Abstract
The effect of exposure to ultra‐low temperature (liquid nitrogen, LN) on viability of seeds desiccated to various water contents was investigated in 9 coffee species. Three groups of species could be distinguished based on seed survival after LN exposure. In group 1 species, no seedling production could be obtained after LN exposure due to endosperm injury. In group 2 species, recovery was very low or nil after rapid cooling, and only moderate after slow cooling. In group 3 species, very high percentages of seedling development were observed after both rapid and slow cooling. A high interspecific variability for the high moisture freezing limit was observed within the species of groups 2 and 3, since it ranged from 0.14 to 0.26 g H2O g−1 dry weight. A very highly significant correlation was found for those species between the unfreezable water content, as determined from DSC analysis, and the high moisture freezing limit of their seeds. No significant correlation was found between seed lipid content, which varied from 9.8 to 34.6% dry weight, and survival after LN exposure. However, a negative relationship was found between seed unfreezable water content and lipid content. Interspecific differences in fatty acid composition of seed lipids resulted in a high variability in the percentage of unsaturated fatty acids, which ranged from 28.7 to 54.4% among the 9 species studied. For all species studied, a highly significant correlation was found between the percentage of unsaturated fatty acids and the percentage of seedling recovery after rapid or slow cooling.
Abbreviations
- DSC
differential scanning calorimetry
- LN
liquid nitrogen
Introduction
Seeds of Coffea species can withstand partial dehydration (Ellis et al. 1990; Dussert et al. 1999; Eira et al. 1999a), but they cannot be stored under conventional genebank conditions, i.e. 3–7% seed moisture content at −18°C (FAO/IPGRI 1994), because they are cold‐sensitive (Van der Vossen 1977; Ellis et al. 1990; Eira et al. 1999a) and desiccation does not increase their longevity (Van der Vossen 1977; Ellis et al. 1990). For species whose seeds exhibit this particular storage behavior, termed ‘intermediate’ (Ellis et al. 1990), cryopreservation is the only technique available for long‐term germplasm conservation.
It is generally accepted that the range of seed water contents conducive to tolerance to liquid nitrogen (LN) exposure is limited on the one hand by desiccation sensitivity – the lower limit – and on the other by the occurrence of intracellular ice formation – the higher limit (Becwar et al. 1983; Kuranuki and Yoshida 1996; Vernon et al. 1999; Sun 1999). However, this simple rationale for seed tolerance to LN exposure has been questioned by the demonstration that intracellular ice formation in seed tissues during cooling to LN temperature is not always a lethal event. In pea seeds, the unfreezable water content, as determined from DSC heating thermograms, was found to be lower than the critical water content for freezing injury, i.e. 0.26 and 0.33 g H2O g−1 dry weight, respectively (Vertucci 1989a). Since then, it has been shown that embryos/embryonic axes of 3 desiccation‐sensitive seed species could tolerate the presence, to a certain extent, of freezable water in their tissues during exposure to sub‐freezing temperatures (Vertucci et al. 1991; Wesley‐Smith et al. 1992; Vertucci et al. 1995). However, with seeds of some species survival to LN exposure seems to be unconditionally dependent on the avoidance of intracellular ice formation: in soybean seeds, the high moisture freezing limit was shown to coincide with the unfreezable water content (Vertucci 1989a).
In orthodox seeds, the lower limit of the hydration window for tolerance to LN exposure is generally ignored from a practical standpoint because orthodox seeds are tolerant to desiccation to very low moisture contents. However, it has been shown that there is a negative interaction between high cooling rates (i.e. direct immersion in LN) and low water contents on survival of lipid‐rich orthodox seeds to LN exposure (Stanwood 1987, 1989a, 1989b). Thus, the presence of lipid reserves in seed tissues appears to be another key factor involved in survival of seeds after exposure to ultra‐low temperature. To our knowledge, the influence of the amount and the composition of reserve lipids on the sensitivity of seeds to LN exposure have never been investigated.
With non‐orthodox seeds species, the width of the potential hydration window for cryopreservation is dramatically reduced due to their sensitivity to desiccation. It is therefore necessary to optimize their moisture content before cryopreservation (Becwar et al. 1983; Vertucci et al. 1991; Wesley‐Smith et al. 1992; Vertucci et al. 1995; Kuranuki and Yoshida 1996; Pritchard and Manger 1998; Sun 1999). Successful cryopreservation of embryonic axes of several recalcitrant seeds has been achieved by lowering their level of desiccation sensitivity through the use of very high drying rates – i.e. the ‘flash drying’ technique (Vertucci et al. 1991; Wesley‐Smith et al. 1992). However, due to their large size, such high drying rates are not easily achieved with whole seeds of most non‐orthodox seed species.
Species of the sub‐genus Coffea display a broad variability in sensitivity of their seeds to desiccation and to rapid cooling (100–200°C min−1) to LN temperature (Becwar et al. 1983, 1998, 1999, 1999a). With C. arabica seeds, which exhibit a very high sensitivity to rapid cooling to LN temperature (Becwar et al. 1983, 1997, 1998, 2000, 1999a), a slow precooling step (1°C min−1 to −50°C) prior to immersion in LN had a beneficial effect on seed survival (Dussert et al. 1997, 1998). Furthermore, it has been shown that sensitivity of whole C. arabica seeds to LN exposure was due to damage caused to the endosperm only, since all embryos extracted from frozen seeds after thawing developed into normal seedlings when cultured in vitro (Dussert et al. 1997, 2000). In the present study, experiments involving two cooling regimes down to LN temperature were carried out with whole seeds of 9 coffee species showing differences in desiccation sensitivity. To study whether intracellular ice formation was associated with freezing injury, the unfreezable water content of endosperm of seeds of these 9 coffee species was determined by DSC analysis. Moreover, endosperm lipid content and composition were studied to determine their influence on sensitivity of seeds to LN exposure.
Materials and methods
Plant material
Fresh mature seeds of Coffea arabica L. (typica variety) were provided from CATIE, Turrialba, Costa Rica. Bulks of seeds of C. brevipes Hiern, C. canephora Pierre, C. eugenioides Moore, C. liberica Hiern., C. pseudozanguebariae Bridson, C. racemosa Lour., C. sessilflora Bridson and C. stenophylla G. Don. were provided from the field collections of CNRA‐IRD, Divo and IRD, Man, Côte‐d'Ivoire. Before desiccation treatments, seed lots were stored in the dark at ambient temperature for 1–2 weeks. The seed lots of C. arabica, C. brevipes, C. canephora, C. eugenioides, C. humilis, C. liberica and C. pseudozanguebariae were the same as those used in a previous study (Dussert et al. 1999).
Desiccation and cooling procedures
After the testa (endocarp) was removed, seeds were desiccated by equilibration for 3 weeks at 25°C over various saturated salt solutions, as previously described by Dussert et al. (1999). The water content of seeds (expressed in g H2O g−1 dry weight) was estimated using 3 replicates of 10 seeds and their dry weight measured after 2 days of desiccation in an oven at 105°C. The effect of desiccation alone on seed viability was studied after desiccation over 6–12 salt solutions, depending of the species, and desiccation sensitivity was quantified by the water content at which 50% of the initial viability was reached, WC50, using the desiccation sensitivity model developed in a previous study (Dussert et al. 1999). Exposure of seeds to LN temperature was performed after desiccation over K2SO4, KNO3, MgCl2, Na2CO3, NH4Cl and NH4NO3 saturated solutions. Before cooling, seeds were hermetically sealed in 10‐ml polypropylene tubes. The initial temperature of the seeds was around 25°C (room temperature). Seeds were either immersed directly into LN (rapid cooling treatment, average of 200°C min−1) or precooled to −50°C at 1°C min−1 prior to immersion into LN (slow cooling treatment), as described by Dussert et al. (1997). Frozen seeds were stored for several months at −196°C before thawing. Thawing was carried out by plunging the cryotubes in a 40°C waterbath for 2 min.
Survival assessment
For each of the 3 treatments (desiccation alone, desiccation and slow cooling, desiccation and rapid cooling) and each saturated salt solution employed, seed viability was assessed using 2–3 replicates of 24–25 seeds, depending on the species. Seed culture was carried out according to the method described by Dussert et al. (1998). Seed survival was assessed using 3 developmental stages: emergence of the hypocotyl and radicle (germination sensu stricto), radicle growth (>20 mm) and normal seedling development (opening of cotyledonary leaves). With one of the replicates of C. liberica and C. stenophylla seeds, zygotic embryos were extracted from frozen seeds after thawing and cultured in vitro according to the method described by Dussert et al. (1997). Excised embryos were considered viable when they stood upright on the culture medium and the first pair of leaves was developed.
Determination of seed lipid content and fatty acid composition
Seeds dried for 3 weeks over silica gel (final water content between 0.02–0.04 g H2O g−1 dry weight) were used for lipid analysis. Total lipids were solvent‐extracted from 1 g samples of ground seeds according to Folch et al. (1957). Extracted lipids were quantified gravimetrically after complete solvent evaporation under a nitrogen stream at 40°C. The total lipid content was determined in triplicate and analyzed using a one‐way ANOVA and the Newman and Keuls test.
Fatty acid methyl esters (FAME) were obtained by trans‐methylation of about 50 mg of total lipids with 2 ml of 7% boron‐trifluoride in methanol, in a waterbath heated at 90°C for 10 min. FAME were extracted with 2 ml of hexane and washed twice with 2 ml of deionized water for pH neutralization. FAME were separated on a Helwett Packard 6890 Gas Chromatograph equipped with a split (50:1) injector, a flame ionization detector, and a BPX‐70 bonded fused‐silica capillary column (SGE, France). Both the injector and the detector were kept at 230°C for FAME separation, while the column was maintained at 185°C. Helium at 1 ml min−1 was the carrier gas. Commercial standards chromatographed under the same conditions were used for FAME identification. Fatty acid composition of seed lipids was expressed as a percentage of total fatty acids (w/w). Peaks lower than 0.1% of the total area were omitted. For each coffee species, the fatty acid composition was analyzed in triplicate (i.e. from three different lipid extracts).
Differential scanning calorimetry
In order to obtain a broad range of seed water contents for DSC analysis, seeds were equilibrated over the 6 saturated salt solutions used for cryopreservation experiments and then partially dehydrated (over silica‐gel) or rehydrated (in a 100% RH atmosphere) for 0–60 min prior to DSC analysis. Thermal transitions in thin slices of endosperm (10–15 mg dry weight) were measured using a Perkin‐Elmer Pyris‐1 calibrated daily with n‐octane and n‐octadecane. Empty pans were also scanned daily before seed sample analysis to correct the baseline curvature. Seed samples sealed in aluminum pans were cooled to −120°C at the maximal cooling rate of the apparatus (about 320°C min−1), then heated at 10°C min‐1 from −120 to +20°C. Only heating thermograms were recorded. After DSC analysis, pans were punctured, and the sample dry weight was determined. In C. arabica, 3–5‐mg samples of total seed lipid extract obtained as described above were analyzed in triplicate in order to confirm the identification of an endothermic lipid transition in seed sample thermograms. Heating thermograms were analyzed for the determination of the temperature and the enthalpy of endothermic transitions measured. Transition enthalpies, expressed in J g−1 dry weight, were determined from the area above the baseline and plotted against seed sample water content, expressed in g H2O g−1 dry weight, for the determination of unfreezable water content. Two lines of regression were then determined for each species, according to the method described by Vertucci (1989a) for oily seeds. The heat of fusion of freezable water was estimated by the difference in the slopes of the two regression lines.
Results
Tolerance to LN exposure
Three groups of species could be distinguished as regards seedling recovery after LN exposure (Table 1). In species of the first group, C. brevipes, C. canephora, C. liberica and C. stenophylla, no seed germination, and consequently, no seedling production, could be obtained after LN exposure, independently of the seed water content and of the cooling procedure. In contrast to species of group 1, with the 5 species of groups 2 and 3, C. arabica, C. eugenioides, C. racemosa, C. pseudozanguebariae and C. sessiliflora, seedlings could be recovered after LN exposure when seeds were sufficiently dehydrated (Table 1; 1, 2 for C. eugenioides and C. pseudozanguebariae, respectively; all data not shown for other species). For each of these 5 species, there was a specific water content above which no survival was observed after LN exposure. This threshold water content for LN exposure is named in this study the high moisture freezing limit (HMFL), and is indicated by a dashed line in 1, 2. With the 4 species of groups 2 and 3, where seedlings could be recovered after both rapid and slow cooling, the HMFL value was the same for the two cooling protocols (1, 2; data not shown for C. racemosa and C. sessiliflora). A high interspecific variability for the HMFL was observed within the five coffee species of groups 2 and 3 (Table 1): it ranged from 0.14 g H2O g−1 dry weight in C. pseudozanguebariae to 0.26 in C. eugenioides. In all species of groups 2 and 3, the HMFL corresponded also to the optimal water content for LN exposure. Regardless of the criterion used for survival assessment, there was a decline in seed survival after LN exposure with dehydrating seeds to water contents below the specific HMFL value (1, 2; data not shown for C. arabica, C. racemosa and C. sessiliflora).
Table 1.
Desiccation sensitivity, as estimated by the water content at which 50% of the initial viability was reached, WC50, high moisture freezing limit (HMFL), percentages of seedlings recovered after desiccation to HMFL and direct immersion into LN (rapid cooling) or by a precooling to −50°C at 1°C min−1 prior to immersion in LN (slow cooling), unfreezable water content of endosperm, WCu, and corresponding water activity, awu, and water potential, Ψ u, and percentage of unsaturated fatty acids in total lipids of seeds of 9 coffee species classified in 3 groups according the percentages of seedling recovery after LN exposure (g g−1=g H2O g−1 DW; ND=not determined). a The desiccation sensitivity model could not be applied because no decline in seed viability was observed at the lowest water content tested, 0.14 g H2O g−1 DW.
| Group | Species | WC50 (g g−1) | HMFL (g g−1) | Normal seedlings (%) | WCu (g g−1) | awu | Ψ u (MPa) | Unsaturated fatty acids (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Rapid cooling | Slow cooling | ||||||||
| 1 | C. brevipes | 0.20 | – | 0 | 0 | ND | ND | ND | 47.2 |
| C. canephora | 0.17 | – | 0 | 0 | 0.28 | 0.86 | −20 | 54.4 | |
| C. liberica | 0.29 | – | 0 | 0 | 0.26 | 0.85 | −22 | 48.6 | |
| C. stenophylla | 0.16 | – | 0 | 0 | ND | ND | ND | 49.2 | |
| 2 | C. arabica | 0.11 | 0.21 | 0 | 17 | 0.21 | 0.78 | −34 | 43.9 |
| C. eugenioides | 0.11 | 0.26 | 8 | 19 | 0.26 | 0.86 | −21 | 42.0 | |
| 3 | C. pseudozanguebariae | 0.06 | 0.14 | 73 | 68 | 0.14 | 0.75 | −38 | 38.1 |
| C. racemosa | <0.14a | 0.23 | 67 | 73 | 0.24 | 0.83 | −25 | 40.4 | |
| C. sessiliflora | <0.14a | 0.19 | 81 | 76 | 0.18 | 0.79 | −32 | 35.3 | |
Figure 1.
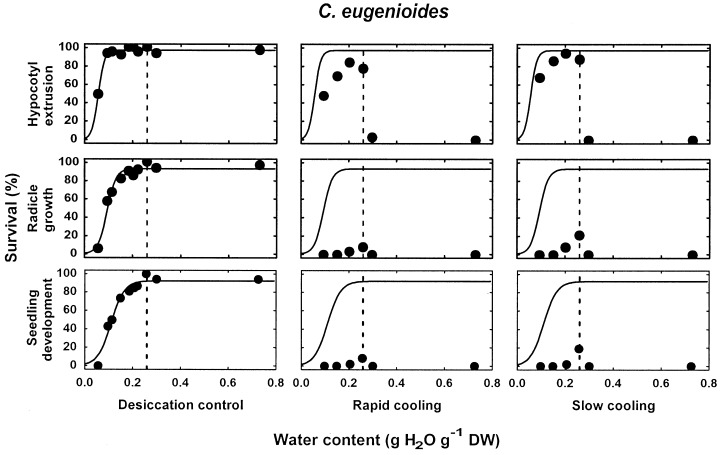
Survival, according to three different criteria of survival assessment (hypocotyl and radical extrusion, radicle growth and normal seedling development), of C. eugenioides seeds after desiccation to various water contents followed or not (desiccation control) by direct immersion into LN (rapid cooling) or by a precooling to −50°C at 1°C min−1 prior to immersion in LN (slow cooling). The HMFL is indicated by a vertical dashed line. For each criterion of survival assessment, the solid line represents the pattern of the desiccation sensitivity model fitted to desiccation control data.
Figure 2.
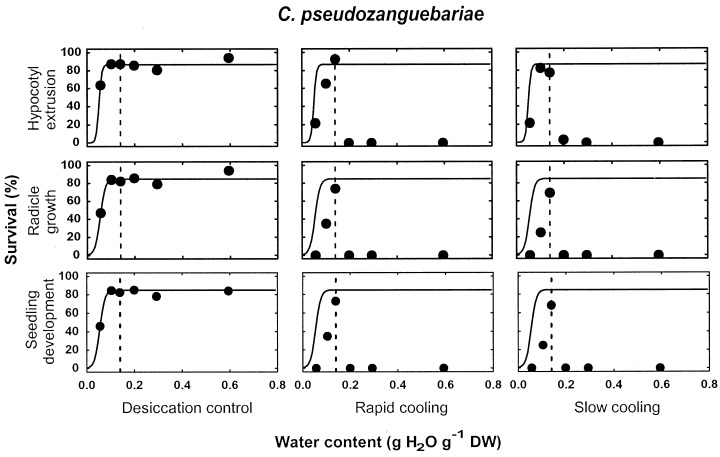
Survival, according to three different criteria of survival assessment (hypocotyl extrusion, radicle growth and normal seedling development), of C. pseudozanguebariae seeds after desiccation to various water contents followed or not (desiccation control) by direct immersion into LN (rapid cooling) or by a precooling to −50°C at 1°C min−1 prior to immersion in LN (slow cooling). The HMFL is indicated by a vertical dashed line. For each criterion of survival assessment, the solid line represents the pattern of the desiccation sensitivity model fitted to desiccation control data.
Species of groups 2 and 3 differed by the percentage of seedling recovery observed after LN exposure (Table 1). In the two species of group 2, C. arabica and C. eugenioides (Fig. 1), recovery was very low or nil after rapid cooling and only moderate (17–19%) after slow cooling (Table 1). In these two species, the percentage of seeds showing hypocotyl extrusion after LN exposure was always higher than that of seeds exhibiting radicle growth, and of seeds that developed into normal seedlings. In species of group 3, C. pseudozanguebariae (Fig. 2), C. racemosa and C. sessiliflora, very high percentages of germination, radicle growth and normal seedling development were observed after both rapid and slow cooling (Table 1).
To verify whether both the endosperm and the embryo of seeds of species of group 1 were damaged by cooling to LN temperature, embryos were extracted after thawing from frozen seeds of two of the 4 species of this group, C. liberica and C. stenophylla. In both cases, patterns of survival of embryos extracted from frozen seeds were very similar to those observed for whole seeds of species of group 3 (Fig. 3 for C. stenophylla, data not shown for C. liberica): there was a specific water content at which no significant damage to embryos could be detected after both rapid and slow cooling (seed water content of 0.27 and 0.25 g H2O g−1 dry weight for C. liberica and C. stenophylla, respectively), and below which survival of embryos rapidly declined with lowering seed water content. From this, we concluded that there was a range of water contents for which the complete loss of viability after LN exposure of whole seeds was due to freezing injury to the endosperm only.
Figure 3.
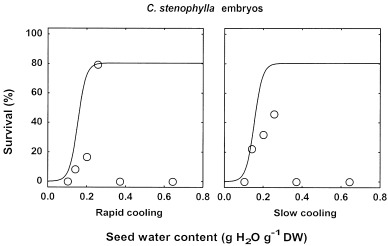
Survival, according to the criterion of normal seedling development, of zygotic embryos extracted after thawing from C. stenophylla seeds after desiccation to various water contents followed by direct immersion into LN (rapid cooling) or by a precooling to −50°C at 1°C min−1 prior to immersion in LN (slow cooling). The solid line represents the pattern of the desiccation sensitivity model fitted to desiccation control data, according to the criterion of normal seedling development.
Differential scanning calorimetry
In the seven coffee species for which DSC analysis was performed, similar global changes were observed in heating thermograms with varying seed water content. Heating thermograms of 5 C. arabica samples at different water contents are given in Fig. 4 in order to illustrate these global changes. At water contents lower than 0.20 g H2O g−1 dry weight (0.13 g H2O g−1 dry weight shown in Fig. 4), a broad but small endotherm (peak 1) was detected (onset temperature of −11°C), without any apparent increase in its size, nor change in its temperature with varying seed water contents. At water contents higher than 0.22 g H2O g−1 dry weight, a second endotherm (peak 2) appeared with an onset temperature of −20°C. With continuing seed rehydration, the size and the onset temperature of peak 2 increased and, at the highest water contents experimented, the peak 1 appeared only as a small shoulder at the end of peak 2 (Fig. 4). DSC analysis of C. arabica lipid extract revealed the presence of one main endotherm whose characteristics were identical to those of peak 1 of seed sample thermograms. Its onset temperature was of −11°C and its transition enthalpy of 43 J g−1, a value very similar to the transition enthalpy of peak 1 measured in dry C. arabica seeds if taking in account their lipid content (16.1% dry weight). Thus, peaks 1 and 2 were attributed to the melting transition of lipids and water, respectively. Even if the characteristics of the peak corresponding to the melting of lipids varied between the different coffee species studied, the unfreezable water content could be determined using the same method. Because the two events partly overlapped at high water contents, the sum of the areas of the two peaks was used for regression against seed sample water content as performed by Vertucci (1989a) in oily seeds.
Figure 4.
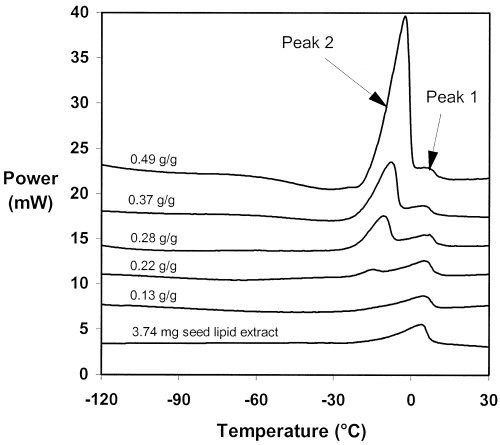
DSC heating thermograms of endosperm samples of C. arabica seeds at various water contents (g/g=g H2O g−1 DW) and of a sample of total lipids extracted from dry C. arabica seeds.
With each of the 7 species for which DSC analysis was performed, this method led to the determination of two regression lines (Fig. 5) whose characteristics are given in Table 2. A highly significant linear correlation between the melting enthalpy of water and lipids and seed water content was found within the region of high water contents with the 7 species (r2 >0.84; P slope<0.001; P Y origin<0.05). In the region of low water contents, the Y value at origin of the regression line, which corresponds to the transition enthalpy of lipids in dry seeds, was always highly significant (Table 2) and, as expected, was significantly correlated to seed lipid content (P=0.026).
Figure 5.
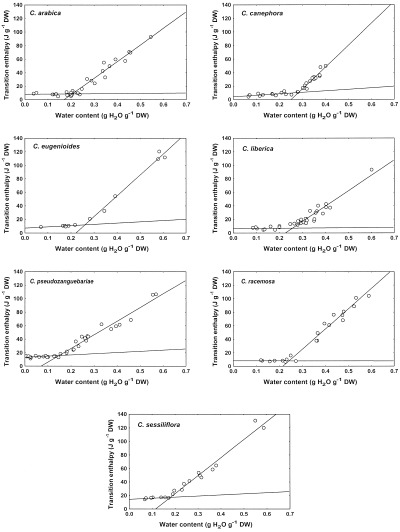
Determination of the unfreezable water content of endosperm of seeds of seven coffee species as calculated by the X‐intercept of the intersection between the two lines of regression which best fitted the relationship between water content and enthalpy of the melting transitions of seed samples dried to various water contents.
Table 2.
Regression parameters, within the two regions of water contents (absence/presence of freezable water in seed tissues), of the relationship between water content and enthalpy of melting transitions of endosperm samples of seven coffee species, proportion of variance explained by the regression, r2, and probability P of significance of each parameter.
| Linear regression at low water contents | Linear regression at high water contents | Heat of fusion of freezable | |||||
|---|---|---|---|---|---|---|---|
| Y value at origin | Slope (J g−1 H2O) | r2 | Y value at origin | Slope (J g−1 H2O) | r2 | water (J g−1 H2O) | |
| C. arabica | 8.0 | 10.3 | 0.03 | −41.8 | 245.3 | 0.92 | 235.0 |
| P | 0.0031 | 0.4448 | 0.0003 | 0.0000 | |||
| C. canephora | 4.7 | 17.5 | 0.29 | −76.8 | 310.9 | 0.92 | 293.4 |
| P | 0.0040 | 0.0486 | 0.0000 | 0.0000 | |||
| C. eugenioides | 7.3 | 18.3 | 0.78 | −68.1 | 307.5 | 0.98 | 289.2 |
| P | 0.0009 | 0.0194 | 0.0025 | 0.0001 | |||
| C. liberica | 6.6 | 2.4 | 0.00 | −51.3 | 227.4 | 0.90 | 225.0 |
| P | 0.0335 | 0.8900 | 0.0000 | 0.0000 | |||
| C. pseudozanguebariae | 13.2 | 18.0 | 0.28 | −11.2 | 190.9 | 0.84 | 172.9 |
| P | 0.0000 | 0.0298 | 0.0147 | 0.0000 | |||
| C. racemosa | 8.2 | −0.4 | 0.00 | −60.5 | 286.6 | 0.89 | 287.0 |
| P | 0.0099 | 0.9734 | 0.0004 | 0.0000 | |||
| C. sessiliflora | 14.4 | 16.2 | 0.39 | −30.5 | 266.2 | 0.97 | 250.0 |
| P | 0.0000 | 0.1313 | 0.0003 | 0.0000 | |||
A very broad variability was found among the seven coffee species whose seeds were analyzed by DSC for the unfreezable water content, WCu, as determined by the X‐intercept of the intersection between the two regression lines: it ranged from 0.13 g H2O g−1 dry weight for C. pseudozanguebariae to 0.28 g H2O g−1 dry weight for C. canephora. Using the water sorption model previously developed (Dussert et al. 1999) and sorption data obtained from the same seed lots (Dussert et al. 1999), the water activity, awu, and the water potential, Ψ u, corresponding to WCu was calculated: awu ranged from 0.75 to 0.88 and Ψ u from −19 to −38 MPa (Table 1).
Interspecific correlation between WCu and HMFL
A very highly significant correlation (P=0.0000; r2=0.99) was found between WCu and HMFL values within the five coffee species of groups 2 and 3 for which DSC analysis was performed: C. arabica, C. eugenioides, C. pseudozanguebariae, C. racemosa and C. sessiliflora (Fig. 6). The slope of the line of regression was not significantly different from unity (tobs=2.62<t3,0.025=3.28), showing that HMFL was not significantly different from WCu in these five coffee species.
Figure 6.
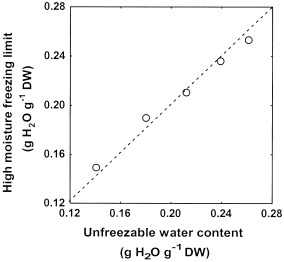
Relationship between the unfreezable water content of endosperm, as determined from DSC analysis, and the HMLF of seeds of the five coffee species with which normal seedlings could be recovered from seeds cooled to LN temperature: C. arabica, C. eugenioides, C. pseudozanguebariae, C. racemosa and C. sessiliflora.
Tolerance to liquid nitrogen exposure and seed lipid composition
We observed a very high interspecific variability for endosperm lipid content and lipid composition (Table 3). Seed lipid content varied from 9.8% dry weight in C. brevipes to 34.6% dry weight in C. pseudozanguebariae but we found no significant (P>0.05) correlation between seed lipid content and survival percentages after rapid and slow cooling treatments within the 8 species where WC50 was lower than WCu.
Table 3.
Total lipid content (% DW) and fatty acids composition (%) in seeds of the 9 coffee species studied (n−?=position of the double bound unknown). Results of one‐way ANOVAs: F and P. For each variable, means with a common letter were not significantly different at P=0.05 according to the Newman and Keuls test.
| Species | Lipid content | Fatty acid composition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (% DW) | C14:0 | C15:0 | C15:1, n−? | C16:0 | C17:0 | C18:0 | C18:1, n−9 | C18:2, n−6 | C18:3, n−3 | C20:0 | C20:1, n−9 | C22:0 | |
| C. arabica | 16.1 a | 0.2 | 0.1 | 0.0 | 40.4 d | 0.2 | 9.8 de | 7.7 b | 34.7 c | 1.1 b | 4.3 b | 0.4 d | 1.2 b |
| C. brevipes | 9.8 b | 0.2 | 0.1 | 0.0 | 35.7 b | 0.1 | 9.2 d | 13.6 e | 31.0 b | 1.5 c | 6.0 c | 1.1 f | 1.5 c |
| C. canephora | 11.0 c | 0.2 | 0.1 | 0.1 | 34.2 a | 0.1 | 6.3 a | 10.2 cd | 42.9 e | 0.8 ab | 3.7 b | 0.4 d | 1.2 b |
| C. eugenioides | 15.0 d | 0.2 | 0.1 | 0.0 | 45.9 e | 0.2 | 8.2 c | 5.3 a | 34.7 c | 1.8 d | 2.7 a | 0.2 c | 0.7 a |
| C. liberica | 13.7 e | 0.1 | 0.0 | 0.0 | 37.9 c | 0.2 | 8.2 c | 10.4 cd | 36.9 d | 0.8 ab | 4.0 b | 0.5 e | 1.0 ab |
| C. pseudozanguebariae | 34.6 f | 0.2 | 0.0 | 0.0 | 40.0 d | 0.2 | 16.3 f | 9.1 c | 28.3 a | 0.5 a | 4.2 b | 0.2 c | 0.8 ab |
| C. racemosa | 13.9 e | 0.2 | 0.1 | 0.0 | 45.0 e | 0.1 | 10.4 e | 10.6 cd | 29.3 a | 0.5 a | 2.9 a | 0.0 a | 1.0 ab |
| C. sessiliflora | 21.4 g | 0.2 | 0.0 | 0.0 | 38.3 c | 0.2 | 19.5 g | 6.6 ab | 28.2 a | 0.5 a | 5.6 bc | 0.0 a | 1.0 ab |
| C. stenophylla | 13.4 e | 0.2 | 0.0 | 0.0 | 39.2 cd | 0.0 | 7.3 b | 11.6 d | 37.0 d | 0.5 a | 3.3 a | 0.1 b | 0.9 ab |
| F | 519.9 | 19.5 | 61.6 | >105 | 81.2 | 26.4 | 353.8 | 36.1 | 78.1 | 29.9 | 34.5 | 129.4 | 7.8 |
| P | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Palmitic, stearic, oleic and linoleic acids were the major fatty acids in all the studied species (Table 3). However, there were very large variations between species as regards the relative proportions of these 4 fatty acids: palmitic acid varied from 34.2% in C. canephora to 45.9% in C. eugenioides, stearic acid ranged from 6.3% in C. canephora to 19.5% in C. sessiliflora, oleic acid varied from 5.3% in C. eugenioides to 13.6% in C. brevipes, and linoleic acid varied from 28.2% in C. sessiliflora to 42.9% in C. canephora. Interspecific differences in fatty acid composition of seed lipids resulted in a high variability for the percentage of unsaturated fatty acids, which ranged from 28.7% in C. sessiliflora seeds to 54.4% in C. canephora seeds (Table 1). Among the 8 species for which WC50 was lower than WCu, a highly significant correlation was found between the percentage of unsaturated fatty acids and the percentage of seedling recovery after rapid (P=0.012) or slow (P=0.0048) cooling.
Interspecific relationship between WCu and seed lipid content
Whatever the basis of the expression of the unfreezable water content (total dry weight or non‐lipid dry weight), a negative relationship was found between the unfreezable water content and the lipid content of seeds of the seven coffee species for which DSC analysis was performed (Fig. 7). Even if the linear regression between WCu and the seed lipid content was significant (P=0.0025 and P=0.0072, respectively), the apparent relationship between these two traits did not appear to be linear and many curvilinear models, e.g. y = ae(bx) + c, fitted the data better than the linear regression (comparison of a curvilinear model to the linear one was achieved by comparing the residual variance ratio to the corresponding theoretical F value).
Figure 7.
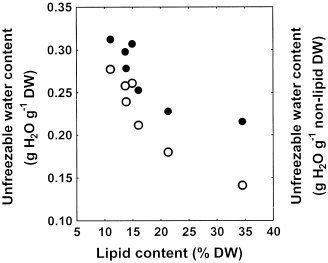
Relationship between the lipid content and the unfreezable water content of endosperm of seeds of seven coffee species, as determined from DSC analysis and expressed on a dry weight basis (○) or a non‐lipid dry weight basis (●).
Discussion
With the five coffee species for which seedling recovery was achieved after seed LN exposure, the HMFL value coincided with that of the unfreezable water content, indicating that seeds of those species were not able to withstand intracellular ice formation during the freezing/thawing process. Seed cryopreservation studies involving determination of the unfreezable water content from DSC analysis are scarce. However, from these few studies, it can be established that some seed materials can withstand the presence of some freezable water during exposure to sub‐freezing temperatures, while others can not. It was first demonstrated with pea seeds that formation of intracellular ice crystals did not always limit survival (Vertucci 1989a). Vertucci (1989a) suggested that, below a threshold water content (about 0.33 g H2O g−1 dry weight in pea seeds while the unfreezable water content was of 0.26 g H2O g−1 dry weight), the ice crystals that form in pea cotyledons and embryos during cooling are too small to cause significant damage and do not coalesce during rewarming. By contrast, whole soybean seeds did not withstand intracellular ice formation during LN exposure (Vertucci 1989a). Since the shift in tolerance of soybean seeds to the presence of some freezable water in their tissues was observed in the range of temperatures where phase transitions of storage lipids occurred (between −30 and −50°C), it was suggested by Vertucci (1989a) that the interaction between storage lipids and water somehow promoted formation of ice crystals large enough to cause lethal damage. Seed lipid contents of the different coffee species studied here were of the same order of soybean seeds (about 20% dry weight). Thus, the present results are consistent with the earlier study of Vertucci (1989a) with orthodox soybean seeds, and confirm that intracellular ice formation seems to be lethal in lipid‐rich seeds, independent of their level of desiccation sensitivity. Furthermore, these data indicate that the unfreezable water content does correspond to the upper limit of the hydration window allowing the successful cryopreservation of such seeds. In addition, the susceptibility of seeds to the presence of some freezable water during LN exposure does not seem to be related to desiccation sensitivity per se because some of the seed materials that were shown to tolerate it, such as Landolphia kirkii (Vertucci et al. 1991) and Zizania (Vertucci et al. 1995) embryos, were also shown to be considerably less tolerant to desiccation than coffee seeds.
At least two possibilities, which may not be mutually exclusive, can be proposed to explain the low survival after LN exposure observed in C. arabica, C. canephora and C. eugenioides at water contents where no more freezable water is present in seed tissues and where no desiccation damage was detected: (1) some of the cooling, thawing, and post‐thawing conditions were sub‐optimal; (2) desiccation sensitivity as quantified at room temperature in terms of water content was not the real lower limit of the hydration window for LN exposure. These two proposals have to be explored, taking in account the strong association found between the level of unsaturation of fatty acids and the sensitivity to LN exposure in seeds of the coffee species studied here.
The first proposal is supported by the observation of a beneficial effect of a slow precooling prior to immersion in LN in species of group 2 (present results and Dussert et al. 1997, 1998), as also observed in other oily seeds (Stanwood 1987; Vertucci 1989b), and the beneficial effect of a post‐thawing osmoconditioning treatment in frozen C. arabica seeds (Dussert et al. 2000), which may be related to reduced oxidative stress.
The second proposal, i.e. that the critical water content for desiccation sensitivity measured at room temperature does not correspond to the lower limit of the hydration window for LN exposure, is derived from observations and suggestions of Eira et al. (1999a). These authors observed an increase in the critical water content for desiccation sensitivity of Coffea spp. seeds with decreasing temperature from +15 to −20°C. A theoretical basis for this phenomenon has been proposed by Vertucci et al. (1994). This assumes that a single critical water activity determines the desiccation sensitivity of seeds of a given species. Since, at given water content, seed water activity decreases with decreasing temperature (Vertucci and Roos 1993, 1994, 1995), it is possible that the water activity of seeds cooled to −196°C is decreased to a value lower than the critical water activity for desiccation sensitivity. Consequently, even if at room temperature the water activity of seeds desiccated to WCu is higher than the critical water activity for desiccation sensitivity, when frozen these seeds undergo desiccation damage. The relationship between the water activity, aw, and the temperature, T (in Kelvin), is described by the following equation derived from the van't Hoff isochore (Vertucci and Roos 1993; Vertucci et al. 1995; Eira et al. 1999b): d(ln(aw))/d(1/T)=ΔHsorp(T)/R, where ΔHsorp(T) is the enthalpy of sorption and R is the ideal gas constant. Assuming a constant ΔHsorp with temperature (Vertucci and Roos 1993), in C. arabica seeds at 0.2 g H2O g−1 dry weight (the unfreezable water content), ΔHsorp should not be lower than −0.5 kJ mol−1 of water in order to avoid a decrease of seed water activity at −196°C below the critical water activity for desiccation tolerance, i.e., about 0.45, as quantified at room temperature (Dussert et al. 1999). The value of ΔHsorp measured between 5 and 25°C at 0.2 g H2O g−1 dry weight in C. arabica seeds by Eira et al. (1999b) was lower than −0.5 kJ mol−1 of water, indicating clearly that desiccation damage could occur at LN temperature in C. arabica seeds if assuming the existence of a single water activity for desiccation sensitivity and a constant value of ΔHsorp with temperature. Therefore, the prediction of the existence of an hydration window for LN exposure in non‐orthodox oily seeds should not be done by the comparison of the unfreezable water content with the critical water content for desiccation sensitivity as measured at room temperature, but by comparing the critical water activity for desiccation damage, measured at room temperature, to the calculated water activity at LN temperature of seeds desiccated to the unfreezable water content.
The unfreezable water content in coffee species was found to be negatively correlated to seed lipid content. To our knowledge, this is the first study that investigates the nature of this relationship. Additional data, previously reported by other authors, fit remarkably well with the relationship shown on Fig. 7: the unfreezable water contents of seeds of Quercus rubra, Q. robur and Azadirachta indica were of 0.32, 0.21 and 0.14 g H2O g−1 dry weight, respectively, whereas their lipid contents are 3, 20 and 43% dry weight, respectively (Pritchard and Manger 1998; Sun 1999; Sacandé et al. 2000). The relationship between the lipid content and WCu appeared not to follow a linear model, even when WCu was expressed on a non‐lipid dry weight basis. Since this relationship was not linear, it is a good indication that either the interaction between non‐lipid components and water varies among species or that lipids interfere with the freezing properties of water. The interspecific variability observed in coffee seeds for the water activity corresponding to the unfreezable water content ranged between 0.75 and 0.88 (water potentials of between −38 and −19 MPa), and was found to be independent of the seed lipid content. These values of awu are remarkably consistent with those observed in orthodox seeds of pea and soybean (Vertucci 1990). However, the interspecific variance for the water activity corresponding to unfreezable water content is certainly associated with the non‐linear relationship observed between the lipid content and the unfreezable water content of seeds: in other words, components other than lipids influence the value of awu.
Acknowledgement
The authors gratefully acknowledge Christina Walters for her precious advice regarding DSC analysis and the Bureau des Ressources Génétiques (Paris, France) for providing partial financial support to this study.
References
- Becwar MR, Stanwood PC, Lehonardt KW (1983) Dehydration effects on freezing characteristics and survival in liquid nitrogen of desiccation‐tolerant and desiccation‐sensitive seeds. J Am Soc Hortic Sci 108: 613–618. [Google Scholar]
- Dussert S, Chabrillange N, Engelmann F, Anthony F, Hamon S (1997) Cryopreservation of coffee (Coffea arabica L.) seeds: importance of the precooling temperature. Cryo-Lett 18: 269–276. [Google Scholar]
- Dussert S, Chabrillange N, Engelmann F, Anthony F, Louarn J, Hamon S (1998) Cryopreservation of seeds of four coffee species (Coffea arabica, C. costatifructa, C. racemosa and C. sessiliflora): importance of water content and cooling rate. Seed Sci Res 8: 9–15. [Google Scholar]
- Dussert S, Chabrillange N, Engelmann F, Hamon S (1999) Quantitative estimation of seed desiccation sensitivity using a quantal response model: application to nine species of the genus Coffea L. Seed Sci Res 9: 135–144. [Google Scholar]
- Dussert S, Chabrillange N, Vasquez N, Engelmann F, Anthony F, Guyot A, Hamon S (2000) Beneficial effect of post‐thawing osmoconditioning on the recovery of cryopreserved coffee (Coffea arabica L.) seeds. Cryo Lett 21: 47–52. [PubMed] [Google Scholar]
- Eira MTS, Walters C, Caldas LS, Fazuoli LC, Sampaio JB, Dias MC (1999a) Tolerance of Coffea spp. seeds to desiccation and low temperature. Rev Bras Fisiol Veg 11: 97–105. [Google Scholar]
- Eira MTS, Walters C, Caldas LS (1999b) Water sorption properties in Coffea spp. seeds and embryos. Seed Sci Res 9: 321–330. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH (1990) An intermediate category of seed storage behaviour? I. Coffee. J Exp Bot 41: 1167–1174. [Google Scholar]
- FAO/IPGRI (1994) Genebank Standards. Food and Agriculture Organization of the United Nations/International Plant Genetic Resources Institute, Rome. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- Kuranuki Y & Yoshida S (1996) Different responses of embryonic axes and cotyledons from tea seeds to desiccation and cryoexposure. Breed Sci 46: 149–154. [Google Scholar]
- Pritchard HW & Manger KR (1998) A calorimetric perspective on desiccation stress during preservation procedures with recalcitrant seeds of Quercus robur L. Cryo Lett Suppl 1: 23–30. [Google Scholar]
- Sacandé M, Buitink J, Hoekstra FA (2000) A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. J Exp Bot 51: 635–643. [DOI] [PubMed] [Google Scholar]
- Stanwood PC (1987) Survival of sesame seeds at the temperature (−196°C) of liquid nitrogen. Crop Sci 27: 327–331. [Google Scholar]
- Sun WQ (1999) State and phase transition behaviors of Quercus rubra seed axes and cotyledonary tissues: relevance to the desiccation sensitivity and cryopreservation of recalcitrant seeds. Cryobiology 38: 372–385.DOI: 10.1006/cryo.1999.2180 [DOI] [PubMed] [Google Scholar]
- Van der Vossen HAM (1977) Methods of preserving the viability of coffee seed in storage. Kenya Coffee 45: 31–35. [Google Scholar]
- Vernon P, Vannier G, Arondel V (1999) Supercooling capacity of seeds and seedlings in Arabidopsis thaliana . Cryobiology 39: 138–143.DOI: 10.1006/cryo.1999.2192 [DOI] [PubMed] [Google Scholar]
- Vertucci CW (1989a) Relationship between thermal transitions and freezing injury in pea and soybean seeds. Plant Physiol 90: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW (1989b) Effects of cooling rate on seeds exposed to liquid nitrogen temperatures. Plant Physiol 90: 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW (1990) Calorimetric studies of the state of water in seed tissues. Biophys J 58: 1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertucci CW & Roos EE (1993) Theoretical basis of protocols for seed storage II. The Influence of temperature on optimal moisture levels. Seed Sci Res 3: 201–213. [Google Scholar]
- Vertucci CW, Berjak P, Pammenter NW, Crane J (1991) Cryopreservation of embryonic axes of an homeohydrous (recalcitrant) seed in relation to calorimetric properties of tissue water. Cryo-Lett 12: 339–350. [Google Scholar]
- Vertucci CW, Crane J, Porter RA, Oelke EA (1994) Physical properties of water in Zizania embryos in relation to maturity status, water content and temperature. Seed Sci Res 4: 211–224. [Google Scholar]
- Vertucci CW, Crane J, Porter RA, Oelke EA (1995) Survival of Zizania embryos in relation to water content, temperature and maturity status. Seed Sci Res 4: 31–40. [Google Scholar]
- Wesley‐Smith J, Vertucci CW, Berjak P, Pammenter NW, Crane J (1992) Cryopreservation of desiccation‐sensitive axes of Camellia sinensis in relation to dehydration, freezing rate and the thermal properties of tissue water. J Plant Physiol 14: 596–604. [Google Scholar]


