Abstract
PURPOSE
Five percent to 9% of pancreatic ductal adenocarcinomas (PDACs) develop in patients with a germline BRCA1/2 or PALB2 (gBRCA/PALB2+) mutation. Phase IB data from a trial that used cisplatin, gemcitabine, and veliparib treatment demonstrated a high response rate (RR), disease control rate (DCR), and overall survival (OS) in this population. We designed an open-label, randomized, multicenter, two-arm phase II trial to investigate cisplatin and gemcitabine with or without veliparib in gBRCA/PALB2+ PDAC.
PATIENTS AND METHODS
Eligible patients had untreated gBRCA/PALB2+ PDAC with measurable stage III to IV disease and Eastern Cooperative Oncology Group performance status of 0 to 1. Treatment for patients in arm A consisted of cisplatin 25 mg/m2 and gemcitabine 600 mg/m2 intravenously on days 3 and 10; treatment for patients in arm B was the same as that for patients in arm A, and arm A also received veliparib 80 mg orally twice per day on days 1 to 12 cycled every 3 weeks. The primary end point was RRs of arm A and arm B evaluated separately using a Simon two-stage design. Secondary end points were progression-free survival, DCR, OS, safety, and correlative analyses.
RESULTS
Fifty patients were evaluated by modified intention-to-treat analysis. The RR for arm A was 74.1% and 65.2% for arm B (P = .55); both arms exceeded the prespecified activity threshold. DCR was 100% for arm A and 78.3% for arm B (P = .02). Median progression-free survival was 10.1 months for arm A (95% CI, 6.7 to 11.5 months) and 9.7 months for arm B (95% CI, 4.2 to 13.6 months; P = .73). Median OS for arm A was 15.5 months (95% CI, 12.2 to 24.3 months) and 16.4 months for arm B (95% CI, 11.7 to 23.4 months; P = .6). Two-year OS rate for the entire cohort was 30.6% (95% CI, 17.8% to 44.4%), and 3-year OS rate was 17.8% (95% CI, 8.1% to 30.7%). Grade 3 to 4 hematologic toxicities for arm A versus arm B were 13 (48%) versus seven (30%) for neutropenia, 15 (55%) versus two (9%) for thrombocytopenia, and 14 (52%) versus eight (35%) for anemia.
CONCLUSION
Cisplatin and gemcitabine is an effective regimen in advanced gBRCA/PALB2+ PDAC. Concurrent veliparib did not improve RR. These data establish cisplatin and gemcitabine as a standard approach in gBRCA/PALB2+ PDAC.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth-leading cause of cancer mortality; more than 50% of patients are diagnosed with locally advanced or metastatic disease.1 Combination cytotoxic chemotherapies such as leucovorin, fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX) and gemcitabine with nab-paclitaxel have improved outcomes in PDAC, but even so, the 5-year overall survival (OS) rate remains under 10%, and median survivals are typically less than 1 year.2-4 The identification of actionable genomic alterations in cancer cells has enabled an unprecedented era of precision medicine.5-7 Mutations in BRCA1/2 and PALB2 genes are present in approximately 5% to 9% of patients with PDAC; these genes code for proteins critical for homologous recombination repair of DNA.8,9 Cancers arising in the setting of a BRCA mutation are associated with increased sensitivity to platinum agents because of ineffective DNA repair, and they also demonstrate a synthetically lethal interaction with poly(ADP-ribose) polymerase inhibitors (PARPi’s) because of the accumulation of double-strand DNA breaks arising in the setting of unrepaired single-strand DNA breaks during cell replication.10,11 The utility of PARPi’s in BRCA-mutated cancers has been established in breast and ovarian cancer, with the recent VELIA/GOG-3005 (ClinicalTrials.gov identifier: NCT02470585) trial. which demonstrated significantly improved outcomes in patients who had high-grade serous ovarian carcinomas with cytotoxic therapy and concurrent/sequential veliparib, particularly in patients with homologous recombination deficiency (HRD).12 Recent data from the phase III POLO (Olaparib in gBRCA Mutated Pancreatic Cancer Whose Disease Has Not Progressed on First Line Platinum-Based Chemotherapy; ClinicalTrials.gov identifier: NCT02184195) trial has established proof of principle for the use of a PARPi as a maintenance therapy for germline BRCA-mutated metastatic PDAC.13-15
We designed a series of trials to investigate combining the PARPi veliparib with cisplatin and gemcitabine in patients with PDAC who have a germline BRCA1/2 or PALB2 (gBRCA/PALB2+) mutation. Results from our initial phase I trial established the dose of veliparib given daily for 12 days in combination with cisplatin and gemcitabine dosed days 3 and 10 every 3 weeks. This triplet demonstrated a high response rate (RR) in this genetically selected population, with 78% of patients with gBRCA/PALB2+ PDAC achieving an objective tumor response and a median OS of 23.3 months.16,17
The strategy of combining platinum therapy and a PARPi is of significant interest in BRCA-mutated PDAC to enhance tumor response, delay resistance, and improve survival. Herein, we report on a multicenter, multinational, randomized, two-arm, open-label phase II trial evaluating cisplatin and gemcitabine with or without veliparib in patients with gBRCA/PALB2+ advanced PDAC. This study was designed in conjunction with the National Cancer Institute’s (NCI’s) Cancer Therapeutics and Evaluation Program (CTEP) and was supported in part by the Lustgarten Foundation.
PATIENTS AND METHODS
Study Design and Treatment
The primary objective was to evaluate the overall response rate for the combination of cisplatin, gemcitabine, and veliparib (arm A) and for cisplatin and gemcitabine (arm B). This trial was reviewed by the institutional review boards and privacy boards at all sites. All participants provided written informed consent.
Treatment was administered in 21-day cycles. For arms A and B, the cisplatin dose was 25 mg/m2 and the gemcitabine dose was 600 mg/m2 infused over 30 minutes on days 3 and 10. Patients in arm A received veliparib at 80 mg orally twice per day on days 1 to 12 every 21 days. Growth factors were used per ASCO guidelines. Germline genetic testing was conducted locally per site institutional policy.
Patient Population
Patients with untreated locally advanced or metastatic (American Joint Committee on Cancer stage III to IV) gBRCA/PALB2+ PDAC were eligible. Only patients with pathogenic germline mutations in BRCA/PALB2 were included. Patients with previous adjuvant therapy with gemcitabine or a fluoropyrimidine with or without radiation were eligible if therapy was completed more than 6 months before recurrence. No previous platinum agent or PARPi was allowed. Other key eligibility criteria were measurable disease (Response Evaluation Criteria in Solid Tumors v1.1 [RECIST 1.1]), age 18 years or older, Eastern Cooperative Oncology Group performance status (ECOG PS) 0 to 1, life expectancy more than 3 months, and normal major organ and marrow function.
Key exclusion criteria were the presence of active CNS metastases, other concurrent investigational agents, contraindication to platinum compounds, a history of seizure or allergic reaction attributed to compounds such as veliparib, pregnancy, active cardiovascular disease, or serious psychiatric illness.
Participants were removed from study if they experienced disease progression, intercurrent illness precluding treatment administration, unacceptable toxicity, or withdrawal of consent. Individuals in arm A for whom cytotoxic therapy was discontinued but who were otherwise benefiting from therapy were permitted to continue veliparib as single-agent maintenance at the full single-agent dose of 400 mg twice per day. A maximum of three dose reductions were allowed in each arm.
Biostatistical Design
The primary end point was to evaluate the RECIST RR for arms A and B. Patients were randomly assigned to arm A or arm B, and a Simon’s two-stage minimax design was used in arm A and arm B separately.18 We assumed an unacceptable RR of 10% and a promising rate of 30% with type I and II error rates of 10% each, based on activity of cisplatin and gemcitabine in sporadic PDAC.19
In each arm, 16 patients were planned for the first stage. If two or more responses were observed, an additional 9 patients were to be accrued for a total of 25 patients. If five or more of the 25 patients in an arm achieved an objective response, then that arm was designated worthy of additional investigation. The probability of early termination was 0.51. In the event that both arms showed promising activity at the end of the second stage, the trial permitted an additional 10 patients to be enrolled in each arm for a total of 35 patients per arm. This would allow distinction between true response rates of 20% and 40% with 83% power at the one-sided 0.17 significance level, or between true response rates of 30% and 50% with 79% power at the one-sided 0.16 level. These power calculations were predicated on the successful completion of the Simon’s two-stage design in both arms.
Radiologic imaging was conducted every 6 weeks (two cycles) from the start of therapy with a window of ± 5 days to assess treatment response irrespective of treatment delays. After 6 and 18 months on study, restaging imaging was moved to every 9 and 12 weeks, respectively. Toxicity was assessed with the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. The revised CTCAE version 5.0 was used for adverse event reporting beginning April 1, 2018.
The secondary end points included progression-free survival (PFS), OS, safety and tolerability, disease control rate (DCR), and duration of response. Exploratory analyses included evaluation of PFS and OS for patients who received 4 months or more of platinum followed by a PARPi and the association between BRCA status, ECOG PS, cancer stage, treatment center, and outcomes. All participants were observed for survival until death. A series of correlative studies was incorporated that included acquisition of archival tissue and pretreatment, on treatment, and post-treatment tumor biopsies, and serial blood samples were collected for all participants. Analyses are ongoing and will be reported separately.
RESULTS
Patient Characteristics
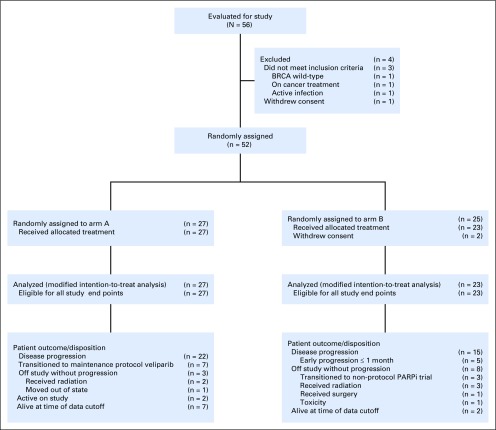
Baseline characteristics are listed in Table 1. Fifty-two patients were enrolled between January 2014 and November 2018 at six sites in three countries: the United States (Memorial Sloan Kettering Cancer Center [MSKCC], University of Chicago Medical Center, University of Michigan Medical Center), Canada (Princess Margaret-University Health Network), and Israel (Sha’are Zedek Medical Center, and Chaim Sheba Medical Center at Tel HaShomer). Two patients withdrew consent and were never treated because of random assignment to arm B. A modified intention-to-treat principle was applied for all randomly assigned and treated patients. The cutoff for data analysis was July 31, 2019. Random assignment and patient disposition are listed in the CONSORT diagram (Fig 1).
TABLE 1.
Baseline Patient Characteristics
FIG 1.
CONSORT diagram. PARPi, poly(ADP-ribose) polymerase inhibitor.
Fifty patients with a median age of 64 years (range, 37 to 82 years) and of whom 28 (56%) were female were included in the final analysis. Arm A had 27 patients (54%), and arm B had 23 patients (46%). Forty-two patients (84%) had stage IV disease, and 30 (60%) were of Ashkenazi Jewish descent. Twelve (24%) had BRCA1 mutations, 35 (70%) had BRCA2 mutations, and 3 (6%) had PALB2 mutations. Twenty-eight enrollees (56%) were treated at MSKCC (16 in arm A, and 12 in arm B), and 22 patients (46%) were treated at external centers (11 in arm A, and 11 in arm B). Cancer stage, ECOG PS, and BRCA mutation distribution were comparable in both arms. Within arm A, 7 patients (25.9%) transitioned to maintenance protocol-specified veliparib.
Tumor Response
Tumor response data and duration are listed in Table 2 and Figure 2A-B. Twenty patients (74%; one-sided 90% lower bound, 60%) in arm A had a partial response (PR), and 15 patients (65.2%; one-sided 90% lower bound, 50%) in arm B (P = .55) had a PR. DCR at any time point (complete response [CR], PR, and stable disease) was 27 (100%) in arm A and 18 (78%) in arm B (P = .02). Of specific note, the trial underwent an NCI audit, including response assessment, which served as an independent review of the primary end point. Both arms A and B significantly exceeded prespecified levels of tumor response. In November 2018 in discussion with NCI-CTEP and the study statisticians, a decision was made to close the study to further recruitment after 50 were enrolled. If the study enrolled 35 patients per arm (N = 70), the conditional power to detect a difference in RR between arms would be less than 20% to observe a 10% difference, less than 30% to observe a 20% difference, and less than 60% to observe a 30% difference; two-sided type 1 error is 0.15.
TABLE 2.
Best Response to Treatment
FIG 2.
(A) Best target responses in arm A and arm B. A waterfall plot depicts the percent change in tumor size from baseline in all patients in the study (N = 47). Three patients (arm B) had clinical progression before their first scan and are omitted from this figure. The number of patients with a partial response was 35 (70%). (B) Treatment duration and survival. A swimmer plot depicts the overall survival and indicates the treatment duration. One patient was consented but did not receive treatment and was omitted from the figure. Two patients from arm A remain on study treatment. (*) Denotes patients who were alive at the time of data analysis.
Survival Outcomes
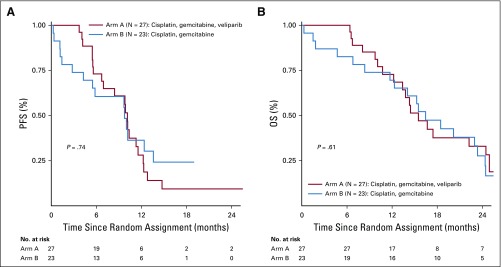
Nine patients (18%) were alive at the final data cutoff time point: seven in arm A and two in arm B. PFS and OS data are listed in Table 2 and Figure 3A-B. Median PFS was 10.1 months (95% CI, 6.7 to 11.5 months) for arm A and 9.7 months (95% CI, 4.2 to 13.6) for arm B (P = .73). Median OS was 15.5 months (95% CI, 12.2 to 24.3 months) for arm A and 16.4 months (95% CI, 11.7 to 23.4) for arm B (P = .6). Two patients from arm A remained on study at the time of data lock, one receiving maintenance veliparib 3 years after diagnosis. Treatment duration and survival are listed in Figure 3. Two-year OS rate for the entire cohort was 30.6% (95% CI, 17.8% to 44.4%), and 3-year OS rate for the entire cohort was 17.8% (95% CI, 8.1% to 30.7%).
FIG 3.
Kaplan-Meier curves for (A) progression-free survival (PFS) and (B) overall survival (OS).
Hematologic Toxicity
Hematologic toxicities are listed in Table 3. We observed more than double the number of total grade 3 to 4 hematologic toxicities in arm A compared with arm B (53 v 22). Eighty-one percent of patients (22 of 27) enrolled in arm A had at least one grade 3 to 4 hematologic toxicity versus 73% in arm B. Fourteen patients in arm A (52%) experienced grade 3 to 4 anemia, and 15 (55%) in arm A experienced grade 3 to 4 thrombocytopenia. Eight patients in arm B (35%) experienced grade 3 anemia and two (9%) experienced grade 3 thrombocytopenia; no grade 4 hematologic toxicity was observed in arm B.
TABLE 3.
Hematologic Toxicities (maximum grade per patient)
Nonhematologic Toxicity
Nonhematologic toxicities are listed in Table 4. The total number of grade 3 to 4 nonhematologic toxicities was 39 in arm A and 35 in arm B. There were two cases of non-neutropenic grade 4 infection or sepsis. One patient in arm A experienced seizures shortly after being transitioned from chemotherapy and veliparib to the higher dose of single-agent veliparib and was removed from the trial in view of toxicity and disease progression.
TABLE 4.
Nonhematologic Toxicities (maximum grade per patient)

Dose Reductions, Dose Delays, and Dose Intensity
Twenty patients (74%) in arm A had at least one dose reduction or drug discontinuation as a result of toxicity compared with six patients (26%) in arm B. In arm A, 18 patients (90%) received dose reductions as a result of hematologic toxicity compared with four patients (67%) in arm B. Patients in arm A received a median of 942 mg (interquartile range [IQR], 526 to 1,370 mg) cisplatin and 18,955 mg (IQR, 12,645 to 29,160 mg) gemcitabine, both over a median of 7 months. Patients in arm B received a median of 766 mg (IQR, 502 to 1,271 mg) cisplatin and a median of 23,900 mg (IQR, 15,000 to 38,400 mg) gemcitabine over a median of 8 and 9 months of treatment duration, respectively. Thirty-seven patients (74%) came off trial because of radiographic or clinical progression of disease. Eleven patients (22%) discontinued study treatment in the absence of disease progression (Fig 1). Of these, five patients (45.5%) received radiation therapy and three (27.3%) received nonprotocol PARPi’s.
After study therapy was discontinued, 36 patients (72%) received additional therapy. The average number of additional lines of therapy received after study treatment was 2.4 (range, 1 to 6). Twenty-eight (77.8%) received platinum-based therapy, including 13 (46.4%) FOLFIRINOX, four (14.3%) received leucovorin, fluorouracil, and oxaliplatin (FOLFOX), 10 (35.7%) received cisplatin-based therapy, and one (3.6%) received carboplatin-based therapy. Nine patients (25%) received gemcitabine and nab-paclitaxel. Eighteen patients (50%) received a PARPi, and 12 (33.3%) received radiation therapy. One patient (2.8%) in arm B had a near CR, proceeded to surgery after 15 months of trial therapy, and had subsequent adjuvant FOLFIRINOX therapy followed by olaparib, and a checkpoint inhibitor some time later, ultimately living for 4 years after enrolling on the study.
Exploratory Analyses
In light of emerging data in the field and from the POLO trial, we conducted several exploratory analyses of participants who received 4 or more months of platinum therapy and then continued or received a PARPi as an immediate next line of therapy in the absence of disease progression.14 In this subset of patients (n = 10; eight with stage IV) combined from both arms, the median OS was 23.4 months (95% CI, 6.5 to 53.9 months).
We investigated the association between BRCA status, ECOG PS, cancer stage at diagnosis, center (MSKCC vs non-MSKCC), and survival outcomes for the entire cohort. The median PFS for BRCA1 (n = 12) was 6.8 months (95% CI, 2.8 to 10.1 months) and for BRCA2 (n = 35), it was 11.3 months (95% CI, 9.8 to 12.8 months). Median OS was 14 months (95% CI, 8.1 to 18.5 months) for BRCA1 and 20.2 months (95% CI, 12.3 to 24.4 months) for BRCA2. Median OS was 23 months (95% CI, 13.8 to 24.5 months) for patients with ECOG PS 0 and 14.3 months (95% CI, 8.1 to 16.4 months) for patients with ECOG PS 1. The median OS was 24.9 months (95% CI, 10.7 months to not reached) for patients (n = 8) with stage III disease and 15.2 months (95% CI, 12.2 to 18.5 months) for patients (n = 42) with stage IV disease. Median PFS was 10.2 months (95% CI, 8.4 to 12.8 months) for the MSKCC site and 6.9 months (95% CI, 4.3 to 10.4 months) for non-MSKCC sites. Median OS was 22.2 months (95% CI, 14.4 to 24.9 months) for the MSKCC site and 14 months (95% CI, 10.1 to 18.5 months) for non-MSKCC sites.
DISCUSSION
Therapy for advanced PDAC continues to evolve and is increasingly being guided by genomic analysis and identification of subgroups of patients who are enriched to benefit from a specific therapeutic strategy; HRD is a prime example in PDAC.20-22 The identification of mutations in DNA repair genes and therapeutic vulnerabilities in PDAC have been established.14,17,23
This trial evaluated an uncommon but important subset of patients in the context of a prospective, multinational, multicenter, randomized trial design. The primary end point of each treatment arm was RR per RECIST 1.1, and the trial was independently audited. Both arm A (triplet) and arm B met, and significantly exceeded, prestudy thresholds of efficacy (RR, 74% for arm A and 65.2% for arm B). This level of activity was comparable to that in the preceding phase I trial.17 Of significant note is the 2-year (30.6%) and 3-year (17.8%) survival rates of the entire cohort, which represent among the longest reported in any randomized trial in PDAC.
Another aim was to understand the value of combining veliparib with cisplatin and gemcitabine. Although both study arms had high RRs, the addition of the PARPi to cisplatin and gemcitabine was not superior in terms of response (P = .55). PFS and OS were high and similar between arms. Arm A had more hematologic toxicities and more dose reductions, although nonhematologic toxicities in arm A were similar compared with those in arm B. Interestingly, we did not observe significant differences in dose intensity between arms, even though treatment duration was longer for arm B. Speculatively, the higher DCR in arm A (100%) may be a signal to investigate for a poor prognostic subgroup (destined to be early progressers) of patients with gBRCA/PALB2 in an effort to overcome de novo therapy resistance.
The OS in an exploratory subset of patients from both treatment arms who received platinum-based therapy followed by PARPi maintenance therapy was 23.4 months and is consistent with OS data from the POLO study, which reported a median OS of 18.9 months (with the addition of approximately 4 to 6 months of therapy before random assignment) in a highly selected subgroup of patients with gBRCA1/2 disease. FOLFIRINOX is an established active therapy in PDAC, although specific data in the gBRCA/PALB2+ setting are lacking. Toxicities, including GI, myelosuppression, fatigue, requirement for infusional therapy, and neuropathy, are often limiting; alternatively, cisplatin and gemcitabine is a tolerable regimen applicable to a broader population with regard to age and functional status.24 Furthermore, combined data from the POLO trial and from this trial suggest that platinum-based therapy followed by sequential maintenance PARPi’s (or continuation of cytotoxic therapy) represents an optimal first-line treatment approach in gBRCA/PALB2+ PDAC.
Germline BRCA1/2 and PALB2 mutations are validated predictive biomarkers for platinum response; however, these mutations are present in approximately 5% to 9% of patients with PDAC.14,25-27 Beyond BRCA, other genes have generated significant interest given their role in HRD, including ARID1A, ATM, RAD51, CHEK2, and the Fanconi anemia genes. Recent sequencing of more than 800 pancreas tumors revealed a frequency of mutations in HRD-related genes to be 15.4% (95% CI, 13.0% to 18.0%).28 Although the therapeutic significance of many of these somatic and germline mutations is unknown and is currently being studied in PDAC, preliminary data suggest that HRD predicts for better OS with platinum therapy in the first-line setting.21,29,30
The development of resistance to cytotoxic and targeted therapies remains a critical challenge in PDAC.31 Checkpoint blockade has demonstrated significant response in numerous cancer types; however, its efficacy in PDAC is limited.32,33 Checkpoint blockade is being investigated in combination with PARPi (ClinicalTrials.gov identifier: NCT02660034) and may exploit the increased neoantigen load of HRD and BRCA-mutated PDAC as a strategy for surmounting resistance.34-36
Strengths of our study design are the prospective, multicenter, multinational, randomized design in a focused biomarker selected subgroup of gBRCA/PALB2+ PDAC. Limitations include the small number of patients in each arm, the imbalance of ECOG PS between arms, the inclusion of a small number of patients with stage III disease, and the lack of a standard control arm.
In conclusion, cisplatin and gemcitabine is an active regimen in advanced gBRCA/PALB2+ PDAC. The addition of veliparib to cisplatin and gemcitabine was not superior to cisplatin and gemcitabine, and the triplet combination was notable for increased hematologic toxicity relative to the doublet. The data reported herein establish cisplatin and gemcitabine as a standard-of-care regimen in gBRCA/PALB2+ PDAC. Further insights into biomarkers of response and resistance will be forthcoming.
ACKNOWLEDGMENT
We thank all the patients and families and the research teams at all the participating sites. MSKCC: Michal Segal, Hayley Estrella, Hannah Maynard, Geoffrey Ku, Sree Chalasani, Erica Kaufmann, and Parisa Momtaz; University of Michigan: Muneez Patel; Princess Margaret-University Health Network: Angela Rodriguez; Chaim Sheba Medical Center at Tel HaShomer: Aliza Ackerstein and Menucha Jukowicz; Sha’are Zedek Medical Center: Amiel Segal, and Dalia Sherizen. We thank AbbVie Inc.
Footnotes
Supported by the Lustgarten Foundation, the David M. Rubenstein Center for Pancreatic Cancer Research, the Reiss Family Foundation, the National Institutes of Health, National Cancer Institute (NCI) Cancer Therapeutics Evaluation Program, and NCI Grants No. P30 CA008748 and R25CA020449.
Listen to the podcast by Dr Renouf at http://ascopubs.org/journal/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Eileen M. O’Reilly, Marinela Capanu, Alice P. Chen, David P. Kelsen
Financial support: Eileen M. O’Reilly
Administrative support: Eileen M. O’Reilly, Jennifer Park, Robin Brenner, Shreya Vemuri, David P. Kelsen
Provision of study materials or patients: Eileen M. O’Reilly, Mark Zalupski, Talia Golan, Esther Tahover, Vaibhav Sahai, Hedy L. Kindler, Kenneth H. Yu, Neesha Dhani, Alice Zervoudakis, David P. Kelsen
Collection and assembly of data: Eileen M. O’Reilly, Jonathan W. Lee, Mark Zalupski, Jennifer Park, Talia Golan, Esther Tahover, Maeve A. Lowery, Vaibhav Sahai, Robin Brenner, Hedy L. Kindler, Kenneth H. Yu, Shreya Vemuri, Richard K.G. Do, Neesha Dhani, David P. Kelsen
Data analysis and interpretation: Eileen M. O’Reilly, Jonathan W. Lee, Mark Zalupski, Marinela Capanu, Talia Golan, Joanne F. Chou, Hedy L. Kindler, Alice Zervoudakis, Zsofia K. Stadler, David P. Kelsen
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Eileen M. O’Reilly
Consulting or Advisory Role: CytomX, BioLineRx, Targovax, Celgene, Bayer, Loxo, Polaris, Ipsen, Merck
Research Funding: AstraZeneca/MedImmune (Inst), Genentech (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), MabVax Therapeutics (Inst), ActaBiologica (Inst), Parker Institute (Inst), AstraZeneca (Inst), Silenseed (Inst)
Mark Zalupski
Research Funding: Halozyme Therapeutics, NewLink Genetics/Pharmatech, OncoMed Pharmaceuticals, MedImmune, AstraZeneca, Merck, Seattle Genetics
Talia Golan
Honoraria: MSD, Rafael Pharmaceuticals
Consulting or Advisory Role: AbbVie, AstraZeneca
Research Funding: AstraZeneca (Inst), MSD Oncology (Inst)
Maeve A. Lowery
Consulting or Advisory Role: Agios, Genentech
Travel, Accommodations, Expenses: Ipsen
Vaibhav Sahai
Consulting or Advisory Role: Celgene, Halozyme Therapeutics, NewLink Genetics, Ipsen, Incyte, QED Therapeutics, KLUS Pharma
Research Funding: Celgene (Inst), Bristol-Myers Squibb (Inst), Agios (Inst), Incyte (Inst), Clovis Oncology (Inst), Debiopharm Group (Inst), FibroGen (Inst), Halozyme Therapeutics (Inst), MedImmune (Inst), Rafael Pharmaceuticals (Inst), Ipsen (Inst)
Hedy L. Kindler
Consulting or Advisory Role: AstraZeneca, Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Ipsen, Erytech Pharma, Five Prime Therapeutics, Paradox Therapeutics, Aldeyra Therapeutics, Kyowa Hakko Kirin, Astellas Pharma
Research Funding: Aduro BioTech (Inst), AstraZeneca (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Merck (Inst), MedImmune (Inst), Verastem (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Polaris Pharmaceuticals (Inst), Deciphera Pharmaceuticals (Inst), Inhibrx (Inst), Genentech (Inst)
Kenneth H. Yu
Consulting or Advisory Role: Ipsen, Halozyme Therapeutics, Bristol-Myers Squibb, Ipsen
Zsofia K. Stadler
Consulting or Advisory Role: Allergan (I), Genentech (I), Regeneron Pharmaceuticals (I), Optos (I), Adverum Biotechnologies (I), Biomarin Pharmaceutical (I), Alimera Sciences (I), Novartis (I), Spark Therapeutics (I), Fortress Biotech (I), REGENXBIO (I)
Richard K. G. Do
Honoraria: Bayer, ALK-Abelló (I)
Consulting or Advisory Role: DBV Technologies (I)
Patents, Royalties, Other Intellectual Property: UptoDate chapters on Food Allergy (I)
Neesha Dhani
Honoraria: Celgene, AstraZeneca
Consulting or Advisory Role: Celgene, Shire Baxalta, AstraZeneca
Research Funding: AstraZeneca, Halozyme Therapeutics, Celgene
Travel, Accommodations, Expenses: Celgene
David P. Kelsen
Consulting or Advisory Role: Steba biotech, Novo Nordisk, Kitov Pharma
Patents, Royalties, Other Intellectual Property: Use of iron-containing particles for imaging by MRI
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Ma H, Hong G, et al. Survival improvement in patients with pancreatic cancer by decade: A period analysis of the SEER database, 1981-2010. Sci Rep. 2014;4:6747. doi: 10.1038/srep06747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowery MA, Wong W, Jordan EJ, et al. Prospective evaluation of germline alterations in patients with exocrine pancreatic neoplasms. J Natl Cancer Inst. 2018;110:1067–1074. doi: 10.1093/jnci/djy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell. 2018;33:125–136.e3. doi: 10.1016/j.ccell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7:60. [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch HT, Deters CA, Snyder CL, et al. BRCA1 and pancreatic cancer: Pedigree findings and their causal relationships. Cancer Genet Cytogenet. 2005;158:119–125. doi: 10.1016/j.cancergencyto.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 11.Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 12.Coleman RL, Fleming GF, Brady MF, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. doi: 10.1056/NEJMoa1909707. [epub ahead of print on September 28, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 14.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: A proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 16.Lowery MA, Kelsen DP, Capanu M, et al. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Reilly EM, Lee JW, Lowery MA, et al. Phase 1 trial evaluating cisplatin, gemcitabine, and veliparib in 2 patient cohorts: Germline BRCA mutation carriers and wild-type BRCA pancreatic ductal adenocarcinoma. Cancer. 2018;124:1374–1382. doi: 10.1002/cncr.31218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 20.Furuse J. Paradigm shifting of systemic chemotherapy for unresectable pancreatic cancer in Japan. J Clin Med. 2019;8 doi: 10.3390/jcm8081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pishvaian MJ, Blais EM, Brody JR, et al. Outcomes in pancreatic adenocarcinoma (PDA) patients (pts) with genetic alterations in DNA damage repair (DDR) pathways: Results from the Know Your Tumor (KYT) program. J Clin Oncol. 2019;37 doi: 10.1200/PO.19.00115. (suppl; abstr 191) [DOI] [PubMed] [Google Scholar]
- 22.Singh RR, Goldberg J, Varghese AM, et al. Genomic profiling in pancreatic ductal adenocarcinoma and a pathway towards therapy individualization: A scoping review. Cancer Treat Rev. 2019;75:27–38. doi: 10.1016/j.ctrv.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of patients with pancreatic cancer: Initial results from the Know Your Tumor Initiative. Clin Cancer Res. 2018;24:5018–5027. doi: 10.1158/1078-0432.CCR-18-0531. [DOI] [PubMed] [Google Scholar]
- 24.Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer. 2014;111:1132–1138. doi: 10.1038/bjc.2014.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalewski A, Szylberg Ł, Saganek M, et al. Emerging strategies in BRCA-positive pancreatic cancer. J Cancer Res Clin Oncol. 2018;144:1503–1507. doi: 10.1007/s00432-018-2666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez-Useros J, Garcia-Foncillas J. The role of BRCA2 mutation status as diagnostic, predictive, and prognosis biomarker for pancreatic cancer. BioMed Res Int. 2016;2016:1869304. doi: 10.1155/2016/1869304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heeke AL, Pishvaian MJ, Lynce F, et al. Prevalence of homologous recombination-related gene mutations across multiple cancer types. JCO Precis Oncol. doi: 10.1200/PO.17.00286. 10.1200/PO.17.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park W, Wong W, Yu KH, et al. Homologous recombination deficiency (HRD): A biomarker for first-line (1L) platinum in advanced pancreatic ductal adenocarcinoma (PDAC) J Clin Oncol. 2019;37 (suppl; abstr 4132) [Google Scholar]
- 30.Pishvaian MJ, Blais EM, Brody JR, et al. Outcomes in patients with pancreatic adenocarcinoma with genetic mutations in DNA damage response pathways: Results from the Know Your Tumor Program. JCO Precis Oncol. doi: 10.1200/PO.19.00115. 10.1200/PO.19.00115. [DOI] [PubMed] [Google Scholar]
- 31.Chand S, O’Hayer K, Blanco FF, et al. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int J Biol Sci. 2016;12:273–282. doi: 10.7150/ijbs.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu ZI, Hellmann MD, Wolchok JD, et al. Acquired resistance to immunotherapy in MMR-D pancreatic cancer. J Immunother Cancer. 2018;6:127. doi: 10.1186/s40425-018-0448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. doi: 10.1001/jamaoncol.2019.1588. O’Reilly EM, Oh DY, Dhani N, et al: Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol [epub ahead of print on July 18, 2019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedlander M, Meniawy T, Markman B, et al. Pamiparib in combination with tislelizumab in patients with advanced solid tumours: Results from the dose-escalation stage of a multicentre, open-label, phase 1a/b trial. Lancet Oncol. 2019;20:1306–1315. doi: 10.1016/S1470-2045(19)30396-1. [DOI] [PubMed] [Google Scholar]
- 35.Gong J, Hendifar A, Tuli R, et al. Combination systemic therapies with immune checkpoint inhibitors in pancreatic cancer: Overcoming resistance to single-agent checkpoint blockade. Clin Transl Med. 2018;7:32. doi: 10.1186/s40169-018-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young K, Hughes DJ, Cunningham D, et al. Immunotherapy and pancreatic cancer: Unique challenges and potential opportunities. Ther Adv Med Oncol. 2018;10:1758835918816281. doi: 10.1177/1758835918816281. [DOI] [PMC free article] [PubMed] [Google Scholar]