Abstract
Highly conserved among species and expressed in various types of cells, numerous roles have been attributed to the cellular prion protein (PrPC). In hematopoiesis, PrPC regulates hematopoietic stem cell self-renewal but the mechanisms involved in this regulation are unknown. Here we show that PrPC regulates hematopoietic stem cell number during aging and their determination towards myeloid progenitors. Furthermore, PrPC protects myeloid progenitors against the cytotoxic effects of total body irradiation. This radioprotective effect was associated with increased cellular prion mRNA level and with stimulation of the DNA repair activity of the Apurinic/pyrimidinic endonuclease 1, a key enzyme of the base excision repair pathway. Altogether, these results show a previously unappreciated role of PrPC in adult hematopoiesis, and indicate that PrPC-mediated stimulation of BER activity might protect hematopoietic progenitors from the cytotoxic effects of total body irradiation.
Introduction
Radiotherapy is commonly used alone or in combination with genotoxic drugs for treatment of numerous solid tumors. Despite progress in its targeting, radiotherapy can be deleterious to two tissues, the gastrointestinal tract and the bone marrow (BM), and can lead to secondary effects commonly defined as Acute Radiation Syndrome.1 Irradiation of the BM damages hematopoietic stem and progenitor cells (HSPC) and perturbs the hematopoietic microenvironment,2,3 resulting in radiation-induced acute myelosuppression4,5 and increased susceptibility to infections.6,7
Numerous types of DNA lesions are induced by cell exposure to ionizing radiation. They include base modifications, apurinic/apyrimidic sites (AP sites), and single- (SSB) and double (DSB)-strand breaks. DSB are the main lesions affecting cell survival. They can arise not only directly by deposition of energy on the DNA, but also as a consequence of the formation of AP sites or SSB.8,9 Indeed, base excision repair (BER) activities, and in particular the processing of abasic sites, have been shown to contribute to radiation-induced DNA damage.10,11
Apurinic/apyrimidic endonuclease-1 (Ape1) is the unique enzyme that converts AP sites into SSB intermediates during BER. Ape1 3’-phosphodieterase and -phosphate activities (for a review, see Laev et al.12) also contribute to the processing of radiation-induced DNA strand break extremities13 during the single strand break repair pathway (SSBR). Accordingly, protection of neuronal cells from radiation-induced damage requires Ape1.14,15
The cellular prion protein (PrPC) is a highly conserved glycoprotein that, when structurally modified, plays a critical role in the pathogenesis of neurodegenerative disorders called prion diseases.16 The normal prion protein was shown to protect cells from oxidative stress.17–20 It also gives protection from DNA damage by promoting Ape1 DNA repair activity and cell survival through an interaction with Ape1.21 During hematopoiesis, PrPC is highly expressed in HSC and hematopoietic progenitors22–24 and PrPC deficiency is associated with decreased HSC self-renewal.25 As oxidative stress and DNA damage help determine HSC cell fate,26 PrPC might participate in the maintenance of the hematopoietic system and its response to cytotoxic stresses. To address these points, we used Prnp knockout mice to study the consequences of PrPC deficiency on hematopoiesis of young and old adult mice, and on the response of hematopoietic stem cells (HSC) and hematopoietic progenitors to gamma-irradiation.
Methods
Mice
Mice experiments were carried out in compliance with the European Community Council Directive (EC/2010/63) and were approved by our institutional ethics committee (CetEA-CEA-DRF–n. 17-096). The B6.129S7-Prnptm1Cwe/Orl mice were from the European Mutant Mouse Archive and bred in our animal facility. We also used Prnp ZH3/ZH3 mice provided by A. Aguzzi (Zurich, Switzerland) and C57BL/6 mice were purchased from Charles River.
Cell sorting and flow cytometry analysis of bone marrow cells
Murine BM cells were flushed out of femurs, tibiae, hip bone and humeruses using a syringe filled with DPBS and filtered through a 70 μm-cell strainer. After red blood cell lysis using NH4Cl solution (STEMCELL Technologies), mononuclear cells were phenotyped using different antibody cocktails from Biolegend, e-Bioscience or Beckton Dickinson. Flow cytometry analysis was performed with a BD FACS LSRIITM flow cytometer (BD Biosciences) and cell sorting with a FACS Influx cell sorter (Becton Dickinson). Data were analyzed with FlowJo software. Antibodies and gating strategies for hematopoietic subset analysis and sorting are described in the Online Supplementary Methods. For RT-PCR and Ape1 endonuclease activity experiments, aliquots of 50,000 myeloid progenitor cells were sorted in PBS whereas aliquots of 10,000 HSC and multi-potent progenitors (MPP) were sorted in PBS/1%BSA.
Ape1 endonuclease activity
Cell extracts were obtained by sonication of pelleted BM sorted cells in 20mM Tris-HCl, pH 7.5, 250mM NaCl, 1mM EDTA, 20mM sucrose, and protease inhibitor cocktail 0.1% (Sigma-Aldrich P2714). For progenitor analysis, 50,000 cells were suspended in 125 μL extraction buffer. For HSC or MEP analysis, 10,000 sorted cells were suspended in 30μL extraction buffer. After sonication, the homogenate was centrifuged at 20,000 × g for 30 minutes (min) at 4°C and aliquots of the supernatant were stored at −80°C. Ape1 endonuclease activity was measured using a 5’-end labeled 34-mer oligonucleotide containing a single tetrahydrofuranyl artificial AP site at position 16 hybridized to its complementary oligonucleotide containing a cytosine opposite the lesion (Eurogentec). To measure Ape1 activity in BM progenitors, 1-4 μL of cell extract were incubated for 30 min at 30°C in 10 μL of reaction buffer containing 25mM Tris-HCl pH 7.5, 5mM MgCl2, 5% glycerol, 52mM NaCl, BSA 1 μg/mL and 150 fmoles of the hybridized oligonucleotide. To determine Ape1 activity in BM HSC and MPP, 1-4 μL of cell extract were incubated for 10 min at 30°C in 10 μL reaction buffer containing 25mM Tris-HCl pH 7.5, 5mM MgCl2, 5% glycerol, 52mM NaCl, and 150 fmoles of the hybridized oligonucleotide. The reaction was stopped by adding 4μL of denaturating buffer (80% formamide, 0.1% bromophenol blue, 10mM EDTA) followed by heating for 5 min at 95°C. The products of the reaction were resolved by denaturating 7M urea-20% polyacrylamide gel electrophoresis. Gels were scanned using a Typhoon 5 (GE Healthcare Life Sciences) and band intensifies were quantified with ImageQuant TL 8.1 (GE Healthcare Life Sciences).
Statistical analyses
Quantitative data are presented as the mean±standard error of mean (SEM). Statistical significance was assayed using the nonparametric Mann Whitney U-test (GraphPad Prism software).
Additional information concerning the materials and methods used are to be found in the Online Supplementary Methods.
Results
Cellular prion protein is differentially expressed in hematopoietic stem cells and myeloid progenitors and is involved in hematopoietic stem cell expansion during aging
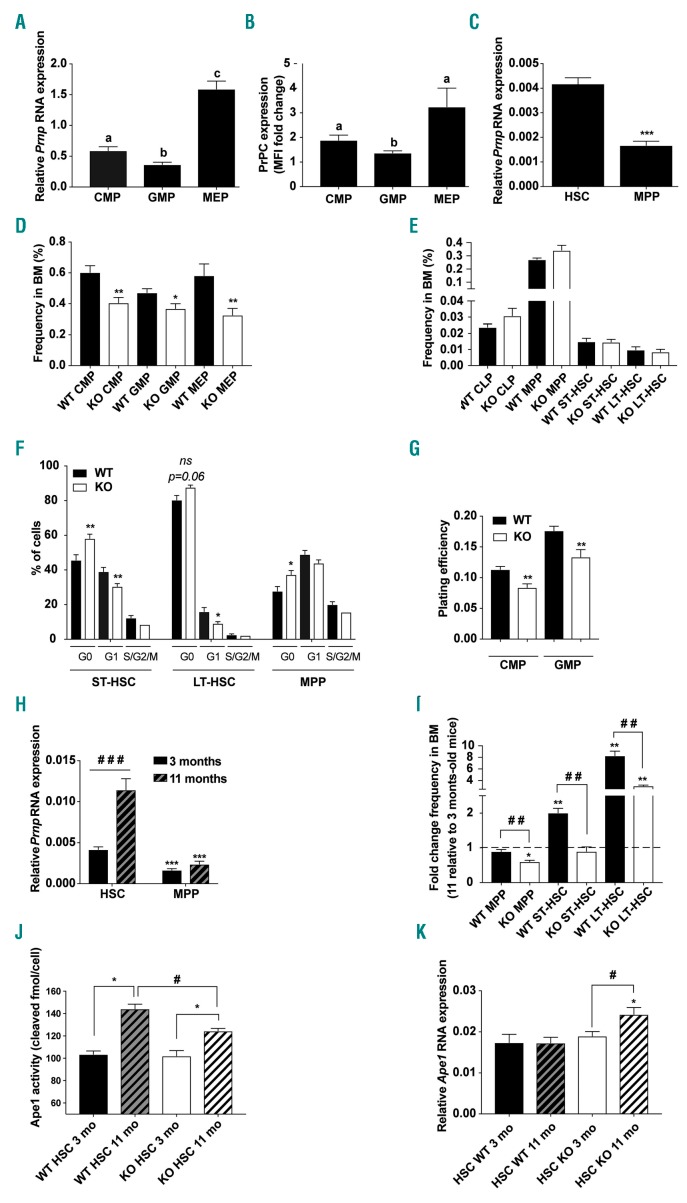
To address the potential functions of PrPC in hematopoietic stem and progenitor cells (HSPC), we first characterized the expression pattern of Prnp in different purified hematopoietic subpopulations, i.e. common myeloid progenitors (CMP), granulocyte-monocyte progenitors (GMP), megakaryocyte-erythrocyte progenitors (MEP), MPP, and hematopoietic stem cells (HSC). The highest level of Prnp mRNA was found in MEP while they were 2.7-fold and 4.3-fold lower in CMP and GMP, respectively (Figure 1A). These differences in mRNA expression were also found at the protein level (Figure 1B and Online Supplementary Figure S1A). The Prnp mRNA level in purified HSC was 2.5-fold higher than in MPP (Figure 1C).
Figure 1.
PrPC contributes to mouse hematopoietic homeostasis. (A) quanta-tive real-time polymerase chain reaction (qRT-PCR) analysis of Prnp expression, normalized to Rplp0 in the indicated bone marrow (BM) subpopulations: CMP: common myeloid progenitor; GMP: granulocyte-macrophage progenitor; MEP: megakaryocyte-erythrocyte progenitor purified by flow cytometry from BM of 3-month old mice (n=7-9). Data are presented as mean±standard error of mean (SEM). Means with different letters are significantly different (P<0.05). (B) Flow cytometry analysis of the PrPC protein expression in the indicated BM supopulations. Graph depicts ratio of median fluorescence intensity (MFI) in wild-type (WT) and knock-out (KO) control cells (n=5-6). Data are presented as mean±SEM. Means with different letters are significantly different (P<0.05) (CMP vs. MEP, P=0.06). (C) qRT-PCR analysis of Prnp expression, normalized to Actb in hematopoietic stem cell (HSC) (LSK CD135−) and in multipotent progenitor (MPP) (LSK CD135+) purified by flow cytometry from BM of 3-month old mice (n=9); Data are presented as mean±SEM. ***P<0.001. (D) Frequencies of myeloid progenitors in WT (black bars) and KO (white bars) BM from 3-month old mice (n=6-10). Data are presented as mean±SEM. *P<0.05; **P<0.01. (E) Frequencies of lymphoid progenitors (CLP), MPP, ST- and LT-HSC in the BM from WT (black bars) and KO (white bars) mice (n=6). Data are presented as mean±SEM. (F) Distribution of WT (black bars) and KO (white bars) HSC and MPP in each phase of the cell cycle. Data are presented as mean±SEM. *P<0.05; **P<0.01. ns: not significant. (G) In vitro plating efficiency of CMP and GMP purified by flow cytometry from BM of WT (black bars) and KO (white bars) mice (n=6-9). Data are presented as mean±SEM. **P<0.01. (H) qRT-PCR analysis of Prnp expression, normalized to Actb in WT and KO HSC and MPP purified by flow cytometry from BM of 3-month and 11-month old mice (n=6-9). Data are presented as the mean±SEM. ***P<0.001 or ###P<0.001. (I) Frequencies of MPP, ST-and LT-HSC in BM from 3-month and 11-month old WT (black bars) and KO (white bars) mice. Data are presented as the mean±SEM fold change of the frequencies in 11-month relative to 3-month old mice (n=6-10). **P<0.01 or ##P<0.01. (J) Ape1 endonuclease activity in 3-month (opened) and 11-month (hatched) old WT (dark) and KO (light) HSC. Data are presented as the mean±SEM. (n=4-5). *P<0.05 or #P<0.05. (K) qRT-PCR analysis of Ape1 expression, normalized to Actb in WT (dark) and KO (light) HSC purified by flow cytometry from BM of 3-month (opened bars) and 11-month (hatched bars) old mice. Data are presented as mean±SEM. (n=7-9). *P<0.05 or #P<0.05.
To determine if PrPC has a role in hematopoiesis, we first compared BM from 3-month old Prnp+/+ (WT) and Prnp−/− (KO) mice. WT and KO mice showed similar peripheral blood counts (data not shown) and BM cellularity (Online Supplementary Figure S1B). However, the frequency of CMP, GMP and MEP was significantly reduced in KO mice compared to WT mice (Figure 1D), whereas CLP, MPP, ST-HSC and LT-HSC frequencies were similar (Figure 1E). The differences between KO and WT myeloid progenitor frequencies were not associated with increased apoptosis (Online Supplementary Figure S1C) or cell cycle alteration (Online Supplementary Figure S1D) but with a higher percentage of quiescent MPP and ST-HSC (Figure 1F). These results suggest a defect of determination of HSPC (MPP and ST-HSC) towards the myeloid lineage in Prnp−/− mice. Finally, clonogenic assay using purified CMP and GMP showed a significantly decreased plating efficiency of Prnp−/− CMP and GMP (Figure 1G). Taken together, these results show intrinsic myeloid differentiation deficiencies in Prnp−/− HSC and progenitors and suggest that the reduction of cycling MPP and ST-HSC contributes to the lower myeloid progenitor content in the BM from KO compared to WT mice.
Aging of HSC is associated with an increased percentage of HSC in BM, decreased HSC self-renewal, and accumulation of DNA damage.27 As PrPC has been implicated in HSC self-renewal25 and in DNA repair,21 we investigated the effect of PrPC loss in HSC numbers and DNA repair capacity during aging in Prnp+/+ and Prnp−/− mice. Prnp mRNA level in HSC was 2.7-fold higher in 11-month old compared to 3-month old mice (Figure 1H) but did not change in MPP (Figure 1H). BM from 11-month old WT and KO mice displayed similar cellularity (Online Supplementary Figure S1E). As in 3-month old mice, a lower frequency of myeloid progenitors but not MPP (Figure 1I) was found in 11-month old KO mice (Online Supplementary Figure S1F). In contrast, compared to their 3-month old counterparts, ST- and LT-HSC frequencies respectively increased 2- and 8.3-fold in 11-month old WT mice but did not change (ST-HSC) or was only 3-fold increased (LT-HSC) in 11-month old KO mice (Figure 1I). DNA repair was slightly dependent on PrPC in aged HSC. A 1.4-fold increased activity of Ape1 in WT HSC was found between three months and 11 months without any change in Ape1 mRNA level. In KO HSC, Ape1 activity also increased between three months and 11 months, but to a lesser extent (1.2-fold) than in WT HSC. Interestingly, this increased activity was associated with an increased Ape1 mRNA level (Figure 1J and K).
Altogether, these results show that PrPC deficiency is associated with decreased HSC determination towards the myeloid lineage, and decreased number of HSC and decreased Ape1 activity in old mice.
Prnp expression is up-regulated in myeloid progenitors and hematopoietic stem cell subpopulations after in vivo radiation exposure
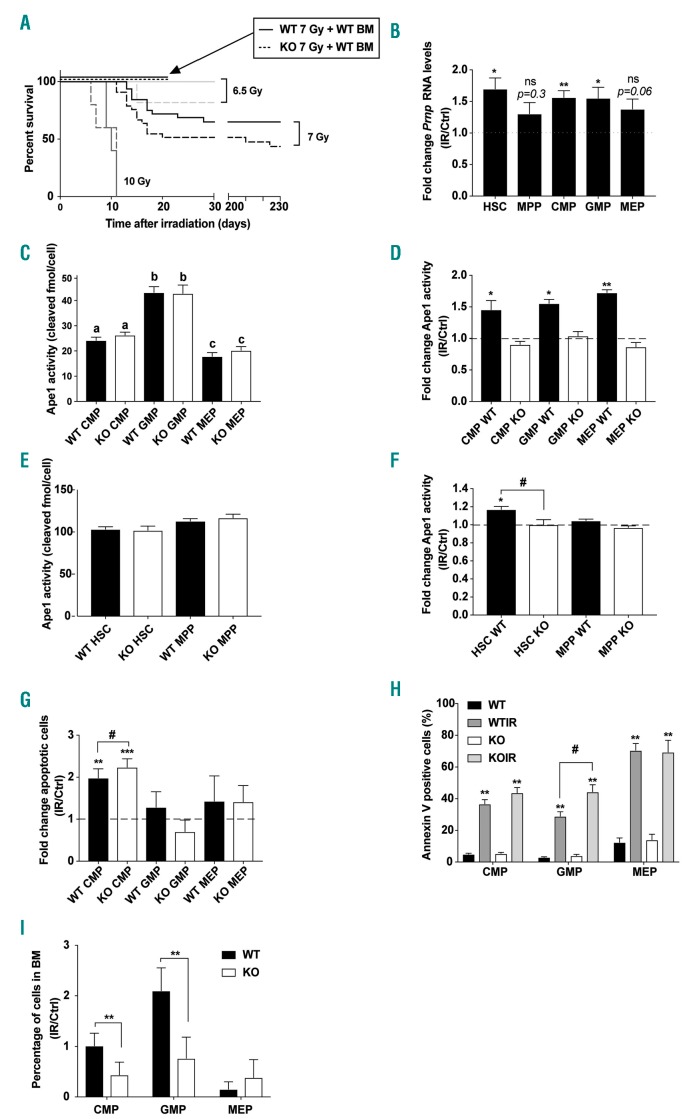
Hematopoietic stem cell aging is associated with increased oxidative stress28 and PrPC has been shown to protect cells from oxidative stress.17–20 To characterize the role of PrPC during the oxidative stress of hematopoiesis, we performed total body irradiation (TBI) on WT and KO mice. Survival curves of WT and KO mice exposed to increasing gamma-radiation doses showed that a higher percentage of irradiated KO mice died earlier than irradiated WT mice even if statistical significance between both genotypes was not reached (P=0.0792 at 7 Gy) (Figure 2A). When KO mice were grafted with BM from non-irradiated WT mice 24 hours (h) after a 7 Gy irradiation, they did not die, indicating that the higher sensitivity of KO mice to TBI was not due to the BM microenvironment of KO mice and that they died from hematopoietic syndrome.
Figure 2.
PrPC favors survival of mice exposed to moderate doses of γ-rays and protects common myeloid progenitors (CMP) from radiation-induced death. (A) Kaplan-Meier survival plots of wild-type (WT) (solid lines) and knock-out (KO) (dashed lines) mouse overall survival after total body irradiation (TBI) at indicated doses: n=5, 10 Gy; n=13, 6.5 Gy; n=28, 7 Gy; for each genotype. Arrow points to the 100% survival of both KO and WT irradiated mice (7 Gy) after transplantation of WT bone marrow (BM) cells (n=5). (B) Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of Prnp expression in hematopoietic stem cell (HSC), multi-potent progenitor (MPP), and myeloid progenitors 1 hour (h) after irradiation (7 Gy) (n=6). Prnp RNA levels were normalized to Actb (HSC and MPP) or Rplp0 (myeloid progenitors). Data are presented as mean±standard error of mean (SEM) fold change of normalized Prnp RNA levels in irradiated relative (IR) to control cells (Ctrl). *P<0.05; **P<0.01. (C) Ape1 endonuclease activity in myeloid progenitor subpopulations from WT (black bars) and KO (white bars) mice (n=5-8). Data are presented as mean±SEM. Means with different letters are significantly different (P<0.05). (D) Ape1 endonuclease activity in myeloid progenitors from WT (black bars) and KO (white bars) mice (n=5-8) 1 h after irradiation (7 Gy). Data are presented as mean±SEM fold change of Ape1 endonuclease in irradiated relative to non-irradiated control cells. *P<0.05; **P<0.01. (E) Ape1 endonuclease activity in HSC and MPP from WT (black bars) and KO (white bars) mice (n=5-8). Data are presented as mean±SEM. (F) Ape1 endonuclease activity in HSC and MPP from WT (black bars) and KO (white bars) mice (n=4-5) 1 h after irradiation (7 Gy). Data are presented as mean±SEM fold change of Ape1 endonuclease in irradiated relative to non-irradiated control cells. *P<0.05 or #P<0.05. (G) Percentage of apoptotic myeloid progenitors (AnnexineV-positive cells) in BM from WT (black bars) and KO (white bars) mice (n=6-8) 1 h after irradiation (7 Gy). Data are presented as mean±SEM fold change of percentage of apoptotic cells in irradiated relative to non-irradiated control myeloid progenitors. **P<0.01; ***P<0.001; #P<0.05. (H) Percentage of apoptotic myeloid progenitors (AnnexineV-positive cells) in BM from WT (dark gray bars, WTIR) and KO (light gray bars, KOIR) mice (n=6-8) 1 h after irradiation (7 Gy). Data are presented as mean±SEM. Non-irradiated control WT (black bars) and KO (white bars) myeloid progenitors are shown. **P<0.01; #P<0.05. (I) Percentage of myeloid progenitors in BM from WT (black bars) and KO (white bars) mice (n=6-7) 18 h after irradiation (7 Gy). Data are presented as mean±SEM of the percentage of cells remaining in BM 18 h after irradiation compared to non-irradiated control. **P<0.01.
One hour after a 7 Gy TBI, a 1.5-fold increase in Prnp mRNA level was found in HSC, CMP and GMP but not in MPP and MEP (Figure 2B). These data are consistent with the observed Prnp upregulation in neuronal tissues after exposure to genotoxic stress,21 and suggest a potential role of PrPC in response to radiation in GMP, CMP, and HSC.
Cellular prion protein-dependent increase in the DNA repair activity of Ape1 is associated with radioprotection of CMP and GMP
Cellular prion protein prevents cell death in response to alkylating agent or H2O2 exposure by directly stimulating the DNA repair activity of Ape1.21 Without irradiation, Ape1 activity was similar in WT and KO progenitors (Figure 2C). One hour after a 7 Gy TBI, Ape1 activity increased in all WT irradiated myeloid progenitors analyzed (from 1.5- to 1.7-fold) but not in their KO counter parts (Figure 2D). In HSC and MPP, Ape1 activity was similar in WT and KO mice (Figure 2E) but increased only in WT HSC after a 7 Gy TBI (Figure 2F). Whatever the subpopulation analyzed, the radiation-induced Ape1 activity was not associated with an increase in Ape1 mRNA level (Online Supplementary Figure S2A and B). These data show that, after irradiation, Ape1 activity in all myeloid progenitor subpopulations and in HSC is stimulated in a PrPC-dependent manner.
As the radiation-induced death of myeloid progenitors is dependent on apoptosis,5 we quantified apoptosis in WT and KO myeloid progenitors 1 and 12 h after TBI at 7 Gy. One hour after irradiation, apoptotic (Annexin V-positive cells) and dead cell (Annexin V-negative and Hoechst-positive cells) fractions increased only in CMP and were higher in KO compared to WT CMP (Figure 2G and Online Supplementary Figure S2C). Twelve hours after irradiation, both apoptotic (Figure 2H) and dead (Online Supplementary Figure S2D) cell fractions increased in all myeloid progenitor subpopulations in both WT and KO mice. However, higher rates of apoptosis and cellular death were observed in irradiated KO compared to WT GMP. In accordance with this, significantly lower frequencies of CMP and GMP were found in KO versus WT irradiated mice 18 h after irradiation (Figure 2I). Myeloid progenitors from Prnp ZH3/ZH3 mice exhibited the same radiation sensitivity than those from Prnp−/− mice, shown by a similar reduced number compared to mice in non-irradiated conditions (Online Supplementary Figure S2E). Altogether, these results suggest that PrPC-dependent stimulation of the DNA repair activity of Ape1 is required for the radioprotection of myeloid progenitors.
Discussion
Despite numerous studies, the physiological role of PrPC remains elusive. Recently, we showed that PrPC can stimulate an important DNA repair pathway, the BER, in neuronal tissues through interaction with and stimulation of its key enzyme, APE1.21 Here we show that the same mechanism can be proposed for the radioprotection of myeloid progenitors, HSC determination, and the expansion of the HSC compartment during aging.
Previous studies22–24,29 indicated a decreased Prnp expression during differentiation of hematopoietic cells. Here, we performed an extended study of Prnp expression in different hematopoietic subpopulations and showed a 3-fold higher Prnp expression level in MEP compared to their progenitors CMP, suggesting that the correlation between Prnp downregulation and cellular differentiation24 may not be a general feature in hematopoiesis. Furthermore, and contrary to a previous study,25 we found that KO mice have less myeloid progenitors. This discrepancy could be explained by the fact that, in the previous study, younger mice were analyzed (7-10-week old mice compared to the 3-month and 11-month old mice used in the present study) and by the number of backcrosses (4 vs. >10) that might influence the phenotype of Prnp knockout mice.30 Finally, we found a higher frequency of KO ST-HSC and KO MPP in the G0 phase. These populations being the direct precursors of myeloid progenitors, the increased quiescence of these cells might account for the decreased myeloid progenitor subpopulations.31 Strikingly, both KO CMP and GMP exhibited a lower plating efficiency despite no significant change in their cell cycle in vivo. Whether the microenvironment of these cells could compensate in vivo for an intrinsic growth deficiency observed in vitro remains to be clarified.
Prnp expression in the HSC compartment increased 8-fold with age. This higher expression was associated with the known elevated frequency of both ST- and LT-HSC.27,32 PrPC deficiency was associated with no increase in ST-HSC and with a diminished increase in LT-HSC with age, suggesting that PrPC plays a role in the age-dependent increase in HSC. Although an aging-associated increase in HSC numbers has been known for a long time and is known to be cell-intrinsic,33,34 the underlying mechanisms are not fully understood. The results presented here indicate a significant involvement of PrPC in the age-dependent increase in HSC frequency. This increase may be accounted for by a PrPC-dependent upregulation of Ape1 repair activity. Independently of its DNA repair activity,35,36 Ape1 has a redox activity shown to be necessary for normal embryonic hematopoiesis,37 stem cell pool maintenance,38,39 and hematopoietic progenitor colony formation.40 Thus, the PrPC-dependent stimulation of the Ape1 DNA repair activity might contribute to hematopoietic homeostasis.
We found a modest but recurrent radiation sensitization of Prnp KO mice that contrasts with a previous study showing that the absence of PrPC protected rather than sensitized mice to an 8 Gy TBI.41 However, this work was performed on a mixed 129/C57BL6 background, whereas we used a pure C57BL6 background. Furthermore, that study used a dose of X rays that was lethal for WT animals, while we used different doses of non-lethal γ-rays. BM myeloid cells are particularly sensitive to chemical and radiation cytotoxicity.4,42,43 Accordingly, we found a dramatic decrease in BM myeloid progenitors within the first 24 h after radiation exposure that was exacerbated in Prnp KO irradiated mice. The reduced frequency of KO irradiated myeloid progenitors was associated with higher CMP and GMP apoptosis, within the first 12 h after irradiation, as well as with an absence of stimulation of Ape1 activity in these subpopulations 1 h after irradiation. In contrast, in WT irradiated myeloid progenitors and HSC, upregulation of Prnp gene expression was associated with an increase in Ape1 activity in these subpopulations. PrPC has been shown to protect HSC from myelotoxic injury by 5-FU, commonly used in chemotherapy,25 and we now extend its myeloprotective role to radiotherapy.
Finally, a similar basal reduced number of myeloid progenitors and a similar radiation sensitivity of myeloid progenitors were found in the co-isogenic PrnpZH3/ZH3 mouse line44 and the Prnp−/− mouse line. These results rule out the involvement of any Prnp flanking gene polymorphism previously described by Nuvolone et al.45
Altogether, these results suggest that PrPC is involved in the homeostasis of steady-state hematopoiesis and that PrPC-dependent activation of base excision repair contributes to the radioprotection of the myeloid progenitors of the mouse bone marrow.
Acknowledgments
The authors thank Véronique Neuville and staff of the IRCM animal facility for animal care and breeding, and Petra Schwarz for managing Prnp ZH3/ZH3 mice supply. Flow cytometry and cell sorting were performed at the IRCM Flow Cytometry Shared Resource, established by equipment grants from DIM-Stem-Pôle, INSERM, Foundation ARC, and CEA.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/105/5/1216
Funding
This work was supported by the French National Electricity Company (EDF), the Transverse Division N°4 (Segment n°4 Radiobiologie – headed by Christophe Carles) and the Radiobiology Program of the French Alternative Energies and Atomic Energy Commission (CEA).
References
- 1.Dörr H, Meineke V. Acute radiation syndrome caused by accidental radiation exposure - therapeutic principles. BMC Med. 2011;9(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonnet AJ, Nehmë J, Vaigot P, Barroca V, Leboulch P, Tronik-Le Roux D. Phenotypic and functional changes induced in hematopoietic stem/progenitor cells after gamma-ray radiation exposure. Stem Cells. 2009;27(6):1400–1409. [DOI] [PubMed] [Google Scholar]
- 3.Shao L, Luo Y, Zhou D. Hematopoietic Stem Cell Injury Induced by Ionizing Radiation. Antioxid Redox Signal. 2014;20(9):1447–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Down JD, Boudewijn A, van Os R, Thames HD, Ploemacher RE. Variations in radiation sensitivity and repair among different hematopoietic stem cell subsets following fractionated irradiation. Blood. 1995; 86(1):122–127. [PubMed] [Google Scholar]
- 5.Mohrin M, Bourke E, Alexander D, et al. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Thimmulappa RK, Kumar V, et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124(2):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zachman DK, Leon RP, Das P, et al. Endothelial cells mitigate DNA damage and promote the regeneration of hematopoietic stem cells after radiation injury. Stem Cell Res. 2013;11(3):1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ensminger M, Iloff L, Ebel C, Nikolova T, Kaina B, Löbrich M. DNA breaks and chromosomal aberrations arise when replication meets base excision repair. J Cell Biol. 2014;206(1):29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer NC, Corbett AH, Doetsch PW. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 2015; 43(21):10083–10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung H, Demple B. Distinct roles of Ape1 protein in the repair of DNA damage induced by ionizing radiation or bleomycin. J Biol Chem. 2011;286(7):4968–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Wang X, Chen G, et al. Distinct roles of Ape1 protein, an enzyme involved in DNA repair, in high or low linear energy transfer ionizing radiation-induced cell killing. J Biol Chem. 2014;289(44):30635–30644. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Laev SS, Salakhutdinov NF, Lavrik OI. Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1). Bioorganic Med Chem. 2017;25(9):2531–2544. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JL, Dianova II, Dianov GL. APE1 is the major 3 -phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 2004;32(12):3531–3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasko MR, Guo C, Thompson EL, Kelley MR. The repair function of the multifunctional DNA repair/redox protein APE1 is neuroprotective after ionizing radiation. DNA Repair (Amst). 2011;10(9):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ströbel T, Madlener S, Tuna S, et al. Ape1 guides DNA repair pathway choice that is associated with drug tolerance in glioblastoma. Sci Rep. 2017;7(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982; 216(4542):136–144. [DOI] [PubMed] [Google Scholar]
- 17.Milhavet O, Lehmann S. Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Brain Res Rev. 2002;38(3):328–339. [DOI] [PubMed] [Google Scholar]
- 18.Rachidi W, Vilette D, Guiraud P, et al. Expression of prion protein increases cellular copper binding and antioxidant enzyme activities but not copper delivery. J Biol Chem. 2003;278(11):9064–9072. [DOI] [PubMed] [Google Scholar]
- 19.McLennan NF, Brennan PM, McNeill A, et al. Prion protein accumulation and neuroprotection in hypoxic brain damage. Am J Pathol. 2004;165(1):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krebs B, Wiebelitz A, Balitzki-Korte B, et al. Cellular prion protein modulates the intracellular calcium response to hydrogen peroxide. J Neurochem. 2007;100(2):358–367. [DOI] [PubMed] [Google Scholar]
- 21.Bravard A, Auvré F, Fantini D, et al. The prion protein is critical for DNA repair and cell survival after genotoxic stress. Nucleic Acids Res. 2015;43(2):904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent DG, Copley MR, Benz C, et al. Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood. 2009;113(25):6342–6350. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Li R, Wong B-S, et al. Normal Cellular Prior Protein Is Preferentially Expressed on Subpopulations of Murine Hemopoietic Cells. J Immunol. 2001;166(6):3733–3742. [DOI] [PubMed] [Google Scholar]
- 24.Panigaj M, Glier H, Wildova M, Holada K. Expression of prion protein in mouse erythroid progenitors and differentiating murine erythroleukemia cells. PLoS One. 2011;6(9):e24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc Natl Acad Sci U S A. 2006;103(7):2184–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss CN, Ito K. DNA damage: A sensible mediator of the differentiation decision in hematopoietic stem cells and in leukemia. Int J Mol Sci. 2015;16(3):6183–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi DJ, Seita J, Czechowicz A, Bhattacharya D, Bryder D, Weissman IL. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6(19):2371–2376. [DOI] [PubMed] [Google Scholar]
- 28.Chen F, Liu Y, Wong NK, Xiao J, So KF. Oxidative Stress in Stem Cell Aging. Cell Transplant. 2017;26(9):1483–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodelet VC, Cashman NR. Prion protein expression in human leukocyte differentiation. Blood. 1998;95(5):1556–1561. [PubMed] [Google Scholar]
- 30.Schmitz M, Greis C, Ottis P, et al. Loss of Prion Protein Leads to Age-Dependent Behavioral Abnormalities and Changes in Cytoskeletal Protein Expression. Mol Neurobiol. 2014;50(3):923–936. [DOI] [PubMed] [Google Scholar]
- 31.Susek KH, Korpos E, Huppert J, et al. Bone marrow laminins influence hematopoietic stem and progenitor cell cycling and homing to the bone marrow. Matrix Biol. 2018;6747–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalczyk MS, Tirosh I, Heckl D, et al. Single-cell RNA-seq reveals changes in cell cycle and differentiation programs upon aging of hematopoietic stem cells. Genome Res. 2015;25(12):1860–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102(26):9194–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger H, De Haan G, Carolina Florian M. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5): 376–389. [DOI] [PubMed] [Google Scholar]
- 35.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The Many Functions of APE1/Ref-1: Not Only a DNA Repair Enzyme. Antioxid Redox Signal. 2008;11(3):601–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Zhang L, Li M, et al. Functional analysis of the involvement of apurinic/apyrimidinic endonuclease 1 in the resistance to melphalan in multiple myeloma. BMC Cancer. 2014;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou GM, Luo MH, Reed A, Kelley MR, Yoder MC. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109(5):1917–1922. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Zhang T, Dong Q, Nice EC, Huang C, Wei Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013;4:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domenis R, Bergamin N, Gianfranceschi G, et al. The redox function of APE1 is involved in the differentiation process of stem cells toward a neuronal cell fate. PLoS One. 2014;9(2):e89232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rohrabaugh SL, Hangoc G, Kelley MR, Broxmeyer HE. Mad2 haploinsufficiency protects hematopoietic progenitor cells subjected to cell-cycle stress in vivo and to inhibition of redox function of Ape1/Ref-1 in vitro. Exp Hematol. 2011;39(4):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strup-Perrot C, Vozenin MC, Monceau V, et al. PrP c deficiency and dasatinib protect mouse intestines against radiation injury by inhibiting of c-Src. Radiother Oncol. 2016;120(1):175–183. [DOI] [PubMed] [Google Scholar]
- 42.Pilzecker B, Buoninfante OA, van den Berk P, et al. DNA damage tolerance in hematopoietic stem and progenitor cells in mice. Proc Natl Acad Sci U S A. 2017;114(33):E6875–E6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth RB, Samson LD. 3-Methyladenine DNA glycosylase-deficient Aag null mice display unexpected bone marrow alkylation resistance. Cancer Res. 2002;62(3):656–660. [PubMed] [Google Scholar]
- 44.Nuvolone M, Hermann M, Sorce S, et al. Strictly co-isogenic C57BL/6J- Prnp −/− mice: A rigorous resource for prion science. J Exp Med. 2016;213(3):313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuvolone M, Kana V, Hutter G, et al. SIRP polymorphisms, but not the prion protein, control phagocytosis of apoptotic cells. J Exp Med. 2013;210(12):2539–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]