Abstract
The aim of this study is to compare the effects of colloidal cardioplegia and blood cardioplegia in patients who underwent cardiac surgical procedures with cardiopulmonary bypass, and to evaluate their influence on hemodilu-tion, bleeding and consumption of donor blood products in a retrospective clinical study. 100 male patients who underwent cardiac surgical procedure were divided into two groups: 50 patients were administered intermittent normotherm or mild hypotherm (34°C) Calafiore blood cardioplegia with potassium chloride 14,9%; 50 patients were administered one initial doses of cold Kirsch – solution followed from intermittent cold colloidal cardioplegia using hydroxyethyl starch (HES 450/0,7). Hemoglobin values after the first dose of cardioplegia were significantly lower in the HES-group than in the Calafiore-group). After the first dose of cardioplegia platelets count was lower in the HES-group than in the Calafiore-group. Hemoglobin and hematocrit values 24h postoperative were lower in the HES-group than in the Calafiore-group. There was no difference in chest-drainage bleeding 12h and 24h postoperative between the groups. The consumption of donor erythrocyte concentrate and fresh frozen plasma was significantly higher in the HES-than in the Calafiore-group. The choice of either colloidal or blood cardioplegia does not influence the postoperative chest-drainage bleeding. The results suggest that high molecular colloidal cardioplegia with HES-solution is associated with higher hemodilution during and after car-diopulmonary bypass and significantly increases the consumption of donor blood products.
Keywords: cardiopulmonary bypass, myocardial protection, cardioplegia, postoperative bleeding, hemodilution
INTRODUCTION
Myocardial state and need for the blood which circulating through coronary artery depend of the functional condition of the heart. As a sub-discipline of cardiac surgery, myocardial protection involves skillful application of techniques designed to protect the operated heart from injury. There are a variety of strategies for myocardial protection. Frequently used and well established strategy is the pharmacologically induced, reversible, electromechanical, diastolic cardiac arrest achieved by direct infusion of cardioplegic crystalline solutions or blood cardioplegia in the coronary circulation. Different possibilities to induce and receive cardiac arrest are warm or cold, antegrade or retrograde, continuous or intermittent application of a cardioplegic solution. Cardioplegia offers selective perfusion to the heart and avoids ischemic injury primarily in that it reduces oxygen demand to less than 10% of that of the working heart (1). Cardioplegic solutions vary greatly concerning their temperature at which they will be infused, their composition, pH-val-ues and osmolality. The frequency of application, the amount of the solution and the style of application are also under discussion. It is common, for all cardioplegic solutions that they change the extra-cellular milieu for electrolytes and the attributes of myocardial cell membranes, either by electrically stabilizing the membranes or sealing up the permeability of myocardial cell membranes for ions. An electromechanical block with distinct decrease in energy-using actions on myocardial cell membranes and in the contractile apparatus is the aim (1). The advantages of every strategic application of cardioplegic technique should be compared against known disadvantages of that technique and a balance should be achieved, which enhances the safety of every cardiac operation as it is harmonized with the patient’s specific physiological needs (1). In our retrospective study we examined a crystalline – colloidal cardioplegia and a blood cardioplegia concerning their systemic influence on hemodilution during and after CPB, postoperative chestdrainage bleeding and the consumption of donor blood products. The transfusion of donor blood or plasma decreases the patient’s safety because of potential risk of infection or allergic reactions and raises the costs of the procedure, which is increasingly important as the clinics are requested to focus on cost effectiveness.
MATERIALS AND METHODS
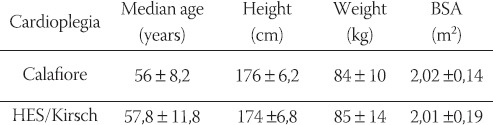
All the patients included in the study were male and underwent either a coronary artery bypass grafting (CABG), a mitralor aortal valve replacement or a combination of these procedures. The differences in patients’ age, weight and height and their body surface area (BSA) were not statistically significant (Table 1). After median sternotomy, systemic heparinization (400 U/kg) and cannulation of the ascending aorta and cavoatrial cannulation or cannulation of superior and inferior vena cava was conducted. Cardiopulmonary bypass was started with a hollow fibre membrane oxygenator (“Monolyth” Sorin Group), a non coated table and machine tubing set with arterial filter (Sorin Group) and a roller pump head (Stöckert). The volume of the priming solution in all patients amounted to 1500 ml (1000 ml NaCl 0, 9% and 500 ml Ringer/Lactate) with 10.000 I.U. Heparin. In all patients cardioplegia was administered through a cannulation in the aortic root, except in the patients who underwent an aortic valve procedure when the ascending aorta was opened and the cardioplegia directly administered in both coronary orifices by special cardioplegic cannulas (12 or 14 French DLP).
TABLE 1.
Patients age, weight and BSA
KIRSCH – SOLUTION
The cardioplegic effect of this solution established by Kirsch, Rodewald and Kalmar in 1972 (2) result from the substances procaine hydrochloride and magnesium aspartate in sorbitol solution with a greatly reduced concentration of sodium and calcium. Magnesium inhibits calcium receptors in the cell membrane. Pro-caine has a membrane stabilizing effect which blocks sodium flow into the cell. Decreased concentrations of sodium and calcium avoid action potential. Increased concentration of magnesium in the Kirsch -solution leads to a rapid diastolic cardiac arrest. In contrast to other cardioplegic methods, Kirsch-solution is administered as a single cold injection immediately after aortic clamping. 100 – 200 ml is injected in the aortic root or directly in the coronary orifices with soft manual pressure over 10 – 20 seconds (Table 2).
TABLE 2.
Application protocol for single injection of cold Kirsch-solution

HES – SOLUTION (BLEESE)
The cardioplegicaly active components of this so-called “Hamburger” solution are magnesium, procaine and a reduced sodium concentration. The solution contains glucose as a substrate. Myocardial metabolism is decreased by inhibition of membrane – ATP. In order to avoid myocardial edema, the solution contains colloid-osmoticaly active hydroxyethylstarch (HES) with molecular weight of 450.000 Dalton, which maintains colloid osmotic pressure of the solution at 33,8 mmHg (4, 5). 250 mg prednisolone was added into 1000 ml of HES – solution as a steroid which decreases capillary permeability and stabilizes lysosomal membrane during ischemia. Also, 20 ml sodium hydrogencarbonate was added to reach a pH-value of 7,4 at 37°C. Application of cold HES solution followed directly after a single injection of Kirsch – solution – as described in Table 3 using roller pump pressure and volume control until myocardial temperature of approximately 15 – 20°C was reached. The coronary perfusion pressure should not exceed 30 -40 mmHg. The total amount of given HES – solution varied from 400 – 1400 ml with a mean of 710 ml.
TABLE 3.
Application protocol for multidose cold HES – solution

CALAFIORE BLOOD CARDIOPLEGIA
Comparing with crystalline cardioplegic solutions blood cardioplegia have serious advantages like higher oxygen transport capacity, higher oncotic pressure, physiological rheology and an antioxidant effect. Blood cardioplegia contains oxygen radical scavengers and natural buffer substances such as proteins of histidine and imidazole – groups (6, 7). Calafiore introduced an intermittent warm blood cardioplegia where a perfuser syringe with 2 mmol/l potassium chloride is connected in line with oxygenated blood via V” – V” LL connector (8). The blood and potassium is administered using a roller pump and bubble trap following the application protocol described in Table 4. After an initial infusion, additional infusions follow respectively when a distal coronary anastomosis is finished or every 15 minutes (8).
TABLE 4.
Calafiore application protocol for blood cardioplegia

STATISTICAL ANALYSIS
Statistical analysis of differences within and between groups was performed with multiple analysis of variance followed by application of Student t-test. Differences were considered significant when p<0,05. All data are expressed as mean ± SD.
RESULTS
Following the first dose of cardioplegia hemoglobin values were significantly lower (p<0,013) in the HES-group (9,36 ± 1,09 g/dl) than in the Calafiore group (9,84 ± 1,04 g/dl); (Table 5, Chart 1). Hemoglobin values 24h postoperative were lower in the HES-group (9, 98 ± 1, 16 g/dl) than in the Calafiore-group (10,30 ± 0,95 g/dl). (Table 5, Chart 1). Also, hematocrit values 24h after the surgery were lower in the HES-group (29,43 ± 3,9%) than in the Calafiore-group (30,26 ± 3,1%); (Table 6, Chart 2). Platelets count after first dose of cardioplegia was lower in the HES-group (139,72 ± 46,3 Ι</μΚ) than in the Calafiore-group (153,32 ± 45,6 K^L); (Table 7, Chart 3). There was no difference in chest-drainage bleeding 12h and 24h postoperative between the HES – group and the Calafiore – group (495,4 ± 274,7 ml vs. 452,9 ± 174,9 ml and 701,5 ± 345,5 ml vs. 696,7 ± 269,9 ml); (Table 8, Chart 4). However, the consumption of donor erythrocyte concentrate and fresh frozen plasma was significantly higher in the HES – than in the Calafiore – group (324 ml vs. 135 ml, p<0,013 and 196 ml vs. 107 ml; p<0, 04); (Table 9, Chart 5).
TABLE 5.
Hemoglobin values pre-bypass, after the first dose of cardioplegia and 24h postoperative
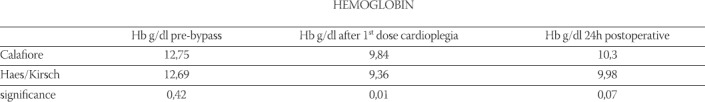
CHART 1.
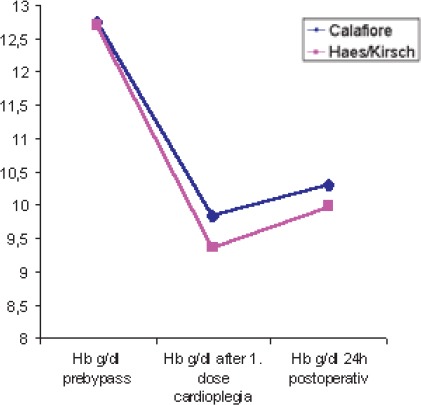
Hemoglobin values pre-bypass, after the first dose of cardioplegia and 24h postoperative
TABLE 6.
Hematocrit values pre-bypass, after the first cardioplegia and 24h postoperative

CHART 2.
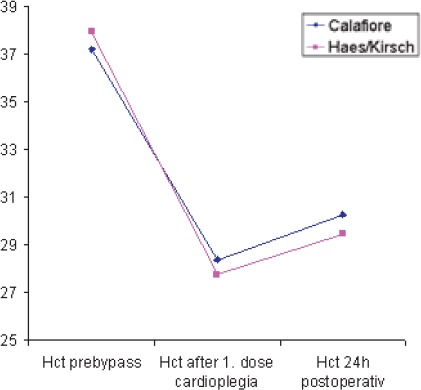
Hematocrit values pre-bypass, after the first cardioplegia and 24h postoperative
TABLE 7.
Platelets count pre-bypass, after the first cardioplegia and 24h postoperative

CHART 3.
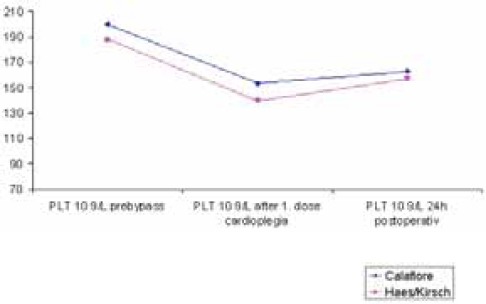
Platelets count pre-bypass, after the first cardioplegia and 24h postoperative
TABLE 8.
Chest-drainage output 12h and 24h postoperative

CHART 4.
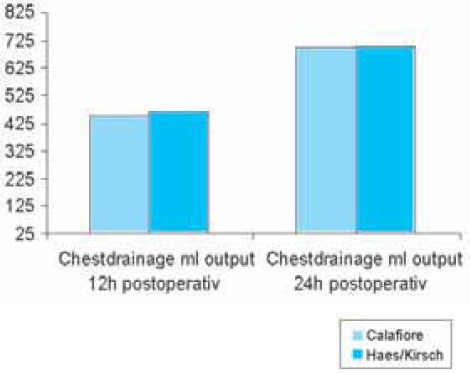
Chest-drainage output 12h and 24h postoperative
TABLE 9.
Consumption of donor erythrocyte concentrate and fresh frozen plasma within 24h postoperative
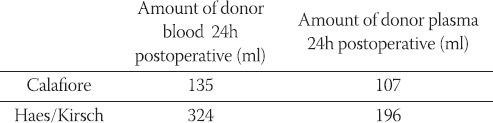
CHART 5.
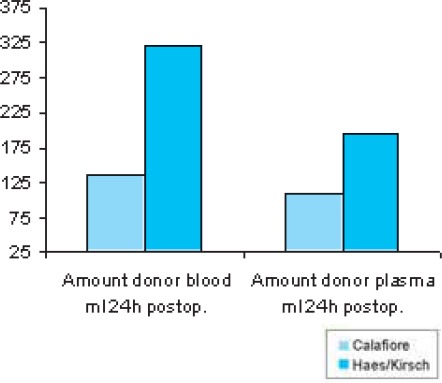
Consumption of donor erythrocyte concentrate and fresh frozen plasma within 24h postoperative
DISCUSSION
We examined two different types of cardioplegia to study the consequences of a volume invasive cardio-plegia concerning hemodilution and the effects on the circulatory system during and after cardiac surgical procedures with cardiopulmonary bypass. Hemodilu-tion affects the pharmacokinetic and pharmacodynamic properties of drugs, predominantly by changing the protein binding by dilution of all plasma proteins (9). Large amounts of colloid cardioplegia dilute circulating catecholamine (9). HES-solution is known to interfere with blood coagulation according to inhibition of thrombocyte aggregation. Although we did not notice significant difference in postoperative blood loss in our patients between the two cardioplegia forms, longer bleeding time may be a potential risk. Large molecules of the HES-solution (450.000 Dalton) increase blood viscosity and may, in hypothermia, have negative influence on microcirculation (10). Higher colloid osmotic pressure resulting from the size of molecules may decrease urine output because glomerular filtration rate developed proportional to the difference between perfusion pressure and colloid osmotic pressure (10).
CONCLUSION
The use of colloidal or blood cardioplegia does not influence the postoperative chest-drainage bleeding. The results show that high molecular colloidal cardioplegia with HES-solution is associated with greater hemodilution during CPB which remains almost 24h postoperative. Consequence is a significantly increased consumption of donor blood and plasma products intra- and postoperative. Blood cardioplegia may have advantages in this sense. Toreiterate, the goal of the operation is not to deliver cardioplegia, but to treat the patient as well as possible.
REFERENCES
- 1.Gravlee GP, Davis RF, Joe R. Cardiopulmonary Bypass: Principles and Practice. Baltimore, Maryland: Williams & Wilkins; 1993. [Google Scholar]
- 2.Kirsch U, Rodewald G, Kalmar P. Induced ischemic arrest. Clinical experience with cardioplegia in open-heart surgery. J. Thoracic. Cardiovasc. Surg. 1972;63:121–130. [PubMed] [Google Scholar]
- 3.Hölscher B. Die Bedeutung des Magnesium-Novocamid als Kardioplegicum für die offene Herzchirurgie. Thoraxchirurgie. 1965;13:446–452. doi: 10.1055/s-0028-1100764. [DOI] [PubMed] [Google Scholar]
- 4.Bleese N, Döring V, Kalmar P, Pokar H, Polonius MJ, Steiner D, Rodewald G. Intraoperative myocardial protection by cardioplegia in hypothermia. Clinical findings. J. Thorac. Cardio-vasc. Surg. 1978;75:405–413. [PubMed] [Google Scholar]
- 5.Bleese N, Döring V, Kalmar P, Lutz G, Pokar H, Steiner D, Rodewald G. Myokardprotektion durch den induzierten Herzstillstand in tiefer Hypothermie. Kardiotechnik. 1977;3:20–24. [Google Scholar]
- 6.Barner HB. Blood cardioplegia: a review and comparison with crystalloid cardioplegia. Ann. Thorac. Surg. 1991;52:1354–1367. doi: 10.1016/0003-4975(91)90034-n. [DOI] [PubMed] [Google Scholar]
- 7.Buckberg GD. Oxygenated cardioplegia: blood is a many splen-dored thing [comment] Ann. Thorac. Surg. 1990;50:175–177. doi: 10.1016/0003-4975(90)90726-m. [DOI] [PubMed] [Google Scholar]
- 8.Calafiore AM, Teodori G, Mezzetti A, Bosco G, Verna AM, DiGiammarco G, Lapenna D. Intermittent antegrad warm blood cardioplegia. Ann. Thorac. Surg. 1995;59:398–402. doi: 10.1016/0003-4975(94)00843-v. [DOI] [PubMed] [Google Scholar]
- 9.Schaper J, Scheld HH, Schmidt U, Hehrlein F. Ultrastructural study comparing the efficacy of five different methods of intraoperative myocardial protection in the human heart. J. Thorac. Cardiovasc. Surg. 1986;92:47–55. [PubMed] [Google Scholar]
- 10.Entholzner EK, Mielke LL, Calatzis AN, Feyh J, Hipp R, Har-gasser SR. Coagulation effects of a recently developed hydroxy-ethyl starch (HES 130/0,4) compared to hydroxyethyl starches with higher molecular weight. Acta Anestesiolog. Scand. 2000;44:1116–1121. doi: 10.1034/j.1399-6576.2000.440914.x. [DOI] [PubMed] [Google Scholar]


