Abstract
Amino acid transporters, which play a vital role in transporting amino acids for the biosynthesis of mammalian cells, are highly expressed in types of tumors. Increasing studies have shown the feasibility of amino acid transporters as a component of tumor-targeting therapy. In this review, we focus on tumor-related amino acid transporters and their potential use in tumor-targeting therapy. Firstly, the expression characteristics of amino acid transporters in cancer and their relationship with tumor growth are reviewed. Secondly, the recognition requirements are discussed, focusing on the “acid-base” properties, conformational isomerism and structural analogues. Finally, recent developments in amino acid transporter-targeting drug delivery strategies are highlighted, including prodrugs and nanocarriers, with special attention to the latest findings of molecular mechanisms and targeting efficiency of transporter-mediated endocytosis. We aim to offer related clues that might lead to valuable tumor-targeting strategies by the utilization of amino acid transporters.
Keywords: Amino acid transporter, Tumor-targeting, Drug delivery, Prodrug, Nanocarriers
Graphical abstract
1. Introduction
Amino acids are indispensable nutrients for all living cells. Both non-essential and essential amino acids play a key role in supporting cell growth and proliferation. In tumor cells, the rapid growth and proliferation of tumor cells have an increased demand for amino acids, which is necessary to support the basic unit of protein synthesis [1].
Amino acids are hydrophilic that cannot freely transfer through mammalian cell membrane [2]. The uptake of amino acids into cells requires the assistance of specific transporters on the cell membrane [3]. To date, a plenty of amino acid transporters have been found to be expressed in mammals. They are differentiated by substrate selectivity, appetency, driving force and coupling ions (e.g., H+, Na+, K+ and Cl−) [3]. Since tumor cells have a unique metabolic demand for amino acids to satisfy rapid growth and proliferation, the expression of amino acid transporters is elevated compared with that of normal tissues [4,5]. Thus, amino acid transporters have been involved as emerging targets for cancer therapy [2].
Traditional targeting strategies have mainly focused on “starving cancer cells to death” by blocking the intake of nutrients [6]. Studies have shown that the blockade of amino acid transporters is more selective in tumor cells and avoids undesirable off-target effects due to different distribution profile of transporters [2,6]. However, from another perspective, this particular distribution might also provide an opportunity for tumor-targeting therapy, such as the application in positron emission tomography (PET), boron neutron capture therapy (BNCT) and chemotherapeutic drug delivery system [7], [8], [9]. Currently, several commercially available drugs, including methyldopa (ActavisⓇ), levodopa (StalevoⓇ), and baclofen (ZentivaⓇ), have been clinically used that utilized the transport function of SLC7A5 and SLC7A8. Moreover, valaciclovir (ValtrexⓇ), as the l-valine ester of acyclovir, has been confirmed to be a substrate for PEPT1 and SLC6A14. Several ongoing clinical trials are also conducted for elucidating the clinical value of amino acid-tracer for diagnosis and staging of cancer based on its high expression level in tumor. For example, a form of tryptophan marked with radiation has been applied for PET scan to recognize and differentiate between various types of brain tumors (NCT02367482) [10]. Understanding tryptophan metabolism in brain tumors will help to find new approaches to treat brain tumors by altering abnormal tryptophan metabolism. Moreover, a phase I study has been carried out to evaluate whether 18F-fluciclovine PET/CT can better stage muscle invasive bladder cancer, and reveal the pathologic grade of the bladder cancer by targeting SLC1A5 and SLC7A5 amino acid transporters, compared to conventional detection methods (NCT04018053) [11].
In this review, we concentrate on tumor-related amino acid transporters and targeting delivery strategies for cancer therapy, including (1) amino acid transporters in tumors; (2) important roles of amino acid transporter in the development and progression of tumors; (3) structural characteristics of typical substrates; (4) amino acid transporter as a target for rational design of prodrugs; and (5) amino acid transporter-targeted nanocarriers.
2. Amino acid transporters in tumors
Due to the hydrophilicity, amino acids cannot diffuse through the biofilm and rely on specific transporters to traverse across biological membranes to satisfy nutritional requirements and participate in cell metabolism. The amino acid transporters are expressed in different levels in types of cancers, and exhibit different properties in substrate selectivity, required coupling ions and transporting dynamics (Table 1).
Table 1.
Amino acid transporters associated with cancer.
| Amino acids transporter | Gene symbol | Location |
Substrates | Km (μM) | Transport mechanism | |
|---|---|---|---|---|---|---|
| Normal organ/tissue | Cancer type | |||||
| LAT1 | SLC7A5 | Brain, spleen, testis, thymus [13] | Breast cancer, cervical cancer, nasopharyngeal cancer, pulmonary cancer, glioma [14], [15], [16] | Large neutral amino acids, l-dopa, thyroid hormones (T3 and T4), melphalan, baclofen, gabapentin [47] | 10–200 [47] | Obligatory exchange; AA1 exchanged for AA |
| ASCT2 | SLC1A5 | Kidney, intestine [3] | Colorectal cancer, prostate cancer, liver cancer, lung cancer, breast cancer, cervical cancer, esophageal cancer, ovarian cancer, renal cancer, brain cancers [19,20] | Alanine, serine, threonine, glutamine [48] | 20–500 [48] | Obligatory exchange; Na+/AA exchanged for Na+/AA |
| xCT | SLC7A11 | Ubiquitous [3] | Lymphoma, glioma, prostate cancer, breast cancer [26] | Cysteine, glutamate [24] | Obligatory exchange; AA exchanged for AA | |
| ATB0,+ | SLC6A14 | Lung, intestine, prostate, mammary gland, salivary gland, eye [29,49] | Colon cancer, cervical cancer, pancreatic cancer, breast cancer [33] | Neutral and cationic amino acids, carnitine [50] | 6–633 [29] | Unidirectional; Na+/Cl−/AA symport |
| CAT1 | SLC7A1 | Ubiquitous [36] | Liver cancer, colorectal cancer, breast cancer [38], [39], [40], [41] | Arginine, histidine, lysine, pyrrolysine, ornithine [51] | 100–150 [51] | Unidirectional |
AA is an abbreviation for amino acid.
2.1. LAT1
LAT1 (large amino acids transporter, SLC7A5), a high-affinity transporter for branched chain amino acids and aromatic amino acids such as leucine and phenylalanine, is covalently attached to 4F2hc (CD98/SLC3A2) for its trafficking to the cytomembrane [12]. LAT1 is a Na+-independent obligatory exchanger between amino acids [12].
Besides normal tissues (such as brain, spleen, testis and thymus), LAT1 is also highly expressed in tumor cells (such as breast cancer, pulmonary pleomorphic cancer and glioblastoma) [13], [14], [15], [16]. The inhibition of LAT1 is suggested to be an effective therapeutic approach in the context of thyroid [12]. Hafliger et al. found that JPH203, a LAT1 inhibitor, markedly inhibited proliferation of tree anaplastic thyroid cancer through suppression of mammalian target of rapamycin (mTOR) signals and blocked cell cycle progression from the G0/G1 phase to the S phase [12].
2.2. ASCT2
ASCT2, known as SLC1A5, is related to the glutamine exchange with other neutral amino acids such as serine, alanine and cysteine as a modulator [17]. It functions as an obligatory exchanger that imports a Na+-coupled amino acid substrate into cells and exports another Na+-coupled amino acid substrate with 1:1 stoichiometry [18].
Particularly, glutamine is primarily imported into cancer cells via ASCT2 [19]. For highly proliferative cells such as inflammatory and stem cells that have a great demand for glutamine, ASCT2 shows a relatively high expression level compared with normal cells [17]. Similarly, ASCT2 is also highly expressed in many human cancers, such as colorectal, prostate, hepatic, lung, breast, cervical, esophageal, ovarian, renal and brain cancers, being a putative therapeutic target for anti-cancer therapy [19,20]. The blocking of ASCT2 has been successfully used to inhibit tumor cell growth both in vitro and in vivo, with rare effect on normal cells [20], [21], [22], [23].
2.3. xCT
System xc− (cysteine/glutamate antiporter) belongs to the family of heterodimeric amino acid transporter [1]. It consists of a light chain xCT (SLC7A11) as transporter subunit and a heavy chain (SLC3A2) as regulatory subunit [24]. System xc− is highly expressed in cancer cells as a Na+-independent transporter for importing extracellular cysteine and exporting glutamate from cells with a stoichiometry of 1:1 [24,25]. System xc− is essential for maintaining the intracellular redox homeostasis of cancers. xCT is upregulated in cancers to promote glutathione (GSH), and thus reduce oxidative stress and cell apoptosis [26]. A decrease of xCT suppresses the growth of lymphoma, glioma, prostate and breast cancer, and inhibits tumor metastasis [27]. xCT-mediated redox balance in tumor cells is supposed to be related to drug- and radiation-resistance, indicating xCT as a therapeutic target for cancer treatment [26,28].
2.4. ATB0,+
ATB0,+ (SLC6A14) is consisted of 638 amino acids with 12 putative transmembrane domains. It was originally cloned from human mammary gland [29]. The transport function is unidirectional, coupled with a Na+/Cl−/amino acid (or amino acid-like substrate) at a stoichiometry of 2-3:1:1 [30,31]. Thus in theory ATB0,+ owns the ability of concentrating its substrates inside the cells and achieve exceeding multiple times of that in extracellular media, in stark contrast to the majority of other amino acid transporters with only membrane potential [32].
High levels of SLC6A14 has been found in many kinds of cancers, including pancreatic, cervical, colon, stomach, thyroid, endometrioid, rectal, liver and bladder cancer [33]. Furthermore, the overexpressed ATB0,+ in colorectal cancer even markedly promoted the metastases in lymph nodes and liver [34]. It's also worth mentioning that ATB0,+ could transport almost all essential amino acids to meet the enormous nutritional demand for the proliferative tumor cells [35]. Meanwhile, ATB0,+could cooperate with several exchange-type transporters. For instance, glutamine could be concentrated to a sufficient intracellular level via ATB0,+, and acts as an exchangeable substrate for LAT1, ASCT2 and xCT to balance the types of amino acids in supporting the growth of tumor cells [6].
2.5. CAT1
CAT1 (cationic amino acid transporter 1, SLC7A1) belongs to the CAT family (SLC7) [36]. CAT1 is a facilitated diffuser and major entry path in most cells for cationic amino acids and play a key role in nitric oxide synthesis, polyamine biosynthesis and interorgan amino acid flow by delivery l-arginine [37]. CAT1 protein exhibited higher levels in hepatitis B virus (HBV)-related hepatocellular carcinomatous tissues than in para-cancerous tumor tissues [38]. CAT1 was also found to promote cell growth, proliferation and metastasis in colorectal and breast cancer [38], [39], [40]. Besides CAT1, CAT family contains CAT2A (SLC7A2A) and CAT2B (SLC7A2B) [36]. In contrast, CAT2A is mainly located in liver while CAT2B is relevant to immune cells [36,41].
2.6. Other amino acid transporters
GLAST (GLutamate ASpartate Transporter, SLC1A3) is a Na+-dependent glutamate-aspartate transporter expressed by astrocytes with a role in glutamate uptake, which is significantly correlated with a shortened overall survival of patients [42]. New research suggests that UCPH-101, the inhibitor of GLAST, limited the progression and invasion of glioblastoma xenografts, indicating that GLAST might have a crucial role in glutamate trafficking as a new therapeutic target [42].
PQLC2, a heptahelical protein with a duplicated PQ loop (PQ loop repeat-containing), specifically mediates the pH-dependent export of the cationic amino acids (arginine, histidine and lysine) from lysosomes [43]. Yun J. demonstrated that the upregulation of PQLC2 might be involved in the development of gastric cancer. PQLC2 promotes cell growth, anchorage independence and tumor formation, possibly by activating MEK/ERK1/2 and PI3K/AKT signaling [43].
SNAT1, also referred to as ATA1 or SLC38A1, has been found to be overexpressed in breast and gastric cancer and contributes to tumor progression [44]. Consequently, suppression of SNAT1 could also lead to cell growth inhibition, cell cycle arrest and cell apoptosis [45].
LAT2 (SLC7A8), a Na+-independent neutral amino acid transporter, overexpressed in gemcitabine-resistant pancreatic cancer cells, and functions as an oncogenic protein [46]. LAT2 could regulate glutamine-dependent mTOR activation to promote glycolysis and decrease the sensitivity of gemcitabine [46].
3. Important roles of amino acid transporter in the development and progression of tumors
Due to the rapid growth, tumor cells have increased demand for amino acid in maintaining one-carbon metabolism, signal transduction, as well as protein and nucleotide synthesis. Glutamine acts as a prominent role in metabolic pathways of tumors, which is called “glutamine addiction” [2]. Glutamine participates in tricarboxylic acid cycle and offers nitrogen in the synthesis of purines and pyrimidines that comprise the human gene [52]. Glutamine, leucine and arginine also function as activating mTOR that regulates cell growth and cell cycle progression [52,53]. Cysteine is the rate-limiting amino acid in synthesizing GSH against oxidative stress and chemoresistance to certain drugs [54,55]. xCT is the main transporter for cystine that followed converting to cysteine in cells. The blockade of xCT may interfere with tumor growth and reverse resistance to chemotherapy [56,57].
Amino acid transporters expression levels vary to different degrees among cancer types. According to the report of the Cancer Cell Line Encyclopedia project (Fig. S1A–E), the mRNA expression of SLC7A11 showed the highest level in giant cell tumor and the lower levels in leukemia. Besides, the mRNA expression of SLC7A5 is higher than other four amino acid transporters (SLC1A5, SLC7A11, SLC7A1 and SLC6A14) in upper aerodigestive tumor. These data can help us choose suitable targets for tumor-targeted delivery system. Additionally, the expression of amino acid transporters also depends on the molecular subtypes of cancers. Take breast cancer as a sample, ASCT2 is upregulated in human epidermal growth factor receptor 2 (HER-2) type breast cancer [58]. Moreover, ATB0,+ showed 9.8-fold higher expression in estrogen receptor (ER) positive breast cancer specifically than adjacent normal tissues, since it is induced by estrogen [59]. Besides, LAT1 were found in HER-2 and triple-negative breast cancer (TNBC) with higher levels than luminal A and B subtypes [60].
The expression of amino acid transporter is strongly associated with tumor size, tumor grade or metastasis [14,61,62]. According to the data from Kaplan-Meier Plotter, the level of ATB0,+ expression in cancer affects the overall survival (OS) and relapse-free survival (RFS) of patients (Fig. S1F-I), and always represents poor prognosis of cancer. Compared with patients expressing high level of SLC6A14, the patients with low level of that exhibited a longer OS and RFS in kidney renal papillary cell carcinoma and lung adenocarcinoma.
4. Structural characteristics of typical substrates
Specialized amino acid transporters mediate the transfer of corresponding substrates across the cell membrane depending on their typical properties.
4.1. Properties of amino acids
The ionic equilibrium of substrates depends on the pH in blood and extracellular environment. Based on the “acid-base” property of amino acids, transporters are classified into acidic transporter system (e.g., XAG−), basic transporter system (e.g., y+), neutral transporter system (e.g., ASC, B0, L) and basic/neutral transporter system (e.g., B0,+, y + L, b0,+) [3]. For example, ASCT2, which belongs to system ASC, preferred to transporting neutral amino acids with Km value in the range of 20–500 µM [48]. CAT1 (system y+) is verified to mediate cationic amino acid-preferring substrates, showing Km value at a range of 100–150 μM for l-arginine, l-lysine and l-ornithine, and easily triggered by substrates at the trans-side of the membrane [51].
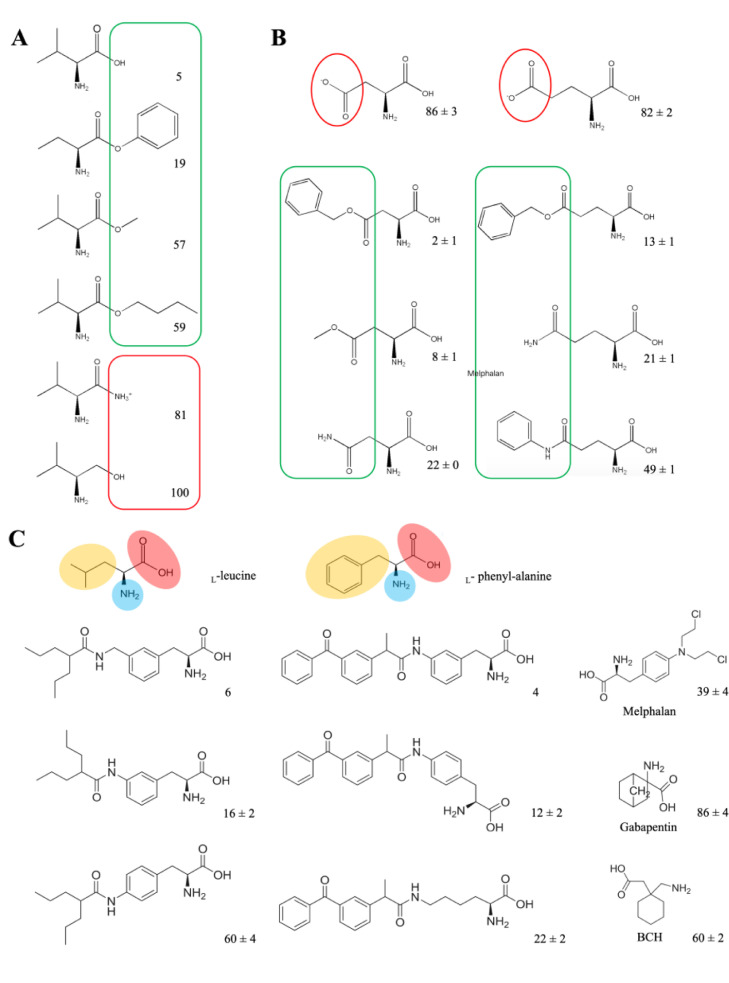
In terms of substrate selectivity, different amino acid transporters have different recognition ranges for substrates. ATB0,+ (system B0,+) showed a wide selectivity of substrates, transporting 20 naturally essential amino acids (except proline, aspartate and glutamate) with Km value ranging from 6 to 633 µM [29]. When the α-carboxyl group of the neutral amino acid was amidated or replaced by hydroxymethyl group, the subsequent structures obstructed the recognition of ATB0,+ [31]. However, when the α-carboxyl group was esterified, the binding force with ATB0,+ still existed (Fig. 1A) [31]. Furthermore, the recognition of the esterification of α-carboxyl group by ATB0,+ is not restricted to small aliphatic group. The choice of esters is broad, such as large aliphatic esters and aromatic esters. Among the derivatives (L-val-methyl ester, l-val-butyl ester and l-val-benzyl ester), l-val-benzyl ester showed outstanding affinity with ATB0,+ [31]. In addition, the neutral amino acids for esterification can choose from small aliphatic amino acids (e.g., glycine, alanine), bulky hydrophobic amino acids (valine, leucine, isoleucine) and aromatic amino acids (phenylalanine) [31]. ATB0,+ is confirmed to accept not only cationic amino acids, but also some amino acid derivatives with basic features [30]. Thus, some amino acids that cannot be transported by ATB0,+ could be recognized through esterification or acylation of carboxyl group. For example, aspartate and glutamate were not substrates of ATB0,+ owing to their anionic side chains, however, β-carboxyl derivatives of aspartate and γ-carboxyl derivatives of glutamate exhibited different interaction efficiency with ATB0,+(Fig. 1B) [30]. The interaction efficiency possibly depends on the electron-donating ability and steric hindrance of substituents. These results indicated that the modification of anionic side chain of amino acids to neutral or cationic ones might help to be recognized as substrates by ATB0,+.
Fig. 1.
The structure characteristics of substrates for ATB0,+ and LAT1. (A) Neutral amino acid (valine) and its derivatives. Adapted from Umapathy et al. [31]. (B) Aspartate/glutamate and their derivatives. The values given in (A)&(B) represent the percent of glycine uptake percentage in present of the amino acid or corresponding derivatives as competitors. Glycine uptake measured in the absence of any competitors is taken as 100%. In the structures, the moieties in favor of binding with ATB0,+ is highlighted in green line, otherwise in red. Adapted from Hatanaka et al. [30]. (C) Structure requirement of substrates for LAT1. The values given represent the percent of leucine uptake percentage in present of the analogs as competitors. Leucine uptake measured in the absence of any competitors is taken as 100%. In the structures of l-leucine and l-phenyl-alanine, the positive-charged amino group is highlighted in blue, the negative-charged carboxyl group is highlighted in red and the hydrophobic/aromatic moiety is highlighted in yellow. Adapted from Karkkainen et al. [66].
Additionally, the affinity between anionic amino acids and transporters might also be affected by the pH in the environment. For instance, the Km for glutamate of ASCT2 decreased as pH got lower [3]. The uptake of β-alanine via ATB0,+ also showed a downward trend when the extracellular pH changed from pH 7.4 to 5.5, suggesting that the affinity of the substrates to transporters might be affected by the microenvironmental acidity [3,63].
Most amino acid transporters transport substrates in the form of l-enantiomers. d-amino acids, generally considered foreign to metabolic pathway in mammals, are abundant in colon and ileum where bacteria colonization is widespread [64]. They have the potential of modulating various biological functions in human. Hatanaka found that LAT1 and ATB0,+ were capable of mediating transport of both l-enantiomers and d-enantiomers [64]. However, not all amino acids can be transported as d-enantiomers, and some of them are only transportable in the form of as l-enantiomers [64].
4.2. Amino acid analogs
A wide variety of structural analogs of amino acids can be regarded as transportable substrates for specific transporters in cancer cells. For instance, LAT1 transported several amino acid analogues, such as l-dopa, thyroid hormones (T3 and T4), melphalan, baclofen and gabapentin with a large maximal transport capacity (Vmax≈40–60 nmol/min/g) and binding affinity (Km≈10–200 µM) at blood brain barrier (BBB) [47]. Additionally, substrates of LAT1 request the structure containing both a positive-charged amino group and a negative-charged carboxyl group, as well as a hydrophobic or an aromatic moiety next to a hydrogen bond acceptor in the center, similar to natural amino acids (e.g., l-phenyl-alanine and l-leucine) (Fig. 1C) [65]. Compared with para-conjugation and aliphatic amino acid analog, the meta-conjugation of l-phenyl-alanine had an increased affinity with LAT1 [66]. A large, rigid and aromatic meta-substituted l-phenyl-alanine derivative could achieve more selective and efficient cellular uptake than a smaller, flexible and aliphatic conjugate in LAT1-mediated endocytosis. The selectivity and transport efficacy could also be elevated by linking a methylene group between the aromatic ring of meta-substituted l-phenyl-alanine and the substituent [66]. Nagamori found that carboxylic ester and hydroxamic acid derivatives of amino acid analogs were also the preferred substrates of LAT1 [67,68].
5. Amino acid transporter as a target for rational design of prodrugs
The differential distribution of amino acid transporters confers important properties as a key point to targeted therapy. By utilizing prodrug approach, it is possible to convert a drug molecule to amino acid-mimicking substrates that has the possibility to make use of amino acid-mediated selective delivery into the target cells (Table 2).
Table 2.
Amino acid transporter mediated prodrugs for tumor-targeting therapy.
| Prodrug | Structure | Amino acid transporter | Cancer type | Efficacy | Reference |
|---|---|---|---|---|---|
| Methionine-doxorubicin conjugate | 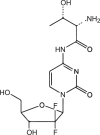 |
LAT1 | Human liver cancer, lung cancer, breast cancer; mouse sarcoma | Low toxicity of gold nanoclusters (Au-NCs) Long circulation time High tumor affinity High NIR fluorescence intensity High anti-tumor activity of DOX |
[73] |
| Gemcitabine-threonine amide prodrug | 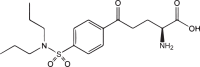 |
LAT1 | Human pancreatic cancer | High toxicity to cancer cells Long circulation time |
[74] |
| Aliphatic/aromatic amino acid-propenecid conjugate | 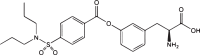 |
LAT1 | Human breast cancer | High cellular accumulation of anti-cancer agent Low multidrug resistance (MDR) |
[72] |
| Tyrosine-conjugated P(NIPAAm-co-DMAAm)-fluorescein-5-maleimide | 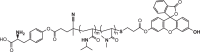 |
LAT1 | Human cervical cancer | Thermoresponsitivity High cellular uptake |
[81] |
| Valine-SN-38 | 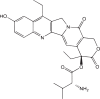 |
ATB0,+ ATA1 ATA2 ASCT2 |
Human breast cancer | High cellular accumulation No MDR | [77] |
| Glutamine-Pt (IV) conjugate | 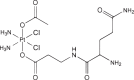 |
ASCT2 | Human lung cancer | Low extrusion by LAT1 High cellular accumulation High antiproliferation activity Low Pt-associated side effects |
[79] |
| Boronophenylalanine | 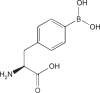 |
LAT1 LAT2 ATB0,+ |
Human glioblastoma, pancreatic cancer, cervical cancer, breast cancer |
High tumor accumulation | [7,80] |
LAT1-utilizing prodrugs were designed to achieve both targeted brain delivery and low peripheral distribution of the parent drug by taking advantage of specific high expression of LAT1 in the BBB and brain parenchyma [69,70]. The aspartate-modified doxorubicin (Asp-DOX), in which aspartate was attached to the N-terminal of DOX via an amide bond with the free carboxyl of Asps, obtained the ability of actively targeting to HepG-2 cells (a human hepatoma cancer cell line overexpressing LAT1) [71]. This targeting property further facilitated the uptake of Asp-DOX in cancer cells and thus showed significant inhibition of tumor growth [71]. Besides, LAT1 has also been applied to facilitating the accumulation of inhibitors of efflux transporters, maintaining the uptake of cytotoxic agents at effectively high concentrations for therapeutic use [72]. Probenecid (PRB, an efflux pump inhibitor), as one of LAT1-utilizing prodrugs, interacted with either multiresistant proteins (MRPs), P-glycoprotein (P-gp) or breast cancer resistant protein (BCRP), and thus increased the cellular accumulation of vinblastine as well as its anti-proliferative efficacy [72]. In addition, several LAT1-based prodrugs were designed to improve the metabolic stability of therapeutic agents [73,74]. For instance, gemcitabine-threonine amide prodrug showed not only elevated anticancer effect in vitro, but also better pharmacokinetic characteristics than gemcitabine, indicating its great potential in treating pancreatic cancer in vivo [74].
Quantities of amino acids and their derivatives can be recognized and transported by ATB0,+, such as nitric oxide synthase inhibitors and carnitine, and even short-chain fatty acid esters of carnitine [32,75,76]. Some studies revealed that several prodrugs are potent substrates for ATB0,+ as a conjugation of amino acid by esterification or acylation, such as valacyclovir (L-valyl ester of acyclovir), Acv-Glu (L-glutamate ester γ-ester of acyclovir) and valganciclovir (L-valyl ester of ganciclovir) [30]. Moreover, SN-38, an active ingredient of irinotecan, was introduced to valine as Val-SN-38, which resulted in increased intracellular accumulation approximately 5-fold in MCF7 cells compared with SN-38 [77]. Furthermore, it was found that ATB0,+ and other Na+-dependent neutral amino acid transporters, such as ATA1(SLC38A1), ATA2 (SLC38A2) and ASCT2, all participated in the uptake of Val-SN-38 to varying degrees [77]. This strategy might contribute to classic nucleoside analogues (cytarabine and decitabine), which led to several times in cellular permeability and bioavailability by conjugating to valine [78].
Glutamine-like Pt (IV) conjugates showed better cellular accumulation and antiproliferative activities by glutamine addiction [79]. In particular, the complex that linked glutamine through the α-carboxylic group acted as a Trojan horse in ASCT2-overexpressing cells [79]. Disguised as glutamine, it entered the cells via the transporter and immediately released cisplatin and glutamine [79]. In boron neutron capture therapy, the efficacy relies on selective delivery of boron-10 to malignant cells. ATB0,+ and LAT1 were found to transport P-boronophenylalanine (BPA) with Km values of 137.4 ± 11.7 and 20.3 ± 0.8 µM, respectively [80]. Both of them transported BPA at affinities comparable with their endogenous substrates and contributed significantly to the tumor accumulation of BPA at clinical doses [80].
6. Amino acid transporter-targeted nanocarriers
For most anticancer agents without reactive group, the conjunction with amino acids is hard to realized. Surprisingly, some functional nanocarriers could be modified with transporter ligands and target to tumors by interacting with corresponding transporter, such as OCTN2 (SLC22A5) [82,83], PEPT1 [84,85] and SVCT1 [86]. So far, increasing studies have demonstrated the potential of amino acid transporter targeted nanocarriers for anticancer agents (Table 3).
Table 3.
Amino acid transporter assisted nanocarriers for tumor-targeting therapy.
| Amino acid transporter | Targeting moiety | Carrier | Drug | Cancer type | Efficacy | Reference |
|---|---|---|---|---|---|---|
| LAT1 | Dopa | Liposomes | WP1066 | Glioma | High brain accumulation (i.v.) High OS (60% higher vs. untreated) High growth inhibition of orthotopically glioblastoma |
[87] |
| Multi-branched gold nanoparticles; AuNPs | —— | Breast cancer | Elevated sensitivity of cancer cells to anticancer drugs High accumulation and cytotoxicity in cancer cells |
[99] | ||
| Glutamate | TPGS Liposomes | Docetaxel | Mouse glioma | High brain accumulation (i.v.) | [88] | |
| Polyoxyethylene stearate PLGA nanoparticle; SPG NPs | Paclitaxel | Human and mouse breast cancer; human cervical cancer | High tumor accumulation High antitumor efficiency in vivo (i.v.) |
[89] | ||
| L-tyrosine | Poly (N-isopropylacrylamide- co-N,N- dimethylacryl amide) liposomes; P (NIPAAm- co-DMAAm) liposomes | —— | Human cervical cancer | Temperature responsibility High cellular uptake |
[90] | |
| L-methionine | grafted hyaluronic-acid-N-acetyl-l-methinoine; HA-ADH-Met | —— | —— | High binding affinity with LAT1 | [100] | |
| ATB0,+ | Lysine | Liposomes | Docetaxel | Human liver cancer, mouse liver cancer | High TIR Low systemic toxicity | [91] |
| Aspartate | Liposomes | Docetaxel | Human lung cancer | Enhanced cellular uptake Low vascular irritation |
[92] | |
| L-carnitine | PLGA nanoparticles | 5-Fluorouracil | Human colon cancer, breast cancer | High cellular uptake High cytotoxicity | [50] | |
| ASCT2 | Glutamine | Polyplexs | siRNA | Human lung cancer | Intracellular glutamine exhaustion and SLC1A5 upregulation Elevated sensitivity of cancer cells to cisplatin |
[94] |
| β-cyclodextrin inclusion complex | Doxorubicin | Human triple-negative breast cancer | High antitumor efficiency in vivo (i.v.) Minimizing the toxicity in main organs |
[93] |
6.1. Targeting to LAT1
LAT1 has been confirmed to be overexpressed on brain capillary endothelial cells (BCECs) and glioma cells [87]. Hence in the therapy of central nervous system diseases, some studies utilized the special properties of LAT1 in BBB to facilitate the transport of drugs in the brain by preparing LAT1 substrate-modified nanoparticles [87]. A novel l-3,4-dihydroxyphenylalanine (L-DOPA) functionalized amphiphile (Amphi-DOPA) was synthesized to modify liposomes to enhance the BBB penetration in glioma therapy [87]. WP1066-loaded liposomes with Amphi-DOPA modification showed preferential accumulation in brain and higher overall survivability of mice bearing orthotopical glioblastoma by about 60% vs. untreated group [87]. Glutamate-D-α-tocopherol polyethylene glycol 1000 succinate (Glu-TPGS) has also been proved to enhance the BBB penetration, glioma accumulation and cellular uptake of docetaxel (DTX)-loaded liposomes via LAT1 [88]. These results implicated that LAT1-assisted strategy contributed to an optional direction for the development in brain glioma-targeting nano-drug delivery system.
Besides BCECs and glioma cells, LAT1 is also found to be abundant in breast cancer [89]. Glutamate was modified on poly (lactic-co-glycolic acid) (PLGA) particles to enhance the cellular uptake and cytotoxicity of paclitaxel (PTX) in breast cancer cells [89]. As expected, Glu-TPGS modified nanoparticles demonstrated improved tumor site accumulation and antitumor effects compared with unmodified ones in a subcutaneous 4T1 breast cancer mouse model [89]. Similarly, l-tyrosine-conjugated thermo-responsive liposomes led to the elevated cellular uptake of liposomes in Hela cells by enhanced affinity between l-tyrosine and the overexpressed LAT1 [90].
6.2. Targeting to ATB0,+
Amino acid transporter ATB0,+ mediates cellular uptake of its substrates with a broad selectivity and high transporting concentrative capability, which has attracted increasing attention for assisting tumor-targeting nano-drug delivery system. Amino acids with different electrical properties, such as glycine, aspartate and lysine, were covalently linked with polyoxyethylene stearate and then modified on nanocarriers to target overexpressed ATB0,+ on cancer cells [91]. Among them, lysine-modified liposomes (LPS-Lips) exhibited superior tumor-targeting properties. In vivo studies demonstrated that DTX-loaded LPS-Lips showed 1.45- and 1.91-fold higher tumor inhibition rate (TIR) than unmodified liposomes and TaxotereⓇ, indicating strongest suppression effect of tumor [91]. In terms of safety, ATB0,+-mediated nanoparticles showed less weight loss and no obvious structural disturbances in liver or kidney, and greatly alleviated vascular irritation of TaxotereⓇ, suggesting that ATB0,+-utilizing drug delivery could also minimize local and system toxic effects [91,92].
6.3. Targeting to ASCT2
The glutamine uptake pathway via ASCT2 could be exploited as an alternative transport approach for the therapy of glutamine-addicted cancers. For example, glutamine was conjugated with 6-hydroxy of β-cyclodextrin to load DOX as inclusion complex (DOX@GLN-CD) for TNBC [93]. GLN-CD showed similar ASCT2-binding sites compared with glutamine, and specifically accumulated in ASCT2-expressed TNBC cells [93]. Consequently, DOX@GLN-CD improved the antitumor effects and minimized the toxic effects of DOX on main organs in MDA-MB-231 breast cancer-bearing mice [93]. In another study, glutamine macromolecular analog polyglutamine (PGS) was utilized to deliver therapeutic siRNA to glutamine-addicted cancer cells. The modified nanocarrier exhibited a high binding affinity to ASCT2 and increased intracellular accumulation [94].
6.4. Dual-targeting strategies
It is known that tumors are consisted of heterogeneous tumor cells, in which a series of specific amino acids are expressed with different expression levels [9]. Furthermore, there is also a difference between expression level of a certain transporter according to different tumor sites, histopathologic types or neoplasm staging [50,95]. Therefore, dual-targeting strategies seem to be an effective approach to overcome tumor heterogeneity [96], [97], [98]. For example, the development of l-carnitine-modified nanoparticles was designed to target both OCTN2 and ATB0,+ that highly expressed in colon cancer cells [50]. The modified nanoparticles made progress in cellular uptake and cytotoxicity of 5-fluorouacil by providing more chances for the multivalent binding between nanoparticles and tumor cell surface [50]. Wang Z. synthesized polyethylene glycol stearate with the conjugation of glutamate, lysine and tyrosine to modify irinotecan-loaded liposomes for the targeting of LAT1 and ATB0,+ simultaneously. The dual-targeting liposomes exhibited a better anti-tumor effect than OnivydeⓇ and single-targeting liposomes both in vitro and in vivo [98].
6.5. Molecular mechanisms of amino acid transporter-mediate uptake of nanocarriers
As discussed above, it is sufficiently compelling that the use of amino acid transporter might be a promising approach to increase the efficacy of amino acid transporter-mediated antitumor drugs and a novel therapeutic approach in the context of cancers. Numbers of studies have focused on the mechanisms of how amino acid transporters effectively mediate nanocarriers into cells with specific modification [101].
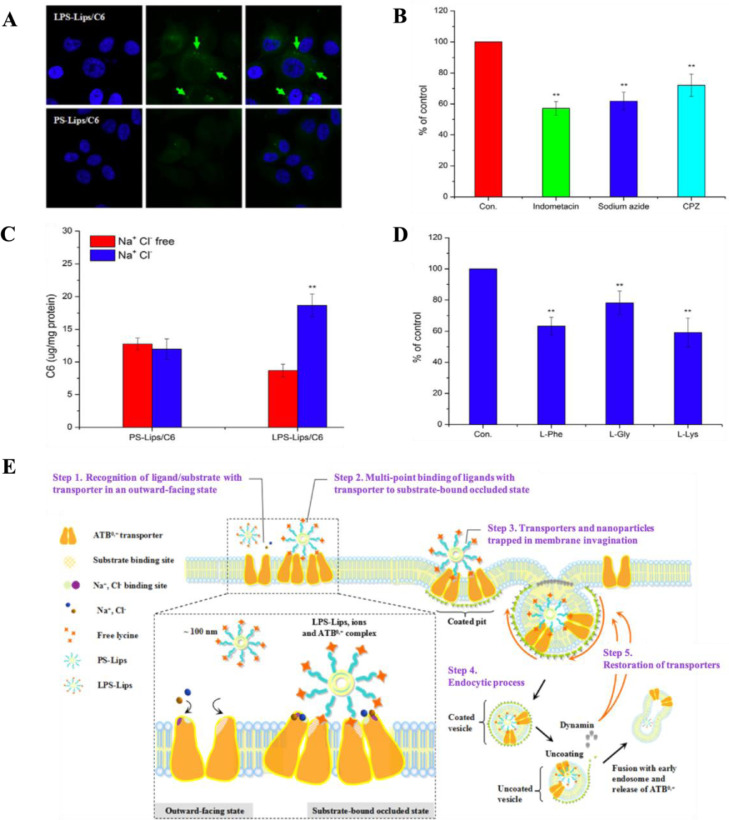
The mechanism of amino acid transporter-mediated uptake of nanocarriers has been speculated as a receptor-mediated process [91]. Previous results indicated that the uptake efficiency mediated by transporter had a positive correlation with incubation time, ligand ratio, ions and affinity of nanocarriers to membrane, but might be inhibited by the presence of endocytic inhibitors or free amino acids (Fig.2A–2D) [88,91,94]. A better attachment capability was suggested to be derived from the intensive interaction with cell membrane where transporter serving as an anchoring point for particles with ligands/substrates. Depending on the factors affecting the uptake efficiency mediated by amino acid transporters, the transport process possibly involves five steps (take ATB0,+ mediated uptake of lysine-modified liposome as an example): (1) recognition of ligand/substrate with transporter in an outward-facing state; (2) multi-point binding of ligands with transporter to substrate-bound occluded state; (3) transporters and nanoparticles trapped in membrane invagination; (4) endocytic process; (5) restoration of transporters (Fig. 2E) [91].
Fig. 2.
Affecting factors involved in the uptake of lysine-modified coumarin 6-loaded liposomes (LPS-Lips/C6) mediated by ATB0,+. (A) More liposomes attached on cell membrane of LPS-Lips/C6 compared with unmodified liposomes (PS-Lips/C6) were observed in confocal microscope images (50 ×); (B) The cellular uptake of LPS-Lips/C6 was suppressed in the presence of endocytic inhibitors; (C) The cellular uptake of LPS-Lips/C6 was enhanced in the presence of Na+ and Cl− ions; (D) The cellular uptake of LPS-Lips/C6 was suppressed in the presence of amino acids; (E) The speculative process of ATB0,+-mediated uptake of nanocarriers. Adapted from Luo et al. [91].
To sum up, it is definite about the positive role of amino acid transporters in efficient tumor-targeting drug delivery. However, it is unclear how these nanocarriers in turn affect the restoration of corresponding amino acid transporters. According to recent studies, two possible restoration pathways were discovered on amino acid transporters: (1) recycling back to cell membranes, (2) degradation through endosome/lysosome routes. In the former, once the substrate-modified nanocarriers along with endocytic cargos dissociate from the amino acid transporters, these transporters return back to cell membrane. During the entire process, the total transporter protein remains constant, suggesting the sufficient levels of amino acid transporters. Li L. indicated LAT1-targeting nanoparticles endocytosis caused the level of LAT1 protein decrease on the cell membrane but increase in the cytoplasm at the beginning [89]. While LAT1 protein diminished from cytoplasm, it recovered on cell membrane, confirming the original aspect [89]. On the contrary, ATB0,+-targeted nanocarriers endocytosis led to a gradual reduction of ATB0,+ protein, verifying the degradation of the transporter during the uptake [50,91].
6.6. Effects of nanocarriers themselves on transporter-targeting efficiency
The physicochemical properties of nanocarriers, including sharp, size and charges, determine their in vivo fates in the circulation. The shape of nanocarriers could be classified as spherical, cubic, rod-like and worm-like ones, which influences the uptake efficiency to a large extent [102]. Rod-like and worm-like nanoparticles showed higher uptake than spherical polymeric nanoparticles [102]. The size of nanocarriers for intravenous administration is in a range of 10–200 nm, which is in favor of tumor penetration and cellular uptake for tumor therapy by taking advantage of the EPR effect [102]. Undersized particles (about 10 nm) would undergo filtration at the renal glomerulus and accordingly get fast eliminated [103]. For nanocarriers with ligands, a size of ∼25 nm is the enthalpic limit for spherical nanoparticles binding to target proteins on the surface of cancer cells in close proximity to drive the membrane-wrapping process [104]. However, oversized particles (greater than 200 nm) would activate the complement system and be removed fast from the blood stream, resulting in the accumulation in liver and spleen [105]. In the case of intravenous administration, a negative or neutral nanoparticle is more stable in the system circulation, while positive charged nanoparticle showed better reaction with negative charged cell membrane for uptake [106].
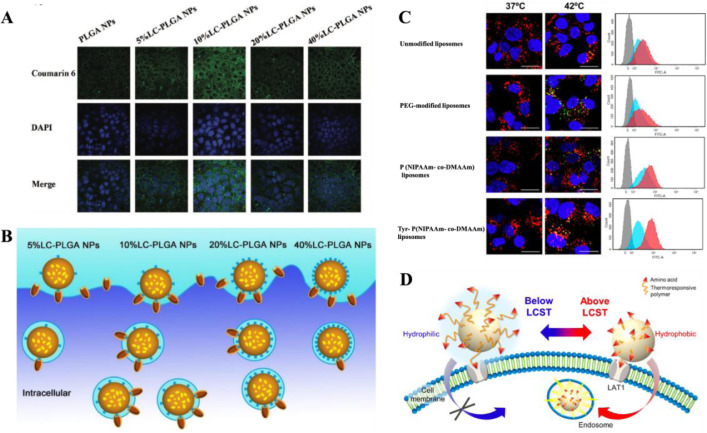
Furthermore, the density of substrate/ligand modification also affects the targeting efficiency of amino acid transporter-targeting nanoparticles. Li found that the uptake of glutamate-modified nanoparticles was growing with the increase of glutamate density [89]. Similarly, the uptake of aspartate-modified liposomes was improved with the enriching aspartate density in the range of 0–10% and reached maximal effect at 10% of aspartate on liposome. Whereas, the uptake declined as soon as the aspartate density continued to be 20% [83,92]. It could be explained in two aspects: (1) the cellular uptake is increased within a certain range of substrate/ligand density; (2) when the density is exceeded, the subsequent increased ones become too crowded on the surface to compete for transporters, thus decrease cellular endocytosis of nanoparticles (Fig. 3A and 3B) [83,107]. The cellular uptake was improved with the increase of PEG chain length from 10 EGs (ethylene glycol) to 25 EGs, but weaken at 40 EGs [89]. A synthetic action of substrate/ligand density and linker length defines the attachment capacity of nanocarriers on cells [108]. As we discovered, a better attachment capacity of nanocarriers on cell surface leads to a greater cellular uptake of them [91].
Fig. 3.
(A) Confocal microscope images of Caco-2 cells incubated with LC-PLGA NPs at 37 °C for 1 h. Blue: DAPI for nuclei, Green: coumarin 6. (B) The effects of the substrate/ligand density on the nanoparticle absorption. Adapted from Kou et al. [83] (C) Confocal microscope images and fluorescence intensity for flow cytometry of Hela cells incubated with liposomes for 4 h at 37 °C (left or blue) and 42 °C (right or red). Nuclei were stained blue (DAPI), CF appeared green, and lysosomes were stained red (LysoTracker Red DND-99). (D) Schematic illustration showing the LAT1-targeting thermoresponsive liposomes. Adapted from Maekawa-Matsuura et al. [90].
Additionally, the inherent states of substrate/ligand determine the final properties of amino acid transporter mediated nanocarriers. The interaction force between the substrate/ligand and amino acid transporters would be strengthened or suppressed, according to charge transfer and temperature variation. In our study, free aspartate is not a typical substrate of ATB0,+ due to the anionic β-carboxyl group on its side chain, once the resultant side chain loses negative charge via the esterification with PEG, it is recognized as a substrate of ATB0,+ [91]. By contrast, although free glycine is originally a substrate of ATB0,+, it loses the ability to be recognized when conjugated to PEG [91]. In the study of LAT1-targeting liposomes, the cellular uptake of liposomes was enhanced by external temperature rising (Fig. 3C). The Tyr-P (NIPAAm- co-DMAAm) liposomes could be more effectively taken up by Hela cells at 42 °C compared with 37 °C [90]. At temperature above the lower critical solution temperature (LCST) of P(NIPAAm- co-DMAAm), the uptake by cancer cells was enhanced through improving the hydrophobicity of liposome surface and its interaction with cell membrane [90]. The synergetic effect of the hydrophobic interaction between the polymer and cell membranes, and the affinity between polymer terminal ligand and LAT1, improved the cellular uptake of the liposomes (Fig. 3D) [90]. To sum up, these integrated factors determine the attachment capacity of nanocarriers, by which a greater cellular uptake might show up [91,108].
7. Summary and outlook
Tumor cells overexpress various types of transporters to meet the enormous demand for proliferation. Amino acid transporters are markedly located in certain specific tumors and contribute an efficient way to deliver anticancer agents selectively to tumors via prodrugs and nanocarriers. However, several problems need to be settled in the future research, including: (1) amino acid transporters are also equally important to normal cells, thus putting forward the challenge of how to avoid the transporter-mediate uptake of anticancer agents in normal cells; (2) the definite effects of transporter-mediate prodrugs/nanocarriers on transporters is still unclear, raising the question of the design of multiple dosing and its curative effect; (3) despite the success in the safety and effectiveness on animal models, its future clinical effects needs further confirmation, challenging us to unlock the underlying regularity and mechanism of transporter-mediate prodrugs/nanocarriers. Amino acid transporter-mediate tumor-targeting drug delivery strategy opens a new field in tumor treatment, and needs continuous research in the validation of its feasibility.
Conflicts of interest
The authors confirm that this article content has no conflicts of interest.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (Nos. 81803442 and 81703425).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2019.12.002.
Appendix. Supplementary materials
References
- 1.Ganapathy V., Thangaraju M., Prasad P.D. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Bhutia Y.D., Babu E., Ramachandran S., Ganapathy V. Amino acid transporters in cancer and their relevance to "glutamine addiction": novel targets for the design of a new class of anticancer drugs. Cancer Res. 2015;75:1782–1788. doi: 10.1158/0008-5472.CAN-14-3745. [DOI] [PubMed] [Google Scholar]
- 3.Broer S., Fairweather S.J. Amino acid transport across the mammalian intestine. Compr Physiol. 2018;9:343–373. doi: 10.1002/cphy.c170041. [DOI] [PubMed] [Google Scholar]
- 4.Szefel J., Danielak A., Kruszewski W.J. Metabolic pathways of l-arginine and therapeutic consequences in tumors. Adv Med Sci. 2018;64:104–110. doi: 10.1016/j.advms.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Cha Y.J., Kim E.S., Koo J.S. Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19030907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutia Y.D., Babu E., Prasad P.D., Ganapathy V. The amino acid transporter SLC6A14 in cancer and its potential use in chemotherapy. Asian J Pharm Sci. 2014;9:293–303. [Google Scholar]
- 7.Detta A., Cruickshank G.S. l-amino acid transporter-1 and boronophenylalanine-based boron neutron capture therapy of human brain tumors. Cancer Res. 2009;69:2126–2132. doi: 10.1158/0008-5472.CAN-08-2345. [DOI] [PubMed] [Google Scholar]
- 8.Wei L., Tominaga H., Ohgaki R., Wiriyasermkul P., Hagiwara K., Okuda S. Specific transport of 3-fluoro-l-alpha-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 2016;107:347–352. doi: 10.1111/cas.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su H., Wang Y., Liu S., Wang Y., Liu Q., Liu G. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm Sin B. 2019;9:49–58. doi: 10.1016/j.apsb.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://clinicaltrials.gov/ct2/show/NCT02367482?term=transporter&cond=cancer&draw=8&rank=62.
- 11.https://clinicaltrials.gov/ct2/show/NCT04018053?term=LAT1&rank=4.
- 12.Hafliger P., Graff J., Rubin M., Stooss A., Dettmer M.S., Altmann K.H. The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. J Exp Clin Cancer Res. 2018;37:234. doi: 10.1186/s13046-018-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien H.-.C., Colas C., Finke K., Springer S., Stoner L., Zur A.A. Reevaluating the substrate specificity of the l-type amino acid transporter (LAT1) J Med Chem. 2018;61:7358–7373. doi: 10.1021/acs.jmedchem.8b01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Ansari R., Craze M.L., Miligy I., Diez-Rodriguez M., Nolan C.C., Ellis I.O. The amino acid transporter SLC7A5 confers a poor prognosis in the highly proliferative breast cancer subtypes and is a key therapeutic target in luminal B tumours. Breast Cancer Res. 2018;20:21. doi: 10.1186/s13058-018-0946-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaira K., Kawashima O., Endoh H., Imaizumi K., Goto Y., Kamiyoshihara M. Expression of amino acid transporter (LAT1 and 4F2hc) in pulmonary pleomorphic carcinoma. Hum Pathol. 2018;84:142–149. doi: 10.1016/j.humpath.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 16.An S., Lu X., Zhao W., Sun T., Zhang Y., Lu Y. Amino acid metabolism abnormity and microenvironment variation mediated targeting and controlled glioma chemotherapy. Small. 2016;12:5633–5645. doi: 10.1002/smll.201601249. [DOI] [PubMed] [Google Scholar]
- 17.Scalise M., Pochini L., Console L., Losso M.A., Indiveri C. The human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garibsingh R.A., Otte N.J., Ndaru E., Colas C., Grewer C., Holst J. Homology modeling informs ligand discovery for the glutamine transporter ASCT2. Front Chem. 2018;6:279. doi: 10.3389/fchem.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scalise M., Pochini L., Galluccio M., Console L., Indiveri C. Glutamine transport and mitochondrial metabolism in cancer cell growth. Front Oncol. 2017;7:306. doi: 10.3389/fonc.2017.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J., Yang T., Peng Z., Xiao H., Jiang N., Zhang L. SLC1A5 silencing inhibits esophageal cancer growth via cell cycle arrest and apoptosis. Cell Physiol Biochem. 2018;48:397. doi: 10.1159/000491769. [DOI] [PubMed] [Google Scholar]
- 21.Nakaya M., Xiao Y., Zhou X., Chang J.H., Chang M., Cheng X. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masle-Farquhar E., Broer A., Yabas M., Enders A., Broer S. ASCT2 (SLC1A5)-deficient mice have normal B-cell development, proliferation, and antibody production. Front Immunol. 2017;8:549. doi: 10.3389/fimmu.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulte M.L., Fu A., Zhao P., Li J., Geng L., Smith S.T. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24:194–202. doi: 10.1038/nm.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fotiadis D., Kanai Y., Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Robert S.M., Sontheimer H. Glutamate transporters in the biology of malignant gliomas. Cell Mol Life Sci. 2014;71:1839–1854. doi: 10.1007/s00018-013-1521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habib E., Linher-Melville K., Lin H.X., Singh G. Expression of xCT and activity of system xc− are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R.S., Song Y.M., Zhou Z.Y., Tong T., Li Y., Fu M. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene. 2009;28:599–609. doi: 10.1038/onc.2008.414. [DOI] [PubMed] [Google Scholar]
- 28.Koppula P., Zhang Y., Zhuang L., Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun. 2018;38:12. doi: 10.1186/s40880-018-0288-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan J.L., Mager S. Cloning and functional expression of a human Na+ and Cl–dependent neutral and cationic amino acid transporter B0+ J Biol Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- 30.Hatanaka T., Haramura M., Fei Y.J., Miyauchi S., Bridges C.C., Ganapathy P.S. Transport of amino acid-based prodrugs by the Na+- and Cl− -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2004;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- 31.Umapathy N.S., Ganapathy V., Ganapathy M.E. Transport of amino acid esters and the amino-acid-based prodrug valganciclovir by the amino acid transporter ATB0,+ Pharm Res. 2004;21:1303–1310. doi: 10.1023/b:pham.0000033019.49737.28. [DOI] [PubMed] [Google Scholar]
- 32.Hatanaka T., Nakanishi T., Huang W., Leibach F.H., Prasad P.D., Ganapathy V. Na+ - and Cl− -coupled active transport of nitric oxide synthase inhibitors via amino acid transport system B0,+ J Clin Invest. 2001;107:1035–1043. doi: 10.1172/JCI12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sikder M.O.F., Yang S., Ganapathy V., Bhutia Y.D. The Na+/Cl−-coupled, broad-specific, amino acid transporter SLC6A14 (ATB0,+): emerging roles in multiple diseases and therapeutic potential for treatment and diagnosis. AAPS J. 2017;20:12. doi: 10.1208/s12248-017-0164-7. [DOI] [PubMed] [Google Scholar]
- 34.Gupta N., Miyauchi S., Martindale R.G., Herdman A.V., Podolsky R., Miyake K. Upregulation of the amino acid transporter ATB0,+ (SLC6A14) in colorectal cancer and metastasis in humans. Biochim Biophys Acta. 2005;1741:215–223. doi: 10.1016/j.bbadis.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Coothankandaswamy V., Cao S., Xu Y., Prasad P.D., Singh P.K., Reynolds C.P. Amino acid transporter SLC6A14 is a novel and effective drug target for pancreatic cancer. Br J Pharmacol. 2016;173:3292–3306. doi: 10.1111/bph.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chafai A., Fromm M.F., Konig J., Maas R. The prognostic biomarker l-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2A and CAT2B. Sci Rep. 2017;7:4767. doi: 10.1038/s41598-017-04965-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatzoglou M., Fernandez J., Yaman I., Closs E. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 2004;24:377–399. doi: 10.1146/annurev.nutr.23.011702.073120. [DOI] [PubMed] [Google Scholar]
- 38.Dai R., Peng F., Xiao X., Gong X., Jiang Y., Zhang M. Hepatitis B virus X protein-induced upregulation of CAT-1 stimulates proliferation and inhibits apoptosis in hepatocellular carcinoma cells. Oncotarget. 2017;8:60962–60974. doi: 10.18632/oncotarget.17631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelmagid S.A., Rickard J.A., McDonald W.J., Thomas L.N., Too C.K. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J Cell Biochem. 2011;112:1084–1092. doi: 10.1002/jcb.23022. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y., Wang W., Wang J., Yang C., Mao H., Fu X. Overexpression of arginine transporter CAT-1 is associated with accumulation of l-arginine and cell growth in human colorectal cancer tissue. PLoS ONE. 2013;8:e73866. doi: 10.1371/journal.pone.0073866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang J., Nicolas E., Marks D., Sander C., Lerro A., Buendia M.A. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 42.Corbetta C., Di Ianni N., Bruzzone M.G., Patane M., Pollo B., Cantini G. Altered function of the glutamate-aspartate transporter GLAST, a potential therapeutic target in glioblastoma. Int J Cancer. 2019;144:2539–2554. doi: 10.1002/ijc.31985. [DOI] [PubMed] [Google Scholar]
- 43.Jeung Y.J., Lee K., Lee H.J., Kim E., Son M.J., Ahn J. Cationic amino acid transporter PQLC2 is a potential therapeutic target in gastric cancer. Cancer Sci. 2019;110:1453–1463. doi: 10.1111/cas.13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie J., Li P., Gao H.F., Qian J.X., Yuan L.Y., Wang J.J. Overexpression of SLC38A1 is associated with poorer prognosis in Chinese patients with gastric cancer. BMC Gastroenterol. 2014;14:70. doi: 10.1186/1471-230X-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang K., Cao F., Fang W., Hu Y., Chen Y., Ding H. Activation of SNAT1/SLC38A1 in human breast cancer: correlation with p-Akt overexpression. BMC Cancer. 2013;13:343. doi: 10.1186/1471-2407-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng M., Xiong G., Cao Z., Yang G., Zheng S., Qiu J. LAT2 regulates glutamine-dependent mTOR activation to promote glycolysis and chemoresistance in pancreatic cancer. J Exp Clin Cancer Res. 2018;37:274. doi: 10.1186/s13046-018-0947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scalise M., Galluccio M., Console L., Pochini L., Indiveri C. The human SLC7A5 (LAT1): the intriguing histidine/large neutral amino acid transporter and its relevance to human health. Front Chem. 2018;6:243. doi: 10.3389/fchem.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Utsunomiya-Tate N., Endou H., Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 49.Gupta N., Prasad P.D., Ghamande S., Moore-Martin P., Herdman A.V., Martindale R.G. Up-regulation of the amino acid transporter ATB0,+ (SLC6A14) in carcinoma of the cervix. Gynecol Oncol. 2006;100:8–13. doi: 10.1016/j.ygyno.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Kou L., Yao Q., Sivaprakasam S., Luo Q., Sun Y., Fu Q. Dual targeting of l-carnitine-conjugated nanoparticles to OCTN2 and ATB0,+ to deliver chemotherapeutic agents for colon cancer therapy. Drug Deliv. 2017;24:1338–1349. doi: 10.1080/10717544.2017.1377316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Closs E.I., Boissel J.P., Habermeier A., Rotmann A. Structure and function of cationic amino acid transporters (CATs) J Membr Biol. 2006;213:67–77. doi: 10.1007/s00232-006-0875-7. [DOI] [PubMed] [Google Scholar]
- 52.Vanhove K., Derveaux E., Graulus G.J., Mesotten L., Thomeer M., Noben J.P. Glutamine addiction and therapeutic strategies in lung cancer. Int J Mol Sci. 2019;20:252. doi: 10.3390/ijms20020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boutouja F., Stiehm C.M., Platta H.W. mTOR: a cellular regulator interface in health and disease. Cells. 2019;8:18. doi: 10.3390/cells8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu S.C. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 55.Franco R., Cidlowski J.A. Apoptosis and glutathione: beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 56.Zhang P., Wang W., Wei Z., Xu L.I., Yang X., Du Y. xCT expression modulates cisplatin resistance in Tca8113 tongue carcinoma cells. Oncol Lett. 2016;12:307–314. doi: 10.3892/ol.2016.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyoshi S., Tsugawa H., Matsuzaki J., Hirata K., Mori H., Saya H. Inhibiting xCT improves 5-fluorouracil resistance of gastric cancer induced by CD44 variant 9 expression. Anticancer Res. 2018;38:6163–6170. doi: 10.21873/anticanres.12969. [DOI] [PubMed] [Google Scholar]
- 58.Kim S., Kim D.H., Jung W.H., Koo J.S. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr Relat Cancer. 2013;20:339–348. doi: 10.1530/ERC-12-0398. [DOI] [PubMed] [Google Scholar]
- 59.Karunakaran S., Ramachandran S., Coothankandaswamy V., Elangovan S., Babu E., Periyasamy-Thandavan S. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. 2011;286:31830–31838. doi: 10.1074/jbc.M111.229518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furuya M., Horiguchi J., Nakajima H., Kanai Y., Oyama T. Correlation of l-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- 61.Lu X. The role of large neutral amino acid transporter (LAT1) in cancer. Curr Cancer Drug Targets. 2019;19:863–876. doi: 10.2174/1568009619666190802135714. [DOI] [PubMed] [Google Scholar]
- 62.Koshi H., Sano T., Handa T., Yanagawa T., Saitou K., Nagamori S. l-type amino acid transporter-1 and CD98 expression in bone and soft tissue tumors. Pathol Int. 2015;65:460–467. doi: 10.1111/pin.12323. [DOI] [PubMed] [Google Scholar]
- 63.Anderson C.M., Ganapathy V., Thwaites D.T. Human solute carrier SLC6A14 is the beta-alanine carrier. J Physiol. 2008;586:4061–4067. doi: 10.1113/jphysiol.2008.154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hatanaka T., Huang W., Nakanishi T., Bridges C.C., Smith S.B., Prasad P.D. Transport of d-serine via the amino acid transporter ATB0,+ expressed in the colon. Biochem Biophys Res Commun. 2002;291:291–295. doi: 10.1006/bbrc.2002.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rautio J., Gynther M., Laine K. LAT1-mediated prodrug uptake: a way to breach the blood-brain barrier? Ther Deliv. 2013;4:281–284. doi: 10.4155/tde.12.165. [DOI] [PubMed] [Google Scholar]
- 66.Karkkainen J., Gynther M., Kokkola T., Petsalo A., Auriola S., Lahtela-Kakkonen M. Structural properties for selective and efficient l-type amino acid transporter 1 (LAT1) mediated cellular uptake. Int J Pharm. 2018;544:91–99. doi: 10.1016/j.ijpharm.2018.04.025. [DOI] [PubMed] [Google Scholar]
- 67.Nagamori S., Wiriyasermkul P., Okuda S., Kojima N., Hari Y., Kiyonaka S. Structure-activity relations of leucine derivatives reveal critical moieties for cellular uptake and activation of mTORC1-mediated signaling. Amino Acids. 2016;48:1045–1058. doi: 10.1007/s00726-015-2158-z. [DOI] [PubMed] [Google Scholar]
- 68.Zur A.A., Chien H.C., Augustyn E., Flint A., Heeren N., Finke K. LAT1 activity of carboxylic acid bioisosteres: evaluation of hydroxamic acids as substrates. Bioorg Med Chem Lett. 2016;26:5000–5006. doi: 10.1016/j.bmcl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Puris E., Gynther M., Huttunen J., Auriola S., Huttunen K.M. l-type amino acid transporter 1 utilizing prodrugs of ferulic acid revealed structural features supporting the design of prodrugs for brain delivery. Eur J Pharm Sci. 2019;129:99–109. doi: 10.1016/j.ejps.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Puris E., Gynther M., Huttunen J., Petsalo A., Huttunen K.M. l-type amino acid transporter 1 utilizing prodrugs: how to achieve effective brain delivery and low systemic exposure of drugs. J Control Release. 2017;261:93–104. doi: 10.1016/j.jconrel.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 71.Wu W., Dong Y., Gao J., Gong M., Zhang X., Kong W. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci. 2015;106:747–756. doi: 10.1111/cas.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huttunen J., Gynther M., Huttunen K.M. Targeted efflux transporter inhibitors - a solution to improve poor cellular accumulation of anti-cancer agents. Int J Pharm. 2018;550:278–289. doi: 10.1016/j.ijpharm.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 73.Chen H., Li B., Ren X., Li S., Ma Y., Cui S. Multifunctional near-infrared-emitting nano-conjugates based on gold clusters for tumor imaging and therapy. Biomaterials. 2012;33:8461–8476. doi: 10.1016/j.biomaterials.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 74.Hong S., Fang Z., Jung H.Y., Yoon J.H., Hong S.S., Maeng H.J. Synthesis of gemcitabine-threonine amide prodrug effective on pancreatic cancer cells with improved pharmacokinetic properties. Molecules. 2018;23:2608. doi: 10.3390/molecules23102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakanishi T., Hatanaka T., Huang W., Prasad P.D., Leibach F.H., Ganapathy M.E. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB0,+ from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532:297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Srinivas S.R., Prasad P.D., Umapathy N.S., Ganapathy V., Shekhawat P.S. Transport of butyryl-l-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB0,+ Am J Physiol Gastrointest Liver Physiol. 2007;293:G1046–G1053. doi: 10.1152/ajpgi.00233.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwak E.Y., Shim W.S., Chang J.E., Chong S., Kim D.D., Chung S.J. Enhanced intracellular accumulation of a non-nucleoside anti-cancer agent via increased uptake of its valine ester prodrug through amino acid transporters. Xenobiotica. 2012;42:603–613. doi: 10.3109/00498254.2011.646339. [DOI] [PubMed] [Google Scholar]
- 78.Tao W., Zhao D., Sun M., Wang Z., Lin B., Bao Y. Intestinal absorption and activation of decitabine amino acid ester prodrugs mediated by peptide transporter PEPT1 and enterocyte enzymes. Int J Pharm. 2018;541:64–71. doi: 10.1016/j.ijpharm.2018.02.033. [DOI] [PubMed] [Google Scholar]
- 79.Ravera M., Gabano E., Tinello S., Zanellato I., Osella D. May glutamine addiction drive the delivery of antitumor cisplatin-based Pt(IV) prodrugs? J Inorg Biochem. 2017;167:27–35. doi: 10.1016/j.jinorgbio.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 80.Wongthai P., Hagiwara K., Miyoshi Y., Wiriyasermkul P., Wei L., Ohgaki R. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015;106:279–286. doi: 10.1111/cas.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsuura M., Ohshima M., Hiruta Y., Nishimura T., Nagase K., Kanazawa H. LAT1-targeting thermoresponsive fluorescent polymer probes for cancer cell imaging. Int J Mol Sci. 2018:19. doi: 10.3390/ijms19061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kou L., Hou Y., Yao Q., Guo W., Wang G., Wang M. l-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif Cells Nanomed Biotechnol. 2018;46:1605–1616. doi: 10.1080/21691401.2017.1384385. [DOI] [PubMed] [Google Scholar]
- 83.Kou L., Yao Q., Sun M., Wu C., Wang J., Luo Q. Cotransporting ion is a trigger for cellular endocytosis of transporter-targeting nanoparticles: a case study of high-efficiency SLC22A5 (OCTN2)-mediated carnitine-conjugated nanoparticles for oral delivery of therapeutic drugs. Adv Healthc Mater. 2017;6:1700165. doi: 10.1002/adhm.201700165. [DOI] [PubMed] [Google Scholar]
- 84.Gourdon B., Chemin C., Moreau A., Arnauld T., Baumy P., Cisternino S. Functionalized PLA-PEG nanoparticles targeting intestinal transporter PepT1 for oral delivery of acyclovir. Int J Pharm. 2017;529:357–370. doi: 10.1016/j.ijpharm.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 85.Du Y., Tian C., Wang M., Huang D., Wei W., Liu Y. Dipeptide-modified nanoparticles to facilitate oral docetaxel delivery: new insights into PepT1-mediated targeting strategy. Drug Deliv. 2018;25:1403–1413. doi: 10.1080/10717544.2018.1480675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Luo Q., Jiang M., Kou L., Zhang L., Li G., Yao Q. Ascorbate-conjugated nanoparticles for promoted oral delivery of therapeutic drugs via sodium-dependent vitamin C transporter 1 (SVCT1) Artif Cells Nanomed Biotechnol. 2017:1–11. doi: 10.1080/21691401.2017.1417864. [DOI] [PubMed] [Google Scholar]
- 87.Bhunia S., Vangala V., Bhattacharya D., Ravuri H.G., Kuncha M., Chakravarty S. Large amino acid transporter 1 selective liposomes of l-DOPA functionalized amphiphile for combating glioblastoma. Mol Pharm. 2017;14:3834–3847. doi: 10.1021/acs.molpharmaceut.7b00569. [DOI] [PubMed] [Google Scholar]
- 88.Li L., Di X., Zhang S., Kan Q., Liu H., Lu T. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf B Biointerfaces. 2016;141:260–267. doi: 10.1016/j.colsurfb.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 89.Li L., Di X., Wu M., Sun Z., Zhong L., Wang Y. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: toward a novel active targeting strategy in breast cancer therapy. Nanomedicine. 2017;13:987–998. doi: 10.1016/j.nano.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Maekawa-Matsuura M., Fujieda K., Maekawa Y., Nishimura T., Nagase K., Kanazawa H. LAT1-targeting thermoresponsive liposomes for effective cellular uptake by cancer cells. ACS Omega. 2019;4:6443–6451. [Google Scholar]
- 91.Luo Q., Gong P., Sun M., Kou L., Ganapathy V., Jing Y. Transporter occluded-state conformation-induced endocytosis: amino acid transporter ATB0,+-mediated tumor targeting of liposomes for docetaxel delivery for hepatocarcinoma therapy. J Control Release. 2016;243:370–380. doi: 10.1016/j.jconrel.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 92.Luo Q., Yang B., Tao W., Li J., Kou L., Lian H. ATB0,+ transporter-mediated targeting delivery to human lung cancer cells via aspartate-modified docetaxel-loading stealth liposomes. Biomater Sci. 2017;5:295–304. doi: 10.1039/c6bm00788k. [DOI] [PubMed] [Google Scholar]
- 93.Zhou P., Liang X., Zhou C., Qin J., Hou C., Zhu Z. Glutamine-beta-cyclodextrin for targeted doxorubicin delivery to triple-negative breast cancer tumors via the transporter ASCT2. J Mater Chem B. 2019;7:5363–5375. doi: 10.1039/c9tb01225g. [DOI] [PubMed] [Google Scholar]
- 94.Wang C., Wu J., Wang Z., Yang Z., Li Z., Deng H. Glutamine addiction activates polyglutamine-based nanocarriers delivering therapeutic siRNAs to orthotopic lung tumor mediated by glutamine transporter SLC1A5. Biomaterials. 2018;183:77–92. doi: 10.1016/j.biomaterials.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 95.Luo C., Sun J., Sun B., He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol Sci. 2014;35:556–566. doi: 10.1016/j.tips.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Chen Q., Liu G., Liu S., Su H., Wang Y., Li J. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends Pharmacol Sci. 2018;39:59–74. doi: 10.1016/j.tips.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 97.Luo C., Sun J., Liu D., Sun B., Miao L., Musetti S. Self-assembled redox dual-responsive prodrug-nanosystem formed by single thioether-bridged paclitaxel-fatty acid conjugate for cancer chemotherapy. Nano Lett. 2016;16:5401–5408. doi: 10.1021/acs.nanolett.6b01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z., Chi D., Wu X., Wang Y., Lin X., Xu Z. Tyrosine modified irinotecan-loaded liposomes capable of simultaneously targeting LAT1 and ATB0,+ for efficient tumor therapy. J Control Release. 2019;316:22–33. doi: 10.1016/j.jconrel.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 99.Ong Z.Y., Chen S., Nabavi E., Regoutz A., Payne D.J., Elson D.S. Multibranched gold nanoparticles with intrinsic LAT-1 targeting capabilities for selective photothermal therapy of breast cancer. ACS Appl Mater Interfaces. 2017;9:39259–39270. doi: 10.1021/acsami.7b14851. [DOI] [PubMed] [Google Scholar]
- 100.Waddad A.Y., Ramharack P., Soliman M.E.S., Govender T. Grafted hyaluronic acid N-acetyl-l-methionine for targeting of LAT1 receptor: in-silico, synthesis and microscale thermophoresis studies. Int J Biol Macromol. 2019;125:767–777. doi: 10.1016/j.ijbiomac.2018.12.104. [DOI] [PubMed] [Google Scholar]
- 101.Kou L., Bhutia Y.D., Yao Q., He Z., Sun J., Ganapathy V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:27. doi: 10.3389/fphar.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoshyar N., Gray S., Han H., Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11:673–692. doi: 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zuckerman J.E., Choi C.H., Han H., Davis M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc Natl Acad Sci USA. 2012;109:3137–3142. doi: 10.1073/pnas.1200718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yuan H., Li J., Bao G., Zhang S. Variable nanoparticle-cell adhesion strength regulates cellular uptake. Phys Rev Lett. 2010;105 doi: 10.1103/PhysRevLett.105.138101. [DOI] [PubMed] [Google Scholar]
- 105.Kulkarni S.A., Feng S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013;30:2512–2522. doi: 10.1007/s11095-012-0958-3. [DOI] [PubMed] [Google Scholar]
- 106.Zhang L., Tian B., Li Y., Lei T., Meng J., Yang L. A copper-mediated disulfiram-loaded pH-triggered PEG-shedding TAT peptide-modified lipid nanocapsules for use in tumor therapy. ACS Appl Mater Interfaces. 2015;7:25147–25161. doi: 10.1021/acsami.5b06488. [DOI] [PubMed] [Google Scholar]
- 107.Elias D.R., Poloukhtine A., Popik V., Tsourkas A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine. 2013;9:194–201. doi: 10.1016/j.nano.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stefanick J.F., Ashley J.D., Kiziltepe T., Bilgicer B. A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptide-targeted liposomes. ACS Nano. 2013;7:2935–2947. doi: 10.1021/nn305663e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.