Abstract
Sepsis causes an activation of the human contact system, an inflammatory response mechanism against foreign surfaces, proteins and pathogens. The serine proteases of the contact system, factor XII and plasma kallikrein, are decreased in plasma of septic patients, which was previously associated with an unfavorable outcome. However, the precise mechanisms and roles of contact system factors in bacterial sepsis are poorly understood. We, therefore, studied the physiological relevance of factor XII and plasma kallikrein in a mouse model of experimental sepsis. We show that decreased plasma kallikrein concentration in septic mice is a result of reduced mRNA expression plasma prekallikrein gene, indicating that plasma kallikrein belong to negative acute phase proteins. Investigations regarding the pathophysiological function of contact system proteases during sepsis revealed different roles for factor XII and plasma kallikrein. In vitro, factor XII decelerated bacteria induced fibrinolysis, whereas plasma kallikrein supported it. Remarkably, depletion of plasma kallikrein (but not factor XII) by treatment with antisense-oligonucleotides, dampens bacterial dissemination and growth in multiple organs in the mouse sepsis model. These findings identify plasma kallikrein as a novel host pathogenicity factor in Streptococcus pyogenes sepsis.
Introduction
Sepsis and severe sepsis are life-threatening complications caused by a dysregulated host response to bacterial infection and activation of coagulation. The liver plays a key role in these events due to the acute phase protein response, an increased or decreased synthesis of host defense and coagulation proteins. Increased production of acute phase proteins contribute to a procoagulant state in sepsis, especially by enhancing production of procoagulants such as fibrinogen, and by decreasing liver synthesis of antithrombin.1 A procoagulant state is thought to be protective against bacterial dissemination, as local activation of coagulation traps bacteria in a fibrin mesh and activates inflammatory reactions.2,3 Inhibition of fibrinolysis may support this process further, since highly invasive pathogens exploit the host fibrinolytic system to degrade fibrin clots and overcome tissue barriers.2 Streptococcus pyogenes is a Gram-positive major human pathogen causing mainly local infections of the skin and mucous membranes such as erysipelas or tonsillitis. Local infections occasionally develop into serious systemic complications, of which streptococcal toxic shock syndrome and necrotizing fasciitis are associated with high morbidity and mortality.3 Virulence factors of S. pyogenes have been studied intensively, and conversion of human plasminogen to plasmin by bacterial streptokinase is a mechanism which supports bacterial dissemination.4 Streptokinase-activated plasmin also activates the human contact system, an inflammatory response mechanism against artificial material and pathogens.5 The human contact system consists of two proteases, factor XII (FXII) and plasma prekallikrein (PPK), as well as the co-factor high molecular weight kininogen (HK). The proteins are produced in the liver and circulate as zymogens in the blood stream or are assembled on endothelial cells, neutrophils, and platelets. When blood is exposed to foreign artificial or biological surfaces, contact factors bind to it, and FXII becomes auto-activated and converts PPK to plasma kallikrein (PK). PK, which circulates in a non-covalent complex with HK,6 cleaves HK and the proinflammatory peptide bradykinin is released.7 In severe sepsis, activation of the contact system is archetypal8 and multiple animal studies with different pharmacological interventions that inhibit FXII, bradykinin receptors or the interaction of contact factors with the bacterial surface9 were carried out to evaluate potential therapeutic options.10 However, surprisingly little is known about the precise role of contact factors during microbial sepsis. Here, therefore, we studied the physiological role of FXII- and PK in a mouse model of experimental sepsis. We found that hepatic expression of F12 and Klkb1 genes after infection with S. pyogenes is quickly reduced upon streptococcal infection. Moreover, a knockdown of Klkb1 gene expression by anti-sense-oligonucleotide (ASO) technology prior to infection diminishes bacterial spreading, but knockdown of F12 did not influence bacterial dissemination. Our data indicate different in vivo roles for FXII and PK in streptococcal sepsis.
Methods
A detailed description of materials and methods with additional information is provided in the Online Supplementary Appendix.
Antisense-oligonucleotides
Antisense-oligonucleotides (ASO) for Klkb1 or F12 mRNA knockdown in vivo were provided by Ionis Pharmaceuticals and have been described previously.11
Infection of HepG2 cells
Details are provided in the Online Supplementary Appendix.
mRNA analysis
Total RNA was isolated from HepG2 cells or homogenized mouse liver with RNeasy Plus Mini Kit (Qiagen). RNA quality was checked with Agilent RNA 6000 Nano Kit (Agilent Technologies) and RNA concentration determined with QubitTM RNA HS Assay Kit (Invitrogen). All analyses were performed according to the manufacturer’s instructions. 800 ng total RNA was converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and the complementary DNA obtained used for real-time quantitative polymerase chain reaction (PCR). Reaction mixture (20 ML) containing gene specific nuclease assay (Taqman Universal PCR Master Mix; Applied Biosystems) and cDNA was amplified as follows: denaturation at 95°C for 10 minutes (min) and 45 cycles at 95°C for 15 seconds (sec) and 60°C for 1 min. GAPDH (human or rodent) was used as housekeeping gene. Relative expression was calculated using the 2− ct method.
Clotting assays
Details are provided in the Online Supplementary Appendix.
Clot lysis time
A clot was generated in human normal, FXII- or PK-deficient plasma by addition of PT-Reagent. In some experiments, CTI (75 μg/mL), PKSI (10 M), FXIIa (50 μg/mL) or PK (50 μg/mL) was added before clot formation was induced. The clot was incubated for 5 min at 37°C before Streptokinase (100 Units), uPA (10 μg/mL g) or tPa (10 μg) was added. Time until clot lysis was determined in a coagulometer.
Measurement of FXII and plasma kallikrein in plasma
Details are provided in the Online Supplementary Appendix.
Proteolytic potential for plasma kallikrein/factor XIIa activity in mouse plasma
Pooled plasma from four mice/group was incubated with Dapptin and FXIIa/PK activity was determined in a microplate reader by chromogenic substrate S-2302 (Chromogenix). (See Online Supplementary Appendix).
Plasma clot escape experiments
See Online Supplementary Appendix.
Light and scanning electron microscopy
See Online Supplementary Appendix and Oehmcke et al.12 and Isenring et al.13
Fibrinogen degradation
Fibrinogen was mixed with either plasmin, plasminogen and streptokinase, FXII, PPK, or PBS as negative control. (See Online Supplementary Appendix). The mix was incubated at 37°C and at indicated time points samples were analyzed by SDS-Page and western blot using fibrinogen antibody (Santa Cruz). Relative fibrinogen levels were determined by densitometry analysis (ImageStudioLite 5.2.5).
Animal experiments
Eight-week-old female BALB/c mice (weight 16-18g) (Charles River Laboratories) were treated with ASO through intraperitoneal injections, with a dose of 800 μg/mouse, twice per week for three weeks (total 7 injections, each with 800 μg ASO/mouse).
The subcutaneous infection model with S. pyogenes AP1 strain and determination of bacterial dissemination were performed as described previously.12 (See also Online Supplementary Appendix). This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments the Landesveterinär- und Lebensmitteluntersuchungsamt Rostock (Permit n. 7221.3-1-002/16).
Plasma proteome
The methods are provided in the Online Supplementary Appendix.
Patient samples
Patients with sepsis, severe sepsis, or septic shock were enrolled from the Intensive Care Medicine Unit at University Medical Center of Rostock, as described previously.14 The protocol had been approved by our Institutional Ethics committee (A 201151), and informed consent was obtained from the patients or their caring relatives.
Results
Plasma proteome from septic mice
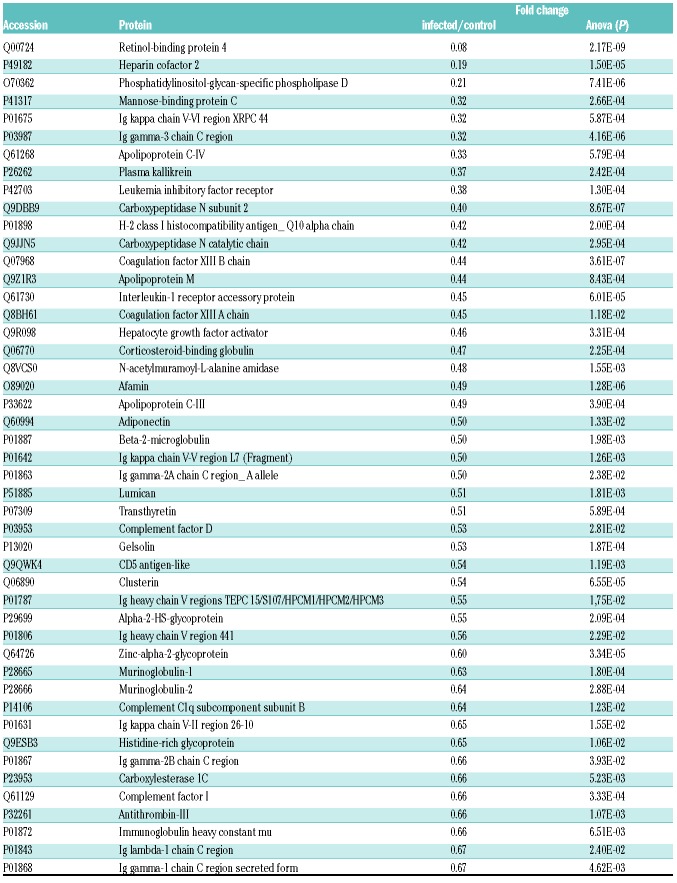
In order to gain more information about contact factors in sepsis, we performed a quantitative proteome analysis of plasma from healthy or S. pyogenes-infected mice. The subcutaneous infection model has been shown to become septic and activate the contact system,9 which is similar to the situation encountered in human streptococcal toxic shock syndrome.15 Plasma was collected 24 hours (h) after infection and a total number of 137 proteins including PK, kininogen-1 and FXII could be quantified based on the abundance of at least two peptides (Online Supplementary Table S1). There was a rise in the concentration of 38 proteins due to infection (Online Supplementary Table S2). Within this set of elevated proteins, typical positive acute phase proteins such as serum amyloid, C-reactive protein or fibrinogen were detected. In addition, we also identified 47 proteins with significantly decreased concentration due to infection (Table 1). Here, again, we found classical negative acute phase proteins such as retinol binding protein or antithrombin III. PK concentrations were significantly reduced in infected animals (Table 1). Kininogen-1 and FXII levels were also reduced; however, this was not statistically significant (Online Supplementary Table S1).
Table 1.
Plasma proteins from infected mice detected by mass spectrometry analysis that were significantly down-regulated, compared to healthy mice.
F12 and Klkb1 mRNA levels decline in vitro and in vivo after infection with S. pyogenes
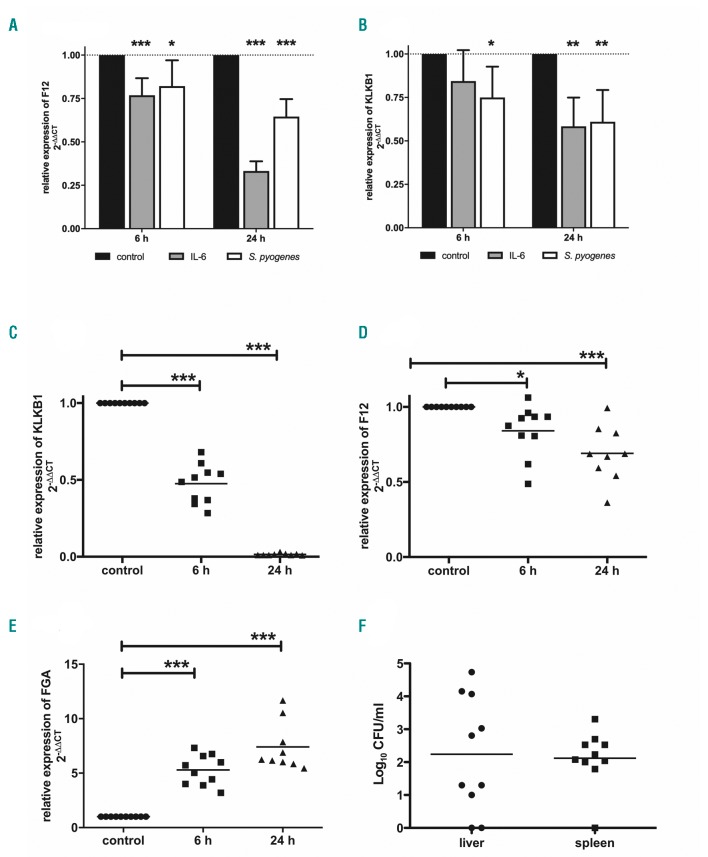
We next investigated in vitro how mRNA expression of contact factors is affected in liver cells in response to infection. HepG2 cells were treated with IL6 or living bacteria and mRNA was analyzed by quantitative real-time PCR. In accordance with Citarella et al.,16 F12 mRNA levels were significantly decreased in cells treated with IL6 for 6 and 24 h (Figure 1A). The same was observed in cells infected with S. pyogenes (Figure 1A). Klkb1 mRNA levels also significantly declined upon treatment with either IL6 or S. pyogenes (Figure 1B).
Figure 1.
Decreased mRNA levels of F12 and Klkb1 in vitro and in vivo after infection with S. pyogenes. (A and B) HepG2 cells (2×105 cells/mL) were incubated with IL6 (50 ng/mL) or S. pyogenes [2×106 colony forming units (CFU)/mL] for 6 hours (h). After incubation, cells were washed and the medium was replaced with fresh medium containing 1% PenStrep. After 6 and 24 h, cells were harvested, total RNA was isolated, and real-time polymerase chain reaction (PCR) TaqMan® gene expression assays were performed. N≥9. (*P≤0.05; **P≤0.01; ***P≤0.001). (C-F) Groups of mice (n=8-10) were subcutaneously (sc.) infected with 2×107 CFU/mouse of S. pyogenes AP1. Animals were killed 6 and 24 h after infection, and liver tissue was collected for total RNA isolation (C-E) and real-time PCR TaqMan® gene expression assays were performed. (F) Spleen and liver were homogenized and the number of CFU was quantified 6 h after infection. *P≤0.05; ***P≤0.001.
To investigate the hepatic expression of Klkb1 and F12 mRNA in vivo, we used the streptococcal murine sepsis model. Mice were infected subcutaneously with 1.5-2×107 colony forming units (CFU) of S. pyogenes and samples collected 6 and 24 h after infection. Six h after infection, Klkb1 mRNA levels were reduced by approximately 50% compared to non-infected controls (Figure 1C). Twenty-four h after infection, Klkb1 mRNA levels dropped down to undetectable levels (Figure 1C), indicating that Klkb1 mRNA production was discontinued upon bacterial spreading. Relative expression of F12 was also significantly reduced at 6 and 24 h after infection (Figure 1D); however, this effect was not as pronounced as for the Klkb1 gene. We also measured fibrinogen alpha (FGA) mRNA levels and found significantly increased FGA expression at 6 and 24 h after infection (Figure 1E), which is consistent with the data from proteome analysis. Ninety-percent of mice were bacteremic at 6 h after infection, containing bacteria in their liver and/or spleen (Figure 1F).
Plasma kallikrein concentration decline significantly in vivo after infection with S. pyogenes
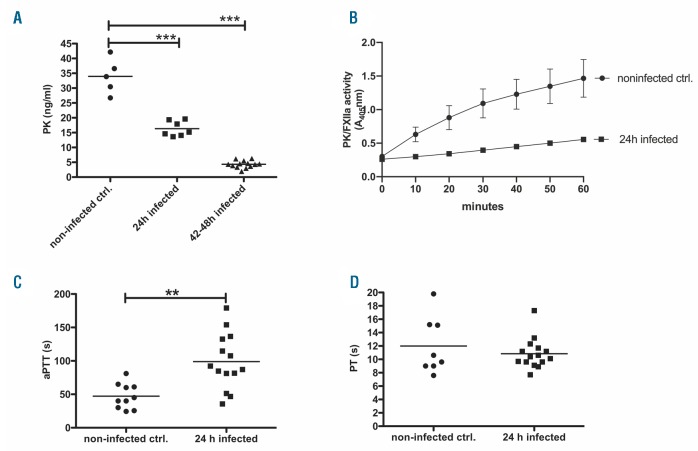
In accordance with the quantitative proteome analysis and Klkb1 mRNA data, we detected a significant decrease in PK levels in mice 24 h after infection, and an even greater decrease 48 h after infection (Figure 2A). This was accompanied by a decreased proteolytic potential of PK/FXIIa in plasma after activation with Dapptin reagent (Figure 2B), and a significantly prolonged activated partial thromboplastin time (aPTT) (Figure 2C) but not PT (Figure 2D). In accordance with mass spectrometry data, FXII plasma levels did not change significantly in infected mice (data not shown).
Figure 2.
Analysis of plasma samples from mice after infection with S. pyogenes. Groups of mice (n=4-10) were subcutaneously (sc.) infected with 2×107 colony forming units (CFU)/mouse of S. pyogenes. (A) Plasma prekallikrein (PPK) concentration in plasma was measured after 24 and 42-48 hours (h) of infection by a specific sandwich enzyme-linked immunosorbent assay. (B) Plasma samples from four mice per group were pooled and proteolytic potential of plasma kallikrein (PK)/factor XIIa (FXIIa) activity was measured using chromogenic substrate S-2302 for PK and FXIIa. (C) Activated partial thromboplastin time (aPTT) and (D) prothrombin time (PT) in plasma of infected and non-infected animals were measured using a coagulometer. *P≤0.05; **P≤0.001; ***P≤0.0001. ctrl: control.
Knockdown of plasma prekallikrein diminishes streptococcal dissemination, dampens kidney damage, cyto- and chemokines, but raises RANTES
Such quick downregulation of protein expression during sepsis implicates that the protein is not required, or is even harmful, under these conditions. To investigate the functional contribution of PK and FXII during sepsis, we inhibited Klkb1 or F12 gene expression by antisense oligonucleotides (ASO), using established protocols,11,17 prior to infection with S. pyogenes. As expected, pre-treatment of female BALB/c mice with PPK ASO (Online Supplementary Figure S1A) or FXII ASO (Online Supplementary Figure S1B) reduces relative expression levels more than 90%, which is in accordance with previous studies in male BALB/c mice. As shown before,11,17 plasma protein level of PPK on FXII depletion, or FXII on PPK depletion, were increased (Online Supplementary Figure S1C-F). This indicates stabilization of FXII or PPK as response to decreased basal activation. As a consequence of PPK or FXII knockdown, decreased proteolytic potential of PK/FXIIa (Online Supplementary Figure S1G) and prolongation of activated partial thromboplastin time (aPTT) (Online Supplementary Figure S1H) in plasma was demonstrated, compared to mice treated with control ASO. Moreover, as expected, PT was not affected by the treatment with either ASO (data not shown).
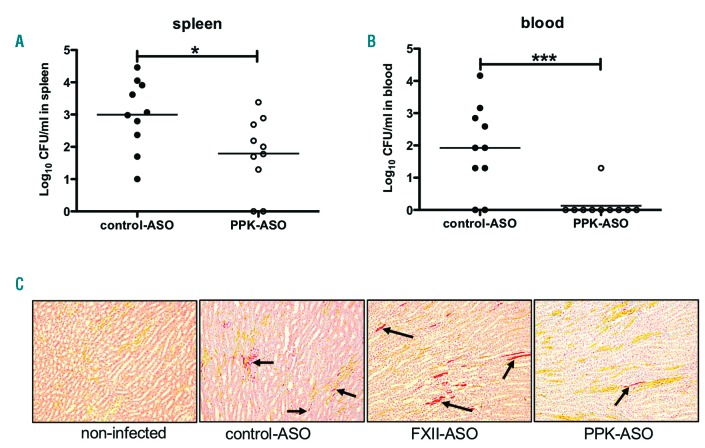
The PPK and FXII-depleted mice were challenged with S. pyogenes as described above. Bacterial dissemination and histopathology of the kidneys was determined 24 h after infection. PPK-depleted mice had significantly fewer bacteria in the spleen and blood compared to control-ASO mice (Figure 3A and B). Intriguingly, there was no difference in bacterial loads in spleen, blood or kidneys between FXII-depleted mice and controls (data not shown).
Figure 3.
Bacterial spreading and histopathology of kidneys from plasma prekallikrein (PPK)- or factor XII (FXII)-depleted mice infected with S. pyogenes. Groups of mice were infected subcutaneously (sc.) with 1.6-2×107 colony forming units (CFU)/mouse S. pyogenes AP1. Twenty-four hours after infection, samples were collected and (A) spleen or (B) blood of infected control-antisense-oligonucleotide (ASO) or PPK-ASO treated mice were homogenized and the number of CFU was quantified. Data are presented as means of ten mice per group and were obtained from two independent experiments. *P≤0.05; ***P≤0.0001. (C) Representative kidney tissue sections showing the medullary rays, from non-infected, control-, PPK, or FXII-ASO animals. Sections were stained (MSB-Lendrum) and fibrin depositions (marked by arrows) were detected and scored as described in the Methods section (10 × magnification).
Formation of microvascular fibrin deposition in kidneys was described in lethal human sepsis.18 In our animal model, fibrin was detected 24 h after subcutaneous (sc.) infection in kidneys from septic animals (Figure 3C). A quantification of fibrin areas (> 5 μm) on a scale from 0 to 3 (0: absent; 1: ≤ 20 fibrin areas; 2: 20-50 fibrin areas; 3: >50 fibrin areas) revealed that the mean score in PPK-ASO treated animals was lower (1.7) than that of the control-ASO treated group (2.0) or the FXII-ASO treated group (2.5). This was reflected by increased creatinine values in plasma from control-ASO (8±2.9 μmol/L) and FXII-ASO (8.25±1.9 μmol/L) mice, compared to values in PPK-ASO mice (6±1 μmol/L).
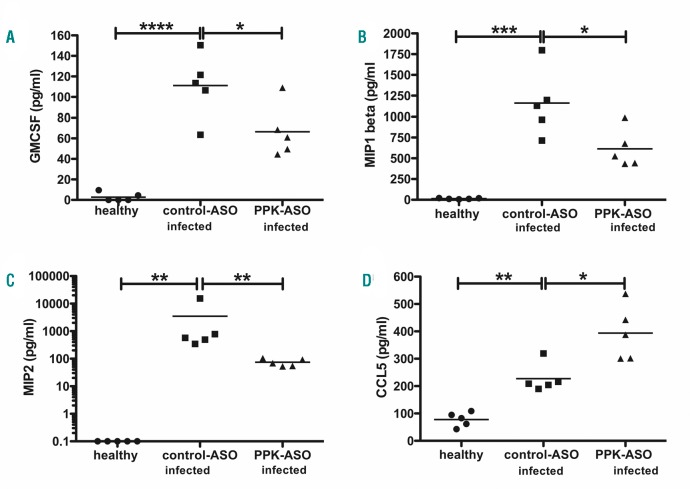
In addition, a panel of 20 cytokines, chemokines and growth factors was measured in healthy, infected control-ASO and PPK-ASO treated mice. Infection boosted the pro-inflammatory response yielding a robust increase of 17 cytokines/chemokines. In agreement with lower bacterial loads, infected PPK-ASO treated mice had significantly lower levels of Gm-CsF (Figure 4A) MIP-1 beta (Figure 4B), and MIP-2 (Figure 4C) compared to infected control mice. Interestingly, CCL5 was significantly increased in infected PPK-ASO mice compared to infected controls (Figure 4D).
Figure 4.
Proinflammatory response in plasma prekallikrein (PPK)-depleted mice infected with S. pyogenes. Groups of mice (n=5 per group) were infected subcutaneously (sc.) with 2×107 colony forming units (CFU)/mouse S. pyogenes AP1. Twenty-four hours after infection animals were collected and EDTA plasma were analyzed for GmCSF (A), MIP-1 beta (B), MIP-2 (C), CCL5 (RANTES) (D), using a Multi-Plex immunoassay. *P≤0.05; **P≤0.01; ***P≤0.0002; ***P≤0.0001.
Role of contact factors in bacterial-triggered fibrinolysis
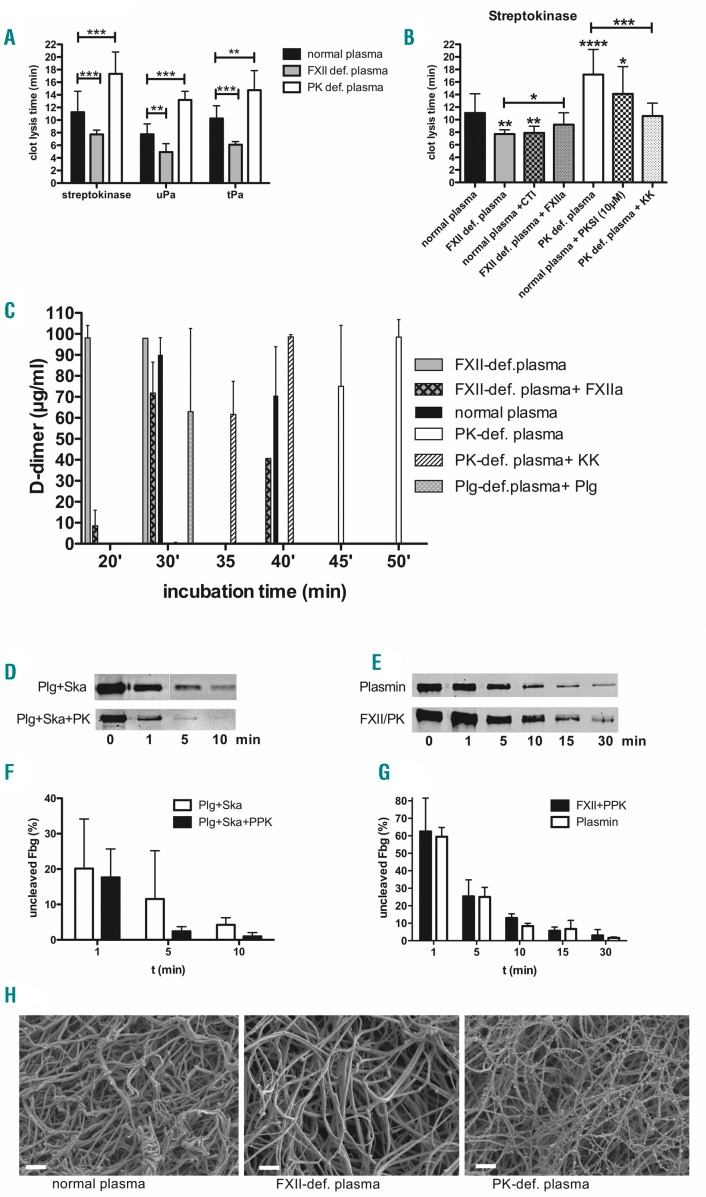
S. pyogenes activates plasminogen to escape from the local site of infection;4 however, knockdown of PPK decelerated bacterial spreading in mice, implicating a role of PK in bacteria-induced fibrinolysis. To further explore the human relevance to our findings, we employed in vitro plasma clotlysis assays in normal human plasma and congenital FXII- or PPK-deficient plasma, followed by incubation with streptokinase or the host plasminogen activators uPA and tPA. Clot lysis in PPK-deficient plasma was significantly longer, regardless of the fibrinolysis activator used (Figure 5A). In contrast, clot lysis in FXII-deficient plasma was significantly shorter compared to normal plasma. When corn trypsin inhibitor (CTI), a FXII inhibitor, was added to normal plasma, the clot lysis, induced by streptokinase, was again significantly shorter compared to normal plasma (Figure 5B). Similarly, the addition of the PK inhibitor PKSI19 to normal plasma prolonged clot lysis induced by streptokinase (Figure 5B). Complementation of human congenital FXII- or PPK-deficient plasma with activated enzymes (FXIIa or PK) could reverse shortening or prolongation of clot lysis by streptokinase (Figure 5B). Plasminogen content was slightly reduced in FXII-deficient plasma (97±5 μg/mL) comparing to pooled normal plasma (141μ8 μg/mL) or PPK deficient plasma (123μ28 μg/mL). However, plasmin activity after activation with streptokinase was similar in all plasma types (Online Supplementary Figure S2A), providing proof that the streptokinase/plasmin activity is not inhibited due to different donors.
Figure 5.
Effect of human congenital factor XII (FXII) or plasma prekallikrein (PPK) deficiency on fibrinolysis induced by streptokinase or S. pyogenes bacteria. (A) A clot was derived in normal, FXII- or PPK-deficient plasma by thromboplastin (PT)-reagent and time until clot lysis was measured after addition of streptokinase, uPA or tPA. (B) A clot was derived in normal, FXII- or PPK-deficient (def) plasma that was, if indicated, pre-incubated with CTI (75 μg/mL), PKSI (10 μM), FXIIa (50 μg/mL) or PK (50 μg/mL). Time until clot lysis was measured after addition of streptokinase. (C) Growing S. pyogenes (2×108 CFU/mL) were mixed with plasma, and thrombin was used to form a stable clot. The plasma clot was overlaid with PBS and D-dimer concentration in the supernatant was measured after different time points, using an ELISA. (D and E) Representative western blot analysis of fib-rinogen incubated for up to 30 minutes with plasminogen+streptokinase (Plg/Ska), plasminogen + streptokinase + PPK (Plg/Ska/PPK), Plasmin, or FXII+PPK (FXII/PPK) (F and G) relative levels of uncleaved fibrinogen (Fbg), quantified by densitometry from three independent experiments. (H) Plasma clots were induced by thrombin, fixed and analyzed by Scanning electron microscopy. Bars represent 2 μm. *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. min: minutes.
Activation of fibrinolysis results in the release of D-dimer from cross-linked fibrin; thus, we measured the content of D-dimer in the supernatant from human plasma clots, which contained S. pyogenes bacteria. Twenty minutes after incubation, D-dimer were detected in samples from clots in FXII-deficient plasma, but not in samples from clots in normal or PPK-deficient plasma (Figure 5C). Thirty minutes after incubation, D-dimer could be detected from clots in normal plasma and FXII-deficient plasma complemented with FXIIa, but still not from clots in PPK-deficient plasma. Forty-five minutes after incubation, D-dimer were detected in the supernatant of PPK-deficient plasma, and this time-lag could be reversed by complementation with PK (Figure 5C). As expected, in plasminogen-depleted plasma, no D-dimer were detected within 180 min, but after complementation with plasminogen D-dimer, release occurred after 30 min. Complete clot lysis by the bacteria could be observed when D-dimer concentration was at the highest level, i.e. after 20 min in FXII-deficient plasma, after 30 min in normal plasma, and after 45-50 min in PPK-deficient plasma.
As clot lysis time and D-dimer production were decelerated in PPK-def. plasma, we investigated whether PK might accelerate plasmin degradation of fibrinogen. Western blot analysis shows that pure fibrinogen is degraded by the streptokinase/plasminogen complex within 5- 10 min, and addition of PPK supports fibrinogen degradation further (Figure 5D and F). Of note, PPK was activated by Ska/plasminogen in the presence of fibrinogen (Online Supplementary Figure S2C and D). If PPK and FXII were added to fibrinogen it was degraded within 30 min, comparable to plasmin (Figure 5E and G). PPK or FXII alone had marginal effects on fibrinogen degradation, and blocking the proteases by PKSI or CTI inhibited degradation (Online Supplementary Figure S2B). Intriguingly, the degradation pattern of PK cleaved alpha chain of fibrinogen was different, compared to the FXII or the plasmin cleavage pattern (Online Supplementary Figure S2E). We conclude that PPK, activated either by streptokinase/plasminogen or by FXIIa supports plasmin mediated degradation of fibrinogen.
We also investigated the structure of plasma clots by scanning electron microscopy. Clots derived from PPK-deficient plasma had thinner fibrin strands than clots from normal or FXII-deficient plasma (Figure 5H).
Plasma kallikrein promotes bacterial escape from mouse plasma clots
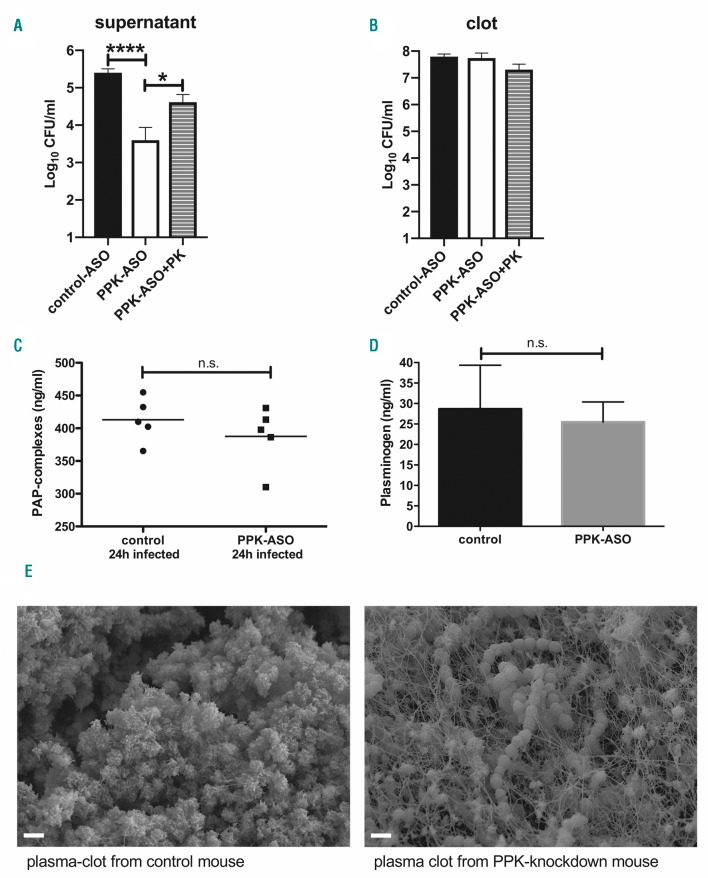
Streptokinase was shown to specifically activate human plasminogen,20 but S. pyogenes is able to escape from mouse (C57BL/6)-derived plasma clots.21 To test whether S. pyogenes can survive in, and escape from, plasma clots of BALB/c mice, plasma samples from control- or PPK-ASO treated animals were clotted with thrombin in the presence of S. pyogenes. Clots were covered with 1% plasma and incubated for up to 4 h at 37°C. Viable S. pyogenes count assays from the supernatants and the clots were performed. Two hours after incubation, no bacteria could be detected in the supernatant. Four hours after incubation, the supernatants of PPK-depleted clots contained significantly fewer viable bacteria compared to supernatants from control-ASO clots (Figure 6A). Thus, S. pyogenes is able to escape from BALB/c mouse plasma clots and PK plays an important role in this process. The relative importance of PK was confirmed in PPK-depleted plasma reconstituted with human PK, where significantly more viable bacteria (compared to PPK-depleted plasma clots) could be detected in the supernatants (Figure 6A). No difference in CFU could be determined inside the clots (Figure 6B).
Figure 6.
Antisense-oligonucleotide (ASO)-mediated plasma prekallikrein (PPK) depletion reduced bacterial escape and inhibited bacteria-triggered fibrinolysis in mouse plasma clots. (A and B) Plasma from four mice per group was pooled and mixed with 1×107 colony forming units (CFU)/mL S. pyogenes, a stable clot was induced by addition of thrombin and CaCl2, and overlaid with PBS, containing 1% plasma. After 4 hours (h), the bacterial loads in the supernatant (A) and homogenized clots (B) were determined by plating. N=4. *P=0.0323; ****P<0.0001. (C) PAP complexes or plasminogen content (D) were determined in EDTA plasma from infected control- or PPK-ASO treated mouse, n=5 per group. (E) Plasma was mixed with 1×109 CFU/mL S. pyogenes and clot formation was induced by addition of thrombin and CaCl2. After 4 h of incubation at 37°C, clots were fixed and analyzed by Scanning electron microscopy. Bars represent 2 μm. PK: plasma kallikrein; n.s. not significant.
It has previously been shown that PK activates plasminogen,22 which would enhance plasmin concentration in control mice. To address this experimentally, circulating plasmin 2-antiplasmin (PAP) complexes were quantified in infected control- and PPK-ASO treated mice (Figure 6C). However, no difference between the two groups was observed, supporting the assumption that PK assist in fibrinogen degradation and might be relevant for the observed bacterial dissemination. Importantly, there was no difference in the plasminogen concentration between healthy control and PPK-ASO treated mice (Figure 6D).
Finally, plasma clots from control ASO or PPK-depleted animals incubated with S. pyogenes were imaged with scanning electron microscopy. Clots derived from control mice and incubated with bacteria lost their structural integrity (Figure 6E), fibrin fibers were degraded, and bacteria could not be detected within the clot (Figure 6E, left). If PPK was absent, intact fibrin fibers were visible, with bacteria trapped inside of them (Figure 6E, right).
Taken together, the data show that PK supports degradation and streptococcal escape from mouse plasma clots leading to increased bacteremia and infection of tissues.
Plasma kallikrein and FXII concentrations are significantly decreased in plasma of septic patients
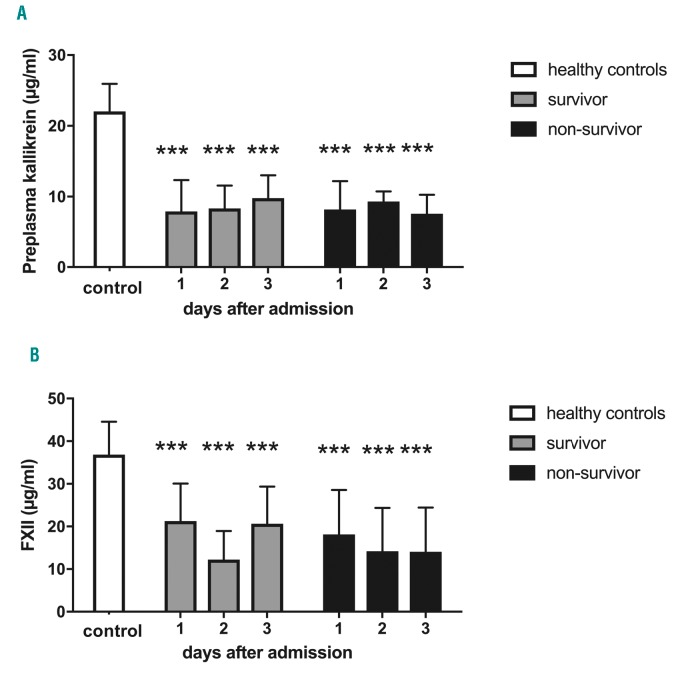
We further investigated PK and FXII concentrations in plasma of 23 patients with sepsis, severe sepsis or septic shock, collected 1, 2 and 3 days after admission to the Intensive Care Unit (ICU). In most of the patients, multiple infectious sources and bacterial isolates were identified and, importantly, non-survivors had significantly prolonged aPTT compared to survivors.14 Plasma samples from 12 healthy persons were used as controls. Both PK and FXII levels are significantly reduced in all patients compared to healthy controls (Figure 7A and B). There is no significant difference between survivors and non-survivors, or between the days of admission. The data support earlier studies, and clearly show that reduction of PK and FXII in plasma is not restricted to S. pyogenes sepsis.
Figure 7.
Significant decrease in plasma prekallikrein and factor XII (FXII) in plasma of patients with sepsis, severe sepsis or septic shock. Blood samples were collected from patients diagnosed with sepsis. Specific sandwich ELISA was performed to detect the levels of (A) plasma kallikrein (PK) and (B) FXII in plasma of sepsis patients on days 1-3. Non-survivor: n=8; survivor: n=15; healthy controls: n=15. ***P≤0.001.
Discussion
Local activation of contact factors on the bacterial surface may be protective against infections, due to the release of antimicrobial peptides and bradykinin from HK, which trigger inflammatory reactions. On the other hand, activation of the system by the pathogen may provoke invasive spreading via bradykinin-induced vascular leakage.5 We previously reported that S. pyogenes triggers activation of the contact system by streptokinase with the liberation of bradykinin. In addition, we showed that S. pyogenes isolates from invasive infections trigger an activation of the contact system more potently than strains isolated from non-invasive infections. Intriguingly, no significant difference was observed when plasmin activation was analyzed,23 supporting the idea that the ability of certain strains to activate contact factors is associated with improved bacterial dissemination. The current study showed that PK, a serine protease of the contact system, is involved in fibrinolysis triggered by S. pyogenes, and supports streptococcal dissemination in mice. In vitro, degradation of fibrinogen and lysis of plasma clots (induced by streptokinase) was impaired in the absence of PK. We therefore, suggest that PK assist in degradation of fibrin by plasmin. This hypothesis is supported by ex vivo and in vivo experiments showing that PK is involved in S. pyogenes escape from mouse plasma clots and dissemination. On the other hand, our data do not exclude a direct plasminogen activation by PK that was shown before in vitro.22 Of note, a knock-out of the Klkb1 gene in mice results in an antithrombotic phenotype.11,24 However, reduced thrombosis in Klkb1 KO mice was not due to defective contact activation but was a result of reduced aortic tissue factor in this mouse;24 thus, this mouse would be not suitable for our investigations. In the present study, we demonstrated that the selective reduction of PPK by ASO-technology decelerated bacterial spreading, dampens inflammatory cytokine and chemokines, and raises CCL5 (RANTES). RANTES acts as a chemoattractant for monocytes, memory Th cells, and eosinophils. As in our animal model, in humans, the level of RANTES was inversely associated with bacteremia25 and the APACHE II score, thus low levels were predictive with poor outcome.26,27
FXII is the main physiological activator of PPK but, surprisingly, a knockdown of F12 gene had no influence on bacterial dissemination in our sepsis model. Beside its function as an activator for PPK, FXII directly increases the fiber density within a clot and makes it more resistant to fibrinolysis.28,29 Consequently, deficiency of FXII in mice or human impairs thrombus stability.30,31 The present study supports these findings, as fibrinolysis, initiated in vitro by bacteria or pure streptokinase, was faster in the absence of FXII. A recent study by Stroo et al. showed that FXII deficiency in mice improved survival and reduced bacterial outgrowth in an airway infection model with the Gram-negative Klebsiella pneumoniae. However, and similar to our data, FXII deficiency did not protect mice when the Gram-positive Streptococcus pneumoniae was used in the airway infection model.32
A depletion of contact factors is often seen in sepsis patients,15,33,34 and this was confirmed in the present study. Depletion of contact factors has been assigned to consumption resulting from massive activation of the contact system. However, although a prolonged aPTT indicated a consumption of FXII and PK in these patients, we could not observe a massive HK cleavage.14 As contact factors are mainly synthesized in the liver, an alternative explanation for low contact factor levels is downregulation of gene expression in the liver, as shown in the present study for F12 and Klkb1 genes in the mouse sepsis model. The knowledge about the pathophysiological role of contact factor gene expression is important for the understanding of their general functions in infection. Expression of Klkb1 was quickly decreased due to invasive infection in mice and accompanied by a fast decrease in the protein in plasma. In addition, an earlier study has shown that the clearance rate of PK by the liver is significantly increased during the acute phase reaction.35 Both mechanisms could contribute to low PK levels in plasma of septic patients, and this suggests that the fast reduction of PK is part of the physiological acute phase response, which supports the antifibrinolytic state. The role of the acute phase response is to enhance host defense to prevent injury of the host within the process of killing bacteria.1 A procoagulant state with inhibition of fibrinolysis may help to contain the bacteria at the primary site of infection. The impairment of fibrinolysis is mediated by different mechanisms, i.e. by decreasing liver synthesis of anticoagulants such as antithrombin III,36 or increasing production of fibrinolysis inhibitors, such as thrombin-activatable fibrinolytic inhibitor (TAFI)37 or plasminogen-activator-inhibitor 1 (PAI1).38,39 Our investigations suggest that down-regulated Klkb1 expression during infection likewise contributes to inhibited fibrinolysis.
The intrinsic coagulation pathway does not contribute to physiological hemostasis, and activation occurs in vivo always under pathological conditions, such as thrombosis, sepsis, or ARDS.40–42 This is the first study that investigated the functional contribution of PK in host defense to bacterial sepsis in rodent and human systems. The study shows that FXII and PK play distinct roles within the infection process. PK supports streptococcal spreading by its profibrinolytic function, whereas, in our model, FXII did not influence bacterial dissemination.
Acknowledgments
We thank Jana Bull (IMIKRO) and Dr. Armin Springer (EMZ) for excellent technical assistance and Dr. Ulf Broschewitz for assessment of histology. We are also grateful to Ionis Pharmaceutical for providing us with ASO.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/5/1424
Funding
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (project OE 547/4-1). SOH was supported by the Federal Excellence Initiative of Mecklenburg Western Pomerania and European Social Fund (ESF) Grant KoInfekt (ESF_14-BM-A55-00xx_16) and by a grant from the Medical Faculty of the University of Rostock in the framework of the FORUN program 2018.
References
- 1.Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: Interaction between coagulation and inflammatory processes. Crit Care Med. 2001;29(7):S42–S47. [DOI] [PubMed] [Google Scholar]
- 2.Lähteenmäki K, Edelman S, Korhonen TK. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 2005;13(2):79–85. [DOI] [PubMed] [Google Scholar]
- 3.Walker MJ, Barnett TC, McArthur JD, et al. Disease manifestations and pathogenic mechanisms of group a Streptococcus. Clin Microbiol Rev. 2014;27(2):264–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Ringdahl U, Homeister JW, et al. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305(5688):1283–1286. [DOI] [PubMed] [Google Scholar]
- 5.Oehmcke-Hecht S, Köhler J. Interaction of the human contact system with pathogens-An update. Front Immunol. 2018;9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandle RJ, Colman RW, Kaplan AP. Identification of Prekallikrein and High-Molecular-Weight Kininogen as a Complex in Human-Plasma. Proc Natl Acad Sci USA. 1976;73(11):4179–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90(10):3819–3843. [PubMed] [Google Scholar]
- 8.Schmaier AH. Physiologic activities of the contact activation system. Thromb Res. 2014;133 Suppl 1:S41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oehmcke S, Shannon O, Köckritz-Blickwede von M, et al. Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood. 2009;114(2):444– 451. [DOI] [PubMed] [Google Scholar]
- 10.Nicola H. The role of contact system in septic shock: the next target? An overview of the current evidence. J Intensive Care. 2017;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revenko AS, Gao D, Crosby JR, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118(19):5302–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oehmcke S, Westman J, Malmström J, et al. A novel role for pro-coagulant microvesicles in the early host defense against streptococcus pyogenes. PLoS Pathog. 2013;9(8):e1003529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isenring J, Köhler J, Nakata M, et al. Streptococcus gallolyticus subsp. gallolyticus endocarditis isolate interferes with coagulation and activates the contact system. Virulence. 2017;9(1):1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trepesch C, Nitzsche R, Glass A, et al. High intravascular tissue factor-but not extracellular microvesicles-in septic patients is associated with a high SAPS II score. J Intensive Care. 2016;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sriskandan S, Cohen J. Kallikrein-kinin system activation in streptococcal toxic shock syndrome. Clin Infect Dis. 2000;30(6):961– 962. [DOI] [PubMed] [Google Scholar]
- 16.Citarella F, Felici A, Brouwer M, et al. Interleukin-6 downregulates factor XII production by human hepatoma cell line (HepG2). Blood. 1997;90(4):1501–1507. [PubMed] [Google Scholar]
- 17.Bhattacharjee G, Revenko AS, Crosby JR, et al. Inhibition of vascular permeability by antisense-mediated inhibition of plasma kallikrein and coagulation factor 12. Nucleic Acid Ther. 2013;23(3):175–187. [DOI] [PubMed] [Google Scholar]
- 18.Aslan A, van den Heuvel MC, Stegeman CA, et al. Kidney histopathology in lethal human sepsis. Crit Care. 2018;22(1):359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanaka K, Okamoto S, Bohgaki M, et al. Effect of a highly selective plasma-kallikrein synthetic inhibitor on contact activation relating to kinin generation, coagulation and fibrinolysis. Thromb Res. 1990;57(6):889–895. [DOI] [PubMed] [Google Scholar]
- 20.Wulf RJ, Mertz ET. Studies on Plasminogen .8. Species Specificity of Streptokinase. Can J Biochem. 1969;47(10):927–931. [DOI] [PubMed] [Google Scholar]
- 21.Shannon O, Rydengård V, Schmidtchen A, et al. Histidine-rich glycoprotein promotes bacterial entrapment in clots and decreases mortality in a mouse model of sepsis. Blood. 2010;116(13):2365–2372. [DOI] [PubMed] [Google Scholar]
- 22.Colman RW. Activation of plasminogen by human plasma kallikrein. Biochem Biophys Res Commun. 1969;35(2):273–279. [DOI] [PubMed] [Google Scholar]
- 23.Nitzsche R, Rosenheinrich M, Kreikemeyer B, Oehmcke-Hecht S. Streptococcus pyogenes triggers activation of the human contact system by streptokinase. Infect Immun. 2015;83(8):3035–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavrou EX, Fang C, Merkulova A, et al. Reduced thrombosis in Klkb1−/− mice is mediated by increased Mas receptor, prostacyclin, Sirt1, and KLF4 and decreased tissue factor. Blood. 2015;125(4):710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosevoll KA, Skrede S, Markussen DL, et al. Inflammatory Mediator Profiles Differ in Sepsis Patients With and Without Bacteremia. Front Immunol. 2018;9:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003;35(9):535–544. [DOI] [PubMed] [Google Scholar]
- 27.Ng PC, Li K, Leung TF, et al. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–1189. [DOI] [PubMed] [Google Scholar]
- 28.Konings J, Govers-Riemslag JWP, Philippou H, et al. Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118(14):3942– 3951. [DOI] [PubMed] [Google Scholar]
- 29.Konings J, Hoving LR, Ariëns RS, et al. The role of activated coagulation factor XII in overall clot stability and fibrinolysis. Thromb Res. 2015;136(2):474–480. [DOI] [PubMed] [Google Scholar]
- 30.Renné T, Pozgajová M, Grüner S, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluthero FG, Ryan C, Williams S, Brandão LR, Kahr WHA. Decreased in vitro thrombin generation and clot stability in human FXII-null blood and plasma. Br J Haematol. 2011;152(1):111–112. [DOI] [PubMed] [Google Scholar]
- 32.Stroo I, Zeerleder S, Ding C, et al. Coagulation factor XI improves host defence during murine pneumonia-derived sepsis independent of factor XII activation. Thromb Haemost. 2017;117(8):1601–1614. [DOI] [PubMed] [Google Scholar]
- 33.Mason JW, Kleeberg U, Dolan P, Colman RW. Plasma kallikrein and Hageman factor in Gram-negative bacteremia. Ann Intern Med. 1970;73(4):545–551. [DOI] [PubMed] [Google Scholar]
- 34.Aasen AO, Smith-Erichsen N, Amundsen E. Plasma kallikrein-kinin system in septicemia. Arch Surg. 1983;118(3):343–346. [DOI] [PubMed] [Google Scholar]
- 35.Martins B, Kouyoumdjian M, Limaos EA, Borges DR. The Clearance Rate of Plasma Kallikrein by the Liver Increases During the Acute-Phase Response to Inflammation. Agents Actions. 1992;37(1-2):111–113. [DOI] [PubMed] [Google Scholar]
- 36.Niessen RW, Lamping RJ, Jansen PM, et al. Antithrombin acts as a negative acute phase protein as established with studies on HepG2 cells and in baboons. Thromb Haemost. 1997;78(3):1088–1092. [PubMed] [Google Scholar]
- 37.Sato T, Miwa T, Akatsu H, et al. Pro-carboxypeptidase R is an acute phase protein in the mouse, whereas carboxypeptidase N is not. J Immunol. 2000;165(2):1053–1058. [DOI] [PubMed] [Google Scholar]
- 38.Renckens R, Roelofs JJTH, Bonta PI, et al. Plasminogen activator inhibitor type 1 is protective during severe Gram-negative pneumonia. Blood. 2007;109(4):1593–1601. [DOI] [PubMed] [Google Scholar]
- 39.Kager LM, Wiersinga WJ, Roelofs JJTH, et al. Plasminogen activator inhibitor type I contributes to protective immunity during experimental Gram-negative sepsis (melioidosis). J Thromb Haemost. 2011;9(10):2020–2028. [DOI] [PubMed] [Google Scholar]
- 40.Renné T, Schmaier AH, Nickel KF, Blomback M, Maas C. In vivo roles of factor XII. Blood. 2012;120(22):4296–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oehmcke S, Herwald H. Contact system activation in severe infectious diseases. J Mol Med. 2010;88(2):121–126. [DOI] [PubMed] [Google Scholar]
- 42.Hess R, Wujak L, Hesse C, et al. Coagulation factor XII regulates inflammatory responses in human lungs. Thromb Haemost. 2017;117(10):1896–1907. [DOI] [PubMed] [Google Scholar]