimers as nanocarriers for nucleic acid drugs in delivery.
However, the cytotoxicity and hemolytic properties of dendrimers hinder the conversion of dendrimer formulations from clinical studies to clinical trials. To solve this problem, it has been found that the cationic charge is blocked by acetylation and hydroxylation, or the conjugation of dendrimer with PEG chain, lauroyl chain, G4 [81], can reduce the dendritic cytotoxicity. Marina A Dobrovolskaia [82] reviews the literature demonstrating how tuning dendrimer physicochemical properties influences their interaction with erythrocytes, platelets, plasma proteins, complement and coagulation systems. We still need to develop biocompatible and less toxic alternatives to overcome this limitation.
3.5. Polymer micelles
Polymer micelles are colloidal particles self-assembled by amphiphilic block copolymers in aqueous media, which typically has a narrow size distribution, Its diameter is about 10–100 nm. The micelles have the characteristics of excellent stability, strong drug-loading property and long cycle time. They can dissolve water-insoluble anticancer drugs and realize sustained release. It has become a research hotspot for cancer treatment and is usually used for the delivery of poorly soluble cytotoxic drugs. The hydrophilic shell structure of the micelle avoids the uptake of the reticuloendothelial system, thereby prolonging the circulation time of the drug in the body [83]. The most commonly used materials are PEG and Polyoxyethylene (PEO) [84]. The polymer blocks can be arranged in different ways: A-B type copolymer (deblock copolymer), A-B-A type copolymer (triblock copolymer) and graft copolymer [85].
Such amphiphilic nanoparticles have been shown to deliver several different types of therapeutic drugs (e.g. chemotherapeutic drugs, proteins, siRNA and DNA) to tumor cells. The polymer micelles can be loaded with drugs by chemical bonding, physical embedding, electrostatic interaction. The coated drugs can exert pharmacological effects through the degradation of the copolymer micelles or the micropore release of the copolymer carrier. The action time increases the bioavailability of the drug. For example, Rashed et al. [86] prepared a radioactive nimodipine lipid-pluronic micelle, after nasal administration, the test showed that the solubility, absorbance and cycle time of the drug in the micelles increased. Besides, polymer micelles have replaced viral vectors as ideal carriers for gene therapy, and micellar delivery enhances cellular uptake of drugs compared to naked gene drugs. Also, we can modify the surface of the micelle to prepare a drug carrier that specifically binds to the tumor cells, and reduce the toxic side effects on normal tissues. Transferrin-binding micelles are a promising brain-targeted drug delivery vehicle. Sonali et al. [87] developed a transferrin-bound tocopherol-polyethylene glycol-succinate copolymer micelle that targets DTX into brain cancer cells. The results showed that the transferrin-modified micelles showed higher drug concentration in brain tissue and significantly increased the BBB transport activity of DTX. Zhang et al. [88] prepared transferrin-modified paclitaxel-loaded polyphosphoester hybrid micelles, which showed strong cell uptake and brain accumulation ability. Usually, micelle stability and the affinity [89] between the loaded drug and the polymer are governed by the physical state of its hydrophobic core, the interactions between the lipophilic fractions and their molecular weight. An interesting approach for optimizing these properties and overcoming some of their disadvantages is the combination of two or more polymers in order to assemble polymeric mixed micelles [90].
3.6. Other drug delivery systems
In addition to the nano-agents described above, the drug-delivery that can enhance oral bioavailability enhancement of a poorly water-soluble drug drew deep attention. Respond to the need, self-emulsifying drug-delivery systems (SEDDS) have gained increasing attention for enhancing oral BA and reducing drug dose. SEDDS [91] have been widely employed to ameliorate the oral bioavailability of P-gp substrate drugs (e.g. curcumin) and to overcome MDR in cancer cells. It is expected that by formulating self-microemulsifying drug-delivery systems (SMEDDS), artemether and lumefantrine (low aqueous solubility) aqueous solubility and absorption will thus be enhanced [92]. Besides, nanocrystals, also known as nanosuspension, is a useful and successful approach for drug delivery. It is a promising method for improving the dissolution rate and enhancing the bioavailability of poorly soluble drugs. One of the most acceptable advantages of nanocrystals is their wide range of applicability such as oral delivery, ophthalmic delivery, pulmonary delivery, transdermal delivery, intravenous delivery and targeting (brain and tumor targeting). Cheng [93] focused on the nanocrystals technology and its application in pharmaceutical science. The downscaling of nanocrystals will enable rapid optimization of nanosuspension formulation in parallel screening design of preclinical developmental stage drug moieties [94]. In addition to new nanotechnology, implantable drug delivery systems offer new strategies for drug therapy, especially microfluidic chips produced using micro or nanotechnology, with flexible release characteristics that can be developed as active or passive delivery systems. It is hopeful to apply to topical therapy [[95],[96]]. This new type of micro-electrochemical system or MEMS-based drug delivery systems has been improved to overcome the problems related to conventional drug delivery [97]. Lee et al. [98] prepared an implantable micro-chip made of poly(methyl methacrylate) for controlled delivery of diclofenac sodium. Currently, implantable microchips still require many improvements, such as biocompatibility, accurate size and shape, patient compliance with drug delivery, and rate of drug delivery [99].
Despite the many advantages of the new drug carriers, only a few have been approved by the FDA, as shown in Table 3. Most polymers-proteins, polymers-drug conjugates, liposome formulations (including immunoliposomes, polymer micelles and polymer nanoparticles) are in clinical trials. Carbon nanotubes and dendrimers are currently not approved due to their unresolved toxicity. Nowadays, the synthesis and discovery of new nontoxic, biocompatible, biodegradable polymer drug carriers is the direction of our efforts.
Table 3.
FDA approved nano preparations.
| FDA approved year | Drug name | Active ingredients | Use |
|---|---|---|---|
| 1997 | Ambisome | Amphotericin B | Fungal infection |
| 2000 | RAPAMUNE® | Sirolimus | Immunosuppression of kidney transplantation |
| 2003 | Zirconium Oxide | Zirconia | Tooth repair |
| 2003 | Estrasorb™ | Estradiol | Menopause |
| 2003 | EMEND® | Aprepitant | feel sick and vomit |
| 2004 | TriCor® | Fenonote | reduce cholesterol |
| 2004 | MEGACE®ES | Megestrol acetate | Treatment of anorexia |
| 2004 | Vitoss | Myelosuppressive substitute | Bone defect repair |
| 2005 | Abraxane™ | Paclitaxel | Advanced breast cancer |
| 2005 | Doxil®, | Adriamycin | Ovarian cancer |
| 2015 | Oniivyde | Irinotecan | Pancreatic cancer |
| 2017 | Vyxeos | Daunorubicin and Cytarabine | Treatment of poor prognosis AML |
| 2018 | ARIKAYCE KIT | Amikacin | Nontuberculous mycobacteria (NTM) lung disease |
4. New delivery system application on targeting drug transporters
A large number of drug candidates have been produced for a variety of disease-causing mechanisms. However, during the use of these drugs, the drug may be affected by the presence of a transporter, which may affect the pharmacokinetics of the drug, and may even have an effect on the efficacy of the drug. It may cause adverse reactions due to the excessive accumulation of drugs in the body. Nano-targeted preparations provide a breakthrough point for drug development based on transport mechanism by virtue of its stability, targeting, slow-release, and long cycle time. In the clinical use of oral preparations, the bioavailability may be affected by the influx and efflux transporters in the intestine. The use of the nanoparticle system can prevent the drug from being recognized and transported by efflux transporters and improve its recognition by influx transporters. At present, many drugs have been made into nano-preparations to change their oral bioavailability. For example, polylactic acid (PLA) and polyethylene glycol graft copolymers are made into nano-particle PLA-g-PEG, which is expected to improve the oral bioavailability of the P-gp substrate (famotidine) [100], [101]. A novel drug delivery system for enoxaparin sodium-PLGA hybrid nanoparticles enhances the encapsulation of DOX hydrochloride efficiency and oral absorption [102]. In the process of studying the MDR mechanism based on the drug transporters, we need to silence the expression of a specific gene to study the expression of related proteins and mRNAs. We must deliver siRNA into the body to silence the target gene, while the nano-formulation exhibits good characteristics in the application of the delivery of the nucleic acid preparation. The application shows good characteristics, and the nano-formulation is less toxic than the traditional viral carrier-loaded nucleic acid drug. In the treatment of brain diseases, nanotechnology also has a good application prospect. Due to the expression of various efflux proteins in the blood-brain barrier, many drugs are difficult to cross the BBB and cannot function in the brain at therapeutic concentrations. The nano-preparation can be modified by the surface of the carrier to target the BBB, and play a role in the treatment of diseases such as Alzheimer's disease (AD), Parkinson`s disease (PD) and glioma.
In the single drug treatment process, drug resistance and the emergence of poor prognosis in each case have brought obstacles to clinical treatment. In order to fully utilize the efficacy of a single drug, a combination of drug-based interactions emerged. Combination therapy involves the incorporation of two active compounds into a single “hybrid” molecule to serve a dual role in increasing drug-target interactions. Combination therapy can achieve drug synergy by regulating multiple signaling pathways, reducing the incidence of adverse reactions, good prognosis, less drug resistance than single drug use, and gradually highlights advantages in disease treatment. In the development of the co-transfer formulation, the nano-formulation exhibits good bioavailability and compatibility. For example, polymeric micelles (PMs) [103] are self-assemblies of block copolymers providing numerous opportunities for drug delivery. Besides, the nano-encapsulated anticancer agent targeting specific tumor tissues can significantly optimize the therapeutic efficacy of the drug. The effectiveness of these preparations is attributed to a controlled metabolic process and longer in vivo circulation, which improves the stability of the drug in the body. Regarding the application of nanotechnology in combination therapy, this review focuses on nano-agents for targeted transporters, which are described in detail below.
4.1. Transporter-based nano-formulation against MDR
MDR as a key factor in the development of resistance to chemotherapeutic agents, is a serious problem that hampers the success of cancer pharmacotherapy. A common mechanism is the overexpression of certain transporters by cancer cells [104], such as P-gp, MRP1 and BCRP. Among the major transporters, P-gp-mediated MDR is the most important, and it has become a major obstacle in the clinical treatment of diseases. In order to control the effect of P-gp on pharmacokinetics, we must fully consider the targeting and stability of the drug. The new nanotechnology has a good application prospect in the fight against P-gp-mediated MDR. On the one hand, we can modify the drug by using nanotechnology to improve its fat solubility, so that the drug can be quickly absorbed and efflux based on P-gp is reduced. On the other hand, it can be combined with P-gp inhibitor drugs to inhibit P-gp efflux ability. In this way, it can improve the bioavailability of therapeutic drugs in the body, and thus ensure drug efficacy, avoid multi-drugs resistance.
To date, numerous P-gp inhibitors of natural as well as synthetic origin have been studied for reversal of acquired drug resistance. Though compared with synthetic origin P-gp inhibitors, P-gp inhibitors of natural origin have lower cytotoxicity such as, flavonoids, coumarins, terpenoids, alkaloids and saponins [105], low potency and unsatisfactory physicochemical properties limit the application of these inhibitors. Existing nano-formulations have improved this limitation to some extent. Taking DOX as an example, DOX is a commonly used anti-cancer drug but is susceptible to P-gp-mediated efflux in clinical use. To make cells resistant to it, the DOX nano preparations co-delivered with the inhibitor, which can improve the efficacy of DOX and avoid the generation of cell resistance. It is proven that natural alkaloids possess P-gp inhibition activity and potential for MDR reversal in cancer [106]. However, the use of alkaloids is limited in clinic due to the high hydrophobicity and poor active form's stability. To solve these drawbacks, the combination between poly(anhydride) nanoparticles and cyclodextrins was evaluated, like CPT cyclodextrin/poly(anhydride) nanoparticles [107]. Multiple studies investigated the reversal effect of cinobufagin (CBF) on P-gp -mediated MDR in colon cancer, and it could be further developed into a safe and potent P-gp modulator for combination use with anticancer drugs in cancer chemotherapy [108]. To achieve the sustained drug release, enhanced anticancer efficacy and low side effects in vivo, we can use near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the CBF [109]. The combination of P-gp inhibitors and drugs has been widely used in the fight against efflux transporter-mediated MDR. The protective effects of curcumin against DOX-induced toxicity and resistance have been proved. Dash et al. [28] formulated colon liposomes loaded with DOX and curcumin, successfully reversing DOX resistance in K562 cells with lower cytotoxicity. It has also been found that maximize P-gp inhibition and enhance DOX cytotoxicity in cancer cells by using a dual functional poly(N-(2-hydroxypropyl)methacrylamide) (PHPMA) conjugate carrying both the anticancer drug DOX and the P-gp inhibitor zosuquidar [110]. Maximize The N-(2-hydroxypropyl) methacrylamide copolymer conjugate is covalently bound to the anticancer drug DOX and the P-gp inhibitor reversin 121 through a degradable pH-sensitive bond, and this prepared nano-formulation can effectively make cells sensitive to DOX and overcomes MDR [111]. Shafiei-Irannejad et al. [112] co-encapsulated adriamycin and metformin in polylactide-co-glycolide-D-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles to reverse P-gp-induced MDR. Etoposide-loaded nanoparticles (ENP) and etoposide quercetin dual-loaded nanoparticles naturally derived (EQNP) flavonoids that inhibit P-gp efflux by a variety of pathways, such as direct cell membrane interactions, gene interference, and reduced transporter expression. The superiority of the EQNP to permeate deeper pharmacokinetic studies on rats revealed that EQNP exhibit a 2.4-fold increase in bioavailability of etoposide than ENP with no quercetin [113].
In addition to P-gp transporters, efflux transporters such as BCRP and MRPs are associated with MDR, and the down-regulation of uptake transporters in tumor cells is also a concern for the development of tumor therapeutics. The stability and slow-release characteristics of nano-formulations in delivery have also highlighted their advantages in the combination of targeting other transporters.
The dual inhibitors of P-gp and BCRP, elacridar, and certain TKIs (gefitinib and imatinib) are not specific for these two transporters and are prone to adverse effects on normal cells. It can be made into β-casein micelles, loaded with N-38 (a BCRP transport substrate) and BCRP efflux transport inhibitors, which can improve the targeting and synergy and overcome P-gp and BCRP-dependent MDR [114]. As a potential substrate for BCRP, mitoxantrone dihydrochloride (MTO) has lower cardiotoxicity than DOX and suits for the treatment of multiple solid tumors and cancers. Non-selective delivery of MTO is prone to various side effects. Therefore, the development of MTO nano-preparation has become particularly important. In the process of preparing nano-preparation, to improve the encapsulation efficiency of MTO, sodium deoxycholate (SDC)/ low molecular weight heparin-sodium deoxycholate conjugate [115] is required to prepare MTO-SDC composite nanoparticles, which are contained in the preparation. The combined delivery system of BCRP inhibitors has a longer cycle time and is effective against BCRP and P-gp-mediated efflux and improves efficacy.
Nanotechnology optimizes multiple mechanisms for drug delivery, which can target solid tumors to deliver drugs, and provide a broader space for gene therapy and the reversal of drug resistance. Nanotechnology has a promising application in reversing the MDR mediated by P-gp and BCRP. It can be used to load drugs through nanomaterials to achieve the targeted release of drugs and reduce the recognition of efflux transporters. In combination with an efflux transporter inhibitor, it can effectively increase the circulation time in the living body and enhance the activity of the drug. In summary, nano-formulations have outstanding contributions in reversing multi-drug resistance and achieving drug co-delivery. It is believed that with the continuous optimization of nano-targeted preparations, the development of tumor treatment drugs with low toxicity, high specificity and high bioavailability is just around the corner.
4.2. Transporter-based nucleic acid nano-preparation against cancer
A large number of studies have shown that the emergence of tumor resistance based on efflux transporters is often associated with the up-regulation of individual genes. To this end, we hope to achieve a reversal of tumor resistance by down-regulating this gene. One method is using siRNA to knock down gene expression, which can inhibit tumor proliferation, reduce the chemical resistance of cancer cells, and enhance the therapeutic effect of chemotherapy drugs. Based on the above mechanism, the combined application of nucleic acid drugs and chemotherapeutic drugs has emerged. Because nucleic acid is easy to be discharged by intravenous injection and is easy to be degraded by nuclease, how to stably deliver nucleic acid drugs [116] and chemotherapeutic drugs have become resistance to the development of such a combination. Nano-liposomes [117] can improve the stability and targeting of nucleic acid drugs through modification, and it can also load chemotherapy drugs to achieve the co-delivery of the two drugs. Studies show that delivery of siRNA in vitro and in vivo by using polyethyleneimine-capped porous silicon nanoparticles can silence MRP1 and inhibit proliferation in glioblastoma [118]. Xu et al. [119] demonstrated that chitosan-MRP1-siRNA nanoparticles can be used for the down-regulation of MRP1 expression in C6/VP16 Cells. In one study, a EphA10 antibody-conjugated pH-sensitive DOX, MDR1-siRNA coloading lipoplexes [120] were successfully loaded, which exhibited an incremental cellular uptake, enhanced P-gp downregulation efficacy, as well as a better cell cytotoxicity in human breast cancer cell. Risnayanti et al. [121] found that siRNA-loaded PLGA nanoparticles for co-delivering MDR1 and BCL2 siRNA provide an efficient combination therapy strategy to overcome the chemoresistance of paclitaxel and CDDP, by which MDR1 and BCL2 genes are silenced. Besides, therapy with siRNA and chemotherapeutics to overcome MDR is also a Hot trend, like PAMAM dendrimers co-loaded with DOX and therapeutic siRNA [122]. In vitro cytotoxicity assay demonstrated that the DOX/P-gp siRNA-loaded nano-micelles [123] showed much higher cytotoxicity in MCF-7 cells than DOX-loaded nano-micelles due to their synergistic killing effect and that the blank nano-micelles had good biocompatibility. Amreddy et al. [124] developed a folic acid (FA) conjugated polyamide amine dendrimer (Den) nanoparticle system, namely Den-PEI-CDDP-HuR-FA nanoparticles. Co-delivery of siRNA targeting human antigen R mRNA and CDDP to folate receptor alpha overexpressed H1299 lung cancer cells, and the results showed that the system was superior to the therapeutic agent alone. Palmerston et al. [76] introduced the role of PLL and PAMAM polymer-carriers in the combined application of DOX paclitaxel and nucleic acid preparations. PAMAM was modified by PEG, plectin-1 peptide, graphene oxide, and hyaluronic acid to increase the drug loading and targeting of the vector, and showed better serum stability and therapeutic effect during the loading of DOX and siRNA. In addition to siRNA, miRNAs are also important in regulating the expression of various proteins in the body, and the use of related miRNA preparations in combination with chemotherapeutic drugs faces the same problem. Zhang et al. [125] encapsulated miR-451 mimetic and poly(ADP-ribose) polymerase 1 (PARP1) inhibitor in the same cationic liposome and found that the combination of PARP inhibitor and phosphatidylinositol 3-kinase modulator can exert synergy compared to PARP inhibition alone. This finding was also confirmed by in vivo anti-tumor results from xenograft tumor models.
4.3. Transporter-based nano-formulation to improve cellular uptake
Nowadays, for the development of drugs, it is necessary to consider its bioavailability and metabolism in the body. The presence of transporters in the body mainly regulates the absorption, distribution, and excretion processes of drugs. It also determines whether the drug can function at the target site. Modification of the drug with the nano-preparation can improve the physicochemical properties of the drug and increase the exudation of the drug at the target site.
In the absence of a combination, the nano-formulation reduces the recognition and transport of the drug by P-gp and avoids the possible adverse effects of the combination. For example, anti-P-gp-mediated etoposide efflux is a major strategy to improve the bioavailability of etoposide. The use of PLGA nanoparticles to support etoposide can effectively improve the oral bioavailability of etoposide [113]. Docetaxel is a cytotoxic taxane, a poorly water-soluble drug, and a polybutylide-co-glycolide coated with a chitosan coating of DTX (PLGA)-nanoparticles can effectively improve the oral bioavailability of DTX [126]. In vitro experiments indicate that nquercetin (Qu)-grafted glyceryl caprylate-caprate (Gcc) are introduced novel lipid nanoparticles (LNPs) can achieve rapid and efficient drug release, and the Qu released concomitantly with the breakdown of disulfide bonds combines with P-gp and inhibits the drug efflux triggered by P-gp [127]. The PHPMA nano preparation loaded with the anticancer drug DOX can realize the sustained release and controlled release of the drug [110]. The thiolated microspheres were prepared by ionic gelation between alginic acid and calcium ions, and the lopinavir microspheres were synthesized using thiolated xyloglucan (TH-MPs) as a carrier. This approach not only improves the oral bioavailability of lopinavir, but also avoids the irritation caused by the combination of lopinavir and ritonavir [128]. Nanoparticles prepared from N-(2-phenoxyacetamide)−6-O-hydroxyacetyl chitosan enable paclitaxel to increase its paclitaxel without the addition of a P-gp inhibitor dissolution in the gastrointestinal tract. These nanoparticles can increase the local concentration of paclitaxel and improve the bioavailability of paclitaxel drugs to some extent [129]. In addition, intravenous injection of pegylated paclitaxel nanoparticles showed marked anticancer efficacy in nude mice bearing resistant NCI/ADR-RES tumors versus all control groups [130].
Platinum drugs have become one of the most widely used chemotherapeutic drugs in the clinic because of their unique anticancer mechanism and extensive anticancer spectrum. As a primary anticancer drug, platinum compounds are substrates for many influx and efflux transporters. However, platinum compounds have shortcomings such as poor pharmacokinetic characteristics, low cellular uptake, and rapid metabolic inactivation during use, which makes the drug concentration of the target site unsatisfactory and affects the chemotherapy effect. The ability of nano-formulations to modify blood circulation and protect therapeutic agents has made it a boon for cancer treatment. Based on the combination of oxaliplatin and nano preparations, the addition of targeting protein conjugation can effectively reduce the transport of oxaliplatin based on drug transporters by improving the activity and targeting of the pharmaceutical preparation, thereby obtaining a better therapeutic effect. For example, the combination of oxaliplatin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [131], which can be made into immune-hybrid nanoparticles. It had shown enhanced apoptotic activity comparable to oxaliplatin and unconjugated nanoparticles. In addition to TRAIL, the combination of oxaliplatin and anti-DR5 antibody enhances anticancer activity in a synergistic and site-specific manner, and the resulting oxaliplatin is produced by oxaliplatin and anti-DR5 antibody. MTT analysis of oxaliplatin immune nanoparticles (Co-Ox-AuNPs) showed that compared with oxaliplatin, the cell activity of the nanoparticles decreased by 3 times [132]. Nowadays, CDDP + DOX establishes a novel dual drug delivery system (DDDS) for cancer chemotherapy by using highly purified large-diameter multi-walled carbon nanotubes as a carrier. CDDP is encapsulated in the nanotube carrier by wet chemical method, and DOX is connected to the outer surface by non-covalent interaction [102]. Besides, in the development of drugs for the treatment of colon adenocarcinoma, the anti-proliferative activity of the nanoparticles can be significantly enhanced by the 5-FU and oxaliplatin in traditional chemotherapy. Not only that, lactoferrin nanoparticles significantly improve the pharmacokinetics, safety parameters and efficacy of 5-FU and oxaliplatin [133].
4.4. Transporter-based brain-targeting nano-formulation
At the BBB, several efflux transporters, such as the P-gp, BCRP and MRPs, are responsible for the transport of xenobiotics from the brain into the bloodstream or vice versa [134]. Organic cation/carnitine transporter 2 (OCTN2) expressed in the BBB has potential to facilitate the transfer of drugs across the biological barriers, and has emerged as a viable target for drug delivery [135]. Because a large number of efflux transporters exist in the blood-brain barrier, it's difficult for brain-targeted drugs to reach the target and produce therapeutic effects. Nanotechnology allows the creation of drug vehicles, functionalized with targeting ligands for binding specific BBB transporters, hence triggering the transport through this bio-barrier. Several lipophilic and cytotoxic drugs have proven to be actively drained by P-gp expressed at the luminal membrane of the brain capillary endothelial cells, resulting in the very low apparent BBB permeation of these P-gp substrates [136]. Nano-enabled delivery systems that utilize lipid-based carriers have been designed to improve drug delivery to the brain. It has been shown that DOX as liposomal encapsulated formulation is effective in treating patients with malignant glioma [137]. As erlotinib is a known substrate of P-gp and BCRP, the brain penetration of the tyrosine kinase inhibitor erlotinib is restricted [138]. To solve this problem, SushantL akkadwala et al. [139] developed a dual functionalized liposomal delivery system, which were modified with transferrin for receptor targeting and cell penetrating peptide PFVYLI to increase the translocation of DOX and Erlotinib across the BBB.
In addition to contributing to transporter-mediated drug transport, nanoparticles can also play a role in optimizing drug absorption in receptor-mediated drug delivery. B6 peptide-modified nanoparticle (B6-NP) [140] shows higher accumulation in brain capillary endothelial cells by lipid raft-mediated and clathrin-mediated endocytosis. If the B6 peptide is conjugated with sialic acid modified selenium nanoparticles, it exhibits higher permeability on the BBB and is expected to be a novel nanomedicine to improve disease in AD [140]. Also, Fan et al. [141] suggested curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide would be of potential and promising use for the treatment of AD. These findings suggest that B6-NP can serve as a promising drug delivery system that facilitates the delivery of neuropeptides to the brain, demonstrating the good performance of nano-drug delivery in delivering across the blood-brain barrier.
5. Conclusions
In summary, transporter-targeted nano-drug delivery system (nano-DDS) has emerged as a promising nanoplatform for efficient drug delivery. The drug transporter can affect the absorption and metabolism of the drug in the body, thereby affecting the effectiveness of the drug; the down-regulation of the uptake transporter and the up-regulation of the efflux transporter can cause cell resistance in the target organ or tumor; drug-transporter-based DDIs have inspired us to develop transporters as therapeutic targets and provide a basis for rational drug use. In order to increase the targeting and specificity of drug formulations that based on the transporter, the development of nano-formulations has improved the physicochemical properties of the corresponding drugs and reduced cytotoxicity. There is also a good delivery effect in the combination of the administration of the transporter. In this paper, we reviewed the classification of drug transporters, development of nano-DDS and recent developments in transporter-targeted nano-DDS.
Despite the potential advantages, the number of nanomedicine products on the market for tumor-targeted therapy is limited. While some products are about to be approved and may be marketed, most products used to treat cancer are still in preclinical development, such as the L-BLP25 liposome vaccine [142] and liposome Vincristine [143]. Due to the low permeability of nano-formulations in the body, a large number of nanomaterials are still in the phase of in vitro research, and a lot of work needs to be done before they can be developed into clinical drugs
Nano-formulations have advantages in both targeted transporter-mediated and receptor-mediated drug absorption. It has become a hotspot in targeted formulation research, which can help improve the bioavailability of traditional drugs and enhance targeting. At the same time, it reduces the toxicity and improves the physical and chemical properties of the drug. In the treatment of tumors, MDR based on transporters hinders the efficacy of anticancer drugs and is a major obstacle in the treatment of tumors. With the targeting and stability of nano-formulations, we can use nano-materials to load drugs to reverse the MDR based on drug transporters. In the development of nanomaterials, we must focus on their safety and targeting to avoid cytotoxicity and off-target distribution of nano-DDS in normal cells. In order to achieve the widespread application of transporter-targeted nano-DDS, we also need to gradually resolve the gap between preclinical animal models and clinical trials. To follow the trend of combined drug use, in the treatment of reversing tumor resistance, we should make full use of the drug-loading capabilities of nano-formulations to achieve combination therapy. Although the research of nano-preparation is less clinical, its excellent delivery performance is still worthy of our attention.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81773805 and 81973390) and the National Key Research and Development Program of China (2017YFC0908600).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2020.02.004.
Appendix. Supplementary materials
References
- 1.Cocucci E., Kim J.Y., Bai Y., Pabla N. Role of passive diffusion, transporters, and membrane trafficking-mediated processes in cellular drug transport. Clin Pharmacol Ther. 2017;101(1):121–129. doi: 10.1002/cpt.545. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto J., Stewart T., Sheng L., Li N., Bullock K., Song N. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Com. 2017;5(1):71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chavda H., Cn P., Anand I.S. Biopharmaceutics classification system. Sys Rev Pharm. 2010;1:62–69. [Google Scholar]
- 4.Lammers T., Kiessling F., Hennink W.E., Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 5.Estudante M., Morais J.G., Soveral G., Benet L.Z. Intestinal drug transporters: an overview. Adv Drug Deliv Rev. 2013;65(10):1340–1356. doi: 10.1016/j.addr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 6.De Boer A.G., Van Der Sandt I.C.J., Gaillard P.J. The role of drug transporters at the blood-brain barrier. Annu Rev Pharmacol Toxicol. 2003;43:629–656. doi: 10.1146/annurev.pharmtox.43.100901.140204. [DOI] [PubMed] [Google Scholar]
- 7.Halilbasic E., Claudel T., Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58(1):155–168. doi: 10.1016/j.jhep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motohashi H., Sakurai Y., Saito H., Masuda S., Urakami Y., Goto M. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13(4):866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- 9.Kalliokoski A., Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.König J., Seithel A., Gradhand U., Fromm M.F. Pharmacogenomics of human OATP transporters. N-S Arch Pharmacol. 2006;372(6):432–443. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z.Z., Li L., Wang L., Xu M.C., An S., Jiang C. siRNA capsulated brain-targeted nanoparticles specifically knock down OATP2B1 in mice: a mechanism for acute morphine tolerance suppression. Sci Rep. 2016;6:33338. doi: 10.1038/srep33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S., Wang L.L., Di Y., Xue C., Duan W., Li C.G. Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem. 2008;15:1981–2039. doi: 10.2174/092986708785132870. [DOI] [PubMed] [Google Scholar]
- 13.Roth M., Obaidat A., Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Brit J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizwan A.N., Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res-dordrv. 2007;24(3):450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- 15.Burckhardt G. Drug transport by organic anion transporters (OATs) Pharmacol Therapeut. 2012;136(1):106–130. doi: 10.1016/j.pharmthera.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Nieskens T.T.G., Peters J.G.P., Schreurs M J., Smits N., Woestenenk R., Jansen K. A human renal proximal tubule cell line with stable organic anion transporter 1 and 3 expression predictive for antiviral-induced toxicity. AAPS J. 2016;18(2):465–475. doi: 10.1208/s12248-016-9871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller J., Lips K.S., Metzner L., Neubert R.H.H., Koepsell H., Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem Pharmacol. 2005;70(12):1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Koepsell H., Lips K., Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res-dordrv. 2007;24(7):1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- 19.Koepsell H. Role of organic cation transporters in drug–drug interaction. Expert Opin Drug Met. 2015;11:1–15. doi: 10.1517/17425255.2015.1069274. [DOI] [PubMed] [Google Scholar]
- 20.Umehara K.I., Iwatsubo T., Noguchi K., Usui T., Kamimura H. Effect of cationic drugs on the transporting activity of human and rat OCT/Oct 1–3 in vitro and implications for drug–drug interactions. Xenobiotica. 2008;38(9):1203–1218. doi: 10.1080/00498250802334409. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Aliaga I., Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol Sci. 2002;23(9):434–440. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- 22.Vasiliou V., Vasiliou K., Nebert D.W. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3(3):281. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demeule M., Régina A., Jodoin J., Laplante A., Dagenais C., Berthelet F. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood–brain barrier. Vasc Pharmacol. 2002;38(6):339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 24.Samanian S., Mahjoubi F., Mahjoubi B., Mirzaee R., Azizi R. MDR1 gene polymorphisms: possible association with its expression and clinicopathology characteristics in colorectal cancer patients. Asian Pac J Cancer P. 2011;12:3141–3145. [PubMed] [Google Scholar]
- 25.Cantore M., Leopoldo M., Berardi F., Perrone R., Colabufo N. Design and synthesis of new selective P-gp substrates and inhibitors. Curr Pharm Des. 2016;22:1. doi: 10.2174/1381612822666160810114008. -1. [DOI] [PubMed] [Google Scholar]
- 26.Cui J., Liu X., Chow L.M.C. Flavonoids as P-gp inhibitors: a systematic review of SARs. Curr Med Chem. 2019;26(25):4799–4831. doi: 10.2174/0929867325666181001115225. [DOI] [PubMed] [Google Scholar]
- 27.Cheon J.H., Kim J.Y., Lee B.M., Kim H.S., Yoon S. P-gp inhibition by XL019, a JAK2 inhibitor, increases apoptosis of vincristine-treated resistant kbv20c cells with increased p21 and pH2AX expression. Anticancer Res. 2017;37(12):6761–6769. doi: 10.21873/anticanres.12136. [DOI] [PubMed] [Google Scholar]
- 28.Dash T.K., Konkimalla V.B. Selection of P-Glycoprotein inhibitor and formulation of combinational nanoformulation containing selected agent curcumin and dox for reversal of resistance in K562 cells. Pharm Res. 2017;34(8):1741–1750. doi: 10.1007/s11095-017-2182-7. [DOI] [PubMed] [Google Scholar]
- 29.Sodani K., Patel A., Kathawala R.J., Chen Z.S. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31(2):58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nies A., Koenig J., Pfannschmidt M., Klar E., Hofmann W., Keppler D. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int J Cancer. 2001;94:492–499. doi: 10.1002/ijc.1498. [DOI] [PubMed] [Google Scholar]
- 31.Leslie E.M., Deeley R.G., Cole S.P.C. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharm. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Scheffer G., Kool M., Heijn M., Haas M., Pijnenborg A., Wijnholds J. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and MDR3 P-glycoprotein with a panel of monoclonal antibodies. Cancer Res. 2000;60:5269–5277. [PubMed] [Google Scholar]
- 33.Berthier J., Arnion H., Saint-Marcoux F., Picard N. Multidrug resistance-associated protein 4 in pharmacology: overview of its contribution to pharmacokinetics, pharmacodynamics and pharmacogenetics. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.06.015. -40. [DOI] [PubMed] [Google Scholar]
- 34.Peterson B.G., Tan K.W., Osa-Andrews B., Iram S.H. High-content screening of clinically tested anticancer drugs identifies novel inhibitors of human MRP1 (ABCC1) Pharmacol Res. 2017;119:313–326. doi: 10.1016/j.phrs.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi T., Ross D.D. Breast cancer resistance protein (BCRP/ABCG2): its role in multidrug resistance and regulation of its gene expression. Chin J Cancer. 2012;31(2):73–99. doi: 10.5732/cjc.011.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickel S., Selo M.A., Fallack J., Clerkin C.G., Huwer H., Schneider-Daum N. Expression and activity of breast cancer resistance protein (BCRP/ABCG2) in human distal lung epithelial cells in vitro. Pharm Res. 2017;34(12):2477–2487. doi: 10.1007/s11095-017-2172-9. [DOI] [PubMed] [Google Scholar]
- 37.Mao Q., Unadkat J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport–an update. AAPS J. 2015;17(1):65–82. doi: 10.1208/s12248-014-9668-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staud F., Pavek P. Breast cancer resistance protein (BCRP/ABCG2) Int J Biochem Cell Biol. 2005;37(4):720–725. doi: 10.1016/j.biocel.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Omote H., Hiasa M., Matsumoto T., Otsuka M., Moriyama Y. The mate proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci. 2006;27(11):587–593. doi: 10.1016/j.tips.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Phan D.D., Chin-Hong P., Lin E.T., Anderle P., Sadee W., Guglielmo B.J. Intra- and interindividual variabilities of valacyclovir oral bioavailability and effect of coadministration of an hPEPT1 inhibitor. Antimicrob Agents Chemother. 2003;47(7):2351–2353. doi: 10.1128/AAC.47.7.2351-2353.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakakariya M., Goto A., Amano N. Appropriate risk criteria for oatp inhibition at the drug discovery stage based on the clinical relevancy between OATP inhibitors and drug-induced adverse effect. Drug Metab Pharmacokinet. 2016;31(5):333–339. doi: 10.1016/j.dmpk.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Yu J., Zhou Z., Tay-Sontheimer J., Levy R.H., Ragueneau-Majlessi I. Intestinal drug interactions mediated by OATPs: a systematic review of preclinical and clinical findings. J Pharm Sci. 2017;106(9):2312–2325. doi: 10.1016/j.xphs.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Ren J., Sun Q., Zhang Z., Lin Y., Deng S. Organic anion transporter 3 (OAT3)-mediated transport of dicaffeoylquinic acids and prediction of potential drug-drug interaction. Eur J Pharm Sci. 2019;133:95–103. doi: 10.1016/j.ejps.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Huo X., Meng Q., Wang C., Zhu Y., Liu Z., Ma X. Cilastatin protects against imipenem-induced nephrotoxicity via inhibition of renal organic anion transporters (OATs) Acta Pharm Sin B. 2019;9(5):986–996. doi: 10.1016/j.apsb.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hua W.J., Hua W.X., Fang H.J. The role of OATP1B1 and BCRP in pharmacokinetics and DDI of novel statins. Cardiovasc Ther. 2012;30(5):e234–ee41. doi: 10.1111/j.1755-5922.2011.00290.x. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y., Zheng X., Yu Q., Wang H., Tan F., Zhu Q. Epigenetic activation of the drug transporter OCT2 sensitizes renal cell carcinoma to oxaliplatin. Sci Transl Med. 2016;8(348):348ra97. doi: 10.1126/scitranslmed.aaf3124. [DOI] [PubMed] [Google Scholar]
- 47.König J., Müller F., Fromm M.F. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65(3):944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 48.Yu H.P., Aljuffali I.A., Fang J.Y. Injectable drug-loaded nanocarriers for lung cancer treatments. Curr Pharm Des. 2017;23(3):481–494. doi: 10.2174/1381612822666161027113654. [DOI] [PubMed] [Google Scholar]
- 49.Wang F., Liu X., Yuan J., Yang S., Li Y., Gao Q. Synthesis and characterization of poly(lactic acid-co-glycolic acid) complex microspheres as drug carriers. J Biomater Appl. 2016;31(4):544–552. doi: 10.1177/0885328216657548. [DOI] [PubMed] [Google Scholar]
- 50.Andhariya J.V., Burgess D.J. Recent advances in testing of microsphere drug delivery systems. Expert Opin Drug Deliv. 2016;13(4):593–608. doi: 10.1517/17425247.2016.1134484. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J., Tang Q., Xu X., Li N. Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int J Pharm. 2013;448(1):168–174. doi: 10.1016/j.ijpharm.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 52.Cheng Q., Du L., Meng L., Han S., Wei T., Wang X. The promising nanocarrier for doxorubicin and siRNA co-delivery by PDMAEMA-based amphiphilic nanomicelles. ACS Appl Mater Interfaces. 2016;8(7):4347–4356. doi: 10.1021/acsami.5b11789. [DOI] [PubMed] [Google Scholar]
- 53.Patil S.B., Sawant K.K. Mucoadhesive microspheres: a promising tool in drug delivery. Curr Drug Deliv. 2008;5(4):312–318. doi: 10.2174/156720108785914970. [DOI] [PubMed] [Google Scholar]
- 54.Lin C.Y., Lin S.J., Yang Y.C., Wang D.Y., Cheng H.F., Yeh M.K. Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases. Hum Vaccin Immunother. 2015;11(3):650–656. doi: 10.1080/21645515.2015.1009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farooq U., Malviya R., Sharma P.K. Advancement in microsphere preparation using natural polymers and recent patents. Recent Pat Drug Deliv Formul. 2014;8(2):111–125. doi: 10.2174/1872211308666140218110520. [DOI] [PubMed] [Google Scholar]
- 56.Young S.W.S., Stenzel M., Yang J.L. Nanoparticle-siRNA: a potential cancer therapy? Crit Rev Oncol Hematol. 2016;98:159–169. doi: 10.1016/j.critrevonc.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 57.Yuliana M., Giovanni T., Barbara R., Daniela B., Antonietta V., Michele Z. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine. 2015;10(11):1735–1750. doi: 10.2217/nnm.15.29. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Tong D., Che D., Pei B., Xia X., Yuan G. Ascorbyl palmitate/d-α-tocopheryl polyethylene glycol 1000 succinate monoester mixed micelles for prolonged circulation and targeted delivery of compound K for antilung cancer therapy in vitro and in vivo. Int J Nanomedicine. 2017;12:605–614. doi: 10.2147/IJN.S119226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rode A., Sharma S., Mishra D.K. Carbon nanotubes: classification, method of preparation and pharmaceutical application. Curr Drug Deliv. 2018;15(5):620–629. doi: 10.2174/1567201815666171221124711. [DOI] [PubMed] [Google Scholar]
- 60.Alhans R., Singh A., Singhal C., Narang J., Wadhwa S., Mathur A. Comparative analysis of single-walled and multi-walled carbon nanotubes for electrochemical sensing of glucose on gold printed circuit boards. Mater Sci Eng C Mater Biol Appl. 2018;90:273–279. doi: 10.1016/j.msec.2018.04.072. [DOI] [PubMed] [Google Scholar]
- 61.Ren J., Shen S., Wang D., Xi Z., Guo L., Pang Z. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33(11):3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Varshosaz J., Taymouri S. Hollow inorganic nanoparticles as efficient carriers for siRNA delivery: a comprehensive review. Curr Pharm Des. 2015;21(29):4310–4328. doi: 10.2174/1381612821666150901103937. [DOI] [PubMed] [Google Scholar]
- 63.Rao L., Xu J.H., Cai B., Liu H., Li M., Jia Y. Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake. Nanotechnology. 2016;27(8) doi: 10.1088/0957-4484/27/8/085106. -06. [DOI] [PubMed] [Google Scholar]
- 64.Gómez-Vallejo V., Puigivila M., Plaza-García S., Szczupak B., Piñol R., Murillo J.L. PEG-copolymer-coated iron oxide nanoparticles that avoid the reticuloendothelial system and act as kidney MRI contrast agents. Nanoscale. 2018;10(29):14153–14164. doi: 10.1039/c8nr03084g. [DOI] [PubMed] [Google Scholar]
- 65.Talkar S., Dhoble S., Majumdar A., Patravale V. Transmucosal nanoparticles: toxicological overview. Adv Exp Med Biol. 2018;1048:37–57. doi: 10.1007/978-3-319-72041-8_3. [DOI] [PubMed] [Google Scholar]
- 66.Agrawal M., Ajazuddin, Tripathi D.K., Saraf S., Saraf S., Antimisiaris S.G. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer's disease. J Control Release. 2017;260:61–77. doi: 10.1016/j.jconrel.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 67.Iqbal J., Anwar F., Afridi S. Targeted drug delivery systems and their therapeutic applications in cancer and immune pathological conditions. Infect Disord Drug Targets. 2017;17(3):149–159. doi: 10.2174/1871526517666170606102623. [DOI] [PubMed] [Google Scholar]
- 68.Elhissi A. Liposomes for pulmonary drug delivery: the role of formulation and inhalation device design. Curr Pharm Des. 2017;23(3):362–372. doi: 10.2174/1381612823666161116114732. [DOI] [PubMed] [Google Scholar]
- 69.Li M., Du C., Guo N., Teng Y., Meng X., Sun H. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Fan Y., Chen C., Huang Y., Zhang F., Lin G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surf B Biointerfaces. 2017;151:19–25. doi: 10.1016/j.colsurfb.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 71.Halevas E., Mavroidi B., Swanson C.H., Smith G.C., Moschona A., Hadjispyrou S. Magnetic cationic liposomal nanocarriers for the efficient drug delivery of a curcumin-based vanadium complex with anticancer potential. J Inorg Biochem. 2019;199:110778. doi: 10.1016/j.jinorgbio.2019.110778. [DOI] [PubMed] [Google Scholar]
- 72.Noble C.O., Kirpotin D.B., Hayes M.E., Mamot C., Hong K., Park J.W. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8(4):335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- 73.Cao H., Fang X., Liu P., Li H., Chen W., Liu B. Magnetic-Immuno-Loop-Mediated isothermal amplification based on dna encapsulating liposome for the ultrasensitive detection of P-glycoprotein. Sci Rep. 2017;7(1):9312. doi: 10.1038/s41598-017-10133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Z.R., Tipparaju S.K., Kirpotin D.B., Pien C., Kornaga T., Noble C.O. Formulation optimization of an ephrin A2 targeted immunoliposome encapsulating reversibly modified taxane prodrugs. J Control Release. 2019;310:47–57. doi: 10.1016/j.jconrel.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Pradhan P., Giri J., Rieken F., Koch C., Mykhaylyk O., Döblinger M. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121. doi: 10.1016/j.jconrel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Palmerston Mendes L., Pan J., Torchilin V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules. 2017;22(9):1401. doi: 10.3390/molecules22091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H., Wang J. Loading IR820 using multifunctional dendrimers with enhanced stability and specificity. Pharmaceutics. 2018;10(3):77. doi: 10.3390/pharmaceutics10030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eichman J.D., Bielinska A.U., Kukowska-Latallo J.F., Baker J.R. The use of pamam dendrimers in the efficient transfer of genetic material into cells. Pharm Sci Technolo Today. 2000;3(7):232–245. doi: 10.1016/s1461-5347(00)00273-x. [DOI] [PubMed] [Google Scholar]
- 79.Li T., Smet M., Dehaen W., Xu H. Selenium-Platinum coordination dendrimers with controlled anti-cancer activity. ACS Appl Mater Interfaces. 2016;8(6):3609–3614. doi: 10.1021/acsami.5b07877. [DOI] [PubMed] [Google Scholar]
- 80.Tambe V., Thakkar S., Raval N., Sharma D., Kalia K., Tekade R.K. Surface engineered dendrimers in sirna delivery and gene silencing. Curr Pharm Des. 2017;23(20):2952. doi: 10.2174/1381612823666170314104619. [DOI] [PubMed] [Google Scholar]
- 81.Somani S., Laskar P., Altwaijry N., Kewcharoenvong P., Irving C., Robb G. PEGylation of polypropylenimine dendrimers: effects on cytotoxicity, DNA condensation, gene delivery and expression in cancer cells. Sci Rep. 2018;8(1):9410. doi: 10.1038/s41598-018-27400-6. -10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dobrovolskaia M.A. Dendrimers effects on the immune system: insights into toxicity and therapeutic utility. Curr Pharm Des. 2017;23(21):3134–3141. doi: 10.2174/1381612823666170309151958. [DOI] [PubMed] [Google Scholar]
- 83.Yousefpour Marzbali M., Yari Khosroushahi A. Polymeric micelles as mighty nanocarriers for cancer gene therapy: a review. Cancer Chemother Pharmacol. 2017;79(4):637–649. doi: 10.1007/s00280-017-3273-1. [DOI] [PubMed] [Google Scholar]
- 84.Rey-Rico A., Cucchiarini M. PEO-PPO-PEO tri-block copolymers for gene delivery applications in human regenerative medicine-an overview. Int J Mol Sci. 2018;19(3):775. doi: 10.3390/ijms19030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oerlemans C., Bult W., Bos M., Storm G., Nijsen J.F.W., Hennink W.E. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589. doi: 10.1007/s11095-010-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rashed H.M., Shamma R.N., Basalious E.B. Contribution of both olfactory and systemic pathways for brain targeting of nimodipine-loaded lipo-pluronics micelles: in vitro characterization and in vivo biodistribution study after intranasal and intravenous delivery. Drug Deliv. 2016;24(1):181–187. doi: 10.1080/10717544.2016.1236848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sonali, Agrawal P., Singh R.P., Rajesh C.V., Singh S., Vijayakumar M.R. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23(5):1788–1798. doi: 10.3109/10717544.2015.1094681. [DOI] [PubMed] [Google Scholar]
- 88.Zhang P., Hu L., Yin Q., Zhang Z., Feng L., Li Y. Transferrin-conjugated polyphosphoester hybrid micelle loading paclitaxel for brain-targeting delivery: synthesis, preparation and in vivo evaluation. J Control Release. 2012;159(3):429–434. doi: 10.1016/j.jconrel.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 89.Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J Drug Target. 2014;22(7):576–583. doi: 10.3109/1061186X.2014.934688. [DOI] [PubMed] [Google Scholar]
- 90.Cagel M., Tesan F.C., Bernabeu E., Salgueiro M.J., Zubillaga M.B., Moretton M.A. Polymeric mixed micelles as nanomedicines: achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 91.Aboulfotouh K., Allam A.A., El-Badry M., El-Sayed A.M. Self-emulsifying drug-delivery systems modulate P-glycoprotein activity: role of excipients and formulation aspects. Nanomedicine (Lond) 2018:0354. doi: 10.2217/nnm-2017-0354. [DOI] [PubMed] [Google Scholar]
- 92.Bhandari S., Rana V., Tiwary A.K. Antimalarial solid self-emulsifying system for oral use: in vitro investigation. Ther Deliv. 2017;8(4):201–213. doi: 10.4155/tde-2016-0092. [DOI] [PubMed] [Google Scholar]
- 93.Zhongyao C., Yumei L., Zul K., Xin M., Jianjun C., Xinbo Z. Nanocrystals technology for pharmaceutical science. Curr Pharm Des. 2018;24(21):2497–2507. doi: 10.2174/1381612824666180518082420. [DOI] [PubMed] [Google Scholar]
- 94.Pardhi V.P., Verma T., Flora S.J.S., Chandasana H., Shukla R. Nanocrystals: an overview of fabrication, characterization and therapeutic applications in drug delivery. Curr Pharm Des. 2018;24(43):5129–5146. doi: 10.2174/1381612825666190215121148. [DOI] [PubMed] [Google Scholar]
- 95.Staples M. Microchips and controlled-release drug reservoirs. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):400–417. doi: 10.1002/wnan.93. [DOI] [PubMed] [Google Scholar]
- 96.Lee M.S., Yao D.J., Hsu W. An implantable drug-delivery system on a chip. Curr Top Med Chem. 2015;15(15):1516–1524. doi: 10.2174/1568026615666150414121427. [DOI] [PubMed] [Google Scholar]
- 97.Sutradhar K.B., Sumi C.D. Implantable microchip: the futuristic controlled drug delivery system. Drug Deliv. 2016;23(1):1–11. doi: 10.3109/10717544.2014.903579. [DOI] [PubMed] [Google Scholar]
- 98.Lee S.H., Park M., Park C.G., Kim B.H., Lee J., Choi S. Implantable micro-chip for controlled delivery of diclofenac sodium. J Control Release. 2014;196:52–59. doi: 10.1016/j.jconrel.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 99.Eltorai A.E.M., Fox H., Mcgurrin E., Guang S. Microchips in medicine: current and future applications. Biomed Res Int. 2016;2016 doi: 10.1155/2016/1743472. -72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mokhtar M., Gosselin P., Lacasse F., Hildgen P. Design of PEG-grafted-PLA nanoparticles as oral permeability enhancer for P-gp substrate drug model famotidine. J Microencapsul. 2017;34(1):91–103. doi: 10.1080/02652048.2017.1290155. [DOI] [PubMed] [Google Scholar]
- 101.Essa S., Rabanel J.M., Hildgen P. Effect of polyethylene glycol (PEG) chain organization on the physicochemical properties of poly(D, L-lactide) (PLA) based nanoparticles. Eur J Pharm Biopharm. 2010;75(2):96–106. doi: 10.1016/j.ejpb.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Yang T., Wu Z., Wang P., Mu T., Qin H., Zhu Z. A large-inner-diameter multi-walled carbon nanotube-based dual-drug delivery system with pH-sensitive release properties. J Mater Sci Mater Med. 2017;28(7):110. doi: 10.1007/s10856-017-5920-9. -10. [DOI] [PubMed] [Google Scholar]
- 103.Thipparaboina R., Chavan R.B., Kumar D., Modugula S., Shastri N.R. Micellar carriers for the delivery of multiple therapeutic agents. Colloids Surf B Biointerfaces. 2015;135:291–308. doi: 10.1016/j.colsurfb.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 104.Choi Y.H., Yu A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abdallah H.M., Al-Abd A.M., El-Dine R.S., El-Halawany A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. doi: 10.1016/j.jare.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joshi P., Vishwakarma R.A., Bharate S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur J Med Chem. 2017;138:273–292. doi: 10.1016/j.ejmech.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 107.Huarte J., Espuelas S., Lai Y., He B., Tang J., Irache J.M. Oral delivery of camptothecin using cyclodextrin/poly(anhydride) nanoparticles. Int J Pharm. 2016;506(1–2):116–128. doi: 10.1016/j.ijpharm.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 108.Qi F., Li A., Inagaki Y., Kokudo N., Tamura S., Nakata M. Antitumor activity of extracts and compounds from the skin of the toad BUFO bufo gargarizans cantor. Int Immunopharmacol. 2011;11(3):342–349. doi: 10.1016/j.intimp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Ren W., Chen S., Liao Y., Li S., Ge J., Tao F. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surf B Biointerfaces. 2019;174:384–392. doi: 10.1016/j.colsurfb.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 110.Battistella C., Klok H.A. Reversion of P-gp-Mediated drug resistance in ovarian carcinoma cells with PHPMA-Zosuquidar conjugates. Biomacromolecules. 2017;18(6):1855–1865. doi: 10.1021/acs.biomac.7b00291. [DOI] [PubMed] [Google Scholar]
- 111.Sivak L., Subr V., Tomala J., Rihova B., Strohalm J., Etrych T. Overcoming multidrug resistance via simultaneous delivery of cytostatic drug and P-glycoprotein inhibitor to cancer cells by HPMA copolymer conjugate. Biomaterials. 2017;115:65–80. doi: 10.1016/j.biomaterials.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 112.Shafiei-Irannejad V., Samadi N., Salehi R., Yousefi B., Rahimi M., Akbarzadeh A. Reversion of multidrug resistance by co-encapsulation of doxorubicin and metformin in poly(lactide-co-glycolide)-D-α-tocopheryl polyethylene glycol 1000 succinate nanoparticles. Pharm Res. 2018;35(6):119. doi: 10.1007/s11095-018-2404-7. -19. [DOI] [PubMed] [Google Scholar]
- 113.Fatma S., Talegaonkar S., Iqbal Z., Panda A.K., Negi L.M., Goswami D.G. Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: an in vitro, ex vivo and in vivo investigations. Drug Deliv. 2016;23(2):500–511. doi: 10.3109/10717544.2014.923956. [DOI] [PubMed] [Google Scholar]
- 114.Bar-Zeev M., Kelmansky D., Assaraf Y.G., Livney Y.D. β-Casein micelles for oral delivery of SN-38 and elacridar to overcome BCRP-mediated multidrug resistance in gastric cancer. Eur J Pharm Biopharm. 2018;133:240–249. doi: 10.1016/j.ejpb.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 115.Hu T., Cao H., Yang C., Zhang L., Jiang X., Gao X. LHD-Modified mechanism-based liposome coencapsulation of mitoxantrone and prednisolone using novel lipid bilayer fusion for tissue-specific colocalization and synergistic antitumor effects. ACS Appl Mater Interfaces. 2016;8(10):6586–6601. doi: 10.1021/acsami.5b10598. [DOI] [PubMed] [Google Scholar]
- 116.Buddolla A.L., Kim S. Recent insights into the development of nucleic acid-based nanoparticles for tumor-targeted drug delivery. Colloids Surf B Biointerfaces. 2018;172:315–322. doi: 10.1016/j.colsurfb.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J., Weng G., Li J., Zhu J., Zhao J. Polyester-based nanoparticles for nucleic acid delivery. Mater Sci Eng C Mater Biol Appl. 2018;92:983–994. doi: 10.1016/j.msec.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 118.Tong W.Y., Alnakhli M., Bhardwaj R., Apostolou S., Sinha S., Fraser C. Delivery of siRNA in vitro and in vivo using PEI-capped porous silicon nanoparticles to silence MRP1 and inhibit proliferation in glioblastoma. J Nanobiotechnology. 2018;16(1):38. doi: 10.1186/s12951-018-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu H., Nie X., Wu L., Zhu X., Yi W., Huang S. Down-regulation of MRP1 expression in C6/VP16 cells by chitosan-mrp1-sirna nanoparticles. Cell Biochem Biophys. 2015;72(1):227–233. doi: 10.1007/s12013-014-0442-2. [DOI] [PubMed] [Google Scholar]
- 120.Zhang J., Du Z., Pan S., Shi M., Li J., Yang C. Overcoming multidrug resistance by codelivery of MDR1-Targeting siRNA and doxorubicin using epha10-mediated pH-Sensitive lipoplexes: in vitro and in vivo evaluation. ACS Appl Mater Interfaces. 2018;10(25):21590–21600. doi: 10.1021/acsami.8b01806. [DOI] [PubMed] [Google Scholar]
- 121.Risnayanti C., Jang Y.S., Lee J., Ahn H.J. PLGA nanoparticles co-delivering MDR1 and BCL2 siRNA for overcoming resistance of paclitaxel and cisplatin in recurrent or advanced ovarian cancer. Sci Rep. 2018;8(1):7498. doi: 10.1038/s41598-018-25930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan J., Mendes L.P., Yao M., Filipczak N., Garai S., Thakur G.A. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur J Pharm Biopharm. 2019;136:18–28. doi: 10.1016/j.ejpb.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu M., Li J., Lin X., Wei Z., Zhang D., Zhao B. Reduction/photo dual-responsive polymeric prodrug nanoparticles for programmed siRNA and doxorubicin delivery. Biomater Sci. 2018;6(6):1457–1468. doi: 10.1039/c8bm00226f. [DOI] [PubMed] [Google Scholar]
- 124.Amreddy N., Ahmed R.A., Munshi A., Ramesh R. Drug delivery systems. Springer New York; New York, NY: 2019. Tumor-Targeted dendrimer nanoparticles for combinatorial delivery of siRNA and chemotherapy for cancer treatment. [DOI] [PubMed] [Google Scholar]
- 125.Zhang H., Yu N., Chen Y., Yan K., Wang X. Cationic liposome codelivering PI3K pathway regulator improves the response of BRCA1-deficient breast cancer cells to PARP1 inhibition. J Cell Biochem. 2019;120(8):13037–13045. doi: 10.1002/jcb.28574. [DOI] [PubMed] [Google Scholar]
- 126.Ahmad N., Alam M.A., Ahmad R., Naqvi A.A., Ahmad F.J. Preparation and characterization of surface-modified PLGA-polymeric nanoparticles used to target treatment of intestinal cancer. Artif Cells Nanomed Biotechnol. 2017;46(2):1–15. doi: 10.1080/21691401.2017.1324466. [DOI] [PubMed] [Google Scholar]
- 127.Chen S.Q., Wang C., Tao S., Wang Y.X., Hu F.Q., Yuan H. Rational design of redox-responsive and P-gp-Inhibitory lipid nanoparticles with high entrapment of paclitaxel for tumor therapy. Adv Healthc Mater. 2018;7(17):e1800485–e1800e85. doi: 10.1002/adhm.201800485. [DOI] [PubMed] [Google Scholar]
- 128.Madgulkar A.R., Bhalekar M.R., Kadam A.A. Improvement of oral bioavailability of lopinavir without co-administration of ritonavir using microspheres of thiolated xyloglucan. AAPS PharmSciTech. 2017;19(1):1–10. doi: 10.1208/s12249-017-0834-x. [DOI] [PubMed] [Google Scholar]
- 129.Soundararajan R., Sasaki K., Godfrey L., Odunze U., Fereira N., Schätzlein A. Direct in vivo evidence on the mechanism by which nanoparticles facilitate the absorption of a water insoluble, P-gp substrate. Int J Pharmaceut. 2016;514(1):121–132. doi: 10.1016/j.ijpharm.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 130.Dong X., Mattingly C.A., Tseng M.T., Cho M.J., Liu Y., Adams V.R. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 2009;69(9):3918–3926. doi: 10.1158/0008-5472.CAN-08-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tummala S., Gowthamarajan K., Satish Kumar M.N., Wadhwani A. Oxaliplatin immuno hybrid nanoparticles for active targeting: an approach for enhanced apoptotic activity and drug delivery to colorectal tumors. Drug Deliv. 2016;23(5):1773–1787. doi: 10.3109/10717544.2015.1084400. [DOI] [PubMed] [Google Scholar]
- 132.Tummala S., Kumar M.N.S., Pindiprolu S.K. Improved anti-tumor activity of oxaliplatin by encapsulating in anti-DR5 targeted gold nanoparticles. Drug Deliv. 2016;23(9):3505–3519. doi: 10.1080/10717544.2016.1199606. [DOI] [PubMed] [Google Scholar]
- 133.Ahmed F., Kumari S., Kondapi A.K. Evaluation of antiproliferative activity, safety and biodistribution of oxaliplatin and 5-fluorouracil loaded lactoferrin nanoparticles for the management of colon adenocarcinoma: an in vitro and an in vivo study. Pharm Res. 2018;35(9):178. doi: 10.1007/s11095-018-2457-7. [DOI] [PubMed] [Google Scholar]
- 134.Chaves C., Remiao F., Cisternino S., Decleves X. Opioids and the blood-brain barrier: a dynamic interaction with consequences on drug disposition in brain. Curr Neuropharmacol. 2017;15(8):1156–1173. doi: 10.2174/1570159X15666170504095823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kou L., Sun R., Ganapathy V., Yao Q., Chen R. Recent advances in drug delivery via the organic cation/carnitine transporter 2 (OCTN2/SLC22A5) Expert Opin Ther Targets. 2018;22(8):715–726. doi: 10.1080/14728222.2018.1502273. [DOI] [PubMed] [Google Scholar]
- 136.Tsuji A. P-Glycoprotein-Mediated efflux transport of anticancer drugs at the blood-brain barrier. Ther Drug Monit. 1998;20(5):588–590. doi: 10.1097/00007691-199810000-00024. [DOI] [PubMed] [Google Scholar]
- 137.Fabel K., Dietrich J., Hau P., Wismeth C., Winner B., Przywara S. Long-term stabilization in patients with malignant glioma after treatment with liposomal doxorubicin. Cancer. 2001;92(7):1936–1942. doi: 10.1002/1097-0142(20011001)92:7<1936::aid-cncr1712>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 138.De Vries N.A., Buckle T., Zhao J., Beijnen J.H., Schellens J.H.M., Van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drug. 2012;30(2):443–449. doi: 10.1007/s10637-010-9569-1. [DOI] [PubMed] [Google Scholar]
- 139.Lakkadwala S., Singh J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloid Surface B. 2019;173:27–35. doi: 10.1016/j.colsurfb.2018.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin T., Yang L., Liu Y., Zhou X., Sun J., Liu J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood-brain barrier permeability peptide-B6 peptide for potential use in Alzheimer's disease. Acta Biomater. 2015;25:172–183. doi: 10.1016/j.actbio.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 141.Fan S., Zheng Y., Liu X., Fang W., Chen X., Liao W. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer's disease. Drug Deliv. 2018;25(1):1091–1102. doi: 10.1080/10717544.2018.1461955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia W., Wang J., Xu Y., Jiang F., Xu L. l-BLP25 as a peptide vaccine therapy in non-small cell lung cancer: a review. J Thorac Dis. 2014;6(10):1513–1520. doi: 10.3978/j.issn.2072-1439.2014.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Douer D. Efficacy and safety of vincristine sulfate liposome injection in the treatment of adult acute lymphocytic leukemia. Oncologist. 2016;21(7):840–847. doi: 10.1634/theoncologist.2015-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.