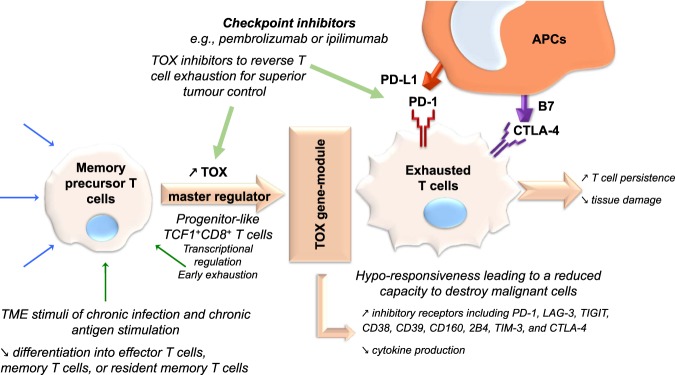
Checkpoint blockade therapy and adoptive therapy with engineered human immune effector cells are paradigm-changing innovations that have already revolutionized the treatment of cancer for which critical advances have been achieved with CTLA-4 or PD-1/PD-L1 inhibitors and with chimeric antigen receptor T (CAR-T) cells, respectively. These paradigm-changing innovations reflect the intricate relationships between inflammatory and immunological events and the pathobiology of cancer. Cancer is, for example, described as “wounds that do not heal”, and deeper exploration of the correspondence between the molecular events that occur in cancer and those that occur in chronic infection constitutes a rich ground for fundamental discoveries. Furthermore, it is the intense collective research effort directed at HTLV and HIV following the onset of the AIDS epidemic that catalyzed the convergence of innovations in the fields of adoptive cell therapy, gene therapy, and immunotherapy to validate the concept of engineered T cells for treating cancers.1,2 Since these early days, remarkable advances have been achieved with CAR-T cells in liquid cancers, particularly in acute lymphoblastic leukemia.3 Although the achievement of durable remission in a significant number of patients was indeed breath-taking, the goal of curing or making cancer a chronic and manageable disease in most cases now appears to be possible—however, this goal remains elusive to date. A first line of research is the exploration of T-cell subsets.2,4 In chronic infection and in cancer, transcription factor T-cell factor 1 (TCF1), coded for by the gene Tcf7, was demonstrated to be required for both long-term CD8+ T-cell-mediated immunity and checkpoint blockade response, as observed in Tcf1+PD-1+CD8+ T cells, which promote tumor control in response to checkpoint blockade or vaccination.5 Further increases in partial and complete remission rates in solid tumor cancers is likely to require the harnessing of multiple mechanisms of action within paradigm-changing treatment algorithms to offensively destroy the bulk of malignant tumors on the one hand and defensively destroy treatment-resistant cells or prevent their emergence on the other hand while ensuring treatment tolerability. It is probable that such transformation in the treatment of solid tumors can be achieved only by implementing well-designed combination therapies that target several underlying mechanisms of the neoplastic process that are collectively encompassed by the concept of “hallmarks of cancer”.6 Here, the ability to modulate the typically hypoxic and immunosuppressive tumor microenvironment by pharmacological intervention is a critical factor of long-term treatment success (Fig. 1).
Fig. 1.
Balancing tissue integrity and T-cell action in chronic inflammation: the physiological role of TOX as a master regulator of T-cell exhaustion. By inducing T-cell exhaustion in conditions of chronic inflammation, TOX limits tissue damage that would result from a persistent acute inflammation process and prevents activation-induced cell death, thereby permitting extended persistence of CD8+ T cells. This extended persistence enables the maintenance of some level of immune control during chronic infection or cancer, akin to a “physiological plan B”, when the acute phase fails to clear the infectious agent. TOX is an exciting new target in immuno-oncology for combination therapy in solid tumors
The cellular immune response to cancer is the result of a complex and partially redundant network of immunoregulatory interactions implemented by three main cell types: antigen-presenting cells (APCs), T cells, and tumor cells. The control of cell fates—notably, those of T cells—exerted by transcriptional regulators in response to extracellular signal sensing and transduction is critical to the adaptive immune system. The release of prosurvival and proinflammatory cytokines such as IL-2 and IFN-γ promotes a cytotoxic CD8+ T-cell response in which debris released from apoptotic tumor cells is subsequently taken up by APCs and presented in a cycle of immunogenic cell death, a process to which the efficacy of conventional chemotherapies can be at least partially ascribed.7 The mechanism of action of checkpoint inhibitors is to counteract the upregulation of immunoinhibitory molecules (e.g., CTLA-4 or PD-1) or their ligands (e.g., B7-1 and B7-2 or PD-L1 and PD-L2, respectively), which is adaptively promoted by tumor cells after prolonged immune activation, ultimately driving escape from immune attack. Although the network of cytokines in clinical cancer has been described in some detail, notably highlighting the critical roles of the immunostimulatory molecules IL-2, IL-12, IL-15, IL-21, and GM-CSF as well as IFN-α, and those of the immunosuppressive cytokines TNF-α, TNF-β, CSF-1, CXCL8/IL-8, VEGF, and CCR2/CCR5,8 much is unknown.
Another prism of analysis in cancer relates to the prominent intratumor dynamics that characterize neoplastic lesions, perhaps best exemplified by the tremendous diversity observed in advanced melanoma. From the initiation of the neoplastic process to the development of metastases, cancer can be considered through the lens of both population dynamics and single-cell oncogenic mutations. Global transcriptomic and proteomic analyses have enabled the discovery of main nodes in the chronic inflammation/cancer response network. Moreover, these analyses have been useful in highlighting a potential critical role of the transcriptional regulator TOX (thymocyte selection associated high-mobility group box) in CD8+ T-cell differentiation and dysfunction.9 However, what has remained insufficiently clarified is the identity of the precise mechanisms that underlie T-cell differentiation and divergence, including the emergence of resident memory T cells,10 during active and chronic infection, notably characterized by a change in the microenvironment to which T cells need to adapt. During acute infection, naive CD8+ T cells proliferate, expand clonally, and differentiate into effector CD8+ T cells, which control infection via direct killing.11 During chronic infection and in cancer, T-cell populations typically become exhausted due to chronic antigen stimulation; that is, their effector functions become attenuated, with decreased cytokine production, whereas their inhibitory receptors, including PD-1, LAG-3, TIGIT, CD38, CD39, CD160, 2B4, TIM-3, and CTLA-4, are upregulated.12 Terminally exhausted T cells thus exhibit a reduced capacity to destroy malignant cells, a phenomenon to which immune dysfunction in cancer can be largely ascribed. Exhausted T-cell populations during chronic infection have been observed to be heterogeneous and hyporesponsive. A plausible explanation, corroborated by recent data, for the selection of this mechanism by the evolutionary process is the prevention of tissue damage owing to overstimulation by cognate antigens, via an appropriate negative feedback loop, during chronic infections, while maintaining a good potential to control infectious agents that have successfully survived the acute phase.12 Identifying rare T-cell subtypes that emerge during chronic infection or cancer and understanding the heterogeneity of exhausted CD8+ T cells is thus expected to yield critically important new knowledge that will offer novel pharmacological intervention avenues, but these discoveries require a different methodology.
Yao et al. have thus deployed the power of single-cell RNA sequencing (scRNA-seq) to unlock the key to the heterogeneity of T cells in chronic infection. To this end, this group has studied virus-specific CD8+ T cells during acute and chronic LCMV infection in a mouse model. This targeted approach enabled the discovery that TOX is a critical regulator of CD8+ T-cell persistence in chronic infection, thus facilitating long-term antiviral CD8+ immunity during chronic infection.13 Notably, this group demonstrated by t-SNE analysis that the transcriptional programs between T cells that respond to acute or chronic infection diverge before the peak of initial expansion and that memory precursor cell fate is ultimately established in a subset of antiviral CD8+ T cells. Even more notably, the outcomes of this study revealed that the genes Tox and Tcf7 are co-expressed in progenitor-like CD8+ T cells but not in memory precursor cells, therefore indicating that these two genes are involved in two separate genetic regulatory circuits. Discovered in 2002 and observed to impact the differentiation program in developing T cells, Tox codes for a transcription factor in the high-mobility group box superfamily that has been shown to regulate the development of CD4+ T cells and innate lymphocytes; this gene is notably aberrantly expressed in a variety of cancers as well as in infectious diseases such as AIDS and pulmonary tuberculosis.14,15 By co-expression network analyses of the scRNA-seq data, Yao et al. identified the module of potential direct targets of the TOX protein; flow cytometric analyses further confirmed the observed differential expression of TOX. Taken together, these results suggest that the high expression of Tox and its associated gene targets in CD8+ T cells responding to chronic viral infection is a characteristic differentiating these T cells from memory precursor T cells, which also differ in their epigenome, as demonstrated by ChIP-seq. Considering this underlying observation, Yao et al. overexpressed TOX to demonstrate that ectopic expression of TOX is sufficient to confer persistence on terminally exhausted T cells during chronic infection but reduces PI3K/Akt/mTOR signaling and cytokine production. TOX-overexpressing cells showed a moderate increase in the levels of PD-1 and immunoreceptors, as well as reduced production of IFN-γ, TNF, and IL-2, but their in vitro cytotoxicity appeared essentially unaffected compared to that of TOX-nonoverexpressing control cells. Moreover, gene set enrichment analysis confirmed that the Tox gene module comprises overexpressed genes (in conditions of TOX overexpression) in the hypoxia pathway and downregulated genes in the oxidative phosphorylation pathway, PI3K/Akt/mTOR signaling, IFN-α response, and DNA repair. Similarly, Tox deficiency was observed to lead to a significant reduction in the frequency of TCF1hiTIM-3lo virus-specific CD8+ T cells without impacting the magnitude of their initial expansion and thus to a defect in long-term persistence.
The findings presented by Yao et al. are corroborated by an array of parallel reports that demonstrate the central role played by TOX in exhausted T cells.9,16–22 The significance of this work lies chiefly in the finding that TOX plays a master regulatory role in enabling the persistence of T cells in chronic infection and is a probable actor in mediating the persistence of therapeutic T cells used as living drugs in cancer; indeed, the persistence of these cells has been clearly linked to their efficacy (Fig. 1). The further understanding of the mechanism of action of TOX and of its network of dependent proteins might thus shed new light on immunotherapy, a critical step towards the development of safer and more efficacious T-cell therapies or combination therapies for solid cancers. For example, the adoptive transfer of LCMV-specific TOX-deficient T cells was reported by Alfei et al.16 to lead to an improved reduction in viral load at the early stages of infection but at the cost of worsened immunopathology. Similarly, CAR-T cells devoid of TOX and TOX2 exhibit increased antitumor effects,20 an observation strengthened by the reports by Khan et al.17 and Wang et al.22, who concluded that these T cells exert superior effects on tumor control. Again, the physiological significance of this finding is that by inducing T-cell exhaustion in conditions of chronic inflammation, TOX limits tissue damage resulting from the acute inflammation process and prevents activation-induced cell death, thus permitting the extended persistence of CD8+ T cells. This fundamental knowledge can now be deployed via conventional pharmaceutical intervention to better offensively destroy the bulk of malignant tumors and defensively destroy treatment-resistant cells while ensuring treatment tolerability. This knowledge can also be deployed in the engineering of universal CAR-T cells or the engineering of vector-mediated CAR-T cells in vivo. The lines of research suggested by this recent body of work on the biology of TOX notably include pharmaceutical intervention with the epigenome of T cells, with members of the TOX family or with the expression of their coding genes or associated transcription factors (such as NR4A, as reported by Seo et al.21). Exhausted T cells are the targets of checkpoint blockade therapies; thus downmodulating the synthesis of TOX proteins or reducing their activity might enhance—with good tolerability—the effects of immunotherapeutic interventions such as ipilimumab, nivolumab, pembrolizumab, durvalumab, atezolizumab, or avelumab. However, new evidence reported by Scott et al.19 suggests that the control of the genetic expression of inhibitory receptors is uncoupled from the observed loss of the effector function of exhausted T cells. TOX genes have been found to be aberrantly expressed in an array of cancers and infectious diseases, and their dysregulation was shown to be linked to downstream effects on the expression of the tumor suppressor gene RUNX3, which is involved in numerous cancers.15,23 Collectively, these discoveries open a new path to dramatically increase remission rates via immunotherapies. Furthermore, the importance of this work highlights that a deeper understanding of the plasticity and transcriptional control of T-cell fate in chronic infection, cancer, and immunosenescence might be the key to unlocking the full potential of immuno-oncology, a viewpoint reinforced by other recent discoveries exemplified by compositional changes in the γδ T-cell pool in aging mice that lead to unbalanced antitumor responses.24 The work by Yao et al. and others thus highlights not only that there are still central molecular mechanisms in immunology left to decipher and that TOX is one of the master regulators of T-cell programs, but also that single-cell analytic methodologies are essential tools for delineating the complex immunological molecular events that occur during both cancer and chronic infection, as well as in immuno-senescence.
References
- 1.Eshhar Z, Waks T, Gross G. The emergence of T-bodies/CAR T cells. Cancer J. 2014;20:123–126. doi: 10.1097/PPO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 2.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Sadelain M. Chimeric antigen receptor therapy. New Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thommen DS, Schumacher TN. T cell dysfunction in cancer. Cancer cell. 2018;33:547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiqui I, et al. Intratumoral Tcf1+PD-1+CD8+T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50:195–211. doi: 10.1016/j.immuni.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Cogdill AP, Andrews MC, Wargo JA. Hallmarks of response to immune checkpoint blockade. Br. J. cancer. 2017;117:1–7. doi: 10.1038/bjc.2017.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berraondo P, et al. Cytokines in clinical cancer immunotherapy. Br. J. cancer. 2019;120:6–15. doi: 10.1038/s41416-018-0328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott A. C., et al. The role of thymocyte selection-associated HMG box protein (TOX) in CD8 T cell differentiation and dysfunction. J. Immunol.200, 57.36 (2018)
- 10.Blanc C, et al. Targeting resident memory T cells for cancer immunotherapy. Front. Immunol. 2018;9:1722. doi: 10.3389/fimmu.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 12.Philip M, Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao C, et al. Single-cell RNA-seq reveals TOX as a key regulator of CD8+T cell persistence in chronic infection. Nat. Immunol. 2019;20:890–901. doi: 10.1038/s41590-019-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Flaherty E, Kaye J. TOX defines a conserved subfamily of HMG-box proteins. BMC Genomics. 2003;4:13. doi: 10.1186/1471-2164-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu X, Li Z. TOX gene: a novel target for human cancer gene therapy. Am. J. cancer Res. 2015;5:3516–3524. [PMC free article] [PubMed] [Google Scholar]
- 16.Alfei F, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–269. doi: 10.1038/s41586-019-1326-9. [DOI] [PubMed] [Google Scholar]
- 17.Khan O, et al. TOX transcriptionally and epigenetically programs CD8+T cell exhaustion. Nature. 2019;571:211–218. doi: 10.1038/s41586-019-1325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim K., et al. 2019. Single-cell transcriptome analysis revealed a role of the transcription factor TOX in promoting CD8+T-cell exhaustion in cancer. bioRxiv10.1101/641316.
- 19.Scott AC, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. 2019;571:270–274. doi: 10.1038/s41586-019-1324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo H, et al. Disruption of TOX transcription factors enhances CAR T cells function in solid tumors. J. Immunol. 2019;202:134.3. [Google Scholar]
- 21.Seo H, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+T cell exhaustion. Proc. Natl. Acad. Sci. USA. 2019;116:12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., et al. 2019. TOX promotes the exhaustion of antitumor CD8+T cells by preventing PD1 degradation in hepatocellular carcinoma. J. Hepatol. In the Press. [DOI] [PubMed]
- 23.Dulmage BO, Akilov O, Vu JR, Falo LD, Geskin LJ. Dysregulation of the TOX-RUNX3 pathway in cutaneous T-cell lymphoma. Oncotarget. 2019;10:3104. doi: 10.18632/oncotarget.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HC, et al. IL-7-dependent compositional changes within the γδ T cell pool in lymph nodes during ageing lead to an unbalanced anti-tumour response. EMBO Rep. 2019;20:e47379. doi: 10.15252/embr.201847379. [DOI] [PMC free article] [PubMed] [Google Scholar]