Abstract
Awarding CO2 offset credits may incentivize seagrass restoration projects and help reverse greenhouse gas (GHG) emissions from global seagrass loss. However, no study has quantified net GHG removal from the atmosphere from a seagrass restoration project, which would require coupled Corg stock and GHG flux enhancement measurements, or determined whether the creditable offset benefit can finance the restoration. We measured all of the necessary GHG accounting parameters in the 7-km2 Zostera marina (eelgrass) meadow in Virginia, U.S.A., part of the largest, most cost-effective meadow restoration to date, to provide the first seagrass offset finance test-of-concept. Restoring seagrass removed 9,600 tCO2 from the atmosphere over 15 years but also enhanced both CH4 and N2O production, releasing 950 tCO2e. Despite tripling the N2O flux to 0.06 g m−2 yr−1 and increasing CH4 8-fold to 0.8 g m−2 yr−1, the meadow now offsets 0.42 tCO2e ha−1 yr−1, which is roughly equivalent to the seagrass sequestration rate for GHG inventory accounting but lower than the rates for temperate and tropical forests. The financial benefit for this highly successful project, $87 K at $10 MtCO2e−1, defrays ~10% of the restoration cost. Managers should also consider seagrass co-benefits, which provide additional incentives for seagrass restoration.
Subject terms: Ecosystem ecology, Ecosystem services, Marine biology
Introduction
Seagrass meadows have been identified as important sinks in the global carbon cycle, because they are highly productive systems that bury organic carbon (Corg)1–4. Seagrass meadows potentially contain 4,200-8,400 Tg Corg in bed sediments and an additional 151 Tg Corg in above- and belowground biomass5—a significant global carbon stock threatened by accelerating seagrass habitat loss from coastal development, eutrophication, climate change, and other anthropogenic impacts6,7. Seagrass bed erosion following meadow collapse accelerates oxidation and remineralization of this sediment Corg8–10. Global meadow loss may, therefore, release 50–330 Tg CO2 yr−1 back to the atmosphere11. Seagrass restoration transfers Corg back to the sediment9,12,13. However, despite increasing interest in seagrass ‘blue carbon’ and studies reporting seagrass sediment Corg stocks5,13–15, including several from restored meadows13,16,17, a study has yet to quantify the net greenhouse gas (GHG) removal from the atmosphere resulting from a seagrass restoration project18. Tokoro et al.3 provide, perhaps, the closest approximation, a net GHG removal estimate for natural seagrass meadows based on carbon flux measurements and a one-time sediment Corg burial rate. However, identifying the creditable GHG offset benefit requires isolating seagrass-enhanced Corg sequestration over time18, accounting for sequestered Corg turnover19, and determining whether seagrass presence also increases GHG emissions of CH4, N2O, and CO2 evasion associated with CaCO3 buried in seagrass sediment, all of which would reduce the GHG benefit from seagrass-enhanced carbon sequestration20–22. Seagrass GHG flux measurements, coupled with repeated measurements of Corg stock enhancement over time to account for Corg turnover within sediment and biomass carbon pools (i.e., GHG ‘stock change’), would enable calculation of seagrass-enhanced sequestration; however, there are questions about the feasibility of applying a stock change approach in a seagrass system18.
Prospective seagrass restoration projects currently face uncertainty about the magnitude of the GHG offset benefit they can generate, and perhaps as a result, a seagrass project has not yet applied for voluntary carbon offset-credits to help finance additional seagrass restoration23. Seagrass projects have been eligible to receive offset-credits since 2015, when the Verified Carbon Standard Program (the VCS, now administered by Verra) published the first seagrass offset-credit accounting framework, VM0033: Methodology for Tidal Wetland and Seagrass Restoration24. The framework has been used by countries seeking to incorporate seagrass meadows into national GHG inventories but not by individual projects. Under this methodology, the certifiable GHG offset benefit only corresponds to the net CO2 (or CO2 equivalent GHG: CO2e) removal from the atmosphere that can be directly attributed to a restoration project in a recognized carbon pool (i.e., negative emissions over time), minus any GHG emission increases. It is important to emphasize that this enhanced sequestration equals the CO2 sequestered by the restoration project (i.e., the ‘with project’ scenario) minus the background sequestration that would occur if the project did not exist (i.e., in the status quo baseline: the ‘without project’ scenario)24,25. The former can be measured directly; the latter must be estimated by extrapolating pre-project conditions or by comparing project and control sites over time.
For seagrass restoration projects, the net GHG benefit equals CO2 sequestrated as enhanced sediment Corg (see Fig. 1: gross meadow sediment stock minus an equivalent area bare sediment stock) and the long-term average Corg sequestered in above- and belowground biomass within the project area, minus any enhanced GHG production24,25—specifically CH4, N2O, and CO2 evasion associated with CaCO3 buried in seagrass sediment20–22,26. Community respiration does not affect the GHG offset benefit for meadow restoration projects, because CO2 fixed through photosynthesis and then returned to the atmosphere through respiration is not a net flux of CO2 to the atmosphere. Enhanced respiration could, however, adversely affect a seagrass conservation project attempting to avoid the remineralization of sequestered Corg stocks. As noted above, the offset benefit from seagrass biomass sequestration over interannual timescales corresponds to the average, annual standing biomass stock, not peak biomass. This average reflects loss and turnover due to herbivory, senescence, export, and, in some cases, harvest or other disturbances. Some of the exported seagrass carbon may remain sequestered at deep ocean depositional sites27, and some is deposited along the coastline as wrack on beaches, marshes, and on tidal flats. The VCS and other offset crediting standards conservatively assume that exported biomass is decomposed and returns to the atmosphere as CO2.
Figure 1.
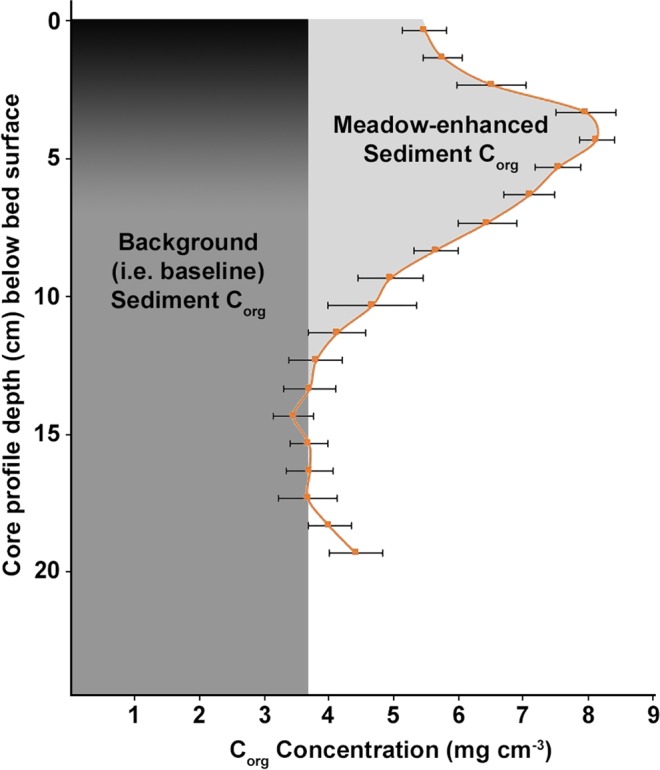
Seagrass meadow sediment Corg concentrations are typically highest below the surface in a region corresponding with the rhizosphere and approach the background concentration observed at unvegetated sites with increasing depth (data adapted from Greiner et al.12 and used with permission). The seagrass-enhanced sediment Corg stock (light gray) can be quantified by integrating the area under the profile and subtracting the background Corg stock that one would expect to find absent the meadow (dark gray); note that this approach does not require establishing a reference plane or quantifying bed accretion (black gradient) attributable to the meadow by sediment dating.
The offset-credit methodology recommends measuring the sediment Corg stock repeatedly over time to quantify sequestered Corg enhancement (i.e., stock change), rather than measuring the Corg stock to an arbitrary depth on a single occasion or estimating Corg accumulation from burial rates25. This is because seagrass sediment Corg stock estimates15,28,29 and burial rates2,30,31 likely overestimate net CO2 removal from the atmosphere due to uncertainties with dating techniques for sediment accretion over relatively short time scales (decades)18. These estimates also include allochthonous carbon (Corg fixed outside the project area) that is excluded from GHG offset accounting methodologies and background Corg that would be sequestered in the area in the baseline scenario (see Supplement)18,32. This study shows how repeated stock change measurements can provide a more reliable approach for assessing how meadow presence enhances sediment Corg accumulation and how remineralization, especially in the upper mixed layer of the sediment18,19,33, affects this Corg to determine sequestration for offset-credit accounting34.
Uncertainty about how seagrass restoration affects CH4 and N2O fluxes represents a data gap for prospective restoration projects. The VCS defines the de minimis threshold at <5% of the GHG benefit; fluxes of CH4 and N2O lower than this are discounted in offset accounting24. Given their higher global warming potentials relative to CO2, a marginal increase in either CH4 or N2O production could substantially reduce the net GHG benefit from meadow restoration35–37. Emissions of CH4 and N2O from seagrass systems were earlier assumed to be negligible38,39, because H2S produced by sulfate reduction oxidizes CH4 in marine sediments40,41 and seagrass nitrogen demand limits N2O efflux42. Oremland43 and Moriarty et al.44 reported very low seagrass methane fluxes, and studies have documented high sulfate reduction in seagrass beds44–46. However, several recent studies have determined that CH4 and N2O enhancement partially offsets the ‘blue carbon’ benefit in mangrove and marsh systems37,47–49. A recent review found that CH4 fluxes in seagrass systems varied considerably, from 1.25–401.50 μmol CH4 m−2 d−1, and were lower on average than mangrove and salt marsh habitats48 (Table 1). One study has suggested that seagrass sediments may limit N2O release (Table 2), but the only available N2O data from a seagrass system derives from sediment core incubations50.
Table 1.
Reported CH4 flux data for seagrass systems.
| Location | Seagrass | Method | CH4 Flux (µmol m−2 hr−1) | Notes | Reference |
|---|---|---|---|---|---|
| Florida Keys, FL, USA | Thalassia testudinum | Benthic chambers and incubations | 1.81–1.86 | 43 | |
| Bimini, Bahama Island | Syringodium sp. | Benthic chambers and incubations | 0.14–0.47 | 43 | |
| Moreton Bay, Australia | Zostera capricorni | In vitro incubations | 14.5 | Est. for top 20 cm of bed | 44 |
| Red Sea | Multispecies: Thalassodendron ciliatum, Cymodocea serrulata, Halodule uninervis, etc. | Core incubations | 0.004–23.6 | Salinity range = 37.98–42.29 | 49 |
| Ria Formosa Lagoon, Portugal | Zostera noltii | Benthic chambers | 4.4 | Aerial exposure at night | 82 |
| Ria Formosa Lagoon, Portugal | Zostera noltii | Benthic chambers | 6.9 | Aerial exposure during day | 82 |
| Ria Formosa Lagoon, Portugal | Zostera noltii | Benthic chambers | 9.0–30 | During tidal flooding | 82 |
| Ria Formosa Lagoon, Portugal | Zostera noltii | Benthic chambers | 4.4–71(mean = 12.8) | 82 | |
| Florida Bay, FL, USA | Thalassia testudinum | Benthic chambers and porewater samples | 0.567 | Dead seagrass areas in winter | 83 |
| Florida Bay, FL, USA | Thalassia testudinum | Benthic chambers and porewater samples | 14.21 | Live seagrass areas in fall | 83 |
| Cape Lookout Bight, NC, USA | Zostera marina and Halodule sp. | Core extraction, centrifuging, porewater sampling | 20–2000 | Seagrass not specifically studied but occurs in the general study area | 84 |
| Arcachon Bay, France | Zostera noltii | Benthic chambers | 1.6–32.7 | Sed-water flux with seasonal variation | 85 |
| Chilika Lagoon, India | Multispecies: Halodule spp., Halophila spp. | Open water and sediment samples | 4.17, 5.6 | Wet and dry season averages | 86 |
| Tomales Bay, CA, USA | (Zostera marina) | Benthic chambers | 2.08 | Summer eelgrass bed | 87 |
| Tomales Bay, CA, USA | (Zostera marina) | Benthic chambers | 0.896 | Winter eelgrass bed | 87 |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 13.110 ± 4.570 | Seagrass spring average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 3.136 ± 1.307 | Seagrass summer average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 0.845 ± 0.255 | Seagrass fall average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 5.697 | Seagrass 9-month average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 1.778 ± 0.930 | Bare spring average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 0.050 ± 0.021 | Bare summer average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 0.387 ± 0.104 | Bare fall average | This study |
| South Bay, VA, USA | Zostera marina | Benthic chambers | 0.739 | Bare 9-month average | This study |
Table 2.
Reported N2O flux data for seagrass systems.
| Location | Seagrass | Method | N2O Flux (µmol m−2 hr−1) | Notes | Reference |
|---|---|---|---|---|---|
| Nanwan Bay, Taiwan | Thalassia hemprichii, Halodule uninervis | Sediment incubations | 0.3–2.2* | 12-hr incubations | 42 |
| Lake Akkeshi, Japan | Zostera marina | Sediment incubations | (0.009–0.022 µmol L−1) | Concentrations following 7-day incubations | 50 |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.378 ± 0.184 | Seagrass spring average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.043 ± 0.013 | Seagrass summer average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.039 ± 0.007 | Seagrass fall average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.153 | Seagrass 9-month average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.120 ± 0.073 | Bare spring average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.003 ± 0.002 | Bare summer average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.046 ± 0.013 | Bare fall average | This study |
| South Bay, Virginia, USA | Zostera marina | Benthic chambers | 0.057 | Bare 9-month average | This study |
*µmol g wet wt−1 hr−1.
Without adequate data to quantify the net GHG benefit from seagrass restoration, the VCS allows projects to use the emission factor for seagrass established by the Intergovernmental Panel on Climate Change (IPCC) for national GHG inventory accounting, 0.43 t C ha−1 yr−1 51, even in areas where regional or local estimates for some parameters are available24,34. This default factor may over/underestimate the net GHG benefit. The number derives from only two studies of Posidonia oceanica, a seagrass species that generates unusually high sediment Corg stocks, and does not account for the baseline sediment Corg stock, allochthonous carbon, or the enhancement of GHG fluxes52,53.
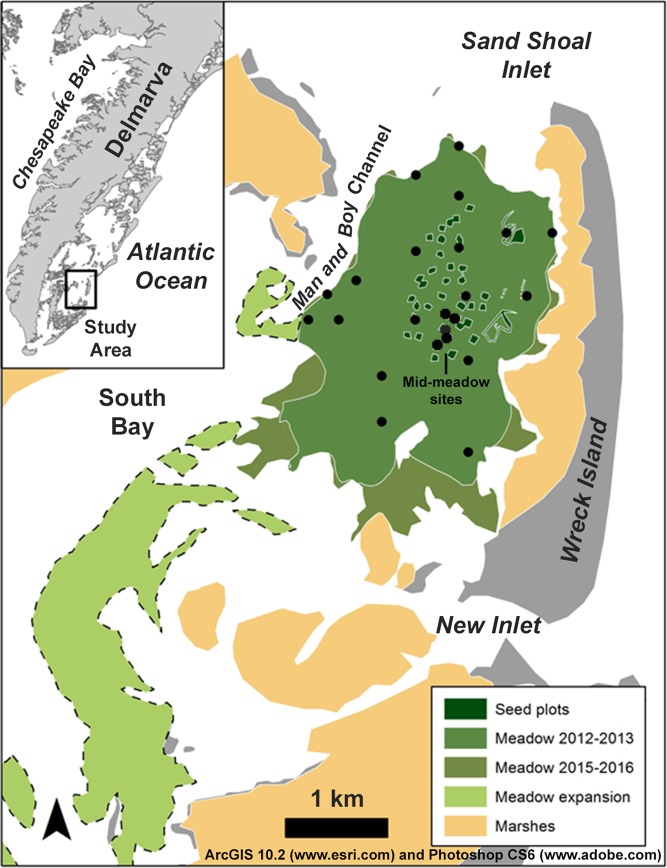
This study is the first study to calculate net GHG removal by a seagrass restoration project based on measured data for all of the parameters required by the VCS accounting framework23,24, making this the first verification that seagrass systems provide a creditable GHG offset benefit. We leveraged the long-term seagrass restoration and monitoring effort in the Virginia, U.S.A., coastal bays, which is acknowledged as the world’s largest successful seagrass restoration to date. Our study focused on the 7 km2 Zostera marina (eelgrass) meadow in South Bay (Fig. 2). We undertook this work to address two urgent GHG accounting questions: 1) does seagrass restoration increase GHG fluxes that adversely impact the net GHG benefit, and 2) is the IPCC seagrass restoration default factor conservative for GHG accounting51? No other study has attempted to apply these comprehensive GHG accounting methods to a seagrass system before. This study, therefore, establishes a benchmark for expectations about seagrass ‘blue carbon’ finance potential, because the South Bay meadow likely remains the least expensive meadow restoration on a cost per area basis17,54. It represents a best-case scenario for potentially financing restoration through offset-crediting.
Figure 2.
The South Bay, Virginia, study area, showing the locations of biomass and sediment Corg sample sites (black circles), original restoration seed plots (established in 2000–2001: Orth et al.70, the central meadow extent prior to sampling in 2013, and the expanded meadow extent prior to sampling in 2016. Meadow expansion areas to the west and south (light green areas enclosed by dashed lines) were excluded from the net GHG benefit calculations in this study. The figure was created in ArcGIS 10.2 (www.esri.com) and Photoshop CS6 (www.adobe.com).
Results
Enhanced carbon sequestration
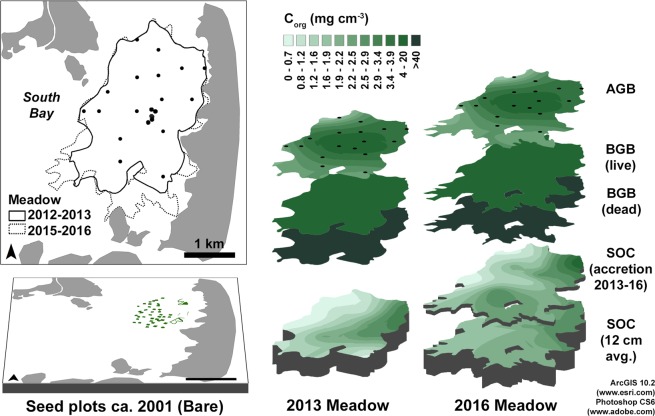
With repeated stock change measurements, we observed significant Corg stock enhancement at the meadow scale resulting from increasing Corg concentrations within the bed, seagrass-enhanced bed accretion, and meadow expansion. The meadow-wide, net sediment Corg sequestration attributable to the restoration increased from 1,130 t Corg in 2013 to 2,010 t in 2016 (Table 3; Fig. 3). Note that these values are stocks relative to a known baseline that represents the ‘without restoration project’ scenario, not rates, which can be obtained by dividing the stock by a time interval. Approximately 280 t of this 880 t Corg stock increase occurred in the top 2 cm of the bed, which was likely deposited between 2013 and 2016 (see Supplement discussion of accretion); the remaining 600 t accumulated within the bed between 2013 and 2016. The 2013 meadow stored an average of 196 g Corg m−2 and the 2016 meadow stored an average of 292 g Corg m−2. The 2013 enhanced stock took 12 years to accumulate. Between 2013 and 2016, the enhanced sediment Corg stock almost doubled, indicating that the sequestration rate also increased. Meadow Corg sequestration in sediments was 346 t CO2 yr−1 from 2001–2013 and 1070 t CO2 yr−1 from 2013–2016.
Table 3.
Sequestered CO2 stocks (negative values), cumulative GHG emissions, and the net GHG benefit from the South Bay meadow in 2013 and in 2016; all values are Mt CO2 equivalent units. Gross values = observed seagrass meadow-scale stocks; net values = seagrass meadow stock enhancement above the baseline (gross seagrass stocks – equivalent area bare stocks; aboveground biomass - AGB, belowground biomass - BGB); standard errors reflect error propagation.
| 2001 Start (Bare) | 2013 Gross | 2013 Net | 2016 Gross | 2016 Net | |
|---|---|---|---|---|---|
| Meadow area (km2) | 0.096 | 5.79 | 5.79 | 6.86 | 6.86 |
| AGB | 0 | −710 ± 14.8 | −710 ± 14.8 | −810 ± 17.6 | −810 ± 17.6 |
| Live BGB | 0 | −339 ± 30.0 | −339 ± 30.0 | −401 ± 33.2 | −401 ± 33.2 |
| Dead BGB | 0 | −857 ± 44.3 | −857 ± 44.3 | −1020 ± 52.5 | −1020 ± 52.5 |
| Sediment Corg | −78 ± 6.29a | −13500 ± 792 | −4150 ± 412 | −20400 ± 3440 | −7360 ± 1790 |
| Total GHG Benefit | −6060 | −9590 | |||
| CH4 | 0.5 ± 0.20 | 385 ± 177 | 335 ± 156 | 611 ± 275 | 532 ± 249 |
| N2O | 1.5 ± 0.64 | 420 ± 152 | 264 ± 84.6 | 667 ± 243 | 420 ± 134 |
| CO2 from CaCO3 | 3.8 ± 1.14 | 450 ± 137b | 0c | 623 ± 190c | 0c |
| Total Emissions | 5.7 | 1260 | 599 | 1780 | 952 |
| Net GHG benefit | −5460 | −8630 |
abackground (i.e. baseline) stock within total seed plot area.
bThe CO2 and CaCO3 gas exchange/reaction ratio may vary; we used 0.6, as discussed in the methods26.
cNote that we did not observe seagrass-enhanced CaCO3 burial in this system.
Figure 3.
Sequestered GHG pools (aboveground biomass - AGB, belowground biomass - BGB, and net sediment Corg – SOC) in 2013 and in 2016 resulting from seagrass restoration; maps generated by kriging data measured at sample sites (n = 21: circles in inset map); note that the bed volume has changed over time due to both meadow expansion and accretion (see Supplement). The mid-meadow SOC decline in the 2016 accreted interval reflects a local seagrass die-off event in 2015. The figure was created in ArcGIS 10.2 (www.esri.com) and Photoshop CS6 (www.adobe.com).
The average aboveground biomass standing stock over three years was 109 gdw m−2, equivalent to approximately 40.5 g Corg m−2. This reflects seasonal fluctuations that ranged from 330 g dry weight (gdw) m−2 in August (201.4 ± 29 g live plus 129.7 ± 15 g dead) to 38.5 gdw m−2 in March (19.58 ± 4.8 g live plus 18.86 ± 2.4 g dead) (see Supplement). All reported errors relate standard errors (SE), unless otherwise stated. The average aboveground biomass shoot−1 was 0.4 ± 0.07 gdw. Multiplying the average annual biomass per shoot by the interpolated average annual density values and integrating over the meadow area yielded an aboveground biomass standing stock of 710 t CO2 in 2013 and 810 t CO2 in 2016, due to meadow expansion. This standing stock is the average amount of Corg held in seagrass biomass throughout the year and is less than a third of the peak biomass in summer. Live belowground biomass ranged from 35.51 ± 7.3 gdw m−2 in January to 95.26 ± 13 gdw m−2 in August; the average annual live belowground biomass was 47.1 gdw m−2 (Supplement). Dead belowground biomass ranged from 91.03 ± 17 gdw m−2 in June 2016 to 131.91 ± 12 gdw m−2 in March, yielding an average, annual dead belowground biomass of 119 gdw m−2 (Supplement). Average, annual unit area estimates for live and dead belowground biomass were 16.0 and 40.4 g Corg m−2, respectively. Multiplied by the respective meadow areas, the combined belowground biomass stock sequestered 1,200 t CO2 in 2013 and 1,520 t CO2 in 2016.
Sediment Corg represented the largest sequestered carbon pool in the meadow in both 2013 and 2016, accounting for 68.5% of the total GHG benefit in 2013 and more than three-quarters of the total GHG benefit in 2016 (Table 3). Annual belowground biomass (live + dead) accounted for 14.7% of the total 2016 sequestered stock, and aboveground biomass represented 8.4%. Enhanced sediment Corg and the average, annual seagrass stock sequestered a combined 6,060 t CO2 in 2013 and 9,590 t CO2 in 2016 (Table 3).
The total, cumulative gross primary production (GPP) in the meadow from 2001–2013 was calculated to be 39,700 t CO2. By 2016, this estimate had increased to 84,900 t CO2, due to meadow expansion. Total, enhanced Corg sequestration was, therefore, 15.3% of cumulative GPP in 2013 and 11.3% in 2016.
Enhanced GHG emissions and the net GHG benefit
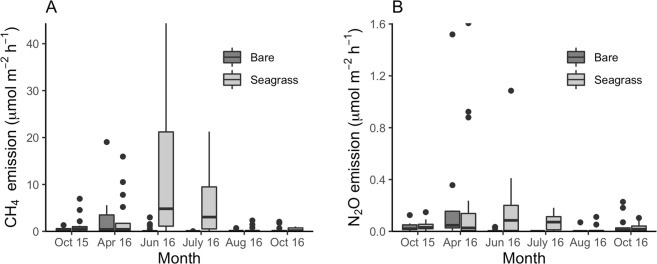
Seagrass presence significantly increased both the CH4 (χ2(1) = 13.1, p < 0.0003) and the N2O fluxes (χ2(1) = 8.46, p < 0.004) (Fig. 4A,B; Table 4). There was seasonal variation with seagrass presence*month interaction significant for both CH4 (χ2(10) = 36.4, p < 7.08e-5) and N2O release (χ2(10) = 35.8, p < 9.09e-5). The seagrass CH4 flux was highest in June, 15.9 ± 6.95 (SE) µmol CH4 m−2 hr−1 and lowest in August, 0.32 ± 0.22 (SE) µmol CH4 m−2 hr−1. The October 2016 flux was also low, 0.38 ± 0.06 (SE) µmol CH4 m−2 hr−1. The average bare site CH4 flux ranged from 3.37 ± 1.60 (SE) µmol CH4 m−2 hr−1 in April to 0.01 ± 0.007 (SE) µmol CH4 m−2 hr−1 in July. The average, annual enhanced CH4 flux was 0.70 ± 0.46 (SE) g CH4 m−2 yr−1. This represents the average, annual fluxes of 0.80 ± 0.53 (SE) g CH4 m−2 yr−1 from vegetated sites minus the average flux (0.10 ± 0.07 (SE) g CH4 m−2 yr−1) in bare sites (Fig. 4A; Table 1).
Figure 4.
CH4 (A) and N2O (B) ebullition flux (μmol m−2 hr−1) box plots (quartiles) at sites (n = 10) by observation month (Oct. 2015–Oct. 2016) and by treatment (bare and seagrass). See Table 4 for log-likelihood ratio test results for assessing the treatment effect.
Table 4.
Log-likelihood ratio test results for assessing a seagrass treatment effect (presence/absence) and a treatment*month interaction effect on benthic CH4 and N2O fluxes; rows relate the null model, reduced model, and full model for CH4 and N2O, respectively.
| Dfa | logLik | deviance | χ2 | Dfb | Pr(>χ2) | |
|---|---|---|---|---|---|---|
| CH4^(0.133) ~ 1 + (1|ID) | 3 | −9.25 | 18.50 | |||
| CH4^(0.133) ~ Treat + (1|ID) | 4 | −2.71 | 5.41 | 13.08 | 1 | 2.98E-04 |
| CH4^(0.133) ~ Treat + Treat*Month + (1|ID) | 14 | 15.51 | −31.02 | 36.44 | 10 | 7.08E-05 |
| N2O^(0.133) ~ 1 + (1|ID) | 3 | 9.70 | −19.39 | |||
| N2O^(0.133) ~ Treat + (1|ID) | 4 | 13.92 | −27.84 | 8.45 | 1 | 3.65E-03 |
| N2O^(0.133) ~ Treat + Treat*Month + (1|ID) | 14 | 31.83 | −63.65 | 35.81 | 10 | 9.09E-05 |
aMixed effects model degrees of freedom determined by lmer function (see Bates et al.78)
bLikelihood ratio test degrees of freedom (the difference between models used in each comparison).
Bulk porewater CH4 concentrations measured at seagrass and bare sites in August and October yielded a negligible diffusive flux (Fig. 5). The highest average CH4 porewater concentration was 0.30 ± 0.25 µmol L−1 at 1.5 cm below the sediment water interface at seagrass sites in October. The highest average concentrations in August were observed at 10.5 cm below the sediment water interface, 0.18 ± 0.14 µmol L−1 at the bare sites and 0.19 ± 0.06 µmol L−1 at the seagrass sites (Fig. 5). Assuming a sediment diffusivity of 0.1 × 10–4 cm2 s−1 and using Fick’s first law of diffusion, a CH4 concentration of 0.02 nmol cm−3 gave a diffusive flux of -0.007 µmol m−2 hr−1. This flux was negligible compared to CH4 emissions captured in the water column and was therefore excluded from subsequent GHG accounting.
Figure 5.
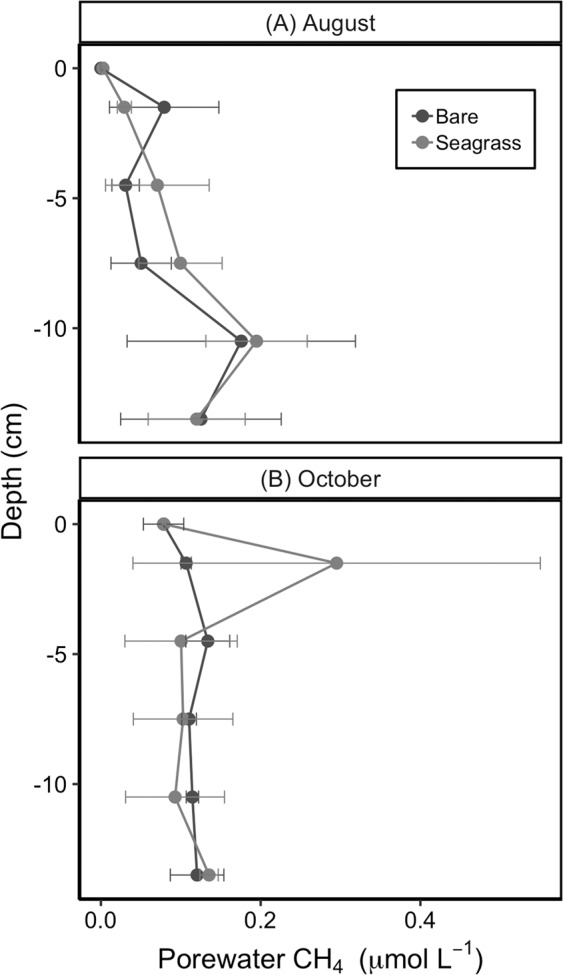
Porewater profile CH4 concentrations measured at bare and seagrass sites in August (A: site n = 6) and in October 2016 (B: site n = 4).
Average N2O fluxes in the seagrass meadow ranged from 0.67 ± 0.42 (SE) in April to 0.01 ± 0.01 (SE) µmol N2O m−2 hr−1 in August. N2O fluxes were also lower at bare sites, ranging from 0.21 ± 0.14 (SE) in April to 0.001 ± 0.0004 (SE) µmol N2O m−2 hr−1 in July (Fig. 4B). The average, annual vegetated flux of 0.06 ± 0.04 (SE) g N2O m−2 yr−1 minus the average, annual bare flux of 0.02 ± 0.01 (SE) g N2O m−2 yr−1 yielded an enhanced flux of 0.04 ± 0.03 (SE) g N2O m−2 yr−1 (Table 2). Scaling the trace GHG fluxes by meadow area over time and by their 100-year global warming potentials36, meadow-enhanced CH4 and N2O fluxes released 530 and 420 t CO2e between 2001–2016, respectively (Table 3; Fig. 6).
Figure 6.
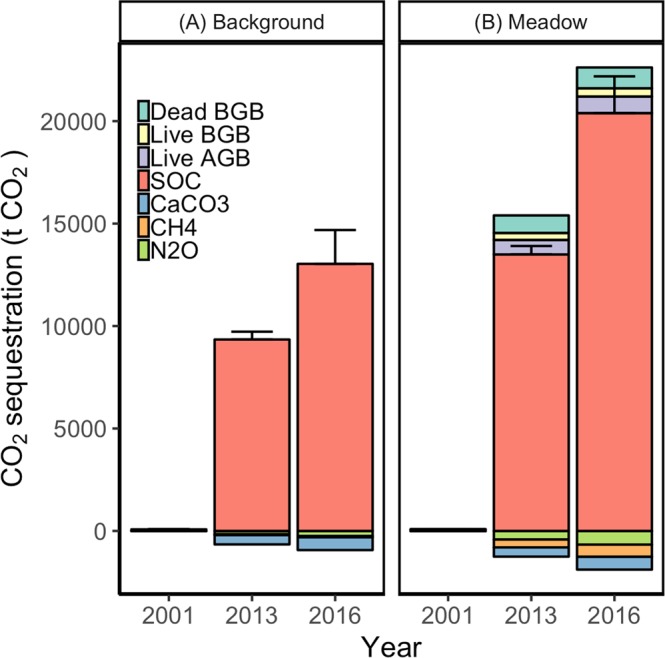
Cumulative background (A) and gross meadow (B) GHG stocks in the meadow areas over time; sequestration (i.e., GHG uptake from the atmosphere) in this figure is shown as positive, GHG release (i.e., a GHG flux to the atmosphere) is negative; CH4 and N2O quantities were standardized to CO2e; ‘CaCO3’ relates CO2 evasion attributable to CaCO3; background stocks were calculated by scaling average bare site values by total meadow area at each time step; net stock enhancement attributable to the meadow (see Table 3) can be calculated by subtracting the bare values (A) from equivalent gross meadow values (B); Error bars represent SE for the sediment Corg (SOC) stock.
We did not find a significant difference between average Cinorg concentrations by paired depth horizon in bare and seagrass sediment cores (t = -0.287, df = 13, p > 0.389). Inorganic carbon concentrations in the top 12 cm of the bed were similar throughout the meadow (site n = 16), averaging 0.11 ± 0.04 mg Cinorg cm−3. Scaling our average concentration from the top 6 cm of the bare site, 0.13 ± 0.04 mg Cinorg cm−3, by meadow area gave estimated CO2 emissions from CaCO3 formation of 450 t CO2 in 2013 and 623 t CO2 by 2016. However, the absence of a significant difference in CaCO3 between bare and seagrass sites meant that there was no net CO2 evasion attributable to the seagrass restoration (Table 3), so seagrass-enhanced CO2 evasion from CaCO3 between 2001–2016 was zero.
Integrating both stock changes and fluxes, this seagrass meadow restoration generated a net GHG benefit, which increased from 0.21 t C ha−1 yr−1 between 2001–2013 to 0.42 t C ha−1 yr−1 from 2013–2016, 12–15 years after restoration started.
Discussion
By applying the VCS GHG accounting methodology for the first time to an actual seagrass restoration project24, this study confirms the generally accepted but essentially untested hypothesis that seagrass restoration results in net GHG removal from the atmosphere—a GHG offset benefit that can potentially finance restoration. We also found that seagrass presence increased both CH4 and N2O release, but these increases had a relatively small effect on the net GHG benefit. Although other studies have reported increases in gross seagrass bed sediment carbon concentrations following seagrass restoration (e.g.9,12,), these reports do not translate directly to an offset benefit18. As we demonstrate in this study, gross Corg stocks determined in previous studies overestimate the GHG offset benefit, because they do not account for background Corg sequestration that would occur in the absence of seagrass or GHG flux increases due to meadow restoration. All of these parameters must be known to determine the GHG offset benefit provided by seagrass restoration. This study also demonstrates the utility of the stock change approach for seagrass GHG offset accounting and addresses questions about stock change feasibility18.
Seagrass-effects on CH4 and N2O release
The enhanced CH4 emissions reported here marginally exceeded the de minimis threshold, as defined by the VCS (<5%), reducing the total GHG benefit by 5.5% in 2013 and by 5.6% in 2016. By comparison, Rosentreter et al.47 estimated a 20.5% offset for methanogenesis in a tropical Australian mangrove forest. The enhanced N2O flux for the seagrass restoration was technically de minimis, 4.7% in 2013 and 4.4% in 2016. However, it is important to note that both seagrass trace gas fluxes reported here, 0.695 g CH4 m−2 yr−1 and 0.037 g N2O m−2 yr−1, exceeded the conservative general default factors for net benefit accounting, 0.56 g CH4 m−2 yr−1 (for salinities>20 ppt) and 0.016 g N2O m−2 yr−1 (Section 8.1.4.3.4 in24). These general default factors may, therefore, underestimate the magnitude of CH4 and N2O fluxes in other seagrass systems.
We observed considerable variability in CH4 and N2O fluxes at seagrass sites, especially during spring and summer months. More work is needed to understand site-specific drivers of CH4 and N2O production to better constrain annual fluxes48. This includes determining whether CH4 production varies with sediment Corg concentrations, whether CH4 and CO2 interactions affect CH4 release, and whether microbial community differences affect CH4 and N2O enhancement. We also note that using benthic chambers may have moderated release rates for both trace gases by inhibiting flow-induced efflux and that using experimentally cleared control sites, rather than bare sites outside the meadow, may have reduced the apparent seagrass enhancement effect. We advise other seagrass blue carbon studies to measure both trace gases directly, until a sufficient number of additional studies suggest conservative release rates for seagrass GHG accounting that are generally applicable.
Identifying the net GHG benefit from seagrass restoration
Studies based on burial rates have suggested that seagrass meadows may sequester more carbon in soils than terrestrial forests55. The net sequestration rate based on sediment and plant stock changes and emissions of CH4 and N2O that we measured in this study, 0.42 t C ha−1 yr−1, is lower than the average rates for temperate and tropical forests, 2.6 and 5.3 t C ha−1 yr−1, respectively51, but generally agrees with the IPCC sequestration rate for seagrass systems, 0.43 t C ha−1 yr−1 51. Similar studies in other systems may also support the use of this default factor, but we note several reasons why this default factor may not be an appropriate rate for all seagrass systems at all times. First, the IPCC rate is double the sequestration rate that we calculated for the first decade of our restoration, 0.21 t C ha−1 yr−1. Long-term research in this restored meadow has shown that it took about a decade for sediment carbon sequestration rates and plant biomass to be equivalent to natural meadows12. Second, sediment accretion may vary throughout the meadow. We assumed uniform sediment accretion, but actual accretion may be lower near the meadow edge, as evidenced by the grain size distribution and reported Reynolds stresses56,57 (see the Supplement). Third, our system has negligible carbonate, because the sediment in the region is siliciclastic, and there are no nearby coral reefs. We did not expect to find a significant difference in CaCO3 at seagrass and bare sites. This is not the case in other seagrass systems, where increased CO2 evasion may be significant (see the comparison between this system and others in Sadrene et al.22). Finally, the South Bay meadow also appears to be metabolically balanced on a decadal time scale, but studies in autotrophic systems may need to determine whether direct plant metabolism increases pCO2 and results in a CO2 flux back to the atmosphere58,59. These caveats point to areas where future research needs to be done to verify how generally the IPCC default factor applies to seagrass ecosystems worldwide.
The stock change approach indicates that the carbon sequestration rate for this meadow is increasing but that net CO2 sequestration as a percentage of meadow-wide community GPP may be declining with meadow age. Cumulative GPP increased by 114% between 2013 and 2016, due largely to meadow expansion, but the enhanced sequestered stock only increased by 78% over this period. The fraction of GPP that is sequestered may increase over time if the meadow stops expanding and GPP reaches a long-term steady state. Recent work at this site has shown that GPP initially exceeded respiration in this meadow but later reached equivalence59,60, a finding that may pertain to eelgrass systems generally61. Studies need to determine whether carbon sequestration as a percentage of GPP changes over time in other systems, including those that appear to be net autotrophic30, and whether seagrass offset benefits continue to accumulate indefinitely.
Given that measuring sediment Corg stock changes in a seagrass system is feasible, we recommend using this method to calculate seagrass net GHG benefits to avoid issues associated with using burial fluxes for this purpose18. Use of 210Pb dating to calculate sedimentation rates in seagrass systems has been criticized where relatively short (decadal) time scales are addressed and where bioturbation could disturb sediment profiles18. A recent study used surface elevation tables (SET) to compare changes in surface elevation between bare and seagrass sites over short (<1 yr) time scales62, but the SETs and marker horizons used widely in salt marshes are generally problematic in seagrass meadows. Subtidal currents re-suspend surface sediments, scouring occurs around vertical objects, including SET pins, and the high-water content of surface sediment makes precise (mm-scale) measurements of surface elevation difficult63. Burial rate sequestration estimates also assume that surface deposition is the primary vector for transferring Corg to the sediment, but we observed considerable Corg accumulation within the bed. This may be due to sediment Corg accumulation from root Corg exudates or from increased preservation of benthic microalgae migrating up and down within the sediment64. The sediment Corg stock increase that we observed, 874 t Corg, exceeded the increase we would have estimated by scaling the Greiner et al.12 surface burial flux reported for this system by meadow area and by the three-year time period, 755 t Corg. However, we also observed sediment Corg declines in 2016 at particular sites, which affected the 2016 sediment Corg spatial distribution (Fig. 3). Random disturbance events will likely affect long-term (i.e. decadal) sediment Corg accumulation rates by periodically removing sequestered sediment Corg stocks. A stock change approach captures these changes. Burial flux rates derived from dated sediment cores may need to be reconsidered, given the magnitude of the within bed Corg accumulation that we observed.
Individual seagrass projects should also take care to avoid overestimating the GHG offset benefit by failing to account for allochthonous Corg. The VCS carbon-offset protocol conservatively requires that carbon fixed outside the project area (allochthonous carbon) be excluded from the GHG offset benefit, because this cannot be unequivocally attributed to the seagrass restoration project18,24. We conservatively deducted the background Corg concentration from the entire seagrass Corg profile to account for possible deposition of allochthonous carbon (see Fig. 1 and the Supplement). Including all of the sediment Corg in the accreted part of the South Bay bed would have almost doubled the apparent project benefit to 10.1 K t CO2e in 2013 and 17.2 K t CO2e in 2016.
Offset-credit finance as an incentive for seagrass restoration
Had this restoration project been able to apply for VCS offset-credits in 2001, it would now receive up to 8,630 credits. The actual allocation of credits would be slightly lower to account for CO2 emissions from project activities (i.e. travel to restoration sites, etc.) and ‘buffer pool’ set aside credits to account for the risk of GHG offset gain reversals24. Investors do not typically consider GHG offset projects viable unless they sequester at least 50,000 tCO2e over the project lifetime (typically 30 years)65. Reaching 50,000 credits by 2031 would require a further increase in the C sequestration rate by this meadow. Future work, including repeated carbon stock change measurements and bed accretion measurements, will be necessary to determine whether the sequestration rate continues to increase.
Given current market prices, carbon offset-credits currently provide a marginal incentive for seagrass restoration. At a price of $10 ton−1, offset-credits would finance approximately 10% of the approximately $800 K South Bay restoration cost17,66. Fully financing a seagrass restoration project with a unit cost equivalent to this South Bay Z. marina restoration would require a voluntary offset price greater than $95 per MtCO2e. This cost-benefit comparison excludes project development costs, which may exceed $100 K, and net present value discounting. We note that the carbon burial rates measured in South Bay are on the low end of those documented for other seagrass meadows globally5. Other species and locations may generate larger sediment Corg stocks than we measured for Z. marina over time (e.g.67). However, the South Bay restoration was accomplished at a unit cost of only $1,200 ha−1 17, and the range for other seagrass projects is $1,900–4,000,000 ha−1 54.
Rather than rely solely on carbon offset-credits to finance meadow restoration, coastal managers should think holistically about the other values that seagrass systems provide, including fisheries support, nutrient removal, and reduced marsh erosion, among other services. Quantifying these values, even absent markets for co-benefit ‘credits,’ would provide further incentive for seagrass restoration, in addition to carbon sequestration.
Methods
Study area
We measured all of the parameters required by the VCS methodology to quantify the GHG offset benefit from the Z. marina restoration in South Bay, VA24. The restoration history68, project cost17, sediment Corg stock enhancement12,57,69, and net ecosystem metabolism58–60 of this meadow have been documented and provide a baseline for stock-change assessment. The South Bay meadow area is shallow, with an average depth at mean sea level of 0.76 ± 0.28 (SD) m, and oligotrophic, with low nutrient loading (Fig. 2)57. For additional background on the Virginia Coast Reserve Long-Term Ecological Research eelgrass restoration, including reseeding methods, see Orth and McGlathery68 and other studies in the Marine Ecology Progress Series v. 44869,70.
Sediment Corg stock enhancement
Meadow sediment Corg stock enhancement was determined for both 2013 and 2016 by subtracting baseline sediment (i.e., bare) Corg stocks from the gross stocks measured within the meadow (Fig. 1). Corg is generally present in subtidal sediment without seagrass meadows, and this background Corg should not be attributed to a seagrass restoration project. The restored meadow was already in existence when we began sampling in 2013, so time = 0 values at sites within the meadow were not available. The sediment Corg baseline scenario (the Emmer et al.24 ‘without project’ scenario) that would represent pre-restoration (time = 0) was, therefore, established by measuring Corg concentrations at bare control sites outside the meadow. The average Corg concentration in cores collected at four bare sites by Greiner et al.12 in 2011 and by Oreska et al.57,64 in 2013 and in 2014 was 3.67 ± 0.55 (SE) mg Corg cm−3 (see Supplement). We verified that this background concentration remained unchanged by collecting new, replicate cores (n = 4) at two of these bare sites in 2016. We deducted this average background sediment Corg concentration from the sediment Corg concentrations measured within the meadow in 2013 and in 2016 to identify the Corg attributable to the seagrass restoration (Fig. 1). This is in accordance with the stock change assessment recommended by the VCS methodology24.
We assessed Corg changes at sites within the meadow in 2016 by resampling 16 randomly-selected meadow sites first sampled by Oreska et al.57 in 2013 (the ‘with project’ scenario). Four 12-cm long, 2.7 cm diameter cores were collected at each site and subdivided into 3-cm intervals. Macroscopic roots and rhizomes were removed from each sample manually, using tweezers. Note that belowground biomass (BGB) was quantified separately, as described in the following section, to avoid double counting. All sediment samples were prepared according to methods used previously in this system12,57,64. We measured %C on a Thermo Scientific Flash 2000 Organic Element Analyzer; %Corg was determined by subtracting %Cinorg, which we determined using element analysis of samples ashed at 500 °C for six hours71. The element analyzer average percent error was 0.48%, based on analysis of lab standards.
Allochthonous Corg may be deposited within the bed due to bed accretion (Fig. 1). Rather than deduct an arbitrary ‘allochthonous compensation factor’ from the meadow sediment Corg stock72,73, we accounted for allochthonous Corg that could have been deposited in the baseline scenario by deducting the bare site sediment Corg average from the entire meadow carbon profile, including the part of the sediment profile that may have resulted from accretion facilitated by the meadow (see Fig. 1 and the Supplement for more explanation).
Total, meadow-enhanced sediment Corg stocks in 2013 and in 2016 were quantified by interpolating the average 2013 and 2016 sediment Corg enhancement at each site in ArcGIS 10.2 Geostatistical Analyst using Ordinary Kriging74. We fitted stable, circular, spherical, Guassian, and exponential semivariogram models to each dataset and selected the sediment Corg distribution maps with the lowest root mean square errors (Supplement). The 2013 data was best fit using a circular model, the equivalent 2016 data was best fit using a Gaussian model, and the uppermost 2-cm interval in 2016, which may be the result of accretion and is shown separately in Fig. 3, was best fit using an exponential model.
Biomass CO2 sequestration
The carbon sequestered in seagrass tissue is periodically lost to export, herbivory, and decomposition, so we calculated and reported the average, annual standing biomass stock based on seasonal measurements from 2014–2016 (see Supplement). This represents a running average that reflects periodic export and other fluctuations, rather than peak observed biomass. This is the same general approach that reforestation GHG offset projects use to address the cyclical harvest and replanting of aboveground biomass (AGB), and it is permitted for seagrass GHG accounting24. Shoot densities ranged from approximately 250 to 617 shoots m−2 in South Bay due to seasonal thinning and export, and biomass ranged from 0.26 to 0.781 gdw shoot−1. We accounted for variability in AGB using existing density measurements (shoots m−2) taken at sites throughout this meadow over time to account for seasonal changes57,75. The average density over the course of a year was approximately half of the peak density observed during July (48%)57,75
We quantified average AGB per shoot and BGB by collecting additional replicate (n ≥ 4) 15.2-cm diameter biomass cores seasonally from June 2014 to June 2016 to a depth of 15 cm at five central meadow sites (see Supplement), following methods employed by past studies in this system69,76. We also collected biomass cores (n ≥ 3) at four additional, systematically located sites during the summer of 2016 (see Supplement). Samples were sieved using a 1-mm mesh, separated the same day into live and dead fractions, and then dried to a constant weight at 60 °C. Biomass data—both live and dead—was averaged by site and then by month to generate seasonal averages, which were used to calculate the average, annual standing stocks. The average, annual shoot densities were multiplied by the average biomass shoot−1, 0.41 ± 0.09 gdw shoot−1 (this study), and by 37.1% C gdw−1 biomass76. The resulting aboveground biomass values (Corg m−2) were interpolated using Ordinary Kriging in ArcGIS 10.2 Geostatistical Analyst and a Gaussian semivariogram to generate average, annual AGB stocks for the 2013 and 2016 meadow extents. Average live and dead BGB values (g m−2) were multiplied by the average Corg fraction in belowground biomass, 33.8% Corg gdw−1 biomass77, and scaled by the 2013 and 2016 meadow areas to generate Corg stocks.
GHG fluxes
We deployed clear plastic, bell-shaped benthic chambers over vegetated and experimentally cleared 2 m x 2 m bare plots at the five central meadow sites to identify changes in benthic CH4 and N2O fluxes attributable to Z. marina presence. Each chamber sat on the sediment surface, covering a 0.046 m2 circular area and enclosing a 10.5 L volume. Comparing fluxes at cleared, central meadow plots allowed us to control for confounding factors at bare sites outside of the meadow. These areas are generally deeper with more sand-sized sediment and experience greater Reynolds stresses, because of area geomorphology56, factors that may affect sediment:water gas exchange. We cleared the bare plots during spring 2015, installed plastic lawn edging to a depth of 8 cm to prevent seagrass rhizome re-colonization, and allowed plots to equilibrate for five months. Comparing seagrass and cleared bare plots to assess a seagrass enhancement effect on CH4 and N2O was conservative, because some seagrass BGB potentially remained at the cleared plots and may have contributed to microbial production of these trace gases. Eight chambers were deployed at each site during each observation, four replicates over seagrass and four over bare sediment. Every deployment exactly bracketed low tide, such that gas accumulation time captured equal parts falling- and rising-tide. Deployment durations ranged from 1 to 5 hours. Trace gases were collected on multiple days per month in October 2015, April 2016, June 2016, July 2016, August 2016, and October 2016. Using chambers allowed us to conduct a controlled experiment in situ to test for a seagrass presence effect, but we acknowledged that using benthic chambers may have introduced container effects that affected release rates, including the elimination of hydrodynamic flow-induced efflux.
The gas that collected in each chamber was syringe extracted and injected into an exetainer filled with 12 ml N2 and 0.2 ml 0.01 M ZnC4H6O4 to prevent microbial activity resulting from the syringe transfer. The total gas volume collected within each chamber was noted and used to calculate the gas flux as a function of time and bed surface area. We also measured bulk CH4 concentrations in replicate porewater samples collected at bare and vegetated sites in August (site n = 6) and October (site n = 4) 2016 to determine the magnitude of the diffusion flux relative to the ebullition flux. We extracted 7 ml of porewater through mini-piezometers (inner diameter 1.8 mm) at 3-cm intervals, from 1.5 cm down to 13.5 cm. The water samples were syringe injected into exetainers filled with 12 ml N2 and fixed with 0.2 ml ZnCl2. The diffusive flux was calculated using Fick’s first law of diffusion:
| 1 |
where the sediment diffusivity, Ds, was assumed to be 0.1 × 10–4 cm2 s−1.
All exetainer samples were analyzed on a Varian 450-Gas Chromatograph with a Bruker GC/MS workstation at the Smithsonian Environmental Research Center. We determined sample CH4 and N2O concentrations using onsite standards and corrected for differences in atmospheric temperature and pressure during each GC analysis. Standard curve R2 values ranged from 0.992 to 0.996.
We tested for an effect of seagrass presence on CH4 and N2O fluxes using linear mixed effect models in R78,79. Replicate results were averaged by site. Seagrass presence/absence and month were treated as fixed effects; individual sites were randomly selected. Tests were run on each GHG dataset using the lmer function (lme4 package version 1.1–14). We expected to find increased GHG fluxes attributable to seagrass presence, as well as a seagrass*month interaction effect. Both the CH4 and N2O datasets required transformation due to heteroskedasticity and the presence of outliers. The optimal transformation (identified using the optim.boxcox function in the boxcoxmix package version 0.14) for the averaged data was λ = 0.133 (Maximum log-likelihood = −77.608). Model p-values were obtained from likelihood ratio tests on the full model and a reduced model without the fixed effects. Average, annual seagrass and bare CH4 and N2O fluxes were determined by first averaging fluxes by season and then averaging the seasonal averages. Note that the early June observations were included as spring values and that we conservatively reported 9-month averages. The difference between seagrass and bare values represented the net fluxes attributable to seagrass presence. All statistics were calculated in R (R stats package version 3.4.2)79.
CO2 evasion attributable to Cinorg was estimated by multiplying the Cinorg stock by a CO2 and CaCO3 gas exchange/reaction ratio of 0.6, following Howard et al.26. We determined whether or not seagrass presence increased Cinorg concentrations by running a paired t-test on average, depth-calibrated Cinorg concentrations from 20-cm cores collected at a representative meadow site and a representative bare site in this system.
Net GHG benefit accounting
Total meadow CO2 sequestration was calculated for both 2013 and 2016 by summing the above- and belowground biomass (both live and dead) and meadow-enhanced sediment Corg stocks measured in each year. Cumulative, enhanced CH4 and N2O emissions attributable to the meadow were estimated by multiplying the average enhanced (i.e., net) fluxes (g m−2 yr−1) by meadow area over time. Meadow area changes were calculated in ArcGIS 10.2 by georeferencing the Virginia Institute of Marine Science aerial photographs for every year after initial reseeding in 2001 and delineating the meadow perimeter74,80. Meadow area was interpolated for the three years where photographs were unavailable. These cumulative, net GHG emissions calculated for 2013 and for 2016 were subtracted from the respective meadow-enhanced CO2 sequestration results to determine the net GHG benefit in each year (note that seagrass-enhanced CO2 emissions from CaCO3 were not observed).
We compared the total meadow sequestration in 2013 and in 2016 with the total, cumulative GPP within the meadow in each of those years to estimate the percentage of total GPP sequestered by the meadow. Cumulative GPP was estimated as a function of shoot density and meadow area. The relationship between meadow age and density was determined by fitting a polynomial regression to existing data from this meadow collected as part of the annual VCR-LTER seagrass survey81. This relationship was observed by Berger et al.59 to be:
| 2 |
where Y was shoot density in shoots m−2, and x was the meadow age in years (R2 = 0.91). GPP was calculated using the following relationship observed in this meadow by Berger et al.59:
| 3 |
where x was density (shoots m−2) and Y was GPP in mmol O2 m−2 d−1 (R2 = 0.69). Calculated GPP values for meadow areas of different age were summed and integrated over time to generate cumulative values.
Supplementary information
Acknowledgements
P. Megonigal, M. Castorani, P. Wiberg, J. Porter, M. White, S. Emmett-Mattox, R. Orth, D. Wilcox, and S. Settelmyer provided advice or helpful feedback. J. Greiner and M.-L. Delgard provided access to data used in analyses. D. Peresta, M. Miller, and A. Schwarzchild provided field and/or lab assistance. We also want to thank the editor and two anonymous reviewers for their helpful feedback. Financial support was provided by Virginia Sea Grant, the University of Virginia Jefferson Scholars Foundation, and by National Science Foundation grants to the Virginia Coast Reserve Long-Term Ecological Research project (DEB-1237733, DEB-1832221). The Nature Conservancy owns and manages land areas in the Virginia Coast Reserve; permissions are not required to conduct research in subtidal areas, which are in the public domain. This project was completed at the University of Virginia. The authors declare no conflicts of interest.
Author contributions
M.P.J.O., K.J.M. and L.R.A. designed the study. M.P.J.O., L.R.A., A.C.B. and L.M. collected the data. All authors analyzed the data. M.P.J.O., K.J.M., L.R.A. and P.B. wrote the main manuscript text. All authors reviewed the manuscript.
Data availability
Data reported and analyzed in this study is available in the Supplement and on the LTER Network Data Portal (https://portal.lternet.edu/nis/home.jsp).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64094-1.
References
- 1.Duarte CM, Middelburg JJ, Caraco N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences. 2005;2:1–8. doi: 10.5194/bg-2-1-2005. [DOI] [Google Scholar]
- 2.Champenois W, Borges AV. Seasonal and interannual variations of community metabolism rates of a Posidonia oceanica seagrass meadow. Limnol. Oceanogr. 2012;57(1):347–361. doi: 10.4319/lo.2012.57.1.0347. [DOI] [Google Scholar]
- 3.Tokoro T, et al. Net uptake of atmospheric CO2 by coastal submerged aquatic vegetation. Glob. Chang. Biol. 2014;20(6):1873–1884. doi: 10.1111/gcb.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gullström M, et al. Blue carbon storage in tropical seagrass meadows relates to carbonate stock dynamics, plant sediment processes, and landscape context: insights from the Western Indian Ocean. Ecosys. 2018;21:511–566. doi: 10.1007/s10021-017-0170-8. [DOI] [Google Scholar]
- 5.Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:505–509. doi: 10.1038/ngeo1477. [DOI] [Google Scholar]
- 6.Orth RJ, et al. A global crisis for seagrass ecosystems. BioSci. 2006;56(12):987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. [DOI] [Google Scholar]
- 7.Waycott M, et al. Accelerating loss of seagrass across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 2009;106(30):12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macreadie PI, et al. Losses and recovery of organic carbon from a seagrass ecosystem following disturbance. Proc. B. 2015;282(1817):20151537. doi: 10.1098/rspb.2015.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marbà N, et al. Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J. Ecol. 2015;103:296–302. doi: 10.1111/1365-2745.12370. [DOI] [Google Scholar]
- 10.Lovelock CE, et al. Assessing the risk of carbon dioxide emissions from blue carbon ecosystems. Front. Ecol. Env. 2017;15(5):257–265. doi: 10.1002/fee.1491. [DOI] [Google Scholar]
- 11.Pendleton L, et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE. 2012;7(9):e43542. doi: 10.1371/journal.pone.0043542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greiner JT, McGlathery KJ, Gunnell J, McKee BA. Seagrass restoration enhances “blue carbon” sequestration in coastal waters. PLoS ONE. 2013;8(8):e72469. doi: 10.1371/journal.pone.0072469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorhaug A, Poulos HM, López-Portillo L, Ku TCW, Berlyn GP. Seagrass blue carbon dynamics in the Gulf of Mexico: Stocks, losses from anthropogenic disturbance, and gains through seagrass restoration. Sci. Total Environ. 2017;605-6:626–636. doi: 10.1016/j.scitotenv.2017.06.189. [DOI] [PubMed] [Google Scholar]
- 14.Nellemann, C., et al. Blue Carbon. A Rapid Response Assessment. United Nations Environment Programme 1-80 (GRID-Arendal (2009).
- 15.Röhr ME, et al. Blue carbon storage capacity of temperate eelgrass (Zostera marina) meadows. Global Biogeochem. Cycles. 2018;32(10):1457–1475. doi: 10.1029/2018GB005941. [DOI] [Google Scholar]
- 16.Russell M, Greening H. Estimating benefits in a recovering estuary: Tampa Bay, Florida. Estuaries Coast. 2015;38(Suppl 1):S9–S18. doi: 10.1007/s12237-013-9662-8. [DOI] [Google Scholar]
- 17.Reynolds LK, Waycott M, McGlathery KJ, Orth RJ. Ecosystem services returned through seagrass restoration. Restor. Ecol. 2016;24(5):583–588. doi: 10.1111/rec.12360. [DOI] [Google Scholar]
- 18.Johannessen SC, Macdonald RW. Geoengineering with seagrasses: is credit due where credit is given? Env. Res. Letters. 2016;11:113001. doi: 10.1088/1748-9326/11/11/113001. [DOI] [Google Scholar]
- 19.Belshe EF, Mateo MA, Gillis L, Zimmer M, Teichberg M. Muddy waters: unintentional consequences of blue carbon research obscure our understanding of organic carbon dynamics in seagrass ecosystems. Front. Mar. Sci. 2017;4:125. doi: 10.3389/fmars.2017.00125. [DOI] [Google Scholar]
- 20.Howard JL, Creed JC, Aguiar MVP, Fourqurean JW. CO2 released by carbonate sediment production in some coastal areas may offset the benefits of seagrass “Blue Carbon” storage. Limnol. Oceanogr. 2018;63(1):160–172. doi: 10.1002/lno.10621. [DOI] [Google Scholar]
- 21.Macreadie PI, Serrano O, Maher DT, Duarte CM, Beardall J. Addressing calcium carbonate cycling in blue carbon accounting. Limnol. Oceanogr. Lett. 2017;2:195–201. doi: 10.1002/lol2.10052. [DOI] [Google Scholar]
- 22.Saderne V, et al. Role of carbonate burial in “blue carbon” budgets. Nat. Commun. 2019;10:1106. doi: 10.1038/s41467-019-08842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verified Carbon Standard. VCS Project Database. Available at: http://www.vcsprojectdatabase.org/ (2017).
- 24.Emmer, I., et al. Methodology for Tidal Wetland and Seagrass Restoration. Verified Carbon Standard, VM0033 Version 1.0. https://verra.org/methodology/vm0033-methodology-for-tidal-wetland-and-seagrass-restoration-v1-0/ (2015).
- 25.Emmer I., von Unger, M., Needelman, B.A., Crooks, S., & Emmett-Mattox, S. Coastal Blue Carbon in Practice: A Manual for Using the VCS Methodology for Tidal Wetland and Seagrass Restoration, V 1.0. 82p (Arlington, VA: Restore America’’s Estuaries (2015).
- 26.Howard J, et al. Clarifying the role of coastal and marine systems in climate mitigation. Front. Ecol. Environ. 2017;15(1):42–50. doi: 10.1002/fee.1451. [DOI] [Google Scholar]
- 27.Duarte CM. Reviews and syntheses: Hidden forests, the role of vegetated coastal habitats in the ocean carbon budget. Biogeosciences. 2017;14:301–310. doi: 10.5194/bg-14-301-2017. [DOI] [Google Scholar]
- 28.Röhr ME, Boström C, Canal-Vergés P, Holmer M. Blue carbon stocks in Baltic Sea eelgrass (Zostera marina) meadows. Biogeosciences. 2016;13:6139–6153. doi: 10.5194/bg-13-6139-2016. [DOI] [Google Scholar]
- 29.Rozaimi M, et al. Carbon stores from a tropical seagrass meadow in the midst of anthropogenic disturbance. Mar. Poll. Bull. 2017;119:253–260. doi: 10.1016/j.marpolbul.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 30.Duarte CM, et al. Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Global Biogeochem. Cycles. 2010;24:GB4032. doi: 10.1029/2010GB003793. [DOI] [Google Scholar]
- 31.Johnson RA, Gulick AG, Bolten AB, Bjorndal KA. Blue carbon stores in tropical seagrass meadows maintained under green turtle grazing. Sci. Rep. 2017;7:13545. doi: 10.1038/s41598-017-13142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oreska MPJ, et al. Comment on Geoengineering with seagrasses: is credit due where credit is given? Environ. Res. Lett. 2018;13(3):038001. doi: 10.1088/1748-9326/aaae72. [DOI] [Google Scholar]
- 33.Mateo, M. A., Cebrián, J., Dunton, K., & Mutchler, T. Carbon flux in seagrass ecosystems in Seagrasses: Biology, Ecology and Conservation (eds. Larkum, A. W. D., Orth, R. J., & Duarte, C. M.) 159-192 (Netherlands: Springer (2006).
- 34.Needelman B, et al. The science and policy of the Verified Carbon Standard Methodology for Tidal Wetland and Seagrass Restoration. Estuaries Coast. 2018;41(8):2159–2171. doi: 10.1007/s12237-018-0429-0. [DOI] [Google Scholar]
- 35.Neubauer SC, Megonigal JP. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems. 2015;18:1000–1013. doi: 10.1007/s10021-015-9879-4. [DOI] [Google Scholar]
- 36.United Nations Framework Convention on Climate Change (UNFCCC). Global Warming Potentials. http://unfccc.int/ghg_data/items/3825.php (2017).
- 37.Roughan BL, Kellman L, Smith E, Chmura GL. Nitrous oxide emissions could reduce the blue carbon value of marshes on eutrophic estuaries. Environ. Res. Lett. 2018;13:044034. doi: 10.1088/1748-9326/aab63c. [DOI] [Google Scholar]
- 38.Pollard PC, Moriarty DJW. Organic carbon decomposition, primary and bacterial productivity, and sulphate reduction, in tropical seagrass beds of the Gulf of Carpentaria, Australia. Mar. Ecol. Prog. Ser. 1991;69:149–159. doi: 10.3354/meps069149. [DOI] [Google Scholar]
- 39.Welsh D, et al. Denitrification, nitrogen fixation, community primary productivity and inorganic-N and oxygen fluxes in an intertidal Zostera noltii meadow. Mar. Ecol. Prog. Ser. 2000;208:65–77. doi: 10.3354/meps208065. [DOI] [Google Scholar]
- 40.Holmer M, Anderson FØ, Nielsen SL, Boschker HTS. The importance of mineralization based on sulfate reduction for nutrient regeneration in tropical seagrass sediments. Aquat. Bot. 2001;71:1–17. doi: 10.1016/S0304-3770(01)00170-X. [DOI] [Google Scholar]
- 41.Poffenbarger HJ, Needelman BA, Megonigal JP. Salinity influence on methane emissions from tidal marshes. Wetlands. 2011;31:831–842. doi: 10.1007/s13157-011-0197-0. [DOI] [Google Scholar]
- 42.Shieh WY, Yang JT. Denitrification in the rhizosphere of the two seagrasses Thalassia hemprichii (Ehrenb.) Aschers and Halodule uninervis (Forsk.) Aschers. J. Exp. Mar. Biol. Ecol. 1997;218:229–241. doi: 10.1016/S0022-0981(97)00076-2. [DOI] [Google Scholar]
- 43.Oremland RS. Methane production in shallow-water, tropical marine sediments. Appl. Environ. Microbiol. 1975;30(4):602–608. doi: 10.1128/AEM.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriarty DJW, et al. Microbial biomass and productivity in seagrass beds. Geomicrobiology Journal. 1985;4(1):21–51. doi: 10.1080/01490458509385919. [DOI] [PubMed] [Google Scholar]
- 45.Isaksen MF, Finster K. Sulphate reduction in the root zone of the seagrass Zostera noltii on the intertidal flats of a coastal lagoon (Arcachon, France) Mar. Ecol. Prog. Ser. 1996;137:187–194. doi: 10.3354/meps137187. [DOI] [Google Scholar]
- 46.Lee K-S, Dunton KH. Diurnal changes in pore water sulfide concentrations in the seagrass Thalassia testudinum beds: the effects of seagrasses on sulfide dynamics. J. Exp. Mar. Bio. Ecol. 2000;255:201–214. doi: 10.1016/S0022-0981(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 47.Rosentreter JA, Maher DT, Erler DV, Murray RH, Eyre BD. Methane emissions partially offset “blue carbon” burial in mangroves. Sci. Adv. 2018;4:eaao4985. doi: 10.1126/sciadv.aao4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Haj, A. N., & Fulweiler, R. W. A synthesis of methane emissions from shallow water coastal ecosystems. Global Change Biol. 10.1111/gcb.15046 (2020). [DOI] [PubMed]
- 49.Garcias-Bonet N, Duarte CM. Methane production by seagrass ecosystems in the Red Sea. Front. Mar. Sci. 2017;4:340. doi: 10.3389/fmars.2017.00340. [DOI] [Google Scholar]
- 50.Nakagawa T, Tsuchiya Y, Ueda S, Fukui M, Takahashi R. Eelgrass sediment microbiome as a nitrous oxide sink in brackish Lake Akkeshi, Japan. Microbes Environ. 2019;34(1):13–22. doi: 10.1264/jsme2.ME18103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Intergovernmental Panel on Climate Change (IPCC). 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands (eds. Hirashi, T., et al.). 354p. (Switzerland: IPCC Press (2014).
- 52.Mateo MA, Romero J. Detritus dynamics in the seagrass Posidonia oceanica: elements for an ecosystem carbon and nutrient budget. Mar. Ecol. Prog. Ser. 1997;151:43–53. doi: 10.3354/meps151043. [DOI] [Google Scholar]
- 53.Serrano O, Mateo MA, Renom P, Julià R. Characterization of soils beneath a Posidonia oceanica meadow. Geoderma. 2012;185-186:26–36. doi: 10.1016/j.geoderma.2012.03.020. [DOI] [Google Scholar]
- 54.Paling E. I., Fonseca, M., van Katwijk, M. M., & van Keulen, M. Seagrass restoration in CoastalWetlands: An Integrated Ecosystems Approach (eds. Perillo, G. M. E., Wolanski, E., Cahoon, D. R., & Brinson, M.) 687–713 (Amsterdam: Elsevier (2009).
- 55.Mcleod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011;9(10):552–560. doi: 10.1890/110004. [DOI] [Google Scholar]
- 56.Hansen JCR, Reidenbach MA. Wave and tidally driven flows in eelgrass beds and their effect on sediment suspension. Mar. Ecol. Prog. Ser. 2012;448:271–287. doi: 10.3354/meps09225. [DOI] [Google Scholar]
- 57.Oreska MPJ, McGlathery KJ, Porter JH. Seagrass blue carbon accumulation at the meadow-scale. PLoS ONE. 2017;12(4):e0176630. doi: 10.1371/journal.pone.0176630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berg, P. et al Dynamics of benthic metabolism, O2, and pCO2 in a temperate seagrass meadow. Limnol. Oceanogr. 64: 2586-2604 (2019).
- 59.Berger, A. E., Berg, P., McGlathery, K. J. & Delgard, M. L. Long-term trends and resilience of seagrass metabolism: a decadal aquatic eddy covariance study. Limnol. Oceanogr. 10.1002/lno.11397 (2020).
- 60.Rheuban JE, Berg P, McGlathery KJ. Ecosystem metabolism along a colonization gradient of eelgrass (Zostera marina) measured by eddy correlation. Limnol. Oceanogr. 2014;59(4):1376–1387. doi: 10.4319/lo.2014.59.4.1376. [DOI] [Google Scholar]
- 61.Ferguson AJP, et al. Oxygen and carbon metabolism of Zostera muelleri across a depth gradient – Implications for resilience and blue carbon. Estuar. Coast. Shelf Sci. 2017;187(5):216–230. doi: 10.1016/j.ecss.2017.01.005. [DOI] [Google Scholar]
- 62.Potouroglou M, et al. Sci. Reports. 2017;7:11917. doi: 10.1038/s41598-017-12354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lefebvre A, Thompson CEL, Amos CL. Influence of Zostera marina canopies on unidirectional flow, hydraulic roughness and sediment movement. Continental Shelf Res. 2010;30:1783–1794. doi: 10.1016/j.csr.2010.08.006. [DOI] [Google Scholar]
- 64.Oreska MPJ, Wilkinson GM, McGlathery KJ, Bost M, McKee BA. Non-seagrass carbon contributions to seagrass sediment blue carbon. Limnol. Oceanogr. 2018;63(S1):S3–S18. doi: 10.1002/lno.10718. [DOI] [Google Scholar]
- 65.Kollmuss, A., Lazarus, M., Lee, C., LeFranc, M., & Polycarp, C. Handbook of Carbon Offset Programs: Trading Systems, Funds, Protocols and Standards. 210p. (London.: Earthscan (2010).
- 66.Forest Trends. Unlocking Potential: State of the Voluntary Carbon Markets 2017. 52p (Washington, D.C.: Forest Trends’ Ecosystem Marketplace (2017).
- 67.Marbà N, et al. Growth and population dynamics of Posidonia oceanica on the Spanish Mediterranean coast: elucidating seagrass decline. Mar. Ecol. Prog. Ser. 1996;137:203–213. doi: 10.3354/meps137203. [DOI] [Google Scholar]
- 68.Orth RJ, McGlathery KJ. Eelgrass recovery in the coastal bays of the Virginia Coast Reserve, USA. Mar. Ecol. Prog. Ser. 2012;448:173–176. doi: 10.3354/meps09596. [DOI] [Google Scholar]
- 69.McGlathery KJ, et al. Recovery trajectories during state change from bare sediment to eelgrass dominance. Mar. Ecol. Prog. Ser. 2012;448:209–221. doi: 10.3354/meps09574. [DOI] [Google Scholar]
- 70.Orth RJ, Moore KA, Marion SR, Wilcox DJ, Parrish DB. Seed addition facilitates eelgrass recovery in a coastal bay system. Mar. Ecol. Prog. Ser. 2012;448:177–195. doi: 10.3354/meps09522. [DOI] [Google Scholar]
- 71.Fourqurean, J. W., et al. Field sampling of soil carbon pools in coastal ecosystems in Coastal blue carbon: methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrass meadows (eds. Howard, J., Hoyt, S., Isensee, K., Pidgeon, E., Telszewski, M.) 39-66 (Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature (2014).
- 72.Kennedy H, et al. Seagrass sediments as a global carbon sink: Isotopic constraints. Global Biogeochem. Cycles. 2010;24:1–8. doi: 10.1029/2010GB003848. [DOI] [Google Scholar]
- 73.Howard, J., Hoyt, S., Isensee, K., Pidgeon, E., & Telszewski, M. Coastal blue carbon: methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and seagrass meadows (eds. Howard, J., Hoyt, S., Isensee, K., Pidgeon, E., Telszewski, M.) 15-24 (Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature. Arlington, Virginia, USA (2014).
- 74.Environmental Systems Research Institute. ArcGIS Desktop: Release 10.2. https://www.esri.com (2014).
- 75.Thomas, E. Influence of Zostera marina on wave dynamics, sediment suspension, and bottom boundary layer development within a shallow coastal bay. Thesis submitted to the University of Virginia 28-30 (2014).
- 76.McGlathery, K. J. Above- and Below-Ground Biomass and Canopy Height of Seagrass in Hog Island Bay and South Bay, VA 2007-2017. Environmental Data Initiative. 10.6073/pasta/09a0ce35bb3fc72113b5a16ad5b0d6bd (2017).
- 77.McGlathery K. J. Carbon and Nitrogen in Seagrass Tissue from Virginia Coastal Bays, 2010-2017. Environmental Data Initiative. 10.6073/pasta/b4d1f74041d329386591a32e9ea202b2 (2017).
- 78.Bates, D., Mächler, M., Bolker, B., & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67(1); 10.18637/jss.v067.i01 (2015).
- 79.R Core Team. R: The R Project for Statistical Computing. https://www.r-project.org (2017).
- 80.Virginia Institute of Marine Science (VIMS). SAV in Chesapeake Bay and Coastal Bays. Available at: http://web.vims.edu/ bio/sav/ (2016).
- 81.McGlathery, K. J. Density of seagrass in Hog Island Bay and South Bay, VA 2007-2017. Environmental Data Initiative. 10.6073/pasta/5a6ea442cf59cabb3112bb634a968ae5 (2017).
- 82.Bahlmann E, et al. Tidal controls on trace gas dynamics in a seagrass meadow of the Ria Formosa lagoon (southern Portugal) Biogeosci. Discuss. 2014;11:10571–10603. doi: 10.5194/bgd-11-10571-2014. [DOI] [Google Scholar]
- 83.Barber, T. R. & Carlson, P. R. Effects of seagrass die-off on benthic fluxes and porewaterconcentrations of ∑CO2, ∑H2S, and CH4 in Florida Bay sediments in Biogeochemistry of Global Change: Radiatively Active Trace Gases (ed. Oremland, R. S.) 530-550 (New York: Chapman & Hall (1993).
- 84.Crill PM, Martens CS. Spatial and temporal fluctuations of methane production in anoxic coastal marine sediments. Limnol. Oceanogr. 1983;28(6):1117–1130. doi: 10.4319/lo.1983.28.6.1117. [DOI] [Google Scholar]
- 85.Deborde J, et al. Methane sources, sinks, and fluxes in a temperate tidal lagoon: the Archachon lagoon (SW France) Estuar. Coast. Shelf Sci. 2010;89(4):256–266. doi: 10.1016/j.ecss.2010.07.013. [DOI] [Google Scholar]
- 86.Banerjee, K., et al Seagrass and macrophyte mediated CO2 and CH4 dynamics in shallow coastal waters. PLoS ONE e0203922; 10.1371/journal.pone.0203922 (2018). [DOI] [PMC free article] [PubMed]
- 87.Sansone FJ, Rust TM, Smith SV. Methane distribution and cycling in Tomales Bay, California. Estuaries. 1998;21(1):66–77. doi: 10.2307/1352547. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported and analyzed in this study is available in the Supplement and on the LTER Network Data Portal (https://portal.lternet.edu/nis/home.jsp).