Key Points
Question
What is the clinical activity of nivolumab in patients with advanced refractory biliary tract cancer?
Findings
In this multicenter phase 2 study of 54 patients with advanced refractory biliary tract cancer, 10 patients achieved an objective response with a disease control rate of 59% by investigator assessment. Five patients experienced an objective response with a disease control rate of 50% by blinded central independent radiologic review.
Meaning
Further exploration of nivolumab is warranted for patients with advanced refractory biliary tract cancer.
Abstract
Importance
Currently, there is no established second-line systemic treatment for biliary tract cancer (BTC). Preclinical data have demonstrated that the presence of tumor-infiltrating CD8 T cells and programmed cell death 1 ligand 1–expressing tumor cells in the tumor microenvironment of BTC supports the rationale of using programmed cell death 1 protein blockade immunotherapy in BTC.
Objective
To evaluate anticancer activity of nivolumab in patients with advanced refractory BTC.
Design, Setting, and Participants
In this single-group, multicenter phase 2 study of nivolumab, 54 patients with histologically confirmed BTC whose disease progressed while undergoing treatment with at least 1 line but no more than 3 lines of systemic therapy were enrolled between October 5, 2016, and December 26, 2018. Analysis was performed on an intention-to-treat basis.
Interventions
Nivolumab, 240 mg, was delivered intravenously every 2 weeks for 16 weeks, and then 480 mg was delivered intravenously every 4 weeks until disease progression or unacceptable toxic effects occurred.
Main Outcomes and Measures
The primary end point was investigator-assessed objective response rate, and the secondary end points were progression-free survival, overall survival, and incidence of adverse events.
Results
A total of 54 patients (27 men and 27 women; median age, 65 years [range, 28-86 years]) enrolled, and 46 (22 men and 24 women; median age, 65 years [range, 28-86 years]) were examined for objective response with radiologic imaging. The investigator-assessed objective response rate was 22% (10 of 46), including 1 unconfirmed partial response, with a disease control rate of 59% (27 of 46). Central independent review found an objective response rate of 11% (5 of 46), including 1 unconfirmed partial response, with a disease control rate of 50% (23 of 46). All patients who responded to treated (hereafter referred to as responders) had mismatch repair protein–proficient tumors. The median duration of investigator-assessed response was not reached, with a median follow-up of 12.4 months. Among the intention-to-treat population, median progression-free survival was 3.68 months (95% CI, 2.30-5.69 months) and median overall survival was 14.24 months (95% CI, 5.98 months to not reached). Programmed cell death 1 ligand 1 expression in tumors was associated with prolonged progression-free survival (hazard ratio, 0.23; 95% CI, 0.10-0.51; P < .001). The most common treatment-related grade 3 or 4 toxic effects were hyponatremia (3 of 54 [6%]) and increased alkaline phosphatase (2 of 54 [4%]).
Conclusions and Relevance
This study found that nivolumab was well tolerated and showed modest efficacy with durable response in patients with refractory BTC. Further studies are warranted to verify the findings and evaluate biomarkers for improved treatment selection for patients.
Trial Registration
ClinicalTrials.gov Identifier: NCT02829918
This multicenter phase 2 study evaluates the anticancer activity of nivolumab in patients with advanced refractory biliary tract cancer.
Introduction
Biliary tract cancers (BTCs) comprise malignant intrahepatic and extrahepatic cholangiocarcinomas and gallbladder cancers. They are uncommon malignant neoplasms: an estimated 12 360 patients received a diagnosis of extrahepatic cholangiocarcinoma and gallbladder cancer in 2019 in the United States.1 The exact incidence of intrahepatic cholangiocarcinoma is unknown since it is grouped with primary liver cancer. The prognosis of BTCs is dismal, with a 5-year survival rate of only 10%.2 Surgical resection is the only curative modality for localized disease. The treatment of unresectable, recurrent, or metastatic disease remains challenging. Although gemcitabine plus cisplatin has demonstrated significant antitumor activity as first-line therapy for metastatic BTC,3 to our knowledge there is no established systemic therapy after failure of gemcitabine plus cisplatin. In the recent ABC-06 (Advanced Biliary Cancer-06) study, FOLFOX (folinic acid, fluorouracil, and oxaliplatin) was evaluated as second-line treatment after progression when given gemcitabine plus cisplatin, and it demonstrated only a modest 1-month survival benefit vs active symptom control.4 Novel effective therapeutic approaches are needed for improvement in the clinical outcomes of patients with BTCs.
Nivolumab is a human immunoglobulin G4 monoclonal antibody that blocks the ligation of programmed cell death 1 protein (PD-1) and programmed cell death 1 ligand 1 (PD-L1). Programmed cell death 1 protein is a transmembrane protein expressed on T cells, and it interacts with its ligand PD-L1 expressed on cancer cells, leading to inhibition of T-cell proliferation and activation and apoptosis of antigen-specific T cells.5 With the success of PD-1 blockade immunotherapy in melanoma, nivolumab has been extensively studied in the treatment of multiple tumor types, and emerging clinical data have demonstrated durable clinical activity and safety of nivolumab in various cancers. However, only limited data are reported on the role of immune checkpoint inhibitors in BTC owing to the rarity of the disease. Programmed cell death 1 ligand 1 expression and infiltration of CD8 T cells in the tumor microenvironment have been reported as potential biomarkers of PD-1 blockade immunotherapy in several cancers.6,7 A recent study demonstrated the presence of tumor-infiltrating CD8 T cells and PD-L1–expressing tumor cells in the tumor microenvironment of BTC.8 These data support the rationale of using PD-1 blockade immunotherapy in BTC. On the basis of preclinical data, we conducted a phase 2 study to evaluate the safety and efficacy of nivolumab for patients with advanced refractory BTC.
Methods
The study was an investigator-initiated, multi-institutional (Moffitt Cancer Center, City of Hope, and Winship Cancer Institute of Emory University), open-label, single-group phase 2 study designed to evaluate the safety, tolerability, and efficacy of nivolumab for patients with advanced BTCs, who were enrolled between October 5, 2016, and December 26, 2018. The study was approved by the Moffitt Cancer Center, City of Hope, and Winship Cancer Institute of Emory University institutional review boards, and all patients provided written informed consent prior to enrollment. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline. The study was conducted in compliance with the trial protocol (Supplement 1).
Patient Selection and Treatment
Pertinent eligibility criteria were histologically confirmed unresectable or metastatic cholangiocarcinoma or gallbladder cancers, refractory or intolerant to at least 1 line of systemic therapy but no more than 3 prior lines of systemic therapy, age 18 years or older, Eastern Cooperative Oncology Group performance status of 0 or 1, and adequate organ function. All patients received a flat dose of nivolumab, 240 mg, intravenously every 2 weeks for 16 weeks and then 480 mg intravenously every 4 weeks until disease progression or unacceptable toxic effects occurred.
Evaluation
Tumor assessment was performed with computed tomography and/or magnetic resonance imaging at baseline and every 8 weeks until disease progression or treatment discontinuation. Objective response rate (ORR) was evaluated using Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1)9 and iRECIST10 criteria by the local investigators and centrally reviewed by independent radiologists. Survival was monitored every 12 weeks after discontinuation of treatment. Toxic effects were assessed according to Common Terminology Criteria for Adverse Events, version 4.0.11
Immunohistochemical Staining
For patients with available archival tissues, PD-L1 expression on tumor cells and PD-1 expression on tumor-infiltrating lymphocytes (TILs) were assessed in pretreatment tumor biopsy samples. Tissue sections (4 μm thick) were immersed in citrate buffer solution for antigen retrieval and boiled in a microwave for 10 minutes and washed in buffer solution. They were incubated with anti–PD-L1 antibody (E1L3N; Cell Signaling) and anti–PD-1 antibody (NAT105; Abcam) for 1 hour at room temperature and then washed in buffer solution. After 1 hour of incubation in the secondary antibody, the sections were incubated with streptavidin-biotin complex (DAKO). Appropriate positive and negative controls were used. Stained tumor samples were reviewed and enumerated by 2 gastrointestinal pathologists (including B.H.K.). For estimation of positive reaction, only tumor cells with membranous staining at any intensity were counted. Samples with 1% or more tumor cells for PD-L1 and any TILs for PD-1 exhibiting membranous staining were considered positive.
Mismatch repair protein (MMR) deficiency was evaluated as a standard practice. Briefly, staining of slides was performed by Benchmark Ultra immunohistochemistry and in situ hybridization system (Roche Diagnostics) according to the manufacturer’s instructions. Antibodies used in this immunohistochemical staining are as follows: MLH1 (M1; Ventana), MSH2 (G219-1129; Ventana), MSH6 (SP93; Ventana), and PMS2 (A1604; Ventana). Stained slides were examined by experienced gastrointestinal pathologists (including B.H.K.). When there was loss of expression in 1 or more MMRs, the tumor was classified as MMR deficient.
Statistical Analysis
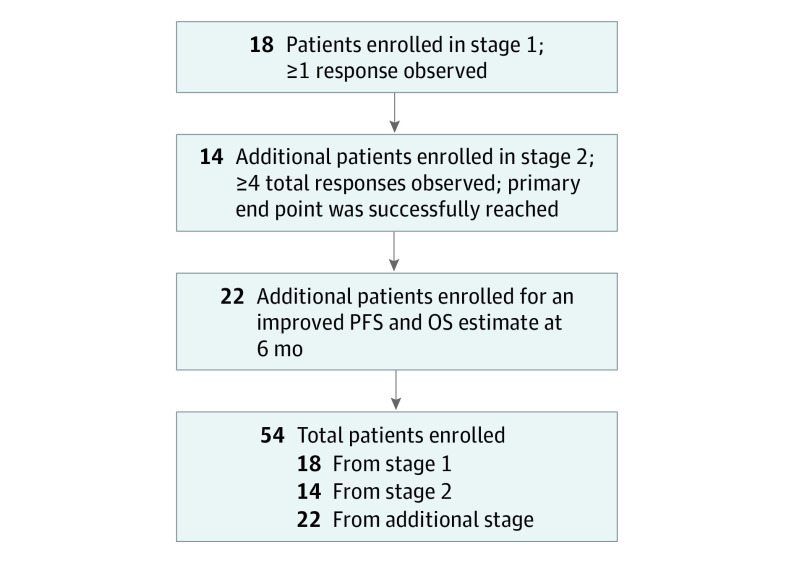
The primary end point of the study was investigator-assessed ORR by RECIST v1.1 criteria. Secondary end points were progression-free survival (PFS), overall survival (OS), and incidence of adverse events (AEs). Simon 2-stage design was used to assess ORR based on local radiologic assessment, with a null hypothesis ORR rate of 5% and an alternative hypothesis ORR rate of 20%, with α = .10 and 90% power. In the first stage, 18 patients were accrued and if 1 response was observed, the plan was to accrue an additional 14 patients, with 4 or more responses needed among the 32 patients. After complete accrual of 32 patients, the primary end point was successfully reached, with 5 partial responses (PRs), including 2 PRs in the first 18 patients. Given the positive finding and for more precise PFS and OS estimates at 6 months, 22 additional patients were recruited per the amended study protocol. A study flow diagram is shown in Figure 1. Log-rank tests were used to identify variables that were statistically significantly associated with OS or PFS. Cox proportional hazards regression models were used for multivariable analyses. All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc); all P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Figure 1. Study Flow Diagram.
OS indicates overall survival; PFS, progression-free survival.
Results
Baseline Characteristics
A total of 54 patients were enrolled between October 5, 2016, and December 26, 2018. Baseline characteristics are summarized in eTable 1 in Supplement 2. Median patient age was 65 years (range, 28-86 years) and 27 (50%) were male. Most patients (36 [67%]) had an Eastern Cooperative Oncology Group performance status of 1. Thirty-two patients (59%) had intrahepatic cholangiocarcinoma, 5 (9%) had extrahepatic cholangiocarcinoma, and 17 (31%) had gallbladder cancer. Forty-four patients (81%) had distant metastatic disease and 10 (19%) had locally advanced disease. Most patients were white (35 [65%]), followed by Hispanic (8 [15%]) and African American (8 [15%]). Twenty-seven patients (50%) progressed after receiving 1 line of systemic therapy and 27 (50%) had received 2 or more lines of therapy prior to study enrollment.
Treatment
A total of 54 patients received at least 1 dose of nivolumab. As of data lock on May 1, 2019, the median number of doses was 7 (range, 1-32). Five patients were still receiving active treatment with nivolumab, 38 patients discontinued treatment owing to disease progression, 6 patients discontinued treatment owing to an AE, and 5 patients discontinued treatment owing to withdrawal of consent. Eight patients did not have their tumor response evaluation owing to clinical progression (6 patients), withdrawal of consent (1), and gastrointestinal bleeding (1) not related to nivolumab.
Efficacy
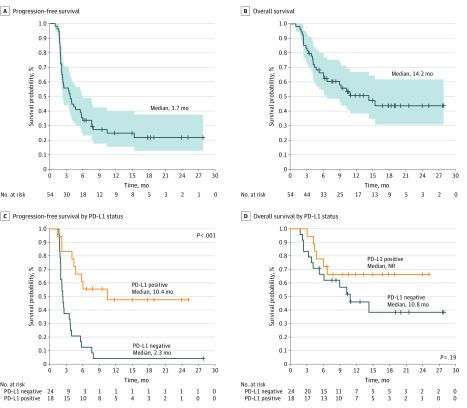
As the primary end point of this study was ORR, examined patients were defined as those with more than 1 baseline radiologic image. Among 46 examined patients who had tumor response evaluation with radiologic imaging, 10 (22%) achieved an investigator-assessed objective response (all PRs), including 1 unconfirmed PR per RECIST v1.1 criteria (Table 1). Seventeen of 46 patients (37%) achieved stable disease, with a disease control rate of 59% (n = 27), per investigator assessment (Table 1; eFigure 1 in Supplement 2). Disease control for 16 weeks or more was achieved in 24 of 46 patients (52%). Objective response was observed in 6 of 28 patients (21%) with intrahepatic cholangiocarcinoma, 2 of 5 patients (40%) with extrahepatic cholangiocarcinoma, and 2 of 13 patients (15%) with gallbladder cancer. The median time to response was 4.6 months (range, 1.9-11.2 months). Two patients who responded to treatment (hereafter referred to as responders) achieved a delayed PR 11 months after starting nivolumab, and 1 of them had shown a 19% increase in target lesions on the first scans at 8 weeks (eFigure 1 in Supplement 2). The median duration of response was not reached, with a median follow-up of 12.4 months (eFigure 2 in Supplement 2). Four responders (40%) achieved durable objective response lasting at least 1 year. Among 10 patients with locally advanced disease, 1 patient developed distant metastatic disease involving bone and subcutaneous tissue and 6 patients developed local progression. Three patients with local progression experienced abnormal liver function, including elevated bilirubin (1 patient with grade 2 and 1 patient with grade 1) and elevated alanine aminotransferase (1 patient with grade 1). None of the patients developed major biliary obstruction. The tumor response was evaluated by blinded independent radiologists centrally, and central review revealed 5 PRs (11%), including 1 unconfirmed PR, with a disease control rate of 50% (n = 23) per RECIST v1.1 criteria (Table 1). Tumor response was also evaluated by iRECIST criteria.10 Although 10 patients achieved investigator-assessed immune PR, with a disease control rate of 61% (n = 28), 6 patients achieved investigator-assessed immune PR, with a disease control rate of 61% (n = 28) per iRECIST criteria (Table 1). All responders had MMR-proficient cancer. Among the intention-to-treat population, median PFS was to 3.68 months (95% CI, 2.30-5.69 months) and median OS was 14.24 months (95% CI, 5.98 months to not reached) (Figure 2). The median PFS of the examined patients was 4.0 months (95% CI, 2.3-7.6 months) and the median OS of the examined patients was not reached at a median follow-up of 16.9 months (eFigure 3 in Supplement 2). The clinical outcome was evaluated for patients who had 1 line of systemic therapy and 2 or more lines of systemic therapy that failed (eFigure 4 in Supplement 2). The median PFS of patients with 1 line of systemic therapy was 4.2 months and of patients with 2 or more lines of systemic therapy was 3.8 months (P = .91). The median OS of patients with 1 line of systemic therapy was not reached and of patients with 2 or more lines of systemic therapy was 14.2 months (P = .69) (eFigure 4 in Supplement 2).
Table 1. Best Overall Response and Disease Control Rate.
| Best overall response | No. (%) (n = 46) | |||
|---|---|---|---|---|
| RECIST, version 1.1 | iRECIST | |||
| Investigator review | Central review | Investigator review | Central review | |
| CR (iCR) | 0 | 0 | 0 | 0 |
| PR (iPR) | 10 (22) | 5 (11) | 10 (22) | 6 (13) |
| 1 Unconfirmed | 1 Unconfirmed | 1 Unconfirmed | 1 Unconfirmed | |
| SD (iSD) | 17 (37) | 18 (39) | 18 (39) | 22 (48) |
| PD (iUPD + iCPD) | 19 (41) | 23 (50) | 18 (39) | 18 (39) |
| Disease control rate | 27 (59) | 23 (50) | 28 (61) | 28 (61) |
Abbreviations: CR, complete response; iCPD, immune confirmed progressive disease; iCR, immune complete response; iPR, immune partial response; iRECIST, immunotherapy Response Evaluation Criteria in Solid Tumors; iSD, immune stable disease; iUPD, immune unconfirmed progressive disease; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Figure 2. Kaplan-Meier Survival Curves.
A, Kaplan-Meier estimate of progression-free survival in the intention-to-treat population. The shaded area represents the 95% CI. B, Kaplan-Meier estimate of overall survival in the intention-to-treat population. The shaded area represents the 95% CI. C, Kaplan-Meier estimate of progression-free survival by programmed cell death 1 ligand 1 (PD-L1) expression status (≥1% of tumor cells expressing PD-L1 as a cutoff). D, Kaplan-Meier estimate of overall survival by PD-L1 expression status. NR indicates not reported.
Safety
The most common treatment-related AE was increased alkaline phosphatase (13 [24%]), followed by decreased lymphocytes (12 [22%]), increased aspartate aminotransferase (11 [20%]), and fatigue (11 [20%]) (Table 2; eTable 2 in Supplement 2). Grade 3 and grade 4 treatment-related AEs occurred in 9 of 54 patients (17%). Grade 3 or grade 4 treatment-related AEs that occurred in more than 2 patients were hyponatremia (3 [6%]) and increased alkaline phosphatase (2 [4%]). There were no treatment-related grade 5 AEs. Twenty-eight patients (52%) experienced immune-mediated AEs; the most common immune-mediated AEs were increased aspartate aminotransferase (11 [20%]), increased alanine aminotransferase (9 [17%]), diarrhea (6 [11%]), rash (5 [9%]), infusion-related reaction (4 [7%]), and pruritus (4 [7%]). Grade 3 immune-mediated AEs were observed in 2 patients (colitis and adrenal insufficiency). One patient received systemic corticosteroids, 6 patients experienced treatment interruption, and 1 patient discontinued therapy owing to immune-mediated AEs, per investigator.
Table 2. Treatment-Related Adverse Events.
| Adverse eventa | Grades, No. (%) (N = 54) | |
|---|---|---|
| ≥3 | All | |
| Alkaline phosphatase increase | 2 (4) | 13 (24) |
| Lymphopenia | 1 (2) | 12 (22) |
| Aspartate aminotransferase increase | 0 | 11 (20) |
| Fatigue | 1 (2) | 11 (20) |
| Anemia | 1 (2) | 10 (19) |
| Alanine aminotransferase increase | 0 | 9 (17) |
| Vomiting | 0 | 9 (17) |
| Diarrhea | 0 | 6 (11) |
| Nausea | 0 | 6 (11) |
| Rash | 0 | 5 (9) |
| Hyponatremia | 3 (6) | 4 (7) |
| Infusion reaction | 0 | 4 (7) |
| Pruritus | 0 | 4 (7) |
| Elevated bilirubin | 1 (2) | 3 (6) |
| Hypothyroidism | 0 | 3 (6) |
| Thrombocytopenia | 0 | 3 (6) |
Reported in 5% or more of all patients, any grade.
Biomarkers
Programmed cell death 1 ligand 1 expression is one of the potential biomarkers of anti–PD-1 inhibitors in diverse cancers,12,13,14 and PD-L1 expression was investigated as a potential biomarker in this study (eFigure 5 in Supplement 2). Among 42 available tumor samples, 18 (43%) were positive for PD-L1 staining in tumor cells (≥1% of tumor cells expressing PD-L1 as a cutoff). Although 9 of 10 investigator-assessed responders had tumors expressing PD-L1, all 5 centrally assessed responders had PD-L1 expression. Among 18 patients with PD-L1–positive BTC, 9 (50%) achieved investigator-assessed objective response and 5 (28%) achieved centrally assessed objective response. The clinical outcomes including OS and PFS were evaluated by 2 different PD-L1 cutoff values (≥1% and >10%) (Figure 2; eFigure 6 in Supplement 2). Programmed cell death 1 ligand 1 expression in tumor samples was associated with statistically significant prolonged PFS across the expression level of PD-L1 (hazard ratio, 0.23; 95% CI, 0.10-0.51; P < .001 with cutoff ≥1%; hazard ratio, 0.37; 95% CI, 0.17-0.84; P = .02 with cutoff >10%). However, there was no correlation between PD-L1 expression and OS regardless of the expression level of PD-L1 (Figure 2; eFigure 6 in Supplement 2).
We also evaluated the potential prognostic value of PD-1 expression on TILs in this study. Programmed cell death protein 1–expressing TILs were observed in the margin of PD-L1–expressing cancer cells (eFigure 5 in Supplement 2), and 22 of 42 tumor samples (52%) demonstrated positive PD-1–expressing TILs, including 7 responders. In contrast to PD-L1 expression, there was no correlation between PD-1–expressing TILs and clinical outcome (PFS: hazard ratio, 0.58; 95% CI, 0.28-1.18; P = .12; OS: hazard ratio, 0.62; 95% CI, 0.24-1.57; P = .31). The combination of PD-L1 expression on tumor and PD-1 expression on TIL was also evaluated as a potential biomarker. However, the combined expression did not show any significant predictive value for clinical outcome compared with PD-L1 expression on tumor alone.
Discussion
There are limited data available for evaluating the role of immune checkpoint inhibitors in BTC. In our phase 2 study, nivolumab monotherapy was well tolerated and demonstrated durable objective response lasting at least 1 year in 4 of the 10 responders (40%). The 4 responders are still experiencing ongoing response with nivolumab treatment. The safety and toxic effect profile of nivolumab in these patients was manageable and similar to that reported in patients with other tumor types.15,16,17 No unexpected or unanticipated AEs were observed in this study.
In our study, there was a discrepancy between investigator-assessed and centrally reviewed ORR. Although 10 patients achieved PR by investigator assessment, on blinded central review, only 5 patients were considered to achieve PR by RECIST v1.1 criteria. The discrepancy is due to new lymphadenopathy and enlargement of lymph nodes, for which 1 patient was considered to have progressive disease and 4 were considered to have stable disease by blinded central review. Similarly, based on iRECIST criteria, there was still a discrepancy between investigator-assessed and centrally reviewed ORR. Only 1 patient who was considered to have progressive disease by RECIST v1.1 criteria was considered to have immune PR by iRECIST criteria.
However, 4 of the 5 nonresponders on central review who were classified as responders by investigator review received nivolumab treatment for more than 18 months. Because lymphadenopathy is frequently observed during treatment with immune checkpoint inhibitors,18,19 the patients may have developed benign or reactive lymphadenopathy from nivolumab instead of new metastatic disease lymph nodes.
The durable response and safety profiles of nivolumab in our study are consistent with other PD-1 and PD-L1 blockade agents, including durvalumab and pembrolizumab, for patients with BTCs.20,21,22 In the KEYNOTE-158 study, 104 patients were enrolled, and in the KEYNOTE-028 study, 24 patients were enrolled to evaluate the role of pembrolizumab for patients with refractory BTCs.20,22 In the KEYNOTE-158 study, the ORR was 6%, with a median PFS of 2 months and a median OS of 7.4 months.22 The KEYNOTE-028 study reported an ORR of 13% with OS of 6.2 months.22 Grade 3 or higher treatment-related AEs occurred in 14% and 17% of patients in the 2 studies, consistent with rates of treatment-related AEs in our trial.22 Durvalumab was evaluated in a cohort of 42 Asian patients with advanced BTCs.21 Only 2 patients had PR and median OS was 8.1 months. The median duration of response was 9.7 months. In contrast to the above studies that demonstrated ORRs of 5% in unselected patients and 13% in selected patients with PD-L1 and median OS of 6 to 8 months,20,21,22 our study showed ORRs of 11% by central review and 22% by investigator review in unselected patients. In addition, the median OS was not reached in the patients who were examined and was 14.2 months in the intention-to-treat population. At this time, it is unclear why our data demonstrated better clinical outcomes, but it may be related to the patient population or type of BTCs. None of the other studies of PD-1 and PD-L1 blockade agents are published and exist only in abstract forms; therefore, detailed information cannot be obtained. In our study, most patients (59%) had intrahepatic cholangiocarcinoma, and based on molecular profiling, intrahepatic, hilar, and distal cholangiocarcinoma and gallbladder cancer have distinct molecular pathogenesis.23 Once the final data of the KEYNOTE-028,22 KEYNOTE-158,20 and durvalumab21 studies are published, we may have a better explanation of the difference in the clinical outcome. All responders to anti–PD-1 immunotherapy demonstrated durable response with median duration of response not reached in the abovementioned studies20,22 as well as in our study, demonstrating the significant clinical benefit of anti–PD-1 immunotherapy in selected patients with advanced BTC and suggesting identification of potential predictive biomarkers.
Mismatch repair protein deficiency or microsatellite instability–high cancer is a consistently reliable biomarker for PD-1 and PD-L1 blockade immunotherapy.24 However, MMR deficiency or microsatellite instability–high cancer is extremely rare, seen in only 1% to 2% of patients with cholangiocarcinoma,24,25 and none of the responders in our study had MMR-deficient tumors, suggesting the necessity of identifying better predictive biomarkers in cholangiocarcinoma. Although PD-L1 status is likely not a sufficient and comprehensive biomarker, several studies12,13,14 have reported the correlation between PD-L1 expression in tumors and ORR to PD-L1 and PD-1 blockade immunotherapy. In our study, PD-L1 (≥1% of tumor cells expressing PD-L1 as a cutoff) was expressed on tumor cells in 9 of 10 (90%) investigator-assessed responders and all 5 centrally reviewed responders. Programmed cell death t ligand 1 expression correlated with prolonged PFS but not significantly with OS, likely owing to the small sample size. Our study suggests that PD-L1 expression in tumor cells should be assessed in future clinical trials with checkpoint inhibitors in BTC.
In addition, we evaluated the potential prognostic value of PD-1 expression on TILs in this study. Programmed cell death 1 protein–expressing TILs are associated with shorter OS in diverse malignant neoplasms, including extrahepatic cholangiocarcinoma,26 breast cancer,27 and synovial carcinoma.28 However, it is unclear whether PD-1–expressing TILs have a prognostic effect on PD-1 and PD-L1 blockade immunotherapy. Although PD-1–expressing TILs were observed in 22 of 42 tumor samples (52%), there was no correlation between PD-1–expressing TILs and clinical outcome in our study.
The recent ABC-06 study demonstrated that FOLFOX was an option for second-line treatment, with improvement of survival benefit vs active symptom control.4 The median OS was 6.2 months, and 59% of patients experienced grade 3 or 4 toxic effects. Although direct comparison of these studies should be interpreted cautiously, our findings of a median OS of 14.2 months and grade 3 or 4 toxic effects in 17% of patients are encouraging, because our study included a more heavily pretreated patient population than in the ABC-06 study.
Limitations
This study had some limitations, including a relatively small sample cohort with a mixed population of intrahepatic BTC, extrahepatic BTC, and gallbladder cancer and the lack of a control group. Our findings need to be validated in larger clinical trials.
Conclusions
This study suggests that nivolumab can provide modest but durable clinical efficacy with a manageable safety profile for patients with refractory BTC. A future randomized clinical trial is warranted to verify our findings and to evaluate biomarkers for improved treatment selection for patients.
Trial Protocol
eFigure 1. Waterfall Plot of Best Percentage Change in Tumor Lesion Size From Baseline and Spider Plot of Percentage Change in Tumor Lesion Size From Baseline Over Time by Investigator Assessment
eFigure 2. Kaplan-Meier Estimates of Duration of Response in Responders
eFigure 3. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival in Evaluable Patients
eFigure 4. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival by Failed Line of Systemic Therapy
eFigure 5. Representative Immunohistochemical Staining of PD-L1 and PD-1
eFigure 6. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival by PD-L1 Expression Status (Cutoff >10%)
eTable 1. Patient Characteristics
eTable 2. Treatment-Related Adverse Events
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134-1144. doi: 10.1053/j.gastro.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 3.Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 4.Lamarca A, Palmer DH, Wasan HS, Ross PJ, May YT, Arora A ABC-06: a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. Presented at: 2019 ASCO Annual Meeting; June 2, 2019; Chicago, IL. [Google Scholar]
- 5.Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9):3384-3391. doi: 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumeh PC, Harview CL, Yearley JH, et al. . PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568-571. doi: 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon B, Young RJ, Bressel M, et al. . Prognostic significance of PD-L1+ and CD8+ immune cells in HPV+ oropharyngeal squamous cell carcinoma. Cancer Immunol Res. 2018;6(3):295-304. doi: 10.1158/2326-6066.CIR-17-0299 [DOI] [PubMed] [Google Scholar]
- 8.Kim R, Coppola D, Wang E, et al. . Prognostic value of CD8CD45RO tumor infiltrating lymphocytes in patients with extrahepatic cholangiocarcinoma. Oncotarget. 2018;9(34):23366-23372. doi: 10.18632/oncotarget.25163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer EA, Therasse P, Bogaerts J, et al. . New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 10.Seymour L, Bogaerts J, Perrone A, et al. ; RECIST working group . iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. doi: 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Dept of Health and Human Services Common terminology criteria for adverse events (CTCAE): version 4.0. Published May 28, 2009. Accessed March 17, 2020. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 12.Daud AI, Wolchok JD, Robert C, et al. . Programmed death-ligand 1 expression and response to the anti–programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34(34):4102-4109. doi: 10.1200/JCO.2016.67.2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borghaei H, Paz-Ares L, Horn L, et al. . Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs CS, Doi T, Jang RW, et al. . Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Khoueiry AB, Sangro B, Yau T, et al. . Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overman MJ, McDermott R, Leach JL, et al. . Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F. Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract. 2017;23(8):620-624. doi: 10.1177/1078155216667635 [DOI] [PubMed] [Google Scholar]
- 19.Tetzlaff MT, Nelson KC, Diab A, et al. . Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6(1):14. doi: 10.1186/s40425-018-0323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno M, Chung HC, Nagrial A, et al. Pembrolizumab for advanced biliary adenocarcinoma: results from the multicohort, phase 2 KEYNOTE-158 study. Presented at: ESMO 2018 Congress; October 19, 2018; Munich, Germany. [Google Scholar]
- 21.Ioka T, Ueno M, Oh D, et al. Evaluation of safety and tolerability of durvalumab (D) with or without tremelimumab (T) in patients (pts) with biliary tract cancer (BTC). Presented at: Gastrointestinal Cancer Symposium 2019; January 18, 2019; San Francisco, CA. [Google Scholar]
- 22.Bang YJ, Ueno M, Malka D, et al. Pembrolizumab (pembro) for advanced biliary adenocarcinoma: results from the KEYNOTE-028 (KN028) and KEYNOTE-158 (KN158) basket studies. Presented at: 2019 ASCO Annual Meeting; June 3, 2019; Chicago, IL. [Google Scholar]
- 23.Mahipal A, Kommalapati A, Tella SH, Lim A, Kim R. Novel targeted treatment options for advanced cholangiocarcinoma. Expert Opin Investig Drugs. 2018;27(9):709-720. doi: 10.1080/13543784.2018.1512581 [DOI] [PubMed] [Google Scholar]
- 24.Le DT, Durham JN, Smith KN, et al. . Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkelmann R, Schneider M, Hartmann S, et al. . Microsatellite instability occurs rarely in patients with cholangiocarcinoma: a retrospective study from a German tertiary care hospital. Int J Mol Sci. 2018;19(5):1421. doi: 10.3390/ijms19051421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma K, Wei X, Dong D, Wu Y, Geng Q, Li E. PD-L1 and PD-1 expression correlate with prognosis in extrahepatic cholangiocarcinoma. Oncol Lett. 2017;14(1):250-256. doi: 10.3892/ol.2017.6105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muenst S, Soysal SD, Gao F, Obermann EC, Oertli D, Gillanders WE. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139(3):667-676. doi: 10.1007/s10549-013-2581-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nowicki TS, Akiyama R, Huang RR, et al. . Infiltration of CD8 T cells and expression of PD-1 and PD-L1 in synovial sarcoma. Cancer Immunol Res. 2017;5(2):118-126. doi: 10.1158/2326-6066.CIR-16-0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Waterfall Plot of Best Percentage Change in Tumor Lesion Size From Baseline and Spider Plot of Percentage Change in Tumor Lesion Size From Baseline Over Time by Investigator Assessment
eFigure 2. Kaplan-Meier Estimates of Duration of Response in Responders
eFigure 3. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival in Evaluable Patients
eFigure 4. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival by Failed Line of Systemic Therapy
eFigure 5. Representative Immunohistochemical Staining of PD-L1 and PD-1
eFigure 6. Kaplan-Meier Estimates of Progression-Free Survival and Overall Survival by PD-L1 Expression Status (Cutoff >10%)
eTable 1. Patient Characteristics
eTable 2. Treatment-Related Adverse Events