Abstract
Satellite cells are the myogenic stem and progenitor population found in skeletal muscle. These cells typically reside in a quiescent state until called upon to support repair, regeneration, or muscle growth. The activities of satellite cells are orchestrated by systemic hormones, autocrine and paracrine growth factors, and the composition of the basal lamina of the muscle fiber. Several key intracellular signaling events are initiated in response to changes in the local environment causing exit from quiescence, proliferation, and differentiation. Signals emanating from Notch, wingless-type mouse mammary tumor virus integration site family members, and transforming growth factor-β proteins mediate the reversible exit from growth 0 phase while those initiated by members of the fibroblast growth factor and insulin-like growth factor families direct proliferation and differentiation. Many of these pathways impinge upon the myogenic regulatory factors (MRF), myogenic factor 5, myogenic differentiation factor D, myogenin and MRF4, and the lineage determinate, Paired box 7, to alter transcription and subsequent satellite cell decisions. In the recent past, insight into mouse transgenic models has led to a firm understanding of regulatory events that control satellite cell metabolism and myogenesis. Many of these niche-regulated functions offer subtle differences from their counterparts in livestock pointing to the existence of species-specific controls. The purpose of this review is to examine the mechanisms that mediate large animal satellite cell activity and their relationship to those present in rodents.
Keywords: livestock, myogenesis, paired box 7, satellite cell, skeletal muscle
Introduction
Satellite cells were first discovered in 1961 by Alexander Mauro, who reported finding a unique population of cells located adjacent to the muscle fiber in frogs (Mauro, 1961). Distinct from the muscle fiber, the cells exhibited a chromatin dense nucleus with little cytoplasmic volume. Mauro suggested that these satellite cells may underlie “the vexing problem of regeneration” in skeletal muscle. Building upon the initial finding, Mauro discovered that satellite cells are mitotically active during growth in rats and proposed that these cells may serve as a source of myonuclei for the growing muscle fiber (Shafiq et al., 1968). Importantly, the work revealed that nuclei within the individual fibers are mitotically inactive, thereby rendering them unable to contribute to the accumulating myonuclei found during fiber hypertrophy. Tritiated thymidine labeled cells become part of the Neofiber syncytium following muscle damage suggesting that the myonuclei originated from satellite cells (Snow, 1977) and transplantation of minced, labeled muscle into hosts recapitulate myogenesis with Neofibers containing labeled nuclei (Snow, 1978). Further support for satellite cells as the source of myonuclei was provided through the culture of isolated myofibers in vitro (Bischoff, 1975). By timed microscopic appraisal, Bischoff (1975) reported that fibers physically damaged undergo necrosis with myonuclei experiencing pyknotic death leaving behind an endomysial tube structure. Satellite cells under the basal lamina become mitotically active, repopulating the lamina vestige with myoblasts that ultimately reform the multinucleated structure. A similar finding was reported for quail with satellite cells serving as the only source of myogenic cells capable of differentiating into myotubes (Konigsberg et al., 1975). These early studies provided the foundation for satellite cells as the muscle stem and progenitor population responsible for skeletal muscle growth and repair.
Although substantial information exists for rodent satellite cells, publications describing their counterparts in domestic livestock represent fewer than 4% of the total satellite cell papers published to date (www.nlm.nih.gov/pubmed, accessed June 25, 2019). This is somewhat surprising given that skeletal muscle growth is paramount to production agriculture and the means to improve its deposition and composition are intimately connected to satellite cell biology. The purpose of this review is to provide an overview of autocrine and paracrine factors that regulate satellite cell activity in large domestic animals.
The Origins of Satellite Cells
The identity, function, and necessity of satellite cells to muscle growth, repair, and regeneration have been advanced substantially through the use of mouse genetic models. A seminal discovery was the identification of Paired box 7 (Pax7), a homeobox-containing transcription factor, as a lineage marker of satellite cells (Seale et al., 2000). Using differential screening, Pax7 was present in proliferating mouse satellite cells and absent from myotubes. Genetic ablation of Pax7 resulted in early neonatal lethality in a majority of pups (Mansouri et al., 1996). Examination of the survivors, however, revealed a 50% reduction in muscle mass as well as a complete absence of satellite cells as determined by electron micrography, mass cell culture, and individual fiber isolates (Seale et al., 2000). Pax7 is expressed in the dermomyotome and presumptive myoblasts of the mouse embryo with a partially overlapping expression pattern with Pax3, a paralog (Relaix et al., 2004; Horst et al., 2006). Pax3 is expressed in a subpopulation of adult satellite cells (Conboy and Rando, 2002; Kuang et al., 2006) but the transcription factor is unable to substitute for Pax7 in either embryonic or adult muscle precursor cells (Relaix et al., 2004, 2005, 2006). For a thorough review of transcriptional control of embryonic Pax3 and Pax7 muscle progenitors, please see Dumont et al. (2015) and Sincennes et al. (2016).
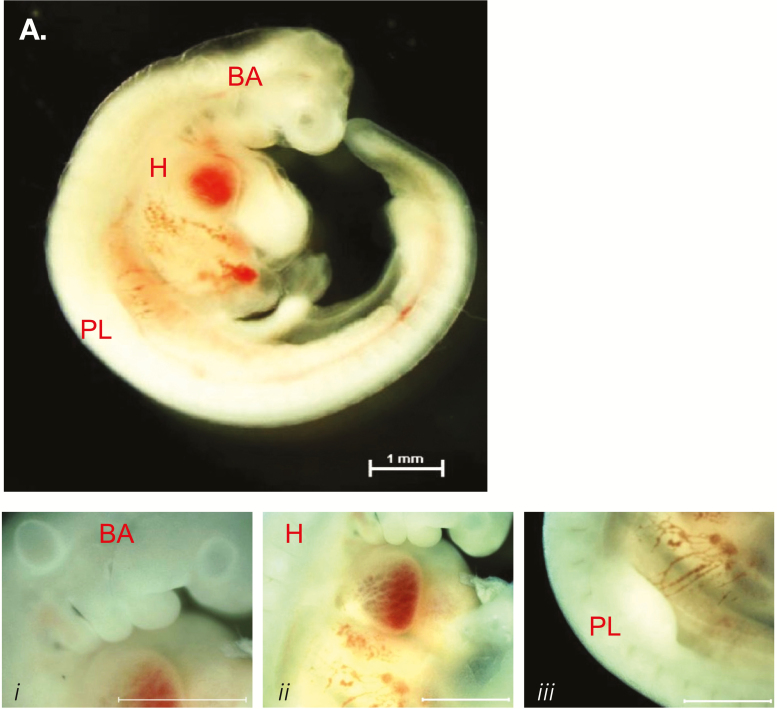
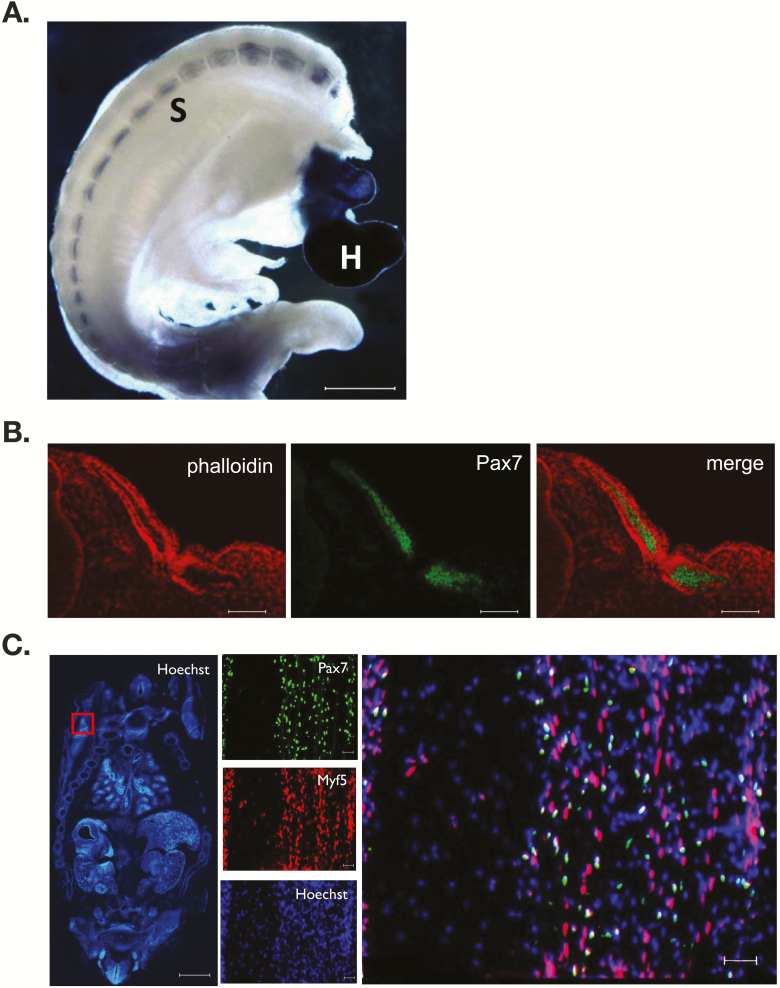
Transcriptome analysis reveals that similar developmental patterns of gene expression exist between mice and cattle. Shortly after the commencement of gastrulation (embryonic day 14), Pax3 mRNA is detected in the bovine conceptus signifying the initial stages of mesoderm formation (Pfeffer et al., 2017). Neither the myogenic factor 5 (Myf5), myogenic differentiation factor D (MyoD), myogenin, myogenic regulatory factor 4 (MRF4) nor Pax7 is expressed at this time. Somites are apparent by embryonic day 21 with between 5 and 14 somite pairs evident (Maddox-Hyttel et al., 2003) (Richard et al., 2015). By day 23 of gestation, a minimum of 24 somite pairs, a presumptive forelimb bud, otic and optic placodes, and five visible branchial arches are present (Figure 1). The morphology and developmental landmarks are equivalent to a Hamburger and Hamilton (1951) stage 21 chick embryo and embryonic day 9.5 in the mouse. Whole-mount immunostaining for myosin heavy chain (MyHC) demonstrates myocytes traversing the somite and in parallel to the neural tube (Figure 2). The presence of the MyHC(+) myotome indicates that temporal expression of the MRFs occurred within the dermamyotome during the developmental window spanning gestational days 14 to 23. Analysis of rostral somite cryosections demonstrates the presence of Pax7 immunopositive cells within the developing dermomyotome (Figure 2). Migration of the myogenic precursors into the developing limb and body muscles ensues resulting in the identification of at least three distinct populations of muscle cells within the embryo at day 45 of gestation: a Pax7(+) cell, a Pax7(+):Myf5(+) cell, and a Pax7(−):Myf5(+) cell. The Myf5-only cell may be analogous to the Pax3(+):Myf5(+) cells found in the muscle anlagen of mice (Bajard et al., 2006; Horst et al., 2006). A definitive answer is elusive as the commercially available antibodies to Pax3 fail to react with the bovine antigen. The numbers of Pax7 immunopositive cells are approximately 70% of the total nuclei within the infraspinatus muscle at gestational day 85 and decline to 45% and 15% at days 145 and 245 of gestation, respectively (Gonzalez et al., 2013). A similar decline in Pax7 expression is found in the longissimus, wherein the abundance of Pax7 mRNA at birth is approximately 5% of the value found at day 90 of gestation (Sun et al., 2015). Thus, the Pax7 precursor cells are steadily incorporated into secondary muscle fibers in utero leaving a minor population of satellite cells at birth.
Figure 1.
Morphological features of a bovine embryo at day 23 of gestation. Embryos were harvested at 23 d of gestation and examined for developmental milestones. Gross morphology of the embryo (A) demonstrating the presence of branchial arches (BA, i), a beating four-chamber heart (H, ii), and the presumptive forelimb bud (PL, iii). Bar represents 1 mm.
Figure 2.
Myogenesis in the early bovine embryo. Day 23 and 45 bovine embryos were fixed and immunostained for key myogenic muscle proteins. MyHC (A) expression localized to the somites (S) and heart (H) of a day 23 embryo. Cryosections through the rostral somites were immunostained for Pax7 (green) and counterstained with phalloidin (red) (B). Coronal sections through a day 45 embryo stained with Hoechst 33324 for nuclei (C). The red box denotes a forelimb with magnification in the remaining panels. Pax7 and Myf5 co-localize to distinct limb myoblast subpopulations. Bar represents 1 mm or 50 µm.
A heterogeneous myogenic precursor population is noted in satellite cell isolates from neonatal calves. Approximately, 85% of the satellite cells isolated from the semitendinosus express Pax7-only or Pax7 and Myf5, corresponding to the putative stem and progenitor populations (Li et al., 2011). The majority of the remaining cells were immunonegative for Pax7 and immunopositive for Myf5. Single-cell reverse transcription-polymerase chain reaction for Pax7, Pax3, and Myf5 mRNA suggests that a portion of the Myf5(−) subpopulation expresses Pax3 but a distinct Myf5-only cell type is still present; this subpopulation is not normally found in postnatal mouse skeletal muscle (Kuang et al., 2007). Satellite cell isolates from neonatal pigs (4 d of age) contain two distinct populations: cells of larger size that attach quickly and fuse into large myotubes and smaller cells that attach to the culture plate substratum slower and form smaller myotubes (Miersch et al., 2018). The smaller satellite cell may represent a distinct progenitor cell as a greater percentage of the cells were Pax7 immunopositive (35%) than the larger cell (18%) at equivalent timepoints in vitro. It should be noted that satellite cell isolates from the longissimus of 4- to 6-wk-old pigs contain a large percentage of Pax3 immunopositive cells (Sebastian et al., 2015). Visual appraisal demonstrates greater than 90% of the cells contain both Pax3 and Pax7. In mice, 80% of colonies formed by trunk muscle satellite cell isolates contain Pax3 by comparison to limb muscle isolates wherein 20% of the colonies express the transcription factor (Relaix et al., 2006). The reason for a diverse population of satellite cells remains unknown but may be linked to specific stages of muscle growth. For example, postnatal fiber hyperplasia occurs in the young pig and it is tempting to speculate that a specific subpopulation of satellite cells accounts for the unique increase in total fiber number (Bérard et al., 2011). With the rapid development of large animal transgenesis, future efforts establishing functional roles for subgroups of satellite cells through targeted ablation will be possible.
Postnatal Satellite Cell Myogenesis
Activation and quiescence
Satellite cell function is regulated, in part, by the local fiber environment. Often referred to as the niche, the myofiber milieu is a source of growth factors and macronutrients that contribute to the metabolic and mitotic activities of the muscle progenitor. The normally quiescent satellite cell transits from growth 0 phase (G0) into the cell cycle wherein it can self-renew, proliferate, or differentiate to support repair or growth. Muscle repair, regeneration, and growth are the primary events leading to satellite cell activation or G0 exit (Anderson, 2016). An activation map for mouse satellite cells includes delivery of systemic hepatocyte growth factor (HGF) A, a pro-HGF protease, followed by HGF docking with its receptor (c-Met) to initiate protein kinase B/mechanistic target of rapamycin C1 (mTORC1) signaling that culminates in phosphorylation of S6 signifying a metabolically active [G(alert)] entity capable of proceeding into the cell cycle or returning to G0 (Rodgers et al., 2014, 2017). Disruption of satellite cell and myofiber interactions results in a quiescent state downstream from G(alert) that may represent a primed muscle stem cell (Goel et al., 2017). Thus, the transition from G0 to a fully active cell represents a continuum with multiple arrest points.
Few direct measures of satellite cell activation in large domestic animals are noted. Immunostaining of fresh isolates of porcine satellite cells with neural cell adhesion molecule (NCAM), a surface marker used to identify human satellite cells, and proliferating cell nuclear antigen (PCNA), an auxiliary protein for DNA polymerase, demonstrated that 80% to 90% of NCAM(+) satellite cells contained nuclear PCNA and thus were considered activated (Mesires and Doumit, 2002). Although a direct comparison to tissue was not performed, it is likely that the isolation process resulted in the large percentage of activated cells as slightly fewer than 5% of satellite cells actively incorporate bromodeoxyuridine (BrdU) in pigs of a comparable age (Alexander et al., 2010). Early experimental definition of activation was noted by an initial delayed cell cycle entry upon seeding in mitogen-rich media (Johnson and Allen, 1993). Termed lag phase, this period of mitotic inactivity was shorter in young satellite cell cultures which express PCNA nearly 24 h sooner than cells isolated from adult rats. The lag phase could be shortened by supplementation with HGF or crushed muscle extract, which was later shown to contain HGF (Johnson and Allen, 1993; Allen et al., 1995; Tatsumi et al., 1998). Using an analogous in vitro system, fresh isolates of satellite cells from young calves (3 to 5 d of age) reengage the cell cycle sooner than isolates from adults (Li et al., 2011). The lag period can be shortened by media supplementation with HGF further supporting a role for the growth factor as an activator (Lapin et al., 2013). Interestingly, bovine satellite cells enter S-phase, as indicated by incorporation of a thymidine analog, prior to the expression of MyoD, a confirmed marker of activation in rodent satellite cells (Li et al., 2011). During the lag period, these cells also respond to the chemoattractant, ephrin A5, which precedes HGF-directed migratory responses (Li and Johnson, 2013). Chemoattraction is evident only in satellite cells prior to MyoD expression suggesting that ephrin A5 recruits and positions the cell along the sarcolemma membrane. This is in stark contrast to mouse satellite cells wherein ephrin A5 acts as a chemorepulsive factor to activated, MyoD(+) cells (Stark et al., 2011). The initial lag period remains unaffected by HGF supplementation to adult equine satellite cells suggesting species-specific mechanisms may govern this phase of myogenesis (Brandt et al., 2018b). Isolates from adult horses were cultured with physiological concentrations of HGF with no effect on the 48-h lag period. Although HGF may be involved in the activation of satellite cells in vivo, it is worth noting that mRNA for the growth factor increases after PCNA(+) cells are apparent and coincident with Pax7 during post-exercise damage repair in horses (Kawai et al., 2013). The identity of niche-associated molecules that promote satellite cell entry and exit from G0 in livestock may assist with the design of management strategies that promote more efficient myonuclear accretion.
The activated satellite cell as a muscle stem cell displays self-renewal properties that are governed by Notch signals (Bjornson et al., 2012; Wen et al., 2012). Notch, the receptor component of a transmembrane intercellular communication system, is cleaved to form Notch intracellular domain (NICD) which translocates to the nucleus and interacts with recombination signal-binding protein for immunoglobulin kappa J region (Rbpj) to affect transcription (Bigas and Espinosa, 2018). Chemical inhibition of Notch signals in vitro results in the expansion of the committed myoblast pool at the expense of self-renewal while ectopic expression of NICD causes an increase in Pax7 expression (Kuang et al., 2007; Wen et al., 2012). Blocking Notch activity in vivo by genetic ablation of Rbpj causes spontaneous activation and differentiation of satellite cells while preventing self-renewal (Bjornson et al., 2012). Ectopic expression of NICD in porcine satellite cells causes a 5-fold increase in Pax7 mRNA expression while suppression of Notch-1 activity facilitates differentiation (Qin et al., 2013; Jiao et al., 2018). Although no other reports exist for cattle or sheep, these results are suggestive of a conserved Notch signaling axis underlying satellite cell self-renewal.
Growth factors and proliferation
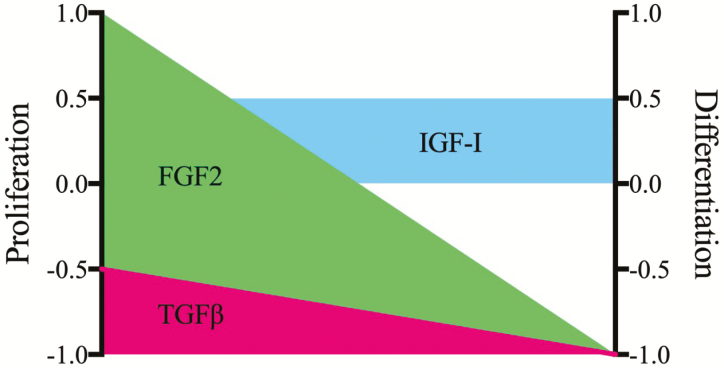
Similar to activation and self-renewal, the fiber niche plays an integral role in proliferation and differentiation (Figure 3). A host of autocrine and paracrine mitogens exist for satellite cells that include fibroblast growth factor (FGF2), HGF, insulin-like growth factor (IGF-1), IGF-II, epidermal growth factor (EGF), and heparin-binding EGF (HB-EGF). FGF 2 serves as a strong stimulant of proliferation while inhibiting morphological differentiation of bovine and porcine satellite cells (Greene and Allen, 1991; Doumit et al., 1993). The growth factor likely acts in a similar capacity on ovine satellite cells, as FGF2 is a component of serum-free media that facilitates survival and increased cell numbers (Dodson and Mathison, 1988). The effective mitogenicity of FGF2 is highlighted by the inability of transforming growth factor-beta (TGF-β 1), a recognized inhibitor of both proliferation and differentiation, to block FGF2-directed bovine satellite cell proliferation (Greene and Allen, 1991). Supplementation of serum-free culture media with IGF-1 (30 ng/mL) does not affect the basal proliferation of bovine satellite cell (Greene and Allen, 1991). By contrast, bovine satellite cells treated with long arginine 3 IGF-1, an IGF-1 analog unable to interact with IGF-binding proteins, were able to stimulate a 2-fold increase in radiolabeled thymidine incorporation (Kamanga-Sollo et al., 2014). Treatment of bovine satellite cells with 500 ng/mL IGF-I in serum-containing media results in a modest (20%) increase in cell proliferation (Ge et al., 2012). Satellite cell isolates from pigs treated with 20 to 100 ng/mL IGF-1 demonstrate a 2-fold increase in DNA content in both a serum-free and low serum basal media (Doumit et al., 1993). Maximal radiolabeled thymidine incorporation into DNA occurred at a concentration of 1 ng/mL with no further increases at 10 or 100 ng/mL (Mau et al., 2008). And, a 30% increase in labeled DNA incorporation is noted following treatment of equine satellite cells with 25 ng/mL IGF-1 in reduced serum media (LaVigne et al., 2015). Others indicate adult sheep satellite cells proliferate in response to autocrine IGF-I production (Oksbjerg et al., 2004). Summation of these studies supports a role for exogenous IGF-1 as a weak mitogen for satellite cells isolated from livestock (Figure 3). However, the functional importance of IGF-1 to the satellite cell remains complex and unresolved as supplementation with 1 to 3 ng/mL IGF-I Ec caused a 2-fold increase in porcine satellite cell numbers while supplementation with 5 ng/mL resulted in a complete inhibition of proliferation (Qin et al., 2012). Because both concentrations are regarded as physiological and IGF-1Ec is produced by the muscle fiber, it is likely the paracrine supply of the IGF-1 splice variant affects the decision of the satellite cell to proliferate, remain quiescent, or possibly fuse with the adjacent fiber.
Figure 3.
Primary regulators of satellite cell proliferation and differentiation in vitro. Satellite cell isolates from meat-producing livestock cultured in permissive media with the FGF2 (green) elicit a strong proliferative response with a corresponding inhibition of differentiation. Modest mitogenic and myogenic actions are found for IGF-I (blue) with TGF-β (red) serving as a general inhibitor of proliferation and a strong suppressor of differentiation. Scale +1 denotes maximal positive response and −1 maximal inhibitory response.
Members of the TGF-β superfamily are key regulators of satellite cell myogenesis and skeletal muscle biology. Generally regarded as negative effectors of muscle growth and satellite cell activity, TGF-β 1 and myostatin (MSTN) bind to their respective extracellular receptors and elicit signals mediated by Sma- and Mad-related (Smad) proteins (for review, see Chen et al., 2016). Originally described as an inhibitor of biochemical and morphological differentiation of mouse myoblasts, TGF-β 1 appeared to be a specific repressor of fusion and contractile protein gene expression as no effects on myoblast division were observed (Olson et al., 1986). In a similar manner, treatment of clonal cultures of ovine satellite cells with TGF-β 1 either in the presence or in the absence of serum had no effect on proliferation (Hathaway et al., 1991). This is in contrast to the treatment of porcine or bovine satellite cells wherein TGF-β 1 acts as an inhibitor of proliferation ( Greene and Allen, 1991; Hathaway et al., 1991). Concentrations as low as 50 pg/mL TGF-β 1 are sufficient to completely suppress bovine and porcine satellite cell proliferation in a growth-permissive, serum-containing media demonstrating the potent growth repressor activities of the cytokine (Hathaway et al., 1991). Interestingly, TGF-β 1 inhibits proliferation of satellite cells isolated from newborn lambs by approximately 10% when compared with non-treated controls with embryonic myoblast proliferation reduced by 30% (Hathaway et al., 1994). These results infer that early muscle progenitors may be affected by the cytokine and become refractile with developmental age.
Mutation of the MSTN gene was discovered as the causative effect of the double-muscled phenotype in Belgian Blue and Piedmontese cattle (McPherron and Lee, 1997). Reduced levels of MSTN mRNA as a result of a mutation in the 3′ untranslated region that creates a microRNA (miRNA) site underlie the increased muscle mass found in Texel sheep (Clop et al., 2006). Genome-wide association studies correlate single-nucleotide polymorphisms on chromosome 18 in proximity to the MSTN locus with a larger body size and likely muscle mass in horses (Tozaki et al., 2017). MSTN is temporally expressed during myogenesis in vitro with no detectable expression in proliferating or confluent bovine satellite cells but the substantial expression in differentiated myotubes (Deveaux et al., 2003). The myokine is transcribed by day 30 of gestation wherein it contributes to the downregulation of RPL3, 11, and 17, all genes involved in ribosome biogenesis and protein synthesis (Potts et al., 2003). Detectable amounts of MSTN are present in the fetal bovine vastus lateralis throughout gestational days 60 to 265 with trace amounts of MSTN mRNA evident at postnatal day 8 (McFarlane et al., 2005). The temporal expression pattern supports elevated levels of MSTN during the expansion of the myoblast pool with lower abundance during periods of muscle fiber hypertrophy. Fetal myoblasts isolated from normal and MSTN null bovine animals do not differ in basal proliferation rate suggesting that autocrine MSTN may not play a substantial role (Thomas et al., 2000). However, proliferation of both wild-type and mutant cell types is inhibited by culture in the presence of 1 to 4 ug/mL recombinant bovine MSTN. These results are consistent with the treatment of human satellite cells with the myokine wherein a 15% to 20% suppression of proliferation is observed (Fakhfakh et al., 2011).
IGF-1 potentiates skeletal muscle growth, development, and function (for a thorough review, see Schiaffino and Mammucari, 2011; Snijders et al., 2015). Rat satellite cells treated with the IGF-1 both proliferate and differentiate to greater levels than that achieved in non-treated controls supporting a dual role for the growth factor (Allen and Boxhorn, 1989). And, ovine satellite cells proliferate in a dose-dependent manner with IGF-I supplementation (Dodson et al., 1985). By contrast, culture of bovine satellite cells in serum-free medium with IGF-I resulted in no change in total cell number (Greene and Allen, 1991). Interestingly, the cells proliferated in response to IGF-I when a low concentration of FGF2 was included in the media. This may explain the mitotic effects observed by others who demonstrate a 1.5-fold increase in bovine satellite cell number following treatment with IGF-I in a serum-containing medium (Han et al., 2008; Ge et al., 2013). The chief intracellular pathways underlying the IGF-I mitotic effects include phosphoinositide 3-kinase upregulation of cyclin D and extracellular regulated kinase 1 and 2 phosphorylation (Ge et al., 2013). It is likely that downstream mTOR-driven signals are important for IGF-I stimulated proliferation; however, the use of rapamycin as an mTOR inhibitor suppressed basal protein synthesis required for viability limiting definitive evidence.
Equally important are the systemic hormones, estrogen, testosterone, growth hormone, and oxytocin, which affect satellite cell mitosis. Porcine satellite cells treated with testosterone demonstrated an increase in androgen receptor content with no effect on proliferation rates or responsiveness to the mitogens, FGF2, or IGF-I (Doumit et al., 1996). By contrast, bovine satellite cells proliferate following treatment with trenbolone acetate, an anabolic testosterone analog. Bovine satellite cells supplemented with physiological concentrations of 17β-estradiol or trenbolone acetate proliferate through a mechanism that involves autocrine synthesis and release of IGF-1 and HB-EGF that stimulate mitosis via IGF-1 and EGF receptors, respectively (Kamanga-Sollo et al., 2008, 2014; Reiter et al., 2014; Thornton et al., 2015). The effects of steroid compounds on bovine satellite cell proliferation remain complex though, as treatment of the cells with either 17β-estradiol or trenbolone acetate and an oxytocin receptor inhibitor suppresses mitotic activity supporting a role for oxytocin as a mitogen (Zhang et al., 2019).
Satellite Cell Differentiation and Fiber Hypertrophy
Differentiation is defined as the acquisition of a specialized function. Myoblast differentiation is the culmination of events from the cessation of myoblast proliferation to contractile protein synthesis and myofibril assembly. The morphological and biochemical events underlying differentiation occur during developmental myogenesis and satellite cell-mediated muscle regeneration. Postnatal satellite cell differentiation contributes to growth as an increase in DNA content in the absence of fiber hyperplasia occurs. Self-renewal of the muscle stem and progenitor population occurs as satellite cells are present throughout the lifetime of the individual. It remains unresolved, however, if a satellite cell requires self-replication prior to fusion into the fiber. Dogma argues that satellite cells as myonuclei donors underlie the hypertrophic response by maintaining a constant myonuclear domain (MND), the cytoplasmic volume controlled transcriptionally by a single myonuclei within the fiber. Early efforts demonstrated that gamma irradiation of skeletal muscle destroys the satellite cell population and prevents the typical increase in fiber diameter found following synergist ablation (Adams et al., 2002). However, challenges to the requirement of satellite cells for fiber hypertrophy and constant MND exist. Administration of the beta-adrenergic agonist, cimaterol, to lambs or ractopamine to cattle increases muscle mass without a concomitant increase in myonuclei numbers suggesting that satellite cells are not essential for muscle fiber hypertrophy (Beermann et al., 1987; Kim et al., 1987; Gonzalez et al., 2007). Insight into the necessity of satellite cells for growth was provided by elegant transgenic experiments in mice. Using a conditional ablation model, satellite cells were eliminated from the tissue of young (<4 mo) and adult (>4 mo) mice and examined following compensatory overload (McCarthy et al., 2011; Murach et al., 2017). Results demonstrate that young mice require the stem cells as indicated by an absence of hypertrophy while adult animals experience an increase in MND size indicative of muscle hypertrophy without satellite cells. Extension of these finding to livestock production imply that satellite cells may only be required for the initial log phase of growth but upon maturity, further increases in muscle mass are achieved primarily through protein accretion.
Growth factor regulation of differentiation
Independent of their unresolved requirement for growth, satellite cell differentiation is acutely affected by niche-localized signaling molecules. The paracrine growth factors, IGF-1 and MSTN, and the myokines, interleukin (IL) 6 and IL15, are the most exhaustively studied effectors of satellite cell differentiation. In general, IGF-1 is regarded as an anabolic effector of differentiation as indicated by a modest increase (15% to 20%) in fusion, myotube diameter, and muscle protein content in human and rat myogenic cells (Allen and Boxhorn, 1989; Jacquemin et al., 2004). Minimal improvements in fusion indices are reported for bovine, equine, and porcine satellite cell isolates (Ge et al., 2012; LaVigne et al., 2015; Chen et al., 2017). In each species, however, IGF-1 plays a major role in protein synthesis and increased contractile protein content in the myotube (Ge et al., 2013; DeBoer et al., 2018). Thus, IGF-1 may not play a substantive role in myonuclear accumulation through satellite cell addition but represent a critical metabolic effector of fiber hypertrophy. In a similar manner, little (<5%) to no effect on fusion or biochemical measures of differentiation are observed with equine and bovine satellite cells, respectively, treated with IL6 (LaVigne et al., 2015; Brandt et al., 2018a). The inability to detect substantial promotional effects on myoblast fusion may be due to the ease with which myotubes form in vitro. Even in the presence of high concentrations of fetal bovine serum (20% v/v), fusion occurs in bovine and equine satellite cell cultures underscoring the difficulty in preventing developmental myogenesis (S. Johnson, unpublished). This is an important point when considering the robust inhibitory actions of TGF-β1 and MSTN. Supplementation of culture media with recombinant MSTN (8 µg/mL) completely abolishes fetal bovine myoblast fusion and MyHC protein expression (Langley et al., 2002). It is worth noting that MSTN inhibition of differentiation requires nearly a 3-fold greater concentration than TGF-β 1 with both eliciting their effects through phosphorylation of Smad2/3 (Trendelenburg et al., 2009).
Genetic predisposition to satellite cell differentiation and fiber hypertrophy
Livestock breeds with a smaller muscle mass likely have inherent differences in satellite cell activity. Wagyu heifers at the same chronological age as Angus heifers contain fewer satellite cells that tend to fuse into smaller myotubes in vitro (Fu et al., 2018). In a similar manner, satellite cells isolated from Holstein bulls form 50% fewer myotubes in vitro than either Hereford or Limousin satellite cell isolates (Sadkowski et al., 2018). Reduced expression of myogenin, the major MRF controlling differentiation, was noted but myogenin/unit of myosin remained similar amongst the breeds indicating that additional mechanisms exist that drive myotube formation. MicroRNAs are short, noncoding RNA molecules that bind to cis regions within mRNA creating hybrid molecules that are either degraded or translationally silenced (Horak et al., 2016). Epigenetic regulation of bovine satellite cell differentiation includes differential expression of several miRNAs that directly target myogenic transcription factors, intracellular signaling components, and metabolism (Table 1). For example, miR-133a, miR-133b, and miR-206 target Pax7, PRDM16, FGFR1, and other critical transcriptional and intracellular signaling genes in human and mouse myogenic cells are expressed developmentally in an equivalent manner during bovine myogenesis indicating a core regulatory network exists independent of species (Mok et al., 2017). The contribution of developmental programming to myotube identity is further noted by contractile gene expression profiles. Satellite cells isolated from white semitendinosus of adult pigs fuse into multinucleated myotubes that express greater amounts of adult MyHC type IIX than their counterparts derived from the red semitendinosus (Zhu et al., 2013). Caution is warranted, however, as 90% of the total myosin expressed by myotubes in culture are the embryonic or fetal isoforms (Perruchot et al., 2012; Zhu et al., 2013).
Table 1.
MicroRNAs differentially expressed during the transition from bovine myoblast to myotube1
1Minimum of 2-fold change.
2Italicized targets based on nlm.nih.gov database searches [accessed October 31, 2018].
Donor age and fusion capacity
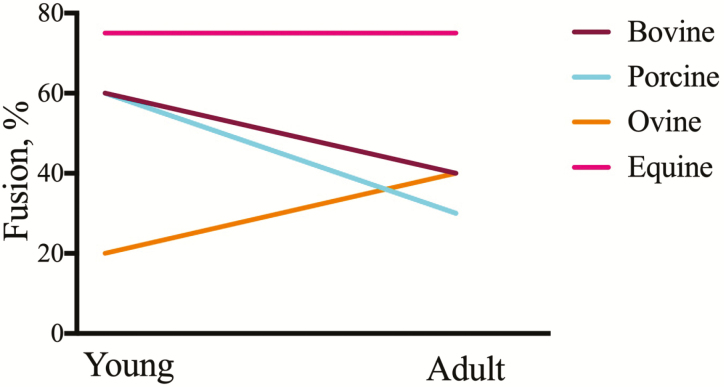
The extent of satellite cell fusion in vitro is both species and age-dependent (Figure 4). Bovine satellite cells isolated from immature calves (<30 d of age) exhibit 60% fusion when cultured in differentiation permissive media by comparison to adult isolates which demonstrate approximately 40% fusion providing evidence for a decline in fusion capacity with advancing age (Ge et al., 2012; Brandt et al., 2018a). Satellite cells isolated from newborn piglets (<5 d of age) or juvenile pigs (5 to 6 wk of age) form large multinucleated syncytia with 60% of the total nuclei contained within the myotube (Zhu et al., 2013; Chen et al., 2017). By contrast, only 30% of satellite cells isolated from adult pigs fuse into myotubes upon culture in differentiation permissive media (Zhu et al., 2013). Others report 20% to 30% fusion rates in isolates of neonatal porcine satellite cells (Miersch et al., 2017, 2018; Vaughn et al., 2018). Possible reasons for lower terminal differentiation capacity may be membrane disruptions as a consequence of liquid nitrogen storage and/or Ficoll density gradient purification. It is noteworthy that cultures of density gradient-purified porcine muscle cells contain 60% to 80% myogenin immunopositive cells at confluence yet fewer than 20% of the total nuclei are contained within a myosin-expressing myotube (Miersch et al., 2017, 2018). This suggests that membrane perturbation affects fusion competence specifically and independent of biochemical differentiation. Ovine satellite cells isolated from adult sheep demonstrate fusion rates of approximately 40% which is greater than values reported for cells isolated at birth (~20% fusion) (Stewart et al., 2001; Raja et al., 2016). Equine satellite cell cultures appear to be different than those isolated from meat-producing livestock. Isolates from 10-d-old foals and adult horses readily form myotubes with fusion rates exceeding 75% independent of donor age (LaVigne et al., 2015; Brandt et al., 2018b; DeBoer et al., 2018). Because horses have a long lifespan, it remains unknown if isolates from horses of advanced age (>30 yr) display the characteristic reduction in fusion capacity.
Figure 4.
Donor age and species differences impact satellite cell myogenic capacity in vitro. Satellite cells were isolated from young and adult cattle, sheep, pigs, and horses, cultured to confluence and examined at various times after fusion. The extent of myoblast fusion reported by authors (see text) graphed as a function of age. Young is equivalent to pre-weaning and adult is considered post-pubertal and beyond the inflection point on a traditional growth curve.
Extracellular matrix and biomechanical properties controlling differentiation
Structural macromolecules and glycoproteins found within the basal lamina represent broad classes of niche factors that play regulatory roles during satellite cell myogenesis. Indeed, culture of satellite cells in vitro requires a supporting matrix (entactin, collagen, laminin, and fibronectin) for cell attachment, proliferation, and differentiation. Fusion of satellite cells isolated from pigs of similar ages and cultured on lysine-fibronectin matrices is 5% (Murray et al., 2018), and 60% when cultured on Matrigel, a complex extracellular matrix (ECM) (Chen et al., 2017), underscoring the importance of the attachment substrate. Support matrices of defined biophysical properties can direct stem cell fate and lineage decisions as well as drive proliferation and differentiation (Lutolf et al., 2009). Culture of bovine satellite cells on pliable polyacrylamide materials with a Young’s modulus that closely mimics that of skeletal muscle (6 kPa) results in fewer actively dividing cells by comparison to conventional culture in plastic tissueware (Lapin et al., 2013). The tensile nature of the myofiber niche is determined by the composition and crosslinking of the primary protein macromolecules, including collagen, laminin, fibronectin, and proteoglycans (Gillies and Lieber, 2011). In addition to its structural properties, collagen 8A1 promotes satellite cell proliferation (Li et al., 2018). It remains unknown if the positive effect is a consequence of altered ECM biophysical properties or a direct effect on intracellular signaling events. Other components of the basal lamina influence satellite cell dynamics by sequestration of growth factors, enzymatic activation of propeptides, and modulation of receptor availability. Extracellular matrix protein 2 (ECM2), which contains a follistatin-like domain, is temporally expressed with differentiation and fusion in cultured bovine satellite cells (Liu et al., 2018). Increased expression of the protein using genetic engineering methodology accelerates myotube formation and muscle-specific protein content by comparison to controls.
Nutrition and Satellite Cell Activity
Intrauterine growth restriction (IUGR) causes long-term consequences on offspring health and performance that are dependent upon the timing of the insult and duration (Barker, 1992). Hyperthermic insult that causes placental insufficiency and subsequent IUGR also disrupts ovine fetal and satellite cell myogenesis (Yates et al., 2014). Although equivalent numbers of Pax7(+) cells are found in the semitendinosus of late gestational (day 134) IUGR and control fetuses, the IUGR fetus contains nearly 50% fewer myogenin(+) cells as measured by cryosection immunohistology. Isolates of IUGR satellite cells proliferate slower and contain fewer MyoD(+) cells during log-phase growth. Although myogenin cell numbers were not measured in vitro, the fewer numbers of MyoD(+) cells point toward early myogenin expression and differentiation. Maternal nutrient restriction (NR) also alters ovine satellite cell myogenesis. Pregnant ewes fed 60% of the NRC recommended caloric intake during gestation causes an increase in fetal semitendinosus fibers/unit area suggestive of smaller fibers at day 135 of gestation with no difference in the percentage of Pax7(+) cells (Gauvin et al., 2020). Satellite cell isolates from NR offspring contain a greater number of MyoD(+) myoblasts that differentiate sooner than control isolates (Raja et al., 2016). This implies that the NR satellite cells are arrested in a state of precocious differentiation and primed for rapid fusion at birth. The epigenetic modification is retained within the satellite cell as isolates from 3-mo-old lambs that originally experienced NR contain a greater percentage of myogenin immunopositive cells that accumulate more rapidly in vitro than their control counterparts. Programming of myogenic precursor cells toward a myoblast state also occurs in bovine fetuses experiencing IUGR. Nutrient restriction of cows during the first 85 d of pregnancy causes a 40% reduction in the numbers of Pax7(+) cells in the fetal infraspinatus with an increase in fiber cross-sectional area (Gonzalez et al., 2013). Thus, IUGR facilitates the progression of Pax7 myogenic precursors toward terminal differentiation. Epigenetic regulation of muscle size and satellite cell function is further noted in littermates that experience divergent growth rates. Gilts classified as small, normal, or heavy in size at 6 wk of age possess equivalent numbers of satellite cells that differentiate to different extents (Nissen and Oksbjerg, 2009). Isolates from smaller pigs form smaller myotubes in vitro as indicated by a lower protein:DNA than their heavier littermates. Neonates appear to behave in a similar manner. Holstein bull calves maintained on a lower plane of nutrition for 8 wk had smaller muscles and muscle fiber cross-sectional areas with no differences in Pax7(+) cell numbers when compared with calves fed a calorie-dense diet (MacGhee et al., 2017). Comparing satellite cell activities over the course of the feeding period reveals a gradual decline in mitotic activity in vitro for calves fed a calorie-dense diet, as predicted. By contrast, the mitotic activity of satellite cells from calves fed a diet to meet their needs was unchanged and greater than those fed a high plane of nutrition after 4 and 8 wk of treatment. This argues that diet can alter satellite cell dynamics during the early postnatal period. Newborn piglets administered a high protein dietary aid or a low protein diet supplemented with β-hydroxy-β-methylbutyrate supports greater proliferation of putative satellite cells in the longissimus by comparison to piglets administered a low protein diet (Kao et al., 2016). A 2-fold reduction in numbers of BrdU(+) satellite cells is found in newborn piglets fed a phosphorus-deficient diet for 2 wk providing evidence for micronutrients as satellite cell effectors (Alexander et al., 2010). Importantly, culture of satellite cells from the subclinical phosphorus-deficient piglets proliferated at rates comparable to controls but expressed less MyoD and myogenin mRNA with no apparent effect on the numbers of immunopositive cells, respectively (Alexander et al., 2012). Although no direct measure of protein content of the MRFs was performed, a disconnect between mRNA and protein content supports a role for metabolic reprogramming of the neonatal satellite cell.
Wagyu cattle are reared for their ability to deposit large amounts of intramuscular fat (marbling) and the regional fat cell precursors may contribute to the localized suppression of satellite cell differentiation. Bovine satellite cells treated with palmitic acid (saturated, long-chain fatty acid), oleic acid (monounsaturated fatty acid), or docosahexaenoic acid (polyunsaturated fatty acid) exhibit a greater fusion capacity and increased expression of myogenin and MyHC(Xu et al., 2018). A possible mechanism for the improved differentiation may include increased beta-oxidation and energy production for morphological differentiation or simply that fusion is enhanced by the insertion of any fatty acid into the sarcolemma.
Summary
Satellite cells are the requisite muscle stem and progenitor for repair and regeneration. Their involvement in muscle hypertrophy, however, may be limited to prepubertal growth. In addition to age, genetics, environment, and diet, all serve as modulators of the global myogenic process through their direct and indirect actions within the fiber niche (Figure 5). Through our reliance on in vitro assay systems, a defined role for growth factors normally found within the fiber niche (FGF2, TGF-β, IGF-I) as key regulators of satellite cell proliferation and differentiation has been established. With advances in genetic technologies and their application to livestock, confirmatory knowledge gains can be made that provide effector targets for satellite cell bioactivity. A better understanding of how the niche interfaces with extrinsic factors to create a regulatory network for satellite cells has the potential to substantially affect muscle growth in meat-producing animals and improve muscle repair, an important contributor to animal health and well-being.
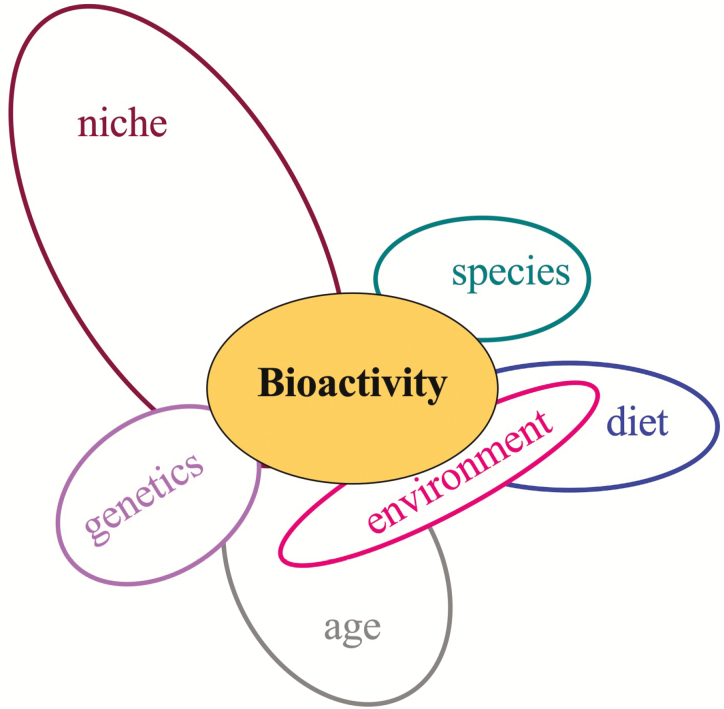
Figure 5.
Satellite cell bioactivity is regulated by multiple factors. Summary of the literature provides evidence for multiple intrinsic (genetics, niche, age) and extrinsic factors (diet, environment) that impinge upon one another to affect the decisions of the satellite cell.
Acknowledgments
The work described herein was supported partially by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2014-67015-21605. Data were presented at the 2018 ASAS-CSAS Growth and Development Symposium: Stem/progenitor cells in animal growth and development, July 9 2018, Vancouver, BC, Canada.
Glossary
Abbreviations
- BrdU
bromodeoxyuridine
- ECM
extracellular matrix
- ERK1/2
extracellular regulated kinase 1 and 2
- FGF
fibroblast growth factor
- G0
growth 0 phase
- HB-EGF
heparin-binding epidermal growth factor
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- IL
interleukin
- IUGR
intrauterine growth restriction
- miR
micro RNA
- MND
myonuclear domain
- MRF
myogenic regulatory factor
- MSTN
myostatin
- mTOR
mechanistic target of rapamycin kinase
- Myf5
myogenic factor 5
- MyHC
myosin heavy chain
- MyoD
myogenic differentiation factor D
- NCAM
neural cell adhesion molecule
- NR
nutrient restriction
- Pax
Paired box
- PCNA
proliferating cell nuclear antigen
- PI3K
phosphoinositide 3-kinase
- Rbpj
recombination signal-binding protein for immunoglobulin kappa J region
- RPL
ribosomal protein L
- Smad
Sma- and Mad-related protein
- TCF
T-cell factor
- TGF-β
transforming growth factor beta
- UTR
untranslated region
Conflict of interest statement
The authors declare no real or perceived conflict of interest.
Literature Cited
- Adams, G. R., Caiozzo V. J., Haddad F., and Baldwin K. M.. . 2002. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am. J. Physiol. Cell Physiol. 283:C1182–C1195. doi: 10.1152/ajpcell.00173.2002 [DOI] [PubMed] [Google Scholar]
- Alexander, L. S., Mahajan A., Odle J., Flann K. L., Rhoads R. P., and Stahl C. H.. . 2010. Dietary phosphate restriction decreases stem cell proliferation and subsequent growth potential in neonatal pigs. J. Nutr. 140:477–482. doi: 10.3945/jn.109.117390 [DOI] [PubMed] [Google Scholar]
- Alexander, L. S., Seabolt B. S., Rhoads R. P., and Stahl C. H.. . 2012. Neonatal phosphate nutrition alters in vivo and in vitro satellite cell activity in pigs. Nutrients. 4:436–448. doi: 10.3390/nu4060436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, R. E., and Boxhorn L. K.. . 1989. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J. Cell. Physiol. 138:311–315. doi: 10.1002/jcp.1041380213 [DOI] [PubMed] [Google Scholar]
- Allen, R. E., Sheehan S. M., Taylor R. G., Kendall T. L., and Rice G. M.. . 1995. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 165:307–312. doi: 10.1002/jcp.1041650211 [DOI] [PubMed] [Google Scholar]
- Anderson, J. E. 2016. Hepatocyte growth factor and satellite cell activation. Adv. Exp. Med. Biol. 900:1–25. doi: 10.1007/978-3-319-27511-6_1 [DOI] [PubMed] [Google Scholar]
- Bajard, L., Relaix F., Lagha M., Rocancourt D., Daubas P., and Buckingham M. E.. . 2006. A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev. 20:2450–2464. doi: 10.1101/gad.382806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, D. J. 1992. The effect of nutrition of the fetus and neonate on cardiovascular disease in adult life. Proc. Nutr. Soc. 51:135–144. doi: 10.1079/pns19920023 [DOI] [PubMed] [Google Scholar]
- Beermann, D. H., Butler W. R., Hogue D. E., Fishell V. K., Dalrymple R. H., Ricks C. A., and Scanes C. G.. . 1987. Cimaterol-induced muscle hypertrophy and altered endocrine status in lambs. J. Anim. Sci. 65:1514–1524. doi: 10.2527/jas1987.6561514x [DOI] [PubMed] [Google Scholar]
- Bérard, J., Kalbe C., Lösel D., Tuchscherer A., and Rehfeldt C.. . 2011. Potential sources of early-postnatal increase in myofibre number in pig skeletal muscle. Histochem. Cell Biol. 136:217–225. doi: 10.1007/s00418-011-0833-z [DOI] [PubMed] [Google Scholar]
- Bigas, A., and Espinosa L.. . 2018. The multiple usages of Notch signaling in development, cell differentiation and cancer. Curr. Opin. Cell Biol. 55:1–7. doi: 10.1016/j.ceb.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Bischoff, R. 1975. Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 182:215–235. doi: 10.1002/ar.1091820207 [DOI] [PubMed] [Google Scholar]
- Bjornson, C. R., Cheung T. H., Liu L., Tripathi P. V., Steeper K. M., and Rando T. A.. . 2012. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells 30:232–242. doi: 10.1002/stem.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, A. M., Kania J. M., Gonzalez M. L., and Johnson S. E.. . 2018b. Hepatocyte growth factor acts as a mitogen for equine satellite cells via protein kinase C δ directed signaling. J. Anim. Sci. 165:307. doi: 10.1093/jas/sky234/5039103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, A. M., Kania J. M., Reinholt B. M., and Johnson S. E.. . 2018a. Human IL6 stimulates bovine satellite cell proliferation through a Signal transducer and activator of transcription 3 (STAT3)-dependent mechanism. Domest. Anim. Endocrinol. 62:32–38. doi: 10.1016/j.domaniend.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Chen, J. L., Colgan T. D., Walton K. L., Gregorevic P., and Harrison C. A.. . 2016. The TGF-β signalling network in muscle development, adaptation and disease. Adv. Exp. Med. Biol. 900:97–131. doi: 10.1007/978-3-319-27511-6_5 [DOI] [PubMed] [Google Scholar]
- Chen, Y., Zhu H., McCauley S. R., Zhao L., Johnson S. E., Rhoads R. P., and El-Kadi S. W.. . 2017. Diminished satellite cell fusion and S6K1 expression in myotubes derived from skeletal muscle of low birth weight neonatal pigs. Physiol Rep. 5:e13075. doi: 10.14814/phy2.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clop, A., Marcq F., Takeda H., Pirottin D., Tordoir X., Bibé B., Bouix J., Caiment F., Elsen J. M., Eychenne F., . et al. 2006. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat. Genet. 38:813–818. doi: 10.1038/ng1810 [DOI] [PubMed] [Google Scholar]
- Conboy, I. M., and Rando T. A.. . 2002. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 3:397–409. doi: 10.1016/s1534-5807(02)00254-x [DOI] [PubMed] [Google Scholar]
- Dai, Y., Zhang W. R., Wang Y. M., Liu X. F., Li X., Ding X. B., and Guo H.. . 2016. MicroRNA-128 regulates the proliferation and differentiation of bovine skeletal muscle satellite cells by repressing Sp1. Mol. Cell. Biochem. 414:37–46. doi: 10.1007/s11010-016-2656-7 [DOI] [PubMed] [Google Scholar]
- DeBoer, M. L., Martinson K. M., Pampusch M. S., Hansen A. M., Wells S. M., Ward C., and Hathaway M.. . 2018. Cultured equine satellite cells as a model system to assess leucine stimulated protein synthesis in horse muscle. J. Anim. Sci. 96:143–153. doi: 10.1093/jas/skx028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveaux, V., Picard B., Bouley J., and Cassar-Malek I.. . 2003. Location of myostatin expression during bovine myogenesis in vivo and in vitro. Reprod. Nutr. Dev. 43:527–542. doi: 10.1051/rnd:2004003 [DOI] [PubMed] [Google Scholar]
- Dodson, M. V., Allen R. E., and Hossner K. L.. . 1985. Ovine somatomedin, multiplication-stimulating activity, and insulin promote skeletal muscle satellite cell proliferation in vitro. Endocrinology 117:2357–2363. doi: 10.1210/endo-117-6-2357 [DOI] [PubMed] [Google Scholar]
- Dodson, M. V., and Mathison B. A.. . 1988. Comparison of ovine and rat muscle-derived satellite cells: response to insulin. Tissue Cell. 20:909–918. doi: 10.1016/0040-8166(88)90032-8 [DOI] [PubMed] [Google Scholar]
- Doumit, M. E., Cook D. R., and Merkel R. A.. . 1993. Fibroblast growth factor, epidermal growth factor, insulin-like growth factors, and platelet-derived growth factor-BB stimulate proliferation of clonally derived porcine myogenic satellite cells. J. Cell. Physiol. 157:326–332. doi: 10.1002/jcp.1041570216 [DOI] [PubMed] [Google Scholar]
- Doumit, M. E., Cook D. R., and Merkel R. A.. . 1996. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137:1385–1394. doi: 10.1210/endo.137.4.8625915 [DOI] [PubMed] [Google Scholar]
- Dumont, N. A., Wang Y. X., and Rudnicki M. A.. . 2015. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development 142:1572–1581. doi: 10.1242/dev.114223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhfakh, R., Michaud A., and Tremblay J. P.. . 2011. Blocking the myostatin signal with a dominant negative receptor improves the success of human myoblast transplantation in dystrophic mice. Mol. Ther. 19:204–210. doi: 10.1038/mt.2010.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, X., Yang Q., Wang B., Zhao J., Zhu M., Parish S. M., and Du M.. . 2018. Reduced satellite cell density and myogenesis in Wagyu compared with Angus cattle as a possible explanation of its high marbling. Animal 12:990–997. doi: 10.1017/S1751731117002403 [DOI] [PubMed] [Google Scholar]
- Gauvin, M. C., Pillai S. M., Reed S. A., Stevens J. R., Hoffman M. L., Jones A. K., Zinn S. A., and Govoni K. E.. . 2020. Poor maternal nutrition during gestation in sheep alters prenatal muscle growth and development in offspring. J. Anim. Sci. 98:166. doi: 10.1093/jas/skz388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge, X., Yu J., and Jiang H.. . 2012. Growth hormone stimulates protein synthesis in bovine skeletal muscle cells without altering insulin-like growth factor-I mRNA expression. J. Anim. Sci. 90:1126–1133. doi: 10.2527/jas.2011-4358 [DOI] [PubMed] [Google Scholar]
- Ge, X., Zhang Y., and Jiang H.. . 2013. Signaling pathways mediating the effects of insulin-like growth factor-I in bovine muscle satellite cells. Mol. Cell. Endocrinol. 372:23–29. doi: 10.1016/j.mce.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Gillies, A. R., and Lieber R. L.. . 2011. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 44:318–331. doi: 10.1002/mus.22094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel, A. J., Rieder M. K., Arnold H. H., Radice G. L., and Krauss R. S.. . 2017. Niche cadherins control the quiescence-to-activation transition in muscle stem cells. Cell Rep. 21:2236–2250. doi: 10.1016/j.celrep.2017.10.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, J. M., Camacho L. E., Ebarb S. M., Swanson K. C., Vonnahme K. A., Stelzleni A. M., and Johnson S. E.. . 2013. Realimentation of nutrient restricted pregnant beef cows supports compensatory fetal muscle growth. J. Anim. Sci. 91:4797–4806. doi: 10.2527/jas.2013-6704 [DOI] [PubMed] [Google Scholar]
- Gonzalez, J. M., Carter J. N., Johnson D. D., Ouellette S. E., and Johnson S. E.. . 2007. Effect of ractopamine-hydrochloride and trenbolone acetate on longissimus muscle fiber area, diameter, and satellite cell numbers in cull beef cows. J. Anim. Sci. 85:1893–1901. doi: 10.2527/jas.2006-624 [DOI] [PubMed] [Google Scholar]
- Greene, E. A., and Allen R. E.. . 1991. Growth factor regulation of bovine satellite cell growth in vitro. J. Anim. Sci. 69:146–152. doi: 10.2527/1991.691146x [DOI] [PubMed] [Google Scholar]
- Hamburger, V., and Hamilton H. L.. . 1951. A series of normal stages in the development of the chick embryo. J. Morphol. 88:49–92. [PubMed] [Google Scholar]
- Han, B., Tong J., Zhu M. J., Ma C., and Du M.. . 2008. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol. Reprod. Dev. 75:810–817. doi: 10.1002/mrd.20832 [DOI] [PubMed] [Google Scholar]
- Hathaway, M. R., Hembree J. R., Pampusch M. S., and Dayton W. R.. . 1991. Effect of transforming growth factor beta-1 on ovine satellite cell proliferation and fusion. J. Cell. Physiol. 146:435–441. doi: 10.1002/jcp.1041460314 [DOI] [PubMed] [Google Scholar]
- Hathaway, M. R., Pampusch M. S., Hembree J. R., and Dayton W. R.. . 1994. Transforming growth factor beta-1 facilitates establishing clonal populations of ovine muscle satellite cells. J. Anim. Sci. 72:2001–2007. doi: 10.2527/1994.7282001x [DOI] [PubMed] [Google Scholar]
- Horak, M., Novak J., and Bienertova-Vasku J.. . 2016. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 410:1–13. doi: 10.1016/j.ydbio.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Horst, D., Ustanina S., Sergi C., Mikuz G., Juergens H., Braun T., and Vorobyov E.. . 2006. Comparative expression analysis of Pax3 and Pax7 during mouse myogenesis. Int. J. Dev. Biol. 50:47–54. doi: 10.1387/ijdb.052111dh [DOI] [PubMed] [Google Scholar]
- Jacquemin, V., Furling D., Bigot A., Butler-Browne G. S., and Mouly V.. . 2004. IGF-1 induces human myotube hypertrophy by increasing cell recruitment. Exp. Cell Res. 299:148–158. doi: 10.1016/j.yexcr.2004.05.023 [DOI] [PubMed] [Google Scholar]
- Jiao, Y., Huang B., Chen Y., Hong G., Xu J., Hu C., and Wang C.. . 2018. Integrated analyses reveal overexpressed notch1 promoting porcine satellite cells’ proliferation through regulating the cell cycle. Int. J. Mol. Sci. 19:271. doi: 10.3390/ijms19010271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, S. E., and Allen R. E.. . 1993. Proliferating cell nuclear antigen (PCNA) is expressed in activated rat skeletal muscle satellite cells. J. Cell. Physiol. 154:39–43. doi: 10.1002/jcp.1041540106 [DOI] [PubMed] [Google Scholar]
- Kamanga-Sollo, E., Thornton K. J., White M. E., and Dayton W. R.. . 2014. Role of G protein-coupled estrogen receptor-1, matrix metalloproteinases 2 and 9, and heparin binding epidermal growth factor-like growth factor in estradiol-17β-stimulated bovine satellite cell proliferation. Domest. Anim. Endocrinol. 49:20–26. doi: 10.1016/j.domaniend.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Kamanga-Sollo, E., White M. E., Chung K. Y., Johnson B. J., and Dayton W. R.. . 2008. Potential role of G-protein-coupled receptor 30 (GPR30) in estradiol-17beta-stimulated IGF-I mRNA expression in bovine satellite cell cultures. Domest. Anim. Endocrinol. 35:254–262. doi: 10.1016/j.domaniend.2008.06.001 [DOI] [PubMed] [Google Scholar]
- Kao, M., Columbus D. A., Suryawan A., Steinhoff-Wagner J., Hernandez-Garcia A., Nguyen H. V., Fiorotto M. L., and Davis T. A.. . 2016. Enteral β-hydroxy-β-methylbutyrate supplementation increases protein synthesis in skeletal muscle of neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 310:E1072–E1084. doi: 10.1152/ajpendo.00520.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, M., Aida H., Hiraga A., and Miyata H.. . 2013. Muscle satellite cells are activated after exercise to exhaustion in Thoroughbred horses. Equine Vet. J. 45:512–517. doi: 10.1111/evj.12010 [DOI] [PubMed] [Google Scholar]
- Kim, Y. S., Lee Y. B., and Dalrymple R. H.. . 1987. Effect of the repartitioning agent cimaterol on growth, carcass and skeletal muscle characteristics in lambs. J. Anim. Sci. 65:1392–1399. doi: 10.2527/jas1987.6551392x [DOI] [PubMed] [Google Scholar]
- Konigsberg, U. R., Lipton B. H., and Konigsberg I. R.. . 1975. The regenerative response of single mature muscle fibers isolated in vitro. Dev. Biol. 45:260–275. doi: 10.1016/0012-1606(75)90065-2 [DOI] [PubMed] [Google Scholar]
- Kuang, S., Chargé S. B., Seale P., Huh M., and Rudnicki M. A.. . 2006. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 172:103–113. doi: 10.1083/jcb.200508001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang, S., Kuroda K., Le Grand F., and Rudnicki M. A.. . 2007. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 129:999–1010. doi: 10.1016/j.cell.2007.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley, B., Thomas M., Bishop A., Sharma M., Gilmour S., and Kambadur R.. . 2002. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277:49831–49840. doi: 10.1074/jbc.M204291200 [DOI] [PubMed] [Google Scholar]
- Lapin, M. R., Gonzalez J. M., and Johnson S. E.. . 2013. Substrate elasticity affects bovine satellite cell activation kinetics in vitro. J. Anim. Sci. 91:2083–2090. doi: 10.2527/jas.2012-5732 [DOI] [PubMed] [Google Scholar]
- LaVigne, E. K., Jones A. K., Londoño A. S., Schauer A. S., Patterson D. F., Nadeau J. A., and Reed S. A.. . 2015. Muscle growth in young horses: effects of age, cytokines, and growth factors. J. Anim. Sci. 93:5672–5680. doi: 10.2527/jas.2015-9634 [DOI] [PubMed] [Google Scholar]
- Li, J., Gonzalez J. M., Walker D. K., Hersom M. J., Ealy A. D., and Johnson S. E.. . 2011. Evidence of heterogeneity within bovine satellite cells isolated from young and adult animals. J. Anim. Sci. 89:1751–1757. doi: 10.2527/jas.2010-3568 [DOI] [PubMed] [Google Scholar]
- Li, J., and Johnson S. E.. . 2013. Ephrin-A5 promotes bovine muscle progenitor cell migration before mitotic activation. J. Anim. Sci. 91:1086–1093. doi: 10.2527/jas2012-5728 [DOI] [PubMed] [Google Scholar]
- Li, X., Wang Z., Tong H., Yan Y., and Li S.. . 2018. Effects of COL8A1 on the proliferation of muscle-derived satellite cells. Cell Biol. Int. 42:1132–1140. doi: 10.1002/cbin.10979 [DOI] [PubMed] [Google Scholar]
- Liu, C., Tong H., Li S., and Yan Y.. . 2018. Effect of ECM2 expression on bovine skeletal muscle-derived satellite cell differentiation. Cell Biol. Int. 42:525–532. doi: 10.1002/cbin.10927 [DOI] [PubMed] [Google Scholar]
- Lutolf, M. P., Gilbert P. M., and Blau H. M.. . 2009. Designing materials to direct stem-cell fate. Nature. 462:433–441. doi: 10.1038/nature08602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGhee, M. E., Bradley J. S., McCoski S. R., Reeg A. M., Ealy A. D., and Johnson S. E.. . 2017. Plane of nutrition affects growth rate, organ size and skeletal muscle satellite cell activity in newborn calves. J. Anim. Physiol. Anim. Nutr. (Berl). 101:475–483. doi: 10.1111/jpn.12568 [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel, P., Alexopoulos N. I., Vajta G., Lewis I., Rogers P., Cann L., Callesen H., Tveden-Nyborg P., and Trounson A.. . 2003. Immunohistochemical and ultrastructural characterization of the initial post-hatching development of bovine embryos. Reproduction. 125:607–623. [PubMed] [Google Scholar]
- Mansouri, A., Stoykova A., Torres M., and Gruss P.. . 1996. Dysgenesis of cephalic neural crest derivatives in Pax7-/- mutant mice. Development. 122:831–838. [DOI] [PubMed] [Google Scholar]
- Mau, M., Kalbe C., Wollenhaupt K., Nürnberg G., and Rehfeldt C.. . 2008. IGF-I- and EGF-dependent DNA synthesis of porcine myoblasts is influenced by the dietary isoflavones genistein and daidzein. Domest. Anim. Endocrinol. 35:281–289. doi: 10.1016/j.domaniend.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Mauro, A. 1961. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9:493–495. doi: 10.1083/jcb.9.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, J. J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A. B., Srikuea R., Lawson B. A., Grimes B., Keller C., . et al. 2011. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 138:3657–3666. doi: 10.1242/dev.068858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane, C., Langley B., Thomas M., Hennebry A., Plummer E., Nicholas G., McMahon C., Sharma M., and Kambadur R.. . 2005. Proteolytic processing of myostatin is auto-regulated during myogenesis. Dev. Biol. 283:58–69. doi: 10.1016/j.ydbio.2005.03.039 [DOI] [PubMed] [Google Scholar]
- McPherron, A. C., and Lee S. J.. . 1997. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. U. S. A. 94:12457–12461. doi: 10.1073/pnas.94.23.1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesires, N. T., and Doumit M. E.. . 2002. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am. J. Physiol. Cell Physiol. 282:C899–C906. doi: 10.1152/ajpcell.00341.2001 [DOI] [PubMed] [Google Scholar]
- Miersch, C., Stange K., Hering S., Kolisek M., Viergutz T., and Röntgen M.. . 2017. Molecular and functional heterogeneity of early postnatal porcine satellite cell populations is associated with bioenergetic profile. Sci. Rep. 7:45052. doi: 10.1038/srep45052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch, C., Stange K., and Röntgen M.. . 2018. Separation of functionally divergent muscle precursor cell populations from porcine juvenile muscles by discontinuous Percoll density gradient centrifugation. BMC Cell Biol. 19:2. doi: 10.1186/s12860-018-0156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miretti, S., Volpe M. G., Martignani E., Accornero P., and Baratta M.. . 2017. Temporal correlation between differentiation factor expression and microRNAs in Holstein bovine skeletal muscle. Animal 11(2):227–235. doi: 10.1017/S1751731116001488 [DOI] [PubMed] [Google Scholar]
- Mok, G. F., Lozano-Velasco E., and Münsterberg A.. . 2017. microRNAs in skeletal muscle development. Semin. Cell Dev. Biol. 72:67–76. doi: 10.1016/j.semcdb.2017.10.03 [DOI] [PubMed] [Google Scholar]
- Murach, K. A., White S. H., Wen Y., Ho A., Dupont-Versteegden E. E., McCarthy J. J., and Peterson C. A.. . 2017. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet. Muscle. 7:14. doi: 10.1186/s13395-017-0132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, R. L., Zhang W., Iwaniuk M., Grilli E., and Stahl C. H.. . 2018. Dietary tributyrin, an HDAC inhibitor, promotes muscle growth through enhanced terminal differentiation of satellite cells. Physiol. Rep. 6:e13706. doi: 10.14814/phy2.13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen, P. M., and Oksbjerg N.. . 2009. In vitro primary satellite cell growth and differentiation within litters of pigs. Animal 3:703–709. doi: 10.1017/S1751731109003929 [DOI] [PubMed] [Google Scholar]
- Oksbjerg, N., Gondret F., and Vestergaard M.. . 2004. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 27:219–240. doi: 10.1016/j.domaniend.2004.06.00 [DOI] [PubMed] [Google Scholar]
- Olson, E. N., Sternberg E., Hu J. S., Spizz G., and Wilcox C.. . 1986. Regulation of myogenic differentiation by type beta transforming growth factor. J. Cell Biol. 103:1799–1805. doi: 10.1083/jcb.103.5.1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchot, M. H., Ecolan P., Sorensen I. L., Oksbjerg N., and Lefaucheur L.. . 2012. In vitro characterization of proliferation and differentiation of pig satellite cells. Differentiation. 84:322–329. doi: 10.1016/j.diff.2012.08.001 [DOI] [PubMed] [Google Scholar]
- Pfeffer, P. L., Smith C. S., Maclean P., and Berg D. K.. . 2017. Gene expression analysis of bovine embryonic disc, trophoblast and parietal hypoblast at the start of gastrulation. Zygote. 25:265–278. doi: 10.1017/S0967199417000090 [DOI] [PubMed] [Google Scholar]
- Potts, J. K., Echternkamp S. E., Smith T. P., and Reecy J. M.. . 2003. Characterization of gene expression in double-muscled and normal-muscled bovine embryos. Anim. Genet. 34:438–444. doi: 10.1046/j.0268-9146.2003.01055.x [DOI] [PubMed] [Google Scholar]
- Qin, L. L., Li X. K., Xu J., Mo D. L., Tong X., Pan Z. C., Li J. Q., Chen Y. S., Zhang Z., Wang C., . et al. 2012. Mechano growth factor (MGF) promotes proliferation and inhibits differentiation of porcine satellite cells (PSCs) by down-regulation of key myogenic transcriptional factors. Mol. Cell. Biochem. 370:221–230. doi: 10.1007/s11010-012-1413-9 [DOI] [PubMed] [Google Scholar]
- Qin, L., Xu J., Wu Z., Zhang Z., Li J., Wang C., and Long Q.. . 2013. Notch1-mediated signaling regulates proliferation of porcine satellite cells (PSCs). Cell. Signal. 25:561–569. doi: 10.1016/j.cellsig.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Raja, J. S., Hoffman M. L., Govoni K. E., Zinn S. A., and Reed S. A.. . 2016. Restricted maternal nutrition alters myogenic regulatory factor expression in satellite cells of ovine offspring. Animal. 10:1200–1203. doi: 10.1017/S1751731116000070 [DOI] [PubMed] [Google Scholar]
- Reiter, B. C., Kamanga-Sollo E., Pampusch M. S., White M. E., and Dayton W. R.. . 2014. Epidermal growth factor receptor is required for estradiol-stimulated bovine satellite cell proliferation. Domest. Anim. Endocrinol. 48:48–55. doi: 10.1016/j.domaniend.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Relaix, F., Montarras D., Zaffran S., Gayraud-Morel B., Rocancourt D., Tajbakhsh S., Mansouri A., Cumano A., and Buckingham M.. . 2006. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 172:91–102. doi: 10.1083/jcb.200508044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix, F., Rocancourt D., Mansouri A., and Buckingham M.. . 2004. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev. 18:1088–1105. doi: 10.1101/gad.301004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix, F., Rocancourt D., Mansouri A., and Buckingham M.. . 2005. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 435:948–953. doi: 10.1038/nature03594 [DOI] [PubMed] [Google Scholar]
- Richard, C., Hue I., Gelin V., Neveux A., Campion E., Degrelle S. A., Heyman Y., and Chavatte-Palmer P.. . 2015. Transcervical collection of bovine embryos up to Day 21: an 8-year overview. Theriogenology. 83:1101–1109. doi: 10.1016/j.theriogenology.2014.12.005 [DOI] [PubMed] [Google Scholar]
- Rodgers, J. T., King K. Y., Brett J. O., Cromie M. J., Charville G. W., Maguire K. K., Brunson C., Mastey N., Liu L., Tsai C. R., . et al. 2014. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert). Nature. 510:393–396. doi: 10.1038/nature13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers, J. T., Schroeder M. D., Ma C., and Rando T. A.. . 2017. HGFA is an injury-regulated systemic factor that induces the transition of stem cells into GAlert. Cell Rep. 19:479–486. doi: 10.1016/j.celrep.2017.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadkowski, T., Ciecierska A., Oprządek J., and Balcerek E.. . 2018. Breed-dependent microRNA expression in the primary culture of skeletal muscle cells subjected to myogenic differentiation. BMC Genomics. 19:109. doi: 10.1186/s12864-018-4492-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino, S., and Mammucari C.. . 2011. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle. 1:4. doi: 10.1186/2044-5040-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale, P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P., and Rudnicki M. A.. . 2000. Pax7 is required for the specification of myogenic satellite cells. Cell. 102:777–786. doi: 10.1016/s0092-8674(00)00066-0 [DOI] [PubMed] [Google Scholar]
- Sebastian, S., Goulding L., Kuchipudi S. V., and Chang K. C.. . 2015. Extended 2D myotube culture recapitulates postnatal fibre type plasticity. BMC Cell Biol. 16:23. doi: 10.1186/s12860-015-0069-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafiq, S. A., Gorycki M. A., and Mauro A.. . 1968. Mitosis during postnatal growth in skeletal and cardiac muscle of the rat. J. Anat. 103(Pt 1):135–141. [PMC free article] [PubMed] [Google Scholar]
- Sincennes, M. C., Brun C. E., and Rudnicki M. A.. . 2016. Concise Review: Epigenetic Regulation of Myogenesis in Health and Disease. Stem Cells Transl. Med. 5:282–290. doi: 10.5966/sctm.2015-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders, T., Nederveen J. P., McKay B. R., Joanisse S., Verdijk L. B., van Loon L. J., and Parise G.. . 2015. Satellite cells in human skeletal muscle plasticity. Front. Physiol. 6:283. doi: 10.3389/fphys.2015.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow, M. H. 1977. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat. Rec. 188:201–217. doi: 10.1002/ar.1091880206 [DOI] [PubMed] [Google Scholar]
- Snow, M. H. 1978. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res. 186:535–540. doi: 10.1007/bf00224941 [DOI] [PubMed] [Google Scholar]
- Stark, D. A., Karvas R. M., Siegel A. L., and Cornelison D. D.. . 2011. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development. 138:5279–5289. doi: 10.1242/dev.068411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, N. T., Byrne K. M., Ragle C. A., Vierck J. L., and Dodson M. V.. . 2001. Patterns of expression of muscle-specific markers of differentiation in satellite cell cultures: determination by enzyme-linked immunoculture assay and confocal immunofluorescent assay. Cell Biol. Int. 25:873–884. doi: 10.1006/cbir.2001.0787 [DOI] [PubMed] [Google Scholar]
- Sun, X., Li M., Sun Y., Cai H., Li R., Wei X., Lan X., Huang Y., Lei C., and Chen H.. . 2015. The developmental transcriptome landscape of bovine skeletal muscle defined by Ribo-Zero ribonucleic acid sequencing. J. Anim. Sci. 93:5648–5658. doi: 10.2527/jas.2015-9562 [DOI] [PubMed] [Google Scholar]
- Tatsumi, R., Anderson J. E., Nevoret C. J., Halevy O., and Allen R. E.. . 1998. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 194:114–128. doi: 10.1006/dbio.1997.8803 [DOI] [PubMed] [Google Scholar]
- Thomas, M., Langley B., Berry C., Sharma M., Kirk S., Bass J., and Kambadur R.. . 2000. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 275:40235–40243. doi: 10.1074/jbc.M004356200 [DOI] [PubMed] [Google Scholar]
- Tong, H., Jiang R. Y., Zhang W. W., and Yan Y. Q.. . 2017. MiR-2425-5p targets RAD9A and MYOG to regulate the proliferation and differentiation of bovine skeletal muscle-derived satellite cells. Sci. Rep. 7:418. doi: 10.1038/s41598-017-00470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, H., Jiang R., Liu T., Wei Y., Li S., and Yan Y.. . 2018. bta-miR-378 promote the differentiation of bovine skeletal muscle-derived satellite cells. Gene 668:246–251. doi: 10.1016/j.gene.2018.03.102 [DOI] [PubMed] [Google Scholar]
- Thornton, K. J., Kamange-Sollo E., White M. E., and Dayton W. R.. . 2015. Role of G protein-coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor-like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate-stimulated bovine satellite cell proliferation. J. Anim. Sci. 93:4291–4301. doi: 10.2527/jas.2015-9191 [DOI] [PubMed] [Google Scholar]
- Tozaki, T., Kikuchi M., Kakoi H., Hirota K. I., and Nagata S. I.. . 2017. A genome-wide association study for body weight in Japanese Thoroughbred racehorses clarifies candidate regions on chromosomes 3, 9, 15, and 18. J. Equine Sci. 28:127–134. doi: 10.1294/jes.28.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg, A. U., Meyer A., Rohner D., Boyle J., Hatakeyama S., and Glass D. J.. . 2009. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009 [DOI] [PubMed] [Google Scholar]
- Vaughn, M. A., Phelps K. J., and Gonzalez J. M.. . 2018. In vitro supplementation with the porcine plasma product, betaGRO®, stimulates activity of porcine fetal myoblasts and neonatal satellite cells in a divergent manner. Animal. 12:1912–1920. doi: 10.1017/S1751731117003329 [DOI] [PubMed] [Google Scholar]
- Wang, Y. M., Ding X. B., Dai Y., Liu X. F., Guo H., and Zhang Y.. . 2015. Identification and bioinformatics analysis of miRNAs involved in bovine skeletal muscle satellite cell myogenic differentiation. Mol. Cell. Biochem. 404:113–122. doi: 10.1007/s11010-015-2371-9 [DOI] [PubMed] [Google Scholar]
- Wen, Y., Bi P., Liu W., Asakura A., Keller C., and Kuang S.. . 2012. Constitutive Notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol. Cell. Biol. 32:2300–2311. doi: 10.1128/MCB.06753-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J., Liu D., Yin H., Tong H., Li S., and Yan Y.. . 2018. Fatty acids promote bovine skeletal muscle satellite cell differentiation by regulating ELOVL3 expression. Cell Tissue Res. 60:1571–10. doi: 10.1007/s00441-018-2812-3 [DOI] [PubMed] [Google Scholar]
- Yates, D. T., Clarke D. S., Macko A. R., Anderson M. J., Shelton L. A., Nearing M., Allen R. E., Rhoads R. P., and Limesand S. W.. . 2014. Myoblasts from intrauterine growth-restricted sheep fetuses exhibit intrinsic deficiencies in proliferation that contribute to smaller semitendinosus myofibres. J. Physiol. 592:3113–3125. doi: 10.1113/jphysiol.2014.272591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. W., Tong H. L., Sun X. F., Hu Q., Yang Y., Li S. F., Yan Y. Q., and Li G. P.. . 2015. Identification of miR-2400 gene as a novel regulator in skeletal muscle satellite cells proliferation by targeting MYOG gene. Biochem. Biophys. Res. Commun. 463:624–631. doi: 10.1016/j.bbrc.2015.05.112 [DOI] [PubMed] [Google Scholar]
- Zhang, W. W., Sun X. F., Tong H. L., Want Y. H., Li S. F., Yan Y. Q. and Li G. P.. . 2016. Effect of differentiation on microRNA expression in bovine skeletal muscle satellite cells by deep sequencing. Cell Mol. Biol. Lett. 21:8. doi: 10.1186/s11658-016-0009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. R., Zhang H. N., Wang Y. M., Dai Y., Liu X. F., Li X., Ding X. B., and Guo H.. . 2017. miR-143 regulates proliferation and differentiation of bovine skeletal muscle satellite cells by targeting IGFBP5. In Vitro Cell. Dev. Biol. Anim. 53:265–271. doi: 10.1007/s11626-016-0109-y [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Zhao L. D., Johnson S. E., Rhoads M. L., Jiang H., and Rhoads R. P.. . 2019. Oxytocin is involved in steroid hormone-stimulated bovine satellite cell proliferation and differentiation in vitro. Domest. Anim. Endocrinol. 66:1–13. doi: 10.1016/j.domaniend.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Zhu, H., Park S., Scheffler J. M., Kuang S., Grant A. L., and Gerrard D. E.. . 2013. Porcine satellite cells are restricted to a phenotype resembling their muscle origin. J. Anim. Sci. 91:4684–4691. doi: 10.2527/jas.2012-5804 [DOI] [PubMed] [Google Scholar]