Abstract
Objective:
To examine whether a combined aerobic exercise and resistance exercise is more effective than either AE or RE alone in improving insulin sensitivity and reducing total adiposity and ectopic fat in adolescents.
Study design:
118 sedentary adolescents with overweight/obesity (BMI≥85th percentile, 12–17 years) were recruited from October 2013 through April 2017 at Children’ Hospital of Pittsburgh. Participants were randomized to one of the following 6-month exercise groups (3 days/week, 180 min/week): AE (n=38), RE (n=40) and combined AE and RE (n=40). The primary outcome was the change in insulin-stimulated glucose disposal (Rd) by a 3-hour hyperinsulinemic-euglycemic clamp. The secondary outcomes were changes in liver fat by proton magnetic resonance spectroscopy and inter-muscular adipose tissue (IMAT) by computed tomography.
Results:
Of the 118 participants randomized, 85 participants (72%) completed the study with 90% exercise attendance. Total adiposity reduced similarly in all groups (−2%, P < .05). After adjusting for age and sex, Rd increased (P<0.05) in all groups, with the increase in the AE group being greater than the RE group (1.7 ± 0.1 versus 0.7 ± 0.1 mg/kg/min, P<0.05), but not different from the combined group (1.2 ± 0.1 mg/kg/min). Liver fat was reduced (P<0.05) in the AE (−0.6%) and combined (−0.6%) groups, but not in the RE group (−0.3%, P>0.05). IMAT decreased (P<0.05) similarly in all groups.
Conclusion:
Combined AE and RE and AE alone are similarly beneficial in improving insulin sensitivity and reducing ectopic fat in adolescents with obesity.
Keywords: childhood obesity, exercise, insulin sensitivity, liver fat, intermuscular fat
Recent national data indicate that one in three U.S. children and adolescents are either overweight or obese (1). In parallel with the increasing prevalence of childhood obesity, the prevalence of pre-diabetes (2), type 2 diabetes mellitus (T2DM) (3) and nonalcoholic fatty liver disease (4) are also increasing in youth. Given that physical activity level decreases substantially between childhood and adolescence (5) and that physically active youth are more likely to remain active in adulthood (6), the enhancement of physical activity during adolescence is of importance.
The 2008 Physical Activity Guidelines for Americans (7) recommend that children and adolescents should do 60 minutes or more of physical activity per day. The guidelines suggest that the activity should include both moderate-or vigorous-intensity physical activity at least 3 days a week and muscle strengthening physical activity at least 3 days a week to obtain health benefits (7). Further, adult studies have shown that the combination of aerobic (AE) and resistance exercise (RE) is a better strategy than either AE or RE alone for improving glycemic control and insulin sensitivity (8–10). However, it is important to note that the exercise dose in terms of exercise duration in these interventions (8, 10) were not matched between the exercise groups and the combined AE and RE group generally exercised for a significantly longer time than the RE or AE only groups. In adults with T2DM, Church et al reported that when similar exercise duration was maintained, only the combined exercise, but not either exercise modality alone, was associated with significant reduction in HbA1C level.(9)
Previously, we and others reported significant reductions in liver fat following a 3-month of AE (11–13) or RE (12) in adolescents with obesity. Additionally, we demonstrated that in girls with obesity, both 3 months of AE and RE resulted in significant reductions in inter-muscular adipose tissue (IMAT) (13), a strong correlate of insulin resistance in adolescents (14). To our knowledge, the independent and combined effects of AE and RE on liver fat and IMAT have not been examined in adolescents.
Currently, whether a combination of AE and RE is a better exercise strategy as compared to a similar duration of either AE or RE alone for reducing total adiposity, and ectopic fat in the liver and skeletal muscle and improving insulin sensitivity is unknown in youth. Therefore, we conducted a randomized trial to examine whether a combined AE and RE is more effective than either AE or RE alone in improving insulin sensitivity and reducing total % body fat, fatty liver and skeletal muscle lipid in previously sedentary adolescents with overweight and obesity.
METHODS
We conducted a 6-month, single-center, randomized trial (ClinicalTrials.gov NCT01938950) with a parallel group design. Participants were recruited from November 2013 through April 2017 at Children’s Hospital of Pittsburgh (CHP) of the University of Pittsburgh Medical Center. The final follow-up evaluation was on June 21, 2017. Adolescents with overweight and obesity (BMI≥85th percentile and <40 kg/m2) were recruited via flyers posted in public transportation venues, the university campus and hospitals, and from the Weight Management and Wellness Center at CHP. Parental informed consent and child assent were obtained from all participants before participation. Inclusion criteria included that the subjects be 12–17 years of age, pubertal (Tanner stages II-V), non-smokers, and physically inactive (no participation in structured physical activity for past three months before study entry except school physical education classes). Exclusion criteria included participation in structured exercise, significant weight change (Δ BMI>2–3 kg/m2) in the past three months before study entry, endocrine disorders (eg, polycystic ovary syndrome, diabetes), syndromic obesity, psychiatric disorders or the use of chronic medications which influence glucose metabolism (ie, insulin sensitizers) or body composition.
Pubertal development was assessed according to Tanner criteria (15) by a certified nurse practitioner. All participants underwent a complete physical examination and routine hematological and biochemical tests at the Pediatric Clinical and Translational Research Center (PCTRC) at CHP. Baseline and post-intervention evaluations were completed during an overnight inpatient admission at CHP. The investigation was approved by the University of Pittsburgh Institutional Review Board (PRO12080401).
Randomization
Participants were randomly assigned to one of three exercise training groups for 24 weeks: aerobic exercise (AE, n=38), resistance exercise (RE, n=40) or combined AE and RE (n=40) group (Figure 1; available at www.jpeds.com). A block randomization was done based on sex and age (12–15 years, 15–17 years), which was performed by the study biostatistician. Randomization was performed after completing all baseline evaluations. Participants and exercise trainers could not be blinded to group assignment after randomization due to the nature of the intervention. However, the main study outcome (insulin sensitivity) and the secondary study outcomes (liver fat and IMAT) were measured and analyzed by staff and investigators who were blinded to group assignment.
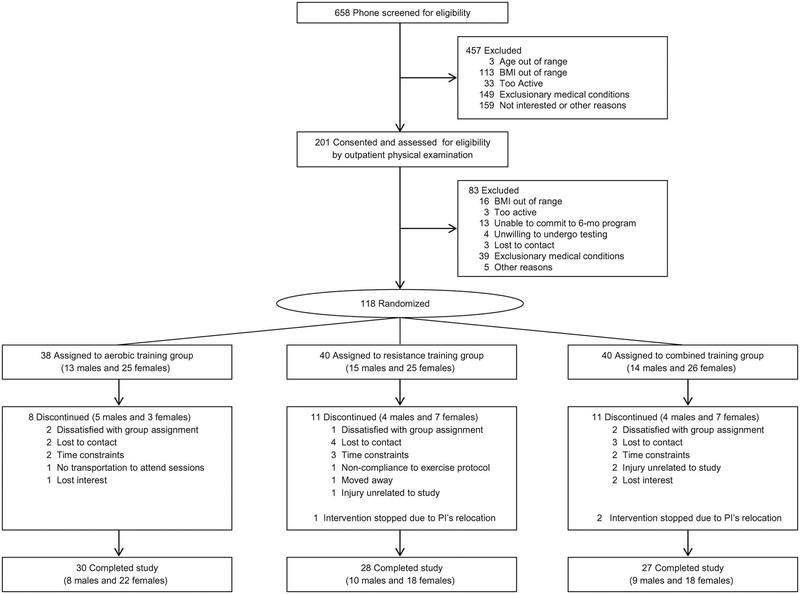
FIGURE 1.
Participant flow diagram. All subjects assigned to each treatment (including those who discontinued the study) were included in intent-to-treat analyses.
Dietary intervention
Prior to baseline evaluations, participants attended two one-hour individual nutrition counseling sessions on proper food selection with the study dietitian. During this dietary run-in period (2–4 weeks), participants were asked to follow a healthy weight maintenance calorie intake (55–60% carbohydrate, 15–20% protein, and 20–25% fat), which was determined using the Harris-Benedict equation (16). During the 6-month exercise intervention period, participants were asked to follow the calorie intake targets determined at baseline to ensure that the negative energy balance was induced by regular exercise alone and not from dieting. Adherence to dietary regimen was monitored by examination of body weight prior to each exercise session and a monthly 24-hour dietary recall was performed by the study dietitian using a computerized program (Food Processor, Esha Research, Salem, OR).
Exercise intervention
Exercise sessions took place at two locations in Pittsburgh: downtown Pittsburgh PNCYMCA and CHP exercise laboratory. All exercise sessions were by appointment and were supervised by an exercise physiologist or exercise science students. All participants were asked to attend exercise sessions, 3 times/week, 60 minutes/session, for 6 months. Exercise training sessions were delivered in small group settings (1–2 participants: 1 trainer ratio) to ensure participant’s safety and compliance. Missed exercise sesssions due to sickness and vacations were recheduled by appointment. Detailed exercise regimens are described in Table I (available at www.jpeds.com). Participants in the AE and combined groups wore a heart rate monitor (Polar Oy, Kempele, Finland) during AE sessions to ensure achievement of the target heart rate (50–65% of peak VO2). Energy expenditure (EE) was estimated using the heart rate-VO2 relationship obtained from the maximal graded treadmill test. The heart rates were translated into estimated EE using the assumption that 1 L O2 equals 5 kcal of EE (17). The relationship between the heart rate and VO2 was re-evaluated during the subsequent maximal treadmill tests at week 4 and week 12 to re-assess the heart rate-EE relationship.
Table 1.
Description of exercise interventions
| Aerobic Exercise | Combined Exercise | Resistance Exercise | |
|---|---|---|---|
| # of weekly exercise session | 3 | 3 | 3 |
| Type of exercise | |||
| Aerobic | 60 minutes | 30 minutes | None |
| moderate-intensity (measured heart rate* to ensure 50–65% of peak VO2†) using a treadmill or an elliptical machine | |||
| Resistance ‡ | 1 set (30 min per session) | 2 sets (60 min per session) | |
| None | 8 exercises (12–15 reps per set) using weight machines, each to volitional fatigue: leg press, leg extension, leg flexion, chest press, latissimus pull down, seated row, bicep curl, and triceps extension. modified push-ups and abdominal crunches to exhaustion |
||
| Total weekly exercise duration | 180 min | 180 min | 180 min |
Aerobic exercise intensity was monitored every 5 minutes using an automated heart rate monitor (Polar Oy, Kempele, Finland).
Obtained from graded maximal treadmill test.
Proper lifting techniques was emphasized and when subjects were able to perform 15 repetitions at a given weight with a proper form, the weight was gradually increased to maintain adequate loads within 12–15 repetition ranges.
Total fat and anthropometric measurements
Total fat and fat free mass (FFM) was assessed by dual energy X-ray absorptiometry using lunar iDXA (GE Healthcare, Madison, WI, USA) (18). Body weight was measured in a hospital gown to the nearest 0.1 kg using a distal scale and height was measured to the nearest 0.1 cm using a fixed wall stadiometer. Waist circumference was measured in a standing position at the superior edge of the iliac crest and the average of two measures was used in the analyses.
Two-hour oral glucose tolerance test (OGTT)
Participants reported to the PCTRC after an overnight fast (minimum 10 hours) for a 2-hour OGTT (1.75 g/kg, max 75 g) to assess for glucose tolerance status (19). Arterialized heated-hand venous blood samples were obtained at −15, 0, 15, 30, 60, 90 and 120 minutes for determination of plasma glucose with a glucose analyzer (YSI, Inc, Yellow Springs, OH).
Following the OGTT, participants were admitted and stayed overnight in the hospital for the hyperinsulinemic-euglycemic clamp the next morning. Participants were fed a standardized lunch and dinner and fasted overnight. One participant in the combined group did not undergo OGTT test at 6 months due to scheduling issues.
Insulin-stimulated whole-body glucose disposal (Rd) by the hyperinsulinemic-euglycemic clamp
Insulin sensitivity was measured as the rate of peripheral insulin-stimulated glucose disposal (Rd) during the 3-h 80 mU/m2/min hyperinsulinemic–euglycemic clamp performed from 09:30 am-12:30 pm as reported by us previously (20). Briefly, intravenous crystalline insulin (Humulin; Lilly Indianapolis, IN) was infused at a constant rate of 80 mU/m2 per min, and plasma glucose was clamped at 5.6 mmol/l with a variable-rate infusion of 20% dextrose based on arterialized plasma glucose determinations using a glucose analyzer (YSI, Inc, Yellow Springs, OH) every 5 min. Rd was calculated by using the average exogenous glucose infusion rate during the last 30 min of steady state. The clamp experiment was performed by a certified nurse practitioner under the direction of pediatric endocrinologists. Post-exercise intervention evaluations and clamp test were performed at least 48–72 hours after the last exercise session to control for the well-known effects of acute exercise on glucose uptake (21). Among 118 randomized participants, 2 subjects’ (1 RE, 1 combined) clamp test at baseline could not be completed due to difficulty with IV access. One participant in the combined group did not undergo clamp test at 6 months as she refused to stay overnight at CHP.
Liver fat content by 1H-MRS
Proton magnetic resonance spectroscopy (1H-MRS) was performed with a 3.0 Tesla MR system (Siemens, Tim Trio, Erlangen, Germany) using a body matrix coil and a spine matrix (Siemens, Erlangen, Germany) as shown by us previously (12, 13). A total of 8 spectra (TR = 4000 ms, TE = 30 ms) were obtained in a measuring time of 32 sec without water suppression and averaged for the determination of intracellular water and lipid content. The AMARES algorithm in the Java-based magnetic resonance user interface (jMRUI) software package (22) was used to quantify the area under the curve of the methylene peak of lipid at 1.3 ppm and the water peak at 4.7 ppm. At baseline, four subjects (2 AE, 2 combined) could not complete 1H MRS due to claustrophobia. One subject in the RE group was excluded in the liver fat data analyses as this subject had 27.8% of liver fat (9 SDs above the mean). Excluding this subject did not alter the significance of our results.
Skeletal muscle composition
CT image was obtained on a GE Scanner (GE Medical Systems, Milwaukee, Wisconsin) using 170 mA, 120 kV, a 512 × 512 matrix, and 48-cm field of view as shown by us previously (14). Using an external landmark, 1 axial image was obtained at the mid-point between the inguinal crease and superior edge of the patella. IMAT area was defined as AT area beneath the fascia lata surrounding skeletal muscle and AT area between muscle bundles (23). Mean muscle attenuation, a measure of muscle density, was measured as the mean attenuation value for all pixels within the range of 0–150 HU using commercially available software (Slice-O-Matic, Tomovision Inc, Montreal, QC).
Cardiorespiratory fitness (CRF) and muscular strength
CRF was determined using a maximal graded treadmill test with the use of standard open-circuit spirometry techniques (True One 2400, Parvo Medics, Sandy, UT) until volitional fatigue as shown previously (12, 13). For the initial 2 min, the grade was set at 0%, after which time it was increased to 2% for the third minute and increased by 1% increments every minute thereafter. Muscular strength was assessed with a one-repetition maximum (1-RM) test for the chest press and leg press using weight stack equipment (Precor, Woodinville, WA) (12, 13). Muscular strength index was calculated as the sum of the 1-RM scores for the chest and leg press expressed per kg of body weight (24). For post-intervention evaluations, two subjects refused to undergo peak VO2 and 1-RM tests and one subject could not perform these tests due to muscle pain.
Changes in study protocol
We terminated the study early on June 30th, 2017 because the principal investigator relocated to Korea. We shortened the length of exercise interventions to 20 weeks-22 weeks for 4 participants in order to complete their post-intervention evaluations in June 2017 before study termination. Three participants who completed the baseline evaluations could not have post intervention evaluations because of the early termination of the trial, which was approved by the IRB.
Statistical analyses
The primary outcome of this study was the change in insulin-stimulated whole-body glucose disposal (Rd) measured by a 3-hour hyperinsulinemic-euglycemic clamp. The power calculations were based on our previous studies (12, 13) to provide a statistical power of 80% to detect a difference in Rd of 0.71 mg/kg/min (SD: 1.1 mg/kg/min) between two exercise groups at 6 months.
A one-way analysis of variance (ANOVA) and Fisher exact test was performed to examine group differences at baseline. When the ANOVA P-value was <0.05, a Tukey post hoc comparison test was used for group differences. All randomized subjects were included in the analysis and the effect of treatment was evaluated by intent-to-treat analysis. The missing data in the 6-month outcomes were imputed using the conditional mean method based on the treatment status, baseline age and sex. Pairwise comparisons of changes in primary and secondary outcome values among groups were conducted using a General Linear Regression model adjusting for baseline age and sex. The adjusted means and standard errors were calculated using the same model for each exercise group. Paired t-tests were performed to compare the pre and post values within each exercise group. Relationships between changes in glucose disposal, and total and ectopic fat were determined using Pearson correlation coefficients. Statistical procedures were conducted in R version 3.4.1 (25) and SPSS (version 24).
RESULTS
Of the 118 participants randomized, 85 participants (30 AE, 28 RE and 27 combined) completed the study (completion rate 72%). Reasons for discontinuation after randomization included dissatisfaction with group assignment, lack of interest, time constraint, lost to contact, or injury unrelated to study (Figure 1). Participants who did not complete the study did not differ significantly in any baseline anthropometric variables from those who completed the study.
Average attendance at exercise sessions in study completers was 90% (SD: ± 10%) and did not differ (P>0.1) by exercise group (AE: 91%, RE: 89%, and combined: 89%). The average (± SD) AE intensity did not differ (P>0.1) between the AE and the combined groups (60.9 ± 8.7% of peak VO2 versus 57.2 ±10.8% of peak VO2) and the average heart rate per session was 145.6 ± 8.7 bpm and 146.1 ± 11.9 bpm in the AE and the combined group, respectively. However, due to longer duration of aerobic exercise regimen (AE group: 60 minutes/session versus combined group: 30 minutes/session), average EE during aerobic exercise sessions was higher (P<0.01) in the AE group compared with the combined group (451 ± 131 kcal/session versus 206 ± 49 kcal/session).
Baseline characteristics
There were no significant differences between groups with respect to age, anthropometric measurements, total fat, liver fat, skeletal muscle composition, and CRF and muscular strength (Table 2). Insulin-stimulated glucose disposal rate (Rd, mg/kg/min) tended to be higher (P=0.05) in the combined exercise group compared with the AE group. However, when the whole-body glucose disposal rate was expressed per FFM, no significant differences were found among groups.
Table 2.
Subject characteristics at baseline
| Aerobic Exercise (n=38) | Resistance Exercise (n=40) | Combined Exercise (n=40) | |
|---|---|---|---|
| Male/Female, n | 13/25 | 15/25 | 14/26 |
| Race, n | |||
| Black | 18 | 26 | 19 |
| White | 18 | 11 | 12 |
| Mixed | 2 | 1 | 6 |
| Other | 0 | 2 | 3 |
| Age, years | 14.4 ± 1.6 | 14.4 ± 1.6 | 14.5 ± 1.7 |
| Tanner stage †† | |||
| II-III | 5 | 6 | 6 |
| IV-V | 33 | 33 | 34 |
| Height, cm | 167.2 ± 8.1 | 165.4 ± 9.1 | 166.3 ± 8.2 |
| Weight, kg | 94.9 ± 16.3 | 91.9 ± 16.8 | 89.9 ± 16.1 |
| BMI, kg/m2 | 33.7 ± 4.0 | 33.4 ± 3.8 | 32.3 ± 4.1 |
| BMI, percentile | 98.3 ± 1.7 | 98.3 ± 1.2 | 97.5 ± 2.6 |
| Waist circumference, cm | 108.4 ± 10.3 | 108.1 ± 10.6 | 105.8 ± 10.7 |
| DXA | |||
| Total fat, % | 42.3 ± 5.2 | 42.4 ± 4.5 | 41.5 ± 5.1 |
| Total fat, kg | 40.3 ± 9.2 | 38.9 ± 8.9 | 37.4 ± 8.9 |
| Fat free mass, kg | 54.1 ± 9.0 | 52.5 ± 9.6 | 52.2 ± 9.5 |
| Liver fat, % | 2.5 ± 2.4 | 3.0 ± 5.1 | 2.2 ± 2.6 |
| Skeletal muscle composition | |||
| IMAT, cm2 | 48.2 ± 19.5 | 47.8 ± 18.0 | 44.0 ± 18.7 |
| Thigh subcutaneous fat, cm2 | 332.1 ± 105.5 | 312.8 ± 88.5 | 318.5 ± 80.4 |
| Muscle attenuation, HU | 52.5 ± 3.0 | 52.2 ± 2.6 | 53.0 ± 2.4 |
| Fitness | |||
| CRF, ml/kg/min | 23.0 ± 3.6 | 22.9 ± 4.1 | 23.5 ± 4.5 |
| 1-RM chest press, kg | 44.8 ± 18.0 | 44.7 ± 18.0 | 44.9 ± 16.1 |
| 1-RM leg press, kg | 86.5 ± 36.0 | 92.1 ± 39.1 | 88.9 ± 30.1 |
| Muscular strength index | 1.4 ± 0.5 | 1.5 ± 0.4 | 1.5 ± 0.4 |
| Metabolic profiles | |||
| Fasting glucose, mg/dL | 92.2 ± 7.1 | 91.0 ± 8.3 | 92.0 ± 7.7 |
| 2-h glucose, mg/dL | 128.7 ± 22.0 | 118.6 ± 22.3 | 119.7 ± 21.1 |
| Rd, mg/kg/min † | 5.6 ± 1.9 | 6.4 ± 2.3 | 6.9 ± 2.6 |
| Rd, mg/kg•FFM/min | 9.8 ± 3.2 | 11.1 ± 3.8 | 11.7 ± 3.9 |
| Energy intake, kcal/day | 1950±482 | 2108±591 | 2103 ±613 |
Data are means ± SD.
DXA, dual energy X-ray absorptiometry; IMAT, inter-muscular adipose tissue;
Rd, insulin-stimulated glucose disposal.
P=0.054 between groups.
P=0.05 between groups.
One subject’s Tanner stage was missing in RE. Liver fat: n= 36 in AE and n= 38 in combined groups.
Rd: n= 39 in RE and n= 39 in combined groups.
Changes in primary outcome
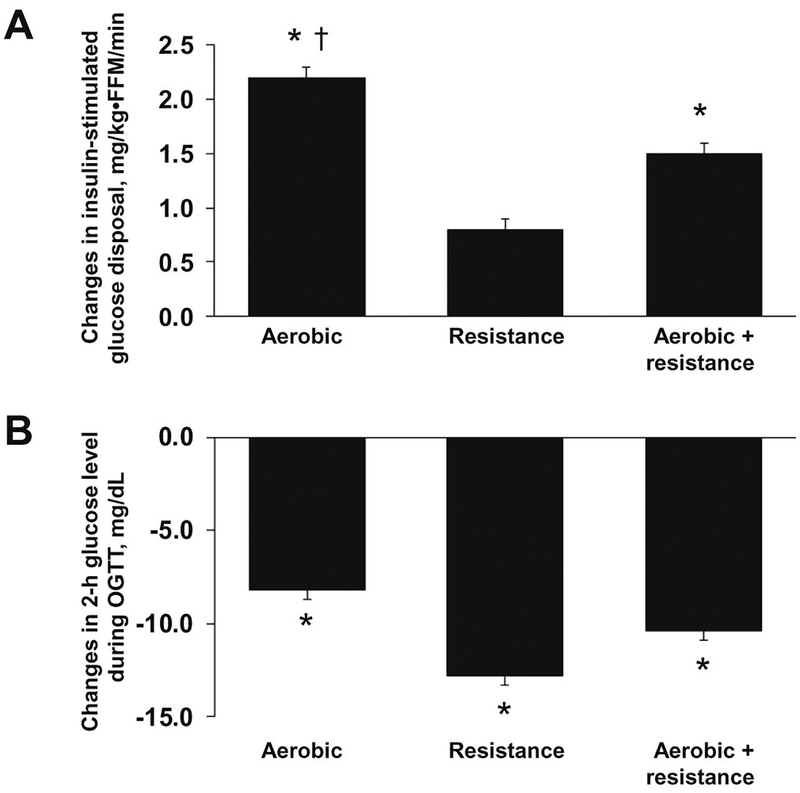
Insulin-stimulated glucose disposal increased significantly in all groups (Table 3). The improvement in Rd in the AE group was greater (P<0.05) than in the RE group (1.7 ± 0.1 versus 0.7 ± 0.1 mg/kg/min), but not different from the combined group (1.2 ± 0.1 mg/kg/min). After accounting for the differences in FFM, the improvements in Rd was still higher in the AE group than in the RE group (2.2 ± 0.1 versus 0.8 ± 0.1 mg/kg•FFM/min, P<0.05) (Figure 2, A). Collapsed across groups, the improvement in Rd was associated (P<0.05) with reductions in body weight (r = −0.53), percent body fat (r = −0.58), liver fat (r = −0.32) and IMAT (r = −0.25).
Table 3.
Changes in study outcome variables using intent-to-treat (ITT) analysis
| Study Group, Mean ± SE | Pairwise Significance † | |||||
|---|---|---|---|---|---|---|
| Aerobic Exercise (n=38) | Resistance Exercise (n=40) | Combined Exercise (n=40) | Aerobic vs. Resistance | Aerobic vs. Combined | Resistance vs. Combined | |
| Weight, kg | −4.0 ± 0.5 * | −1.1 ± 0.5 | 1.0 ± 0.5 | NS | P<0.05 | NS |
| BMI, kg/m2 | −1.4 ± 0.1 * | −0.4 ± 0.1 | −0.4 ± 0.1 | NS | NS | NS |
| BMI percentile | −1.0 ± 0.1 * | −0.6 ± 0.1 * | −0.7 ± 0.1 * | NS | NS | NS |
| Waist circumference, cm | −3.2 ± 0.2 * | −1.6 ± 0.2 | −1.6 ± 0.2 | NS | NS | NS |
| DXA | ||||||
| Total fat, % | −2.3 ± 0.2 * | −2.0 ± 0.19* | −1.7 ± 0.2 * | NS | NS | NS |
| Total fat, kg | −3.7 ± 0.3 * | −2.1 ± 0.3 | −0.9 ± 0.3 | NS | P<0.05 | NS |
| Fat free mass, kg | −0.1 ± 0.2 | 0.9 ± 0.2 | 2.2 ± 0.3 * | NS | P<0.05 | NS |
| Liver fat, % | −0.61 ± 0.03* | −0.27 ± 0.03 | −0.62 ± 0.03 * | NS | NS | NS |
| Skeletal muscle | ||||||
| composition | ||||||
| IMAT, cm2 | −10.7 ± 0.2 * | −8.5 ± 0.2 * | −7.2 ± 0.2 * | NS | NS | NS |
| Thigh subcutaneous fat, cm2 | −40.5 ± 3.2 * | −15.7 ± 3.2 | −8.4 ± 3.4 | NS | P<0.05 | NS |
| Muscle attenuation, HU | 1.19 ± 0.03 * | 1.07 ± 0.03* | 0.64 ± 0.03* | NS | NS | NS |
| Fitness | ||||||
| CRF, ml/kg/min | 5.5 ± 0.2 * | 2.1 ± 0.2 * | 5.4 ± 0.2 * | P<0.05 | NS | P<0.05 |
| 1-RM chest press, kg | 6.8 ± 0.4 * | 26.7 ± 0.4 * | 18.8 ± 0.4 * | P<0.05 | P<0.05 | P<0.05 |
| 1-RM leg press, kg | 20.0 ± 0.4 * | 32.5 ± 0.4 * | 31.6 ± 0.4 * | NS | NS | NS |
| Muscular strength index | 0.35 ± 0.01 * | 0.73 ± 0.01* | 0.57 ± 0.01 * | P<0.05 | P<0.05 | NS |
| Metabolic profiles | ||||||
| Fasting glucose, mg/dL | −0.5 ± 0.2 | −1.6 ± 0.2 | 0.9 ± 0.2 | NS | NS | NS |
| 2-hr glucose, mg/dL | −8.2 ± 0.5 * | −12.8 ± 0.5 * | −10.4 ± 0.5 * | NS | NS | NS |
| Rd, mg/kg/min | 1.7 ± 0.1 * | 0.7 ± 0.1 * | 1.2 ± 0.1 * | P<0.05 | NS | NS |
| Rd, mg/kg•FFM/min | 2.2 ± 0.1 * | 0.8 ± 0.1 | 1.5 ± 0.1 * | P<0.05 | NS | NS |
| Energy intake, kcal/d | 133 ± 16 | −288 ± 16 * | −206± 18 * | P<0.05 | P<0.05 | NS |
Values are expressed as predicted means and standard errors (SE) of differences in pre-intervention versus post-intervention values adjusting for age and sex. The missing data in the 6-month outcomes were imputed using conditional mean method on the treatment status, baseline age and sex.
DXA, dual energy X-ray absorptiometry; IMAT, inter-muscular adipose tissue;
Rd, insulin-stimulated glucose disposal.
Significant pre-intervention versus post-intervention differences within group, P<0.05.
Pairwise comparisons of changes in outcome variables using General Linear Regression models adjusted for age and sex. NS, not significant P>0.05.
FIGURE 2.
A. Improvements in insulin-stimulated glucose disposal within group. Data are shown as predicted means and standard errors (SE) of differences in pre-intervention versus post-intervention values adjusting for age and sex.
* Significant pre-intervention versus post-intervention differences within group, P<0.05.
† Significant pre-intervention versus post-intervention differences compared with the resistance group, P<0.05.
B. Reductions in 2-hr glucose level during a 2-hr oral glucose tolerance test within group. Data are shown as predicted means and standard errors (SE) of differences in pre-intervention versus post-intervention values adjusting for age and sex.
* Significant pre-intervention versus post-intervention differences within group, P<0.05.
Fasting glucose levels did not change in any of the exercise groups. However, there were significant reductions (P<0.05) in 2-hr glucose level in all groups, and these reductions were not different (P>0.05) between exercise groups (Figure 2, B).
Changes in secondary outcomes
A moderate but significant weight loss was observed in the AE group (−4.0 ± 0.5 kg), and the change in body weight in the AE group was greater (P<0.05) than those in the combined group (1.0 ± 0.5 kg) (Table 3). Significant reductions in BMI (−1.4 ± 0.1 kg/m2) and waist circumference (−3.2 ± 0.2 cm) were observed in the AE group only. Total adiposity (%) was reduced (P<0.05) in all groups by a similar magnitude. However, the reduction in total fat mass (kg) was significantly greater in the AE than those in the combined group.
Liver fat was reduced (P<0.05) within the AE (−0.61 ± 0.03%) and combined (−0.62 ± 0.03%) group, but not in the RE group (−0.27 ± 0.03%, P>0.05). IMAT was reduced (P<0.05) within all groups, but these changes were not different between groups. Muscle attenuation (HU) increased (P<0.05) in all groups by a similar magnitude.
CRF and muscular strength index increased (P<0.05) independent of exercise group. The increases in CRF in the AE and combined exercise groups were greater (P<0.05) than those in the RE group. Compared with the AE group, the improvements in muscular strength index was higher (P<0.05) in the RE and the combined exercise groups. Total energy intake assessed by 24-hour dietary recall was reduced (P<0.05) in the RE and combined groups, and not in the AE group.
DISCUSSION
We examined whether a combined aerobic and resistance exercise is more effective than either AE or RE alone (without caloric restriction) in improving insulin sensitivity and reducing liver fat and skeletal muscle lipid in previously sedentary adolescents with overweight or obesity. Our results demonstrate that all 3 types of regular exercise are beneficial in reducing percent body fat and skeletal muscle lipid, and improving insulin sensitivity, OGTT 2-hour glucose, and fitness levels (both cardiorespiratory fitness and muscular strength), combined AE and RE and AE alone are equally effective in reducing ectopic fat in the liver and skeletal muscle, and improving insulin sensitivity and OGTT 2-hr glucose level, and AE is more effective than RE at improving insulin sensitivity.
Although it is well accepted that regular exercise confers substantial health benefits, the optimal mode of exercise for reducing T2DM risk is unclear in youth. A meta-analysis reported that in youth with overweight and obesity, aerobic exercise is typically associated with reductions in fasting insulin and HOMA-IR compared with the control (26). in one study the effects of all 3 exercise modality (AE, RE and combined AE and RE exercise) were evaluated in the pediatric populations. Sigal et al examined the effects of caloric restriction (daily energy deficit 250 kcal) with either AE, RE, or combined exercise on total fat (%) and cardiometabolic risk factors in obese adolescents.(27) In that study (27), all exercise groups similarly reduced total adiposity (−1.1% in the AE, −1.6% in the RE, and −1.4% in the combined group), but no significant group differences were found in fasting glucose and 2-hour glucose levels. The present study randomized trial examined the independent and the combined effects of AE and RE on insulin sensitivity and ectopic fat in the liver and skeletal muscle using the gold standard methods including hyperinsulinemic-euglycemic clamp, 1H-MRS and computed tomography in the pediatric population. Our findings extend the previous finding by Sigal et al, and provide evidence that in the absence of caloric restriction, combined AE and RE and AE alone are similarly effective in improving insulin sensitivity and OGTT 2-hr glucose, and AE alone is superior to RE alone in improving insulin sensitivity in adolescents with overweight and obesity.(27)
Unlike the previous studies in adults reporting greater improvements in T2DM risk factors in response to the combined exercise than either exercise alone (8–10), we did not find substantial benefits of adding RE to a moderate-intensity AE in improving insulin sensitivity in obese adolescents. Although this discrepancy is uncertain, it is important to note that in these adult studies (8, 10), exercise duration was significantly longer in the combined exercise group than either exercise alone group. Therefore, it is unclear whether greater improvements in insulin resistance observed in response to the combined AE and RE regimen are due to a synergistic effect of AE and RE or simply due to a greater exercise volume.
We observed that for a similar exercise duration (approximately 180 min/week), AE alone is more effective than the combined exercise in reducing body weight and total fat mass in adolescents with overweight and obesity. The greater reductions in body weight and total fat in response to AE alone versus combined exercise could be due to the differences in energy expenditure between groups as oxygen consumption during RE is estimated to be less than 50% of maximal VO2 (28). Our findings that collapsed across groups, the improvement in insulin-stimulated glucose disposal was significantly associated with reductions in body weight, total adiposity, liver fat, and IMAT reinforce the importance of reducing total adiposity and ectopic fat in the liver and muscle in order to improve insulin sensitivity in adolescents with overweight and obesity. These findings suggest that for overweight and obese adolescents who want to lose weight and improve insulin resistance and fatty liver, AE may be the most effective and time-efficient exercise strategy.
It has been suggested that skeletal muscle is the major site for whole-body insulin-stimulated glucose disposal (29). Skeletal muscle of adults with obesity and T2DM is characterized by reduced muscle attenuations and an increased IMAT as compared with normal-weight individuals and both reduced muscle attenuation and increased IMAT are associated with insulin resistance (30). Similarly, we previously demonstrated that both IMAT and muscle attenuation are significantly associated with total adiposity in children and adolescents (14). Our current study demonstrates that regular exercise independent of exercise modality is effective in reducing IMAT and increasing muscle attenuation, and highlights the importance of regular exercise as a strategy to reduce skeletal muscle lipid in adolescents with overweight and obesity.
In this study, CRF increased in all three exercise modalities, and the increases in the AE and the combined groups were significantly greater than those in the RE group. With respect to muscular fitness, although all groups increased whole-body muscular strength index, the greatest improvement was observed in the RE group. Based on these observations, we recommend that adolescents with overweight and obesity who want to maximize muscular strength and fitness should incorporate RE in their intervention strategies.
The strengths of our study include the randomized study design including three exercise interventions in the same study, excellent adherence to the exercise regimens (90%), direct supervision of all exercise training sessions with automated monitoring of heart rate during each AE session, and monitoring of proper form and technique during each RE session, and measuring insulin sensitivity and ectopic fat content using the-state-of-the-art methodologies such as the 3-hr hyperinsulinemic–euglycemic clamp and 1H-MRS techniques.
The limitations of this study warrant mention. Our study consists of predominantly black and white adolescents. Whether similar findings would be observed in other ethnic groups (e.g., Asian youth) and youth with T2DM and other metabolic conditions are unknown. We did not include the non-exercising control group as the goal of the study was to examine whether the combined AE and RE is more effective than either exercise alone in reducing obesity-related health risk factors, and the benefits of regular exercise compared with non-exercising controls were clearly documented in obese youth. Further, 64% of our study participants was female and due to small sample size, we were unable to examine the influence of sex and race on the changes in insulin sensitivity in response to different exercise modalities. Currently, it is unknown whether differing distributions of males and females played a role in our findings. Lastly, our findings may not be generalized to the general population of adolescents with overweight and obesity because exercise training sessions, access to exercise facilities and diet counseling were offered for 6 months at no cost, which may have helped to improve exercise compliance.
In conclusion, we found that all three exercise modalities are beneficial in reducing total fat and skeletal muscle lipid, and improving risk factors for T2DM (insulin sensitivity and OGTT 2-hour glucose level) in adolescents with overweight and obesity. The data observed here demonstrate important public health implications for understanding the benefits of different exercise modalities, and provide therapeutic strategies to health care professionals when prescribing the optimal exercise interventions to improve specific health outcomes of interest in adolescents with overweight and obesity.
ACKNOWLEDMENTS
We thank the study participants and their parents, the Weight Management and Wellness Center research staff (Nicole Mullarkey, Jenna Spector, Nancy Guerra, Resa Stauffer), and many undergraduate and graduate students in the Department of Health and Physical Activity at the University of Pittsburgh.
Funded by the National Heart, Lung, and Blood Institute (R01HL114857 [to S.L.]), and the National Center for Advancing Translational Sciences Clinical and Translational Science Award (UL1 RR024153 and UL1TR000005) to the Pediatric Clinical and Translational Research Center. The authors declare no conflicts of interest.
Trial registration ClinicalTrials.gov NCT01938950
LIST OF ABBREVIATIONS
- BMI
body mass index
- CT
computed tomography
- EE
energy expenditure
- FFM
fat free mass
- IMAT
intermuscular adipose tissue
- MRS
magnetic resonance spectroscopy
- OGTT
oral glucose tolerance test
- T2DM
type 2 diabetes mellitus
- 1-RM
one-repetition maximum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama. 2014. February 26;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012. June;129(6):1035–41. [DOI] [PubMed] [Google Scholar]
- 3.Writing Group for the SfDiYSG, Dabelea D, Bell RA, D’Agostino RB Jr., Imperatore G, Johansen JM, et al. Incidence of diabetes in youth in the United States. Jama. 2007. June 27;297(24):2716–24. [DOI] [PubMed] [Google Scholar]
- 4.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. The Journal of pediatrics. 2013. March;162(3):496–500 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and science in sports and exercise. 2008. January;40(1):181–8. [DOI] [PubMed] [Google Scholar]
- 6.Telama R, Yang X, Leskinen E, Kankaanpaa A, Hirvensalo M, Tammelin T, et al. Tracking of physical activity from early childhood through youth into adulthood. Medicine and science in sports and exercise. 2014. May;46(5):955–62. [DOI] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services 2008 Physical Activity Guidelines for Americans; 2008. https://health.gov/paguidelines/guidelines/chapter3.aspx.
- 8.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007. September 18;147(6):357–69. [DOI] [PubMed] [Google Scholar]
- 9.Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of Aerobic and Resistance Training on Hemoglobin A1c Levels in Patients With Type 2 Diabetes. JAMA: The Journal of the American Medical Association. 2010. November 24, 2010;304(20):2253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Archives of internal medicine. 2009. January 26;169(2):122–31. [DOI] [PubMed] [Google Scholar]
- 11.van der Heijden GJ, Wang ZJ, Chu ZD, Sauer PJ, Haymond MW, Rodriguez LM, et al. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity. 2010. February;18(2):384–90. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes. 2012. November;61(11):2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Deldin AR, White D, Kim Y, Libman I, Rivera-Vega M, et al. Aerobic exercise but not resistance exercise reduces intrahepatic lipid content and visceral fat and improves insulin sensitivity in obese adolescent girls: a randomized controlled trial. American journal of physiology Endocrinology and metabolism. 2013. November 15;305(10):E1222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Guerra N, Arslanian S. Skeletal muscle lipid content and insulin sensitivity in black versus white obese adolescents: is there a race differential? The Journal of clinical endocrinology and metabolism. 2010. May;95(5):2426–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981. February;39(2):43–55. [DOI] [PubMed] [Google Scholar]
- 16.Harris JA, Benedict FF. A Biometric Study of Basal Metabolism in Man. Washington DC: Carnegie Institution of Washington; 1919. [Google Scholar]
- 17.McArdle WD, Katch FI, Katch VL. Essentials of Exercise Physiology. 3rd ed Philadephila: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 18.Lee S, Kuk JL. Changes in fat and skeletal muscle with exercise training in obese adolescents: comparison of whole-body MRI and dual energy X-ray absorptiometry. Obesity. 2013. October;21(10):2063–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diagnosis and classification of diabetes mellitus. Diabetes care. 2004. January;27 Suppl 1:S5–S10. [DOI] [PubMed] [Google Scholar]
- 20.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. The Journal of clinical endocrinology and metabolism. 2001. January;86(1):66–71. [DOI] [PubMed] [Google Scholar]
- 21.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996. October 31;335(18):1357–62. [DOI] [PubMed] [Google Scholar]
- 22.Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001. May;12(2–3):141–52. [DOI] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000. July;89(1):104–10. [DOI] [PubMed] [Google Scholar]
- 24.Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Medicine and science in sports and exercise. 2004. August;36(8):1301–7. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing V, Austria: URL https://www.R-project.org/. [Google Scholar]
- 26.Marson EC, Delevatti RS, Prado AK, Netto N, Kruel LF. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Preventive medicine. 2016. December;93:211–8. [DOI] [PubMed] [Google Scholar]
- 27.Sigal RJ, Alberga AS, Goldfield GS, Prud’homme D, Hadjiyannakis S, Gougeon R, et al. Effects of aerobic training, resistance training, or both on percentage body fat and cardiometabolic risk markers in obese adolescents: the healthy eating aerobic and resistance training in youth randomized clinical trial. JAMA pediatrics. 2014. November;168(11):1006–14. [DOI] [PubMed] [Google Scholar]
- 28.Hurley BF, Hagberg JM, Allen WK, Seals DR, Young JC, Cuddihee RW, et al. Effect of training on blood lactate levels during submaximal exercise. Journal of applied physiology: respiratory, environmental and exercise physiology. 1984. May;56(5):1260–4. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. The American journal of physiology. 1979. September;237(3):E214–23. [DOI] [PubMed] [Google Scholar]
- 30.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. The American journal of clinical nutrition. 2000. April;71(4):885–92. [DOI] [PubMed] [Google Scholar]