Abstract
This follow-up study of a randomized, prospective trial included 192 patients with newly diagnosed severe aplastic anemia receiving antithymoglobulin and cyclosporine, with or without granulocyte colony-stimulating factor (G-CSF). We aimed to evaluate the long-term effect of G-CSF on overall survival, event-free survival, probability of secondary myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML), clinical paroxysmal nocturnal hemoglobinuria, relapse, avascular osteonecrosis and chronic kidney disease. The median follow-up was 11.7 years (95% CI, 10.9-12.5). The overall survival rate at 15 years was 57±12% in the group given G-CSF and 63±12% in the group not given G-CSF (P=0.92); the corresponding event-free survival rates were 24±10% and 23±10%, respectively (P=0.36). In total, 9 patients developed MDS or AML, 10 only a clonal cytogenetic abnormality, 7 a solid cancer, 18 clinical paroxysmal nocturnal hemoglobinuria, 8 osteonecrosis, and 12 chronic kidney disease, without any difference between patients treated with or without G-CSF. The cumulative incidence of MDS, AML or isolated cytogenetic abnormality at 15 years was 8.5±3% for the G-CSF group and 8.2±3% for the non-G-CSF group (P=0.90). The cumulative incidence of any late event including myelodysplastic syndrome or acute myeloid leukemia, isolated cytogenetic abnormalities, solid cancer, clinical paroxysmal nocturnal hemoglobinuria, aseptic osteonecrosis, chronic kidney disease and relapse was 50±12% for the G-CSF group and 49±12% for the non-G-CSF group (P=0.65). Our results demonstrate that it is unlikely that G-CSF has an impact on the outcome of severe aplastic anemia; nevertheless, very late events are common and eventually affect the prognosis of these patients, irrespectively of their age at the time of immunosuppressive therapy (NCT01163942).
Introduction
Acquired aplastic anemia is a rare disease defined by peripheral pancytopenia associated with hypocellularity of the bone marrow. The aim of treatment of aplastic anemia is to improve peripheral blood counts and obtain transfusion independency. First-line treatment for younger patients (≤40 year old) with a matched sibling donor is allogeneic stem cell transplantation (SCT). The standard of care for adult patients not eligible for SCT is immunosuppressive therapy (IST), including a combination of horse antithymocyte globulin (ATG) and cyclosporine (CSA).1 In contrast to patients undergoing SCT, those treated with IST are not cured from their disease and are at risk of late complications such as relapse and development of late clonal diseases, including paroxysmal nocturnal hemoglobinuria (PNH), myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML).2 Furthermore, the delayed recovery of peripheral blood counts exposes patients to infectious and hemorrhagic complications.3
Immunosuppression remains a suboptimal treatment, since about 30% of the patients fail to respond and even in responding patients, blood counts often remain subnormal, possibly requiring maintenance IST with CSA. Efforts have been made for 40 years to improve the standard horse ATG plus CSA treatment.1 Other immunosuppressive combinations as well as the use of high-dose cyclophosphamide have been evaluated, without showing the expected breakthrough.4–7 Great hopes have been placed in the development of hematopoietic growth factors. The role of granulocyte colony-stimulating factor (G-CSF) added to standard IST with ATG and CSA, tested in six small prospective randomized trials, was inconclusive.8–13 Therefore, in 2001 a prospective randomized study was initiated to evaluate the short- and long-term effects of G-CSF added to standard IST. Patients with newly diagnosed severe aplastic anemia (SAA) were randomized to treatment with ATG and CSA, with or without G-CSF (NCT01163942). The study demonstrated that G-CSF added to ATG and CSA decreases the rate of early infectious episodes and days of hospitalization in patients with very SAA patients, but has no significant impact on overall survival (OS), event-free survival (EFS), relapse, or death rates.14 The role of G-CSF in triggering late clonal evolution to a hematologic malignancy has been debated for years.15–18 and we lacked follow-up in our previous study for a meaningful assessment of this risk.14
Early death occurs secondary to infection, bleeding, or complications of severe anemia. Limited data are available on late malignant and non-malignant complications after IST. Today, 16 years after initiation, this randomized controlled study is a unique opportunity to assess the long-term outcome of SAA patients treated with IST. We thus aimed to evaluate the durability of response to treatment, survival outcomes, and the risk of long-term complications of patients treated with ATG and CSA, with or without G-CSF.
Methods
Design
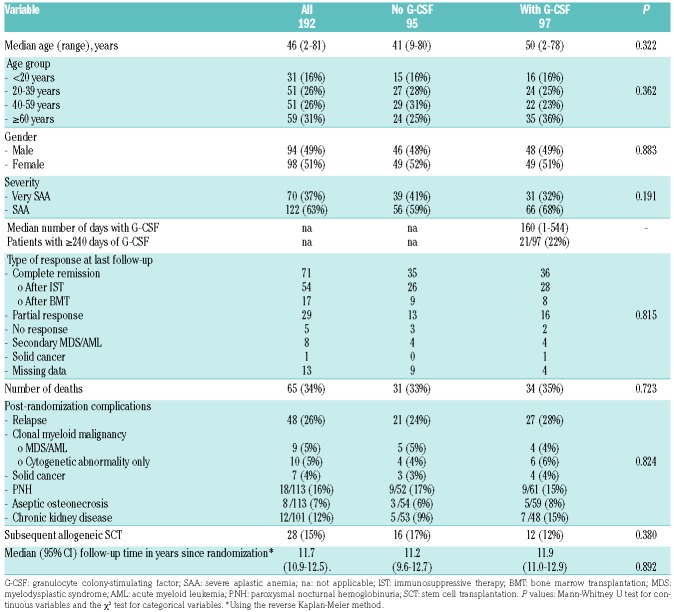
The design and methodology of the randomized study have been described previously.14 It was an open-label, multicenter randomized study conducted by the Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Disease severity was assessed with the use of standard criteria and categorized into SAA and very SAA. Patients of any age were included, but patients with congenital SAA, such as Fanconi anemia, as well as patients with hypoplastic MDS were excluded. A total of 192 patients with newly diagnosed SAA, not eligible for SCT, were randomly assigned in a multicenter trial to receive horse ATG and CSA with (49.5%) or without G-CSF (50.5%). Patients randomized to receive G-CSF were given a dose of 150 mg/m2/day from day 8 through day 240 except for subjects who achieved complete remission before. Methylprednisolone (or prednisone) 1 mg/kg/day was administered on days 1-14. After 14 days, corticosteroids were tapered off over the subsequent 14 days. In the case of serum sickness, a longer tapering schedule, as clinically indicated, was allowed. Complete response was defined as transfusion independency with a hemoglobin level ≥110 g/L, a neutrophil count ≥1.5×109/L and a platelet count ≥150×109/L. Partial response was defined as no longer meeting the criterial of SAA and no transfusion dependence for platelets and/or red blood cells. Continuous transfusion dependency was classified as no response. Relapse was defined as a decrease in blood counts to values either requiring transfusions or needing re-treatment of the aplastic anemia with immunosuppression or SCT. For late complications, the participating centers were asked to report the date of first appearance of a clonal evolution to a hematologic malignancy (MDS or AML, whichever appeared first; or an isolated cytogenetic abnormality), solid cancer, clinical PNH, osteonecrosis and chronic kidney disease. The diagnosis of clinical PNH was retained in patients with a measurable PNH clone who developed either thromboembolic complications or active intravascular hemolysis. Chronic kidney disease was defined as persistence of abnormal creatinine or glomerulation filtration rates more than 1 year after randomization. The present analysis included all 192 randomized patients (Table 1). The study was approved by the ethics committee of each center including patients in the study. All patients gave informed written consent to inclusion in the study.
Table 1.
Characteristics of the study patients, overall and according to randomization to treatment with or without granulocyte colony-stimulating factor.
Outcome measures
Given that at the time of first publication 44 of the original 192 patients had died, the follow-up was done for the remaining 148 patients. Endpoints of the present study were OS, EFS, causes of death, and probability of clonal evolution to a hematologic malignancy (including secondary MDS/AML and an isolated cytogenetic abnormality), solid cancer, clinical PNH, relapse, avascular osteonecrosis and chronic kidney disease by 15 years, comparing for each endpoint patients treated with or without G-CSF. Causes of deaths were classified as related to aplastic anemia (infection, bleeding, undefined), to secondary neoplasm (MDS, AML, solid cancer), transplantation related in patients who received SCT for treatment failure, unrelated to aplastic anemia, or of unknown cause. We analyzed the risk of osteonecrosis because of the use of steroids,19–21 and of chronic kidney disease because of the treatment with ATG and CSA.22,23 Furthermore, for patients with aseptic osteonecrosis we compared those who needed more than one course of ATG and were therefore more exposed to steroids, to those treated with a single course of ATG; for patients with chronic kidney disease we compared patients who were either dependent on CSA or needed a subsequent course of CSA to those who received a single course of ATG without being dependent on CSA. Time to an event started from the day of randomization, except for survival of patients treated with SCT. For OS, patients were censored either at the time of last follow-up or at the time of transplantation, used as salvage therapy. For EFS analysis, events were defined as relapse, non-response at day 120, subsequent SCT, the occurrence of MDS/AML, solid cancer, clinical PNH or death.
Statistical analysis
Group differences were analyzed with the use of the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. Survival probabilities were calculated with the use of the Kaplan-Meier estimator. Time at risk started from the date of randomization and ended on the date of death for OS, and on the date of an event for EFS, or the date of last known assessment, whichever came first. For the cumulative incidence of a late complication, death from other cause was considered as a competing risk. The log-rank test with a two-sided significance level was used for comparison in the Kaplan-Meier estimates. The time to an event was computed from the date of randomization to the date of death or the date of last contact. Univariate competing risk analyses were performed using the Gray test. Multivariate analysis was performed to calculate hazard ratios and their 95% confidence intervals, adjusted for all covariates, using a Cox proportional hazards regression model. Factors considered were age at randomization, severity of aplastic anemia and the use of G-CSF. All P-values are two-sided with a type I error rate fixed at 0.05. Statistical analyses were performed with SPSS Statistic 25 software (IBM Corp., Chicago, IL, USA); cumulative incidence curves were constructed with NCSS 2004 (Statistics and Systems, Kaysville, UT, USA).
Results
Overall survival and event-free survival
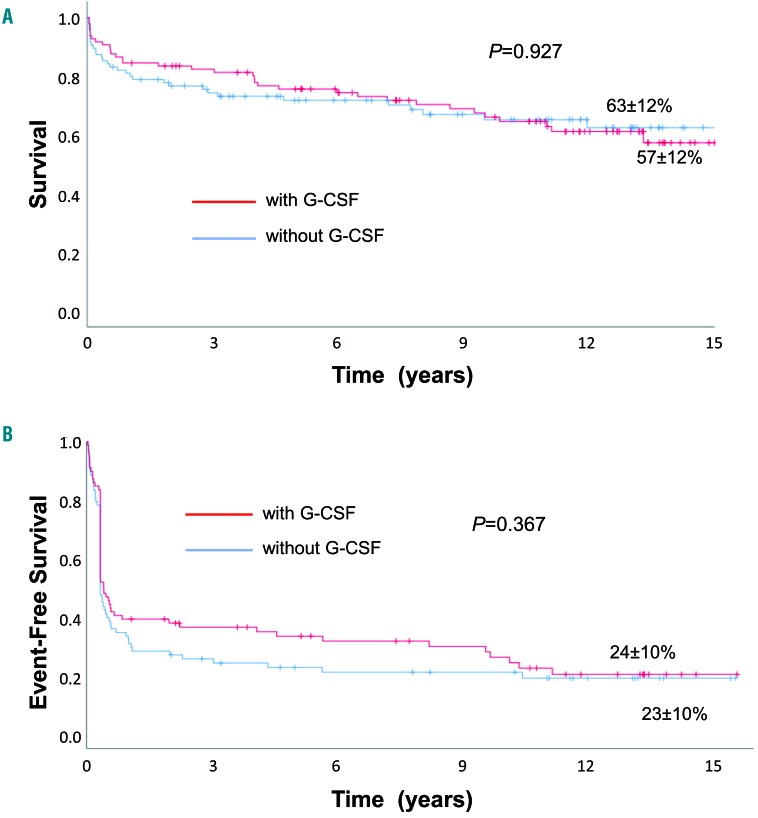
OS and EFS rates at 15 years for all patients were 60±9% and 24±7%, respectively. The OS rate was 57±12% for the G-CSF group and 63±12% for the non-G-CSF group (P=0.92) (Figure 1A). The EFS rate was 24±10% for the G-CSF group and 23±10% for the non-G-CSF group (P=0.36) (Figure 1B). At last follow-up, among 127 alive patients, 71 were in complete remission (54 after IST, 17 after subsequent transplantation), 29 in partial response and five had not responded. Data on remission status of SAA were missing for 13 patients, and not applicable in nine cases (8 with secondary MDS/AML and 1 with solid cancer). There was no difference with respect to remission state at last follow-up between the patients in the G-CSF and non-G-CSF groups (P=0.81). In the 65 patients who died, cause of death was infection (n=26), bleeding (n=3), SAA not further specified (n=3), MDS/AML (n=4), solid cancer (n=4), transplant-related mortality (n=8), cardiovascular/aging (n=9), or unspecified (n=8). There was no difference in the causes of death between patients treated with or without G-CSF.
Figure 1.
Outcomes of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. (A, B) Overall survival (A) and event-free survival (B) of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. Events included relapse, non-response at day 120, subsequent stem cell transplantation, myelodysplastic syndrome/acute myeloid leukemia, solid cancer, paroxysmal nocturnal hemoglobinuria or death. G-CSF: granulocyte colony-stimulating factor.
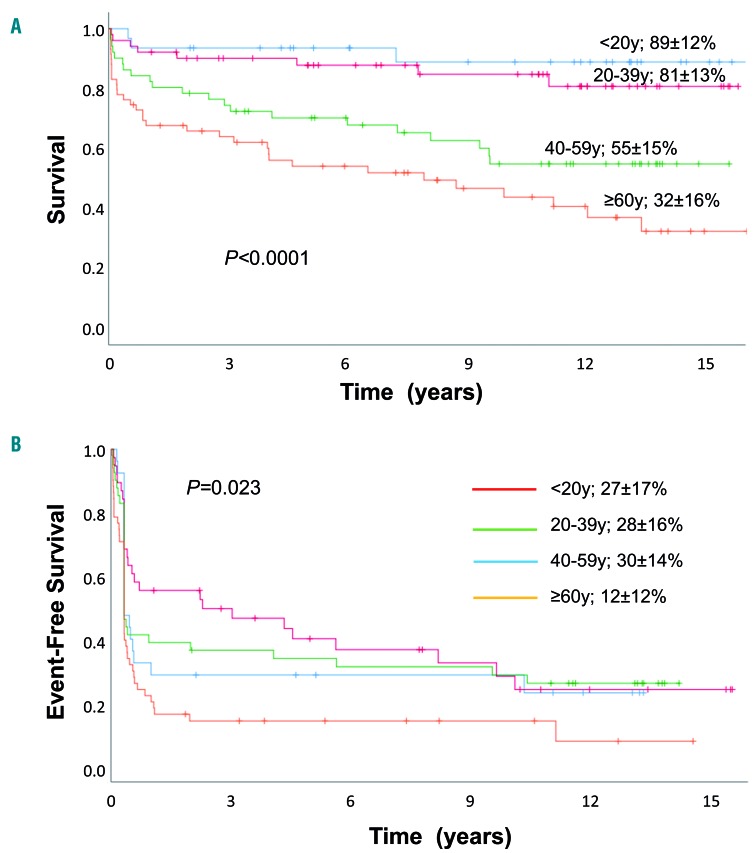
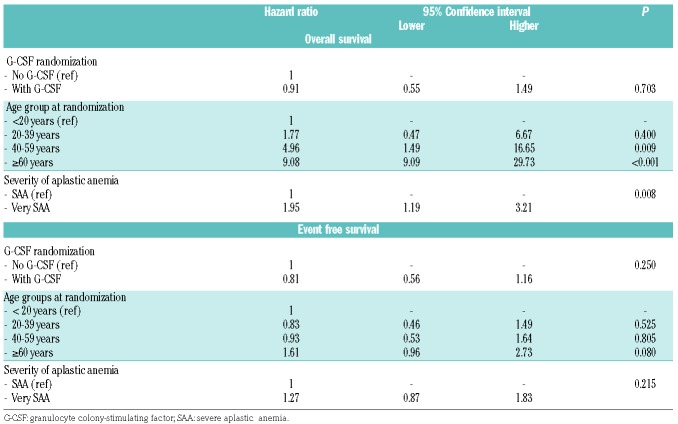
The most important risk factors for OS of patients treated with horse ATG and CSA with or without G-CSF were age and severity of the disease at randomization: the OS rate at 15 years was 89±12% for patients aged <20 years, 81±13% for patients 20-39 years old, 55±15% for patients 40-59 years old, and 32±16% for patients ≥60 years old (P<0.001) (Figure 2A). The OS rate for patients with SAA was 64±11% and that for patients with very SAA was 52±13% (P=0.021). However, for patients surviving 1 year or longer after first IST, there was no longer any difference in survival according to disease severity: the OS rate is 71±11% for patients with SAA and 74±16% for patients with very SAA (P=0.636) (Online Supplementary Figure S1A, B). In multivariate analysis including age, severity and randomization for G-CSF as variables, treatment with G-CSF was not associated with better survival [G-CSF; relative risk (RR) 0.91, 95% confidence interval (95% CI): 0.55-1.49; P=0.70]; the relative risk was increased for very SAA (RR 1.95, 95% CI 1.19-3.21, P=0.008) and older age (reference age <20 years; 20-39 years, RR 1.77, 95% CI: 0.48-6.67, P=0.40; age 40-59 years, RR 4.96, 95% CI: 1.48-16.65, P=0.009; age ≥60 years, RR 9.08, 95% CI 2.78-29.73, P<0001). EFS at 15 years according to age group was as follows: 27%±17% for patients aged <20 years, 28±16% for patients 20-39 years old, 30±14% for patients 40-59 years old, and 12±12% for patients 60 years or older (P=0.023) (Figure 2B). In multivariate analysis, age group was no longer significantly different for EFS (Table 2), although a notable, non-significant trend remained for patients 60 years or older.
Figure 2.
Overall survival and event-free survival according to age groups. (A, B) Overall survival (A) and event-free survival (B) of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor according to age groups at time of randomization: patients <20 years, patients 20-39 years, patients 40-59 years, patients 60 years or older. Events included relapse, non-response at day 120, subsequent stem cell transplantation, myelodysplastic syndrome/acute myeloid leukemia, solid cancer, paroxysmal nocturnal hemoglobinuria or death. G-CSF: granulocyte colony-stimulating factor.
Table 2.
Multivariate analysis for overall survival and event free-survival.
Relapse, non-response to immunosuppression and need for subsequent stem cell transplantation
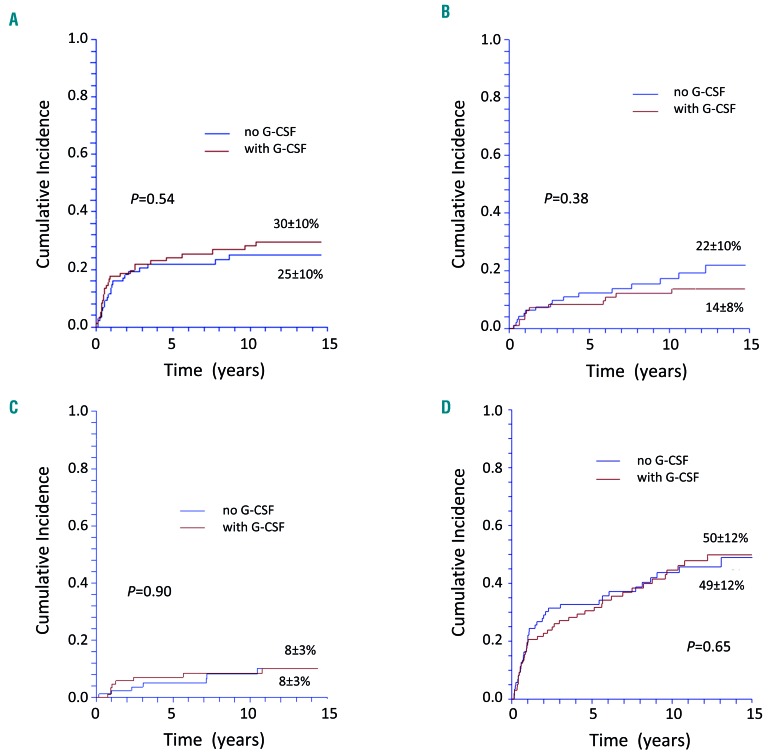
We evaluated relapse, non-response to immunosuppression and the need for either subsequent SCT or subsequent courses of IST. There was no difference between patients treated with or without G-CSF with respect to relapse and the need for second-line treatment: the cumulative incidence of relapse for patients responding at day 120 was 30±10% for the G-CSF group, and 25±10% for the non-G-CSF group (P=0.54) (Figure 3A). Forty patients needed a second-line therapy for relapse (n=17), refractory disease (n=8), or incomplete response (cyclosporine dependency, n=7; decreasing values but still in partial remission, n=8), 24 in the G-CSF group, and 16 in the non-G-CSF group (P=0.35). Sixteen patients needed a third-line therapy for relapse (n=5), refractory disease (n=7), or incomplete response (decreasing values but still in partial remission, n=4), 12 in the G-CSF group, and four in the non-G-CSF group (P=0.60). Twenty-eight patients needed allogeneic SCT as second-line or subsequent treatment, 12 in the G-CSF group, and 16 in the non-G-CSF group, because of non-response/relapse (n=23), or MDS/AML (n=5). The cumulative probability of being treated with SCT at 15 years was 14±8% for patients who had been given G-CSF, and 22%±10% for those who had not (P=0.38) (Figure 3B). The 10-year survival after transplantation was 63±18%.
Figure 3.
Cumulative incidence of late complications of patients with severe aplastic anemia treated with horse antithymocyte globulin and cyclosporine with or without granulocyte colony-stimulating factor. (A) Cumulative incidence of relapse of patients with severe aplastic anemia (SAA) treated with horse antithymocyte globulin (ATG) and cyclosporine (CSA) with or without granulocyte colony-stimulating factor (G-CSF). (B) Cumulative probability of being transplanted (death without transplant is the competing event) of patients with SAA treated in first-line with horse ATG and CSA with or without G-CSF. (C) Cumulative incidence of clonal evolution to a hematologic malignancy (myelodysplastic syndrome/acute myeloid leukemia or isolated cytogenetic abnormality) of patients with SAA treated with horse ATG and CSA with or without G-CSF. (D) Cumulative incidence of any late event (relapse, myelodysplastic syndrome/acute myeloid leukemia, isolated cytogenetic abnormality, solid cancer, clinical paroxysmal nocturnal hemoglobinuria, aseptic osteonecrosis, chronic kidney disease) of patients with SAA treated with horse ATG and CSA with or without G-CSF.
Long-term follow-up
Next, we evaluated the probability of malignant and non-malignant late complications. During the follow-up, 52 of the 192 patients developed a late complication. Some of them developed more than one late event: 44 patients developed one, five developed two, and three developed three or more late complications. Nine patients developed clinical or morphological signs of MDS/AML, ten developed isolated cytogenetic abnormalities (2 cases with del(7q), 1 in the G-CSF group and 1 in the non-G-CSF group, and 1 case with each of the following abnormalities: del(13q), del(9), loss of chromosome X in a female patient, loss of chromosome Y in a male patient, anomaly of chromosome 11 and translocation t(6;10); 2 cases with undefined abnormality), seven developed a solid cancer (colon, pancreas, glioblastoma, gastric, adenocarcinoma and squamous cell carcinoma of unknown origin, unspecified, 1 of each), and 19 developed clinical PNH. At 15 years, the cumulative incidence of MDS/AML or isolated cytogenetic abnormalities was 8.5±3% in the G-CSF group, and 8.2±3% in the non-G-CSF group (P=0.90) (Figure 3C); the cumulative incidence of clinical PNH was 10.1±5% in the G-CSF group, and 13.3±7% in the non-G-CSF group (P=0.499). With regards to non-clonal late complications, there were eight cases of avascular osteonecrosis, and 12 of chronic kidney disease. The cumulative incidence of chronic kidney failure at 15 years was 13%±11% in the G-CSF group, and 16±11% in the non-G-CSF group (P=0.51). Likewise, there was not a difference for aseptic osteonecrosis, although there were not enough events to provide an estimate. Patients needing longer CSA treatment had a higher risk of chronic kidney disease (6/22; 27%) compared to patients who received a single course of CSA (6/79; 7.6%; P=0.021). In contrast, patients needing more steroids (1/13; 7.7%) did not have a higher risk of osteonecrosis than patients needing steroids only for one ATG course (7/100;6.2%; P=0.453). The cumulative incidence of all late events at 15 years (i.e., including MDS/AML, isolated cytogenetic abnormalities, solid cancer, clinical PNH, osteonecrosis, kidney disease, relapse) was 50±12% in the G-CSF group, and 49±12% in the non-G-CSF group (P=0.65) (Figure 3D).
We also evaluated a possible effect of duration of treatment with G-CSF on long-term events. Patients randomized to receive G-CSF, were given the growth factor for a median of 160 days (range, 1-544 days). Of these patients, 21 received G-CSF for ≥240 days. Patients who received G-CSF for ≥240 days were not more likely to develop MDS/AML, isolated cytogenetic abnormalities, second solid cancers, clinical PNH, aseptic osteonecrosis or chronic kidney disease.
Discussion
G-CSF had been shown to reduce infections and days of hospitalization in the first 3 months of its administration in patients with SAA.14 The expectation from adding G-CSF to standard IST was to improve the long-term outcome and sustainability of remission, as has been envisaged recently with the use of eltrombopag.24 We show here that the addition of G-CSF to horse ATG and CSA has no impact on the long-term outcome of patients with acquired SAA: OS, EFS, non-response, relapse and need for a subsequent SCT were similar in the groups that did or did not receive G-CSF. G-CSF treatment during IST is an option of effective supportive care, possibly to combat or prevent infectious complications, even if it does not have any beneficial effect as an adjunct to standard IST in the long-term.25 Clonal malignant evolution was always a concern with the use of G-CSF in SAA patients receiving IST. We could not demonstrate a higher risk for the development of MDS/AML or cytogenetic abnormalities in patients randomized to receive G-CSF. Patients were analyzed for the risk of clonal evolution to a hematologic malignancy according to their initial randomization group (i.e. with or without G-CFS). However, data on patients in the non-G-CSF group who eventually received G-CSF (dose and duration) later in the course of their disease are not available. Thus, it is fair to state that G-CSF given as part of the initial treatment within this randomized clinical trial does not seem to increase the risk of clonal evolution to MDS/AML. Patients with SAA had a baseline risk of developing clonal evolution to a hematologic malignancy; this risk was not increased for patients treated with G-CSF, when compared to those in the non-G-CSF arm.
The strength of our trial is the prospective, randomized design with a follow-up time (median 11.7 years) that is longer than the interval, 10 years after IST,15 within which secondary MDS/AML usually occur. Previous studies were less powerful to investigate this issue for various reasons. They were either retrospective,15,18,26 not randomized, randomized without a non-G-CSF arm16,17 or had a lower number of patients and shorter followup.10,12 However, it is not obvious why few among these studies showed a relationship between the use of G-CSF and a clonal evolution to MDS/AML. Factors explaining the difference from our results could be patient selection (more patients with pre-existing clonal karyotype at diagnosis of SAA).18 ethnic reasons, differences in dose and duration of G-CSF,16 or the retrospective nature of the analysis.15 The present study goes a step further in the long-term observation than most of the previous studies on SAA patients treated with ATG and CSA. We, too, could not demonstrate a higher risk of clinical PNH (the archetype of a benign clonal evolution), second solid cancer or non-malignant late events such as chronic kidney disease and aseptic osteonecrosis in the G-CSF group. Second solid cancers in SAA patients treated with immunosuppression have been shown to be more frequent than expected in a general population27 and to affect OS strongly.15 However, we found that G-CSF has no impact on the development of second solid cancers.
This study highlights two significant messages. First, G-CSF is unlikely to be linked with an increased risk of clonal transformation into a hematologic malignancy; however, available clinical data do not support the routine unse of G-CSF along with IST. G-CSF can be considered as an effective supportive care to combat or prevent infectious complications.25 Second, irrespectively of G-CSF, SAA patients receiving IST are particularly vulnerable to a number of late malignant and non-malignant complications, either because of an intrinsic pre-cancerous nature of the disease or alternatively because of long-term stressed hematopoiesis and prolonged immunosuppression. Severity of the disease and age of the patient at first IST are the most important risk factors for survival. Interestingly, severity seems to have an impact only during the early phase after treatment, due to the higher risk of death from infections. In patients surviving 1 year or longer severity no longer has any impact on survival. It is somewhat disappointing that in this carefully followed cohort, irrespective of the age, less than 25% of patients are alive and event-free 15 years after initial treatment, and about 20% of them required an allogeneic SCT. Among the late events, relapses remain the most common, since, quite surprisingly, they continue to occur for at least 10 years after the initial treatment. Despite an excellent OS, young adults, have a similar risk of malignant and non-malignant complications after IST as older patients. The only difference is that the mortality rate in younger patients (aged <40 years) is lower, likely due to other salvage treatment options (mostly SCT) which are associated with different mortality based on age.28
Our study has a number of limitations. Firstly, despite it being the largest randomized study on the use of G-CSF in patients treated with horse ATG and CSA, because of the slow accrual for this rare disease and the withdrawal of horse ATG in Europe, the EBMT was forced to close the study early.14 However, it is unlikely that a larger number of patients would have changed the findings. Secondly, we do not have the cumulative dose of G-CSF, particularly for non-responding and relapsed patients who have been retreated with immunosuppression. The study design is based on the principle of an intention-to-treat analysis in order to provide unbiased assessments of treatment efficacy.29 Thirdly, not all late events have the same impact on the outcome of the patients. The occurrence of secondary malignancy, MDS, AML or solid cancer, strongly affect-OS.2 Relapse of aplastic anemia does not have the same poor prognosis as relapse of a malignant disease. Although relapse is common, the majority of relapsed patients respond to the reintroduction of IST and relapse does not influence survival.30 Finally, another limitation of our work is the non-exhaustiveness of the cytogenetic analysis in the context of clonal evolution, mainly related to failure to obtain results and also because the long-term evaluation had not been part of the original protocol.
Taken together, our data suggest that G-CSF added to standard IST has no impact on long-term outcome of patients with acquired aplastic anemia and is not directly related with late effects. However, regardless of the use of G-CSF, SAA patients treated with immunosuppression are particularly vulnerable to a number of late malignant and non-malignant complications. In particular, SAA patients treated with IST continue to relapse even at 10 years after initial treatment; therefore, alternative non-transplant treatment strategies are more than welcome. The addition of eltrombopag on top of standard IST resulted in an increased response rate in a phase II study31,32 and is presently being evaluated in a randomized trial (EudraCT number 2014-000363-40). Furthermore, given the dramatic improvement of outcome after SCT, the possibility of early front-line SCT with an alternative donor might be considered for selected young patients who lack a matched sibling donor.33 Clinical trials in this setting remain the only opportunity to investigate the best strategies to improve the rate of cure in SAA, possibly minimizing the risk of early and late events that affect survival.
Acknowledgments
The authors thank all the patients and the centers whose participation made this study possible. The list of participating centers is shown in the appendix of the Online Supplementary Material.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/5/1223
References
- 1.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120(6):1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Socie G, Rosenfeld S, Frickhofen N, Gluckman E, Tichelli A. Late clonal diseases of treated aplastic anemia. Semin Hematol. 2000;37(1):91–101. [PubMed] [Google Scholar]
- 3.Bacigalupo A. Antithymocyte globulin and cyclosporin: standard of care also for older patients with aplastic anemia. Haematologica. 2019;104(2):215–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisdale JF, Dunn DE, Geller N, et al. High-dose cyclophosphamide in severe aplastic anaemia: a randomised trial. Lancet. 2000;356(9241):1554–1559. [DOI] [PubMed] [Google Scholar]
- 5.Scheinberg P, Wu CO, Nunez O, Young NS. Predicting response to immunosuppressive therapy and survival in severe aplastic anaemia. Br J Haematol. 2009;144(2):206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheinberg P, Wu CO, Nunez O, et al. Treatment of severe aplastic anemia with a combination of horse antithymocyte globulin and cyclosporine, with or without sirolimus: a prospective randomized study. Haematologica. 2009;94(3):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cle DV, Atta EH, Dias DSP, et al. Rabbit antithymocyte globulin dose does not affect response or survival as first-line therapy for acquired aplastic anemia: a multi-center retrospective study. Ann Hematol. 2018;97(11):2039–2046. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E, Rokicka-Milewska R, Hann I, et al. Results and follow-up of a phase III randomized study of recombinant human-granulocyte stimulating factor as support for immunosuppressive therapy in patients with severe aplastic anaemia. Br J Haematol. 2002;119(4):1075–1082. [DOI] [PubMed] [Google Scholar]
- 9.Gordon-Smith EC, Yandle A, Milne A, et al. Randomised placebo controlled study of RH-GM-CSF following ALG in the treatment of aplastic anaemia. Bone Marrow Transplant. 1991;7 Suppl 2:78–80. [PubMed] [Google Scholar]
- 10.Kojima S, Hibi S, Kosaka Y, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96(6):2049–2054. [PubMed] [Google Scholar]
- 11.Shao Z, Chu Y, Zhang Y, Chen G, Zheng Y. Treatment of severe aplastic anemia with an immunosuppressive agent plus recombinant human granulocyte-macrophage colony-stimulating factor and erythropoietin. Am J Hematol. 1998;59(3):185–191. [DOI] [PubMed] [Google Scholar]
- 12.Teramura M, Kimura A, Iwase S, et al. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporin A with or without G-CSF in adults: a multi-center randomized study in Japan. Blood. 2007;110(6):1756–1761. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y, Liu Y, Chu Y. Immunosuppressive therapy for acquired severe aplastic anemia (SAA): a prospective comparison of four different regimens. Exp Hematol. 2006;34(7):826–831. [DOI] [PubMed] [Google Scholar]
- 14.Tichelli A, Schrezenmeier H, Socie G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117(17):4434–4441. [DOI] [PubMed] [Google Scholar]
- 15.Socie G, Mary JY, Schrezenmeier H, et al. Granulocyte-stimulating factor and severe aplastic anemia: a survey by the European Group for Blood and Marrow Transplantation (EBMT). Blood. 2007;109(7):2794–2796. [DOI] [PubMed] [Google Scholar]
- 16.Kojima S, Ohara A, Tsuchida M, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100(3):786–790. [DOI] [PubMed] [Google Scholar]
- 17.Locasciulli A, Bruno B, Rambaldi A, et al. Treatment of severe aplastic anemia with antilymphocyte globulin, cyclosporine and two different granulocyte colony-stimulating factor regimens: a GITMO prospective randomized study. Haematologica. 2004;89(9):1054–1061. [PubMed] [Google Scholar]
- 18.Ohara A, Kojima S, Hamajima N, et al. Myelodysplastic syndrome and acute myelogenous leukemia as a late clonal complication in children with acquired aplastic anemia. Blood. 1997;90(3):1009–1013. [PubMed] [Google Scholar]
- 19.Marsh JC, Zomas A, Hows JM, Chapple M, Gordon-Smith EC. Avascular necrosis after treatment of aplastic anaemia with anti-lymphocyte globulin and high-dose methylprednisolone. Br J Haematol. 1993;84(4):731–735. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Jun J, Kim Y, Lee J, Kim C, Hahn S. Osteonecrosis of the hip in patients with aplastic anemia. J Korean Med Sci. 2002;17(6):806–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Socie G, Cahn JY, Carmelo J, et al. Avascular necrosis of bone after allogeneic bone marrow transplantation: analysis of risk factors for 4388 patients by the Societe Francaise de Greffe de Moelle (SFGM). Br J Haematol. 1997;97(4):865–870. [DOI] [PubMed] [Google Scholar]
- 22.Barakat RK, Schmolck JP, Finkel KW, Foringer JR. Prolonged renal failure secondary to antithymocyte globulin treatment in severe aplastic anemia. Ann Pharmacother. 2007;41(5):895–898. [DOI] [PubMed] [Google Scholar]
- 23.Gupta N, Mahapatra M, Rathi S, et al. Acute renal failure following antithymocyte globulin therapy for aplastic anaemia-report of two cases and review of literature. Ann Hematol. 2011;90(2):239–241. [DOI] [PubMed] [Google Scholar]
- 24.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dufour C, Svahn J, Bacigalupo A, Severe Aplastic Anemia-Working Party of the EBMT Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48(2):174–177. [DOI] [PubMed] [Google Scholar]
- 26.Imashuku S, Hibi S, Nakajima F, et al. A review of 125 cases to determine the risk of myelodysplasia and leukemia in pediatric neutropenic patients after treatment with recombinant human granulocyte colony-stimulating factor. Blood. 1994;84(7):2380–2381. [PubMed] [Google Scholar]
- 27.Socie G, Henry-Amar M, Bacigalupo A, et al. Malignant tumors occurring after treatment of aplastic anemia. European Bone Marrow Transplantation-Severe Aplastic Anaemia Working Party. N Engl J Med. 1993;329(16):1152–1157. [DOI] [PubMed] [Google Scholar]
- 28.Giammarco S, Peffault de Latour R, Sica S, et al. Transplant outcome for patients with acquired aplastic anemia over the age of 40: has the outcome improved? Blood. 2018;131(17):1989–1992. [DOI] [PubMed] [Google Scholar]
- 29.Montori VM, Guyatt GH. Intention-to-treat principle. CMAJ. 2001;165(10):1339–1341. [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–1135. [DOI] [PubMed] [Google Scholar]
- 31.Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheinberg P. Activity of eltrombopag in severe aplastic anemia. Blood Adv. 2018;2(21):3054–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol. 2015;171(4):585–594. [DOI] [PubMed] [Google Scholar]