Abstract
Purpose of review
To review recent developments in the field of gastroduodenal mucosal defense.
Recent findings
Research in the field of gastroduodenal mucosal defense has focused on continued elucidation of molecular mechanisms that protect the mucosa and influence healing at the cellular level. Review of literature over the past year reveals focus on familiar processes such as superoxide dismutase, nitric oxide, heme oxygenase-1, neutrophil infiltration, cysteamine, mucin, hydrogen sulfide, ghrelin, adiponectin and the influence of Helicobacter pylori, but also brings into light new processes such as the balance between apoptosis and cellular proliferation, as well as the influence of other organ systems such as the bone marrow and central nervous system on the gastrointestinal tract.
Summary
These new published findings contribute to our overall understanding of gastroduodenal defense and suggest innovative avenues of future research and possible novel therapeutic targets.
Keywords: apoptosis, gastrointestinal defense, Helicobacter pylori, mucins, oxidative stress
INTRODUCTION
The gastroduodenal mucosa has multiple defense mechanisms to protect itself from injury due to oxidative stress, infection-induced injury due to Helicobacter pylori, ischemic and reperfusion injuries, and drug-induced injury primarily due to NSAIDs. During the past year, we have reviewed recent developments in gastroduodenal defense that focused on the mechanisms behind many of the now well known factors in mucosal defense, such as antioxidants, factors contributing to cellular apoptosis, and those that contribute to gastric mucosal healing, the molecular mechanisms underlying mucin secretion and continued research into the pathogenesis of H. pylori. Although some well known molecules and mediators are discussed such as superoxide dismutase (SOD), glutathione (GSH), nitric oxide, heme oxygenase-1 (HO-1), and mucin, there are several new peptides, proteins, and mediators to discuss, in addition to highlighting further research into the influence of bone marrow and the central nervous system on the gastrointestinal tract.
OXIDATIVE STRESS
Oxidative stress is defined as an imbalance in the body’s ability to cope with reactive oxygen species (ROS) secondary to increased production or decreased detoxification. ROS are injurious and pro-inflammatory due to DNA damage, lipid peroxidation, oxidation of amino acids, and oxidation of enzymatic co-factors. How oxidative stress affects the pathogenesis of many diseases is well established. The complex interplay among many of the factors which promote and prevent oxidative stress has been the focus of much research throughout the previous year, which we will discuss below.
Mitochondrial superoxide dismutase and glutathione
SOD is a well known enzyme that converts superoxide, a ROS, into hydrogen peroxide and oxygen. GSH is a tripeptide, membrane-bound, nonenzymatic antioxidant which can be produced by all cells within the human body. Adaptive cytoprotection is a term used to describe protective adaptations that defend against mild irritants, such as ethanol and HCl. Although the mechanism of this process is poorly understood, Kawano et al. [1] provided some insight when they reported decreased adaptive cytoprotection to ethanol-induced injury in portal hypertensive rats. These rats produced significantly lower levels of SOD and GSH, implicating antioxidants as primary factors in gastric mucosal defense to mild irritants.
Alpha-lipoic acid (ALA) is an important vitamin present in all prokaryotic and eukaryotic cells that has antioxidant properties via its ability to directly or indirectly bind free radicals and its ability to interact with other antioxidants. Kaplan et al. [2] reported that ALA exerts gastroprotective effects against indomethacin-induced gastric ulcers through multiple antioxidant pathways such as decreasing the activities of myeloperoxidase and catalase, in addition to increasing activities of SOD and GSH S-transferase in a dose-dependent manner. This study is the first to report that the well known gastroprotective effects of ALA occur via increased antioxidant and decreased lipid peroxidation pathways.
Nitric oxide
Nitric oxide is an important gastroduodenal defense mechanism due to its vasodilatory properties and through reduction of acid secretion. An important alternative source of endogenous nitric oxide discovered in the past year is salivary nitrate and nitrite, which are actively secreted by parotid and submandibular salivary glands in stress, and are metabolized to nitric oxide in the acidic environment of the stomach [3■].
Kwiecien et al. [4■] reported that asymmetric dimethylarginine (ADMA), an endogenous competitive inhibitor of nitric oxide synthase, contributes to the pathogenesis of stress ulcers. ADMA exerts its effects not only through nitric oxide inhibition, but also by decreasing expression of SOD and GSH peroxidase mRNA, thus serving as a potentially important therapeutic target.
Ghrelin, a peptide hormone ligand for the growth hormone secretagogue receptor, exerts gastroprotective effects via release of endogenous nitric oxide. Plasma ghrelin concentrations were elevated in patients infected with H. pylori. Warzecha et al. [5■] reported that ghrelin enhances defense mechanisms such as duodenal blood flow, increasing mucosal activity of SOD, and decreasing lipid peroxidation rather than simply increasing endogenous release of nitric oxide.
Heme oxygenase-1
HO-1 metabolizes pro-oxidant iron-containing heme to carbon monoxide and biliverdin, which is then converted into bilirubin. In the past year’s review, we discussed the role of HO-1 in ulcer prevention. Within the past year, Takagi et al. [6■] have reported that hemopexin (HPX), an acute-phase reactant with the highest known affinity for heme, was up-regulated in small intestinal injury, although its protective mechanism of action was previously unknown. Data suggest that the heme-HPX complex binds to the lipoprotein receptor-related protein, up-regulating HO-1 gene expression.
Despite the reported cytoprotective effects of HO-1, Ibrahim et al. [7] reported the protective effects of zinc protoporphyrin (ZnPP), a HO-1 inhibitor. Although seemingly contradictory, zinc itself, rather than HO-1 inhibition, may be the gastroprotective entity [7], consistent with the well established cytoprotective activity of zinc in many tissues. Zinc may thus be included in mucosally protective drugs.
Hydrogen sulfide
Hydrogen sulfide (H2S) is the third gas, along with nitric oxide and carbon monoxide, to be implicated as a transmitter substance in mammalian tissues. H2S, with its numerous anti-inflammatory and antioxidant activities, is gastroprotective. Liu et al. [8■■] reported that ACS14, an H2S-releasing aspirin derivative, decreased the aspirin-induced up-regulation of cyclooxogenase (COX)-2 expression, but did not have any effect on gastric prostaglandin E2 (PGE2) concentrations. ACS14 also attenuated aspirin suppression of SOD and GSH [8■■]. Their study demonstrated the potential pharmacologic benefits of H2S-releasing drugs in addition to helping elucidate some of the mechanisms by which H2S functions.
Hydrogen
The hydroxyl radical (•OH) is among the causes of stress ulceration. Hydrogen, which functions as a potent selective (•OH) scavenger, can protect animals against ROS-induced damage. Liu et al. [9■] reported that intravenous administration of hydrogen-rich saline decreased stress-induced lipid peroxidation (Fig. 1). Hydrogen also mitigated the inflammatory response to induced stress, including neutrophil infiltration and apoptosis [9■].
FIGURE 1.
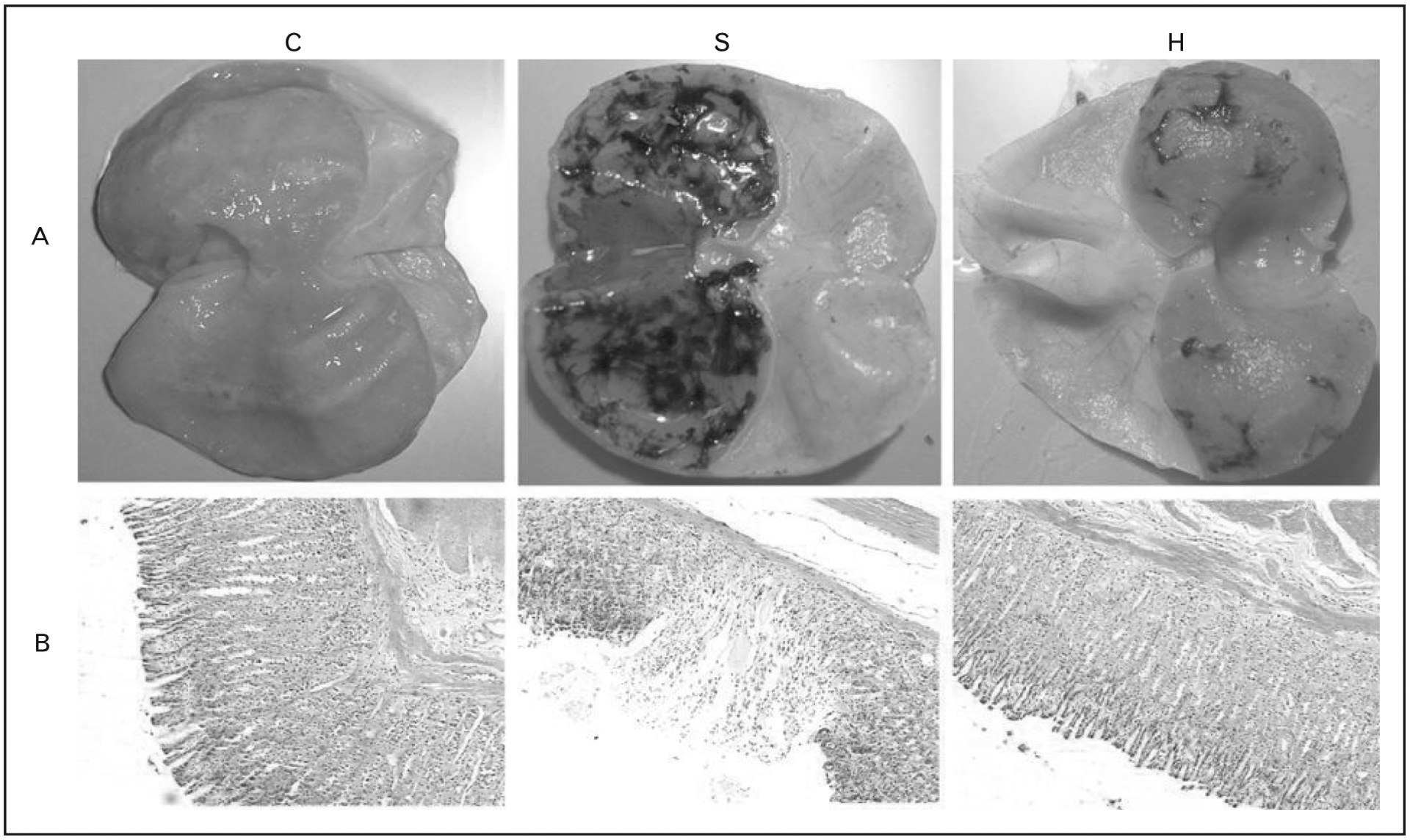
(A) Gross appearance of gastric mucosa from control mice (C), stress-ulcer-induced mice (S), and mice treated with intravenous hydrogen prior to stress (H). (B) Light micrographic images of the specimens which show the mitigating effects of hydrogen administration. Adapted from [9■].
Cysteamine and iron
Cysteamine has been used for experimental induction of duodenal ulcers for many years, although its mechanism of action remains unclear. Khomenko et al. [10] reported that cysteamine-induced ulcer formation preferentially acts in the duodenum and is aggravated by iron overload, but is decreased by iron deficiency. Cysteamine uptake is also modified by organic cation transport (OCT) inhibitors as OCT-1 knockout mice had reduced cysteamine absorption [10]. This study demonstrates that iron and OCTs help mediate cysteamine-induced ulcerogenesis and may also determine the localization of ulcers during in-vivo ulcerogenesis perhaps due to the duodenal localization of the iron uptake mechanism, the divalent metal transporter.
Sensory afferent nerves
Whereas the detrimental effects of COX inhibition on the gastric mucosa are well established, Kwiecien et al. [11] reported that functional ablation of sensory afferent nerves by the selective neurotoxin capsaicin also renders the gastric mucosa more susceptible to injury. This injury was further augmented by rofecoxib, a selective COX-2 inhibitor, which decreased SOD activity and GSH expression [11]. Although functional ablation of sensory afferent nerves by capsaicin impairs the augmentation of foregut protective mechanisms such as mucosal blood flow, and mucus and bicarbonate secretion in response to luminal acid, this is the first description of how sensory afferent nerves affect antioxidant-induced gastric ulceration, in particular, relative to COX-2 activity.
THE INFLAMMATORY RESPONSE
Several of the essential components of maintaining gastric mucosal integrity occur via the inflammatory cascade, most importantly, by production of PGEs. PGEs are synthesized by COX, an enzyme which has constitutive (COX-1) and inducible (COX-2) isoforms.
Adiponectin
Adiponectin is an anti-inflammatory molecule released from adipocytes. Serum levels of adiponectin are lower in obesity, correlating with increased gastric erosions in mouse models. PGE2 expression was reduced in adiponectin knockout mice. Furthermore, exogenous adiponectin administration increased the expression of PGE2 and COX-2 in ethanol-treated rat gastric mucosal cells. Simultaneous administration of celecoxib, a selective COX-2 inhibitor, and adiponectin inhibited efficient wound repair. Taken together, these findings suggest that adiponectin enhances gastric mucosal repair from injury via increased PGE2 synthesis [12].
Melanoma differentiation associated gene 5
Melanoma differentiation associated gene 5 (MDA5) is a doube stranded RNA (dsRNA) receptor which activates the innate immune system in the defense against viral pathogens. Although H. pylori activates the innate immune system, it was unknown if dsRNA receptors were involved. Tatsuta et al. [13■■] reported that MDA5-induced activation of the innate immune system is not only antiviral, but antibacterial as well.
Proteinase-activated receptors
Proteinase-activated receptors (PARs) have many important functions including activation of inflammatory cascades and gastric mucosal protection. PARs are activated by endogenous enzymes and by bacterial components. Sekiguchi et al. [14] reported that, unlike many bacteria, extracts of H. pylori failed to activate PARs in rat gastric mucosal cells. Their work suggested the potential pathogenicity of H. pylori may lie within lipopolysaccharide receptors rather than PAR receptors.
Neutrophils, tumor necrosis factor-α, histamine and the effects of aging
Although the prosecretory effect of histamine via activation of H2 histamine receptors is well known, Adami et al. [15] reported data implicating the H4 histamine receptor (H4R) in ulcerogenesis. Blockade of the H4R, via the antagonist JNJ7777120, ameliorated the ulcerogenic activity of indomethacin co-administered with the muscarinic agonist bethanechol. The likely mechanism is through blockade of H4R-induced neutrophil chemotaxis and activation, which fails to harm the gastric mucosa in the absence of additional harmful stimuli. Interestingly, H4R agonists alone were insufficient to induce ulcers [15].
Further insight into the function of neutrophils in ulcerogenesis comes from Seo et al. [16] in a study on the effects of NSAIDs in the aging gastric mucosa. Rats were more sensitive to the deleterious effects of the NSAIDs indomethacin and diclofenac in an age and dose-dependent manner via neutrophilmediated pathways, which were measured with the neutrophil-derived enzyme myeloperoxidase, and production of leukotriene B4, a chemotactic factor for leukocytes [16].
Yadav et al. [17] provided more insight into the mechanism of indomethacin-induced ulceration. They reported that many inflammatory molecules were up-regulated after indomethacin administration, with tumor necrosis factor (TNF)-α up-regulation preceding gastric ulceration. Abrogation of TNF-α signaling may offer a potential solution to indomethacin-induced gastropathy [17].
Hong et al. [18] reported that concentrations of total oxidants and apoptotic executors were significantly increased in older stomachs, which correlated with decreased levels of lipoxin A4 and antiapoptotic proteins such as B-cell lymphoma (Bcl)-2. This rendered older mouse stomachs more susceptible to the effects of indomethacin-induced gastric injury [18].
Collagenase
Takeuchi et al. [19] identified potential serum biomarkers for gastric ulceration induced by NSAIDs, reporting that NSAID-induced activation of collagenases and decreased SOD activity correlated with an increased expression of four biomarkers in stomach and plasma. These biomarkers, cis-aconitate, o-acetyl carnitine, 3-hydroxybutanoic acid and proline, not only could serve as potential biomarkers for NSAID-induced gastric mucosal injury, but may be involved in mucosal defense.
APOPTOSIS
Several factors examined throughout the past year have been implicated in cellular death via apoptotic pathways rather than inflammatory-cascade-mediated necrotic pathways.
NSAID-activated gene-1
NSAID modulation of the expression of NSAID-activated gene-1 (NAG-1), a protein in the same family as transforming growth factor (TGF)-β, is associated with apoptotic cell death. Colucci et al. [20■] demonstrated that COX-1 expression is decreased and COX-2 expression is increased in ulcerated tissues. COX inhibitors affected gastric ulcer healing independent of their COX isoform inhibition [20■]. These results support ongoing evidence that NAG-1 induction, followed by activation of a pro-apoptotic pathway, rather than COX inhibition, inhibits ulcer healing, providing potential pharmacologic targets.
Ceramide metabolites
Sphingolipids, such as ceramide, are highly bioactive compounds involved in a diverse number of cellular processes including apoptosis. Ceramide accumulation on cell surfaces is a well known phenomenon in apoptotic cells that has recently been demonstrated in the gastric mucosa. Nakashita et al. [21] demonstrated that it is not ceramide itself, but rather its metabolites, glucosylceramide, monosialoganglioside 3 and disialoganglioside 3, that induce gastric mucosal apoptosis.
High mobility group box 1
NSAIDs injure the mucosa in part via activation of a Toll-like receptor 4 (TLR4)-mediated pathway. Whereas TLR4 functions as part of the innate immune system, activating in response to gramnegative lipopolysaccharides, it is also capable of responding to endogenous ligands. Nadatani et al. [22] showed that small intestinal mucosal damage in response to NSAIDs worsens via an increase in high mobility group box 1 (HMGB1) expression. Up-regulation of HMGB1 expression did not correlate with an increase in small intestinal mucosal damage in TLR4-knockout mice [22]. Blocking TLR4-dependent pathways may serve as a potential therapeutic option against NSAID-induced ulceration.
CELLULAR PROLIFERATION
Several studies have addressed the mechanisms by which the damaged gastrointestinal mucosa repairs itself. These newly discovered factors implicated thus are described below.
Musashi-1 and m-Numb
Musashi-1 (Msi-1) is an RNA-binding protein up-regulated in injured gastric mucosa. Takahashi et al. [23] were the first to demonstrate that injured gastric tissue expresses Msi1, which acted to increase m-Numb transcription, which then increased expression of prostate stem cell antigen and metallothionein-2. The ultimate effect was enhanced cellular proliferation and resistance to H2O2-induced cell death [23].
Cytoglobin
Cytoglobin (Cygb) is a recently discovered member of the globin family up-regulated during hypoxia. It scavenges nitric oxide and ROS, transports oxygen and senses the adequacy of blood oxygenation. Expression of hypoxia-inducible factor (HIF)-1α, a Cygb promoter, increases 7–11 days after ulcer formation, followed by increased Cygb expression which peaks 11–18 days after ulcer formation. These two proteins peaked in fibroblasts at the edges of ulcers at day 11 (Fig. 2). Nevertheless, vascular endothelial growth factor (VEGF) mRNA levels were first detectable at day 11 and gradually increased. These results demonstrate that Cygb and HIF-1α are important factors in gastric mucosal healing prior to the initiation of angiogenesis [24■■].
FIGURE 2.
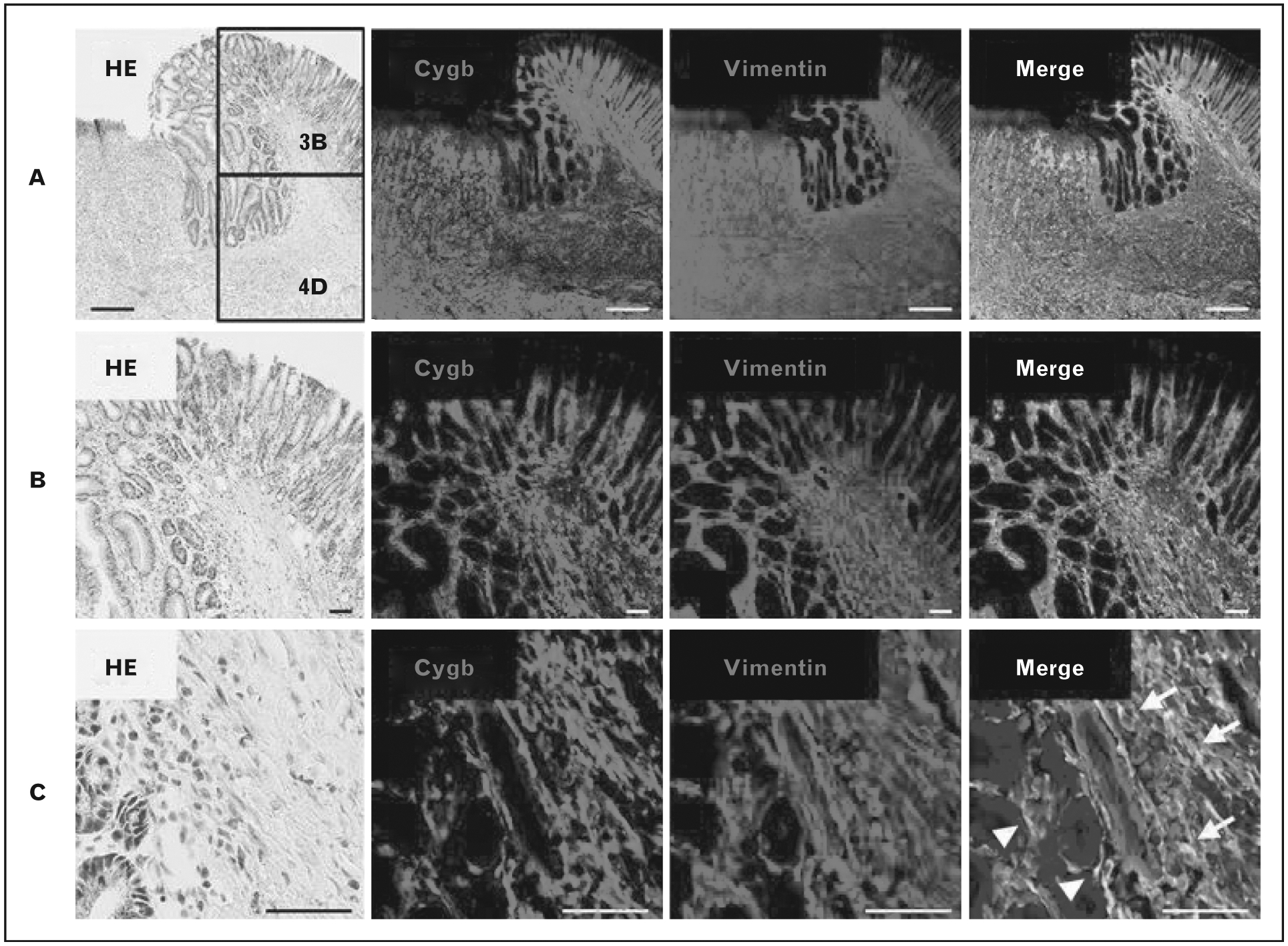
(A) Colocalization of cytoglobin and vimentin in the cytoplasm of mesenchymal cells in the regenerative area and ulcer bed. (B) Colocalization of cytoglobin and vimentin is more pronounced in the regenerative areas demonstrated by this medium magnification view. (C) High magnification image showing that colocalization was observed in fibroblasts (arrows) and myofibroblasts (arrowheads). Adapted from [24■■].
Epidermal growth factor receptor-extracellular signal-regulated kinase pathway
Clopidogrel and ticlopidine, ADP-receptor antagonists, inhibit the epidermal growth factor (EGF)-receptor-extracellular signal-regulated kinase (ERK) pathway in vitro and in vivo, delaying gastric epithelial healing [25]. This research provides a compelling rationale for many further studies including elucidation of the exact mechanism of interaction between ADP-receptor antagonists and the EGF-receptor, and their effects on angiogenesis.
MUCINS
Mucin secretion, strongly implicated in gastroduodenal mucosal defense, serves multiple functions, including protection of the epithelial surface from digestive enzymes, acids, and abrasion from food particles and pathogens. Involvement of mucin secretion in the pathogenesis of small intestinal ulceration was only recently reported. Chang et al. [26■] demonstrated that small intestinal ischemia in rats accelerated the metabolism of the two main forms of intestinal mucin: mucin 2 (secreted) and mucin 13 (membrane bound). This occurred independently of the presence of pancreatic enzymes and was correlated with loss of membrane receptors such as E-cadherin, increasing epithelial permeability to pancreatic enzymes and TLR4 expression [26■].
Niv et al. [27] compared the expression of membrane-bound mucins in H. pylori-associated, NSAID, and idiopathic gastric ulcers, reporting that MUC17 was significantly more expressed in H. pylori associated ulcers than idiopathic ulcers, whereas the opposite was true with MUC1 expression, which was also suppressed in NSAID-induced ulcers. In a second study, Muc1 expression was reportedly reduced by H. pylori infection [28]. These findings demonstrate that the different mucin isoforms have different protection efficiency against different insults to gastroduodenal mucosal integrity.
Unsurprisingly, pharmacologic agents have profound effects on mucin integrity. H2 receptor antagonists have long been used in the treatment of ulcers. Despite similarities in their antisecretory activity, famotidine reduced biosynthesis and accumulation of gastric mucin, whereas these properties were not reduced by roxatidine [29]. Nebivolol, a third-generation highly selective β−1 adrenergic receptor antagonist, improved gastric mucin integrity, presumably via an increase in nitric oxide production via nitric oxide-mediated activation of guanalyl cyclase [30].
HORMONES AND STEM CELLS
Many studies have been performed which examined the intrinsic defense mechanisms of the gastric and duodenal mucosa; however, this year saw several studies that focused on the influence of outside factors on gastroduodenal mucosal defense.
Substance P
Substance P is abundant in intrinsic enteric neurons, where it functions as a neurotransmitter and neuromodulator. Injection of substance P into the dorsal motor nucleus of the vagus nerve inhibits gastric motor activity and gastric acid secretion. Brancati et al. [31] reported that intracerebroventricular injection of substance P reduced the formation of ethanol-induced gastric ulcers via activity of endomorphin-2 centrally and peripheral vagalmediated effects on PGEs, nitric oxide and calcitonin-gene related peptide.
Bone marrow mesenchymal stem cells
The known adverse effects of long-term proton pump inhibition, such as increased susceptibility to H. pylori infection and increased risk of community-acquired pneumonia, led Chang et al. [32■] to study the role of bone marrow mesenchymal stem cells (BMMSCs) in gastric ulcer healing. Labeled BMMSCs transplanted into rats were present in the injured gastric mucosa 48–72 h after transplantation, correlating with significantly higher concentrations of VEGF and EGF-receptor expression [32■]. Although this is a promising and exciting study, one of the greatest limitations was the lack of long-term follow-up as the rats were only studied for 72 h.
Gastrin and the cholecystokinin 2 receptor
Gastrin, a highly selective cholecystokinin 2 (CCK2) receptor ligand, increases the rate of gastric acid secretion while augmenting gastric mucosal growth. Barrett et al. [33■■] showed that a novel CCK2R antagonist inhibited acid secretion similar to a proton pump inhibitor (PPI), however without PPI-induced acid rebound, presumably due to the lack of stimulation of enterochromaffin-like cells. Importantly, PPI-like hypoplastic thinning of the gastric mucosa was not observed with CCK2R antagonist therapy, demonstrating its potential as an acid-suppressing therapy in humans [33■■].
CONCLUSION
Well studied proteins and mediators such as SOD, nitric oxide, H2S, HO-1, and mucin have been further implicated in the mechanism of gastroduodenal defense. Many newly discovered proteins and mediators such as substance P, Msi-1, m-numb, Cygb, MDA5, and HMGB1 have been implicated as well. There have been many exciting and enlightening findings throughout the past year that have contributed significantly towards our understanding of gastroduodenal mucosal defense. Our further understanding will help in the development of novel pharmacologic approaches towards ulcer management and helping to maintain the integrity of the gastrointestinal mucosa.
KEY POINTS.
Oxidative stress continues to play a prominent role in gastroduodenal ulcer formation, which is influenced by numerous mediators.
Throughout the past year, further steps have been taken towards understanding the mechanisms behind the initiation of the inflammatory cascade which results in gastroduodenal mucosal damage.
Several studies throughout the past year have addressed the mechanisms by which the damaged gastrointestinal mucosa repairs itself.
Mucin, with its many isoforms, plays a critical role in maintaining the integrity of the gastroduodenal mucosa.
Acknowledgements
The authors would like to thank Ms Bea Palileo for her assistance with manuscript preparation.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Kawano Y, Ohta M, Eguchi H, et al. Increased oxidative stress may lead to impaired adaptive cytoprotection in the gastric mucosa of portal hypertensive rat. J Gastroenterol Hepatol 2013; 28:639–644. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan KA, Odabasoglu F, Halici Z, et al. Alpha-lipoic acid protects against indomethacin-induced gastric oxidative toxicity by modulating antioxidant system. J Food Sci 2012; 77:H224–H230. [DOI] [PubMed] [Google Scholar]
- 3.■.Jin L, Qin L, Xia D, et al. Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic Biol Med 2013; 57:61–67. The first study to demonstrate that stress-induced production of salivary nitrite is an important innate factor in ulcer defense.
- 4.■.Kwiecien S, Ptak-Belowska A, Krzysiek-Maczka G, et al. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, interacts with gastric oxidative metabolism and enhances stress-induced gastric lesions. J Physiol Pharmacol 2012; 63:515–524. This study introduces a potentially new therapeutic target, asymmetric dimethylarginine, which serves as an endogenous competitive inhibitor to nitric oxide synthase.
- 5.■.Warzecha Z, Ceranowicz D, Dembinski A, et al. Ghrelin accelerates the healing of cysteamine-induced duodenal ulcers in rats. Med Sci Monit 2012; 18:BR181–BR187. This study demonstrates that ghrelin has multiple roles in gastroduodenal defense beyond its known ability to increase nitric oxide synthase activity.
- 6.■.Takagi T, Naito Y, Okada H, et al. Hemopexin is upregulated in rat intestinal mucosa injured by indomethacin. J Gastroenterol Hepatol 2012; 27 (Suppl 3):70–75. This is the first study to discover that hemopexin is up-regulated in rat intestinal mucosa after NSAID injury.
- 7.Ibrahim I, El-Sayed S, Bdel-Hakim S, et al. Inhibition of endogenous CO by ZnPP protects against stress-induced gastric lesion in adult male albino rats. J Physiol Biochem 2012; 68:319–328. [DOI] [PubMed] [Google Scholar]
- 8.■■.Liu L, Cui J, Song CJ, et al. H(2)S-releasing aspirin protects against aspirin-induced gastric injury via reducing oxidative stress. PLoS One 2012; 7: e46301.This study demonstrates that H2S-releasing medications can help to ameliorate the ulcerogenic effects of NSAIDs by mitigating NSAID-induced up-regulation of COX-2 expression without an effect on PGEE2 levels.
- 9.■.Liu X, Chen Z, Mao N, Xie Y. The protective of hydrogen on stress-induced gastric ulceration. Int Immunopharmacol 2012; 13:197–203. This study showed that administration of hydrogen-rich intravenous saline has the potential to ameliorate stress-induced ulcers.
- 10.Khomenko T, Kolodney J, Pinto JT, et al. New mechanistic explanation for the localization of ulcers in the rat duodenum: role of iron and selective uptake of cysteamine. Arch Biochem Biophys 2012; 525:60–70. [DOI] [PubMed] [Google Scholar]
- 11.Kwiecien S, Konturek PC, Sliwowski Z, et al. Interaction between selective cyclooxygenase inhibitors and capsaicin-sensitive afferent sensory nerves in pathogenesis of stress-induced gastric lesions. Role of oxidative stress. J Physiol Pharmacol 2012; 63:143–151. [PubMed] [Google Scholar]
- 12.Yamamoto S, Watabe K, Araki H, et al. Protective role of adiponectin against ethanol-induced gastric injury in mice. Am J Physiol Gastrointest Liver Physiol 2012; 302:G773–G780. [DOI] [PubMed] [Google Scholar]
- 13.■■.Tatsuta T, Imaizumi T, Shimoyama T, et al. Expression of melanoma differentiation associated gene 5 is increased in human gastric mucosa infected with Helicobacter pylori. J Clin Pathol 2012; 65:839–843.This study discovered that H. pylori induces the innate immune system via activation of the dsRNA receptor, MDA5. This is a novel function for MDA5, which was previously thought to function only in antiviral defense.
- 14.Sekiguchi F, Matsumoto Y, Maeda Y, et al. Biological activity of Helicobacter pylori components in mammalian cells: is it independent of proteinase-activated receptors? J Physiol Pharmacol 2012; 63:571–576. [PubMed] [Google Scholar]
- 15.Adami M, Pozzoli C, Menozzi A, et al. Effects of histamine H4 receptor ligands in a mouse model of gastric ulceration. Pharmacol 2012; 89:287–294. [DOI] [PubMed] [Google Scholar]
- 16.Seo PJ, Kim N, Kim JH, et al. Comparison of indomethacin, diclofenac and aspirin-induced gastric damage according to age in rats. Gut Liver 2012; 6:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav SK, Adhikary B, Chand S, et al. Molecular mechanism of indomethacin-induced gastropathy. Free Radic Biol Med 2012; 52:1175–1187. [DOI] [PubMed] [Google Scholar]
- 18.Hong H, Kim EH, Lee HJ, et al. Molecular mechanisms elucidating why old stomach is more vulnerable to indomethacin-induced damage than young stomach. Dig Dis Sci 2013; 58:61–71. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi K, Ohishi M, Ota S, et al. Metabolic profiling to identify potential serum biomarkers for gastric ulceration induced by nonsteroid anti-inflammatory drugs. J Proteome Res 2013; 12:1399–1407. [DOI] [PubMed] [Google Scholar]
- 20.■.Colucci R, Antonioli L, Bernardini N, et al. Nonsteroidal anti-inflammatory drug-activated gene-1 plays a role in the impairing effects of cyclooxygenase inhibitors on gastric ulcer healing. J Pharmacol Exp Ther 2012; 342:140–149. This study supports ongoing evidence that NSAIDs cause more harm than just the well known COX-mediated effects. They also promote pro-apoptotic pathways and inhibit ulcer healing.
- 21.Nakashita M, Suzuki H, Miura S, et al. Attenuation of acetic acid-induced gastric ulcer formation in rats by glucosylceramide synthase inhibitors. Dig Dis Sci 2013; 58:354–362. [DOI] [PubMed] [Google Scholar]
- 22.Nadatani Y, Watanabe T, Tanigawa T, et al. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol 2012; 181:98–110. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Suzuki H, Imai T, et al. Musashi-1 posttranscriptionally enhances phosphotyrosine-binding domain-containing m-Numb protein expression in regenerating gastric mucosa. PLoS One 2013; 8:e53540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.■■.Tanaka F, Tominaga K, Sasaki E, et al. Cytoglobin may be involved in the healing process of gastric mucosal injuries in the late phase without angiogenesis. Dig Dis Sci 2013; 58:1198–1206. This is the first study to show that cytoglobin and HIF-1α are important factors in gastric mucosal healing prior to the initiation of angiogenesis.
- 25.Luo JC, Huo TI, Hou MC, et al. Clopidogrel delays gastric ulcer healing in rats. Eur J Pharmacol 2012; 695:112–119. [DOI] [PubMed] [Google Scholar]
- 26.■.Chang M, Alsaigh T, Kistler EB, Schmid-Schonbein GW. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One 2012; 7:e40087. Discovery that ischemia, rather than enzymatic breakdown, can serve as the initial step in ulcerogenesis by inducing breakdown of secreted and membrane-bound mucins.
- 27.Niv Y, Boltin D, Halpern M, et al. Membrane-bound mucins and mucin terminal glycans expression in idiopathic or Helicobacter pylori, NSAID associated peptic ulcers. Dig Dis Sci 2012; 57:2535–2544. [DOI] [PubMed] [Google Scholar]
- 28.Navabi N, Johansson ME, Raghavan S, Linden SK. Helicobacter pylori infection impairs the mucin production rate and turnover in the murine gastric mucosa. Infect Immun 2013; 81:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shikama N, Ichikawa T, Iwai T, et al. Different effects of two types of H2-receptor antagonists, famotidine and roxatidine, on the mucus barrier of rat gastric mucosa. Biomed Res 2012; 33:45–51. [DOI] [PubMed] [Google Scholar]
- 30.Morsy MA, Heeba GH, Abdelwahab SA, Rofaeil RR. Protective effects of nebivolol against cold restraint stress-induced gastric ulcer in rats: role of NO, HO-1, and COX-1,2. Nitric Oxide 2012; 27:117–122. [DOI] [PubMed] [Google Scholar]
- 31.Brancati SB, Zadori ZS, Nemeth J, Gyires K. Substance P induces gastric mucosal protection at supraspinal level via increasing the level of endomorphin-2 in rats. Brain Res Bull 2013; 91:38–45. [DOI] [PubMed] [Google Scholar]
- 32.■.Chang Q, Yan L, Wang CZ, et al. In vivo transplantation of bone marrow mesenchymal stem cells accelerates repair of injured gastric mucosa in rats. Chin Med J (Engl) 2012; 125:1169–1174. Identification of a new potential therapeutic avenue in ulcer healing via the use of bone marrow mesenchymal stem cells.
- 33.■■.Barrett TD, Lagaud G, Wagaman P, et al. The cholecystokinin CCK2 receptor antagonist, JNJ-26070109, inhibits gastric acid secretion and prevents omeprazole-induced acid rebound in the rat. Br J Pharmacol 2012; 166:1684–1693. This study demonstrated that CCK2 receptor antagonists can serve as a potential therapeutic target which can suppress acid production like PPIs, without causing hypoplastic thinning of the gastric mucosa.


