Abstract
Citrus canker, caused by Xanthomonas citri subsp. citri (Xcc), is a serious bacterial disease that affects citrus production worldwide. Citron C-05 (Citrus medica) is the only germplasm in the Citrus genus that has been identified to exhibit strong resistance to Xcc. However, it has not been determined when, where, and how Xcc is restricted in the tissues of Citron C-05 during the infection process. In the present study, we investigated the spatiotemporal growth dynamics of an eGFP-labeled virulent Xcc (eGFP-Xcc) strain in Citron C-05 along with five susceptible biotypes (i.e., lemon, pummelo, sour orange, sweet orange, and ponkan mandarin) upon inoculation via the spraying or leaf infiltration of a bacterial suspension. The results from extensive confocal laser scanning microscopy analyses showed that while Xcc grew rapidly in plants of all five susceptible genotypes, Xcc was severely restricted in the epidermal and mesophyll cell layers of the leaves of Citron C-05 in the early stage of infection. Not surprisingly, resistance against Xcc in Citron C-05 was found to be associated with the production of reactive oxygen species and hypersensitive response-like cell death, as well as greater upregulation of several defense-related genes, including a pathogenesis-related gene (PR1) and a glutathione S-transferase gene (GST1), compared with sweet orange as a susceptible control. Taken together, our results not only provide further valuable details of the spatiotemporal dynamics of the host entry, propagation, and spread of Xcc in both resistant and susceptible citrus plants but also suggest that resistance to Xcc in Citron C-05 may be attributed to the activation of multiple defense mechanisms.
Subject terms: Plant breeding, Biotic
Introduction
Citrus canker is a major bacterial disease that affects most commercial citrus varieties worldwide1 and causes serious economic losses in almost all the major citrus-producing areas in China2. The causal agent is Xanthomonas citri subsp. citri (Xcc), a Gram-negative bacterial pathogen capable of colonizing all aboveground tissues of citrus plants, including young leaves, thorns, shoots, and fruits3,4. Under warm temperature and high-humidity conditions, the canker lesions first appear as circular oily spots, usually on the abaxial leaf surface, and then develop into tiny, slightly raised blister-like lesions5. The typical characteristic symptom is the formation of an enlarged hyperplastic lesion with a raised and spongy or corky center surrounded by a yellow chlorotic halo4,6, where the bacteria remain alive and active3 in the late stage of an infection. Severe canker disease causes defoliation, shoot dieback, and premature fruit drop6,7.
Different citrus species and cultivars exhibit different levels of sensitivity to canker disease. Susceptible biotypes of Citrus and its relatives include sweet orange (Citrus sinensis), pummelo (C. grandis), lemon (C. limon), lime (C. aurantifolia), grapefruit (C. paradisi), clementine (C. clementina), trifoliate orange (Poncirus trifoliata), and some mandarin-like hybrids such as “Orah” and “Orri”3,7. Certain citrus biotypes, such as mandarin (C. reticulata), Ichang papeda (C. ichangenesis), and C. junos, usually do not show canker symptoms in the field; however, when they are artificially inoculated with Xcc, they develop typical canker disease symptoms8. Kumquat (Fortunella spp.) and its hybrid calamondin (also known as Citrofortunella) are considered to be resistant to canker disease9,10. Through many years of efforts aimed at screening for Xcc-resistant citrus germplasm, we identified a citron (C. medica) biotype designated “Citron C-05” as highly resistant to Xcc in both field and laboratory conditions8. However, when, where and how Xcc bacteria are restricted in Citron C-05, the sole resistant citrus biotype reported thus far, have yet to be determined.
To establish successful colonization, Xcc bacteria have to penetrate host tissues through stomata or wounds. The length of the latent infection period is temperature dependent. It takes 6–7 days for the bacteria to propagate in several layers of mesophyll cells before the appearance of typical canker symptoms, including yellowing and crater formation, under warm temperature, high-humidity conditions11,12. Conceivably, resistance and tolerance in citrus hosts that do not display typical canker symptoms may involve distinct mechanisms. Preformed or inducible physical barriers may prevent bacterial attachment to the host surface and/or penetration into host tissues before colonization can occur13. Effector-triggered immunity (ETI), which is activated by resistance (R) proteins (normally members of the nucleotide-binding (NB) leucine-rich-repeat (LRR) superfamily), can restrict the propagation of bacteria within host tissue14. ETI is often, though not always, accompanied by a hypersensitive response (HR), consisting of pathogen-induced, localized programmed cell death at the site of infection15. In plants exhibiting tolerance, the bacterial pathogen can propagate in the host tissue, but the infection causes no or less-typical disease symptoms16. Additionally, the developmental stage of the plant or even the age of individual leaves may impact the degree of Xcc infection17,18; thus, the use of plants and leaves in similar developmental stages for the determination of Xcc infection in mesophyll tissues is considered to be critical for the reliable evaluation of germplasms for resistance or susceptibility to citrus canker17,18. For example, based on our observations, the leaves of a susceptible citrus plant may show reduced susceptibility to spray-inoculated Xcc when they turn dark green as they mature (data not shown), presumably because there are age-dependent structural changes such as wax deposition in the leaves that may restrict the epiphytic colonization of bacteria4,18. To more reliably and efficiently assess the resistance or susceptibility of host plants to a particular pathogen and infer likely associated mechanisms, fluorescence protein-labeled pathogen strains have been widely used for studying bacterial colonization and biofilm formation at a much improved spatiotemporal resolution11,19,20. We have thus also successfully developed an eGFP- Xcc strain with comparable pathogenicity to the wild-type strain DL509 isolated by our group21 for studying the Xcc infection process in citrus.
Based on our evaluations conducted in the past 10 years, Citron C-05 is the only resistant biotype in the genus Citrus that displays high resistance to canker disease in the field, as well as under greenhouse conditions upon natural infection or artificial inoculation with Xcc8. In this study, to determine the types of resistance mechanisms by which Xcc is restricted in Citron C-05, we conducted a comparative analysis of the Xcc infection process between Citron C-05 and five selected susceptible citrus biotypes by taking advantage of the availability of an eGFP-Xcc strain and a confocal imaging facility. Our results revealed the spatiotemporal dynamics of Xcc infection in both susceptible and resistant citrus biotypes and suggested that Citron C-05 can mount defense responses to effectively restrict Xcc propagation in mesophyll cells upon host penetration.
Results
Xcc growth and canker symptom development on the leaf surface
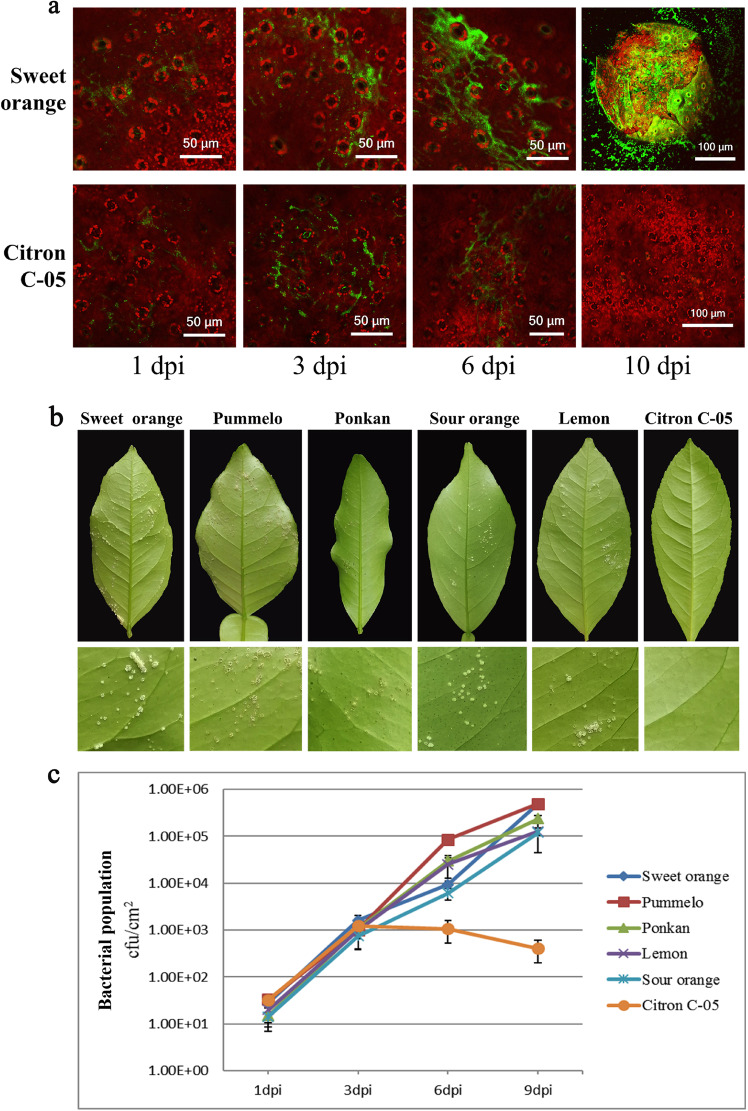
It is believed that successful attachment, adaptation and colonization on the host surface are the first steps for bacterial pathogens such as Xcc to establish a successful infection in the host plant22,23. To assess whether Citron C-05 can inhibit Xcc surface attachment and leaf-surface growth, young leaves of a similar age from Citron C-05 and five citrus biotypes (i.e., sweet orange, lemon, ponkan, pummelo, and sour orange) known to be susceptible to Xcc were inoculated by spraying a 108 cfu/ml eGFP-Xcc suspension on the abaxial leaf surface. Bacterial growth was monitored with a Zeiss confocal laser scanning microscope 710 (CLSM 710) at multiple time points after inoculation. From 1 to 3 days post-inoculation (dpi), similar bacterial growth on the leaf surface, including the intercellular space of the epidermal cells, was observed in all six citrus biotypes (Fig. 1a and Supplementary Fig. S1), suggesting that bacterial attachment and growth on the epidermal surface are not noticeably inhibited in Citron C-05. At 6 dpi, however, while Xcc continued to grow on the five susceptible biotypes, as shown by a significant increase in eGFP fluorescence (Fig. 1a and Supplementary Fig. S1), no further bacterial growth was observed on Citron C-05, whose leaves often showed even less eGFP fluorescence compared to the same leaves at 3 dpi (Fig. 1a). Z-stack images were generated to assess the distribution of bacteria in the spray-inoculated leaves of sweet orange and Citron C-05 at 6 dpi. As shown in Supplementary Fig. S4, Xcc bacteria were mostly detected in the intercellular space between the epidermal cells and some substomatal chambers on the leaf surface of sweet orange; however, Xcc bacteria were rarely or only sporadically observed on the leaf surface of Citron C-05. At 10 dpi, typical canker symptoms and intense fluorescence signals from eGFP-Xcc were detected in all five susceptible biotypes (Fig. 1a, b and Supplementary Fig. S1). By contrast, no canker symptoms were visible in Citron C-05 (Fig. 1b). Strikingly, eGFP fluorescence was hardly detectable either on the leaf surface or in mesophyll layers of Citron C-05 (Fig. 1a). Altogether, these observations suggest that resistance against Xcc in Citron C-05 leaves mostly occurs inside the mesophyll tissues at a post-penetration stage after 3 dpi.
Fig. 1. Leaf-surface bacterial growth and canker system development in citrus leaves spray-inoculated with Xcc.
Leaves of plants from Citron C-05 and sweet orange (and four other biotypes, Supplementary Fig. S1) were sprayed with ~ 108 cfu/ml eGFP-Xcc on the abaxial surface of fully expanded young leaves of six citrus biotypes. The inoculated leaves were subjected to confocal imaging for the observation of Xcc growth and the visual assessment of disease symptoms. These experiments were repeated at least three times with similar results. a Representative images showing the growth of Xcc on the leaf surface of sweet orange and Citron C-05 at the indicated time points. b Representative leaves (sections) showing the canker disease symptoms of the six indicated biotypes at 10 dpi. c Quantification of Xcc growth in the inoculated leaves of the six indicated citrus biotypes over a time course
Quantification and vertical distribution of Xcc after leaf tissue penetration
After successful penetration into the leaf tissue, especially the mesophyll layer, Xcc bacteria have to suppress host defense to colonize and proliferate in the apoplast of the host tissue, eventually leading to canker symptom development. To confirm that resistance to Xcc in Citron C-05 indeed occurs inside the infected tissue, we quantified Xcc growth in inoculated leaves after surface sterilization with 75% ethanol. No significant differences in bacterial growth were detected among the six citrus biotypes at 1 or 3 dpi (Fig. 1c). However, while Xcc continued to proliferate in all five susceptible citrus biotypes, reaching ~ 105 cfu/ml, Xcc stopped proliferating inside Citron C-05 leaf tissue at 3 dpi, and the bacterial titer actually decreased to 4 × 102 cfu/ml at 9 dpi, corresponding to ~ 1000× fewer bacteria compared to the bacterial levels in the other biotypes (Fig. 1c). These results agreed well with both the amount of Xcc visualized by confocal imaging and canker symptom development on the leaf surface (Fig. 1a, b).
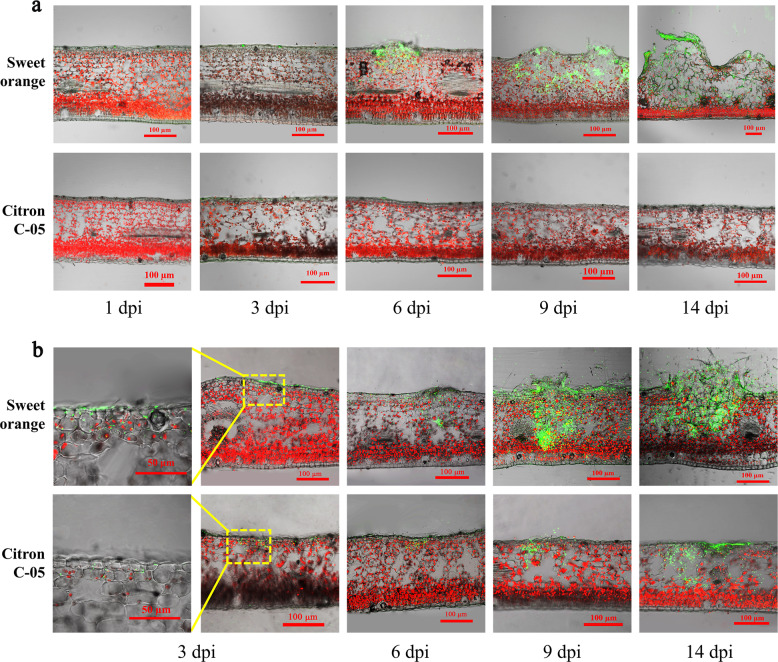
To further validate the above results, we examined the spatiotemporal distribution of Xcc in vertical sections of the inoculated leaves of Citron C-05 and “Bingtang” sweet orange, as a representative susceptible biotype, by CLSM. At 3 dpi with a bacterial suspension of 108 cfu/ml sprayed on fully expanded young leaves, some weak green fluorescence of Xcc was visible in mesophyll cells immediately underneath the epidermis in both “Bingtang” (more) sweet orange and Citron C-05 (less) (Fig. 2a). However, at 6 dpi, while large numbers of Xcc (reflected by more intense green fluorescence) were clearly visible in sweet orange leaf sections, no Xcc (reflected by the lack of fluorescence) was detectable in Citron C-05. Typical symptomatic crater structures concomitant with the spread and release of Xcc were visible in the leaf sections of the sweet orange at 9 dpi, which became more obvious at 14 dpi, whereas no leaf structural changes were seen in Citron C-05 (Fig. 2a). In an enhanced assay in which younger leaves (only half the size of fully expanded leaves) were sprayed with 109 cfu/ml Xcc, more fluorescent Xcc were visible in the leaf sections of Citron C-05 at 3 dpi, which continued to increase over time, reaching a considerable level (as reflected by the increased amount of green fluorescence) by 14 dpi, yet there was still no crater formation observed (Fig. 2b). By contrast, at 9 and 14 dpi, there was a massive explosion of Xcc with the rupture of the craters in the leaf sections of sweet orange (Fig. 2b). These observations support the conclusion that Citron C-05 exhibits post-penetration resistance against Xcc and imply that Xcc fails to manipulate host cell growth and development in Citron C-05 to form craters as it does in susceptible citrus biotypes, even though it accumulates at a fairly high level upon artificial inoculation at a high concentration.
Fig. 2. Spatiotemporal growth dynamics of Xcc visualized by CLSM in vertical sections of spray-inoculated leaves of sweet orange and Citron C-05.
a Fully expanded young leaves of the indicated biotypes were inoculated by spraying 108 cfu/ml eGFP-Xcc. b Younger leaves (half the size of fully expanded leaves) were sprayed with 109 cfu/ml eGFP-Xcc. Leaf vertical sections were monitored using CLSM. Representative images are shown
Xcc infection phenotypes of six citrus biotypes recapitulated by infiltration inoculation
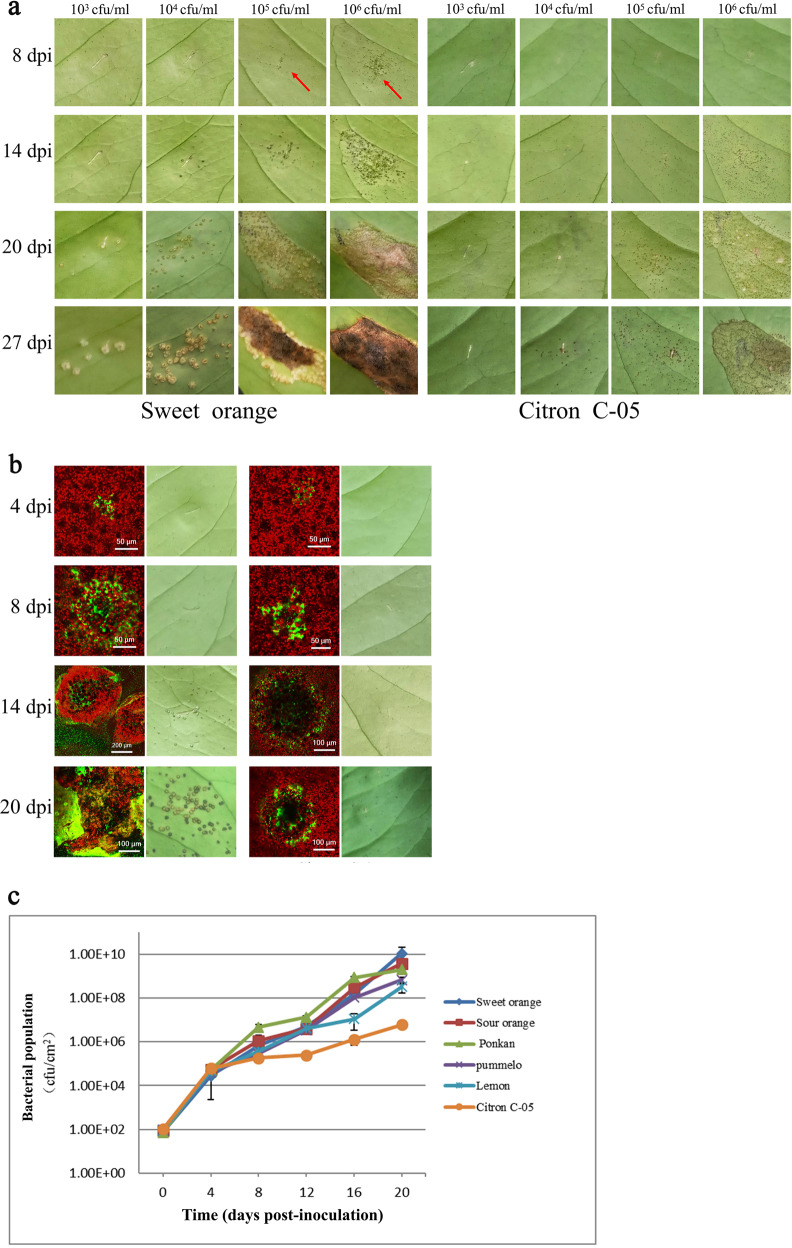
To evaluate the relationship between the bacterial load (i.e., quantity) and canker symptom development and to further assess the resistance level of Citron C-05 relative to those of the susceptible biotypes, we infiltrated the leaves of the six biotypes with Xcc bacterial suspensions at different concentrations (103, 104, 105, and 106 cfu/ml). Small blister-like lumps on the leaves appeared as the earliest symptom visible to the naked eye at ~8 dpi in all five susceptible genotypes, including sweet orange, when infiltrated with 106 cfu/ml Xcc. These lumps gradually spread and merged to produce water-soaked symptoms at 20 dpi and became erumpent and necrotic, with yellow margins surrounding the infected sites, at 27 dpi (Fig. 3a and Supplementary Fig. S2). Small blister-like lumps also occurred on the leaves of sweet orange, ponkan and sour orange infiltrated with 105 cfu/ml Xcc at 8 dpi, but at a lower density compared to the leaves infiltrated with 106 cfu/ml Xcc (Fig. 3a and Supplementary Fig. S2). Small cankers were visible on these three biotypes at 14 dpi and coalesced to form larger necrotic lesions with yellow margins at 20 dpi, which became more severe at 27 dpi. By contrast, the leaves of Citron C-05 showed only tiny black spots at 14 dpi, 20 dpi, and even 27 dpi, and there was no canker formation or significant necrosis when Citron C-05 leaves were infiltrated with Xcc at these two bacterial concentrations (Fig. 3a).
Fig. 3. Infection phenotypes of sweet orange and Citron C-05 following infiltration inoculation.
Xcc bacterial suspensions at different concentrations (103, 104, 105, and 106 cfu/ml) were infiltrated from the abaxial surface into fully expanded young leaves of the six citrus biotypes, and disease symptoms were monitored and documented from 1 to 27 dpi. Images of sweet orange and Citron C-05 are shown here. Images of the other biotypes are shown in Supplementary Fig. S2. a Xcc infection symptoms of sweet orange and Citron C-05 leaves infiltrated with the four indicated Xcc concentrations at the four indicated time points. b Visualization of Xcc after infiltration (at 104 cfu/ml) of the leaves of sweet orange and Citron C-05 by CLSM at the indicated time points. The infection symptoms of the same leaves were photographed and are shown in the right panels. c Quantification of Xcc in the leaves of the six citrus biotypes infiltrated with 104 cfu/ml Xcc
When the leaves of the six biotypes were infiltrated with 103 and 104 cfu/ml Xcc, small blister-like lumps (at a lower density) appeared 4–6 days later on the susceptible biotypes, and typical canker lesions were visible at 27 dpi (Fig. 3a). By contrast, no symptoms were visible in the leaves of Citron C-05 infiltrated with 103 or 104 cfu/ml Xcc except for small black spots at 27 dpi in the case of infiltration with 104 cfu/ml Xcc (Fig. 3a). These results were further validated by CLSM of leaf sections of Citron C-05 and sweet orange (Fig. 3b) along with four other susceptible biotypes (Supplementary Fig. S2) infiltrated with 104 cfu/ml Xcc.
To further evaluate the relationship between the growth dynamics of Xcc in citrus leaf tissues and canker symptom development, we quantified Xcc in the leaves of the six biotypes upon infiltration with 104 cfu/ml Xcc. At 4 dpi, the bacteria grew almost equally well in the tissues of all the tested citrus biotypes, reaching a concentration of 104 cfu/cm2. At 8 dpi, Xcc reached a concentration of 1.8 × 105 cfu/cm2 in Citron C-05, whereas the five susceptible biotypes showed higher concentrations, ranging from 3.0 × 105 to 4.7 × 106 cfu/cm2. The differences in bacterial growth between Citron C-05 and the other five biotypes continued to increase at 12 and 16 dpi, reaching a maximum of ~ 1000× at 20 dpi, with Citron C-05 exhibiting a bacterial load of 5.9 × 106 cfu/cm2, and the loads in the five susceptible biotypes reaching 108–109 cfu/cm2 (Fig. 3c). Additionally, the bacterial load seemed to be positively correlated with canker disease severity among the susceptible biotypes; differences were first observed among the susceptible biotypes at 8 dpi and became more obvious at 20 dpi, with sweet orange, sour orange and ponkan being more susceptible than pummelo and lemon (Fig. 3c).
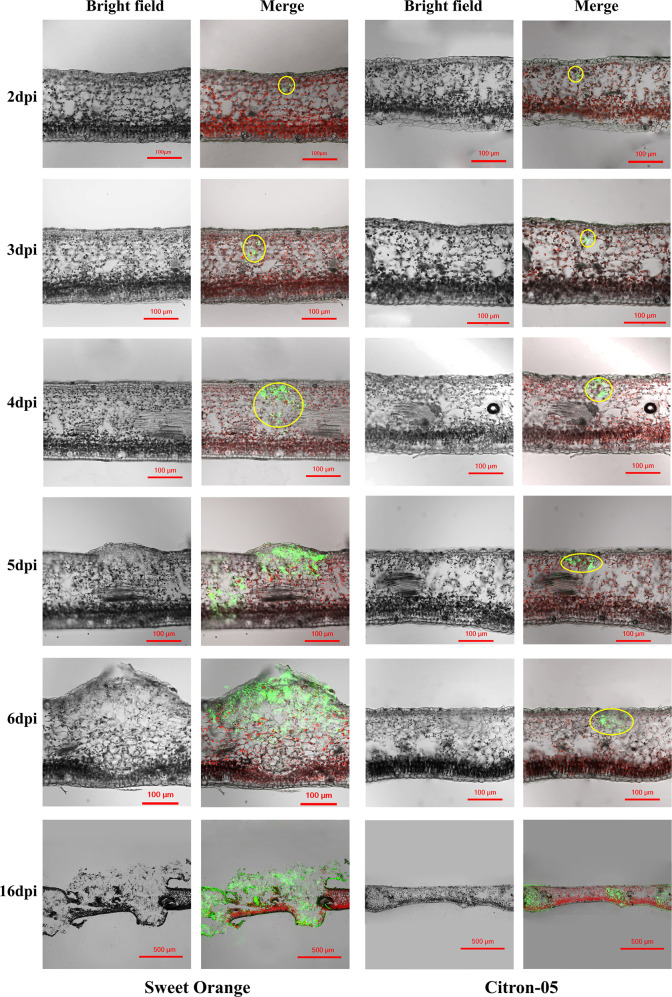
CLSM of vertical sections of the leaves of Citron C-05 and “Bingtang” sweet orange inoculated with 105 cfu/ml eGFP-Xcc showed that there was no clear difference between these two biotypes at 2 dpi (Fig. 4). However, starting from 3 dpi, more Xcc was observed in “Bingtang” sweet orange than in Citron C-05, and the difference became more dramatic as time went on. In the leaves of “Bingtang” sweet orange, Xcc penetrated deep into the spongy tissue at 4 dpi, spread across the entire leaf vertical section at 5 dpi, and formed a crater at 6 dpi; the craters ruptured, destroying the local infected tissues at 16 dpi (Fig. 4). By contrast, in the leaves of Citron C-05, Xcc exhibited limited proliferation, and there was no crater formation even at 16 dpi, when Xcc had spread across the leaf vertical sections (Fig. 4). These results are in accord with our observations following spray inoculation and further support the hypothesis that Citron C-05 restricts Xcc proliferation after penetration into leaf mesophyll layers.
Fig. 4. CLSM of leaf vertical sections showing Xcc proliferation in the leaves of sweet orange and Citron C-05 infiltrated with 105 cfu/ml eGFP-Xcc.
The yellow circles indicate the areas where Xcc aggregated
Resistance to Xcc infection in Citron C-05 is associated with the induction of defense-related genes
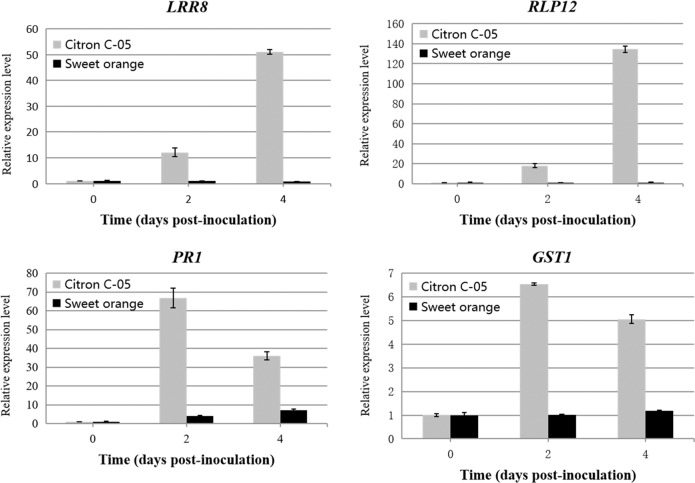
To assess the possible defense mechanisms activated in Citron C-05 when attacked by Xcc, we checked the expression of a number of genes whose homologs in model plants are known to be involved in disease resistance24,25. We infiltrated the leaves of Citron C-05 and Sweet orange with 105 cfu/ml Xcc and measured the expression of PR1 (encoding a pathogenesis-related protein), RLP12 (encoding an LRR receptor-like protein), and LRR8 (encoding an LRR receptor-like protein kinase) by quantitative reverse transcription PCR (RT-PCR). Consistent with the disease infection phenotypes, PR1 was strongly induced (~67×) in Citron C-05 at 2 dpi, while it showed only a slight induction (~4×) in sweet orange (Fig. 5). Although the expression level of PR1 decreased by approximately half in Citron C-05 at 4 dpi, it was still significantly higher (~4×) than that in sweet orange. More interestingly, while RLP12 and LRR8 were highly induced in Citron C-05, they were not induced in sweet orange at all (Fig. 5). Similarly, GST1, which is known to be involved in reactive oxygen species (ROS)-dependent defense26, was significantly upregulated in Xcc-infected Citron C-05 leaves but not in sweet orange (Fig. 5), suggesting that there may be massive ROS production in the Xcc-infected leaf tissues of Citron C-05. We thus examined ROS production in situ by DAB staining and found sporadic and localized ROS accumulation in the Xcc-inoculated leaves of Citron C-05 but not in those of sweet orange at 5 dpi (Fig. 6). ROS production often precedes programmed cell death in resistance gene-mediated resistance27,28. To determine whether the induction of ROS by Xcc in the leaves of Citron C-05 indeed leads to HR, we stained the leaves with trypan blue. As expected, small dead or dying cell clusters stained blue were also visible at 5 dpi only in Xcc-infected Citron C-05 leaves but not in infected sweet orange leaves (Fig. 6). These observations suggest that the resistance of Citron C-05 to Xcc may be attributable to the activation of multiple defense mechanisms.
Fig. 5. Induction of four defense-related genes in Citron C-05 upon Xcc inoculation.
Quantitative RT-PCR was used to check the expression levels of the four indicated genes in the leaves of Citron C-05 and sweet orange infiltrated with a 105 cfu/ml Xcc suspension at 0, 2, and 4 dpi
Fig. 6. Xcc induces ROS accumulation and cell death in Citron C-05 cells.

The leaves of Citron C-05 and sweet orange were infiltrated with 105 cfu/ml Xcc or buffer. At 5 dpi, infiltrated leaves were subjected to DAB and Trypan blue staining. Leaf sections were examined under a Leica DMi8 scope. Reddish-brown staining indicates ROS accumulation, and blue staining indicates dead or dying cells
Discussion
In this study, we conducted a detailed spatiotemporal comparative analysis of the Xcc infection dynamics in five susceptible and one resistant citrus biotype. Our results showed that Citron C-05, the only resistant germplasm identified to date in the Citrus genus8, exhibited post-penetration resistance that was associated with the induction of at least four defense-related genes, ROS production and programmed cell death in most cases.
Type A Xcc is the main type causing severe symptoms in citrus orchards in Asian regions29,30. By using eGFP-Xcc pathotype A21 and CLSM as well as conventional means of bacterial growth determination and symptom examination, we were able to monitor the leaf-surface multiplication, penetration and proliferation inside the host tissue of Xcc in the leaves of six selected citrus biotypes. It has been well established that the stomata, as natural openings on the leaf surface, are exploited by pathogens to enter into host tissue and are thus subjected to tight regulation as part of a plant innate immune mechanism for restricting the entry of bacteria31. The results obtained via spray or infiltration inoculation showed that the inoculated leaves of all five susceptible biotypes exhibited typical canker symptoms (i.e., crater formation), whereas no crater formation was found in the inoculated leaves of Citron C-05, confirming that Citron C-05 is highly resistant to canker disease8. However, we noticed some differences in the kinetics of Xcc growth in Citron C-05 leaves inoculated with these two methods. Xcc growth in Citron C-05 leaves inculcated by spraying 108 cfu/ml Xcc was completely arrested after 3 dpi, and a decreasing amount of the bacteria was over time (Fig. 1c), while Xcc that infiltrated into the leaf tissue multiplied ~100× in the period from 4 dpi to 20 dpi (Fig. 3c). In addition, when a high concentration (105 cfu/ml) of Xcc was infiltrated into the leaves of Citron C-05, the bacteria proliferated to a greater extent at 16 dpi, as shown by CLSM images in Fig. 4. Altogether, these results suggest that while Citron C-05 is able to restrict Xcc inside leaf tissue to prevent its massive proliferation, the overall resistance of Citron C-05 to Xcc may in part be attributable to its ability to inhibit the leaf-surface multiplication and stomatal penetration of Xcc.
Mandarin is usually considered to be moderately resistant to Xcc6, and ponkan, as a hybrid of mandarin × pomelo, exhibits a low level of susceptibility to Xcc17,32,33. We found that ponkan was very susceptible in the experiments using either spray or infiltration inoculation in this study. This discrepancy might be caused by the absolute initial amount of Xcc that entered the inoculated leaf tissue, regardless of the specific inoculation method, even though leaf infiltration has been shown to induce canker disease development more readily5. In spray inoculation, because 104 cfu/ml is reported to be the minimum inoculum concentration for causing symptom development in intact leaves that are less than 75% expanded34, a much higher Xcc concentration is often used to ensure more even disease development33,35. We thus used Xcc suspensions with concentrations as high as 108 cfu/ml for spray inoculation. At such a high dose of inoculum, one may speculate that many more Xcc bacteria penetrate the leaf mesophyll layers of ponkan, causing typical canker symptoms. Since the infiltration of 103 cfu/ml or higher doses of Xcc also induced typical symptoms in all five susceptible citrus biotypes, we can further reason that (i) mandarin and some of its hybrids, such as ponkan, may possess relatively higher levels of stomatal immunity, capable of limiting Xcc entry into the leaf mesophyll under natural growth conditions when the amount of Xcc inoculum is rather low and heterogeneous or when leaves are inoculated with lower concentrations of Xcc and that (ii) ponkan, unlike Citron C-05, does not exhibit post-penetration resistance. Therefore, caution needs to be exercised when we evaluate the resistance and susceptibility of different citrus germplasms treated with different dosages of Xcc. Nevertheless, we considered spraying 108 cfu/ml or infiltrating 103 cfu/ml Xcc to be a safer inoculation method for revealing the distinct nature of the resistance of Citron C-05, which will be particularly useful for determining the phenotypes of segregating progenies derived from Citron C-05 x sweet orange for mapping resistance gene(s) in Citron C-05.
Kumquat and calamondin are considered to be resistant to canker disease, and the defense response to Xcc is associated with programmed cell death, H2O2 production and the induction of defense-related genes7,9,10. Currently, the genetic control mechanism of resistance to Xcc in Citron C-05 (as well as Kumquat and calamondin) is not known, nor is its molecular basis. The observed strong induction of four different defense-related genes (Fig. 5) is compatible with a scenario in which multiple layers of resistance may exist in Citron C-05. Specifically, while the induction of LRR8 and RLP12, two genes encoding proteins involved in the perception of pathogen-associated molecular patterns (PAMPs)24, is suggestive of the activation of pattern-triggered immunity (PTI) in Citron C-05 (Fig. 5), the strong induction of PR1 may imply the existence of R gene-mediated, salicylic acid-dependent resistance and systemic acquired resistance25,36. Consistent with the latter speculation, the resistance of Citron C-05 was also associated with GST1 induction, ROS production and HR-like programmed cell death (Figs. 5 and 6). Future efforts will be directed toward the mapping and identification of the gene(s) underlying the remarkable resistance of Citron C-05 against Xcc.
Materials and methods
Plant materials
The genotypes used in the present study were “Citron C-05” (C. medica), “Zaomi” ponkan (C. reticulate), “Bingtang” sweet orange (C. sinensis), sour orange (C. aurantium), lemon (C. limon) and “Shatian” pummelo (C. grandis). All the citrus plants were grafted onto trifoliate orange (Poncirus trifoliata) rootstock and maintained for 2 years in the greenhouse of the National Center for Citrus Improvement, Changsha, China under the same conditions with standard irrigation and fertilization. Almost fully expanded young leaves from healthy plants were chosen for pathogenicity assays.
Xcc culture and pathogenicity assays
The original Xcc strain DL509 used in this study was isolated from diseased sweet orange leaves showing typical cankers. eGFP-Xcc was then generated by triparental mating, and the bacterial expression of eGFP was confirmed by fluorescence microscopy and pathogenicity tests, indicating that the eGFP-Xcc strain exhibited the same pathogenicity as the wild-type strain21. eGFP-Xcc was plated on Luria-Bertani (LB) solid medium and incubated at 28 °C for 14 h. Individual colonies were cultured separately in LB liquid medium at 28 °C on a shaker at 200 rpm for 18 h. Bacterial cells were harvested and centrifuged at 8000 rpm at room temperature. The cell pellets were resuspended in sterile distilled water, and the concentration was measured by using a spectrophotometer at 600 nm. The concentration of the bacterial suspension was further confirmed by counting colonies on LB plates following appropriate dilution. The inocula were adjusted to concentrations of 103, 104, 105, and 106 cfu/ml for infiltration and 108 cfu/ml for spray inoculation.
Spray inoculation was performed by uniformly distributing a 108 cfu/ml bacterial suspension with 0.05% Silwet-L7 on the abaxial leaf blade. Infiltration inoculation was also performed on the abaxial surface. The leaves were carefully wounded with a needle, and then four concentrations of Xcc suspensions were separately infiltrated into the mesophyll of the same leaf by pressure using a 1 ml syringe. Distilled water was used as a control in both spray and infiltration inoculation. The inoculated plants were kept in a greenhouse under a controlled environment at ≥28 °C with ≥70% relative humidity. Lesion expansion and symptom development were recorded periodically after inoculation.
Periodic quantification of Xcc in the host mesophyll after inoculation
Leaves inoculated by spray inoculations (108 cfu/ml) and infiltration inoculation (104 cfu/ml) were randomly sampled for quantification. Spray-inoculated leaves were collected at 1, 3, 6, and 9 days post-inoculation (dpi), and infiltration-inoculated leaves were collected at 0, 4, 8, 12, 16, and 20 dpi. The sampled leaves were surface disinfected with 75% ethanol. Eight leaf discs were collected within the inoculation area from each of the three inoculated leaves and homogenized in 1 ml of sterile distilled water. The isolation suspension was gradient diluted with sterile distilled water. Then, 20 μl of a diluted suspension from each sample was incubated on plates with solid LB medium at 28 °C for 48 h. The bacteria were quantified according to the formation of colonies on the LB agar plates. All quantifications were repeated three times.
Monitoring the penetration and colonization of Xcc in citrus tissues
The penetration and colonization of Xcc in vivo was examined following the inoculation of eGFP-Xcc and observed under an inverted confocal laser scanning microscope as described previously21. Spray inoculation was performed at a concentration 108 cfu/ml on fully expanded young leaves or 109 cfu/ml on 1/2 expanded young leaves, and 105 cfu/ml Xcc was directly infiltrated into fully expanded young leaves. Six rectangular leaf pieces 0.5 × 1.0 cm2 were sampled from each inoculated leaf and directly frozen in a cryostat (Leica CM1900, Berlin, Germany) at −20 °C. In addition, 10 μm serial frozen sections were cut along the vertical discs and blocked in 20% glycerol (v/v). For bacterial observations, eGFP fluorescence was imaged at an excitation wavelength of 488 nm and emission wavelength of 500–530 nm. The autofluorescence signal from chlorophyll was collected simultaneously at light wavelengths between 650 and 700 nm.
Xcc growth and disease development on the leaf surface and in the mesophyll
eGFP-Xcc was inoculated onto fully expanded young leaves by spraying (108 cfu/ml) and infiltration (104 cfu/ml) to monitor bacterial growth and disease development. The inoculated plants were maintained in a greenhouse at 27 ± 1 °C with high humidity. The presence of bacteria was examined under a Zeiss 710 confocal laser scanning microscope (CLSM) at ×10 magnification, and canker disease symptoms were recorded at regular intervals. Disease development after spray inoculation was observed at 1, 3, 6, and 10 dpi, and the bacterial colonization of Xcc was monitored at 4, 8, 14, and 20 dpi. Four leaves were sampled from each citrus genotype, and more than three discs of ~0.5 cm2 were cut from the sampled leaves. Then, the abaxial leaf surface was mounted for viewing under glass coverslip and observed with a Zeiss 710 confocal laser scanning microscope.
ROS and trypan blue staining
Fully expanded young healthy leaves were infiltrated with 105 cfu/ml Xcc bacteria. DAB (3,3’-diaminobenzidine) staining was performed for ROS analysis as reported by Piazza et al.37. The infiltration-inoculated leaves were submerged in a 1% (w/v) DAB solution and shaken at 100 rpm overnight. The leaves were cleaned in ethanol and excised at 5 dpi for DAB staining and destaining. Then, the leaf samples were examined for brown coloration under a Leica DMi8 microscope (Germany). Programmed cell death was visualized in inoculated leaves after staining with trypan blue at 5 dpi as described by Xiao et al.27.
RNA extraction and real-time quantitative PCR analysis
The expression of defense genes (PR1, RLP12, GST1, and LRR8) induced by Xcc was evaluated. Young leaves were infiltrated with 105 cfu/ml Xcc, and leaves were collected at 0, 2 and 4 dpi. Total leaf RNA was isolated using TRIzol reagent (Invitrogen, Grand Island, NY, USA) according to the protocol described previously38, and the RNA concentration was determined by using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The synthesis of complementary DNA (cDNA) was performed with oligo (dT) primers using the M-MLV reverse transcriptase system (Promega, USA) according to the manufacturer’s instructions. The cDNA products were used for quantitative real-time PCR analysis in a Bio-Rad CFX96 qPCR system. All qPCR assays were performed in duplicate using the SYBR Green protocol. Primer sequences for PR1, RLP12, GST1, and LRR8 were selected from the reported literature24,25.
Data analysis
All of the presented data are shown as average values and standard errors. Differences between the means were evaluated using one-way analysis of variance.
Supplementary information
Acknowledgements
This work was supported by the Key Project of International Cooperation and Exchange of the National Natural Science Foundation of China (No. 31720103915), a Project of the National Natural Science Foundation of China (No. 31572111) and the Key Laboratory of Crop Germplasm Innovation and Resource Utilization Science Foundation (No. 16KFXM01).
Author contributions
Z.D. and X.M. conceived the study, designed the experiment, helped analyze the research results, and edited the manuscript. H.F. conducted the experiment, performed the data analysis, and prepared the manuscript. M.Z., L.T., and J.X. assisted in the experiments. J.H., D.L., and M.W. provided suggestions for the experiments. S.X. and X.M. provided suggestions for the experiments and edited the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Xianfeng Ma, Email: ma8006@hunau.edu.cn.
Ziniu Deng, Email: deng7009@163.com.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-020-0278-4).
References
- 1.Dalio RJD, et al. PAMPs, PRRs, effectors and R-genes associated with citrus-pathogen interactions. Ann. Bot. 2017;119:749–774. doi: 10.1093/aob/mcw238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao TS, Zhou Y, Zhou CY. Research development of the differentiation and control of citrus bacterial canker disease. Acta Horticulturae Sin. 2015;42:1699–1706. [Google Scholar]
- 3.Gottwald TR, Graham JH, Schubert TS. Citrus Canker: the pathogen and its impact. Plant Health Prog. 2002;3:15–49. doi: 10.1094/PHP-2002-0812-01-RV. [DOI] [Google Scholar]
- 4.Graham JH, Gottwald TR, Cubero J, Achor DS. Xanthomonas axonopodis pv. citri: factors affecting successful eradication of citrus canker. Mol. Plant Pathol. 2004;5:1–15. doi: 10.1046/j.1364-3703.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 5.Christiano RSC, Dalla Pria M, Jesus Junior WC, Amorim L, Bergamin Filho A. Modelling the progress of Asiatic citrus canker on Tahiti lime in relation to temperature and leaf wetness. Eur. J. Plant Pathol. 2009;124:1–7. doi: 10.1007/s10658-008-9389-8. [DOI] [Google Scholar]
- 6.Brunings AM, Gabriel DW. Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 2003;4:141–157. doi: 10.1046/j.1364-3703.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen PS, et al. Understanding cellular defence in kumquat and calamondin to citrus canker caused by Xanthomonas citri subsp. citri. Physiological Mol. Plant Pathol. 2012;79:1–12. doi: 10.1016/j.pmpp.2012.03.001. [DOI] [Google Scholar]
- 8.Deng ZN, et al. Screening citrus genotypes for resistance to canker disease (Xanthomonas axonopodis pv. citri) Plant Breed. 2010;129:341–345. doi: 10.1111/j.1439-0523.2009.01695.x. [DOI] [Google Scholar]
- 9.Reddy MRS. Sources of resistance to bacterial canker in citrus. J. Mycol. Plant Pathol. 1997;27:80–81. [Google Scholar]
- 10.Khalaf A, Moore GA, Jones JB, Gmitter FG. New insights into the resistance of nagami kumquat to canker disease. Physiological Mol. Plant Pathol. 2007;71:240–250. doi: 10.1016/j.pmpp.2008.03.001. [DOI] [Google Scholar]
- 11.Cubero J, Gell I, Johnson EG, Redondo A, Graham JH. Unstable green fluorescent protein for study of Xanthomonas citri subsp. citri survival on citrus. Plant Pathol. 2011;60:977–985. doi: 10.1111/j.1365-3059.2011.02450.x. [DOI] [Google Scholar]
- 12.Favaro MA, et al. Surface barriers of mandarin cv. ‘Okitsu’ leaves make a major contribution to canker disease resistance. Phytopathology. 2014;104:970–976. doi: 10.1094/PHYTO-10-13-0277-R. [DOI] [PubMed] [Google Scholar]
- 13.Ade, J. & Innes, R. W. Resistance to bacterial pathogens in plants. in Encyclopedia of Life Sciences, 1–6 (John Wiley & Sons Ltd., 2007). 10.1002/9780470015902.a0020091.
- 14.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 15.Michèle CH. Hypersensitive response-related death. Plant Mol. Biol. 2000;44:321–334. doi: 10.1023/A:1026592509060. [DOI] [PubMed] [Google Scholar]
- 16.Sarkar J, Saha J, Panja BN, Das A. Evaluation of Citrus species and their relatives of tolerance to bacterial canker disease and prediction of canker incidence based on leaf morphological character. J. Mycopathological Res. 2007;45:175–181. [Google Scholar]
- 17.Gottwald TR, Graham JH, Civerolo EL, Barrett HC, Hearn CJ. Differential host range reaction of citrus and citrus relatives to citrus canker and citrus bacterial spot determinated by leaf mesophyll susceptiblity. Plant Dis. 1992;77:1004–1009. doi: 10.1094/PD-77-1004. [DOI] [Google Scholar]
- 18.Graham JH, Gottwald TR, Riley TD, Achor D. Penetration through leaf stomata and strains of Xanthomonas campestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology. 1992;82:1319–1325. doi: 10.1094/Phyto-82-1319. [DOI] [Google Scholar]
- 19.Malamud F, et al. The Xanthomonas axonopodis pv. citri flagellum is required for mature biofilm and canker development. Microbiology. 2011;157:819–829. doi: 10.1099/mic.0.044255-0. [DOI] [PubMed] [Google Scholar]
- 20.Han SW, Park CJ, Lee SW, Ronald PC. An efficient method for visualization and growth of fluorescent Xanthomonas oryzae pv. oryzae in planta. Bmc Microbiol. 2008;8:164–172. doi: 10.1186/1471-2180-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu LP, et al. Construction of EGFP-labeling system for visualizing the infection process of Xanthomonas axonopodis pv. citri in planta. Curr. Microbiol. 2012;65:304–312. doi: 10.1007/s00284-012-0155-y. [DOI] [PubMed] [Google Scholar]
- 22.Rigano LA, et al. Biofilm formation, epiphytic fitness, and canker development in Xanthomonas axonopodis pv. citri. Mol. Plant-Microbe Interact. 2007;20:1222–1230. doi: 10.1094/MPMI-20-10-1222. [DOI] [PubMed] [Google Scholar]
- 23.Bogino PC, Oliva MdeL, Sorroche FG, Giordano W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013;14:15838–15859. doi: 10.3390/ijms140815838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi QC, Febres VJ, Jones JB, Moore GA. Responsiveness of different citrus genotypes to the Xanthomonas citri subsp. Citri derived PAMP flg22 correlates with resistance to citrus canker. Mol. Plant Pathol. 2015;16:507–520. doi: 10.1111/mpp.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitino M, Armstrong CM, Duan Y. Rapid screening for citrus canker resistance employing pathogen-associated molecular pattern-triggered immunity responses. Horticulture Res. 2015;2:15042–15051. doi: 10.1038/hortres.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant JJ, Yun BW, Loake GJ. Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J. 2000;24:569–582. doi: 10.1046/j.1365-313x.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 27.Xiao SY, Brown S, Patrick E, Brearley C, Turner JG. Enhanced transcription of the Arabidopsis disease resistance genes RPW8.1 and RPW8.2 via a salicylic acid-dependent amplification circuit is required for hypersensitive cell death. Plant Cell. 2003;15:33–45. doi: 10.1105/tpc.006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar N, Ebel RC, Roberts PD. H2O2 metabolism during sweet orange (Citrus sinensis L. Osb.) ‘Hamlin’ Xanthomonas axonopodis pv. citri interaction. Sci. Horticulturae. 2011;128:465–472. doi: 10.1016/j.scienta.2011.02.022. [DOI] [Google Scholar]
- 29.Gordon JL, et al. Comparative genomics of 43 strains of Xanthomonas citri pv.citri reveals the evolutionary events giving rise to pathotypes with different host range. BMC Genomics. 2015;16:1098–1108. doi: 10.1186/s12864-015-2310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou HS, Rou W, Wu W. Research progress on the resistance and susceptible mechanisms of citrus bacterial canker disease. J. For. Environ. 2018;38:234–239. [Google Scholar]
- 31.Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Hu XR, et al. The investigation of different varieties of citrus to the disease resistance of canker. Zhejiang Citrus. 2014;31:28–30. [Google Scholar]
- 33.Amaral AM, Carvalho SA, Machado MA, Silva LFC. Reaction of genotypes of citrus species and varieties to Xanthomonas citri subsp. citri under greenhouse conditions. J. Plant Pathol. 2010;92:519–524. [Google Scholar]
- 34.Christiano RSC, et al. Effect of citrus leaf-miner damage, mechanical damage and inoculum concentration on severity of symptoms of Asiatic citrus canker in Tahiti lime. Crop Prot. 2007;26:59–65. doi: 10.1016/j.cropro.2006.03.016. [DOI] [Google Scholar]
- 35.Raza MM, Khan MA, Atiq M, Binyamin R, Javaid M. Prediction of citrus canker epidemics generated through different inoculation methods. Arch. Phytopathol. Plant Prot. 2013;47:1335–1348. doi: 10.1080/03235408.2013.840107. [DOI] [Google Scholar]
- 36.Durrant WE, Dong X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 37.Piazza A, et al. The dual nature of trehalose in citrus canker disease: a virulence factor for Xanthomonas citri subsp. citri and a trigger for plant defence responses. J. Exp. Bot. 2015;66:2795–2811. doi: 10.1093/jxb/erv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan JW, Yuan FR, Long GY, Qin L, Deng ZN. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 2012;39:1831–1838. doi: 10.1007/s11033-011-0925-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.