Abstract
This nationwide, retrospective, matched cohort study was designed to investigate the risk of corneal ulcer in patients with diabetes mellitus (DM). It included 238,701 patients with DM, recruited between 2003 and 2005 from the Longitudinal Cohort of Diabetes Patients database. The control group included the same number of age- and sex-matched non-DM patients selected from the Taiwan Longitudinal Health Insurance Database, 2000. The data of each patient were collected from the index date until December 2013. The incidence of corneal ulcer was compared between the two groups. In total, 2,549 patients with DM and 1,988 controls developed corneal ulcer during the follow-up period, resulting in an incidence rate for corneal ulcers that was 1.27 times (95% confidence interval [CI] = 1.20–1.35; P < 0.001) higher in patients with DM than in controls. After adjustment for potential confounders, including hyperlipidemia, hypertension, congestive heart failure, coronary artery disease, and chronic renal disease, patients with DM were 1.31 times (95% CI, 1.24–1.40; P < 0.05) more likely than the cohort to develop corneal ulcers. In conclusion, this study shows that DM increases the risk of corneal ulcer. Therefore, close collaboration between ophthalmologists and endocrinologists is important to ensure timely ophthalmology visits.
Subject terms: Diseases, Endocrinology, Risk factors
Introduction
The global increase in the prevalence of diabetes mellitus (DM) is an important public health burden related to its accompanying morbidity and mortality1–3. The ocular complications resulting from DM are leading causes of blindness and have become general public health problems4–6. Although diabetic retinopathy is the most well-known ocular complication, DM may also result in corneal ulceration.
Corneal ulcers caused by infection from pathogens, including bacteria, viruses, and fungi, are a leading cause of visual impairment and blindness worldwide7–9. The most frequent clinical presentation of corneal ulcer includes redness, severe pain, photophobia, watering of eyes, pus formation, or blurred visual acuity. Contact lens use is a major risk factor and the most common aetiology for the pathogenesis of corneal ulcers10–12. It may also occur because of dry eyes resulting from an unbalanced and unstable tear film, compromised ocular surface associated with corneal epithelium abnormality, and ocular surface injury associated with decreased corneal sensitivity7–9.
The reduction in the secretion and stability of the precorneal tear film may be a result of the decreased trophic effect of the trigeminal sensory nerves on the cornea in patients with DM13,14. Compromised ocular surface is common in patients with DM and leads to increased prevalence of epithelial fragility, punctate keratopathy, and persistent epithelial defects15–17. Decreased corneal sensitivity and abnormal neural regulation is known to result in delayed epithelial wound healing and increased risk of corneal ulcer following corneal trauma in patients with DM18. Considering the common pathogenic mechanisms in DM and corneal ulcer, it is important to determine whether DM is a predictor of corneal ulcer.
Several previous studies have discussed the association between DM and corneal ulcer, but they were limited by the small number of patients or the absence of comparative control data19–21. Therefore, this study used a nationwide population-based dataset to design a cohort study for assessing the association between DM and risk of corneal ulcer in Taiwan.
Materials and Methods
Database
The data for the cohort study were obtained from the Taiwan National Health Insurance Research Database (NHIRD), which includes the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnoses, prescriptions details, procedure codes, and expenses. NHIRD also provides details regarding encrypted patient identification, age, gender, birthday, and dates of admission and discharge. The Institutional Review Board of Chi Mei Medical Center exempted the review of the study because no identifiable personal information could be analysed and the informed consent was waived. In this study, all procedures were performed in accordance with the ethical standards of the institutional research committee and with the latest version of the declaration of Helsinki.
Selection of patients and variables
This retrospective cohort study included two groups: a new-onset DM group and a matched non-DM (control) group; participants of both groups were recruited between 2003–2005. In total, 238,701 DM patients (code 250) were diagnosed between January 1, 2003 and December 31, 2005. These new-onset DM patients were chosen from the Longitudinal Cohort of Diabetes Patients Database, which is a subdivision data of the NHIRD and it has a random sample of 120,000 DM patients per year from 1996 to 2013. The database was released by Taiwan National Health Research Institutes and applied by the public through formal application. Many research studies used the same database and later had many publications22–24. Patients with missing data, age less than 20 years, and those being diagnosed with corneal ulcer before DM were excluded. A diagnosis of corneal ulcer was defined as conditions diagnosed under the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 370.0, including 370.01 (marginal corneal ulcer), 370.02 (ring corneal ulcer), 370.03 (central corneal ulcer), 370.04 (hypopyon ulcer), 370.05 (mycotic corneal ulcer), and 370.06 (perforated corneal ulcer), but not 370.07 (Mooren’s ulcer).
For each DM patient, one non-DM control was selected from a sub-data of the NHIRD, Longitudinal Health Insurance Database, 2000. This data includes the information of 1 million beneficiaries (4.34% of the total population) randomly selected in 2000. In total, 238,701 non-DM controls were matched to DM patients by age, gender and index date. The index date for DM patients was the date of initial diagnosis. Controls diagnosed with DM or corneal ulcer before the index date were excluded. To determine the incidence of corneal ulcer, each patient was tracked until death or the end of year 2013, whichever was earlier.
To discriminate patients who developed corneal ulcer following DM, each patient with demographic data was traced from index date until December 2013. The data about the comorbidities of patients, including hyperlipidemia (code 272), hypertension (codes 401–405), coronary artery disease (codes 410–414), congestive heart failure (code 428), and chronic renal diseases (codes 582–588 except 587 and 584) were also gathered. The inclusion criterion for hyperlipidemia, hypertension, coronary artery disease, congestive heart failure, and chronic renal disease was as follows: medical record at least once in hospitalization or ≥3 times in outpatient visits within 1 year before the index date.
Statistical analysis
SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA) was used for statistical analyses. Pearson’s chi-square tests were employed to compare the demographics and comorbidities between the DM and control groups. The incidence of corneal ulcer was determined as the patient quantity with corneal ulcer identified during the tracking period divided by the sum of person-years (PY) for each group by age, gender, and relevant comorbidities. The incidence rate ratio (IRR), which compared the corneal ulcer risk between the DM and control groups, was estimated using Poisson regression analysis. The cumulative incidence rates for corneal ulcers and their differences were calculated and analyzed by Kaplan–Meier analyses and log-rank tests, respectively. Cox proportional hazards regression was employed to calculate the adjusted hazard ratios (HRs) for the development of corneal ulcer. The data are exhibited as means and their standard deviations (SDs), with 95% confidence intervals (CIs) where applicable. Statistical significance was defined as P < 0.05. Similar statistical analysis and method description were used in our previous study22–24.
Results
Demographic data
Between 2003 and 2005, 238,701 patients with DM and non-DM 238,701 controls were enrolled after eliminating unqualified subjects. Table 1 reveals the demographics for DM patients and matched controls. The mean age of the DM patients and controls was 55.11 (SD, 14.83) years. Of the 238,701 patients with DM, 132,772 (55.62%) were men and 105,929 (44.38%) women; further, of the 238,701 patients, 90,568 (37.94%) were aged 20–50 years, 85,453 (35.80%) were aged 50–64 years, and 62,680 (26.26%) were aged ≥65 years. The DM patients group exhibited a remarkably higher prevalence of comorbidities, including hyperlipidemia, hypertenstion, coronary artery disease, congestive heart failure, and chronic renal disease, than did the control group.
Table 1.
Demographic characteristics and comparison of comorbid disorders between the diabetes mellitus (DM) and control groups.
| DM N = 238701 | Control N = 238701 | P-value | |
|---|---|---|---|
| Age at index date (years; mean ± SD) | 55.11 ± 14.83 | 55.11 ± 14.83 | 1.0000 |
| Age at index date (years) | |||
| 20–50 | 90568 (37.94) | 90568 (37.94) | 1.0000 |
| 50–64 | 85453 (35.80) | 85453 (35.80) | |
| ≥65 | 62680 (26.26) | 62680 (26.26) | |
| Sex | |||
| Male | 132772 (55.62) | 132772 (55.62) | 1.0000 |
| Female | 105929 (44.38) | 105929 (44.38) | |
| Baseline comorbidities | |||
| HTN | 73890 (30.96) | 26089 (10.93) | <0.0001 |
| HPL | 24722 (10.36) | 5952 (2.49) | <0.0001 |
| CHF | 6411 (2.69) | 1780 (0.75) | <0.0001 |
| CAD | 21418 (8.97) | 7683 (3.22) | <0.0001 |
| CRD | 5912 (2.48) | 2215 (0.93) | <0.0001 |
Note: Demographic characteristics and comorbid disorders were compared between the DM and control groups by using Pearson’s chi-square tests. Abbreviations: HTN, hypertension; HPL, hyperlipidemia; CHF, congestive heart failure; CAD, coronary heart disease; CRD, chronic renal disease
Incidence rates for corneal ulcer
During the tracking period, 4,537 (4,537/477,402, 0.950%) patients developed corneal ulcer, with the proportion being significantly higher in the DM group (2,549/238,701, 1.068%) than in the control group (1,988/238,701, 0.833%; Table 2). In addition, a remarkable intergroup difference was observed in the corneal ulcer incidence rate (DM, 12.19/10000 PY; control, 9.57/10000 PY) and IRR (1.27, 95% CI = 1.20–1.35, P < 0.0001; Table 2).
Table 2.
Risk of corneal ulcer in the diabetes mellitus (DM) and control groups.
| DM | Control | IRR (95% CI) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Corneal ulcer | PY | Incidence ratea | N | Corneal ulcer | PY | Incidence ratea | |||
| All | 238701 | 2549 | 2090353 | 12.19 | 238701 | 1988 | 2078030 | 9.57 | 1.27 (1.20-1.35) | <0.0001 |
| Age at index date (years) | ||||||||||
| 20–50 | 90568 | 1102 | 832987 | 13.23 | 90568 | 864 | 843326 | 10.25 | 1.29 (1.18-1.41) | <0.0001 |
| 50–64 | 85453 | 862 | 768153 | 11.22 | 85453 | 732 | 763355 | 9.59 | 1.17 (1.06-1.29) | 0.0018 |
| ≥65 | 62680 | 585 | 489212 | 11.96 | 62690 | 392 | 471349 | 8.32 | 1.44 (1.27-1.63) | <0.0001 |
| Sex | ||||||||||
| Male | 132772 | 1500 | 1144252 | 13.11 | 132772 | 1142 | 1136159 | 10.05 | 1.30 (1.21-1.41) | <0.0001 |
| Female | 105929 | 1049 | 946101 | 11.09 | 105929 | 846 | 941871 | 8.98 | 1.23 (1.13-1.35) | <0.0001 |
| Baseline comorbidities | ||||||||||
| HTN | 73890 | 686 | 622764 | 11.02 | 26089 | 200 | 223190 | 8.96 | 1.23 (1.05-1.44) | 0.0102 |
| HPL | 24722 | 238 | 219681 | 10.83 | 5952 | 59 | 52681 | 11.20 | 0.97 (0.73-1.29) | 0.8195 |
| CHF | 6411 | 47 | 45760 | 10.27 | 1780 | 12 | 13284 | 9.03 | 1.14 (0.60-2.14) | 0.6915 |
| CAD | 21418 | 188 | 174828 | 10.75 | 7683 | 66 | 64325 | 10.26 | 1.05 (0.79-1.39) | 0.7429 |
| CRD | 5912 | 48 | 41102 | 11.68 | 2215 | 16 | 15401 | 10.9 | 1.12 (0.64-1.98) | 0.6854 |
Note: A Poisson regression analysis was performed to calculate the incidence rate ratio.
Abbreviations: PY, person-years; IRR, incidence rate ratio; CI, confidence interval; HTN, hypertension; HPL, hyperlipidemia; CHF, congestive heart failure; CAD, coronary heart disease; CRD, chronic renal disease.
aIncidence rate: per 10,000 person-years.
DM patients aged 20–50 years exhibited the highest corneal ulcer incidence (13.23/10000 PY), followed by those aged ≥65 years (11.96/10000 PY) and 50–64 years (11.22/10000 PY). The values of IRR were significantly higher for the three different DM age groups than for the age-matched control groups (Table 2).
The corneal ulcer incidence rate was 13.11/10000 PY for male DM patients and 10.05/10000 PY for male controls (IRR = 1.30; 95% CI = 1.21–1.41; P < 0.0001). A significant difference was also observed between female DM patients and female controls (IRR = 1.23; 95% CI = 1.13–1.35; P < 0.0001; Table 2).
In the DM patients, the corneal ulcer incidence rates decreased in the following order according to the comorbidities: chronic renal disease (11.68/10000 PY), hypertension (11.02/10000 PY), hyperlipidemia (10.83/10000 PY), coronary artery disease (10.75/10000 PY), and congestive heart failure (10.27/10000 PY). In particular, the incidence was 1.23 times higher in patients with DM and hypertension than in age-matched controls (IRR = 1.23; 95% CI = 1.05–1.44; P = 0.0102). The IRR for corneal ulcer in DM patients with other comorbidities did not indicate a statistically greater risk than in the corresponding controls. Additionally, by using Kaplan–Meier analyses, DM group showed higher cumulative incidence rate of corneal ulcer than the control group; the log-rank test findings were also significant (P < 0.0001; Fig. 1).
Figure 1.
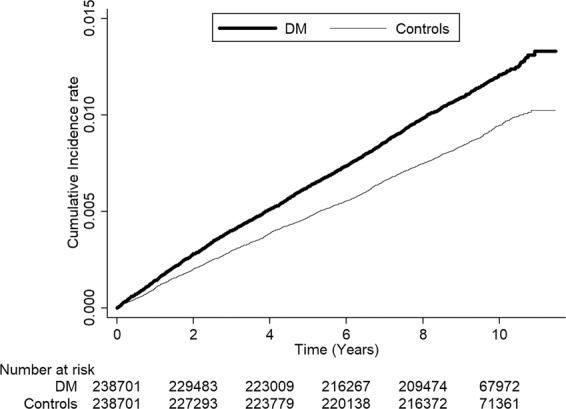
Cumulative incidence of corneal ulcer in patients with diabetes mellitus (DM) and controls during the follow-up period.
Hazard ratios for corneal ulcers
Table 3 reveals the crude and adjusted HRs for corneal ulcer during the tracking period. After adjustment for age, sex, and the selected comorbidities, DM remained an independent risk for corneal ulcer (adjusted HR = 1.31; 95% CI = 1.24–1.40). In both groups, patients aged between 20−50 years (adjusted HR, 1.15; 95% CI = 1.06–1.25; P < 0.05) and of the male sex (adjusted HR, 1.14; 95% CI = 1.08–1.21; P < 0.05) were at higher risk for the development of corneal ulcer. All selected comorbidities were not independent risks for corneal ulcer.
Table 3.
Crude and adjusted hazard ratios and 95% confidence intervals (CI) from Cox proportional hazard regression analysis for corneal ulcer during the follow-up period in the study cohort.
| Crude hazard ratio (95% CI) | Adjusted hazard ratio (95% CI) | |
|---|---|---|
| DM | ||
| Yes | 1.29* (1.22-1.37) | 1.31* (1.24-1.40) |
| No | 1.00 | 1.00 |
| Age at index date | ||
| 20–50 | 1.18* (1.10-1.28) | 1.15* (1.06-1.25) |
| 50–64 | 1.05 (0.97-1.13) | 1.03 (0.95-1.12) |
| ≥65 | 1.00 | 1.00 |
| Sex | ||
| Male | 1.15* (1.08-1.22) | 1.14* (1.08-1.21) |
| Female | 1.00 | 1.00 |
| Baseline comorbidities | ||
| HTN | ||
| Yes | 0.96 (0.89-1.03) | 0.93 (0.86-1.01) |
| No | 1.00 | 1.00 |
| HPL | ||
| Yes | 1.01 (0.90-1.14) | 0.97 (0.85-1.09) |
| No | 1.00 | 1.00 |
| CHF | ||
| Yes | 0.92 (0.71-1.19) | 0.92 (0.71-1.20) |
| No | 1.00 | 1.00 |
| CAD | ||
| Yes | 0.98 (0.86-1.11) | 1.00 (0.88-1.15) |
| No | 1.00 | 1.00 |
| CRD | ||
| Yes | 1.04 (0.81-1.33) | 1.04 (0.81-1.34) |
| No | 1.00 | 1.00 |
Note: The adjusted hazard ratio for developing corneal ulcer was calculated using a Cox proportional hazard regression analysis.
Abbreviations: DM, diabetes mellitus; HTN, hypertension; HPL, hyperlipidemia; CHF, congestive heart failure; CAD, coronary heart disease; CRD, chronic renal disease.
*P-value <0.05.
Discussion
This study is the largest population-based study that has been conducted to explore the relationship between DM and subsequent corneal ulcer. The authors analyzed 238,701 patients with DM and 238,701 control subjects. The incidence rate of corneal ulcer in patients with DM was found to be 1.27 times higher than that in controls, and the relative risk of corneal ulcer in these patients was 1.31 times higher than that in the total cohort, after adjustment for age, sex, and relevant comorbidities.
Several studies have reported the occurrence of DM in patients with infectious keratitis. Badawi et al. studied the prevalence of DM in 245 patients with corneal ulcer and suggested that DM was a predisposing factor (present in 15.1% of the patients)21. Inoue et al. screened for DM in 30 culture-proven cases of corneal ulcer due to Moraxella infection and found that the occurrence of DM was 23.8% in these patients; hence, in line with earlier study findings, they reported that DM was the predominant systemic predisposing factor for Moraxella keratitis19–21. These studies were limited because of their small sample size; in contrast, the present study is the largest nationwide, population-based cohort study to investigate the risk of corneal ulcer following DM in Taiwan.
The possible associated pathogenic mechanisms for corneal ulcer in patients with DM include dryness of eye, compromised ocular surface, and decreased corneal sensitivity. Dry eye symptoms due to reduced stability, secretion, and quality of the precorneal tear film are common in patients with DM. Dryness of the eye in patients with DM render them at greater risk of compromised ocular surface, including superficial punctate keratitis, recurrent corneal erosions, and persistent epithelial defects18,25. In addition, several studies found that the density of the corneal sub-basal nerve plexus, which is responsible for maintaining corneal sensitivity and normal epithelial metabolism, is reduced in patients with DM26,27. Once a patient with DM develops dry eye, compromised ocular surface, and reduced corneal sensitivity, the vulnerability to mild trauma, which is the leading cause of corneal ulcer, increases. These abnormalities in patients with DM play an important role in corneal ulcer development.
In this study, male patients were at a significant higher risk of developing corneal ulcer, after accounting for age and comorbidities in both groups (Table 3). The observation of male predominance is found in several reports21,28–30. The male predominance in corneal ulcer could be attributed to the greater extent of outdoor activities among men. This likely makes them more prone to corneal injury by external agents21,28,31.
The study results show that patients aged between 20–50 years showed a significantly greater incidence of corneal ulcer development in the cohort (Table 3). A similar observation was made in earlier studies21,28,31. This finding could be explained by the fact that younger individuals are more likely to engage in rigorous physical activities or wear contact lenses, thus increasing the risk of ocular surface trauma and subsequent corneal infection.
This study has several merits. These include a high statistical power and accuracy of risk assessment is in our nationwide, population-based study, because a large sample size of DM patients was included in our dataset. Additionally, because patients with visual disturbances visit ophthalmologists rather than general practitioners in Taiwan, selection bias in referral centers and chances of misdiagnosis were low in this study. Finally, this study has a strong evidence level because this cohort study assessed the incidence of corneal ulcer in the DM and control groups with a maximum longitudinal data of 10 years.
This study also has some limitations. Because the medical histories of the sampled patients could only be traced back to 1996, a history of DM before January 1996 could not be confirmed. The influence of blood sugar control on the risk of developing corneal ulcer could not be evaluated, because the insurance claims data did not include information on the hemoglobin A1C level or the current blood sugar value. Several important confounding factors including occupation, contact lens use, plant or mud exposure, and mild ocular trauma could not be evaluated. It is likely that significantly less patients with diabetes used contact lenses owing to the general belief that wearing contact lenses is contradicted in diabetics. If this were true, the current study might actually be underestimating the risk of keratitis in patients with diabetes. This is a retrospective study, and therefore, the results may be less robust in establishing an epidemiological risk or a clear definition of keratitis. Finally, the diagnosis of DM, corneal ulcer, and other comorbid disorders were based on the ICD-9-CM codes, which may lead to disease misclassification.
In summary, this study shows that the risk of corneal ulcer is higher in patients with DM than in those without DM. DM was identified as an independent risk after adjusting for relevant comorbidities in the total cohort. Although the study reports a 1.31-fold increased risk of keratitis in patients with diabetes, which is of minor clinical importance, it is an important finding from a pathophysiological perspective, and is consistent with known compromise of the ocular surface in patients with diabetes.
Acknowledgements
Data from the National Health Insurance Research Database, including the Taiwan Longitudinal Health Insurance Database 2000 and the Longitudinal Cohort of Diabetes Patients Database, was originally provided by the Taiwan Bureau of National Health Insurance and Department of Health. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Author contributions
All authors conceived the study. Y.S. Chang, M.C. Tai, S.H. Tseng, and R.L. Jan conducted the study. Y.S. Chang, M.C. Tai, C.H. Ho, and R.L. Jan analyzed the results. C.H. Ho, C.C. Chu, and J.J. Wang provided materials. Y.S. Chang, M.C. Tai and RL Jan wrote the article. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yuh-Shin Chang and Ming-Cheng Tai.
References
- 1.Blair M. Diabetes Mellitus Review. Urologic nursing. 2016;36:27–36. doi: 10.7257/1053-816X.2016.36.1.27. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Diabetes: Advances in Diagnosis and Treatment. Jama. 2015;314:1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Vanhoutte PM. Macro- and Microvascular Endothelial Dysfunction in Diabetes. Journal of diabetes. 2017;9:434–449. doi: 10.1111/1753-0407.12521. [DOI] [PubMed] [Google Scholar]
- 5.Orchard TJ, Costacou T. Cardiovascular complications of type 1 diabetes: update on the renal link. Acta diabetologica. 2017;54:325–334. doi: 10.1007/s00592-016-0949-7. [DOI] [PubMed] [Google Scholar]
- 6.Khalil H. Diabetes microvascular complications-A clinical update. Diabetes & metabolic syndrome. 2017;11:S133–S139. doi: 10.1016/j.dsx.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Prakash, G., Avadhani, K. & Srivastava, D. The three faces of herpes simplex epithelial keratitis: a steroid-induced situation. BMJ case reports2015 (2015). [DOI] [PMC free article] [PubMed]
- 8.Nielsen SE, et al. Incidence and clinical characteristics of fungal keratitis in a Danish population from 2000 to 2013. Acta Ophthalmol. 2015;93:54–58. doi: 10.1111/aos.12440. [DOI] [PubMed] [Google Scholar]
- 9.Vazirani J, Wurity S, Ali MH. Multidrug-Resistant Pseudomonas aeruginosa Keratitis: Risk Factors, Clinical Characteristics, and Outcomes. Ophthalmology. 2015;122:2110–2114. doi: 10.1016/j.ophtha.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Efron N, Morgan PB. Impact of differences in diagnostic criteria when determining the incidence of contact lens-associated keratitis. Optometry and vision science: official publication of the American Academy of Optometry. 2006;83:152–159. doi: 10.1097/01.opx.0000204519.92436.70. [DOI] [PubMed] [Google Scholar]
- 11.Jeng BH, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–1028. doi: 10.1001/archophthalmol.2010.144. [DOI] [PubMed] [Google Scholar]
- 12.Lim CH, et al. Risk factors for contact lens-related microbial keratitis in Singapore. Eye (Lond) 2016;30:447–455. doi: 10.1038/eye.2015.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001;108:586–592. doi: 10.1016/S0161-6420(00)00599-6. [DOI] [PubMed] [Google Scholar]
- 14.Misra SL, et al. Peripheral neuropathy and tear film dysfunction in type 1 diabetes mellitus. Journal of diabetes research. 2014;2014:848659. doi: 10.1155/2014/848659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz RO, Van Horn DL, Peters MA, Klewin KM, Schutten WH. Diabetic keratopathy. Transactions of the American Ophthalmological Society. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 16.Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. 1977;95:2193–2196. doi: 10.1001/archopht.1977.04450120099012. [DOI] [PubMed] [Google Scholar]
- 17.Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. American journal of ophthalmology. 2005;139:498–503. doi: 10.1016/j.ajo.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg ME, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Investigative ophthalmology & visual science. 2000;41:2915–2921. [PubMed] [Google Scholar]
- 19.Mian SI, Malta JB. Moraxella keratitis: risk factors, presentation, and management. Acta ophthalmologica. 2011;89:e208–209. doi: 10.1111/j.1755-3768.2009.01780.x. [DOI] [PubMed] [Google Scholar]
- 20.Das S, Constantinou M, Daniell M, Taylor HR. Moraxella keratitis: predisposing factors and clinical review of 95 cases. The British journal of ophthalmology. 2006;90:1236–1238. doi: 10.1136/bjo.2006.095182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badawi AE, Moemen D, El-Tantawy NL. Epidemiological, clinical and laboratory findings of infectious keratitis at Mansoura Ophthalmic Center, Egypt. International journal of ophthalmology. 2017;10:61–67. doi: 10.18240/ijo.2017.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu CL, Shen HN, Hu SC, Wang JD, Li CY. A Population-Based Study of All-Cause Mortality and Cardiovascular Disease in Association With Prior History of Hypoglycemia Among Patients With Type 1 Diabetes. Diabetes care. 2016;39:1571–1578. doi: 10.2337/dc15-2418. [DOI] [PubMed] [Google Scholar]
- 23.Huang CC, et al. Long-term Mortality Risk After Hyperglycemic Crisis Episodes in Geriatric Patients With Diabetes: A National Population-Based Cohort Study. Diabetes care. 2015;38:746–751. doi: 10.2337/dc14-1840. [DOI] [PubMed] [Google Scholar]
- 24.Chang YS, et al. Risk of retinal artery occlusion in patients with diabetes mellitus: A retrospective large-scale cohort study. PloS one. 2018;13:e0201627. doi: 10.1371/journal.pone.0201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz RO, Peters MA, Sobocinski K, Nassif K, Schultz KJ. Diabetic keratopathy as a manifestation of peripheral neuropathy. American journal of ophthalmology. 1983;96:368–371. doi: 10.1016/S0002-9394(14)77829-8. [DOI] [PubMed] [Google Scholar]
- 26.Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366:1340–1343. doi: 10.1016/S0140-6736(05)67546-0. [DOI] [PubMed] [Google Scholar]
- 27.Achtsidis V, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes care. 2014;37:e210–211. doi: 10.2337/dc14-0860. [DOI] [PubMed] [Google Scholar]
- 28.Tewari A, Sood N, Vegad MM, Mehta DC. Epidemiological and microbiological profile of infective keratitis in Ahmedabad. Indian journal of ophthalmology. 2012;60:267–272. doi: 10.4103/0301-4738.98702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeed A, et al. Risk factors, microbiological findings, and clinical outcomes in cases of microbial keratitis admitted to a tertiary referral center in ireland. Cornea. 2009;28:285–292. doi: 10.1097/ICO.0b013e3181877a52. [DOI] [PubMed] [Google Scholar]
- 30.Gebremariam TT. Bacteriology and Risk Factors of Bacterial Keratitis in Jimma, Southwest Ethiopia. Ethiopian medical journal. 2015;53:191–197. [PubMed] [Google Scholar]
- 31.Kibret T, Bitew A. Fungal keratitis in patients with corneal ulcer attending Minilik II Memorial Hospital, Addis Ababa, Ethiopia. BMC ophthalmology. 2016;16:148. doi: 10.1186/s12886-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


