Abstract
Introduction:
Thymic epithelial tumors (TETs) including thymoma and thymic carcinoma are rare tumors with little data available to guide treatment. Immunotherapy with checkpoint blockade has shown promising activity, but data regarding the expression patterns and prognostic implications of programmed death 1 (PD-1) and its ligand (PD-L1) in TETs have yielded conflicting results. Intratumoral heterogeneity of PD-1/L1 expression has been shown in other cancers, but has not been described in the TET literature.
Methods:
We performed a retrospective single-center review of 35 patients with resected TET. PD-1/L1 expression was assessed by immunohistochemistry using PD-1 clone: NAT105 and PD-L1 clone: 22C3. Tumor samples from 35 patients were evaluated including 32 patients with thymoma and 3 patients with thymic carcinoma.
Results:
PD-L1 expression was detected in 83% (29 of 35) tumor samples, including 100% (3 of 3) of thymic carcinoma patients and 81% (26 of 32) of thymoma patients. PD-1 expression was detected in 77% (27 of 35), including 33% (1 of 3) of thymic carcinoma patients and 81% (26 of 32) thymoma patients. High PD-1 expression was associated with lower grade tumors. Unlike prior studies, PD-L1 expression was not associated with higher grade tumors or higher stage. Neither PD-L1 nor PD-1 expression was significantly associated with survival. Three patients with thymoma had multiple tumor sections evaluated for expression of PD-1/L1, with differing expression patterns of both PD-L1 and PD-1 observed in two patients.
Conclusions:
This study confirms high expression of PD-L1 and PD-1 in TET and shows for the first time intratumoral heterogeneity of PD-L1 and PD-1 in thymoma patients.
Keywords: Thymoma, Thymic carcinoma, Immunotherapy, Intratumoral heterogeneity
Introduction
Thymic epithelial tumors (TETs), including thymoma and thymic carcinoma, are rare tumors that originate in the thymus.1,2 The WHO classification divides TET into thymoma (types A, AB, B1, B2, and B3) and thymic carcinoma (type C in the 2004 classification).3–5 Thymic carcinomas represent 15% to 20% of all thymic neoplasms, and the 5-year survival rate of 53% is significantly lower than that for thymoma which is close to 90%.2,6–8 Surgical resection is the only curative intervention for patients with TET, and completeness of surgery is the most important predictor of survival.7,9,10 Radiation is typically offered in combination with chemotherapy for patients with unresectable disease.11,12 Patients with metastatic disease are treated with platinum-based chemotherapy regimens.13–16 There are no well-studied standard treatment options for patients in the secondline setting, although the tyrosine kinase inhibitor sunitinib has shown activity in patients who progressed after platinum-based chemotherapy.17 There is clearly an unmet need for additional treatment options for this patient population. Immunotherapy with antibodies to programmed death 1 (PD-1) and its ligand, programmed death ligand 1 (PD-L1), has changed the landscape of treatment options for patients with many tumors, including melanoma, head and neck carcinoma, and NSCLC.18–20 The search for additional treatment options for patients with TET has led to several studies evaluating the expression of PD-L1 in TET, as high expression of PD-L1 has been found to be predictive of benefit in patients with NSCLC treated with checkpoint inhibitors in the first-line setting.20 Case reports have shown responses in both thymoma and squamous cell thymic carcinoma to the checkpoint inhibitor pembrolizumab.21,22 The efficacy of immunotherapy is currently being evaluated in a phase 2 trial of pembrolizumab in patients with thymic carcinoma, with early results presented at the American Society of Clinical Oncology Meeting 2016 showing promising activity with a 22.5% response rate.23 Studies have thus far shown high rates of PD-L1 expression in TET, but have reported conflicting results in terms of the prognostic and predictive value of PD-L1 and PD-1 expression, perhaps due to variabilities of antibodies used and methods of PD-L1 expression scoring. Two studies that have shown a prognostic value of PD-L1 demonstrated opposing results, with Padda et al.24 finding that high PD-L1 expression was associated with a worse prognosis and Yokoyama et al.25 finding that high PD-L1 was associated with a better prognosis. Additionally, there are several limitations of PD-L1 expression as a biomarker including variations between assays as well as interand intratumoral heterogeneity. The most common assays used include 22C3 (Dako North America, Carpinteria, California), 28–8 (Dako), SP263 (Ventana Medical Systems, Tucson, Arizona) and SP142 (Ventana). The recently published Blueprint PD-L1 Immunohistochemistry (IHC) Assay Comparison Project offers an extensive review of these assays, and shows considerable variability in expression patterns between some of the PD-L1 assays in NSCLC, especially SP-142.26 Separately, intratumoral heterogeneity in PD-L1 expression has been reported in NSCLC.27 Therefore, we set out to study the expression patterns, prognostic value, and heterogeneity of PD-L1 and PD-1 expression by IHC among resected TET samples at our institution using the 22C3 antibody which is used in the predictive, U.S. Food and Drug Administration (FDA)–approved companion diagnostic assay for use in detecting PD-L1 expression in NSCLC, where pembrolizumab is approved in the first-line setting in patients with high PD-L1 expression.20 We also sought to examine associations between PD-1 and PD-L1 expression and stage, grade, survival, and diagnosis of myasthenia gravis (MG), as the potential for exacerbating autoimmune conditions associated with TET may limit the utility of checkpoint inhibitors in patients with these diseases.
Materials and Methods
Patients
A retrospective review was performed at The Ohio State University of 35 consecutive patients with thymoma and thymic carcinoma undergoing surgical resection from 2000 – 2010 with tumor specimens available at our institution. Clinicopathologic characteristics assessed included age, grade, stage, overall survival (OS), smoking status, and diagnosis of MG. The 2004 WHO Classification was used.4 The study was approved by the institutional review board.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue specimens obtained from surgical resections were examined. PD-1 and PD-L1 expression patterns were assessed by IHC on tumor samples by a pathologist blinded to clinical outcomes. The PD-1 antibody used was NAT105 (1:100, Abcam, Cambridge, United Kingdom) and PD-L1 antibody used was 22C3 (Merck Research Laboratories, Palo Alto, California). Expression was graded in a semiquantitative scoring system from 0 to 5 (0 indicating no expression, 5 indicating high expression) as described elsewhere.28–30
Statistical Considerations
Demographic and clinical factors were summarized separately by PD-L1 (positive versus negative, high/moderate versus low) and PD-1 (positive versus negative, high/moderate versus low) status separately. High/moderate positivity was determined as an expression score of 3 or greater by IHC, and low expression of 1 or 2 by IHC. Selected factors (WHO grade, stage, smoker, MG diagnosis, and recurrence) were compared between groups using chi-square tests; p values were adjusted for multiple comparisons using Hochberg’s procedure. Estimates of OS were calculated using the Kaplan-Meier method and differences in the curves between groups were assessed using the log-rank test. In addition, hazard ratios (HRs) and 95% confidence intervals (CIs) from univariable (unadjusted) Cox proportional hazards models were calculated; due to the small sample sizes (both overall and number of events), additional covariate adjustment was not performed. All analyses were performed using SAS/STAT software, Version 9.4 of the SAS System for Windows (SAS Institute Inc., Cary, North Carolina).
Results
Patient Characteristics
Tumor specimens from 35 patients with thymoma and thymic carcinoma undergoing surgical resection were collected at our institution. Patient characteristics are shown in Table 1. As would be expected from a cohort of patients undergoing resection, the majority of patients had early-stage disease; they included predominantly thymoma with only 3 thymic carcinoma patients. Median follow-up time was 6.2 years (25th, 57th percentile: 2, 8 years, respectively), at which time 25 patients had died and 10 patients were alive.
Table 1.
Clinical Characteristics and Overall Expression Patterns of PD-L1 and PD-1
| Clinical Characteristic | N (%) |
|---|---|
| Age (median, y) | 55 (range, 33–71) |
| Gender | |
| Male | 18 (51) |
| Female | 17 (49) |
| Pathologic Masaoka stage | |
| I | 15 (43) |
| II | 5 (14) |
| III | 7 (20) |
| IV | 7 (20) |
| Unknown | 1 (3) |
| WHO histology | |
| A | 6 (17) |
| AB | 8 (23) |
| B1 | 4 (11) |
| B2 | 5 (14) |
| B3 | 9 (26) |
| Thymic carcinoma | 3 (9) |
| PD-L1 expression (≥1+ by IHC) | |
| All (N = 35) | 29 (83) |
| Thymoma (n = 32) | 26 (81) |
| Thymic carcinoma (n = 3) | 3 (100) |
| PD-1 Expression (≥1+ by IHC) | |
| All (N = 35) | 27 (77) |
| Thymoma (n = 32) | 26 (81) |
| Thymic Carcinoma (n = 3) | 1 (33) |
| High PD-L1 expression (≥3+ by IHC) | 20 (57) |
| High PD-1 expression (≥3+ by IHC) | 11 (31) |
PD-L1, programmed death ligand 1; IHC, immunohistochemistry; PD-1, programmed death 1.
Immunohistochemical Detection of PD-1 and PD-L1
Expression patterns of PD-L1 and PD-1 by IHC are also depicted in Table 1. PD-L1 was present in the majority of specimens examined, including 81% of thymoma and 100% of thymic carcinoma samples. PD-1 expression was present in 81% of patients with thymoma but only 33% of patients with thymic carcinoma. When only high/moderate levels of PD-L1 and PD-1 were assessed (≥ 3+ by IHC), 57% of patients had high/moderate PD-L1 expression whereas only 31% of all patients had high/moderate PD-1 expression. Unlike prior studies, the expression of PD-L1 was not associated with WHO grade (A, AB, B1 versus B2, B3, C) (p = 0.99) or stage (1/2 versus 3/4) (p = 0.51).31 As seen in Table 2, higher expression of PD-L1 (compared to low or no expression) was not associated with WHO grade (p = 0.94) or stage (p = 0.94). PD-1 positivity was not associated with WHO grade (p = 0.12) or stage (p = 0.18) however, high/moderate expression of PD-1 (compared to low or no expression) was associated with lower WHO grade (p = 0.03). Neither PD-L1 nor PD-1 were associated with tumor size on the 29 patients with preoperative imaging by computed tomography scan available (p = 0.15 and p = 0.23, respectively). Expression of PD-L and PD-1 was not associated with stage at diagnosis, smoking, recurrence, or diagnosis of MG.
Table 2.
Demographic and Clinical Factors by PD-L1 (High Versus Low) and PD-1 (High Versus Low) Expression
| Characteristics | PD-L1 High Expression (N = 20) | p Value | PD-1 High Expression (N = 11) | p Value |
|---|---|---|---|---|
| WHO grade | ||||
| Low (A, AB, B1) | 11 (55) | 0.94 | 10 (91) | 0.03 |
| High (B2, B3, TC) | 9 (45) | 1 (9) | ||
| Stage | ||||
| Early (1,2) | 11 (55) | 0.94 | 9 (82) | 0.27 |
| Late (3,4) | 9 (45) | 2 (18) | ||
| Smoker | ||||
| No | 8 (47) | 0.56 | 4 (40) | >.99 |
| Yes | 9 (53) | 6 (60) | ||
| MG diagnosis | ||||
| No | 14 (70) | >0.99 | 9 (82) | 0.56 |
| Yes | 6 (30) | 2 (18) | ||
| Recurrence | ||||
| No | 16 (80) | 0.94 | 11 (100) | 0.27 |
| Yes | 4 (20) | 0 | ||
WHO grade, stage, smoking history, MG diagnosis, and recurrence were compared between groups; p values were adjusted for multiple comparisons. High PD-1 expression was associated with lower grade tumors (p = 0.03).
Values are expressed as n (%) unless otherwise specified.
TC, thymic carcinoma; MG, myasthenia gravis; PD-L1, programmed death ligand 1; PD-1, programmed death 1.
Prognostic Value of PD-1 and PD-L1
Kaplan-Meier curves for the overall population are shown in Figure 1. There was no significant difference in OS seen in patients with PD-L1 expression (p = 0.10, log-rank test, Fig. 1). Survival was not significantly different for patients with PD-1 expression, high PD-L1 (compared to low PD-L1), and high PD-1 expression. There was no difference in OS based on PD-L1 expression after excluding the three thymic carcinoma patients from the analysis (log-rank p = 0.12). Because of the small number of patients, a wide CI for the HR among patients with positive PD-L1 expression was observed (HR = 3.71, 95% CI: 0.48 – 29.01, p = 0.21).
Figure 1.
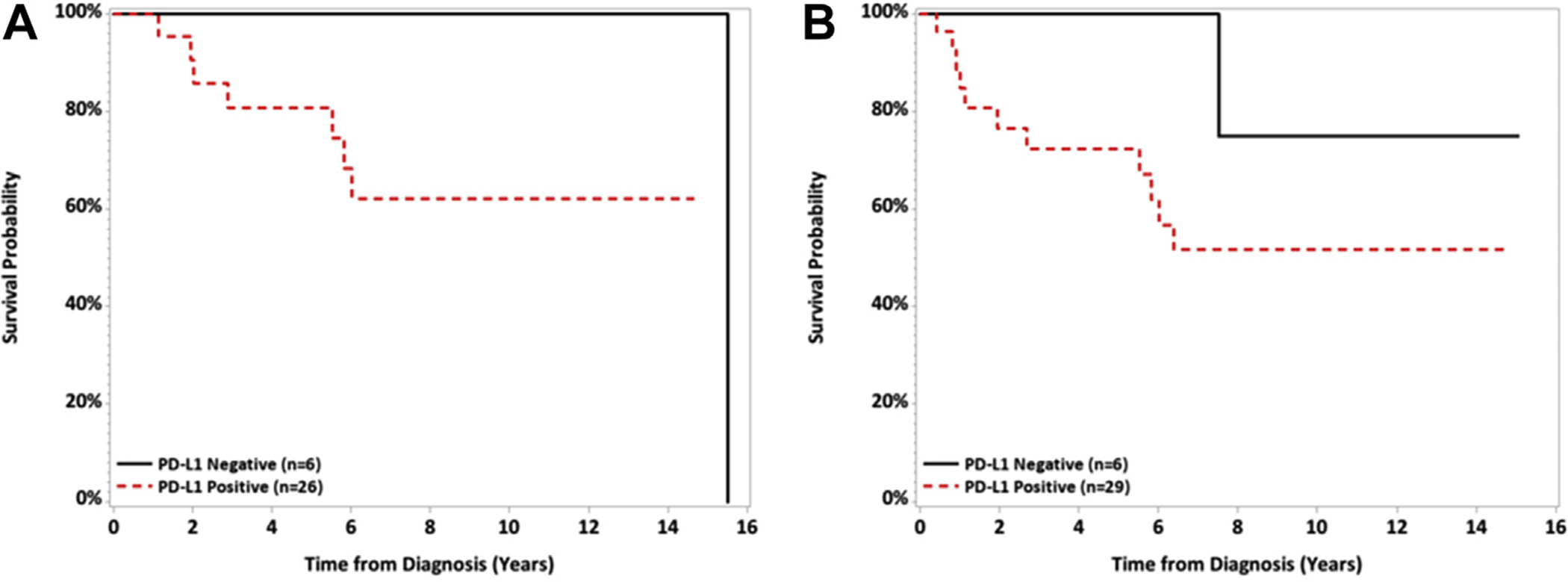
Kaplan Meier curves for overall survival by PD-L1 and PD-1 status. No significant differences in overall survival (A, p = 0.10, log-rank test) or event-free survival (B, p = 0.18, log-rank test) were observed in patients with PD-L1 expression PD-1 expression. PD-L1, programmed death ligand 1; PD-1, programmed death 1.
Intratumor Heterogeneity in PD-L1 and PD-1 Expression
Three patients had multiple slides prepared from the same tumor specimen analyzed for PD-L1 and PD-1 expression by IHC. All three patients had a diagnosis of thymoma. Table 3 and Figure 2 show the results of expression patterns, which were different for two of three patients, including a marked difference in both PD-L1 and PD-1 expression in two of three patients. In comparison, there were relatively minor changes in histology between specimens (patient OSU 07 grade A and B1; patient OSU 26 grade B1 and B2; and patient OSU 32 B2 and B3). This confirms the finding of variable PD-L1 expression in studies in other tumors, and shows for the first time intratumoral heterogeneity in PD-1/L1 expression in thymoma.27
Table 3.
Intratumoral Heterogeneity in PD-1 and PD-L1
| Patient | Diagnosis | IHC | Score #1 | Score #2 |
|---|---|---|---|---|
| OSU 07 | Thymoma AB | PD-L1 | 2 | 3 |
| PD-1 | 0 | 3 | ||
| OSU 26 | Thymoma B1 | PD-L1 | 0 | 0 |
| PD-1 | 2 | 2 | ||
| OSU 32 | Thymoma B3 | PD-L1 | 3 | 5 |
| PD-1 | 2 | 0 |
Three patients had multiple sections of the same tumor analyzed for PD-L1 and PD-1 expression, and two of three patients had markedly different results between samples.
PD-1, programmed death 1; PD-L1, programmed death ligand 1; IHC, immunohistochemistry;
Figure 2.
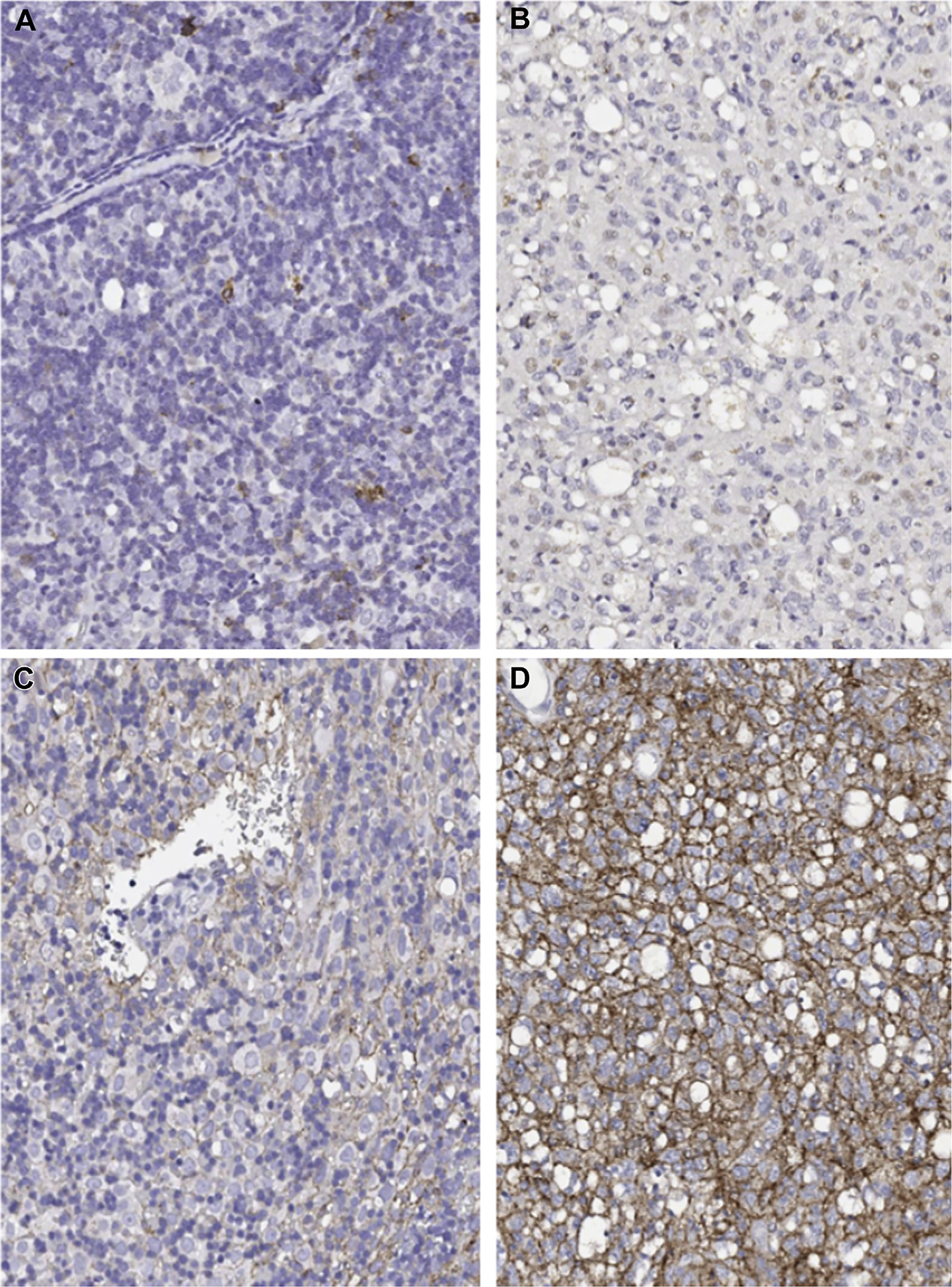
Intratumoral heterogeneity in PD-1 and PD-L1 by immunohistochemistry. Representative slides from multiple sections of the same tumor specimen showing differential expression of PD-1 expression (A, B) and PD-L1 expression (C, D). This patient had a PD-1 expression score of 2 in slide A and 0 in B, with a PD-L1 score of 3 in C and 5 in D within the same tumor. PD-1, programmed death 1; PD-L1, programmed death ligand 1.
Discussion
Immunotherapy with checkpoint blockade has profoundly changed the management of patients with a variety of malignancies, and is now standard of care first-line therapy for select patients with NSCLC whose tumors exhibit PD-L1 expression above a certain threshold (50% tumor proportion score).18–20,32–34 PD-1/L1 expression has been found in a number of different cancers; however, the utility of either PD-1 or PD-L1 as a prognostic biomarker remains unclear. In a meta-analysis including 29 studies covering a total of 7319 patients with 12 types of epithelial-originated malignancies, Zhang et al.35 reported that positive PD-L1 expression and positive PD-1 expression were both associated with shorter OS. However, several other studies have found opposing results for different tumors and even within tumor subtypes. Within early stage lung cancer, studies have shown a correlation with PD-L1 and either poor prognosis or good prognosis, whereas larger studies have shown no prognostic value of PD-L1.36–39 Similarly, there are studies showing prognostic value of PD-L1 and PD-1 in pancreatic cancer, colon cancer, and pulmonary neuroendocrine cancer, among others.40–44
The efficacy and safety of immunotherapy was evaluated in a recently published phase 2 trial of pembrolizumab in patients with thymic carcinoma, with a 22.5% response rate, including one complete response; however, autoimmune-related toxicities developed in several patients.23 Although immunotherapy appears to be a promising treatment for patients with thymoma and thymic carcinoma, the risk of immune-related adverse events and the lack of response in the majority of patients show the necessity of investigating robust biomarkers predictive of response. To date there have been nine prior publications evaluating the expression patterns of PD-L1 in thymoma and thymic carcinoma, and five publications evaluating PD-1 in this population (summarized in Table 4).24,25, 31,45, 46–50 The data so far are conflicting in terms of the prognostic value of either PD-L1 or PD-1 and any association between expression status and tumor grade or stage. Across all studies, high proportions of tumors from patients with thymoma and thymic carcinoma expressed PD-L1 and PD-1. However, significant variation between the studies exists regarding the association between PD-1/L1 expression and OS. For example, Padda et al.24 assessed PD-L1 expression on tissue microarray of 65 thymoma and 4 thymic carcinoma samples and found that higher PD-L1 expression was associated with higher WHO histologic grade (B2, B3, C) and with worse OS in an adjusted analysis (HR 5.40, 95% CI: 1.13 – 25.89, p = 0.035). In a retrospective study of 25 thymic carcinoma patients, Yokoyama et al.25 reported that high PD-L1 expression was associated with a better prognosis, including longer OS and progression-free survival. Katsuya et al.31 reported expression of PD-L1 in 101 thymomas and 38 thymic carcinomas and found higher PD-L1 expression in thymic carcinoma compared to thymoma (p = 0.006), but unlike the prior studies, PD-L1 was not associated with OS (HR 0.99, 95% CI: 0.35–2.73, p = 0.987). The same group later evaluated 30 patients with thymoma and thymic carcinoma who had received chemotherapy and found no association between PD-L1 expression and clinical benefit from chemotherapy.45 Marchevsky and Walts46 reported expression of PD-L1 in 92% of thymomas and 50% of thymic carcinomas and found higher PD-L1 expression in B2/3 thymoma compared to AB and B1 thymoma. The authors also reported that 90% of non-neoplastic thymic tissue was positive for PD-L1, in contrast to the findings from Padda et al.24 Most recently Weissferdt et al.48 reported expression of PD-L1 and PD-1 patterns of 74 thymoma and 26 thymic carcinoma samples and found that PD-1–positive thymic carcinomas were associated with a higher stage, but there was no association between PD-1 and PD-L1 expression with survival in either the thymoma or thymic carcinoma patients.
Table 4.
Studies of PD-L1 and PD-1 in Thymoma and Thymic Carcinoma, Including Expression Patterns and Prognostic Implications
| Source | Thymoma, n | Thymic Carcinoma, n | PD-1 Antibody | PD-L1 Antibody | PD-1 Positivity Thymoma, n (%) | PD-L1 Positivity Thymoma, n (%) | PD-1 Positivity Thymic Carcinoma, n (%) | PD-L1 Positivity Thymic Carcinoma, n (%) | PD-L1 Prognostic of Survival | PD-1 Prognostic of Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Brown et al., 200350 | 26 | 8 | NA | 29E.2A3 29E.5A9 |
NA | 21 (81)a | NA | 7 (88) | NA | NA |
| Katsuya et al., 201531 | 101 | 38 | NAT105 (Abcam, rabbit monoclonal antibody) | E1L3N (Cell Signaling Technology) | NA | 22 (23) | 23 (62) | 26 (70) | No; p = 0.408 |
No; p = 0.836 |
| Yokoyama et al., 201649 | 82 | 0 | NA | EPR1161 (Abcam, rabbit monoclonal antibody) | NA | 44 (53.7) | NA | NA | No; p = 0.957 |
NA |
| Yokoyama et al., 201625 | 0 | 25 | NAT105 (Abcam, rabbit monoclonal antibody) | EPR1161 (Abcam, rabbit monoclonal antibody) | NA | NA | NA | 20 (80) | Yes; high PD-L1 associated with longer OS (p = 0.010) | Yes; PD-1 associated with worse prognosis (HR = 1.496, p = 0.037)b |
| Katsuya et al., 201645 | 12 | 18 | NAT105 (Abcam, rabbit monoclonal antibody) | E1L3N (Cell Signaling Technology) | 4 (44)c | 6 (67) | 8 (47) | 7 (41) | Nod | No |
| Padda et al., 201524 | 65 | 4 | NA | Sino Biological clone 15 (rabbit monoclonal antibody) | NA | 44 (68)e | NA | 3 (75) | Yes; high PD-L1 expression associated with worse OSf | NA |
| Marchevsky and Walts, 201746 | 38 | 8 | MRQ22; Cell Marque | Clone SP142; Spring BioScience | 24 (63) | 35 (92) | 3 (37.5) | 4 (50) | NA | NA |
| Weissferdt et al., 201748 | 74 | 26 | EPR4877 (Abcam, rabbit monoclonal antibody) | E1L3N (Cell Signaling Technology) | 46 (62) | 47 (64) | 6 (23) | 14 (54) | No; thymoma p = 0.4180 or thymic carcinoma p = 0.4628 | No; p = 0.3942 or thymic carcinoma p = 0.2137 |
| Tiseo et al., 201747 | 87 | 20 | NA | E1L3N (Cell Signaling Technology) | NA | 15 (18) | NA | 13 (65) | No; p = 0.604 | No; p = 0.206 |
| Present study | 32 | 3 | NAT105 (Abcam, rabbit monoclonal antibody) | 22C3 (Dako North America) | 26 (81) | 26 (81) | 1 (33) | 3 (100) | No; p = 0.119g | NA; p = 0.469 |
Manufacturers noted are Abcam (Cambridge, United Kingdom), Cell Marque Corporation (Rocklin, California), Cell Signaling Technology (Danvers, Massachusetts), Spring Bioscience (Pleasanton, California), and Dako North America (Carpinteria, California).
Eleven of 11 invasive (100%) thymoma samples and 10 of 15 (67.77%) noninvasive thymoma samples.
Lower PD-1 expression among tumor infiltrating lymphocytes was associated with poorer OS in patients with thymic carcinoma only.
For analysis in this study, evaluable tumor samples included 9 thymomas and 17 thymic carcinomas.
PD-L1 expression was not prognostic in either thymic carcinoma (p = 0.449) or thymoma (p = 0.624). PD-1 expression was not prognostic in either thymic carcinoma (p = 0.650) or thymoma (p = 0.689).
All thymic neoplastic and control samples stained positive for PD-L1; samples were analyzed according to PD-L1 high expression versus PD-L1 low expression.
In unadjusted analysis, no difference in OS was noted, but in age and gender adjusted analysis PD-L1high versus PD-L1 low had worse OS (p = 0.035), but included both thymoma and thymic carcinoma together. When adjusted for stage, no significant association with OS was noted (p = 0.071).
Reported for thymoma cohort, since no difference able to report since three thymic carcinoma patients had PD-L1 expression.
PD-L1, programmed death ligand 1; PD-1, programmed death 1; NA, not available; OS, overall survival; HR, hazard ratio.
One possible explanation for the variable findings of PD-L1 and PD-1 expression between studies is the use of different antibodies for IHC detection. The recently reported data from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project showed significant variation in tumor PD-L1 detection in lung cancer specimens between available PD-L1 assays, especially the SP-142 assay.26 Additionally, four of previously mentioned studies used the E1L3N antibody which has been shown to have lower sensitivity than other available antibodies.51 To determine whether the variations of IHC expression may be due to the different assays used, we used the 22C3 antibody which is employed in an FDA approved assay to guide treatment in NSCLC based on the results of several trials.20,52 Using the 22C3 antibody, we confirmed high expression patterns for the entire population of PD-L1 (n = 29, 83%) and PD-1 (n = 27, 77%). Expression of PD-L1 was present in 81% of thymoma patients and all three thymic carcinoma patients (100%), whereas expression of PD-1 was lower in thymic carcinoma patients (31%) than thymoma patients (81%).
We were unable to replicate the results by Padda et al.24 of an association between high PD-L1 expression and worse clinical outcomes (p = 0.10, log-rank test; p = 0.12 after three thymic carcinoma patients were excluded). Unlike prior studies, neither PD-L1 expression nor high levels of PD-L1 expression was associated with tumor grade.24,31 Whereas Weissferdt et al.48 reported that PD-1 was associated with higher stage thymic carcinomas, we found higher PD-1 expression to be associated with lower WHO histologic grade (p = 0.03). The difference may be due to different assay used and the fact that our trial included mostly thymoma patients. Neither study showed an association between PD-1 and survival.
Heterogeneity between primary tumor site and metastatic site has been reported in melanoma and renal cell carcinoma, and intratumoral heterogeneity has been shown in NSCLC.27, 53–55 Our study adds to the literature regarding heterogeneity of expression patterns of PD-L1 and PD-1 in tumors. By evaluating different sections of the same tumor specimen of three patients, we showed variable expression of both PD-L1 and PD-1 in two of three patients with thymoma. The variation was significant for both PD-L1 and PD-1. This variability further shows the limitations of basing treatment strategies on an assay that is not constant within tumors or over time. For example, Katsuya et al.45 analyzed serial tumor samples from six patients pre-and post-chemotherapy and showed that PD-L1 and PD-1 expression increased after chemotherapy, with all six samples being positive for both PD-L1 and PD-1 after treatment with chemotherapy with doxorubicin and paclitaxel, compared to 67% (4 of 6) for PD-L1 pre-chemotherapy and 33% (2 of 6) of PD-1 samples pre-chemotherapy using the E1L3N antibody.
Our study had several strengths and some limitations. Strengths of this study include the use of resection specimens that assure ample tissue for IHC staining which is not always the case with biopsy specimens that might be limited by lack of tissue and which may impact PD-L1 assessment, as noted in prior studies.45 An additional strength of this study was the use of the 22C3 antibody, which is employed in an FDA-approved assay for use in NSCLC. Limitations of this study include the small sample size and the inclusion of mostly early-stage patients with thymoma. Although the predictive value of PD-L1 expression has been determined by tumor proportion score in NSCLC, this scoring system has not yet been used in thymoma or thymic carcinoma, and the use of expression score by IHC in the current study is consistent with prior studies in thymoma as well as research in melanoma where the melanoma (MEL) scale has been utilized.20,56 Further research is needed to determine whether tumor proportion score has a role in tumors other than NSCLC. Overall, in a rare disease with limited reports in the literature, these data expand upon current knowledge while also adding new information regarding intratumor heterogeneity of PD-L1 and PD-1 expression.
In conclusion, the current study confirmed the finding of high levels of PD-L1 expression in thymoma and thymic carcinoma using an approved assay. Our study also showed no statistically significant differences in survival in patients with high PD-L1 or PD-1 expression. We found that high PD-1 expression was associated with lower histologic grade but found no relationship between either PD-L1 or PD-1 and stage or diagnosis of MG. Finally, in a limited subset of patients there was significant intratumoral heterogeneity in both PD-1 and PD-L1 expression.
Acknowledgments
This work was supported by the National Institutes of Health (grant number P30CA016058), The Ohio State University, Columbus, Ohio, and Merck Research Laboratories, Palo Alto, California.
The authors thank our patients and their families.
Disclosures: Dr. Owen has received grants from Bristol-Myers Squibb; and nonfinancial support from Genentech. Drs. Annamalai and Yearley are employees of Merck Research Laboratories, Palo Alto, California. Dr. Yearley has patents pending related to the subject matter. Dr. Otterson has received grants from Genentech, Pfizer, Bristol-Myers Squibb, Igmyta, and Merck; and consultant fees from Novartis, Pfizer, Amgen, and Genentech. The remaining authors declare no conflict of interest.
References
- 1.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol. 2010;5:S260–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venuta F, Anile M, Diso D, et al. Thymoma and thymic carcinoma. Eur J Cardiothorac Surg. 2010;37:13–25. [DOI] [PubMed] [Google Scholar]
- 3.Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg. 2004;77:1183–1188. [DOI] [PubMed] [Google Scholar]
- 4.Marx A, Strobel P, Zettl A, et al. Thymomas In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Lung, Thymus and Heart. Lyon, France: IARC; 2004;7:152–153. [Google Scholar]
- 5.Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of tumors of the thymus: continuity and changes. J Thorac Oncol. 2015;10:1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg. 2009;138:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litvak AM, Woo K, Hayes S, et al. Clinical characteristics and outcomes for patients with thymic carcinoma: evaluation of Masaoka staging. J Thorac Oncol. 2014;9:1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masaoka A Staging system of thymoma. J Thorac Oncol. 2010;5:S304–S312. [DOI] [PubMed] [Google Scholar]
- 9.Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin. 2011;21:59–67, vi-vii. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Shi J, Fan L, et al. Surgical treatment of thymoma: an 11-year experience with 761 patients. Eur J Cardiothorac Surg. 2016;49:1144–1149. [DOI] [PubMed] [Google Scholar]
- 11.Kim ES, Putnam JB, Komaki R, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. 2004;44: 369–379. [DOI] [PubMed] [Google Scholar]
- 12.Lemma GL, Lee JW, Aisner SC, et al. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol. 2011;29:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furugen M, Sekine I, Tsuta K, et al. Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol. 2011;41: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 14.Giaccone G, Ardizzoni A, Kirkpatrick A, et al. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma. A phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1996;14:814–820. [DOI] [PubMed] [Google Scholar]
- 15.Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol. 2015;26:363–368. [DOI] [PubMed] [Google Scholar]
- 16.Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer. 2010;67: 194–197. [DOI] [PubMed] [Google Scholar]
- 17.Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol. 2015;16:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferris RL, Blumenschein GJ, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 21.Zander T, Aebi S, Rast AC, et al. Response to pembrolizumab in a patient with relapsing thymoma. J Thorac Oncol. 2016;11:e147–e149. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y, Ding L, Wang P. Dramatic response to anti-PD-1 therapy in a patient of squamous cell carcinoma of thymus with multiple lung metastases. J Thorac Dis. 2016;8:E535–E537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol. 2018;19:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumors. J Thorac Oncol. 2015;10:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoyama S, Miyoshi H, Nakashima K, et al. Prognostic value of programmed death ligand 1 and programmed death 1 expression in thymic carcinoma. Clin Cancer Res. 2016;22:4727–4734. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen M, Andreozzi M, Pockaj B, et al. Development and validation of a novel clinical fluorescence in situ hybridization assay to detect JAK2 and PD-L1 amplification: a fluorescence in situ hybridization assay for JAK2 and PD-L1 amplification. Mod Pathol. 2017;30: 1516–1526. [DOI] [PubMed] [Google Scholar]
- 29.Pinto N, Park JR, Murphy E, et al. Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer. 2017;64 10.1002/pbc.26613. [DOI] [PubMed] [Google Scholar]
- 30.Yearley JH, Gibson C, Yu N, et al. PD-L2 Expression in human tumors: relevance to anti-PD-1 therapy in cancer. Clini Cancer Res. 2017;23:3158–3167. [DOI] [PubMed] [Google Scholar]
- 31.Katsuya Y, Fujita Y, Horinouchi H, et al. Immunohistochemical status of PD-L1 in thymoma and thymic carcinoma. Lung Cancer. 2015;88:154–159. [DOI] [PubMed] [Google Scholar]
- 32.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Kang S, Shen J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) expression in epithelial-originated cancer: a meta-analysis. Medicine. 2015;94:e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014;7: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. [DOI] [PubMed] [Google Scholar]
- 38.Sun JM, Zhou W, Choi YL, et al. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–1011. [DOI] [PubMed] [Google Scholar]
- 39.Tsao MS, Le Teuff G, Shepherd FA, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol. 2017;28:882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Lin J, Cui J, et al. Prognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancer. Oncotarget. 2017;8:9354–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee LH, Cavalcanti MS, Segal NH, et al. Patterns and prognostic relevance of PD-1 and PD-L1 expression in colorectal carcinoma. Mod Pathol. 2016;29:1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Y, Ma K, Wang C, et al. Prognostic value of PD-L1 and PD-1 expression in pulmonary neuroendocrine tumors. Onco Targets Ther. 2016;9:6075–6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cho J, Lee J, Bang H, et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. 2017;8:13320–13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han J, Hong Y, Lee YS. PD-L1 Expression and combined status of PD-L1/PD-1-positive tumor infiltrating mononuclear cell density predict prognosis in glioblastoma patients. J Pathol Transl Med. 2017;51:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsuya Y, Horinouchi H, Asao T, et al. Expression of programmed death 1 (PD-1) and its ligand (PD-L1) in thymic epithelial tumors: impact on treatment efficacy and alteration in expression after chemotherapy. Lung Cancer. 2016;99:4–10. [DOI] [PubMed] [Google Scholar]
- 46.Marchevsky AM, Walts AE. PD-L1, PD-1, CD4 and CD8 expression in neoplastic and non-neoplastic thymus. Human Pathol. 2017;60:16–23. [DOI] [PubMed] [Google Scholar]
- 47.Tiseo M, Damato A, Longo L, et al. Analysis of a panel of druggable gene mutations and of ALK and PD-L1 expression in a series of thymic epithelial tumors (TETs). Lung Cancer. 2017;104:24–30. [DOI] [PubMed] [Google Scholar]
- 48.Weissferdt A, Fujimoto J, Kalhor N, et al. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod Pathol. 2017;30:826–833. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama S, Miyoshi H, Nishi T, et al. Clinicopathologic and prognostic implications of programmed death ligand 1 expression in thymoma. Ann Thorac Surg. 2016;101: 1361–1369. [DOI] [PubMed] [Google Scholar]
- 50.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. [DOI] [PubMed] [Google Scholar]
- 51.Smith J, Robida MD, Acosta K, et al. Quantitative and qualitative characterization of Two PD-L1 clones: SP263 and E1L3N. Diagn Pathol. 2016;11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 53.Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2015;28: 245–253. [DOI] [PubMed] [Google Scholar]
- 54.Callea M, Albiges L, Gupta M, et al. Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res. 2015;3:1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rehman JA, Han G, Carvajal-Hausdorf DE, et al. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol. 2017;30:340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the antiprogrammed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]


