Abstract
Bispecific antibodies (bsAbs) refer to a large family of molecules that recognize two different epitopes or antigens. Although a series of challenges, especially immunogenicity and chain mispairing issues, once hindered the development of bsAbs, they have been gradually overcome with the help of rapidly developing technologies in the past 5 decades. In the meantime, an increasing number of bsAb platforms have been designed to satisfy different clinical demands. Currently, numerous preclinical and clinical trials are underway, portraying a promising future for bsAb-based cancer treatment. Nevertheless, bsAb drugs still face enormous challenges in their application as cancer therapeutics, including tumor heterogeneity and mutational burden, intractable tumor microenvironment (TME), insufficient costimulatory signals to activate T cells, the necessity for continuous injection, fatal systemic side effects, and off-target toxicities to adjacent normal cells. Therefore, we provide several strategies as solutions to these issues, which comprise generating multispecific bsAbs, discovering neoantigens, combining bsAbs with other anticancer therapies, exploiting natural killer (NK)-cell-based bsAbs and producing bsAbs in situ. In this review, we mainly discuss previous and current challenges in bsAb development and underscore corresponding strategies, with a brief introduction of several typical bsAb formats.
Keywords: Bispecific antibodies (bsAbs), Tumor microenvironment (TME), Tumor immunotherapy, Monoclonal antibodies (mAbs)
Subject terms: Cancer therapy, Immunology
Introduction
To date, in comparison with conventional anticancer strategies, immunotherapy is considered the most promising systemic cancer treatment, playing an indispensable role in enhancing therapeutic efficacy, especially against refractory cancer types. Emerging cancer immunotherapies comprise cancer vaccines, adoptive transfer of chimeric antigen receptor-armed T cells (CAR-T cells), cytokine administration, immune checkpoint inhibitors, and tumor-targeting monoclonal antibodies (mAbs).1 In general, mAbs are synthetic biotherapeutics generally used to treat or prevent diseases such as infection, cancer, and autoimmune disorders. They are produced based on hybridomas, genetic engineering, phage display, and transgenic mouse technologies to mimic the specificity and functionality of natural antibodies (Abs).2 As such, mAbs have emerged as a crucial and efficacious therapeutic modality in cancer therapeutics due to their ability to specifically target a molecule.3 However, in the sophisticated pathogenesis of a disease, multiple mediators contribute to stimulating different signaling pathways or facilitate overlapping signaling cascades, thus limiting the therapeutic effect of targeting a single molecule.4 In addition, the development of two separate Abs for combination immunotherapy encounters regulatory hurdles, high expense, and inadequate tests for safety or efficacy, thus making this strategy relatively unattainable.5 Therefore, since Nisonoff introduced the revolutionary idea of “recombination of a mixture of univalent Ab fragments of different specificities” in the 1960s, the development of bispecific Abs (bsAbs) has transformed the field of cancer immunotherapy. Later, as genetic engineering techniques progressed rapidly, the generation of versatile bsAb formats received significant attention and has yielded therapeutic potential, making bsAbs readily transferrable into clinical practice, where they may demonstrate better clinical efficacy than mAbs or other conventional antitumor therapies. This is exemplified by some large-scale, multicenter clinical studies of blinatumomab (Amgen Inc., a bispecific T-cell engager (BiTE) Ab with specificity for both CD19 on malignant B cells and CD3 on cytotoxic T cells), which demonstrated increased overall survival rates in patients suffering from relapsed or refractory B-cell precursor acute lymphoblastic leukemia compared with standard combination chemotherapy.6,7
With the ability to concurrently target two epitopes on tumor cells or in the tumor microenvironment (TME), bsAbs are progressively interpreted as a prospective and significant component of next-generation therapeutic Abs.8 The majority of bsAbs currently under development are being devised to form an artificial immunological synapse by bringing immune cells, especially cytotoxic T cells, into close proximity with tumor cells, which eventually leads to selective attack and lysis of target tumor cells.9–11 Although various bsAb formats exist, they can be roughly divided into two categories based on the presence or absence of the fragment crystallizable domain (Fc): IgG-like and non-IgG-like. The existence of the Fc fragment notably exerts additional effector functions.10 In this review, we mainly focus on the challenges that hinder more extensive adhibition of bsAbs and strategies to circumvent these problems, including but not limited to producing multispecific Abs, investigating neoantigens, applying bsAbs in combination with other immune strategies, exploiting natural killer (NK)-cell-based bsAbs and generating bsAbs in situ. In addition, we also elaborate on the architecture of different bsAb formats with their respective pros and cons, as well as the history of bsAbs in technical development and their clinical applications.
The design strategies for bsAbs
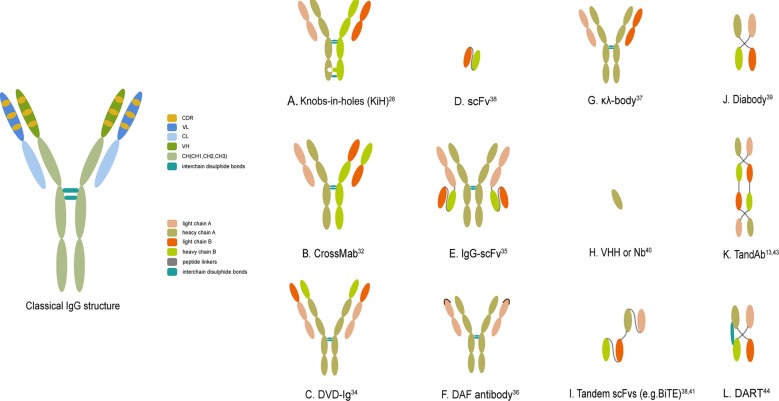
Immunoglobulin (Ig), a classical Ab that is well conserved in mammals, is made up of polypeptide tetramers that contain two identical heavy–light-chain pairs connected via interchain disulfide bonds and noncovalent bonds. The architecture of the Ab resembles the shape of a “Y,” with a total molecular weight of ~150 kDa. Hence, typical Abs are bivalent but monospecific, with two fragments of antigen-binding (Fab) arms binding the same epitope. More specifically, the heavy chain of the Ab consists of one variable region (VH) and three constant regions (CH1, CH2, and CH3), while the light chain encompasses one variable region (VL) and one constant region (CL). Both VH and VL contain three complementarity-determining regions (CDRs), collectively constituting the antigen-binding site of IgG, which shoulders the responsibility of recognizing antigens and determining the binding affinity and specificity. Therefore, two pairs of heavy–light-chain pairs in an Ab molecule contain two Fab arms and one Fc domain, the latter of which binds to complement peptides or Fc receptors (FcRs) on cytotoxic cells such as NK cells, mononuclear phagocytes (especially macrophages), and neutrophils. In addition, the flexible hinge region, which determines different Ig subtypes, is the location where two pairs connect (Fig. 1, left).10,12,13
Fig. 1.
The classical IgG structure and schematic representations of several crucial bsAb formats. a–c, e–g. IgG-like bsAb formats (with Fc region). d, h–l. non-IgG-like bsAb fragments (without Fc region). DVD-Ig dual-variable-domain immunoglobulin, ScFv single-chain variable fragment, VHH variable domain of heavy chain, Nb nanobody, BiTE bispecific T-cell engager, TandAb tandem diabody, DART dual-affinity retargeting molecule
In contrast, bsAbs represent dual specificities through the simultaneous combination of different antigens or epitopes, thus standing on the cusp of Ab-based cancer treatment. They have received significant attention in the field of cancer treatment and mainly function in four ways: (a) redirect specific immune effector cells to selectively destroy cancer cells; (b) target more than one cell surface antigen, thus increasing target specificity; (c) deliver drugs to tumors; and (d) improve the therapeutic potency and persistence via blockade of two biological pathways.12 Among these functions, the most commonly employed one involves engaging immune effector cells in close proximity to cancer cells and thus reducing systemic toxicity, circumventing drug resistance, and improving therapeutic efficacy.8 As mentioned above, bsAbs are roughly divided into two groups: (a) IgG-like (with an Fc region) and (b) non-IgG-like (without an Fc region).
IgG-like bsAbs (with an Fc region)
Owing to its larger size and FcRn-mediated recycling processes, the full-length bsAb with an Fc region has a longer half-life in circulation than bsAbs without an Fc region. It is more convenient to purify and displays increased solubility and stability. More importantly, it may have greater clinical therapeutic potential for retaining a diverse range of Fc-mediated effector functions, including Ab-dependent cellular cytotoxicity, complement-dependent cytotoxicity, and Ab-dependent cellular phagocytosis.
Non-IgG-like bsAb fragments (without an Fc region)
By contrast, the shorter-length non-IgG-like format is made up of Ab moieties, thereby showing relatively low circulation kinetics but better tissue-penetrating capacity, less immunogenicity, and lower nonspecific activation of the innate immune system. This format of bsAb mainly depends on its antigen-binding ability to exert versatile functions.11,14–16
To extend the half-life of non-IgG-like formats while retaining their original bioactivity, safety, and low immunogenicity, several strategies are available to increase their molecular weight and prolong their half-life in serum: (a) multimerization of Ab fragments with peptide linkers; and (b) attachment to other molecules such as human serum albumin, polyethylene glycol (PEG), carbohydrates, N-(2-hydroxypropyl) methacrylamide (HPMA), and dextran. Multimerization of Ab fragments, exemplified by multimeric scFvs, is the core strategy of non-IgG-like formats and will be discussed in detail below. Covalent linkage of scFv-based bsAbs to albumin presents prolonged recycling of bsAbs, which is evidenced by a bispecific scFv-albumin fusion protein MM-111 targeting human epidermal growth factor receptor-2 (HER2) and HER3. PEGylation, introduced 2 decades ago, is conjugating one or more highly flexible, hydrophilic PEG molecules in a random or site-directed manner to enhance the hydrodynamic property of bsAbs. Recombinant PEG mimetics, glycosylated HPMA copolymer and dextran have also been developed to extend Ab circulating time while balancing the need for high bioactivity and safety.17–19 Interestingly, although it is considered that increased size leads to lower tissue penetration, in a study analyzing the biodistribution of an anti-carcinoembryonic antigen (CEA) × CD3 single-chain diabody (scDb), two derivatives (namely, PEGylated scDb and scDb fused to an albumin-binding domain) showed elevated accumulation in CEA+ tumors, implying an overall application advantage.20
The developmental history of multiple platforms of bsAbs
The history of bsAbs can be traced back to 1961, when Nisonoff and Rivers first put forward the concept of producing multispecific Abs by mixing different Ab fragments.21 In the early phases when hybridoma technology and chemical recombination methods were established in 1975 and 1985, respectively,22,23 the production of bsAbs was dependent on somatic fusion of hybridomas to generate a quadroma or chemical heteroconjugation of F(ab′)2 molecules from two different mAbs.23 However, further development of bsAbs was hampered, as chemical conjugation may inactivate, unfold, or aggregate bsAbs. Meanwhile, the quadroma technique often misassembles the heavy and light chains, which consequently leads to low yields of the desired pair and unexpected immune responses like those seen in human anti-mouse Ab and/or anti-rat Ab responses. The correct pairing of heavy–light chains, in essence, often encompasses two aspects: (a) the correct pairing of two different heavy chains and (b) the light chain combining with the correct corresponding heavy chain.12 These issues imposed an urgent necessity for modified methodologies to produce correctly paired bsAbs. Initially, the mispairing problem of heavy/light chains was approached by creating a chimeric rat/mouse-derived quadroma in 1995, which guaranteed the correct pairing of cognate heavy chains.24 For instance, the clinically approved catumaxomab was made up of a murine IgG2a anti-CD3 hapten paired with a rat IgG2b anti-epithelial cell adhesion molecule (EpCAM) hapten.25 Soon after that in 1998, the application of identical light chains to pair both heavy chains also showed efficacy.26 However, the challenges of heavy chain mispairing and nonhuman-related immunogenicity still existed and limited broader clinical applications, calling for the need for better solutions.
The second wave of bsAb production appeared when rapidly developing genetic engineering technologies provided a promising alternative to obviate the aforementioned drawbacks. The production of these newly developed bsAbs mainly depended on recombinant DNA technology, enabling the generation of chimeric or humanized Abs while controlling bsAb attributes such as size, affinity, bispecificity, half-life, stability, solubility, and biodistribution to fulfill the varying demands of desired target products.12,14,15 As a result, more stable and homogeneous bsAb platforms with less immunogenicity were utilized to produce commercially viable biopharmaceuticals.27 In 1996, Ridgway et al. described the “knobs-into-holes” (KiH) methodology utilizing mutations in the CH3 domain to generate a humanized anti-CD3 × CD4-IgG hybrid. This method was realized by replacing a large amino acid with a small one in one heavy chain of the bsAb (the “hole”) and vice versa in another heavy chain of the bsAb (the “knob”), ultimately directing the formation toward being heterodimeric rather than homodimeric on the basis of the electrostatic steering theory. Phage display technology was exploited to select CH3 mutants that formed stable heterodimers.28 To minimize monomer or homodimer contaminants, some researchers further engineered the CH3 domain of heavy chains to add interchain disulfide bonds, further elevating heterodimerization and providing the possibility of purification. This technique was also harnessed to produce various types of heterodimer proteins by fusing peptides, protein ligands, or Ab fragments to two ends of the Fc chains (Fig. 1a).26,29–31 Later, the advent of a new technique referred to as CrossMab further decreased the unwanted side products of heavy/light-chain pairing. This was achieved through swapping heavy- and light-chain domains within one Fab of Abs, resulting in varying molecular structures of the interfaces between VH-VL and CH1-CL. In accordance with these mechanistic insights, a CrossMab bsAb against angiopoietin-2 and vascular endothelial growth factor A was generated with intact Fc and antigen-binding domains that presented higher stability and affinity than its parental Ab. The CrossMab format can be further divided into three subtypes based on the interchange of CH1 with CL (CrossMabCH1-CL), VH with VL (CrossMabVH-VL), or VL-CL with VH-CH1 (CrossMabFab). With no requirements for sequence optimization or additional linkers, CrossMab became an appealing method for the design of new bsAbs. In addition, it could be combined with KiH to ensure the correct pairing of heavy chains (Fig. 1b).32
Gradually, with the advancement of genetic engineering technology as well as the emergence of numerous methods, such as phage display, protein engineering, and transgenic mice, over 100 bsAb formats have been produced at present, providing versatility in clinical applications.33 These newly developed formats circumvent previous manufacturing problems such as instability, low yield rates, and immunogenicity, thus accelerating the leap from bench to bedside. As mentioned above, versatile bsAb formats can be briefly divided into two groups. On the one hand, in addition to quadromas, KiHs and CrossMab, IgG-like formats also encompass dual-variable-domain Ig (DVD-Ig), IgG-single-chain Fv (IgG-scFv), two-in-one or dual action Fab (DAF) Ab, and κλ-body formats, among others. DVD-Ig, a dual-specific, tetravalent IgG-like molecule, is a powerful method to combine two existing Abs via a short peptide linker. This is achieved when four variable domains from one Ab are connected to the N terminus of the heavy and light chains of the second Ab without substantial steric hindrance between the four antigen-binding domains. Consisting of two identical light-heavy-chain pairs, DVD-Ig avoids the possibility of undesired pairing of chains and largely simplifies manufacturing and purification procedures while retaining the optimized antigen-binding activity of the two parental mAbs (Fig. 1c).34 IgG-scFv is a novel format of bsAb generated by fusing a scFv to the C terminus of the light or heavy chain of an IgG. It is regarded as prospective and clinically feasible because it not only shows high expression levels, thermostability, and protease resistance but also retains a similar half-life and similar Fc-mediated functions to its parental IgG (Fig. 1e).35 With a similar appearance to IgG, the DAF Ab has the capacity to recognize two distinct antigens with high affinity. This is achieved by two steps: first, identify a template Ab with specificity to one of the targeted antigens; then, introduce mutation to antigen-binding sites and screen out a modified Ab with dual avidity. Although the DAF Ab cannot bind two distinct antigens concurrently, its dual specificity expands the effective repertoire of traditional Abs with well-established manufacturing procedures (Fig. 1f).36 The κλ-body is constructed by two identical heavy chains paired with different light chains, one κ and one λ. In this way, an unmodified human-derived bsAb is created with the advantages of a mature purifying process and industrial-scale manufacturing (Fig. 1g).37 On the other hand, non-IgG-like formats include scFv-based formats, nanobodies, the dock-and-lock (DNL) method, and other multivalent molecules.38 ScFv refers to the combination of two variable domains from light and heavy chains of the parental Ab, namely, VH and VL. They are connected by a short linker, usually a (G4S)3 sequence (VL1–VH1, 25 kDa) (Fig. 1d). With outstanding antigen specificity and tissue permeability, scFv-based bsAb formats are deemed prospective in further clinical applications.38 Generally, scFv-based formats contain scFvs, single-domain Abs (sdAbs), tandem scFv molecules (ta-scFvs), diabodies, tandem diabodies (TandAbs), and dual-affinity retargeting molecules (DARTs).39 Unlike scFvs, sdAbs only consists of a single variable domain from the heavy chains, such as a variable domain of the heavy chain or a nanobody (12–14 kDa), which is derived from naturally occurring camelid heavy chain Abs with high stability and solubility (Fig. 1h).40 Ta-scFvs comprise two scFvs connected by a peptide linker. Within this format, BiTE is the most typical and widely applied one.38 BiTE, devised to recognize CD3 and target cell-specific antigen simultaneously, brings tumor cells closer to T cells and breaks the restriction of traditional major histocompatibility complex (MHC)-dependent cross-presentation.41 Owing to these advantageous properties, the anti-CD19 × CD3 BiTE blinatumomab (Blincyto; Amgen, Inc.) received accelerated approval by the US Food and Drug Administration (FDA) in December 2014 and the European Medicines Agency (EMA) in December 2015 for treating Philadelphia chromosome-negative relapsed or refractory precursor B-cell acute lymphoblastic leukemia (B-ALLs) (R/R ALL) (Fig. 1i).42 A diabody is composed of two covalently linked polypeptide chains. One chain contains VH from the first Ab and VL from the second Ab, and another chain contains VH from the second Ab and VL from the first Ab (VL1–VH2/VL2–VH1) (Fig. 1j).39 However, since this structure leads to instability, other formats have been developed, although the basis of the diabody is still applied.39 To substantially improve stability, a tetravalent TandAb is constructed by linking two diabodies with peptide linkers.13 Compared with a diabody, a TandAb exhibits higher affinity with a lower dissociation rate from target cells and longer blood retention, thus showing good prospects for clinical application (Fig. 1k).43 Based on the concept of a diabody, a DART is designed to obtain a disulfide bond between two peptide chains. As anticipated, a DART better simulates the spatial position of a natural Ab while sustaining a relatively high degree of stability. In terms of functionality, it also displays a vigorous ability to redirect T cells to target cells and then mediate their lysis (Fig. 1l).44 To date, a wide spectrum of bsAb formats have been elegantly summarized in other comprehensive reviews.33,45
Clinical applications of bsAbs
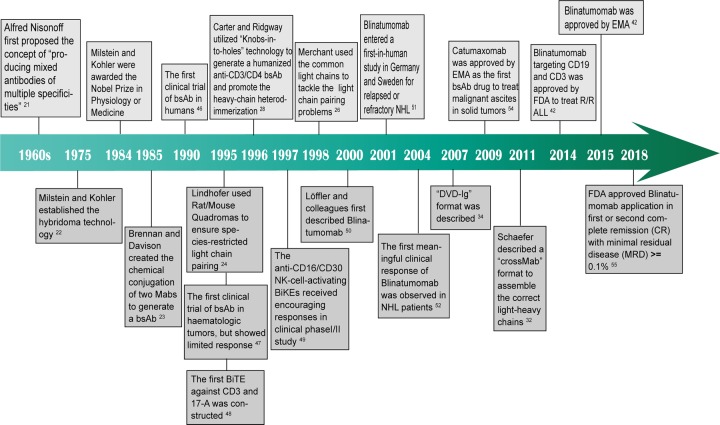
The first clinical trial of bsAbs was conducted in 1990 when Nitta et al. utilized the chemically conjugated anti-CD3 mAb OKT3 and the anti-glioma mAb NE150 to treat patients with malignant glioma.46 In 1995, bsAbs were first applied to hematological tumors through intravenous administration of anti-CD3 × CD19 bsAbs in patients with chemotherapy-resistant non-Hodgkin lymphoma (NHL). Disappointingly, although limited systemic toxicity was observed, this trial demonstrated no clinical response but an increase in tumor necrosis factor (TNF)-α and CD8+ T cells in the serum.47 In the same year, the first BiTE against CD3 and 17-1A, also the precursor of the anti-CD19 × CD3 BiTE blinatumomab, was constructed by linking two scFvs.48 In 1997, an NK-cell-activating bsAb directed at FcγR III (CD16) and the Hodgkin’s-associated antigen CD30 to address refractory Hodgkin’s disease showed encouraging antitumor activity in a clinical phase I/II study.49 Taking advantage of recombinant DNA technology, the first description of blinatumomab was published by Löffler et al., which circumvented the previous issues of low yield, undefined side products, and complicated purification procedures.50 One year later, blinatumomab entered a first-in-human study in Germany and Sweden, where 21 patients with relapsed or refractory NHL were given short-term intravenous infusions.51 It was not until 2004 that the first meaningful clinical response to blinatumomab was observed in NHL patients at a dose of 15 µg/m2 per day.52 Several years later, blinatumomab was used in extensive clinical trials until its final authentication by the FDA and EMA.51 Between 2007 and 2009, several phase I/II clinical trials of catumaxomab, the anti-EpCAM × CD3 trifunctional IgG-like bsAb generated from a rat/mouse quadroma cell, were concurrently carried out in ovarian tumors, gastrointestinal tumors, and other epithelial tumors.53 Finally, in 2009, catumaxomab (Removab®) became the first bsAb drug approved by the EMA for the treatment of malignant ascites in solid tumors.54 Later, in 2014, based on the relatively high complete remission (CR) rate of 30% and moderate side effects, the FDA granted approval of blinatumomab (Blincyto®) for the treatment of patients with R/R ALL.42 Blinatumomab (Blincyto®) also received approval from the EMA in December 2015.42 In the following years, the treatment spectrum of blinatumomab was further broadened, with FDA approval obtained in 2018 for its application in B-ALL at first or second CR with minimal residual disease ≥ 0.1%.55 Since then, the field of bsAbs has experienced explosive growth, yielding tremendous prospects for future clinical development (Fig. 2).
Fig. 2.
Timeline of historical bispecific antibody developments and clinical trials. This timeline demonstrates key points in the development of bispecific antibodies, particularly in the field of oncology. BsAb bispecific antibody, Mab monoclonal antibody, BiTE bispecific T-cell engager, NK cell natural killer cell, NHL non-Hodgkin lymphoma, DVD-Ig dual-variable-domain immunoglobulin, R/R ALL relapsed or refractory precursor B-cell acute lymphoblastic leukemia, EMA European Medicines Agency, FDA US Food and Drug Administration
The solution to antigen escape variants
To address two targets simultaneously while retaining their potency, the first step of generating bsAbs is to determine the appropriate target antigens. Akin to those of conventional Abs, targets of bsAbs should satisfy the following standards: (a) they are distinctively or at least predominantly expressed in target cells rather than adjacent normal cells to avoid nonspecific toxicity (also referred to as “on-target off-tumor” toxicity); and (b) they are intimately related to malignant phenotypes or signal pathways to prevent immune tolerance caused by antigen mutation.16 Currently, it is frustrating to recognize that only a small proportion of tumor-associated antigens (TAAs) have been recognized and strictly meet the above criteria,56 among which CD19 is the most representative. CD19, expressed on most B-ALLs, is indispensable for B-cell development and function, thus serving as a crucial target in CAR-T cell or bsAb-based immunotherapy.57 However, failures of CD19-specific CAR-T cells or anti-CD19 × CD3 BiTEs in B-ALL treatment have been reported to be attributed to the genetic mutation of CD19 resulting in loss of the extracellular domain,58 conformational change,59 impaired trafficking to the cell surface,60 or phenotype transformation from ALL to acute myeloid leukemia (AML).61 Therefore, it is speculated that if only one TAA is targeted on cancer cells, the genetic alteration of the chosen TAA may pose a threat to the efficacy of immunotherapy and culminate in drug resistance.56 This presents us with a formidable challenge.
Strategy 1: Development of multispecific Abs
To obviate single-target-related immune anergy, some researchers proposed the idea of a multitargeted Ab to concurrently recognize multiple antigens on the surface of target cancer cells with improved avidity. For instance, three members of the erythropoietin-producing hepatocellular (Eph) receptor family, EphA2, EphA4, and EphB4, participate in the progression and metastasis of numerous malignant tumors, all serving as appealing antitumor therapeutic targets. Based on that, a trispecific Ab was designed by joining an anti-EphB4/EphA4 diabody to the C terminus of an intact anti-EphA2 Ab, which largely showed improved agonistic activity over its parental Ab without effects on its pharmacokinetic properties.62 Another example of this strategy is the dual-targeting single-chain Fv triple body that simultaneously recognizes CD123 and CD33 on AML cancer cells. It displays evidently stronger antitumor activity than monotargeted agents.63 Moreover, tetraspecific Abs targeting endothelial growth factor receptor (EGFR), HER2, HER3, and VEGF also demonstrate more potent antitumor efficacy than the monotargeted versions both in vitro and in vivo and the ability to disrupt the drug resistance induced by their parental bsAbs.64
Strategy 2: Discovery of neoantigens
Due to the high mutational burden and apparent tropism of resistance to current immune treatment for oncology, it is challenging but also urgent to identify neoantigens and determine the related intrinsic mechanisms to pave the way for improved antitumor strategies.65 In brief, neoantigens, also referred to as tumor-specific antigens (TSAs), are derived from nonsynonymous somatic mutations with exclusive expression in tumor cells and complete absence in normal cells.66–68 Previously, the majority of immunotherapies targeted TAAs because they are commonly overexpressed in a group of tumor types and thus cover a broader patient population, such as CD19 and HER2.67 However, accumulating investigations of TAA-based therapy have reported potential collateral damage to normal tissues accompanied by unsatisfactory clinical efficacy. In contrast, TSAs are selectively expressed in tumor cells and differ among different individuals, thus opening up the opportunity to personalize TSA-dependent immunotherapy.68
Generally, the selection of Ab targets is roughly classified into three generations: (a) the first generation consists of “validated antigens,” which have been verified by extensive experiments and clinical trials; (b) the second generation encompasses modified peptides, which mean either different epitopes from “validated antigens” or identical epitopes with improved attributes; and (c) the third generation includes those newly discovered antigens screened out based on genomics, proteomics or cell-based functional strategies.65 Obviously, the identification of TSAs is closely related to the third generation of Ab targets. Technically, whole-exome sequencing in conjunction with rapidly developing software algorithms represents the most intriguing and promising method to identify neoantigens at present.67 The procedures of whole-exome sequencing, one of the most important genomic-related techniques, include acquiring DNA samples, breaking them into fragments, picking out the coding fraction, amplifying the fragments, sequencing the fragments, and eventually analyzing the results with a reference genome. Based on the DNA codon pairing principle, the mutant DNA sequences are then transformed into amino acid alternations in antigens.69 Later, a variety of algorithms can be applied to predict neoantigens with high binding affinity for MHC molecules. The average scores of multiple algorithms can be further acquired to increase accuracy.67,70,71 Cell-based approaches exploit the entire cancer cell as a platform to selectively generate corresponding mAbs. Those that are able to combine with cancer cell surface antigens at high bioactivity will be screened out. This is based on the theory that Ab targets display specific contexts only in intact and live cancer cells, including conformation, posttranslational modifications, and subcellular locations.72 Since neoantigens show considerable prospects for realizing individualized immunotherapy, further study is required, as reliable and cost-effective techniques are still lacking in predicting practical and clinically acceptable Ab targets.67
Combinational strategies to avoid T-cell anergy-mediated immunotherapy
In the process of host immune surveillance, T cells work as an essential sentinel after antigen-presenting cells present MHC-antigenic peptide complexes to T-cell receptors (TCRs), provide secondary costimulatory signals to activate naive T cells and promote the proliferation of effector T cells. In contrast, if costimulatory molecules are absent or substituted by coinhibitory molecules, namely, immune checkpoints, T cells are functionally inactivated or anergic, and they do not mediate tumor cell-specific eradication.73 Among a wide range of immune checkpoints, programmed death 1 (PD-1, also known as CD279) is one of the most significant and extensively investigated molecules at present. It is expressed on T cells in the TME and interacts with its ligand PD-L1 on tumor cells or tumor-infiltrating lymphatic cells to mediate cancer immunosuppression.74 Meanwhile, it has been observed that the insufficient clinical efficacy of bsAb-dependent treatment may be partially attributed to immunosuppression in the TME, especially regulation by the PD-1/PD-L1 pathway. For instance, a study of anti-AC133 × mCD3 bsAbs in the diabody format reported apoptosis of tumor-infiltrating lymphocytes (TILs) induced by hypofractionated radiotherapy and subsequent outgrowth of tumors, which was mediated by the PD-1 pathway.75 Thus, additional PD-1 blockade is considerably beneficial for increasing the number of TILs, regaining the efficacy of antitumor immunity and improving survival rates. Collectively, combinational strategies mainly include immune checkpoint blockades, CAR-T cells, and other strategies thus far.
Strategy 1: Simultaneous application of bsAbs and immune checkpoint inhibitory mAbs
The renaissance of immune checkpoint inhibitors has fueled the development of T-cell-orchestrated antitumor immune therapy, especially combinational applications to recruit and mobilize T cells against cancer.76 In one study, the application of an anti-CD33 × CD3 BiTE (AMG330) in mouse models led to PD-1 upregulation in tumor cells and displayed significantly impaired T-cell-mediated tumor cell lysis.77,78 By contrast, blocking the PD-1/PD-L1 interaction demonstrated resensitization to AMG330 and enhanced AMG330-mediated cell lysis.77 In another study, dual administration of anti-PD-1 and anti-PD-L1 mAbs prior to anti-CEA × CD3 BiTEs effectively disrupted immunosuppression in the TME and maximized the cytotoxicity of effector T cells.79 In clinical trials, combinational administration of bsAbs and immune checkpoint inhibitors also work synergistically to improve antitumor effects and patient survival. Moreover, in a previous study of drug-resistant HER2+ breast cancer treatment, antitumor benefits were observed when using HER2-TDB, a trastuzumab-based T-cell-recruiting bsAb, along with PD-L1 inhibitors. These benefits included augmented tumor growth inhibition and increased response durability.80 More recently, the construction of novel trivalent T-cell-redirecting bsAbs via the DNL method, namely, (E1)-3s and (14)-3s, which target Trop-2 and CEACAM5, respectively, has displayed enhanced antitumor potency both in vitro and in vivo when combined with PD-L1 blockade.81
Strategy 2: BsAbs that target immune checkpoints
Another attractive option to concurrently utilize immune checkpoint blockade and bsAb technology turns out to be designing bsAbs targeting both immune checkpoints and tumor antigens, which is strategically superior to the combination of immune checkpoint inhibitors and bsAbs.82 Mechanistically, this is achieved through engagement of the PD-1/PD-L1 blockade in TAA-positive cancer cells.82 For example, an anti-PD-L1 × EGFR bsAb was designed to activate the PD-L1 blockade in EGFR-overexpressing tumor cells. It possesses the architecture of the symmetric tetravalent taFv-Fc format with an Fc domain to mediate related functions. As evidenced in this study, anti-PD-L1 × EGFR bsAbs may enhance Ab accumulation at the tumor site and simultaneously prevent severe systemic autoimmune-related adverse events in several epithelial malignancies, such as colorectal cancer and non-small cell lung cancer.83 Similarly, some researchers generated two novel bsAbs: anti-PD-1 × c-Met DVD-Ig and IgG-scFv, both of which showed effective antitumor immune activity in vitro and in vivo.82 Likewise, another bsAb targeting PD-L1 and chondroitin sulfate proteoglycan 4 (CSPG4) also contributes to antitumor efficacy and selectivity while maintaining the safety of PD-1/PD-L1 checkpoint blockade when it is used to treat melanomas and other CSPG4+ malignancies.84 To be more direct and convenient, an anti-CD33 × CD3 BiTE was utilized as a scaffold, and an extracellular domain of PD-1 (PD-1ex) was fused to this scaffold to produce a trispecific checkpoint inhibitory T-cell-engaging Ab that combines T-cell recruitment to CD33+ AML cells with locally restricted blockade of the PD-1 pathway.85
Although the majority of present combinational studies on bsAbs and immune checkpoint blockade focus on the PD-1/PD-L1 pathway, other immune checkpoints, such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4, CD152) and T-cell Ig mucin 3 (TIM-3), also play indispensable roles in the immunosuppressive TME.76 To further reverse the peripheral tolerance and localize the immune checkpoint blockade to the tumor area, a new group of bsAbs is being creatively designed either to concurrently target two different immune checkpoints, such as the anti-PD-1/TIM-3 bsAbs in the CrossMab format,86 or to target a checkpoint inhibitor as well as a T-cell costimulatory receptor, exemplified by the anti-CTLA-4 × OX40 bsAbs in the IgG1 format (named ATOR 1015).87
Strategy 3: Combination with CAR-T cell therapy to provide costimulatory signals
In addition to the abovementioned immunosuppressive signals of T cells, sustained stimulation of TCR-CD3 signals in the absence of costimulatory signals via CD28 or 4-1BB (CD137) molecules serves as another pivotal explanation for bsAb-induced effector T-cell anergy or apoptosis.88 Initially, this problem was addressed by the administration of anti-4-1BB mAbs with an extra domain of 4-1BBL or anti-CD28 mAb as adjuvants to assist in bsAb therapy, which demonstrated prolonged activation of effector T cells.89 In addition, Fellermeier-Kopf et al. generated novel dual-acting, homotrimeric duokines by fusing two members of the TNF superfamily (including CD40L, CD27L, OX40L, and 4-1BBL) to a T-cell-retargeting diabody, ultimately enhancing T-cell stimulation and improving antitumor activity.90 However, these solutions demonstrated relatively short persistence of effector T cells. To tackle this issue, a group of researchers put forward a novel T-cell-retargeting complex to eradicate AML cells, which comprises an anti-CD3 × peptide epitope (E5B9) bsAb, namely, a universal effector module and a target module, which is composed of an anti-CD33 scFv fused with a peptide epitope (E5B9) and an extracellular domain of 4-1BBL. In comparison with conventional anti-CD33 × CD3 bsAbs, this complex shows higher efficacy not only in activating T cells and inducing CD33+ tumor cell lysis but also in potentiating the killing of CD33low cells through an additional costimulatory signal. More importantly, this novel, flexible modular system enables long-lasting and strengthened antitumor function with application potential in a wide range of tumors.91 In addition, to provide long-lasting effector T-cell function, an optimized strategy has been established by other researchers to combine the strategies of bsAbs with CAR-T cells, resulting in a novel group of bsAbs. This novel bsAb, designated frBsAb, is constructed by chemical heteroconjugation of two mAbs, fusing the folate receptor (FR) and a chosen TAA. Correspondingly, the transduced T cell, referred to as BsAb-IR-28z, is engineered as follows: its extracellular FR domain is joined to a CD8 alpha hinge and transmembrane region; and the intracellular CD3z moiety is fused with CD28. Therefore, when frBsAbs cross-link tumor cells (via recognition of the TAA) to transduced T cells, they not only induce transient T-cell activation but also prevent anergy or antigen-induced cell death through concurrent stimulation of the costimulatory molecule CD28, eventually exerting enhanced antitumor capacity.92 In addition to the provision of costimulatory molecules, the combination of CAR-T cells and bsAbs also offers a variety of advantages and is gaining momentum as a promising treatment modality for malignancies. Recently, Choi et al. designed a single-gene-modified T-cell product for the treatment of glioblastoma multiforme (GBM), namely, CAR-T-EGFRvIII. BiTE-EGFR cells which secrete additional anti-EGFR × CD3 BiTEs while maintaining the conventional CAR-T cell backbone with EGFRvIII expression on T-cell surfaces. As both EGFR and EGFRvIII are critical targets of GBM tumor cells, this innovative creation outperforms CAR-T cells and bsAbs as monotherapies because it not only corroborates efficacious antitumor responses in antigen-positive tumor subtypes with relative safety to healthy tissues but also obviates the drawbacks of their parental technologies, such as tumor antigen heterogeneity, systemic application-related nonspecific toxicity, necessity for continuous injection, appearance of immunosuppression, and T-cell exhaustion.93
In essence, to date, an innovative group of universal or modular CAR- (modCAR-) T cells along with their respective adapter molecules (CAR-adapters) have been utilized to circumvent several fatal side effects of conventional CAR-T cells. This strategy splits TAA recognition and T-cell activation into two parts and depends on CAR-adapters to act as a bridge, thus entailing the possibility of titratable regulation of effector T-cell function without the need to eliminate them, retargeting modCAR-T cells against multiple TAAs and using the same modCAR-T cells to target versatile tumor types.94 Currently, CAR-adapters can be divided into several subtypes based on their structure, including small molecules (such as folate and fluorescein isothiocyanate), monovalent and bivalent nanobodies, scFvs, Fabs, and Abs. A detailed description of modCAR-T cells and CAR-adapters has been summarized by Darowski et al. and Minutolo et al. in their reviews.94,95
Bispecific strategies and NK cells
To date, most attempts to enhance antitumor immunity have focused on boosting T-cell responses via CAR-T cells or bsAbs, especially after the success of blinatumomab and catumaxomab. However, it is noteworthy that both T-cell-retargeting therapies are limited by several toxic side effects, and the most challenging one turns out to be the deadly cytokine release syndrome (CRS). Although great efforts have been made to revert CRS by blocking IL-6 or inhibiting monocyte/macrophage activation, it remains a challenge to achieve optimal toxicity management while maintaining full therapeutic potential.96 Furthermore, as most T-cell-retargeting bsAbs are designed to fight against hematological malignancies, bsAb-mediated control of solid tumors demands further investigation. As the first line warriors of innate immunity against tumors, NK cells have also garnered abundant interest in the past several decades. In humans, various studies have shown that impaired or deficient NK-cell function is closely correlated with a higher risk of tumor occurrence, progression, and metastasis. Notably, a higher proportion of NK-cell infiltration in solid tumors is related to better clinical outcome, which has been established in colorectal carcinoma, breast cancer, clear cell renal cancer, head and neck cancer, and pharyngeal cancer.97 As a result, targeting NK cells by immunotherapy serves as an attractive antitumor strategy.
Mechanistically, activated NK cells can eliminate tumor cells through three direct or indirect strategies: (a) release granules, such as secretory lysosomes that contain perforin and granzymes to induce cell membrane lysis or apoptosis; (b) evoke target cell caspase activation via the interaction of TNF-related apoptosis-inducing ligand and Fas ligand on tumor cells; and (c) secrete versatile factors to modulate other immune cell functions to indirectly kill tumor cells.98 Based on these theories, NK-cell engagers (NKCEs) and CAR-NKs are designed to utilize NK-cell-mediated cytotoxicity to fight against tumors. NKCEs are constructed by joining scFvs against an NK-cell receptor (mainly CD16) and a TAA (bispecific killer engagers, BiKEs) or two TAAs (trispecific killer engagers, TriKEs). To date, anti-CD16 BiKEs have been effectively employed to target a wide spectrum of TAAs, including CD19, CD20, CD30, CD33, CD133, and EPCAM.49,99–102 Phase I/II clinical trials of an anti-CD30 × CD16A BiKE (AFM13) have been published on the Clinicaltrials.gov website for the treatment of relapsed or refractory CD30+ Hodgkin or NHL. Interestingly, in a study of myelodysplastic syndrome (MDS), anti-CD16 × CD33 BiKEs not only eradicated CD33+ MDS cells but also targeted CD33+ myeloid-derived suppressor cells to reverse immunosuppression in the TME and enhance antitumor efficacy.99 In contrast, with an additional scFv, TriKEs acquire increased therapeutic benefits by targeting more TAAs or coengaging more NK-cell-activating receptors. Recently, Gauthier et al. introduced a remarkable trifunctional NKCE that cotargets CD16 and NKp46 (a natural cytotoxicity receptor (NCR)) on NK cells as well as a TAA on tumor cells. This ingeniously designed TriKE leads to full activation of NK cells and strengthened cytotoxicity against tumor cells and demonstrates more potent antitumor function than BiKEs activating CD16 and NKp46 separately in mouse models of invasive and solid B-cell lymphoma.103 In addition, Vallera et al. added an IL-15 cross-linker to upgrade an anti-CD16 × CD33 BiKE to a TriKE with increased tumor cell killing efficacy due to self-sustained NK-cell proliferation.104 It is noteworthy that a single-center phase I/II clinical trial of an anti-CD16 × IL-15 × CD33 TriKE (GTB-3550) is already underway for the treatment of CD33+ high-risk MDS, refractory/relapsed AML, or advanced systemic mastocytosis. In addition to superior antitumor effects, BiKEs and TriKEs also demonstrate lower toxicity and higher safety than T-cell engagers, which means a lower risk of CRS or off-target cytotoxicity and broader application prospects in solid tumors.103 Akin to NKCEs, mature NK cells also serve as an intriguing candidate to express CARs against abundant TAAs such as CD19, CD20, CD244, and HER2. This has been comprehensively summarized in other reviews, so we will not discuss it here in detail.98,105,106
BSAB production in situ to circumvent the necessity for continuous injection
Despite the great success achieved by bsAbs, the therapeutic potential of exogenously administered bsAbs is hampered by the short circulation kinetics along with on-target off-tumor toxicities. Thus, researchers have proposed the concept of generating bsAbs in situ to overcome the immunosuppressive TME and avoid the continuous necessity for drug infusion. Until now, methods to produce bsAbs in patient tumor tissues mainly include engineered oncolytic viruses (OVs), transferred T cells, and transfected mesenchymal stem cells (MSCs) (Table 1).11
Table 1.
Current platforms to generate bsAbs in situ
| Type | BsAb vehicles | BsAb types | Specificities | Targeted cancers | References |
|---|---|---|---|---|---|
| Modified oncolytic viruses (OVs) | Double-deleted VVs (vvDD, western reserve strain) | BiTE | EphA2/CD3 | Lung cancer | 109 |
| EnAdenotucirev (EnAd) | BiTE | EpCAM/CD3 | Disseminated cancer | 112 | |
| Adenovirus ICOVIR-15K | BiTE | EGFR/CD3 | Colorectal and head and-neck squamous cell cancer | 110 | |
| Measles virus (MV) | BiTE | CEA or CD20/CD3 | Solid cancers (especially colon cancer spheroids) | 111 | |
| EnAdenotucirev (EnAd) | BiTE | FAP/CD3 | Disseminated caner, solid prostate cancer | 115, 116 | |
| Transduced autologous T cells (ENG T cells) | RD114-pseudotyped retroviral vector | BiTE | CD123/CD3 | Acute myeloid leukemia (AML) | 118 |
| RD114-pseudotyped retroviral vector | BiTE | EphA2/CD3 | Breast, lung, prostate cancer and glioma | 119 | |
| RD114-pseudotyped retroviral vector | BiTE | CD19/CD3 | Leukemia and lymphoma | 120 | |
| CD19 mRNA | BiTE | CD19/CD3 | Leukemia and lymphoma | 121 | |
| Modified mesenchymal stem cells (MSCs) | Lentiviral vector p6NST50 | BiTE | CD33/CD3 | Leukemia | 124 |
| Lentiviral vector | TandAb | CD19/CD3 | B-cell lymphoma | 125 |
Engineered OVs
In 2015, the FDA approved Imlygic, the genetically engineered oncolytic HSV therapy talimogene laherparepvec (T-VEC) with the expression of GM-CSF, for the treatment of advanced melanoma.107 This represented a milestone for OVs and led to numerous preclinical studies and clinical trials.108 With the advantages of tumor-specific expression and virus-mediated T-cell recruitment, OVs are an appealing platform for bsAb delivery. Currently, these OVs that are armed with BiTEs are undergoing preclinical evaluation: EphA2 T-cell engager-armed vaccine virus, which generates an anti-CD3 × EphA2 BiTE;109 an anti-EGFR × CD3 BiTE-armed adenovirus, ICOVIR-15K;110 an anti-CD3 × CEA/CD20 BiTE-armed measles virus (MV-BiTE);111 and the engineered oncolytic group B adenovirus EnAdenotucirev (EnAd) armed with an anti-EpCAM × CD3 BiTE.112 The first OV armed with a BiTE was a double-deleted vaccinia virus (western reverse strain) engineered to encode an EphA2-targeted BiTE, which increased T-cell activation and proliferation in vitro and improved survival in an A549 lung cancer xenograft model with additional exogenous IL-2.109 Later, researchers modified the adenovirus ICOVIR-15K to generate an anti-EGFR × CD3 BiTE, successfully achieving the promotion of T-cell proliferation without additional IL-2.110 Another similar construct, MV-BiTE, provides persistent immune protection for immunocompetent mice and solid tumor xenograft models and promotes long-term tumor regression without relapse.111 The anti-EpCAM × CD3 BiTE-armed EnAd further displays the ability to kill target cells while overcoming the immunosuppressive TME within primary human samples of malignant peritoneal ascites and pleural exudates.112 In general, engineered BiTE-producing OVs can attack and kill carcinoma cells through either nonspecific direct oncolysis or infecting antigen-positive cancer cells, followed by replication-dependent expression of BiTEs, activation of endogenous CD4+ and CD8+ T cells, and consequently immune-mediated cytotoxicity toward those cancer cells. This modified oncolytic virotherapy holds a bright future due to its several advantages over conventional OVs or bsAbs: (a) BiTEs derived from modified OVs selectively target antigen-positive cancer cells while staying independent of MHC/TCR molecule presentation or other costimulatory signals, thus increasing the potency of antitumor T-cell responses and evoking immunity even when cancer cells have lost MHC expression. (b) By restricting continuous BiTE production in virus-infected cancer cells under the control of late viral promoters, not only are systemic toxicities minimized while the concentration of BiTEs in the TME is largely elevated, but the problem of BiTEs' short half-life is also solved simultaneously. (c) OVs mainly infect and replicate in cancer cells and spread from cell to cell, alleviating the collateral damage of adjacent healthy tissues.113,114 (d) BiTEs can target both virus-infected cancer cells and noninfected antigen-positive cancer cells, indicative of bystander killing. (e) Modified OVs can stimulate both CD4+ and CD8+ T cells to mediate immunity while circumventing immunosuppression. (f) This approach has a relatively high level of safety, mild toxicity or side effects, and convenient application.108,109,112 Moreover, analogous to modified OVs targeting tumor antigens, OVs armed with BiTEs targeting stromal cell antigens yield an integrated and more efficacious therapeutic to intransigent, stromal-rich tumors. The engineered EnAds encoding BiTEs that target fibroblast activation protein (FAP) not only kill the tumor cells that they infect and replicate in but also utilize the subsequent production of anti-FAP BiTEs to activate T cells and selectively deplete FAP+ cancer-associated fibroblasts (CAFs). They ultimately reverse CAF-mediated immunosuppression, break stromal barriers for the penetration of OVs into tumor sites and synergistically repolarize the TME toward an immunoreactive state.115,116
Transferred autologous tumor-specific T cells
Currently, transferred autologous tumor-specific T cells also garner particular interest because they realize the theoretical combination of CAR-T cells with bsAbs and circumvent the limitations of both. Lymphocytes were first recognized as bsAb vehicles nearly a decade ago when primary human peripheral blood lymphocytes, especially CD3+ T cells, were transduced by engineered HIV-1-based lentiviral vectors to generate an anti-CEA × CD3 diabody in vivo.117 More recently, this can be exemplified by CD123-ENG T cells, which are transduced by a retroviral vector encoding a bispecific engager molecule that targets CD123+ tumor cells of AML and CD3+ T cells. Mechanistically, ENG T cells not only recognize and kill CD123+ tumor cells but also redirect and activate unmodified bystander T cells to tumor sites via the secretion of BiTEs in an antigen-dependent manner, synergistically leading to potent antitumor immune responses. Furthermore, to avoid undesired and excessive killing of normal hematopoietic stem and progenitor cells, CD20.CD123-ENG T cells are designed to include a suicide gene, CD20, which allows selective depletion in the presence of rituximab and complement.118 Other similar examples of ENG T cells include EphA2-ENG T cells119 and CD19-ENG T cells.120 In addition, some researchers constructed CD19-BiTE-transferred T cells through mRNA electroporation technology and the rapid T-cell expansion protocol, which also show enhanced antitumor immunity both in vitro and in aggressive Nalm6 leukemia mouse models compared with CAR-RNA-transferred T cells.121 The genetically transduced T cells demonstrate obvious superiority over CAR-T cells and bsAbs because once activated, enhanced BiTEs are produced to redirect resident T cells to tumor sites, resulting in continuous and self-amplifying antitumor T-cell responses.119 To further improve antitumor efficiency, costimulatory molecules CD28 and/or 4-1BBL are introduced to the surface of ENG T cells, namely, CD19-ENG.4-1BBL/CD80 T cells, which substantially increase the production of antigen-dependent cytokines (IL-2 and IFNγ) and boost T-cell expansion, ultimately demonstrating optimal responses in vitro and in vivo.122
Mesenchymal stem cells (MSCs)
In addition to cancer cells and T cells, MSCs also serve as an appealing cellular vehicle for constant bsAb production in situ. MSCs demonstrate the unique strengths of less immunogenicity, a tendency to migrate to tumor sites and an ability to track microscopic metastasis, as well as easy transduction by viral vectors.123 In one study, an immortalized human MSC line (SCP-1) was genetically modified via a lentiviral vector to produce fully humanized anti-CD33 × CD3 bsAbs and improve the expression of the costimulatory molecule 4-1BBL. The augmented antigen-specific T-cell responses establish MSCs as a potent conveyor of bsAbs to tumors, with consequently prolonged tumor regression.124 Another study using transduced human umbilical cord-derived MSCs to secrete anti-CD19 × CD3 TandAbs in combination with administration of an indoleamine 2,3-dioxygenase (IDO) pathway inhibitor, D-1MT, demonstrated that this combination was a promising therapeutic strategy.125 In addition, E1A-modified MSCs can also be designed as virus transporters and amplifiers to release engineered adenovirus and reinfect tumor cells after arriving at tumor sites or metastatic niches, where they secrete bsAbs and activate T-cell responses toward tumors. This is exemplified by one dual-virus-loaded MSC (named MSC.CD3-HAC.E1A) that carries a bifunctional fusion protein, CD3-HAC, composed of the anti-CD3 scFv and high-affinity consensus (HAC) PD-1 and ultimately promotes tumor elimination as well as reverses immune tolerance in the TME.126
Minicircle (MC)
MC is another promising alternative to produce bsAbs in vivo. It is a group of nonviral DNA vectors that expresses a high and sustained level of transgene products after being delivered to mouse liver via the hydrodynamic process.127,128 Some researchers took advantage of MC by designing MC bsAb to generate anti-CD20 × CD3 bsAbs, which mediate T-cell killing of human B-cell lymphoma cells both in vitro and in a xenograft mouse model. This method is quite appealing because it is relatively stable, inexpensive, and able to maintain a therapeutic concentration of bsAbs in blood circulation for weeks or even longer after delivery.129
Conclusion and future prospects
The past 3 decades have witnessed a dramatic shift from merely developing and modifying basic Abs with no additional engineering to more sophisticated forms of Ab derivatives in a broad variety of shapes and sizes, especially bsAbs.130 With extraordinary prospects in clinical applications, bsAb technology has attracted the attention of researchers and has extensively evolved into abundant formats, establishing a solid foundation for bsAb-based cancer immunotherapy. Up to September 20, 2019, a total of 183 clinical trials of bsAbs (the majority in the field of cancer) had been published on the official website of the United States National Library of Medicine. However, despite the wide range of aforementioned strategies, the process of commercially producing bsAb-dependent drugs is still hampered by various hurdles. To be more specific, the manufacturing of bsAbs is time consuming and costly. It demands appropriate, safe, and cost-effective cell line production, procedures, and analytical and purifying methods to acquire the desired products.131 In addition, a series of issues after Ab production, including but not limited to the degradation, aggregation, denaturation, fragmentation, and oxidation of Abs, must be solved before patients can enjoy the benefits of this strategy.131 In the meantime, more clinical trials are required to explore the best route and optimum dosage for administration that can lead to higher concentrations in target tissues, as well as decreased systemic side effects and even controlled release formulations.131
On the whole, it is estimated that the majority of bsAbs (67%) under clinical trials currently aim to fight against hematological malignancies.33 In comparison, bsAbs targeting solid tumors merit further investigation because of the inevitable adverse effects on normal tissues or other complicated factors, including an immunotolerant cancer stroma, disordered neovasculature, and inadequate penetration of bsAb drugs.33 Thus, there is real enthusiasm for the ongoing studies of bsAbs in solid tumors, which are supposed to yield promising results in the near future, although translating bsAbs into clinically applicable drugs may be time consuming and requires tremendous effort.
In conclusion, we have mainly focused on the current challenges and strategies of bsAbs, with a brief recapitulation of their varying formats and the history of their development. The results of bsAb studies demonstrate the promise of these molecules in novel drug designs and subsequent clinical applications for cancer treatment.
Competing interests
The authors declare no competing interests.
References
- 1.Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann. Oncol. 2015;26:1813–1823. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 2.Brekke OH, Sandlie I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat. Rev. Drug Discov. 2003;2:52–62. doi: 10.1038/nrd984. [DOI] [PubMed] [Google Scholar]
- 3.Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 4.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 5.Henricks LM, Schellens JH, Huitema AD, Beijnen JH. The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat. Rev. 2015;41:859–867. doi: 10.1016/j.ctrv.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Topp MS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017;376:836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu Y, Choi SH, Shah K. Multifunctional receptor-targeting antibodies for cancer therapy. Lancet Oncol. 2015;16:e543–e554. doi: 10.1016/S1470-2045(15)00039-X. [DOI] [PubMed] [Google Scholar]
- 9.Thakur A, Huang M, Lum LG. Bispecific antibody based therapeutics: strengths and challenges. Blood Rev. 2018;32:339–347. doi: 10.1016/j.blre.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy A, Jimeno A. Bispecific antibodies for cancer therapy: a review. Pharm. Ther. 2018;185:122–134. doi: 10.1016/j.pharmthera.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Viardot A, Bargou R. Bispecific antibodies in haematological malignancies. Cancer Treat. Rev. 2018;65:87–95. doi: 10.1016/j.ctrv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Tiller KE, Tessier PM. Advances in antibody design. Annu. Rev. Biomed. Eng. 2015;17:191–216. doi: 10.1146/annurev-bioeng-071114-040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019;201:103–119. doi: 10.1016/j.pharmthera.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov. Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Ayyar BV, Arora S, O'Kennedy R. Coming-of-age of antibodies in cancer therapeutics. Trends Pharm. Sci. 2016;37:1009–1028. doi: 10.1016/j.tips.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–38. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr. Opin. Biotechnol. 2011;22:868–876. doi: 10.1016/j.copbio.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Saxena A, Sidhu SS, Wu D. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front. Immunol. 2017;8:38. doi: 10.3389/fimmu.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrington-Symes AP, Farys M, Khalili H, Brocchini S. Antibody fragments: prolonging circulation half-life special issue-antibody research. Adv. Biosci. Biotechnol. 2013;4:689–698. [Google Scholar]
- 20.Stork R, Campigna E, Robert B, Muller D, Kontermann RE. Biodistribution of a bispecific single-chain diabody and its half-life extended derivatives. J. Biol. Chem. 2009;284:25612–25619. doi: 10.1074/jbc.M109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nisonoff A, Rivers MM. Recombination of a mixture of univalent antibody fragments of different specificity. Arch. Biochem. Biophys. 1961;93:460–462. doi: 10.1016/0003-9861(61)90296-x. [DOI] [PubMed] [Google Scholar]
- 22.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 23.Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science. 1985;229:81–83. doi: 10.1126/science.3925553. [DOI] [PubMed] [Google Scholar]
- 24.Lindhofer H, Mocikat R, Steipe B, Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J. Immunol. 1995;155:219–225. [PubMed] [Google Scholar]
- 25.Burges A, et al. Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: a phase I/II study. Clin. Cancer Res. 2007;13:3899–3905. doi: 10.1158/1078-0432.CCR-06-2769. [DOI] [PubMed] [Google Scholar]
- 26.Merchant AM, et al. An efficient route to human bispecific IgG. Nat. Biotechnol. 1998;16:677–681. doi: 10.1038/nbt0798-677. [DOI] [PubMed] [Google Scholar]
- 27.Garber K. Bispecific antibodies rise again. Nat. Rev. Drug Discov. 2014;13:799–801. doi: 10.1038/nrd4478. [DOI] [PubMed] [Google Scholar]
- 28.Pande J, Szewczyk MM, Grover AK. Phage display: concept, innovations, applications and future. Biotechnol. Adv. 2010;28:849–858. doi: 10.1016/j.biotechadv.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Ridgway JB, Presta LG, Carter P. ‘Knobs-into-holes' engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996;9:617–621. doi: 10.1093/protein/9.7.617. [DOI] [PubMed] [Google Scholar]
- 30.Gunasekaran K, et al. Enhancing antibody Fc heterodimer formation through electrostatic steering effects: applications to bispecific molecules and monovalent IgG. J. Biol. Chem. 2010;285:19637–19646. doi: 10.1074/jbc.M110.117382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atwell S, Ridgway JB, Wells JA, Carter P. Stable heterodimers from remodeling the domain interface of a homodimer using a phage display library. J. Mol. Biol. 1997;270:26–35. doi: 10.1006/jmbi.1997.1116. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer W, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl Acad. Sci. USA. 2011;108:11187–11192. doi: 10.1073/pnas.1019002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labrijn AF, Janmaat ML, Reichert JM, Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 2007;25:1290–1297. doi: 10.1038/nbt1345. [DOI] [PubMed] [Google Scholar]
- 35.Orcutt KD, et al. A modular IgG-scFv bispecific antibody topology. Protein Eng. Des. Sel. 2010;23:221–228. doi: 10.1093/protein/gzp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eigenbrot C, Fuh G. Two-in-one antibodies with dual action Fabs. Curr. Opin. Chem. Biol. 2013;17:400–405. doi: 10.1016/j.cbpa.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Fischer N, et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat. Commun. 2015;6:6113. doi: 10.1038/ncomms7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan G, Wang Z, Hao M, Li J. Bispecific antibodies and their applications. J. Hematol. Oncol. 2015;8:130. doi: 10.1186/s13045-015-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahamadi-Fesharaki R, et al. Single-chain variable fragment-based bispecific antibodies: hitting two targets with one sophisticated arrow. Mol. Ther. Oncolytics. 2019;14:38–56. doi: 10.1016/j.omto.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannas P, Hambach J, Koch-Nolte F. Nanobodies and nanobody-based human heavy chain antibodies as antitumor therapeutics. Front. Immunol. 2017;8:1603. doi: 10.3389/fimmu.2017.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuraszeck T, Kasichayanula S, Benjamin JE. Translation and clinical development of bispecific T-cell engaging antibodies for cancer treatment. Clin. Pharm. Ther. 2017;101:634–645. doi: 10.1002/cpt.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przepiorka D, et al. FDA approval: blinatumomab. Clin. Cancer Res. 2015;21:4035–4039. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 43.Sergey M, et al. Bispecific tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J. Mol. Biol. 1999;293:41–56. doi: 10.1006/jmbi.1999.3156. [DOI] [PubMed] [Google Scholar]
- 44.Moore PA, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011;117:4542–4551. doi: 10.1182/blood-2010-09-306449. [DOI] [PubMed] [Google Scholar]
- 45.Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017;9:182–212. doi: 10.1080/19420862.2016.1268307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nitta T, Stao K, Yagita H, Okumura K, Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet. 1990;335:368–376. doi: 10.1016/0140-6736(90)90205-j. [DOI] [PubMed] [Google Scholar]
- 47.Gast GCD, et al. CD8 T cell activation after intravenous administration of CD3×CD19 bispecific antibody in patients with non-Hodgkin lymphoma. Cancer Immunol. Immunother. 1995;40:390–396. doi: 10.1007/BF01525390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mack M, Riethmüller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Immunology. 1995;92:7021–7025. doi: 10.1073/pnas.92.15.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hartmann F, et al. Treatment of refractory Hodgkin's disease with an anti-CD16 CD30 bispecific antibody. Blood. 2019;89:2042–2047. [PubMed] [Google Scholar]
- 50.Löffler A, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 51.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharm. Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 52.Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–976. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 53.Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2:129–136. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heiss MM, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J. Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilal T, Prasad V. Eliminating MRD - FDA approval of blinatumomab for B-ALL in complete remission. Nat. Rev. Clin. Oncol. 2018;15:727–728. doi: 10.1038/s41571-018-0087-y. [DOI] [PubMed] [Google Scholar]
- 56.Velasquez MP, Bonifant CL, Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–38. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson HJ, Brentjens RJ. Overcoming antigen escape with CAR T-cell Therapy. Cancer Discov. 2015;5:1238–1240. doi: 10.1158/2159-8290.CD-15-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sotillo E, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–1295. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zaja F, et al. CD38, BCL-2, PD-1, and PD-1L expression in nodal peripheral T-cell lymphoma: Possible biomarkers for novel targeted therapies? Am. J. Hematol. 2017;92:E1–E2. doi: 10.1002/ajh.24571. [DOI] [PubMed] [Google Scholar]
- 60.Braig F, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–104. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 61.Gardner R, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–2410. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimasi N, et al. Development of a trispecific antibody designed to simultaneously and efficiently target three different antigens on tumor cells. Mol. Pharm. 2015;12:3490–3501. doi: 10.1021/acs.molpharmaceut.5b00268. [DOI] [PubMed] [Google Scholar]
- 63.Kugler M, et al. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br. J. Haematol. 2010;150:574–586. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 64.Hu S, et al. Four-in-one antibodies have superior cancer inhibitory activity against EGFR, HER2, HER3, and VEGF through disruption of HER/MET crosstalk. Cancer Res. 2015;75:159–170. doi: 10.1158/0008-5472.CAN-14-1670. [DOI] [PubMed] [Google Scholar]
- 65.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 66.Ton N, Schumacher RDS. Neoantigens in cancer immunotherapy. Science. 2015;348:69–73. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 67.Yarchoan M, Johnson BA, 3rd, Lutz ER, Laheru DA, Jaffee EM. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer. 2017;17:209–222. doi: 10.1038/nrc.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu XS, Mardis ER. Applications of immunogenomics to cancer. Cell. 2017;168:600–612. doi: 10.1016/j.cell.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng SB, et al. Targeted capture and massively parallel sequencing of 12 human exomes. Nature. 2009;461:272–276. doi: 10.1038/nature08250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fritsch EF, et al. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunol. Res. 2014;2:522–529. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 72.Loo DT, Mather JP. Antibody-based identification of cell surface antigens: targets for cancer therapy. Curr. Opin. Pharmacol. 2008;8:627–631. doi: 10.1016/j.coph.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 73.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konstantinidou M, Zarganes-Tzitzikas T, Magiera-Mularz K, Holak TA, Domling A. Immune checkpoint PD-1/PD-L1: is there life beyond antibodies? Angew. Chem. Int. Ed. Engl. 2018;57:4840–4848. doi: 10.1002/anie.201710407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hettich M, Lahoti J, Prasad S, Niedermann G. Checkpoint antibodies but not T cell-recruiting diabodies effectively synergize with TIL-inducing gamma-Irradiation. Cancer Res. 2016;76:4673–4683. doi: 10.1158/0008-5472.CAN-15-3451. [DOI] [PubMed] [Google Scholar]
- 76.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krupka C, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2015;30:484–491. doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 78.Schreiner J, et al. Expression of inhibitory receptors on intratumoral T cells modulates the activity of a T cell-bispecific antibody targeting folate receptor. Oncoimmunology. 2016;5:e1062969. doi: 10.1080/2162402X.2015.1062969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osada T, et al. CEA/CD3-bispecific T cell-engaging (BiTE) antibody-mediated T lymphocyte cytotoxicity maximized by inhibition of both PD1 and PD-L1. Cancer Immunol. Immunother. 2015;64:677–688. doi: 10.1007/s00262-015-1671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Junttila TT, et al. Antitumor efficacy of a bispecific antibody that targets HER2 and activates T cells. Cancer Res. 2014;74:5561–5571. doi: 10.1158/0008-5472.CAN-13-3622-T. [DOI] [PubMed] [Google Scholar]
- 81.Chang CH, et al. Combination therapy with bispecific antibodies and PD-1 blockade enhances the antitumor potency of T cells. Cancer Res. 2017;77:5384–5394. doi: 10.1158/0008-5472.CAN-16-3431. [DOI] [PubMed] [Google Scholar]
- 82.Hou W, et al. A novel tetravalent bispecific antibody targeting programmed death 1 and tyrosine-protein kinase Met for treatment of gastric cancer. Investig. New Drugs. 2019;37:876–889. doi: 10.1007/s10637-018-0689-3. [DOI] [PubMed] [Google Scholar]
- 83.Koopmans I, et al. A novel bispecific antibody for EGFR-directed blockade of the PD-1/PD-L1 immune checkpoint. Oncoimmunology. 2018;7:e1466016. doi: 10.1080/2162402X.2018.1466016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koopmans I, et al. Bispecific antibody approach for improved melanoma-selective PD-L1 immune checkpoint blockade. J. Investig. Dermatol. 2019;139:2343–2351.e3. doi: 10.1016/j.jid.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 85.Herrmann M, et al. Bifunctional PD-1 x alphaCD3 x alphaCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood. 2018;132:2484–2494. doi: 10.1182/blood-2018-05-849802. [DOI] [PubMed] [Google Scholar]
- 86.Herrera-Camacho I, et al. Cancer immunotherapy using anti-TIM3/PD-1 bispecific antibody: a patent evaluation of EP3356411A1. Expert Opin. Ther. Pat. 2019;29:587–593. doi: 10.1080/13543776.2019.1637422. [DOI] [PubMed] [Google Scholar]
- 87.Kvarnhammar AM, et al. The CTLA-4 x OX40 bispecific antibody ATOR-1015 induces anti-tumor effects through tumor-directed immune activation. J. Immunother. Cancer. 2019;7:103. doi: 10.1186/s40425-019-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Appleman LJ, Boussiotis VA. T cell anergy and costimulation. Immunological Rev. 2003;192:161–180. doi: 10.1034/j.1600-065x.2003.00009.x. [DOI] [PubMed] [Google Scholar]
- 89.Guo H, et al. Extracellular domain of 4-1BBL enhanced the antitumoral efficacy of peripheral blood lymphocytes mediated by anti-CD3 x anti-Pgp bispecific diabody against human multidrug-resistant leukemia. Cell Immunol. 2008;251:102–108. doi: 10.1016/j.cellimm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Fellermeier-Kopf S, et al. Duokines: a novel class of dual-acting co-stimulatory molecules acting in cis or trans. Oncoimmunology. 2018;7:e1471442. doi: 10.1080/2162402X.2018.1471442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arndt C, et al. Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: description of a novel modular targeting system. Leukemia. 2014;28:59–69. doi: 10.1038/leu.2013.243. [DOI] [PubMed] [Google Scholar]
- 92.Urbanska K, et al. Targeted cancer immunotherapy via combination of designer bispecific antibody and novel gene-engineered T cells. J. Transl. Med. 2014;12:347. doi: 10.1186/s12967-014-0347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi BD, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 94.Darowski D, Kobold S, Jost C, Klein C. Combining the best of two worlds: highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. MAbs. 2019;11:621–631. doi: 10.1080/19420862.2019.1596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minutolo NG, Hollander EE, Powell DJ., Jr. The emergence of universal immune receptor T cell therapy for cancer. Front. Oncol. 2019;9:176. doi: 10.3389/fonc.2019.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, et al. CD3 bispecific antibody–induced cytokine release is dispensable for cytotoxic T cell activity. Sci. Transl. Med. 2019;11:1–12. doi: 10.1126/scitranslmed.aax8861. [DOI] [PubMed] [Google Scholar]
- 97.Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol. Immunol. 2019;16:415–422. doi: 10.1038/s41423-019-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin. Immunol. 2017;31:37–54. doi: 10.1016/j.smim.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 99.Gleason MK, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123:3016–3026. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmohl JU, Gleason MK, Dougherty PR, Miller JS, Vallera DA. Heterodimeric bispecific single chain variable fragments (scFv) killer engagers (BiKEs) enhance NK-cell activity against CD133+ colorectal cancer cells. Target Oncol. 2016;11:353–361. doi: 10.1007/s11523-015-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vallera DA, et al. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biotherapy Radiopharmaceuticals. 2013;28:274–282. doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gleason MK, et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Therapeutics. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gauthier L, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–1713 e16. doi: 10.1016/j.cell.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 104.Vallera DA, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res. 2016;22:3440–3450. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hodgins JJ, Khan ST, Park MM, Auer RC, Ardolino M. Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Investig. 2019;129:3499–3510. doi: 10.1172/JCI129338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front. Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grigg C, et al. Talimogene laherparepvec (T-Vec) for the treatment of melanoma and other cancers. Semin. Oncol. 2016;43:638–646. doi: 10.1053/j.seminoncol.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2017;3:841–849. doi: 10.1001/jamaoncol.2016.2064. [DOI] [PubMed] [Google Scholar]
- 109.Yu F, et al. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol. Ther. 2014;22:102–111. doi: 10.1038/mt.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fajardo CA, et al. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res. 2017;77:2052–2063. doi: 10.1158/0008-5472.CAN-16-1708. [DOI] [PubMed] [Google Scholar]
- 111.Speck T, et al. Targeted BiTE expression by an oncolytic vector augments therapeutic efficacy against solid tumors. Clin. Cancer Res. 2018;24:2128–2137. doi: 10.1158/1078-0432.CCR-17-2651. [DOI] [PubMed] [Google Scholar]
- 112.Freedman JD, et al. Oncolytic adenovirus expressing bispecific antibody targets T-cell cytotoxicity in cancer biopsies. EMBO Mol. Med. 2017;9:1067–1087. doi: 10.15252/emmm.201707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dyer A, et al. Oncolytic group B adenovirus enadenotucirev mediates non-apoptotic cell death with membrane disruption and release of inflammatory mediators. Mol. Ther. Oncolytics. 2017;4:18–30. doi: 10.1016/j.omto.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Illingworth S, et al. Preclinical safety studies of enadenotucirev, a chimeric group B human-specific oncolytic adenovirus. Mol. Ther. Oncolytics. 2017;5:62–74. doi: 10.1016/j.omto.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Sostoa J, et al. Targeting the tumor stroma with an oncolytic adenovirus secreting a fibroblast activation protein-targeted bispecific T-cell engager. J. Immunother. Cancer. 2019;7:19. doi: 10.1186/s40425-019-0505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Freedman JD, et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852–6865. doi: 10.1158/0008-5472.CAN-18-1750. [DOI] [PubMed] [Google Scholar]
- 117.Compte M, et al. Inhibition of tumor growth in vivo by in situ secretion of bispecific anti-CEA x anti-CD3 diabodies from lentivirally transduced human lymphocytes. Cancer Gene Ther. 2007;14:380–388. doi: 10.1038/sj.cgt.7701021. [DOI] [PubMed] [Google Scholar]
- 118.Bonifant CL, et al. CD123-engager T cells as a novel immunotherapeutic for acute myeloid leukemia. Mol. Ther. 2016;24:1615–1626. doi: 10.1038/mt.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iwahori K, et al. Engager T cells: a new class of antigen-specific T cells that redirect bystander T cells. Mol. Ther. 2015;23:171–178. doi: 10.1038/mt.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Velasquez MP, et al. T cells expressing CD19-specific Engager Molecules for the Immunotherapy of CD19-positive Malignancies. Sci. Rep. 2016;6:27130. doi: 10.1038/srep27130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X, et al. Improved anti-leukemia activities of adoptively transferred T cells expressing bispecific T-cell engager in mice. Blood Cancer J. 2016;6:e430. doi: 10.1038/bcj.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Velasquez MP, et al. CD28 and 41BB costimulation enhances the effector function of CD19-specific engager T cells. cancer. Immunol. Res. 2017;5:860–870. doi: 10.1158/2326-6066.CIR-17-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J. Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 124.Aliperta R, et al. Bispecific antibody releasing-mesenchymal stromal cell machinery for retargeting T cells towards acute myeloid leukemia blasts. Blood Cancer J. 2015;5:e348. doi: 10.1038/bcj.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang X, et al. Mesenchymal stromal cells as vehicles of tetravalent bispecific Tandab (CD3/CD19) for the treatment of B cell lymphoma combined with IDO pathway inhibitor D-1-methyl-tryptophan. J. Hematol. Oncol. 2017;10:56. doi: 10.1186/s13045-017-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang Y, et al. Bispecific CD3-HAC carried by E1A-engineered mesenchymal stromal cells against metastatic breast cancer by blocking PD-L1 and activating T cells. J. Hematol. Oncol. 2019;12:46. doi: 10.1186/s13045-019-0723-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen Z-Y, He C-Y, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol. Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 128.Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat. Biotechnol. 2010;28:1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pang X, et al. Treatment of human B-cell lymphomas using minicircle DNA vector expressing Anti-CD3/CD20 in a mouse model. Hum. Gene Ther. 2017;28:216–225. doi: 10.1089/hum.2016.122. [DOI] [PubMed] [Google Scholar]
- 130.Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the ‘high-hanging fruit'. Nat. Rev. Drug Discov. 2018;17:197–223. doi: 10.1038/nrd.2017.227. [DOI] [PubMed] [Google Scholar]
- 131.Elgundi Z, Reslan M, Cruz E, Sifniotis V, Kayser V. The state-of-play and future of antibody therapeutics. Adv. Drug Deliv. Rev. 2017;122:2–19. doi: 10.1016/j.addr.2016.11.004. [DOI] [PubMed] [Google Scholar]