Graphical abstract
Keywords: Glutamine transporters, Drug delivery, Pharmacokinetics, Screening, Proteoliposome
Abstract
Among the different targets of administered drugs, there are membrane transporters that play also a role in drug delivery and disposition. Moreover, drug-transporter interactions are responsible for off-target effects of drugs underlying their toxicity. The improvement of the drug design process is subjected to the identification of those membrane transporters mostly relevant for drug absorption, delivery and side effect production. A peculiar group of proteins with great relevance to pharmacology is constituted by the membrane transporters responsible for managing glutamine traffic in different body districts. The interest around glutamine metabolism lies in its physio-pathological role; glutamine is considered a conditionally essential amino acid because highly proliferative cells have an increased request of glutamine that cannot be satisfied only by endogenous synthesis. Then, glutamine transporters provide cells with this special nutrient. Among the glutamine transporters, SLC1A5, SLC6A14, SLC6A19, SLC7A5, SLC7A8 and some members of SLC38 family are the best characterized, so far, in both physiological and pathological conditions. Few 3D structures have been solved by CryoEM; other structural data on these transporters have been obtained by computational analysis. Interactions with drugs have been described for several transporters of this group. For some of them, the studies are at an advanced stage, for others, the studies are still in nuce and novel biochemical findings open intriguing perspectives.
1. Introduction
Membrane transporters are primary sites for drug interactions since they are located at the boundary between the intra end extracellular milieu [1], [2], [3], [4], [5], [6]. These interactions can occur at the level of absorbing or re-absorbing epithelia, that is intestine and kidney tubules, as well as at the level of other body districts which are in direct or indirect contact with the bloodstream. Unique cases are those of the blood-brain barrier (BBB) and the placental barrier, responsible for controlling the flux of molecules to specific organs. In these districts, double-membrane barriers are present [7]. From the described scenario it is evident that the absorption and the interaction of drugs with molecular cell systems are complex phenomena and are strongly influenced by the function or dysfunction of specific membrane transporters [8], [9], [10]. Drug-transporter interactions are, then, expected to play key roles in human therapy [11,12] or, in other cases, to trigger side effects, due to the so-called off-target interactions [13]. After some decades of investigations, it is now well accepted that membrane transporters must be taken into account for drug design to improve drug delivery and efficacy. In this respect, the International Transporter Consortium [14] has been founded for establishing: (i) which transporters must be taken into account for improving drug absorption; (ii) the suitable biotechnologies for assaying and screening drug-transporters interactions; (iii) the transporters to consider for off-target effects [15,16]. The Society for Laboratory Automation and Screening (SLAS, https://www.slas.org) too, started to consider membrane transporters in drug discovery applications [17]. The recent methodological advancements for studying transport proteins triggered an exponential growth of the research on membrane transporters and drug-transporter interactions [18], [19], [20]. In this scenario, a great interest is deserved to a special group of membrane transporters: the glutamine transporters. Several reasons are at the basis of the growing interest towards this group of proteins, ranging from the improvement of basic knowledge to the involvement of the glutamine transport in crucial processes for cell life and their role in human pathologies. The last aspect opens new and very promising perspectives in exploiting these proteins as novel targets for human therapy. In this review, the status artis of this rapidly expanding field will be summarized.
2. Glutamine transporters relevant to the interactions with drugs: an overview
Some SLC (SoLuteCarrier) families include glutamine transporters whose role consists in contributing to the complex network of pathways underlying the glutamine metabolism. The main characteristics of glutamine transporters are the apparent redundancy and overlapping functions (Table 1) [21,22]. This phenomenon is explained by the need of maintaining the glutamine homeostasis in the whole body. Glutamine is, indeed, the most abundant amino acid and plays a greater number of roles than the others. A schematic picture of the roles of glutamine as well as of the transporters handling glutamine is summarized in Fig. 1, while a more detailed view of the glutamine pathways together with transporter expression profile under physiological conditions is available in previous papers [21,23,24]. It has to be stressed that several roles of glutamine are tissue-specific, as an example, glutamine is synthesized in muscles or in neurons to scavenge ammonia but is degraded in the liver for urea production. The redundancy of transporters responsible for glutamine traffic in the human body may represent also a challenge in designing specific and safe drugs; however, this aspect may be afforded considering that the pattern of membrane transporter expression varies a lot when comparing normal and pathological conditions and even among different pathologies. As an example, each cancer type is characterized by the expression of only one or a limited number of glutamine transporters that can be exploited for specific drug targeting. Given the pleiotropic role of glutamine, it is not a surprise that even if it is endogenously synthesized, it becomes conditionally essential under increased energy demand. In other words, highly proliferative cells are characterized by a strongly increased utilization of glutamine, together with glucose [11], for energy production bypassing or lowering the canonical mitochondrial production of ATP in the oxidative phosphorylation pathway. The use of glutamine as an energy source by cancer cells is also in line with the higher concentration of this amino acid in cells (from 2 to 20 mM) compared to that of the other amino acids [21,25]. This metabolic rearrangement allows for an efficient ATP production at the substrate level by the TCA (Tricarboxylic Acid Cycle) enzyme succinyl-CoA synthase, besides by glycolysis. In this rewired conditions, the TCA cycle works in a truncated form in which malate is sequestered from the TCA to be exported in the cytosol. In the same scenario, glutamine carbon atoms are used for supporting anaplerotic reactions and for producing reducing equivalents, required for cell growth. It is worth noting that the described phenomenon is typical of cancer cells which are considered, indeed, glutamine addicted [26,27]. In these cells, the endogenous synthesis of glutamine is not sufficient for supporting their high proliferation rate; as a consequence, a strong over-expression of some glutamine transporters is adopted by cancer cells [28]. Therefore, these transporters represent a target for novel anticancer drugs focusing on cell metabolism, rather than on the mechanisms of proliferation as the currently employed chemotherapy. Over the years, efforts have been made to find the right application(s), based on these transporters, to increase the outcome of cancer treatment(s). A specific issue that has been taken into consideration, is the designing of non-covalent (competitive) and covalent (non-competitive) inhibitors (Fig. 2). Indeed, the majority of transporter-based drugs are substrate-analogues and, therefore, act as competitive inhibitors with the consequent increase of the Km for the natural substrate (Fig. 2A). This approach, however, may result in a low efficacy of the treatment due to the displacement of the drug when the substrate concentration rises above the Km. The use of covalent inhibitors may overcome this problem since a covalently bound inhibitor cannot be displaced by the substrate (Fig. 2B). The setting up of this approach implies the knowledge of the structure of the transporter used as a target. In the following paragraphs, the major achievements on each transporter are summarized in terms of historical perspectives, basic functional information and interactions with drugs or potential drugs as principal applications in cancer treatment.
Table 1.
The basic characteristics of membrane transporters that accept glutamine as a substrate. The SLC classification is reported with the common names; the major substrates of transporters are indicated.
| Amino acid Transporter | Common name | Main substrate(s) | |
|---|---|---|---|
| SLC1 | A5 | ASCT2 | A,S,Q,T,A |
| SLC6 | A14 | ATB0,+ | neutral and cationic aa |
| A19 | B0AT1 | neutral aa | |
| SLC7 | A5 | LAT1 | Essential aa, Q |
| A8 | LAT2 | neutral aa, Q | |
| SLC38 | A1 | SNAT1 | A,S,C,N,Q,H |
| A2 | SNAT2 | G,P,A,S,C,N,Q,H,M | |
| A3 | SNAT3 | Q,N,H | |
| A5 | SNAT5 | Q,N,H,A,S | |
| A7 | SNAT7 | Q,N,H,A,S | |
| A9 | Q,R | ||
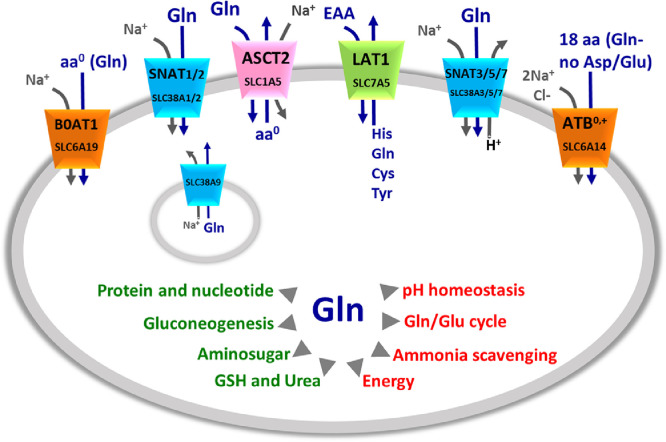
Fig. 1.
Glutamine membrane transporters and their mechanism of transport. The shape of the transporters is consistent with their asymmetry in the membrane. Membrane transporters are indicated by SLC classification and common names (as indicated in Table 1). The colors indicate the different families to which transporters belong: in orange SLC6 family, in green SLC7 family, in pink SLC1 family, in light blue SLC38 family. Lysosome localization of SLC38A9 is also indicated. Arrows indicate the direction of amino acids (blue) and ions (gray) fluxes. In the middle, schematic representation of Gln role in cells, the arrows indicate the main cell pathways in which Gln is involved (red) and the list of molecules synthesized from Gln (green).
Fig. 2.
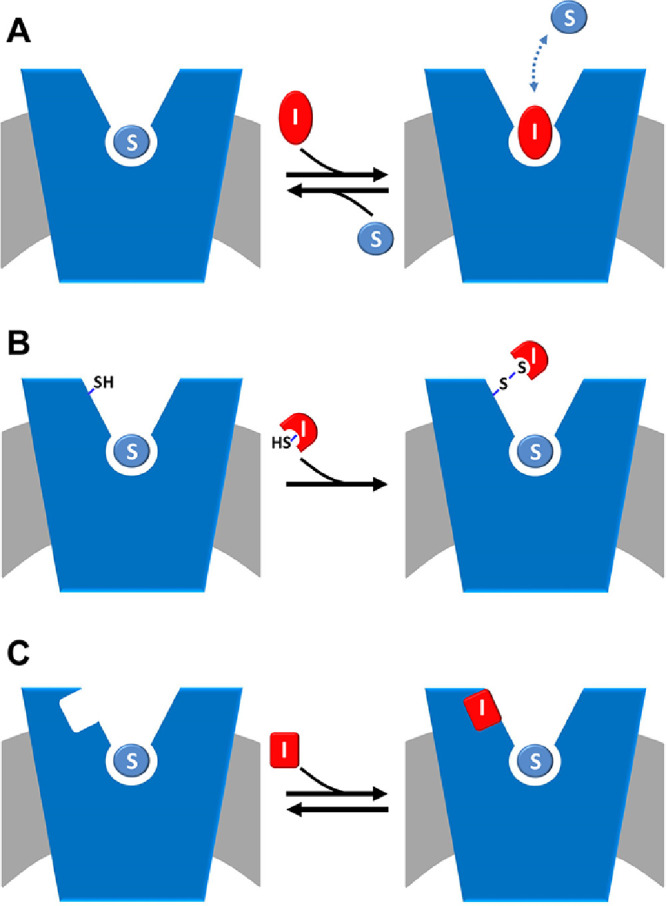
Schematic representation of inhibition kinetics. The membrane is in gray and a generic amino acid transporter is represented in blue and inserted in the membrane. (A) competitive mode is shown: the inhibitor, depicted in red (I), competes with the substrate (S), depicted in light blue, for the binding at the substrate binding site; the interaction is reversible as indicated as the double arrow. (B) covalent non-competitive mode is shown: the inhibitor, depicted in red (I) has a reactive group, exemplified by SH in the figure, that is able to covalently binds the protein in a site, alternative to the substrate binding site where also substrate is present (S, depicted in light blue). (C) non-covalent non-competitive mode is shown: the inhibitor, depicted in red (I) binds the protein in a site, alternative to the substrate binding site where also substrate is present (S, depicted in light blue).
2.1. The sodium-dependent neutral amino acid antiporter ASCT2 (SLC1A5)
ASCT2 (SLC1A5) is a plasma membrane protein [29] with a broad expression profile responsible for an obligatory exchange of neutral amino acid which is strictly Na+-dependent [29], [30], [31], [32] (Fig. 1). Its function has been studied in different experimental models such as intact cells [33] and proteoliposomes reconstituted with the transporter extracted from rat kidney, human cell lines or produced by heterologous expression in P. pastoris [34], [35], [36]. The common name of the protein is an acronym standing for AlanineSerineCysteineTransporter 2 (ASCT2), even though, it is now well assessed that glutamine is a major substrate [37,38] and that cysteine is not transported, but acts as an allosteric regulator [39]. The transport of amino acids is functionally and kinetically asymmetrical, according to the profound structural differences between the extra and intracellular sides of the transporter [37,38]. Under a physiological point of view, ASCT2 is involved in amino acid absorption in several tissues. A peculiar role of ASCT2 is that played in the glutamine/glutamate cycle between neurons and astrocytes. In this pathway, ASCT2 performs an interplay with other glutamine transporters belonging to SLC38 family (Fig. 1) to remove the excess of glutamate from the synaptic cleft to reduce its toxicity [22,30]. The regulation of transport activity is poorly understood. Physical interaction with the scaffold protein PDZK1, whose role is still not known, has been described both in vitro and in vivo [38].
2.1.1. Applications of ASCT2 in drug design
Owing to the above-mentioned involvement of ASCT2 in glutamine absorption in cancer, in the last decades, efforts have been made to increase the knowledge on this transporter [40]. Interestingly, virtually all human cancers are characterized by the over-expression of ASCT2 [28]. This phenomenon is part of the well-known Warburg effect, characterized by the switch towards anaerobic glucose metabolism [26]. These findings stimulated further investigations on new inhibitors of ASCT2 to be used as lead compounds for improving cancer drug design. These studies are performed either by synthesizing compounds whose structures harbor amino acid-like moieties or by predicting interactions of the protein with compounds downloaded from virtual chemical libraries to identify the best hits. This last strategy is based on the procedure of docking virtual chemical compounds to the 3D structure of the ASCT2 (or others) transporter (Fig. 3), allowing high throughput virtual screening. Even though the 3D structure was not available at that time, a homology model has been obtained using specific approaches for improving the quality of the structure. The phylogenetic relationships among SLC1 family members and their prokaryotic homologs have been first analysed. Then, a model of ASCT2 has been generated performing a comparative analysis of the GltPh (glutamate transporter from Pyrococcus horikoshii) solved structure [41]. The good quality of the produced model of ASCT2 has been then confirmed by the strong similarity with the recently solved structure of ASCT2 [37]. The model has been then used for identifying several potential drugs. To evaluate the actual inhibition potency of each compound, drug-transporter interactions must be validated by biochemical assays. In this respect, the proteoliposome tool is very helpful for accurate measurement of IC50 values and type of inhibition. It has to be highlighted that murine and human isoforms of ASCT2 exhibit an extensive difference in a local stretch of 31 amino acids. Even though this segment does not include the substrate-binding site, the difference may give variability in terms of interaction with molecules other than the substrate [38,42]. As a further difference, the rat and the human isoforms harbor 16 and 8 cysteines, respectively [38]. Taken together, this information indicates that the results collected with the murine isoform cannot be unequivocally transferred to the human ASCT2 without proper experimental testing. Notwithstanding, over the years the search for inhibitors have been conducted more or less in parallel with murine and human ASCT2. At first, a study on the rat ASCT2 proposed competitive inhibitors (Fig. 2A) based on glutamine derivatives. These compounds showed, however, relatively low affinity with IC50 of about 500 µM [43] that is not suitable for specific targeting. Later more efficient inhibitors have been identified by combining docking with experimental validation (Fig. 3): small side-chain amino acids with low hydrophobicity (and their derivatives) behaved as substrates of rat ASCT2, while molecules with large, aromatic side chains behave as inhibitors without being transported. Based on these criteria, a potent inhibitor, serine biphenyl-4-carboxylate, has been designed with a Ki of 30 µM. The results have been validated by measuring the transport function of rat ASCT2 following a non-physiological anion conductance in intact cells which is an indirect measurement of the transporter function [44]. Later on, new molecules have been designed as inhibitors of the rat ASCT2 and validation has been performed as anion current or as radiolabeled glutamine uptake measurements in intact cells [45]. It is worth noting that, in this case, the compounds have been designed based on proline structure, even though proline is not an ASCT2 substrate. Surprisingly, some of these compounds activate the transport, while others inhibit it. The best activator revealed to be a cis-3-hydroxyproline derivative while the inhibitor is a larger fluoro-benzylproline- derivative. These compounds, even if designed on rat ASCT2, showed cytotoxic effects also on human melanoma cell line [45]. Moving from this indication, the benzyl proline derivatives constituted the scaffold for designing more efficient inhibitors specific for the human ASCT2 and a Ki in the micromolar range has been measured in human cells for these compounds [46]. In another study, a competitive inhibitor of human ASCT2 has been identified starting from a previous screening conducted on 2-amino-4-bis (aryloxybenzyl)aminobutanoic acid derivatives [47]. The lead compound, V-9302, reached the preclinical phase after in silico prediction and in vitro assays. Mice harbouring a patient-derived xenograft (PDX) tumor revealed sensitive to the treatment with V-9302 that could reduce the tumor size. The molecular mechanism of inhibition of V-9302 is linked to a decrease of mTOR activity, consistent with lowered glutamine transport and metabolism and induction of autophagy and oxidative stress. Even though V-9302 is the first compound for which an in vivo effect has been described, the efficacy and the specificity of this inhibitor are still under debate and some studies showed that the same compound can target also other amino acid transporters in cancer cells acting by a general mechanism on the amino acid metabolism [48,49].
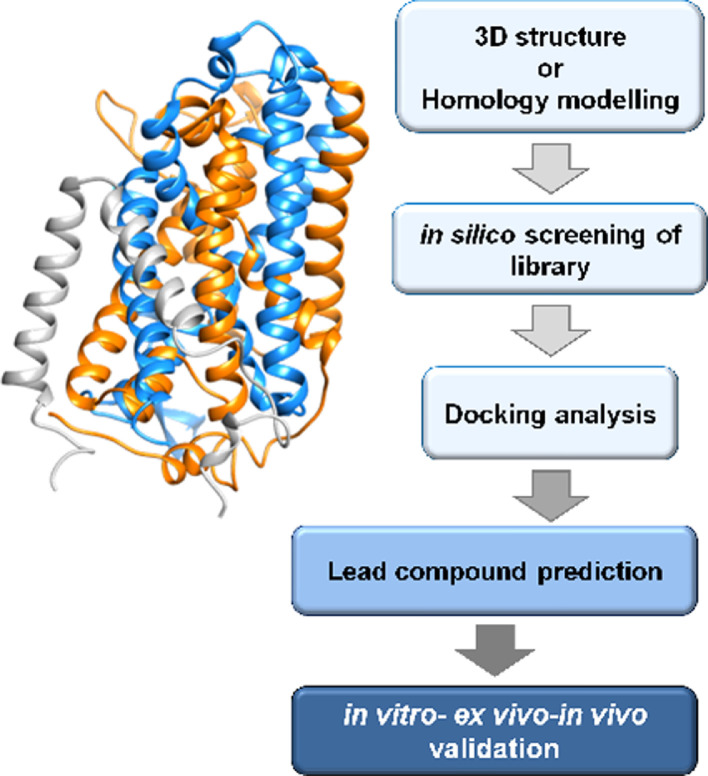
Fig. 3.
Work flow of best ligand prediction by in silico methodology. 3D structure or homology structural model of a transporter (exemplified in the scheme) is required as a first step. Then, virtual high throughput screening of compounds from virtual libraries is realized followed by docking analysis for prediction of lead compounds. As a final step, the prediction is validated employing in vitro, ex vivo and in vivo methodologies.
An alternative approach to the described substrate analogues is that of covalent inhibitors based on chemically targeting the protein of interest (Fig. 2B). This approach would avoid the pitfall of displacement of the substrate analogues by the endogenous amino acids whose concentrations may increase in cancer cells [20]. This strategy, collectively known as targeted covalent inhibitors (TCIs) [50], has been employed for the rat ASCT2 exploiting the presence of cysteine residues which are sensitive to several SH-reagents [51]. Then, thiol reacting compounds based on a dithiazole moiety have been designed with IC50 in the micromolar range, measured in proteoliposomes. The dithiazole ring is able to react with a thiol residue of cysteine forming a stable mixed disulphide (or a trisuphide) with the protein [52]. The possibility of targeting ASCT2 with specific inhibitors with low side effects is indicated by some studies conducted on mice model in which the ASCT2 gene has been knocked out. In this condition, it has been demonstrated that the absence of ASCT2 does not affect the general cell metabolism, growth and proliferation. On the contrary, the KO of ASCT2 negatively affects the growth of cancer cells in which the transporter is overexpressed [53,54]. Importantly, the structure of human ASCT2 has been recently solved [37] opening new perspectives in pharmaceutical studies related to cancer but also to the other pathologies in which ASCT2 is involved. The novel structure will allow performing more suitable high throughput virtual screening accelerating the identification of potent inhibitors of the transporter.
2.2. The sodium/chloride dependent broad amino acid transporter ATB0,+(SLC6A14)
ATB0,+(SLC6A14) is expressed in colon, lung [55], eye [56] and mammary gland [57] (Table 1). This transporter recognizes glutamine together with all the other amino acids except for glutamate and aspartate [58]. Transport is dependent on Na+ and Cl− transmembrane gradients (Fig. 1) and is sensitive to membrane potential [57]. Interestingly, ATB0,+also recognizes carnitine as a low-affinity substrate. The relationships with glutamine metabolism are indirectly demonstrated by its overexpression in human cancers, thus representing a potential target of cancer therapy [59,60].
2.2.1. Application of ATB0,+in drug design
Few are the studies on the interaction with drugs with ATB0,+. This is also due to the scarce functional and structural knowledge of this transporter. Very recently, a novel computational approach has been used for predicting the 3D structure of ATB0,+ allowing a more refined structural model of the transporter with respect to previous predictions [61]. This represents the first step towards the application of the transporter in virtual screening and, hence, will give a further drive in designing possible drugs as ATB0,+ inhibitors. So far, methyl-DL-tryptophan or BCH (2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid) have been exploited as chemical scaffolds for drug design based on substrate analogues (Fig. 2A and Fig. 3) [58]. These studies represent good starting points for further improvements in drug design using ATB0,+ as a target. Interestingly, ATB0,+ expression and function have been associated to cystic fibrosis even if, in this case, the role of ATB0,+ is mostly linked to arginine transport and anion conductance rather than the transport of glutamine. Arginine is, indeed, responsible for nitric oxide production and this seems to be particularly relevant in CFTR mutant mice which are susceptible to ATB0,+ KO, while WT mice are not extremely sensitive to the absence of this transporter in terms of overall metabolic changes [62].
2.3. The sodium-dependent broad amino acid transporter B0AT1 (SLC6A19)
B0AT1 (SLC6A19) is localized mainly in kidney and intestine [63], [64], [65]. It is specific for neutral amino acids, among which glutamine, with half-saturation constants in the micromolar range, while basic and acidic amino acids are not substrate of the transporter (Table 1). Function and regulation have been studied in cells and in vitro models: transport is strictly Na+-dependent (Fig. 1) as expected for a transporter belonging to the SLC6 (Na+-dependent neurotransmitter family). The transport mediated by B0AT1 is electrogenic, with a 1:1 stoichiometry between the amino acid and Na+ [66]. It can be speculated that in vivo B0AT1 is the major responsible for glutamine absorption in the intestine [65,67,68]. The expression of B0AT1 is regulated by the Janus kinase 2 that stimulates its expression at the cell membrane [69], while leptin is a negative regulator. The localization of B0AT1 at the plasma membrane is regulated by ACE2 and by collectrin, a non-peptidase homologue of ACE2, in intestine and kidney, respectively [70]. Collectrin has not any role in the intrinsic transport function [66]; while the role of ACE2 in transport has not been clarified yet, even if it is plausible that the peptidase activity of ACE2 serves as a source of substrates for B0AT1 [71]. Despite its role in glutamine absorption and reabsorption, no variation of B0AT1 expression in cancer cells has been reported, so far.
2.3.1. Application of B0AT1 in drug design
The link of B0AT1 with human pathology relies on the mutation of the coding gene, which is causative of the Hartnup disease (OMIM 234500). The 3D structure of B0AT1 is still not solved. A homology model has been built using the structure of the bacterial transporter LeuT as template [72]. Structure/function relationship studies have been performed in proteoliposomes: B0AT1 harbours two motifs responsible for metal binding, i.e., CXXC and a CXXXC. These two motifs are at the basis of the inhibition exerted by heavy metals which have been demonstrated using proteoliposomes harbouring the rat protein. This interaction may represent one of the mechanisms of toxicity of heavy metals [66]. The application of B0AT1 in drug design starts with discovering the interaction of the transporter with the commonly used drug nimesulide. The interaction has been characterized by combining inhibitor studies with kinetics and computational analysis. Very interestingly, molecular docking of nimesulide suggested that the drug binds to a site different from that of substrate binding located in a more external position producing a steric hindrance on the translocation path (Fig. 2C). This phenomenon has been displayed as a non-competitive inhibition when analysed by inhibition kinetics (Fig. 2C). The test of nimesulide analogues highlighted that minimal structural variations lead to a strong decrease of affinity revealing that the interaction with nimesulide is highly specific [72]. Interestingly, the IC50 for nimesulide is below 30 µM, considered as the threshold for predicting off-site interactions [73]. Some of the side effects reported for nimesulide are similar, even though of lower intensity, to those of the Hartnup disease caused by B0AT1 defects [65]. The side effects of nimesulide such as skin rush may rely on the reduction of absorption of some essential amino acids, such as tryptophan, triggering an impairment of niacin biosynthesis. Interestingly, a link of B0AT1 with fat-induced obesity has been recently proposed. It has been shown that disruption of the B0AT1 encoding gene in mice, induced a greater glycaemic control and resistance to obesity induced by high-fat diet. These data suggested that the pharmacological inhibition of B0AT1 could induce similar benefits in type 2 diabetes and obese patients. Thus, after nimesulide, additional inhibitors have been identified, such as benzotropine. These molecules decreased amino acid absorption in the organ model even though they exhibited an IC50 higher (lower affinity) than that for nimesulide [74]. Another inhibitor has been identified using a two functional cell-based assay employing fluorescence imaging methodology together with isotope-labelled amino acid transport. The identified compound, cinromide, is an amino acid analogue that is also transported by B0AT1, behaving thus as a competitive inhibitor (Fig. 2A) [75].
2.4. The histidine/large neutral amino acid antiporter LAT1 (SLC7A5)
L-type Amino acid Transporter 1 (LAT1) belongs to the large SLC7 family including two subgroups: the first includes cationic amino acid transporters; the second subgroup includes light subunits of heterodimeric amino acid transporters, among which LAT1 (SLC7A5) and LAT2 (SLC7A8) [76].
LAT1 is mainly expressed in testis, bone marrow, brain and placenta [76,77]. In polarized epithelia, LAT1 protein shows a sub localization in basolateral membranes [76,78], while in BBB, this protein is localized also on the apical membrane [79]. In the placenta, it is localized on both maternal and foetal surfaces of syncytiotrophoblasts [80]. Intracellular localization of LAT1 has also been reported in the lysosomal membrane [81]. In the plasma membrane, LAT1 forms a heterodimer with the glycoprotein CD98 (4F2hc, SLC3A2) by the formation of a disulfide between two conserved cysteine residues. From studies conducted in proteoliposomes harbouring the recombinant human protein, it has been demonstrated that CD98 has no role in the intrinsic transport function of LAT1 [82]. LAT1 is a Na+-independent obligatory exchanger of almost all the essential amino acids with a high preference towards histidine (Table 1) [83]. Indeed, in knockout mice, His (histidine) accumulates in the brain demonstrating that it is the main efflux substrate in the brain [84]. The role of LAT1 as glutamine transporter has been proposed in the past linking this activity to the high expression of this protein in human cancer [28,40]. Later, it has been demonstrated that glutamine is a low-affinity substrate of LAT1 (Fig. 1). Therefore, the role of LAT1 in glutamine traffic in cancer has been smoothened suggesting that this protein may be required by cancer cells for supplying essential amino acids [22].
2.4.1. Application of LAT1 in drug design
As in the case of ASCT2, the over-expression of LAT1 in cancers renders this transporter an exciting druggable target. Several reports dealt with chemical reagents designed as inhibitors of LAT1 for chemically knocking out the transporter in cancer cells following the common strategy depicted in Fig. 3.
As in other cases, two main types of inhibitors are the object of active research that is, competitive and non-competitive inhibitors, proposed by virtual screening of drug libraries together with validation in vitro and/or ex vivo models (Figs. 2 and 3). Within the first group, the amino acid analogue BCH, a well-known LAT1 inhibitor, has been firstly proposed as an anticancer drug [85]. Other examples are phenylalanine, tyrosine and tryptophan analogues which showed some efficacy [86,87], as well as triiodothyronine (T3) analogues [88]. Inhibitors based on hydroxamic acids conjugated to substrates of LAT1 have been also designed [89] with IC50 ranging from 1 µM to more than 300 µM. The most effective competitive inhibitor so far designed is the tyrosine analogue JPH203. This inhibitor shows antitumor activity in vitro and in a mouse model of HT-29 tumours [90], [91], [92] as well as in other types of cancers [93], [94], [95], [96], [97]. Very recently, JPH203 has been shown to markedly inhibit the proliferation of Anaplastic Thyroid Cancer (ATC) and to decrease the size of xenograft models [98]. This represents one of most advanced result in the application of a transporter to drug design and cancer treatment. An approach based on computational and proteoliposome assay allowed identifying potent competitive inhibitors (Fig. 2A) based on the typical amino acid scaffold; a set of 30 compounds have been tested and two of them showed IC50 in the low micromolar range [99]. Very recently, a computational analysis has been conducted and several halogen-containing L-phenylalanine-based ligands have been identified displaying high affinity and high selectivity for LAT1; however, these data are still in silico and need further experimental validation [100]. Besides competitive, also non-competitive inhibitors have been designed moving from structure/function relationships studies (Fig. 2B and 2C). Two cysteine residues, i.e., C335 and C407, have been identified within the substrate-binding site of LAT1 [101]. This finding suggested the strategy of designing covalent inhibitors able to react with the thiol groups of the cysteine leading to irreversible inhibition (Fig. 2B). Therefore, compounds harbouring a dithiazole group have been synthesized and tested for inhibition. Some of the compounds led to stable inhibition with a sub-micromolar IC50 value measured in proteoliposomes. The molecular mechanism of such interaction has been demonstrated by employing site-directed cysteine mutants that lost the reactivity towards the compounds. The effect on cancer cell death has been also assessed [102]. Interestingly, the structure of dithiazoles is “histidine-like” in line with minimal requirements for a molecule to interact with the substrate-binding site of LAT1. The His-like property has been recently exploited also by another group that designed 1,2,3-triazolyl analogues [103]. This interesting application links the ability of a molecule to reach the substrate binding site with the capacity to form stable covalent binding. This strategy is expected to increase a lot the potency and efficacy of an inhibitor. The task of designing novel drugs will benefit from the recent solution of the 3D structure of human LAT1 in complex with CD98 [104]. Indeed, the screening of virtual library using the actual 3D structure of the transporters will give more reliable information on the affinity of the compounds identified as good interactors of the transport protein. Moreover, as in the case of ASCT2, the comparison of the previously predicted structures with the actual 3D structure of LAT1 [105] could give information on the validity of the previously identified compounds.
2.5. The neutral amino acids antiporter LAT2 (SLC7A8)
L-type Amino acid Transporter 2 (LAT2) belongs to the large SLC7 transporter family, as LAT1.
LAT2 (SLC7A8) is widely expressed and, in polarized epithelial cells, it is mainly localized at the basolateral membranes. LAT2 works as a heterodimer coupled to the glycoprotein SLC3A2, known as 4F2hc or CD98 [106,107]. The interaction between the two proteins occurs by a disulphide between two conserved cysteine residues, as in the case of LAT1 [76]. The transport mechanism of LAT2 is the same as LAT1 even though the substrate specificity is different. LAT2 is a transporter of small neutral amino acids, including glutamine, with Km in the millimolar range (Table 1) and is inhibited by the non-metabolizable amino acid analogue BCH [108].
A role in cancer growth has been demonstrated for LAT2 that regulates glutamine-dependent activation of mTOR, promoting glycolysis. This molecular mechanism activates chemo-resistance [109]. Therefore, LAT2 represents another potential target in pharmacology, even though no application on interactions with drugs have been described so far for this protein.
2.6. The glutamine transporter SLC38A9
SLC38A9 is a peculiar amino acid transporter belonging to the SLC38 family that includes 11 members, some of which are well-studied, others are still orphans (Fig. 1) [110]. Historically, these transporters are known as SNATs and have been classified in systems A and N, following the features of transport modes and substrate specificity. In particular, System A includes SNAT 1 and 2 (SLC38A1/A2) that mediate the Na+-dependent uniport of small neutral amino acids and are inhibited by MeAIB (MethylAminoIsoButyric acid). System N includes SNAT3, 5 and 7 (SLC38A3/A5/A7) that mediate the uptake of glutamine, histidine and asparagine with a transport mode dependent on both Na+and H+. Despite the importance of these transporters in glutamine disposition, interactions with drugs are not yet investigated. Among the orphan transporters, the SLC38A9 has been recently deorphanized. This protein has been defined “transceptor”, i.e. a transporter with a receptor function [111], [112], [113], [114]. It is localized in the lysosomal membrane (Fig. 1) and at the difference with other canonical transporters, the transceptor SLC38A9 catalyses a glutamine efflux at a relatively low rate. The efflux of glutamine is allosterically regulated by intralysosomal arginine with consequent activation of SLC38A9 receptor function. This consists of the mTORC1 recruitment to the lysosomal membrane and following activation of the mTOR pathway [111], [112], [113], [114]. Given the ability to interact with one of the master protein in cell growth, SLC38A9 represents one of the most promising targets in the future drug design perspective. However, no application is so far described for this transporter.
3. The prodrug approach: exploiting ATB0,+ and LAT1 transporters
The localization of membrane transporters at the boundary with the extracellular environment makes these proteins relevant for mediating drug delivery and not only as drug targets. An interesting strategy is famous as the prodrug approach designed to increase the efficacy of a pharmaceutical compound by improving its pharmacokinetics, i.e. the uptake in the target cell. In particular, the prodrug can be any amino acid recognized by a specific transporter, conjugated with the pharmacological compound (Fig. 4) [115]. This approach is used on the one hand to enhance drug uptake by intestinal absorption as an example [116,117], on the other to reduce drug affinity towards efflux transporters and, thus, to circumvent the multi-drug resistance typical of some pathologies such as cancer [118]. The employment of amino acids has usually the advantage of increasing the water solubility of the prodrug through the presence of ionized carboxylate or amino groups [115]. Furthermore, the use of amino acids is suitable due to the redundancy of the amino acid transporters such as those handling glutamine (Table 1). Among these proteins, ATB0,+ (SLC6A14) and LAT1 (SLC7A5) have been, so far, the most employed in the prodrug approach, for different reasons (Table 2). In the case of ATB0,+, the choice is due to its feature of high capacity transporter and to its localization in the intestine that allows the rapid uptake of prodrugs. In the case of LAT1, the choice is mainly due to its expression in the blood brain barrier, a body district in which the flux of drugs is very limited. Therefore, the possibility of conjugating a drug with a LAT1 substrate increases a lot the possibility of targeting the brain to treat neurological disease, such as Alzheimer's and Parkinson's Diseases [89,[119], [120], [121], [122], [123]. Lastly, the over-expression of ATB0,+ and LAT1 in several human cancers makes the prodrug approach another important possibility for improving the treatment of this multifaceted disease. Examples of prodrugs are the esters of acyclovir and ganciclovir with an alpha-carboxyl group of neutral amino acids that become substrates of ATB0,+ [124]. Furthermore, serine and threonine are also used for delivering coupled drugs into cells via ATB0,+ [56]. Anticancer drugs designed as arginine derivatives and as serine derivatives of irinotecan have been employed as substrates of ATB0,+ in cervix and in breast cancer, respectively [125,126]. Notably, considering that ATB0,+mediates a low efficacy transport of carnitine, the butyrate ester butyryl-L-carnitine revealed to be a prodrug to treat gut inflammation [127]. Concerning LAT1, several reports have dealt with the use of natural amino acids as pro-moiety of L-DOPA, 7-chlorokynurenic acid and ketoprofen ([83,119,128] and refs herein). Furthermore, the protease inhibitors saquinavir, indinavir and nelfinavir, largely used to treat HIV infections, have been conjugated to phenylalanine and leucine for increasing absorption through LAT1 [129]. Interestingly, also prodrugs with chemical structures different from amino acids have been designed to be transported by LAT1 in brain, as dopamine derivatives [130]. In prostate cancer, quinidine derivatives linked to valine and isoleucine cross cell membranes via LAT1 transporter [131]. Prodrug approach, based on LAT1 substrate, has been attempted also to treat brain cancers which are normally characterized by high resistance to chemotherapy [132]. Given the mentioned substrate redundancy for amino acid transporters, is not trivial that the prodrug approach will receive further advancements and that, other amino acid transporters, besides ATB0,+ and LAT1 will be exploited as a target of pro-drug approach. A list of the prodrugs is reported in Table 2.
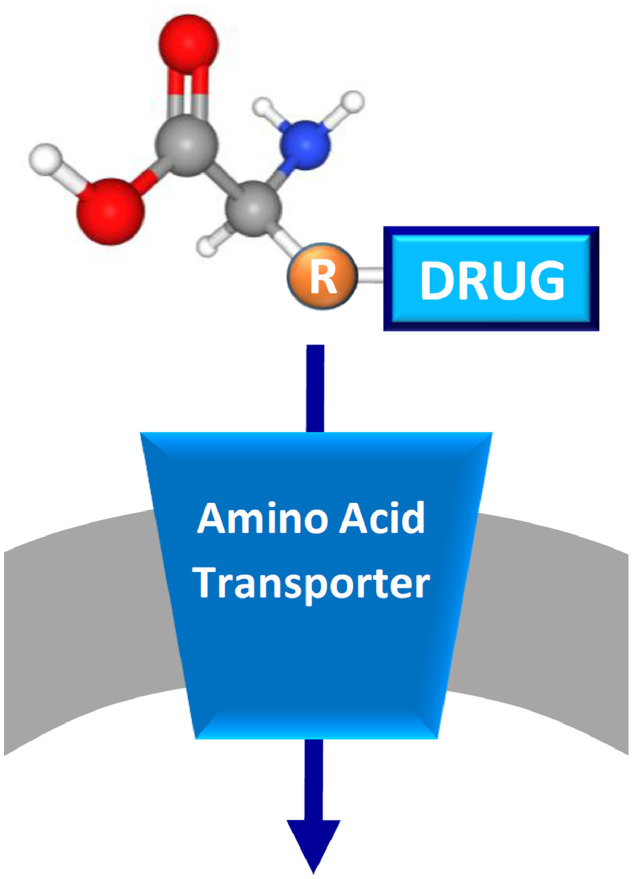
Fig. 4.
Schematic representation of prodrug approach. The membrane is in gray and a generic amino acid transporter is represented in blue and inserted in the membrane. The prodrug is depicted by a generic amino acid scaffold with the coupled drug bound to the side chain (R).
Table 2.
Prodrugs interacting with SLC6A14 and SLC7A5.
| Transporter | Prodrug | Therapeutic application |
|---|---|---|
| SLC6A14 (ATB0,+) | Acyclovir | Anti-cancer |
| Ganciclovir | Anti-cancer | |
| Irinotecan | Anti-cancer | |
| Butyryl-L-carnitine | Inflammation regulation | |
| SLC7A5 (LAT1) | L-DOPA | |
| dopamine derivatives | Parkinson's disease treatment | |
| 7-chlorokynurenic acid | Anti-depressant | |
| Ketoprofen | Anti-Inflammatory | |
| Saquinavir | HIV treatment | |
| Indinavir | HIV treatment | |
| Nelfinavir | HIV treatment | |
| Quinidine derivatives | Anti-cancer |
4. Nanoparticle approach for drug delivery: exploiting glutamine transporters
A more and more frequently used approach for improving drug delivery is that of using nanoparticles as a vector of drugs, that can be associated or covalently bound to such a nanomaterial. Some approaches have been already attempted in the case of transporters and, in particular, transporters dealt with in this review. As an example, glutamate-conjugate paclitaxel nanoparticles or L-dopa conjugated nanoparticles have been exploited to increase the absorption of these drugs via LAT1 [133]. The same transporter has also been exploited to increase the absorption of amino acid-modified drug associates-nanoparticles in the tumours that highly-expressed LAT1 [134]. Another transporter employed in nanoparticle strategy for drug delivery is ATB0,+. However, in this case, the protein has been considered for its capacity to transport L-carnitine besides amino acids. In particular, L-carnitine conjugated nanoparticles have been shown to increase the absorption of some drugs such as 5-fluorouracil [135]. It has to be stressed that the described data represent preliminary investigations and the actual transport processes via the amino acid transporters are still not demonstrated. Anyway, this strategy represents an important starting point for alternative treatments of tumours not responding to the conventional therapies.
5. Conclusion
As described in the previous paragraphs, the interest around membrane transporters and their role in pharmacology increased a lot in the last years as testified by the launch of the International Transporter Consortium and by the FDA (Food and Drug Administration) recommendations and guidelines for studying interactions of transporters with drugs [16]. The involvement of these proteins in common diseases, such as cancer, diabetes and neurological disorders, renders glutamine transporters hot spot targets for improving human therapy. Moreover, the location of specific glutamine transporters in specialized districts of the human body makes these transporters a vehicle for delivering the continuously increasing number of prodrugs. Notwithstanding the importance of the glutamine transporters and their relevance to human health, the interactions with drugs are still not accomplished and a big effort is required to obtain an exhaustive description of the expected involvement of the glutamine transporters in drug delivery and interaction.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgments
This work was supported by PON (Programma Operativo Nazionale) Project No. 01_00937 and by PRIN (Progetti di Ricerca di Rilevante Interesse Nazionale) Project No 2017PAB8EM to CI granted by MIUR (Ministry of Education, University and Research) Italy and by PRIN ( Progetti di Ricerca di Rilevante Interesse Nazionale) Project No 2017PAB8EM.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2020.02.005.
Appendix. Supplementary materials
References
- 1.International Transporter C. Giacomini K.M., Huang S.M., Tweedie D.J., Benet L.Z., Brouwer K.L. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S.M., Strong J.M., Zhang L., Reynolds K.S., Nallani S., Temple R. New era in drug interaction evaluation: US food and drug administration update on CYP enzymes, transporters, and the guidance process. J Clin Pharmacol. 2008;48(6):662–670. doi: 10.1177/0091270007312153. [DOI] [PubMed] [Google Scholar]
- 3.DeGorter M.K., Xia C.Q., Yang J.J., Kim R.B. Drug transporters in drug efficacy and toxicity. Annu Rev Pharmacol Toxicol. 2012;52:249–273. doi: 10.1146/annurev-pharmtox-010611-134529. [DOI] [PubMed] [Google Scholar]
- 4.Han H.K. Role of transporters in drug interactions. Arch Pharm Res. 2011;34(11):1865–1877. doi: 10.1007/s12272-011-1107-y. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi T., Tamai I. Solute carrier transporters as targets for drug delivery and pharmacological intervention for chemotherapy. J Pharm Sci. 2011;100(9):3731–3750. doi: 10.1002/jps.22576. [DOI] [PubMed] [Google Scholar]
- 6.Rives M.L., Javitch J.A., Wickenden A.D. Potentiating slc transporter activity: emerging drug discovery opportunities. Biochem Pharmacol. 2017;135:1–11. doi: 10.1016/j.bcp.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Scalise M., Console L., Galluccio M., Pochini L., Tonazzi A., Giangregorio N. Exploiting cysteine residues of SLC membrane transporters as targets for drugs. SLAS Discov. 2019;24(9):867–881. doi: 10.1177/2472555219856601. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal S., Chinn L., Zhang L. An overview of transporter information in package inserts of recently approved new molecular entities. Pharm Res. 2013;30(3):899–910. doi: 10.1007/s11095-012-0924-0. [DOI] [PubMed] [Google Scholar]
- 9.Mandery K., Glaeser H., Fromm M.F. Interaction of innovative small molecule drugs used for cancer therapy with drug transporters. Br J Pharmacol. 2012;165(2):345–362. doi: 10.1111/j.1476-5381.2011.01618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pardridge W.M. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganapathy V., Thangaraju M., Prasad P.D. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121(1):29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Penmatsa A., Wang K.H., Gouaux E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature. 2013;503(7474):85–90. doi: 10.1038/nature12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pochini L., Scalise M., Galluccio M., Indiveri C. OCTN cation transporters in health and disease: role as drug targets and assay development. J Biomol Screen. 2013;18(8):851–867. doi: 10.1177/1087057113493006. [DOI] [PubMed] [Google Scholar]
- 14.Chambers J.C., Zhang W., Sehmi J., Li X., Wass M.N., Van der Harst P. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat Genet. 2011;43(11):1131–1138. doi: 10.1038/ng.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rask-Andersen M., Masuram S., Fredriksson R., Schioth H.B. Solute carriers as drug targets: current use, clinical trials and prospective. Mol Aspects Med. 2013;34(2–3):702–710. doi: 10.1016/j.mam.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Zamek-Gliszczynski M.J., Taub M.E., Chothe P.P., Chu X., Giacomini K.M., Kim R.B. Transporters in drug development: 2018 ITC recommendations for transporters of emerging clinical importance. Clin Pharmacol Ther. 2018;104(5):890–899. doi: 10.1002/cpt.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scalise M., Jaakola V.P. Membrane proteins: new approaches to probes, technologies, and drug design. SLAS Discov. 2019;24(9):865–866. doi: 10.1177/2472555219876283. [DOI] [PubMed] [Google Scholar]
- 18.Cesar-Razquin A., Snijder B., Frappier-Brinton T., Isserlin R., Gyimesi G., Bai X. A call for systematic research on solute carriers. Cell. 2015;162(3):478–487. doi: 10.1016/j.cell.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Scalise M., Pochini L., Giangregorio N., Tonazzi A., Indiveri C. Proteoliposomes as tool for assaying membrane transporter functions and interactions with xenobiotics. Pharmaceutics. 2013;5(3):472–497. doi: 10.3390/pharmaceutics5030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scalise M., Galluccio M., Pochini L., Console L., Barile M., Giangregorio N. Studying interactions of drugs with cell membrane nutrient transporters: new frontiers of proteoliposome nanotechnology. Curr Pharm Des. 2017;23(26):3871–3883. doi: 10.2174/1381612823666170616083705. [DOI] [PubMed] [Google Scholar]
- 21.Pochini L., Scalise M., Galluccio M., Indiveri C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scalise M., Pochini L., Galluccio M., Indiveri C. Glutamine transport. from energy supply to sensing and beyond. Biochim Biophys Acta. 2016;1857(8):1147–1157. doi: 10.1016/j.bbabio.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Newsholme P., Procopio J., Lima M.M., Pithon-Curi T.C., Curi R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct. 2003;21(1):1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 24.Bode B.P. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131(9 Suppl) doi: 10.1093/jn/131.9.2475S. : 2475S–85S discussion 86S-7S. [DOI] [PubMed] [Google Scholar]
- 25.Cynober L.A. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition. 2002;18(9):761–766. doi: 10.1016/s0899-9007(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 26.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaglio D., Metallo C.M., Gameiro P.A., Hiller K., Danna L.S., Balestrieri C. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol Syst Biol. 2011;7:523. doi: 10.1038/msb.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhutia Y.D., Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863(10):2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kekuda R., Prasad P.D., Fei Y.J., Torres-Zamorano V., Sinha S., Yang-Feng T.L. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271(31):18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- 30.Broer S., Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77(3):705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 31.Gliddon C.M., Shao Z., LeMaistre J.L., Anderson C.M. Cellular distribution of the neutral amino acid transporter subtype ASCT2 in mouse brain. J Neurochem. 2009;108(2):372–383. doi: 10.1111/j.1471-4159.2008.05767.x. [DOI] [PubMed] [Google Scholar]
- 32.Utsunomiya-Tate N., Endou H., Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271(25):14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 33.Torres-Zamorano V., Leibach F.H., Ganapathy V. Sodium-dependent homo- and hetero-exchange of neutral amino acids mediated by the amino acid transporter ATB degree. Biochem Biophys Res Commun. 1998;245(3):824–829. doi: 10.1006/bbrc.1998.8434. [DOI] [PubMed] [Google Scholar]
- 34.Oppedisano F., Pochini L., Galluccio M., Cavarelli M., Indiveri C. Reconstitution into liposomes of the glutamine/amino acid transporter from renal cell plasma membrane: functional characterization, kinetics and activation by nucleotides. Biochim Biophys Acta. 2004;1667(2):122–131. doi: 10.1016/j.bbamem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Oppedisano F., Pochini L., Galluccio M., Indiveri C. The glutamine/amino acid transporter (ASCT2) reconstituted in liposomes: electrical nature of the glutamine/glutamate antiport. Ital J Biochem. 2007;56(4):275–278. [PubMed] [Google Scholar]
- 36.Pingitore P., Pochini L., Scalise M., Galluccio M., Hedfalk K., Indiveri C. Large scale production of the active human ASCT2 (SLC1A5) transporter in Pichia pastoris–functional and kinetic asymmetry revealed in proteoliposomes. Biochim Biophys Acta. 2013;1828(9):2238–2246. doi: 10.1016/j.bbamem.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 37.Garaeva A.A., Oostergetel G.T., Gati C., Guskov A., Paulino C., Slotboom D.J. Cryo-EM structure of the human neutral amino acid transporter ASCT2. Nat Struct Mol Biol. 2018;25(6):515–521. doi: 10.1038/s41594-018-0076-y. [DOI] [PubMed] [Google Scholar]
- 38.Scalise M., Pochini L., Panni S., Pingitore P., Hedfalk K., Indiveri C. Transport mechanism and regulatory properties of the human amino acid transporter ASCT2 (SLC1A5) Amino Acids. 2014;46(11):2463–2475. doi: 10.1007/s00726-014-1808-x. [DOI] [PubMed] [Google Scholar]
- 39.Scalise M., Pochini L., Pingitore P., Hedfalk K., Indiveri C. Cysteine is not a substrate but a specific modulator of human ASCT2 (SLC1A5) transporter. FEBS Lett. 2015;589(23):3617–3623. doi: 10.1016/j.febslet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs B.C., Bode B.P. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15(4):254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Garibsingh R.A., Otte N.J., Ndaru E., Colas C., Grewer C., Holst J. Homology modeling informs ligand discovery for the glutamine transporter ASCT2. Front Chem. 2018;6:279. doi: 10.3389/fchem.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scopelliti A.J., Font J., Vandenberg R.J., Boudker O., Ryan R.M. Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nat Commun. 2018;9(1):38. doi: 10.1038/s41467-017-02444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esslinger C.S., Cybulski K.A., Rhoderick J.F. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg Med Chem. 2005;13(4):1111–1118. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 44.Albers T., Marsiglia W., Thomas T., Gameiro A., Grewer C. Defining substrate and blocker activity of alanine-serine-cysteine transporter 2 (ASCT2) ligands with novel serine analogs. Mol Pharmacol. 2012;81(3):356–365. doi: 10.1124/mol.111.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colas C., Grewer C., Otte N.J., Gameiro A., Albers T., Singh K. Ligand discovery for the alanine-serine-cysteine transporter (ASCT2, SLC1A5) from homology modeling and virtual screening. PLoS Comput. Biol. 2015;11(10) doi: 10.1371/journal.pcbi.1004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh K., Tanui R., Gameiro A., Eisenberg G., Colas C., Schlessinger A. Structure activity relationships of benzylproline-derived inhibitors of the glutamine transporter ASCT2. Bioorg Med Chem Lett. 2017;27(3):398–402. doi: 10.1016/j.bmcl.2016.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulte M.L., Dawson E.S., Saleh S.A., Cuthbertson M.L., Manning H.C. 2-Substituted Ngamma-glutamylanilides as novel probes of ASCT2 with improved potency. Bioorg Med Chem Lett. 2015;25(1):113–116. doi: 10.1016/j.bmcl.2014.10.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broer S. Amino acid transporters as disease modifiers and drug targets. SLAS Discov. 2018;23(4):303–320. doi: 10.1177/2472555218755629. [DOI] [PubMed] [Google Scholar]
- 49.Broer A., Gauthier-Coles G., Rahimi F., van Geldermalsen M., Dorsch D., Wegener A. Ablation of the ASCT2 (SLC1A5) gene encoding a neutral amino acid transporter reveals transporter plasticity and redundancy in cancer cells. J Biol Chem. 2019;294(11):4012–4026. doi: 10.1074/jbc.RA118.006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh J., Petter R.C., Baillie T.A., Whitty A. The resurgence of covalent drugs. Nat Rev Drug Discov. 2011;10(4):307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 51.Oppedisano F., Galluccio M., Indiveri C. Inactivation by Hg2+ and methylmercury of the glutamine/amino acid transporter (ASCT2) reconstituted in liposomes: prediction of the involvement of a CXXC motif by homology modelling. Biochem Pharmacol. 2010;80(8):1266–1273. doi: 10.1016/j.bcp.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 52.Oppedisano F., Catto M., Koutentis P.A., Nicolotti O., Pochini L., Koyioni M. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol Appl Pharmacol. 2012;265(1):93–102. doi: 10.1016/j.taap.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Ni F., Yu W.M., Li Z., Graham D.K., Jin L., Kang S. Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression. Nat Metab. 2019;1(3):390–403. doi: 10.1038/s42255-019-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masle-Farquhar E., Broer A., Yabas M., Enders A., Broer S. ASCT2 (SLC1A5)-deficient mice have normal B-Cell development, proliferation, and antibody production. Front Immunol. 2017;8:549. doi: 10.3389/fimmu.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sloan J.L., Grubb B.R., Mager S. Expression of the amino acid transporter ATB 0+ in lung: possible role in luminal protein removal. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L39–L49. doi: 10.1152/ajplung.00164.2002. [DOI] [PubMed] [Google Scholar]
- 56.Ganapathy M.E., Ganapathy V. Amino acid transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(4):357–364. doi: 10.2174/156800805774912953. [DOI] [PubMed] [Google Scholar]
- 57.Nakanishi T., Hatanaka T., Huang W., Prasad P.D., Leibach F.H., Ganapathy M.E. Na+- and Cl–coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and xenopus oocytes. J Physiol. 2001;532(Pt 2):297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sloan J.L., Mager S. Cloning and functional expression of a human Na(+) and Cl(-)-dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274(34):23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- 59.Karunakaran S., Ramachandran S., Coothankandaswamy V., Elangovan S., Babu E., Periyasamy-Thandavan S. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286(36):31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coothankandaswamy V., Cao S., Xu Y., Prasad P.D., Singh P.K., Reynolds C.P. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br J Pharmacol. 2016;173(23):3292–3306. doi: 10.1111/bph.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palazzolo L., Paravicini C., Laurenzi T., Adobati S., Saporiti S., Guerrini U. SLC6A14, a pivotal actor on cancer stage: when function meets structure. SLAS Discov. 2019;24(9):928–938. doi: 10.1177/2472555219867317. [DOI] [PubMed] [Google Scholar]
- 62.Ahmadi S., Xia S., Wu Y.S., Di Paola M., Kissoon R., Luk C. SLC6A14, an amino acid transporter, modifies the primary CF defect in fluid secretion. Elife. 2018;13:7. doi: 10.7554/eLife.37963. pii: e37963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Broer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem Int. 2006;48(6–7):559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 64.Pramod A.B., Foster J., Carvelli L., Henry L.K. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34(2–3):197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verrey F., Ristic Z., Romeo E., Ramadan T., Makrides V., Dave M.H. Novel renal amino acid transporters. Annu Rev Physiol. 2005;67:557–572. doi: 10.1146/annurev.physiol.67.031103.153949. [DOI] [PubMed] [Google Scholar]
- 66.Oppedisano F., Pochini L., Broer S., Indiveri C. The b degrees AT1 amino acid transporter from rat kidney reconstituted in liposomes: kinetics and inactivation by methylmercury. Biochim Biophys Acta. 2011;1808(10):2551–2558. doi: 10.1016/j.bbamem.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 67.Bohmer C., Broer A., Munzinger M., Kowalczuk S., Rasko J.E., Lang F. Characterization of mouse amino acid transporter B0AT1 (slc6a19) Biochem J. 2005;389(Pt 3):745–751. doi: 10.1042/BJ20050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Broer A., Juelich T., Vanslambrouck J.M., Tietze N., Solomon P.S., Holst J. Impaired nutrient signaling and body weight control in a Na+ neutral amino acid cotransporter (Slc6a19)-deficient mouse. J Biol Chem. 2011;286(30):26638–26651. doi: 10.1074/jbc.M111.241323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhavsar S.K., Hosseinzadeh Z., Merches K., Gu S., Broer S., Lang F. Stimulation of the amino acid transporter SLC6A19 by JAK2. Biochem Biophys Res Commun. 2011;414(3):456–461. doi: 10.1016/j.bbrc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 70.Fairweather S.J., Broer A., O'Mara M.L., Broer S. Intestinal peptidases form functional complexes with the neutral amino acid transporter B(0)AT1. Biochem J. 2012;446(1):135–148. doi: 10.1042/BJ20120307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singer D., Camargo S.M. Collectrin and ACE2 in renal and intestinal amino acid transport. Channels. 2011;5(5):410–423. doi: 10.4161/chan.5.5.16470. [DOI] [PubMed] [Google Scholar]
- 72.Pochini L., Seidita A., Sensi C., Scalise M., Eberini I., Indiveri C. Nimesulide binding site in the B0AT1 (SLC6A19) amino acid transporter. Mechanism of inhibition revealed by proteoliposome transport assay and molecular modelling. Biochem Pharmacol. 2014;89(3):422–430. doi: 10.1016/j.bcp.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 73.Lounkine E., Keiser M.J., Whitebread S., Mikhailov D., Hamon J., Jenkins J.L. Large-scale prediction and testing of drug activity on side-effect targets. Nature. 2012;486(7403):361–367. doi: 10.1038/nature11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng Q., Shah N., Broer A., Fairweather S., Jiang Y., Schmoll D. Identification of novel inhibitors of the amino acid transporter B(0) AT1 (SLC6A19), a potential target to induce protein restriction and to treat type 2 diabetes. Br J Pharmacol. 2017;174(6):468–482. doi: 10.1111/bph.13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Danthi S.J., Liang B., Smicker O., Coupland B., Gregory J., Gefteas E. Identification and characterization of inhibitors of a neutral amino acid transporter, SLC6A19, using two functional cell-based assays. SLAS Discov. 2019;24(2):111–120. doi: 10.1177/2472555218794627. [DOI] [PubMed] [Google Scholar]
- 76.Fotiadis D., Kanai Y., Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34(2–3):139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Prasad P.D., Wang H., Huang W., Kekuda R., Rajan D.P., Leibach F.H. Human LAT1, a subunit of system l amino acid transporter: molecular cloning and transport function. Biochem Biophys Res Commun. 1999;255(2):283–288. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- 78.Verrey F., Closs E.I., Wagner C.A., Palacin M., Endou H., Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflug Arch. 2004;447(5):532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 79.Duelli R., Enerson B.E., Gerhart D.Z., Drewes L.R. Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab. 2000;20(11):1557–1562. doi: 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Ohgaki R., Ohmori T., Hara S., Nakagomi S., Kanai-Azuma M., Kaneda-Nakashima K. Essential roles of l-Type amino acid transporter 1 in syncytiotrophoblast development by presenting fusogenic 4F2hc. Mol Cell Biol. 2017;37(11) doi: 10.1128/MCB.00427-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Milkereit R., Persaud A., Vanoaica L., Guetg A., Verrey F., Rotin D. LAPTM4b recruits the LAT1-4F2hc Leu transporter to lysosomes and promotes mTORC1 activation. Nat Commun. 2015;6:7250. doi: 10.1038/ncomms8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Napolitano L., Scalise M., Galluccio M., Pochini L., Albanese L.M., Indiveri C. LAT1 is the transport competent unit of the LAT1/CD98 heterodimeric amino acid transporter. Int J Biochem Cell Biol. 2015;67:25–33. doi: 10.1016/j.biocel.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Scalise M., Galluccio M., Console L., Pochini L., Indiveri C. The human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018;6:243. doi: 10.3389/fchem.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tarlungeanu D.C., Deliu E., Dotter C.P., Kara M., Janiesch P.C., Scalise M. Impaired amino acid transport at the blood brain barrier is a cause of autism spectrum disorder. Cell. 2016;167(6):1481–1494. doi: 10.1016/j.cell.2016.11.013. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imai H., Kaira K., Oriuchi N., Shimizu K., Tominaga H., Yanagitani N. Inhibition of l-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30(12):4819–4828. [PubMed] [Google Scholar]
- 86.Augustyn E., Finke K., Zur A.A., Hansen L., Heeren N., Chien H.C. LAT-1 activity of meta-substituted phenylalanine and tyrosine analogs. Bioorg Med Chem Lett. 2016;26(11):2616–2621. doi: 10.1016/j.bmcl.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geier E.G., Schlessinger A., Fan H., Gable J.E., Irwin J.J., Sali A. Structure-based ligand discovery for the large-neutral amino acid transporter 1, LAT-1. Proc Natl Acad Sci U S A. 2013;110(14):5480–5485. doi: 10.1073/pnas.1218165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kongpracha P., Nagamori S., Wiriyasermkul P., Tanaka Y., Kaneda K., Okuda S. Structure-activity relationship of a novel series of inhibitors for cancer type transporter l-type amino acid transporter 1 (LAT1) J Pharmacol Sci. 2017;133(2):96–102. doi: 10.1016/j.jphs.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Zur A.A., Chien H.C., Augustyn E., Flint A., Heeren N., Finke K. LAT1 activity of carboxylic acid bioisosteres: evaluation of hydroxamic acids as substrates. Bioorg Med Chem Lett. 2016;26(20):5000–5006. doi: 10.1016/j.bmcl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oda K., Hosoda N., Endo H., Saito K., Tsujihara K., Yamamura M. l-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101(1):173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toyoshima J., Kusuhara H., Wempe M.F., Endou H., Sugiyama Y. Investigation of the role of transporters on the hepatic elimination of an LAT1 selective inhibitor JPH203. J Pharm Sci. 2013;102(9):3228–3238. doi: 10.1002/jps.23601. [DOI] [PubMed] [Google Scholar]
- 92.Wempe M.F., Rice P.J., Lightner J.W., Jutabha P., Hayashi M., Anzai N. Metabolism and pharmacokinetic studies of JPH203, an l-amino acid transporter 1 (LAT1) selective compound. Drug Metab Pharmacokinet. 2012;27(1):155–161. doi: 10.2133/dmpk.dmpk-11-rg-091. [DOI] [PubMed] [Google Scholar]
- 93.Otsuki H., Kimura T., Yamaga T., Kosaka T., Suehiro J.I., Sakurai H. Prostate cancer cells in different androgen receptor status employ different leucine transporters. Prostate. 2017;77(2):222–233. doi: 10.1002/pros.23263. [DOI] [PubMed] [Google Scholar]
- 94.Yothaisong S., Dokduang H., Anzai N., Hayashi K., Namwat N., Yongvanit P. Inhibition of l-type amino acid transporter 1 activity as a new therapeutic target for cholangiocarcinoma treatment. Tumour Biol. 2017;39(3) doi: 10.1177/1010428317694545. [DOI] [PubMed] [Google Scholar]
- 95.Choi D.W., Kim D.K., Kanai Y., Wempe M.F., Endou H., Kim J.K. JPH203, a selective l-type amino acid transporter 1 inhibitor, induces mitochondria-dependent apoptosis in Saos2 human osteosarcoma cells. Korean J Physiol Pharmacol. 2017;21(6):599–607. doi: 10.4196/kjpp.2017.21.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hayashi K., Jutabha P., Maeda S., Supak Y., Ouchi M., Endou H. LAT1 acts as a crucial transporter of amino acids in human thymic carcinoma cells. J Pharmacol Sci. 2016;132(3):201–204. doi: 10.1016/j.jphs.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Rosilio C., Nebout M., Imbert V., Griessinger E., Neffati Z., Benadiba J. l-type amino-acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of T-cell lymphoblastic lymphoma/T-cell acute lymphoblastic leukemia. Leukemia. 2015;29(6):1253–1266. doi: 10.1038/leu.2014.338. [DOI] [PubMed] [Google Scholar]
- 98.Enomoto K., Sato F., Tamagawa S., Gunduz M., Onoda N., Uchino S. A novel therapeutic approach for anaplastic thyroid cancer through inhibition of LAT1. Sci Rep. 2019;9(1):14616. doi: 10.1038/s41598-019-51144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Singh N., Scalise M., Galluccio M., Wieder M., Seidel T., Langer T. Discovery of potent inhibitors for the large neutral amino acid transporter 1 (LAT1) by structure-based methods. Int J Mol Sci. 2018;20(1) doi: 10.3390/ijms20010027. pii: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Singh N., Villoutreix B.O., Ecker G.F. Rigorous sampling of docking poses unveils binding hypothesis for the halogenated ligands of l-type amino acid transporter 1 (LAT1) Sci Rep. 2019;9(1):15061. doi: 10.1038/s41598-019-51455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Napolitano L., Galluccio M., Scalise M., Parravicini C., Palazzolo L., Eberini I. Novel insights into the transport mechanism of the human amino acid transporter LAT1 (SLC7A5). Probing critical residues for substrate translocation. Biochim Biophys Acta Gen Subj. 2017;1861(4):727–736. doi: 10.1016/j.bbagen.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 102.Napolitano L., Scalise M., Koyioni M., Koutentis P., Catto M., Eberini I. Potent inhibitors of human LAT1 (SLC7A5) transporter based on dithiazole and dithiazine compounds for development of anticancer drugs. Biochem Pharmacol. 2017;143:39–52. doi: 10.1016/j.bcp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 103.Hall C., Wolfe H., Wells A., Chien H.C., Colas C., Schlessinger A. l-Type amino acid transporter 1 activity of 1,2,3-triazolyl analogs of l-histidine and l-tryptophan. Bioorg Med Chem Lett. 2019;29(16):2254–2258. doi: 10.1016/j.bmcl.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee Y., Wiriyasermkul P., Jin C., Quan L., Ohgaki R., Okuda S. Cryo-EM structure of the human l-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat Struct Mol Biol. 2019;26(6):510–517. doi: 10.1038/s41594-019-0237-7. [DOI] [PubMed] [Google Scholar]
- 105.Yan R., Zhao X., Lei J., Zhou Q. Structure of the human LAT1-4F2hc heteromeric amino acid transporter complex. Nature. 2019;568(7750):127–130. doi: 10.1038/s41586-019-1011-z. [DOI] [PubMed] [Google Scholar]
- 106.Yoon J.H., Kim I.J., Kim H., Kim H.J., Jeong M.J., Ahn S.G. Amino acid transport system l is differently expressed in human normal oral keratinocytes and human oral cancer cells. Cancer Lett. 2005;222(2):237–245. doi: 10.1016/j.canlet.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 107.Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273(37):23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 108.del Amo E.M., Urtti A., Yliperttula M. Pharmacokinetic role of l-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35(3):161–174. doi: 10.1016/j.ejps.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 109.Kurayama R., Ito N., Nishibori Y., Fukuhara D., Akimoto Y., Higashihara E. Role of amino acid transporter LAT2 in the activation of mTORC1 pathway and the pathogenesis of crescentic glomerulonephritis. Lab Invest. 2011;91(7):992–1006. doi: 10.1038/labinvest.2011.43. [DOI] [PubMed] [Google Scholar]
- 110.Broer S. The SLC38 family of sodium-amino acid co-transporters. Pflug Arch. 2014;466(1):155–172. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 111.Scalise M., Galluccio M., Pochini L., Cosco J., Trotta M., Rebsamen M. Insights into the transport side of the human SLC38A9 transceptor. Biochim Biophys Acta Biomembr. 2019;1861(9):1558–1567. doi: 10.1016/j.bbamem.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 112.Rebsamen M., Pochini L., Stasyk T., de Araujo M.E., Galluccio M., Kandasamy R.K. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519(7544):477–481. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wyant G.A., Abu-Remaileh M., Wolfson R.L., Chen W.W., Freinkman E., Danai L.V. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell. 2017;171(3):642–654. doi: 10.1016/j.cell.2017.09.046. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang S., Tsun Z.Y., Wolfson R.L., Shen K., Wyant G.A., Plovanich M.E. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347(6218):188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vig B.S., Huttunen K.M., Laine K., Rautio J. Amino acids as promoieties in prodrug design and development. Adv Drug Deliv Rev. 2013;65(10):1370–1385. doi: 10.1016/j.addr.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Gaucher B., Rouquayrol M., Roche D., Greiner J., Aubertin A.M., Vierling P. Prodrugs of HIV protease inhibitors-saquinavir, indinavir and nelfinavir-derived from diglycerides or amino acids: synthesis, stability and anti-HIV activity. Org Biomol Chem. 2004;2(3):345–357. doi: 10.1039/b313119j. [DOI] [PubMed] [Google Scholar]
- 117.Rouquayrol M., Gaucher B., Roche D., Greiner J., Vierling P. Transepithelial transport of prodrugs of the HIV protease inhibitors saquinavir, indinavir, and nelfinavir across Caco-2 cell monolayers. Pharm Res. 2002;19(11):1704–1712. doi: 10.1023/a:1020913631309. [DOI] [PubMed] [Google Scholar]
- 118.Huttunen J., Gynther M., Huttunen K.M. Targeted efflux transporter inhibitors - A solution to improve poor cellular accumulation of anti-cancer agents. Int J Pharm. 2018;550(1–2):278–289. doi: 10.1016/j.ijpharm.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 119.Peura L., Malmioja K., Laine K., Leppanen J., Gynther M., Isotalo A. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: new prodrug design ideas for central nervous system delivery. Mol Pharm. 2011;8(5):1857–1866. doi: 10.1021/mp2001878. [DOI] [PubMed] [Google Scholar]
- 120.Huttunen J., Peltokangas S., Gynther M., Natunen T., Hiltunen M., Auriola S. l-Type amino acid transporter 1 (LAT1/Lat1)-utilizing prodrugs can improve the delivery of drugs into neurons, astrocytes and microglia. Sci Rep. 2019;9(1):12860. doi: 10.1038/s41598-019-49009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ylikangas H., Malmioja K., Peura L., Gynther M., Nwachukwu E.O., Leppanen J. Quantitative insight into the design of compounds recognized by the l-type amino acid transporter 1 (LAT1) ChemMedChem. 2014;9(12):2699–2707. doi: 10.1002/cmdc.201402281. [DOI] [PubMed] [Google Scholar]
- 122.Thiele N.A., Karkkainen J., Sloan K.B., Rautio J., Huttunen K.M. Secondary carbamate linker can facilitate the sustained release of dopamine from brain-targeted prodrug. Bioorg Med Chem Lett. 2018;28(17):2856–2860. doi: 10.1016/j.bmcl.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 123.Montaser A., Huttunen J., Ibrahim S.A., Huttunen K.M. Astrocyte-targeted transporter-utilizing derivatives of ferulic acid can have multifunctional effects ameliorating inflammation and oxidative stress in the brain. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/3528148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Umapathy N.S., Ganapathy V., Ganapathy M.E. Transport of amino acid esters and the amino-acid-based prodrug valganciclovir by the amino acid transporter ATB(0,+) Pharm Res. 2004;21(7):1303–1310. doi: 10.1023/b:pham.0000033019.49737.28. [DOI] [PubMed] [Google Scholar]
- 125.Gupta N., Prasad P.D., Ghamande S., Moore-Martin P., Herdman A.V., Martindale R.G. Up-regulation of the amino acid transporter ATB(0,+) (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100(1):8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 126.Kwak E.Y., Shim W.S., Chang J.E., Chong S., Kim D.D., Chung S.J. Enhanced intracellular accumulation of a non-nucleoside anti-cancer agent via increased uptake of its valine ester prodrug through amino acid transporters. Xenobiotica. 2012;42(7):603–613. doi: 10.3109/00498254.2011.646339. [DOI] [PubMed] [Google Scholar]
- 127.Srinivas S.R., Prasad P.D., Umapathy N.S., Ganapathy V., Shekhawat P.S. Transport of butyryl-l-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB(0,+) Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1046–G1053. doi: 10.1152/ajpgi.00233.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh N., Ecker G.F. Insights into the structure, function, and ligand discovery of the large neutral amino acid transporter 1, LAT1. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patel M., Mandava N., Gokulgandhi M., Pal D., Mitra A.K. Amino acid prodrugs: an approach to improve the absorption of HIV-1 protease inhibitor. Lopinavir. Pharm. 2014;7(4):433–452. doi: 10.3390/ph7040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Peura L., Malmioja K., Huttunen K., Leppanen J., Hamalainen M., Forsberg M.M. Design, synthesis and brain uptake of LAT1-targeted amino acid prodrugs of dopamine. Pharm Res. 2013;30(10):2523–2537. doi: 10.1007/s11095-012-0966-3. [DOI] [PubMed] [Google Scholar]
- 131.Patel M., Dalvi P., Gokulgandhi M., Kesh S., Kohli T., Pal D. Functional characterization and molecular expression of large neutral amino acid transporter (LAT1) in human prostate cancer cells. Int J Pharm. 2013;443(1–2):245–253. doi: 10.1016/j.ijpharm.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 132.Ylikangas H., Peura L., Malmioja K., Leppanen J., Laine K., Poso A. Structure-activity relationship study of compounds binding to large amino acid transporter 1 (LAT1) based on pharmacophore modeling and in situ rat brain perfusion. Eur J Pharm Sci. 2013;48(3):523–531. doi: 10.1016/j.ejps.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 133.Hafliger P., Charles R.P. The l-Type amino acid transporter LAT1-an emerging target in cancer. Int J Mol Sci. 2019;20(10) doi: 10.3390/ijms20102428. pii: E2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li L., Di X., Wu M., Sun Z., Zhong L., Wang Y. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: toward a novel active targeting strategy in breast cancer therapy. Nanomedicine. 2017;13(3):987–998. doi: 10.1016/j.nano.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 135.Kou L., Yao Q., Sivaprakasam S., Luo Q., Sun Y., Fu Q. Dual targeting of l-carnitine-conjugated nanoparticles to OCTN2 and ATB(0,+) to deliver chemotherapeutic agents for colon cancer therapy. Drug Deliv. 2017;24(1):1338–1349. doi: 10.1080/10717544.2017.1377316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.