Abstract
PURPOSE
No established treatments exist for relapsed/refractory systemic light-chain (AL) amyloidosis. Bendamustine has shown potential in the treatment of multiple myeloma. We conducted a phase II, multicenter trial to assess the efficacy and safety of bendamustine with dexamethasone (ben-dex) in patients with persistent or progressive AL amyloidosis after ≥ 1 prior therapy.
METHODS
The trial enrolled 31 patients who received bendamustine on days 1 and 2 (100 mg/m2 intravenously) with 40 mg of weekly dexamethasone in 28-day cycles until disease progression or up to 6 cycles after complete hematologic response. The primary objective was the rate of partial hematologic response (PR) or better.
RESULTS
Patients received a median of 4 cycles (range, 2-12 cycles) with 57% of patients achieving a PR or better (11% complete response, 18% very good PR). The overall organ response was 29% among the 24 patients who had measurable organ involvement. Treatment was well tolerated with no grade 5 treatment-related adverse events (AEs). Sixty-five percent of patients had a therapy-related grade 3-4 AE. The most common AEs included myelosuppression, fatigue, and nausea/vomiting. The median overall survival was 18.2 months (95% CI, 11.3 to 43.8 months), and hematologic response was associated with prolonged survival (P = .0291). The median progression-free survival was 11.3 months (95% CI, 5.0 to 15.4 months).
CONCLUSION
Overall, ben-dex is a viable treatment option with substantial efficacy and limited toxicity for patients with pretreated AL amyloidosis who have limited therapeutic options. This trial was registered at (ClinicalTrials.gov identifier: NCT01222260).
INTRODUCTION
Systemic light-chain (AL) amyloidosis is characterized by the production of abnormal immunoglobulin light chains by a plasma cell clone. Abnormal free light chains (FLCs) form toxic misfolded proteins that aggregate and deposit as insoluble fibrils in target organs, leading to organ dysfunction and, ultimately, death.1 Cytotoxic antiplasma cell therapy is used with the aim of eliminating the production of these proteins, thereby allowing organ recovery and prolonged survival. There are currently no Food and Drug Administration (FDA)–approved regimens for AL amyloidosis. All the available therapies, including melphalan, proteasome inhibitors, immunomodulatory agents, and recently, daratumumab, were adapted for AL amyloidosis after activity was established in multiple myeloma (MM).2 Although newer agents have improved the outlook for some patients with AL amyloidosis, limited options exist for patients with relapsed or refractory disease.
Bendamustine is a bifunctional alkylating agent with efficacy in the treatment of several hematologic malignancies, including chronic lymphocytic leukemia, non-Hodgkin lymphoma, and MM. As an alkylating agent, bendamustine induces DNA interstrand crosslinks leading to cytotoxicity.3 It also acts through secondary mechanisms different from other alkylating agents, which may explain its efficacy, even in patients refractory to conventional chemotherapeutic agents.4 Although the mechanisms are not fully understood, studies suggest that bendamustine inhibits several mitotic checkpoints, promotes inefficient DNA repair, and may induce apoptosis via a p53-dependent DNA damage stress response.5
Bendamustine has proven efficacy in both treatment-naïve patients and those with relapsed or refractory MM. It is approved in Europe as a first-line treatment of MM6 based on a phase III study comparing bendamustine and prednisone versus melphalan and prednisone for patients with previously untreated MM. Bendamustine prolonged the time to treatment failure and had a higher rate of complete response (CR).7 Smaller studies also showed efficacy and tolerability of the combination of bendamustine-prednisone-bortezomib in newly diagnosed patients, including those with renal dysfunction.8-11 Additionally, Lentzsch et al12 published data from a multicenter phase I/II clinical trial showing that bendamustine-lenalidomide-dexamethasone was well tolerated and achieved a partial response (PR) rate of 52%, with a very good PR (VGPR) rate of 24% in patients with heavily pretreated MM. Other studies, including several large phase II trials, with different combinations of therapies in relapsed/refractory MM have confirmed the potency of bendamustine as an antiplasma cell therapy with promising results.13-18 Overall, bendamustine is well tolerated, with the most common adverse effects being hematologic events, GI symptoms, fever, and allergic reactions.
Given the efficacy and tolerability of bendamustine in the treatment of MM, we sought to evaluate bendamustine combined with dexamethasone in relapsed/refractory AL amyloidosis (RRAL). We present the data from a multicenter phase II trial using bendamustine-dexamethasone (ben-dex) in this patient population.
METHODS
Patient Eligibility
The study included patients ≥ 18 years old with histopathologically confirmed AL amyloidosis with persistent or progressive hematologic disease after ≥ 1 prior therapy. Inclusion required measurable disease, defined as ≥ 1 of the following: serum monoclonal protein ≥ 0.5 g/dL by serum electrophoresis (SPEP), urine monoclonal protein > 200 mg/dL in 24-hour urine electrophoresis (UPEP), clonal population of plasma cells in the bone marrow, or abnormal FLC ratio. Other eligibility criteria included Eastern Cooperative Oncology Group performance status < 3, ineligible for or declined autologous stem cell transplantation (ASCT) if no previous transplantation, absolute neutrophil count ≥ 1.5 × 109/L, hemoglobin level ≥ 9 g/dL, platelet count ≥ 100 × 109/L, calculated creatinine clearance (CrCl) ≥ 30 mL/min (CrCl ≥ 15 mL/min considered if not in active renal failure and approved by principal investigator), AST and ALT ≤ 2.5 × upper limit of normal (ULN), and serum bilirubin < 1.5 × ULN.
Exclusion criteria included presence of symptomatic MM, myocardial infarction within 6 months, New York Heart Association class IIIB or IV heart failure, uncontrolled angina, severe arrhythmia, active conduction system abnormalities, use of other investigational drug within 14 days before enrollment, serious concurrent illness, HIV infection, pregnancy or breast feeding, and treatment or diagnosis of another malignancy within 3 years of enrollment. Patients with N-terminal pro-brain natriuretic peptide (NT-proBNP) ≥ 1,800 ng/L or brain natriuretic peptide (BNP) ≥ 400 ng/L, or abnormal cTnT or cTnI could only be included after evaluation by a cardiologist to determine the risk associated with treatment. Participating patients provided written informed consent before enrollment, and the study was approved by the institutional review board of all participating sites and registered under ClinicalTrials.gov identifier NCT01222260.
Study Design
This phase IIa trial enrolled patients from 6 sites in the United States using a 2-stage optimal Simon design with an α (type I error) of .1 and power of 0.85. For the treatment to be of further interest, the proportion of patients experiencing hematologic response had to be at least 0.40, over a futile response of 0.2 or less. Thirteen patients were enrolled in the first stage, and if ≥ 3 patients experienced a hematologic PR or better, the trial was to proceed to the second stage. The second stage enrolled an additional 16 patients. If 9 or more of the 29 patients with evaluable response had at least a PR, the treatment was considered worthy of further development. Patients who did not complete 2 cycles were not evaluable for response assessment and were replaced to accrue 29 patients evaluable for response.
Patients received treatment in 28-day cycles with intravenous bendamustine given on days 1 and 2 (100 mg/m2 for CrCl ≥ 60 mL/min, 90 mg/m2 for CrCl 59-30 mL/min, 70 mg/m2 for CrCl 15-30 mL/min), and dexamethasone 40 mg orally, given on days 1, 8, 15, and 22. For patients with good performance status, CrCl ≥ 60 mL/min, and tolerance of treatment, the bendamustine dose could be escalated to 120 mg/m2. Treatment was continued until disease progression per the standard response criteria19 or for up to 6 cycles after hematologic CR. Treatment was stopped for unacceptable toxicity, patient refusal, nonresponse, or noncompliance. The primary objective was to estimate the rate of PR or better. Secondary objectives included the hematologic CR and VGPR rates, organ response rate, progression-free survival (PFS), overall survival (OS), and assessment of toxicity.
Assessment
Patients who received at least 2 complete cycles of treatment were eligible for response assessment. Hematologic response was assessed at the beginning of each cycle according to established consensus criteria based on serum FLCs, SPEP/UPEP, and immunofixation.19 Hematologic CR required negative serum and urine immunofixation and a normal serum FLC ratio. VGPR was defined as a reduction in difference in FLC (dFLC) to < 40 mg/L, and a PR was defined as more than a 50% reduction in dFLC. Progression from CR was defined as any detectable monoclonal protein or abnormal FLC ratio (ratio must have doubled). Progression from PR was a 50% increase in serum M protein to > 0.5 mg/dL or 50% increase in urine M protein to > 200 mg/day. A 50% increase in FLCs to > 100 mg/L also met criteria for progression. No response was defined as no evidence of disease progression and less than a PR.20 For patients with dFLC < 40 mg/L at baseline, standard MM response criteria were applied. Amyloid-related organ response was evaluated starting after the second cycle of therapy on the basis of accepted criteria from Comenzo et al19 for cardiac, hepatic, and neuropathic responses and from Pallidini et al20,21 for renal response. History and physical examination, NT-proBNP/BNP, troponin, echocardiography, 24-hour urine protein, serum creatinine, alkaline phosphatase, and liver imaging were used for organ response assessment. Cardiac response was primarily assessed based on cardiac biomarkers.20 For patients without NT-proBNP measurements, the previously cited formula, log BNP = 0.28 + 0.66 × log NT-proBNP, was used to convert BNP to NT-proBNP.22
After discontinuation of therapy, patients who had not experienced disease progression were observed every 3 months for the first 2 years, every 6 months for years 2-5, and annually thereafter until disease progression for hematologic and organ response evaluation. After hematologic disease progression, patients were observed every 3 months for survival for up to 3 years. Adverse events (AEs) were evaluated on an ongoing basis and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical Analysis
Patient characteristics were summarized using descriptive statistics. Hematologic and organ response rates were calculated as proportions. PFS was defined as time from the first day of treatment until hematologic disease progression or death. OS was defined as time from first day of treatment to death. PFS and OS were estimated using the Kaplan-Meier method with 95% confidence bounds. Median PFS and OS was estimated from the survival function.
RESULTS
Patient Characteristics
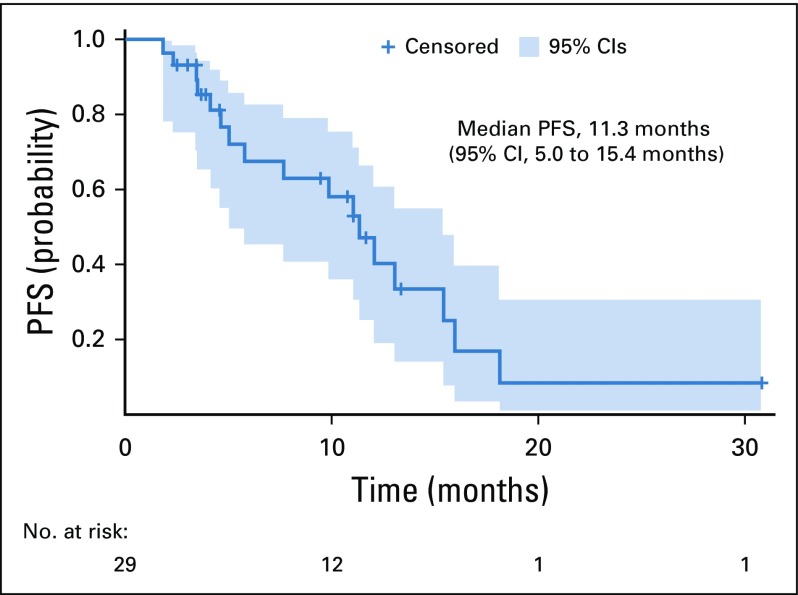
Between January 2013 and March 2016, 31 patients with RRAL were enrolled in the study. Baseline characteristics are summarized in Table 1. Median age was 65 years (range, 42-78 years), with a predominantly male population (71%). The majority of patients had a lambda-clonal plasma cell dyscrasia (68%). All patients had organ involvement, and the median number of involved organs was 2 (range, 1-4). Fifty-eight percent of patients had ≥ 2 organs involved. This included 18 (58%) cardiac, 16 (52%) renal, 8 (26%) neurologic, 9 (29%) GI, and 4 (13%) hepatic organ involvements. Patients had received a median of 2 prior therapies (range, 1-5). Fourteen patients (45%) had previously undergone ASCT. Median time from initial diagnosis to start of treatment was 31.0 months (range, 3.0-167.2 months); 65% of patients had relapsed hematologic disease at enrollment, and the others had refractory disease.
TABLE 1.
Patient Characteristics
Efficacy
Of the 31 patients enrolled, 29 completed at least 2 cycles of treatment and were eligible for response assessment. Their baseline characteristics are summarized in the Data Supplement. Two patients terminated treatment before completion of cycle 2. One patient had a cardiac arrest not attributable to the study drugs, and the other patient developed worsening renal function precluding additional treatment. The 29 patients completed a median of 4 cycles (range, 2-12 cycles). The most common reasons for discontinuation of therapy included AEs in 8 patients (28%), lack of response in 5 (17%), organ disease progression in 3 (10%), and hematologic disease progression in 2 (7%).
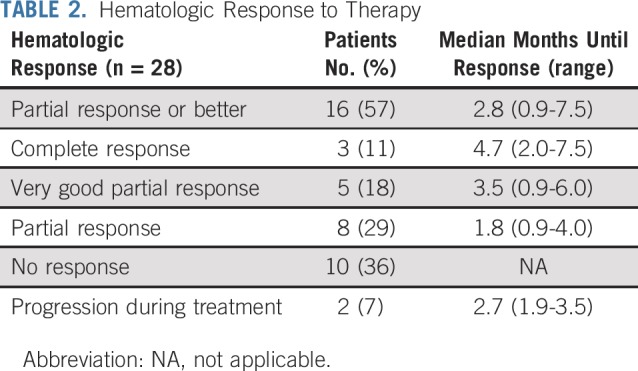
Hematologic responses are summarized in Table 2. One patient was enrolled based on bone marrow involvement without detectable serum monoclonal protein or a dFLC > 40 mg/L and did not undergo repeat bone marrow biopsy at the end of the study. He could not be evaluated for a hematologic response but was assessed for organ response. Of the 28 patients evaluable for hematologic response, the overall hematologic response rate was 57%: 3 patients (11%) with CR, 5 patients (18%) with VGPR, and 8 patients (29%) with PR. Among the hematologic responders, 94% had previously been treated with bortezomib, 50% were treated with lenalidomide, 69% were treated with a melphalan-based therapy, and 50% had undergone ASCT. Median time to best hematologic response was 2.8 months (range, 0.9-7.5 months), and median time to first hematologic response was 1.9 months (range, 0.9-4.0 months). Ten patients (36%) did not respond, and 2 (7%) experienced disease progression during treatment.
TABLE 2.
Hematologic Response to Therapy
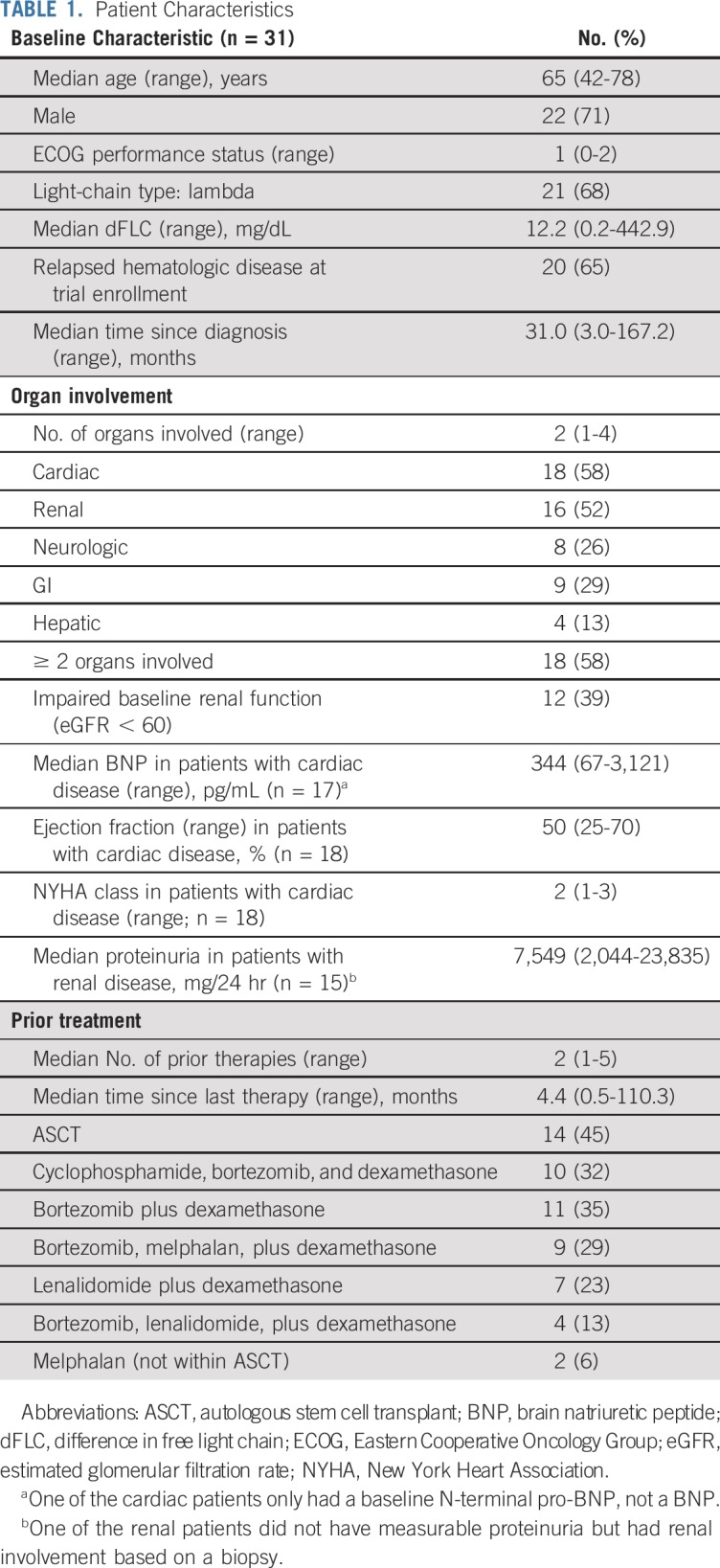
Organ response is summarized in Table 3. Of the 24 patients with measurable organ response, based on established criteria, 7 (29%) achieved an organ response (46% renal response, 13% cardiac response, 0% hepatic response).20,21 Any organ response was reached after a median of 2.8 months (range, 1.9-6.0 months). Ten patients (42%) had organ progression (31% renal, 50% cardiac, 25% hepatic). The majority of patients (62%) who had organ progression never achieved a hematologic response. Eight of the patients with evaluable organ response had multiple organs involved (4 cardiac and renal, 3 cardiac and hepatic, 1 all three); their responses are summarized in the Data Supplement. Only 1 patient had a discordant organ response with cardiac progression but renal response and was counted as having achieved organ response because it was unclear whether treatment with dexamethasone mimicked cardiac organ progression.
TABLE 3.
Organ Response to Therapy
Disease Progression and Survival
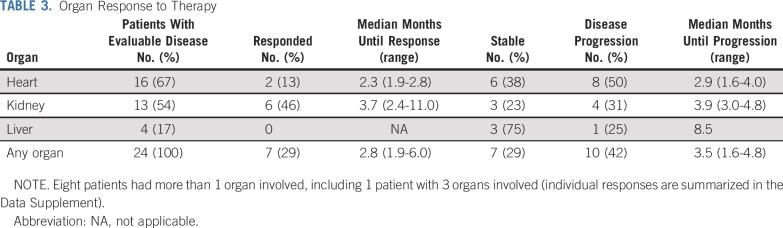
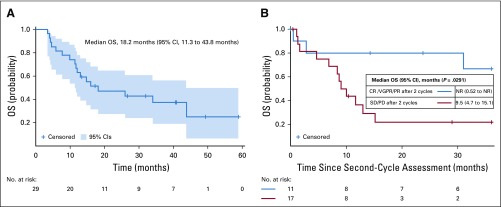
Median duration of patient follow-up was 14.9 months (range, 3.0-59.8 months), with 12 patients alive at the time of data analysis. Median OS was 18.2 months (95% CI, 11.3 to 43.8 months), as shown in Fig 1A. In a landmark analysis after completion of 2 cycles, the median OS among patients who achieved a CR, VGPR, or PR was not reached compared with a median OS of 9.5 months among those without a hematologic response after 2 cycles (P = .0291; Fig 1B). Median PFS was 11.3 months (95% CI, 5.0 to 15.4 months), as illustrated in Fig 2.
FIG 1.
(A) Overall survival (OS) from the start of the first cycle of treatment by Kaplan-Meier estimation with 95% CIs (n = 29). (B) Landmark analysis of OS after completion of 2 cycles of treatment, according to hematologic response category: complete response, very good partial response, or partial response (CR/VGPR/PR) versus stable disease/progression of disease (SD/PD). Hematologic response was assessed at cycle 3 day 1, which is the landmark time (n = 28). Survival functions are compared using the log-rank test with a 2-sided P value. NR, not reached.
FIG 2.
Progression-free survival (PFS) from the start of the first cycle of treatment until hematologic disease progression or time of death by Kaplan-Meier estimation with 95% CIs (n = 29).
Safety and Tolerability
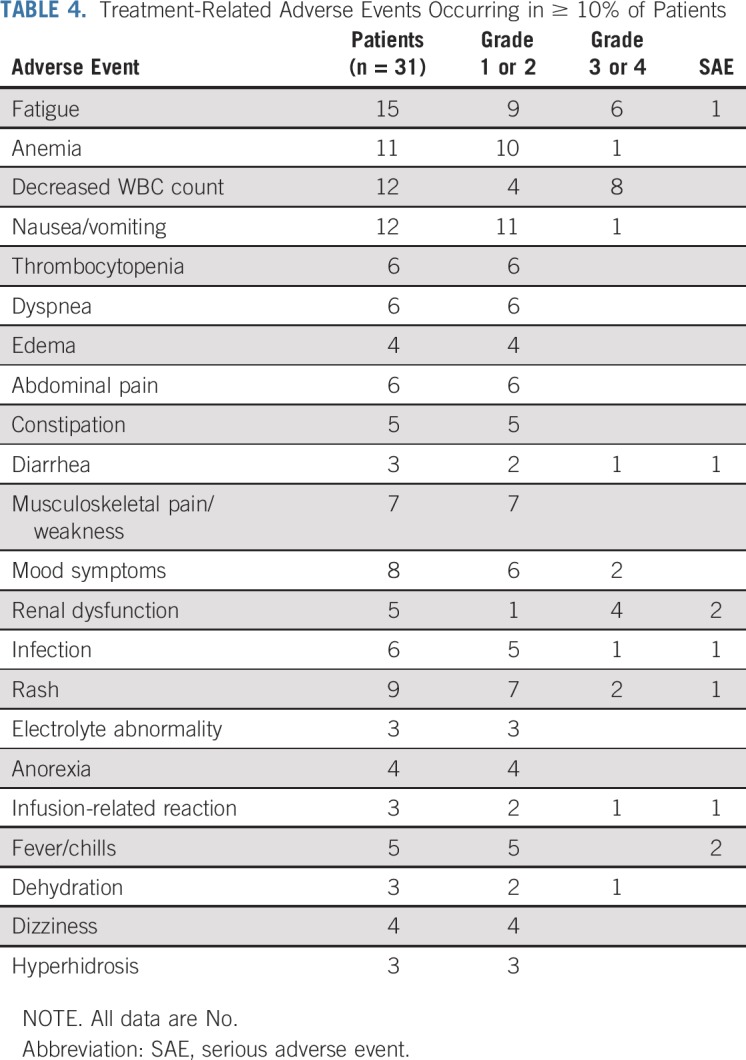
AEs for all 31 enrolled patients are summarized in Table 4. Despite the frail patient population, we did not observe any grade 5 therapy-related serious AEs (SAEs). Overall, 20 of the patients (65%) had at least 1 grade 3-4 therapy-related AE. The most common grade 3 and 4 events included leukopenia (26%), fatigue (19%), renal dysfunction (13%), rash (6%), and mood symptoms (6%). SAEs attributable to the treatment were reported in 10 patients (32%). Only 6 of the patients had more than 1 SAE. The SAEs included atrial fibrillation, colitis, hypothermia, fatigue, fever, hypertension, hypotension, infusion-related reaction, intracranial hemorrhage, pulmonary infection, nephrogenic diabetes insipidus, acute kidney injury, rash, and syncope. One death, which was attributed to infection and underlying disease, occurred during the trial among patients who completed at least 2 cycles of therapy. One patient, who was described previously,23 developed partial nephrogenic diabetes insipidus after the first cycle of ben-dex. He was successfully treated with hydrochlorothiazide, sodium restriction, and desmopressin, with improvement in symptoms while continuing bendamustine. Of the 12 patients with baseline renal dysfunction, only 3 experienced worsening renal function during treatment.
TABLE 4.
Treatment-Related Adverse Events Occurring in ≥ 10% of Patients
Based on comorbidities, bendamustine starting doses included 15 patients at 100 mg/m2, 12 patients at 90 mg/m2, and 4 patients at 70 mg/m2. In 1 patient, the dose was increased to 120 mg/m2. Dose reduction of bendamustine was necessary in 9 patients (29%) because of fatigue, infusion reaction, worsening renal function, fever, or nephrogenic diabetes insipidus. The dose of dexamethasone was reduced in 15 patients (48%) because of fatigue, muscle weakness, edema, weight gain, insomnia, hypertension, GI intolerance, or chest pressure.
DISCUSSION
At present, there are no FDA-approved treatments for AL amyloidosis. Given that many of the available therapies have toxicities that limit their use in patients with amyloid-associated organ dysfunction, there is an unmet need for new therapeutic options. To our knowledge, this is the first prospective phase II trial of ben-dex for the treatment of RRAL. In this study, 57% of patients experienced a hematologic response, with 11% achieving a CR, indicating that the ben-dex regimen has utility in AL amyloidosis, even among frail patients with prior exposure to multiple prior lines of therapy. Furthermore, we found that OS was improved among patients who achieved a hematologic response within 2 cycles, consistent with prior data showing that response correlates with survival.19
In a retrospective study of 122 patients with newly diagnosed and relapsed AL treated with bendamustine and prednisone, the hematologic response rate (HRR) was 35%, with 8% VGPR and 2% CR, which is lower than the response in our prospective trial. We also observed a higher PFS of 11.3 months compared with 8 months in the retrospective study. This is likely because our patients had adequate organ function resulting in a higher total treatment dose of ben-dex. With regard to organ response, 12% of patients had a cardiac response and 31% had a renal response, which was similar to the rates of 13% and 46%, respectively, in our trial.24 Nevertheless, our organ response rate of 29% is relatively low, and we cannot exclude that the high dose of 40 mg dexamethasone in our trial resulted in fluid overload and subsequent increase of NT-proBNP mimicking cardiac progression.
Compared with other treatment regimens used for RRAL, ben-dex has comparable efficacy with good tolerability. Prospective studies of lenalidomide and dexamethasone have shown 41% HRR with no CR in a heavily pretreated population and 67% HRR with 29% CR in a less heavily pretreated group.25,26 Significant nephrotoxicity was seen with 1 study reporting a > 50% increase in creatinine in two thirds of patients, and only half recovered renal function.27 By contrast, only 16% of patients in our trial experienced any increase in creatinine. Additionally, the significant increase of NT-proBNP and BNP associated with worsening of congestive heart failure indicates a potential contraindication of using lenalidomide in patients with cardiac AL amyloidosis.28 Pomalidomide with dexamethasone has also been tested, with comparable HRR rates of 48%-60% and CR of 3%-33%.29-31 The median OS of 26-28 months was higher than what we observed for ben-dex but, unfortunately, pomalidomide is not a feasible option for many patients with amyloidosis because of worsening heart failure.19,31 A trial of carfilzomib reported a high HRR rate of 63% with a comparable CR of 12.5%, but the treatment was associated with several grade 3-4 cardiac or pulmonary AEs, limiting its applicability.32 Ixazomib has shown a similar HRR of 52% with 9% CR but with a longer median PFS of 14.8 months compared with the 11.3 months observed in our study.33 Despite promising data with a high HRR with daratumumab monotherapy (76%), including CR in 36% and VGPR in 24% of patients, the data are limited without prospective trials.34 An ongoing phase III trial is evaluating the role of daratumumab with cyclophosphamide, bortezomib, and dexamethasone in newly diagnosed patients (ClinicalTrials.gov identifier: NCT03201965).
In summary, the HRR induced by ben-dex was competitive with other drug regimens and was well tolerated without significant cardiac, renal, or pulmonary toxicities, which is especially important in this patient population with baseline organ dysfunction. Although higher HRRs are seen with newer drugs, such as daratumumab, these treatments may be moved to the frontline setting, depending on the results of ongoing clinical trials. Given the low tolerance of immunomodulatory derivates and major AEs of carfilzomib in this patient population, the options in the relapsed/refractory setting will be exhausted with the first or second relapse, and potent and well-tolerated drugs like bendamustine would be an excellent alternative.
ACKNOWLEDGMENT
The authors thank the patients and their families, investigators, study coordinators, and support staff.
PRIOR PRESENTATION
Preliminary data previously presented as a poster at The American Society of Hematology 2016 Annual Meeting, San Diego, CA, December 3-6, 2016; and the 2017 American Society of Hematology Annual Meeting, Atlanta, GA, December 9-12, 2017.
SUPPORT
Supported by the Herbert Irving Comprehensive Cancer Center Support Grant awarded by the National Cancer Institute (NCI P30 CA013696) and TEVA Pharmaceuticals.
AUTHOR CONTRIBUTIONS
Conception and design: Suzanne Lentzsch, Raymond L. Comenzo, Jeffrey A. Zonder, Keren Osman, Vaishali Sanchorawala, Heather Landau
Administrative support: Silva Pregja
Provision of study materials or patients: Suzanne Lentzsch, Raymond L. Comenzo, Jeffrey A. Zonder, Keren Osman, Vaishali Sanchorawala, Heather Landau
Collection and assembly of data: Suzanne Lentzsch, Raymond L. Comenzo, Jeffrey A. Zonder, Keren Osman, Vaishali Sanchorawala, Heather Landau, Silva Pregja, Galina G. Lagos
Data analysis and interpretation: Suzanne Lentzsch, Galina G. Lagos, Raymond L. Comenzo, Jeffrey A. Zonder, Keren Osman, Samuel Pan, Divaya Bhutani, Vaishali Sanchorawala, Heather Landau
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Bendamustine With Dexamethasone in Relapsed/Refractory Systemic Light-Chain Amyloidosis: Results of a Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Suzanne Lentzsch
Leadership: Caelum Biosciences
Stock and Other Ownership Interests: Caelum Biosciences
Consulting or Advisory Role: Bristol-Myers Squibb, Janssen, Caelum Biosciences, Bayer Schering Pharma, Takeda, Proclara, AbbVie, Sanofi
Speakers' Bureau: Clinical Care Options/NCCN, PeerView, Sanofi, Karyopharm
Patents, Royalties, Other Intellectual Property: Patent 11-1F4 mAb for use in AL amyloidosis.
Travel, Accommodations, Expenses: Janssen
Other Relationship: Sorrento
Jeffrey A. Zonder
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Prothena, Janssen, Amgen, Takeda, Caelum, Intellia Therapeutics, Alnylam, Oncotherapeutics
Research Funding: Celgene (Inst), BMS (Inst)
Keren Osman
Consulting or Advisory Role: Kite Pharma
Divaya Bhutani
Research Funding: Karyopharm
Silva Pregja
Honoraria: Takeda (I), Celgene (I), Janssen Oncology (I), Caelum Biosciences (I), Intellia Therapeutics (I), Amgen (I), Bristol-Myers Squibb (I), Alnylam (I), Prothena (I)
Consulting or Advisory Role: Intellia (I), Takeda (I), Caelum Biosciences (I), Alnylam (I), Amgen (I), BMS (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Celgene (I), Intellia Therapeutics (I), Takeda (I), Amgen (I), Alnylam (I)
Vaishali Sanchorawala
Consulting or Advisory Role: Proclara, Caelum Biosciences, Janssen Research & Development, AbbVie
Research Funding: Takeda (Inst), Celgene (Inst), Prothena (Inst), Janssen (Inst), Oncopeptides (Inst)
Heather Landau
Consulting or Advisory Role: Celgene, Takeda, Caelum Biosciences, Juno Therapeutics, Karyopharm, Janssen
Research Funding: Takeda
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gertz MA. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91:947–956. doi: 10.1002/ajh.24433. [DOI] [PubMed] [Google Scholar]
- 2.Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128:159–168. doi: 10.1182/blood-2016-01-629790. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Brugger W, Damaj G, et al. Optimal use of bendamustine in hematologic disorders: Treatment recommendations from an international consensus panel—An update. Leuk Lymphoma. 2016;57:766–782. doi: 10.3109/10428194.2015.1099647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Rummel MJ. Bendamustine: Rebirth of an old drug. J Clin Oncol. 2009;27:1492–1501. doi: 10.1200/JCO.2008.18.7252. [DOI] [PubMed] [Google Scholar]
- 5.Leoni LM, Hartley JA. Mechanism of action: The unique pattern of bendamustine-induced cytotoxicity. Semin Hematol. 2011;48(suppl 1):S12–S23. doi: 10.1053/j.seminhematol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6. APA: Levact(R) (bendamustine) Recommended for approval in Europe for treating blood cancers. https://www.ots.at/presseaussendung/OTE_20100319_OTE0022/levactr-bendamustine-recommended-for-approval-in-europe-for-treating-blood-cancers.
- 7.Pönisch W, Mitrou PS, Merkle K, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone—A randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO) J Cancer Res Clin Oncol. 2006;132:205–212. doi: 10.1007/s00432-005-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pönisch W, Andrea M, Wagner I, et al. Successful treatment of patients with newly diagnosed/untreated multiple myeloma and advanced renal failure using bortezomib in combination with bendamustine and prednisone. J Cancer Res Clin Oncol. 2012;138:1405–1412. doi: 10.1007/s00432-012-1212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pönisch W, Holzvogt B, Plötze M, et al. Bendamustine and prednisone in combination with bortezomib (BPV) in the treatment of patients with newly diagnosed/untreated multiple myeloma. J Cancer Res Clin Oncol. 2014;140:1947–1956. doi: 10.1007/s00432-014-1737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateos MV, Oriol A, Rosiñol L, et al. Bendamustine, bortezomib and prednisone for the treatment of patients with newly diagnosed multiple myeloma: Results of a prospective phase 2 Spanish/PETHEMA trial. Haematologica. 2015;100:1096–1102. doi: 10.3324/haematol.2015.124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tessenow H, Holzvogt M, Holzvogt B, et al. Successful treatment of patients with newly diagnosed/untreated light chain multiple myeloma with a combination of bendamustine, prednisone and bortezomib (BPV) J Cancer Res Clin Oncol. 2017;143:2049–2058. doi: 10.1007/s00432-017-2439-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lentzsch S, O’Sullivan A, Kennedy RC, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119:4608–4613. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck J, Schwarzer A, Gläser D, et al. Lenalidomide in combination with bendamustine and prednisolone in relapsed/refractory multiple myeloma: Results of a phase 2 clinical trial (OSHO-#077) J Cancer Res Clin Oncol. 2017;143:2545–2553. doi: 10.1007/s00432-017-2504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenson JR, Yellin O, Bessudo A, et al. Phase I/II trial assessing bendamustine plus bortezomib combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013;160:321–330. doi: 10.1111/bjh.12129. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig H, Kasparu H, Leitgeb C, et al. Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood. 2014;123:985–991. doi: 10.1182/blood-2013-08-521468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mey UJ, Brugger W, Schwarb H, et al. Bendamustine, lenalidomide and dexamethasone (BRd) has high activity as 2nd -line therapy for relapsed and refractory multiple myeloma—A phase II trial. Br J Haematol. 2017;176:770–782. doi: 10.1111/bjh.14481. [DOI] [PubMed] [Google Scholar]
- 17.Pozzi S, Gentile M, Sacchi S, et al. Bendamustine, low-dose dexamethasone, and lenalidomide (BdL) for the treatment of patients with relapsed/refractory multiple myeloma confirms very promising results in a phase I/II study. Leuk Lymphoma. 2017;58:552–559. doi: 10.1080/10428194.2016.1205741. [DOI] [PubMed] [Google Scholar]
- 18.Offidani M, Corvatta L, Maracci L, et al. Efficacy and tolerability of bendamustine, bortezomib and dexamethasone in patients with relapsed-refractory multiple myeloma: A phase II study. Blood Cancer J. 2013;3:e162. doi: 10.1038/bcj.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comenzo RL, Reece D, Palladini G, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. 2012;26:2317–2325. doi: 10.1038/leu.2012.100. [DOI] [PubMed] [Google Scholar]
- 20.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J Clin Oncol. 2012;30:4541–4549. doi: 10.1200/JCO.2011.37.7614. [DOI] [PubMed] [Google Scholar]
- 21.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124:2325–2332. doi: 10.1182/blood-2014-04-570010. [DOI] [PubMed] [Google Scholar]
- 22.Wong SW, Toskic D, Warner M, et al. Primary amyloidosis with renal involvement: Outcomes in 77 consecutive patients at a single center. Clin Lymphoma Myeloma Leuk. 2017;17:759–766. doi: 10.1016/j.clml.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Uwumugambi NA, Sanchorawala V, Shelton AC, et al. Bendamustine-induced nephrogenic diabetes insipidus in a patient with AL amyloidosis. Am J Kidney Dis. 2017;69:317–319. doi: 10.1053/j.ajkd.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Milani P, Schönland S, Merlini G, et al. Treatment of AL amyloidosis with bendamustine: A study of 122 patients. Blood. 2018;132:1988–1991. doi: 10.1182/blood-2018-04-845396. [DOI] [PubMed] [Google Scholar]
- 25.Palladini G, Russo P, Foli A, et al. Salvage therapy with lenalidomide and dexamethasone in patients with advanced AL amyloidosis refractory to melphalan, bortezomib, and thalidomide. Ann Hematol. 2012;91:89–92. doi: 10.1007/s00277-011-1244-x. [DOI] [PubMed] [Google Scholar]
- 26.Sanchorawala V, Wright DG, Rosenzweig M, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 2 trial. Blood. 2007;109:492–496. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- 27.Specter R, Sanchorawala V, Seldin DC, et al. Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol Dial Transplant. 2011;26:881–886. doi: 10.1093/ndt/gfq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tapan U, Seldin DC, Finn KT, et al. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. 2010;116:5071–5072. doi: 10.1182/blood-2010-09-305136. [DOI] [PubMed] [Google Scholar]
- 29.Palladini G, Milani P, Foli A, et al. A phase 2 trial of pomalidomide and dexamethasone rescue treatment in patients with AL amyloidosis. Blood. 2017;129:2120–2123. doi: 10.1182/blood-2016-12-756528. [DOI] [PubMed] [Google Scholar]
- 30.Sanchorawala V, Shelton AC, Lo S, et al. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 1 and 2 trial. Blood. 2016;128:1059–1062. doi: 10.1182/blood-2016-04-710822. [DOI] [PubMed] [Google Scholar]
- 31.Dispenzieri A, Buadi F, Laumann K, et al. Activity of pomalidomide in patients with immunoglobulin light-chain amyloidosis. Blood. 2012;119:5397–5404. doi: 10.1182/blood-2012-02-413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen AD, Landau H, Scott EC, et al. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. 2016;128:645. [Google Scholar]
- 33.Sanchorawala V, Palladini G, Kukreti V, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–605. doi: 10.1182/blood-2017-03-771220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman GP, Schrier SL, Lafayette RA, et al. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900–902. doi: 10.1182/blood-2017-01-763599. [DOI] [PubMed] [Google Scholar]