Abstract
PURPOSE
Observational studies of dietary fat intake and breast cancer have reported inconsistent findings. This topic was addressed in additional analyses of the Women’s Health Initiative (WHI) Dietary Modification (DM) clinical trial that evaluated a low-fat dietary pattern influence on breast cancer incidence.
METHODS
In the WHI DM trial, 48,835 postmenopausal women, ages 50-79 years, with no prior breast cancer, and a dietary fat intake of ≥ 32% of energy were randomly assigned at 40 US centers to a usual diet comparison group (60%) or dietary intervention group (40%). The goals were to reduce fat intake to 20% of energy and increase vegetable, fruit, and grain intake. Breast cancers were confirmed after central medical record review and serial National Death Index linkages to enhance mortality findings.
RESULTS
During 8.5 years of dietary intervention, breast cancer incidence and deaths as a result of breast cancer were nonsignificantly lower in the intervention group, while deaths after breast cancer were statistically significantly lower both during intervention and through a 16.1-year (median) follow-up. Now, after a long-term, cumulative 19.6-year (median) follow-up, the significant reduction in deaths after breast cancer persists (359 [0.12%] v 652 [0.14%] deaths; hazard ratio [HR], 0.85; 95% CI, 0.74 to 0.96; P = .01), and a statistically significant reduction in deaths as a result of breast cancer (breast cancer followed by death attributed to the breast cancer) emerged (132 [0.037%, annualized risk] v 251 [0.047%] deaths, respectively; HR, 0.79; 95% CI, 0.64 to 0.97; P = .02).
CONCLUSION
Adoption of a low-fat dietary pattern associated with increased vegetable, fruit, and grain intake, demonstrably achievable by many, may reduce the risk of death as a result of breast cancer in postmenopausal women.
INTRODUCTION
In the Women’s Health Initiative (WHI) Dietary Modification (DM) trial, 48,835 postmenopausal women were randomly assigned to DM or usual diet comparison groups to test whether a low-fat dietary pattern reduces the incidence of breast cancer and colorectal cancer as separate coprimary end points. The DM program reduced fat intake; increased fruit, vegetable, and grain intake; and was associated with modest weight loss.1-3
At the protocol-specified end of dietary intervention, after 8.5 years (median) follow-up, breast cancer incidence was 8% lower in the intervention group, but the difference was not statistically significant (P = .09).1,4 At that time, the hazard ratio (HR) for dietary intervention influence on deaths as a result of breast cancer was 0.67 (95% CI, 0.43 to 1.06; P = .08).5 However, there was a statistically significant reduction in estrogen receptor (ER)–positive, progesterone receptor (PR)–negative, poor-prognosis breast cancers (HR, 0.64; 95% CI, 0.49 to 0.84) and a statistically significant reduction in deaths after breast cancer in the intervention group (P = .02).5 The significant reduction in deaths after breast cancer was subsequently maintained through cumulative follow-up (P = .01). However, the less common outcome of deaths as a result of breast cancer was nonsignificantly reduced in prior reports of the trial (n = 48,835).1,5
Now, outcome updates through nearly 20 years cumulative follow-up have identified 17% more deaths as a result of breast cancer compared with our most recent report.6 These new data, along with the previously reported trend toward fewer deaths as a result of breast cancer in the dietary intervention group, provided the impetus for the current analyses, which have the potential for more definitive information on the long-term influence of a low-fat dietary pattern on breast cancer mortality.
METHODS
Study Design
Details of the WHI DM trial have been published.1 Briefly, 48,835 postmenopausal women ages 50-79 years with no prior breast cancer, mammogram clearance, and dietary fat intake ≥ 32% of total energy intake by food frequency questionnaire (FFQ) were recruited from 1993 to 1998 at 40 US clinical centers. Participants were randomly allocated at the WHI clinical coordinating center and stratified by age-group and clinical center to a low-fat dietary pattern intervention group (19,541; 40%) or usual diet comparison group (29,294; 60%). Intervention goals included reduction in fat to 20% of calories and increases in vegetables and fruit to 5 servings/d and grains to 6 servings/d. Calorie restriction and weight loss were not intervention goals. Comparison group women received written health-related materials only. Institutional review board approval was obtained at each clinical center, and all participants provided written informed consent.
The frequency of protocol-mandated mammography in the 2 randomization groups was nearly identical through 8 years follow-up, with 92% at years 2 and 4 and 90% at year 6.1 Postintervention, self-reported mammography rates were also closely comparable between randomization groups with median annualized rates of 0.61 (quarter 1 [Q1]-Q3, 0.35-0.78) v 0.60 (Q1-Q3, 0.35-0.78) mammograms/year, respectively.
The dietary intervention program included 18 preplanned group nutritional/behavioral sessions and 1 individual session in the first year followed by quarterly group sessions until the dietary intervention ended after 8.5 years (median). Dietary intake was monitored using FFQs at 1 year and, approximately every 3 years thereafter, in a rotating subgroup. Postintervention follow-up required 2 written reconsents, as previously described.5 National Death Index (NDI) queries, which capture 98% of deaths,7 provided additional mortality information regardless of reconsent status.
Dietary changes associated with the low-fat eating pattern after 1 year included reduced energy from fat to 24.3% (standard deviation [SD], 7.5%) v 35.1% (SD, 6.9%) for the intervention versus comparison groups, respectively, and an increase in fruit, vegetable, and grain consumption, with body weight 3% lower in the intervention group (all P < .001).1,2 Dietary differences were maintained with statistical significance through 5 years of intervention5 and remained statistically significant but were attenuated in late intervention2 and postintervention.4 Women generally continued dietary group activities after breast cancer diagnosis.5 Baseline recreational physical activity did not differ significantly between randomization groups.5
Clinical Outcomes
Clinical outcomes included incident breast cancer, deaths as a result of breast cancer (breast cancer followed by death attributed to breast cancer), and deaths after breast cancer (breast cancer followed by death attributed to any cause), as ascertained for all 48,835 participants’ measurements from random assignment through September 30, 2016. Outcome ascertainment was at 6-month intervals throughout the intervention period, with subsequent updates annually. Breast cancers were confirmed after medical record review by centrally trained clinical center physician-adjudicators. Final adjudication was performed at the clinical coordinating center. All adjudicators were masked to random assignment. Human epidermal growth factor receptor 2 and ER and PR status were based on local laboratory determinations.
Breast cancer therapy was directed by the participants’ physicians. Information on initial breast cancer therapy, including surgery, radiation therapy, and adjuvant chemotherapy, were available from Medicare coding in a subset of participants (n = 1,403) and did not differ between randomization groups.8 Endocrine adjuvant therapy, on the basis of self-report (n = 673), also was balanced between randomization groups (71% v 68% of users in intervention and comparison groups, respectively). Cause of death was determined by medical record or death certificate review at the clinical coordinating center; by NDI findings; and, in some cases, by a relative’s report.
Statistical Analysis
The protocol-defined coprimary end points were incident invasive breast cancer and colorectal cancer, to be analyzed separately. The current analyses were not protocol specified. However, because subsequent funding, beyond that identified in the original protocol, provided support for long-term follow-up, including serial NDI queries, we can now report long-term findings on breast cancer incidence and mortality for the intervention versus comparison groups on the basis of analyses that include all 48,835 randomly assigned participants. All analyses use intention-to-treat and time-to-event methods. Annualized rates for breast cancer events during the dietary intervention period and cumulatively throughout follow-up were assessed by randomization group by dividing the number of events by the corresponding person-time in each period. HRs contrasting the intervention and comparison groups are estimated using Cox regression with baseline rates stratified on age-group in 5-year categories, randomization status and assignment in the WHI hormone therapy trials, hysterectomy status, race/ethnicity (white, black, other), and study phase (intervention and postintervention periods; time dependent). Cumulative results represent findings over the total follow-up periods described earlier. HRs contrasting the intervention and comparison groups by cause of death were estimated using Cox regression models that included cause-specific baseline hazard functions and HRs.9
The Kaplan-Meier method was used for graphical display of death rates. The proportional hazards assumption was assessed graphically. All statistical tests were 2-sided, and nominal P values of ≤ .05 were regarded as significant. Reported P values do not adjust for multiple outcomes or sequential monitoring, which may inflate type I error rates; therefore, results should be interpreted cautiously. In further analyses, we fitted multivariable marginal Cox regression models,10,11 which provide identical HR estimates to the usual estimates presented but allow for a multivariable null hypothesis test of all 4 breast cancer outcomes that accounts for correlated multivariable outcomes, and we presented the evolution of pertinent test statistics for each additional year of follow-up.12,13 All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.4 (R Foundation for Statistical Computing, Vienna, Austria) software.
RESULTS
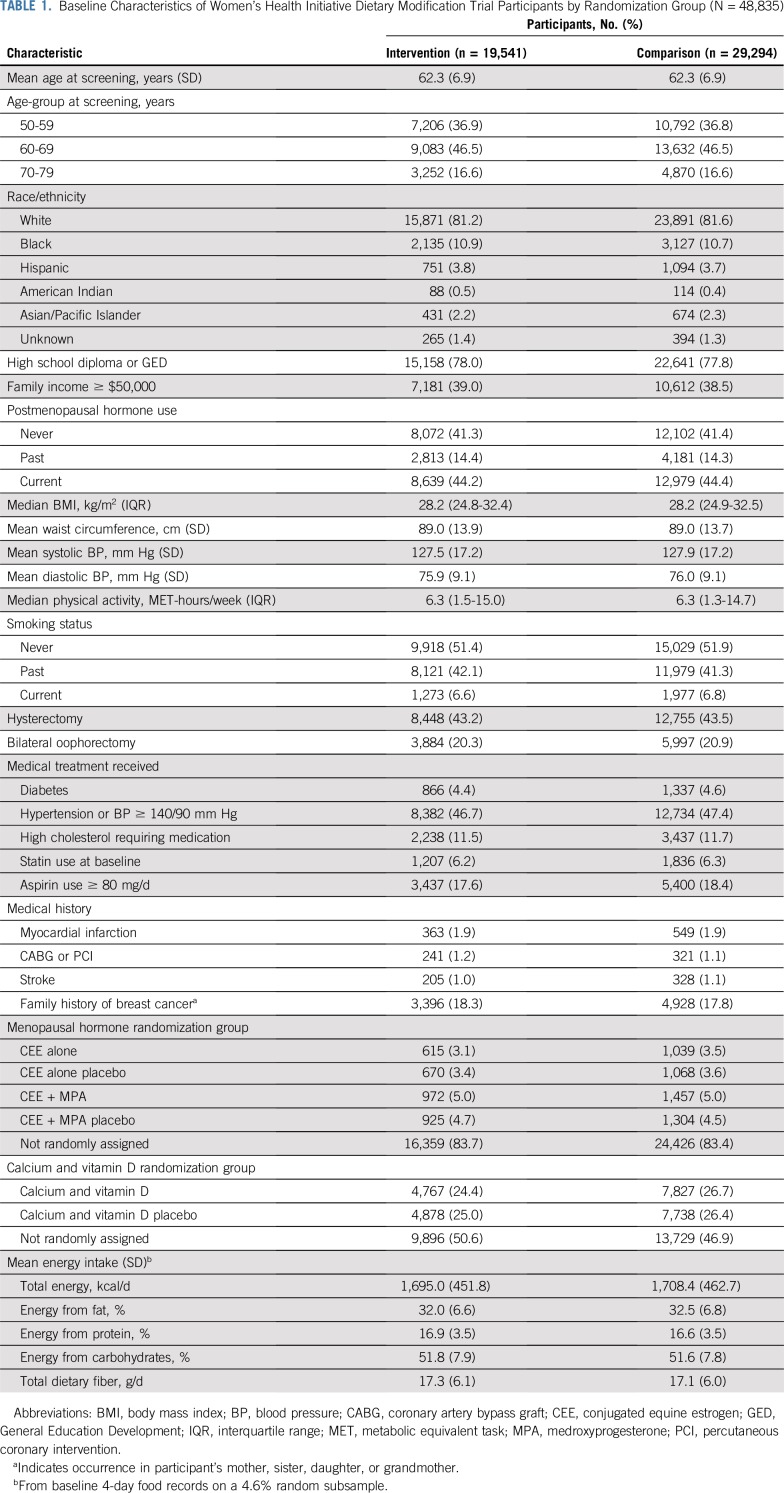
Baseline participant characteristics have been previously described.1 Breast cancer risk factors, demographics, recreational physical activity, socioeconomic status, medical history, and prerandomization dietary intake1 were closely comparable in the 2 randomization groups (Table 1).
TABLE 1.
Baseline Characteristics of Women’s Health Initiative Dietary Modification Trial Participants by Randomization Group (N = 48,835)
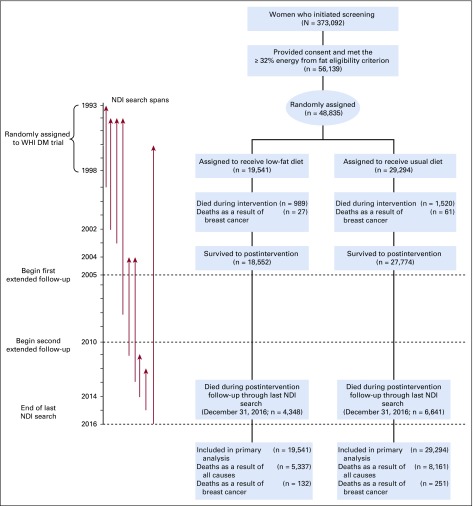
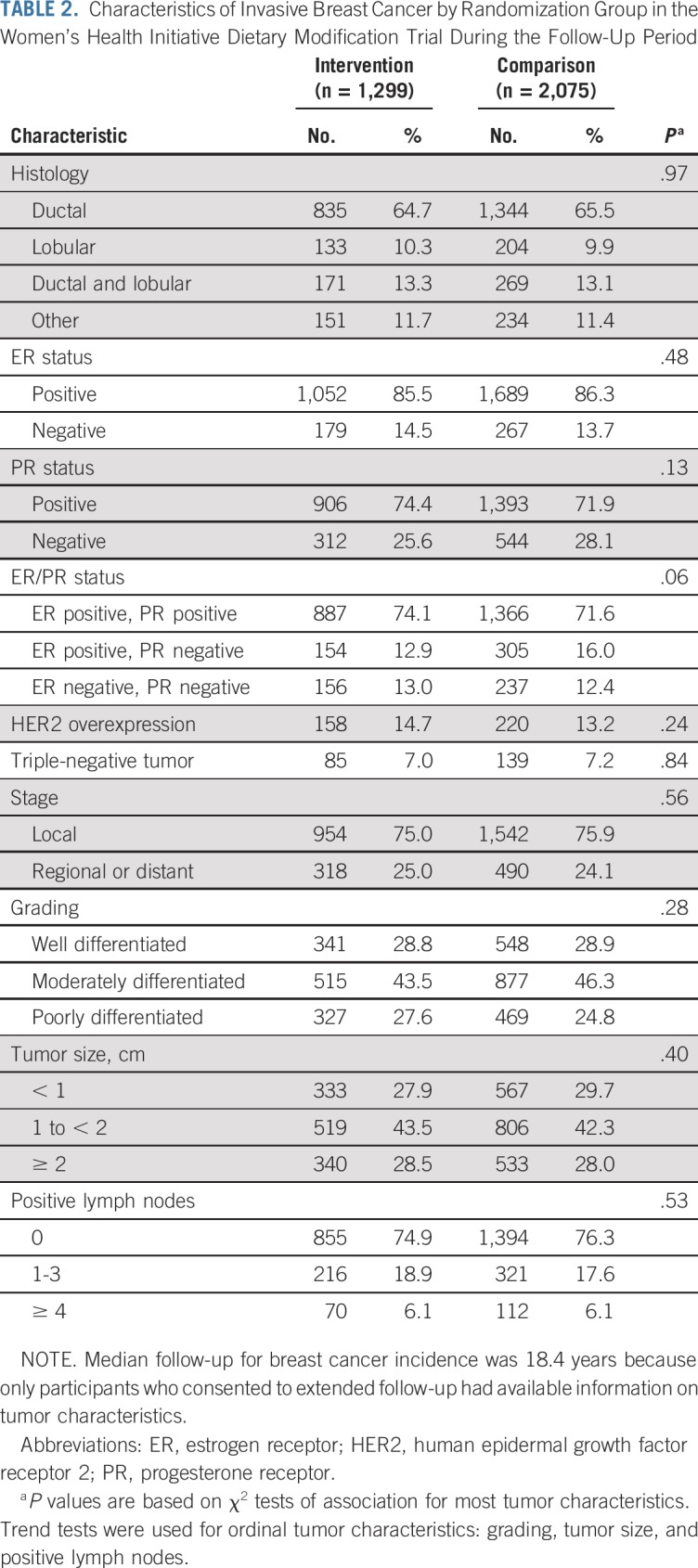
Participant flow during the dietary intervention period and throughout cumulative follow-up is outlined in Figure 1. After cumulative follow-up of 19.6 years, with 3,374 total breast cancers, the HR for breast cancer incidence was 0.95 (95% CI, 0.89 to 1.02). Breast cancer characteristics by randomization group are listed in Table 2. The incidence of ER-positive, PR-negative breast cancers was significantly reduced in the dietary intervention group during the intervention period (HR, 0.69; 95% CI, 0.54 to 0.90) and through cumulative follow-up (HR, 0.77; 95% CI, 0.64 to 0.94).
FIG 1.
Participant flow diagram for the Women’s Health Initiative (WHI) Dietary Modification (DM) trial of a low-fat dietary pattern through extended follow-up. NDI, National Death Index.
TABLE 2.
Characteristics of Invasive Breast Cancer by Randomization Group in the Women’s Health Initiative Dietary Modification Trial During the Follow-Up Period
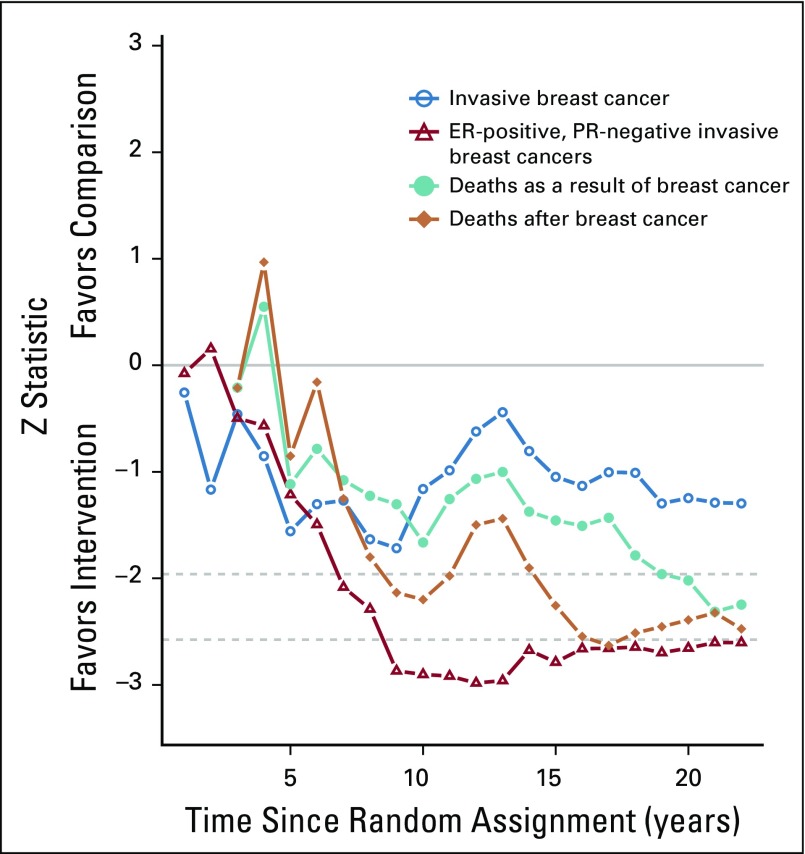
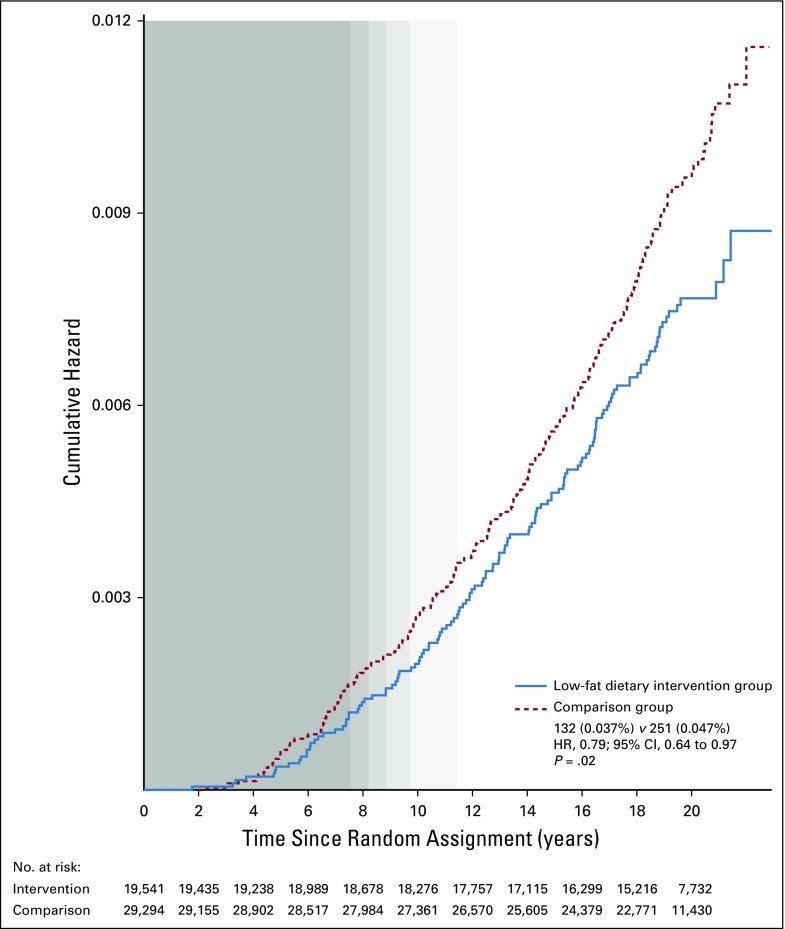
As previously reported, during the intervention period, there was a statistically significant reduction in deaths after breast cancer in the dietary intervention group, which has now persisted through 19.6 years cumulative follow-up (359 [0.12%] v 652 [0.14%], respectively; HR, 0.85; 95% CI, 0.74 to 0.96; P = .01). During the dietary intervention period, there were fewer deaths as a result of breast cancer in the dietary intervention group, but the finding was not statistically significant (27 [0.016%] v 61 deaths [0.024%], respectively; HR, 0.67; 95% CI, 0.43 to 1.06; P = .08).5 Subsequently, evidence for a reduction in deaths as a result of breast cancer has been growing for the past decade as seen in Z statistics for cumulative year-to-year HR estimates (Fig 2). Now, after 19.6 years cumulative follow-up, a statistically significant reduction in deaths as a result of breast cancer is evident (132 [0.037%] v 251 deaths [0.047%], respectively; HR, 0.79; 95% CI, 0.64 to 0.97; P = .02; Fig 3). A multivariable test for the overall null hypothesis of no influence of randomization group on invasive breast cancer; ER-positive, PR-negative breast cancer; death as a result of breast cancer; and death after breast cancer was significant for the intervention phase (P = .01) and over cumulative follow-up (P = .01). This 4-df test is based on the robust log-rank (score) test and accounts for correlation among the 4 event types.
FIG 2.
Z statistics that correspond to hazard ratio estimates, starting at random assignment through each additional year of cumulative follow-up. Dotted lines indicate nominal significance at the .05 and .01 levels. As seen, evidence for deaths as a result of breast cancer, a less common event, has incrementally strengthened over the past decade. ER, estrogen receptor; PR, progesterone receptor.
FIG 3.
Dietary modification influence on deaths as a result of breast cancer during cumulative follow-up. Kaplan-Meier cumulative hazard estimates for death as a result of breast cancer during the 19.6-year (median) cumulative follow-up among all 48,835 trial participants. Background shading shows the distribution for duration of the intervention phase (in quintiles); no shading indicates postintervention follow-up for all participants. Summary statistics are from a Cox proportional hazards regression model stratified by age-group, random assignment in the hormone therapy trials, hysterectomy status, ethnicity (white, black, other), and study period (time dependent). The P value corresponds to a 2-sided score (log-rank) test. HR, hazard ratio.
The HR for deaths as a result of breast cancer was unchanged by analyses incorporating baseline weight (HR, 0.78; 95% CI, 0.63 to 0.97) or time-dependent weight change (HR, 0.78; 95% CI, 0.63 to 0.96). A negligible HR change was observed when physical activity was added as a time-dependent variable or after adjustment for mammogram screening rates. Analyses that added a time-dependent variable for PR status attenuated the HR for death as a result of breast cancer (HR, 0.83; 95% CI, 0.67 to 1.03; P = .09), and this inclusion provided an explanation for 21% of the benefit observed in the unadjusted analyses.
By definition, in all 383 deaths as a result of breast cancer, death was directly attributed to the breast cancer. In comparison, the 1,011 deaths after breast cancer include other mortality outcomes. There, breast cancer was the most common cause of death (33%; HR, 0.78; 95% CI, 0.62 to 0.98) followed by deaths as a result of other cancers (188 deaths; HR, 0.95; 95% CI, 0.71 to 1.28), coronary heart disease (CHD; 81 deaths; HR, 0.56; 95% CI, 0.34 to 0.92), cardiovascular disease other than CHD (124 deaths; HR, 0.92; 95% CI, 0.64 to 1.32), and dementia (76 deaths; HR, 0.70; 95% CI, 0.43 to 1.14). There have been 13,498 deaths among the 48,835 randomly assigned participants. Besides the 383 deaths attributable to breast cancer, there were 3,605 deaths attributable to other cancers (HR, 0.97; 95% CI, 0.91 to 1.04), 4,276 to cardiovascular disease (HR, 0.98; 95% CI, 0.92 to 1.04), and 5,234 deaths to other causes (HR, 1.00; 95% CI, 0.95 to 1.06), accounting for all 13,498 deaths. There was no significant dietary intervention influence on overall mortality (HR, 0.98; 95% CI, 0.95 to 1.01).
DISCUSSION
With long-term follow-up of the WHI DM randomized clinical trial, implementation of a low-fat dietary pattern that included increased vegetable, fruit, and grain consumption and was associated with modest weight loss significantly reduced the risk of death as a result of breast cancer. The finding is based on intention-to-treat comparisons in a secondary analysis between randomization groups and includes all 48,835 participants, which provides evidence that a dietary change may favorably influence a postmenopausal woman’s risk of dying as a result of breast cancer. Although not protocol specified, death as a result of breast cancer is arguably the most clinically relevant breast cancer outcome.
In addition to a statistically significant reduction in death as a result of breast cancer among dietary intervention group participants, a statistically significant reduction in deaths after breast cancer has been consistently seen, even though breast cancer incidence was not significantly reduced. However, there was a substantial and sustained reduction in ER-positive, PR-negative breast cancers, which carry a poor prognosis.14,15 One HR estimate of PR influence on breast cancer–specific mortality was 3.24,14 similar in magnitude to triple-negative breast cancer.16 Thus, the reduction in ER-positive, PR-negative cancers presumably contributed to the favorable findings with regard to deaths as a result of breast cancer. Another potential contributing factor is the previously reported favorable dietary influence on survival after breast cancer diagnosis.8 Of 1,764 women diagnosed with breast cancer during the dietary intervention, postdiagnosis 10-year breast cancer overall survival was significantly greater in the dietary intervention group (82% v 78%; P = .01).8
In addition, the WHI dietary intervention has demonstrated effects of reducing metabolic syndrome risk,17 lowering the need for cholesterol-targeted and hypertensive medicine,18 reducing diabetes progression,19 and reducing estradiol levels,1 all factors that can influence breast cancer progression and mortality20 as well as mortality as a result of other conditions.3,21 Taken together, these findings provide a biologic framework for the dietary intervention to significantly reduce deaths as a result of breast cancer even while not significantly reducing overall breast cancer incidence.
ASCO guidelines since 199922 have supported the use of tamoxifen and later, raloxifene and aromatase inhibition, for breast cancer risk reduction.23-25 However, none of these interventions have been demonstrated to reduce deaths as a result of breast cancer, including tamoxifen trials with the longest follow-up.23,26 These findings are suggestive of tamoxifen influencing mainly favorable-prognosis cancers.27 In contrast, in the WHI DM trial, random assignment to a low-fat dietary pattern resulted in a nominally significant reduction in relatively unfavorable ER-positive, PR-negative cancers and in deaths as a result of breast cancer.
Clinical trials with breast cancer as an end point in generally healthy women without breast cancer cannot be easily compared with findings from breast cancer adjuvant clinical trials. For example, in a meta-analysis of randomized adjuvant trials evaluating tamoxifen in women with early-stage breast cancer, 71% of 3,811 total deaths were a result of breast cancer.28 There, a significant increase in overall survival would be a reasonable expectation because tamoxifen targets the predominant cause of death in the study. In contrast, in the current WHI DM trial involving generally healthy postmenopausal women without breast cancer, while there have been 13,498 total deaths, only 3% were as a result of breast cancer. Considering deaths as a result of all causes, there was not a significant dietary intervention influence on overall mortality (HR, 0.98; 95% CI, 0.43 to 1.01).
The reduction in deaths as a result of breast cancer in the WHI dietary intervention group demonstrates that the reduction in deaths after breast cancer cannot be solely attributed to improvement of cardiovascular disease mortality.29 The WHI DM intervention influence on breast cancer mortality seems to be durable, with favorable influence long after the end of the dietary intervention. Because postintervention information on dietary habits is limited,4 we are unable to determine whether the breast cancer mortality reduction is attributable to continued dietary adherence or solely to dietary changes during the 8.5-year intervention period.
Observational studies of dietary fat and breast cancer have provided inconsistent results,30-33 which likely reflect study limitations, including measurement error associated with self-reported diet and limited dietary variation within study populations. In addition, most reports include a single dietary assessment and are not comparable to a randomized trial with a multiyear nutritional/behavioral intervention. To our review, only 1 other full-scale randomized trial has evaluated a low-fat dietary pattern for breast cancer prevention. In a Canadian trial of 4,690 women with high breast density, many of whom were premenopausal, no effect on breast cancer incidence was seen with 118 incident cases.34
The WHI DM trial low-fat dietary pattern, as implemented in 19,541 postmenopausal women, represents DM. Dietary fat intake was modestly but significantly decreased to 24.7% of energy at 1 year, not far from the current US average intake of postmenopausal women of 33%. In addition, there was some increase in fruit, vegetable, and grain intake with only modest weight loss. Such a program should be achievable by many.5 The WHI DM trial low-fat eating pattern is somewhat similar to the Dietary Approach to Stop Hypertension (DASH) diet identified as an effective strategy to prevent cardiovascular disease.35 Both the DASH diet and the WHI low-fat dietary pattern call for dietary moderation and include increases in fruits, vegetables, and grains, with an additional major focus on total dietary fat intake in the WHI program.
In absolute terms, reductions in deaths as a result of breast cancer in the dietary intervention group were modest. For every 10,000 person-years of women following a low-fat dietary pattern, there would be 1 fewer death as a result of breast cancer and 2 fewer deaths after breast cancer. Because there are > 50 million postmenopausal women in the United States,36 on a population level, such a program could have an appreciable impact if current HR estimates are projected. Alternatively, if the intervention group experienced death rates comparable to the comparison group, there would be an additional 107 deaths in the intervention group, with 35 attributed to breast cancer.
Study strengths include the randomized controlled design with breast cancer incidence as a primary outcome, the enrollment of a large cohort of 48,835 postmenopausal women with ethnic diversity (18.6% minority), closely comparable mammography frequency in randomization groups throughout, dietary adherence supported by body weight and biomarker differences, medical record confirmation of breast cancers, long-term follow-up, and serial NDI linkage to enhance mortality findings. Study limitations include those associated with post hoc analyses and lack of comprehensive breast cancer therapy information. Specifically, reported P values do not adjust for multiple outcomes or sequential monitoring. With regard to multiple outcomes, while breast cancer incidence, rather than breast cancer mortality, is a protocol-specified coprimary outcome, this limitation is partially offset by a nominally significant reduction in ER-positive, PR-negative breast cancer incidence in the intervention group, both during the intervention period and cumulatively. In addition, sequential analyses are expected to lead to some inflation of type I error for comparisons between randomization groups. This limitation is partially offset by test statistic calculations for year-to-year follow-up (Fig 2), with results trending toward greater evidence for breast cancer mortality reduction for some years, with findings not likely reflecting a random high.12,13 However, because these multiple testing limitations cannot be fully addressed in a post hoc fashion, our summary of results is that a low-fat dietary intervention may, rather than does, reduce breast cancer mortality.
In conclusion, adoption of a low-fat dietary pattern that included increased vegetable, fruit, and grain consumption is demonstrably achievable by many postmenopausal women. Such a dietary pattern may reduce the risk of death as a result of breast cancer in postmenopausal women.
ACKNOWLEDGMENT
We thank the WHI investigators, staff, and the trial participants for their outstanding dedication and commitment.
The WHI investigators:
Program office (National Heart, Lung, and Blood Institute, Bethesda, MD): Jacques Roscoe, Shari Ludlum, Dale Burden, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical coordinating center (Fred Hutchinson Cancer Research Center, Seattle, WA): Garnet L. Anderson, Ross L. Prentice, Andrea LaCroix, and Charles Kopperberg
Investigators and academic centers: Brigham and Women’s Hospital, Harvard Medical School, Boston, MA: JoAnn E. Manson; MedStar Health Research Institute/Howard University, Washington, DC: Barbara V. Howard; Stanford Prevention Research Center, Stanford, CA: Marcia L. Stefanick; The Ohio State University, Columbus, OH: Rebecca Jackson; University of Arizona, Tucson/Phoenix, AZ: Cynthia A. Thomson; University at Buffalo, Buffalo, NY: Jean Wactawski-Wende; University of Florida, Gainesville/Jacksonville, FL: Marian Limacher; University of Iowa, Iowa City/Davenport, IA: Robert Wallace; University of Pittsburgh, Pittsburgh, PA: Lewis Kuller; Los Angeles BioMedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA: Rowan T. Chlebowski; and Wake Forest University School of Medicine, Winston-Salem, NC: Sally Shumaker
A full list of all the investigators who have contributed to WHI science appears at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
PRIOR PRESENTATION
Presented during the 2019 American Society of Clinical Oncology Annual Meeting Presscast, May 15, 2019, and at the 2019 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Supported by the National Heart, Lung, and Blood Institute through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221, with additional funding from the National Cancer Institute. The WHI Project Office at the National Heart, Lung, and Blood Institute had no role in the preparation of this report.
Written on behalf of the Women’s Health Initiative.
See accompanying Editorial on page 1375
AUTHOR CONTRIBUTIONS
Conception and design: Rowan T. Chlebowski, Aaron K. Aragaki, Garnet L. Anderson, JoAnn E. Manson, Karen C. Johnson, Jean Wactawski-Wende, Ana Barac, Ross L. Prentice
Financial support: Rowan T. Chlebowski, Jean Wactawski-Wende
Administrative support: Rowan T. Chlebowski, Garnet L. Anderson, Cynthia A. Thomson, Karen C. Johnson, Jean Wactawski-Wende, Linda Snetselaar
Provision of study material or patients: Marian L. Neuhouser, JoAnn E. Manson, Dorothy S. Lane, Karen C. Johnson, Jean Wactawski-Wende
Collection and assembly of data: Rowan T. Chlebowski, Aaron K. Aragaki, Marian L. Neuhouser, JoAnn E. Manson, Cynthia A. Thomson, Dorothy S. Lane, Karen C. Johnson, Jean Wactawski-Wende, Ross L. Prentice
Data analysis and interpretation: Rowan T. Chlebowski, Aaron K. Aragaki, Garnet L. Anderson, Kathy Pan, JoAnn E. Manson, Yasmin Mossavar-Rahmani, Linda Snetselaar, Thomas E. Rohan, Juhua Luo, Ana Barac, Ross L. Prentice
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Rowan T. Chlebowski
Consulting or Advisory Role: Novartis, Pfizer, Genentech, Amgen, AstraZeneca, Immunomedics
Speakers’ Bureau: Novartis, AstraZeneca
Garnet L. Anderson
Research Funding: Mars Symbioscience (Inst)
Yasmin Mossavar-Rahmani
Stock and Other Ownership Interests: Johnson & Johnson, CVS
Thomas E. Rohan
Patents, Royalties, Other Intellectual Property: Patents
Ana Barac
Honoraria: Bristol-Myers Squibb
Research Funding: Genentech (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Bayer AG
No other potential conflicts of interest were reported.
REFERENCES
- 1.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women’s Health Initiative randomized controlled dietary modification trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 2.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: The Women’s Health Initiative Dietary Modification trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Prentice RL, Aragaki AK, Howard BV, et al. Low-fat dietary pattern among postmenopausal women influences long-term cancer, cardiovascular disease, and diabetes outcomes. J Nutr. 2019;149:1565–1574. doi: 10.1093/jn/nxz107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson CA, Van Horn L, Caan BJ, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative Dietary Modification trial. Cancer Epidemiol Biomarkers Prev. 2014;23:2924–2935. doi: 10.1158/1055-9965.EPI-14-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Aragaki AK, Anderson GL, et al. Low-fat dietary pattern and breast cancer mortality in the Women’s Health Initiative randomized controlled trial. J Clin Oncol. 2017;35:2919–2926. doi: 10.1200/JCO.2016.72.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chlebowski RT, Anderson GL, Manson JE, et al: Low-fat dietary pattern and cancer mortality in the Women’s Health Initiative (WHI) randomized controlled trial. JNCI Cancer Spectr 2:pky065, 2019. [DOI] [PMC free article] [PubMed]
- 7.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Aragaki AK, Anderson GL, et al. Association of low-fat dietary pattern with breast cancer overall survival: A secondary analysis of the Women’s Health Initiative randomized clinical trial. JAMA Oncol. 2018;4:e181212. doi: 10.1001/jamaoncol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 10.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 11.Lin DY. Cox regression analysis of multivariate failure time data: The marginal approach. Stat Med. 1994;13:2233–2247. doi: 10.1002/sim.4780132105. [DOI] [PubMed] [Google Scholar]
- 12.Pocock S, White I. Trials stopped early: Too good to be true? Lancet. 1999;353:943–944. doi: 10.1016/S0140-6736(98)00379-1. [DOI] [PubMed] [Google Scholar]
- 13.Montori VM, Devereaux PJ, Adhikari NK, et al. Randomized trials stopped early for benefit: A systematic review. JAMA. 2005;294:2203–2209. doi: 10.1001/jama.294.17.2203. [DOI] [PubMed] [Google Scholar]
- 14.Purdie CA, Quinlan P, Jordan LB, et al. Progesterone receptor expression is an independent prognostic variable in early breast cancer: A population-based study. Br J Cancer. 2014;110:565–572. doi: 10.1038/bjc.2013.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang JL, Kizy S, Marmor S, et al. Tumor grade and progesterone receptor status predict 21-gene recurrence score in early stage invasive breast carcinoma. Breast Cancer Res Treat. 2018;172:671–677. doi: 10.1007/s10549-018-4955-z. [DOI] [PubMed] [Google Scholar]
- 16.Bae SY, Kim S, Lee JH, et al. Poor prognosis of single hormone receptor-positive breast cancer: Similar outcome as triple-negative breast cancer. BMC Cancer. 2015;15:138. doi: 10.1186/s12885-015-1121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuhouser ML, Howard B, Lu J, et al. A low-fat dietary pattern and risk of metabolic syndrome in postmenopausal women: The Women’s Health Initiative. Metabolism. 2012;61:1572–1581. doi: 10.1016/j.metabol.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allison MA, Aragaki AK, Ray RM, et al. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: Tertiary analysis of the WHI Dietary Modification trial. Am J Hypertens. 2016;29:959–968. doi: 10.1093/ajh/hpv196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard BV, Aragaki AK, Tinker LF, et al. A low-fat dietary pattern and diabetes: A secondary analysis from the Women’s Health Initiative Dietary Modification trial. Diabetes Care. 2018;41:680–687. doi: 10.2337/dc17-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–165. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 21.Prentice RL, Aragaki AK, Van Horn L, et al. Low-fat dietary pattern and cardiovascular disease: Results from the Women’s Health Initiative randomized controlled trial. Am J Clin Nutr. 2017;106:35–43. doi: 10.3945/ajcn.117.153270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chlebowski RT, Collyar DE, Somerfield MR, et al. American Society of Clinical Oncology technology assessment on breast cancer risk reduction strategies: Tamoxifen and raloxifene. J Clin Oncol. 1999;17:1939–1955. doi: 10.1200/JCO.1999.17.6.1939. [DOI] [PubMed] [Google Scholar]
- 23.Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152–3165. doi: 10.1200/JCO.19.01472. [DOI] [PubMed] [Google Scholar]
- 24.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235–3258. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Col N, Winer EP, et al. American Society of Clinical Oncology technology assessment of pharmacologic interventions for breast cancer risk reduction including tamoxifen, raloxifene, and aromatase inhibition. J Clin Oncol. 2002;20:3328–3343. doi: 10.1200/JCO.2002.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361:296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 27.Chlebowski RT. IBIS-I tamoxifen update: Maturity brings questions. Lancet Oncol. 2015;16:7–9. doi: 10.1016/S1470-2045(14)71184-2. [DOI] [PubMed] [Google Scholar]
- 28.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ligibel JA: Is it time to give breast cancer patients a prescription for a low-fat diet? JNCI Cancer Spectr 2:pky066, 2019. [DOI] [PMC free article] [PubMed]
- 30.Kroenke CH, Caan BJ. Re: High- and low-fat dairy intake, recurrence, and mortality after breast cancer diagnosis. Response. J Natl Cancer Inst. 2013;105:1761–1762. doi: 10.1093/jnci/djt284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chlebowski RT. Nutrition and physical activity influence on breast cancer incidence and outcome. Breast. 2013;22:S30–S37. doi: 10.1016/j.breast.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Boeke CE, Eliassen AH, Chen WY, et al. Dietary fat intake in relation to lethal breast cancer in two large prospective cohort studies. Breast Cancer Res Treat. 2014;146:383–392. doi: 10.1007/s10549-014-3005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan SF, Woodside JV, Lunny PM, et al. Dietary fat and breast cancer mortality: A systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2017;57:1999–2008. doi: 10.1080/10408398.2012.724481. [DOI] [PubMed] [Google Scholar]
- 34.Martin LJ, Li Q, Melnichouk O, et al. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011;71:123–133. doi: 10.1158/0008-5472.CAN-10-1436. [DOI] [PubMed] [Google Scholar]
- 35.Siervo M, Lara J, Chowdhury S, et al. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. doi: 10.1017/S0007114514003341. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention: Women’s Reproductive Health, 2017. http://www.cdc.gov/reproductivehealth/womensrh/index.htm.