PURPOSE
A powerful consequence of detecting cancer-associated pathogenic variants is the ability to test at-risk relatives (ARRs), termed cascade testing. However, historical studies suggest cascade testing uptake of 30% or less. Here, we tested the feasibility of a novel, streamlined method of cascade testing using telephone genetic counseling and mailed saliva-based genetic testing.
PATIENTS AND METHODS
Probands with newly diagnosed cancer-associated pathogenic variants were offered facilitated cascade testing whereby the genetics team identified and contacted ARRs by telephone to disclose the familial pathogenic variant and offer telephone counseling and mailed saliva testing. Results and guideline-based recommendations were reviewed by telephone and shared with the primary care physician.
RESULTS
Thirty probands were enrolled, and 114 ARRs were identified. Twelve ARRs were excluded (lived outside of the United States, n = 5; proband did not approve of contact, n = 7). Among 102 ARRs telephoned, contact was established with 95 (93%). Among 114 identified ARRs, 66 (58%) completed genetic testing. Among those completing testing, 27 (41%) carried the familial pathogenic variant. Surveys of ARRs at the time of genetic testing and 6 months later demonstrated low levels of anxiety, depression, distress, and uncertainty and high levels of satisfaction with testing. At 6 months, 7 ARRs with pathogenic variants had undergone cancer surveillance interventions and 4 had undergone cancer risk-reducing surgery.
CONCLUSION
Facilitated cascade testing with telephone genetic counseling and mailed saliva kits resulted in high testing uptake among ARRs. Positive genetic testing resulted in utilization of genetically targeted primary disease prevention at short-term follow-up. Facilitated cascade testing is a straightforward, low-cost, easily implemented strategy with significant potential to promote early detection for affected ARRs and reduce cancer mortality and should be evaluated in larger scale clinical trials.
INTRODUCTION
For newly diagnosed patients with cancer, identifying a causative germline pathogenic variant can offer therapeutic and prognostic information. However, the ultimate impact of genetic testing is the ability to identify relatives carrying the pathogenic variant before they develop cancer, maximizing disease prevention and early detection.1 The process of starting with the affected patient (proband) and extending genetic testing to at-risk relatives (ARRs) is termed cascade testing. ARRs found to carry the familial pathogenic variant can take advantage of genetically targeted primary disease prevention through intensive cancer surveillance and risk-reducing surgery, improving morbidity and preventing mortality.2-5
The majority of individuals with a familial cancer syndrome remain unaware of their predisposition. For example, it is estimated that 98% of individuals with Lynch syndrome have not yet been identified.6 The limited literature on oncologic cascade testing suggests that < 30% of ARRs use recommended genetic services.7-11 Traditionally, clinical geneticists have relied on family contact whereby the newly diagnosed patient contacts ARRs and encourages genetic testing. However, direct contact of ARRs by the medical team is faster and more reliable because it does not rely on the proband to mediate the process.12 Alternative care models allowing direct relative contact improve testing rates to 50%-60%; however, there is a paucity of data on oncologic cascade testing.13,14 Evans et al15 reported 56% uptake of genetic testing among first-degree relatives from BRCA1/2 families using proactive directly offered testing. Lessons from other genetic disease sites confirm the importance of direct contact by the medical team and demonstrate that cascade testing is most successful when convenience is maximized, for example, by offering testing in the relative’s home.16,17
Cascade testing has tremendous potential to improve individual and population health outcomes by increasing early awareness of cancer predisposition syndromes, but waiting for patients and families to self-refer or be referred by their clinician to a cancer geneticist is not sufficient on its own. An alternative approach that shifts the genetic testing paradigm allows providers to take on a more active approach in the identification and testing of individuals who are at risk. This pilot study evaluates a novel method of enhanced cascade testing using both facilitation and direct contact by the medical team and convenient testing in the ARR’s home. We hypothesize that this facilitated cascade pathway can improve uptake of genetic testing among relatives of patients with newly diagnosed cancer susceptibility syndromes.
PATIENTS AND METHODS
Patients
Patients (probands) were eligible for the facilitated cascade testing pathway if they were at least 18 years old and diagnosed with an autosomal dominant hereditary cancer syndrome within the preceding 12 months. Probands were treated at a single academic institution where genetic testing occurred following in-person genetic counseling. ARRs are relatives of the proband who are at risk for carrying the familial pathogenic variant. The proband worked with the genetics team to identify ARRs. ARRs were eligible for inclusion if the proband granted permission to contact them, they were ≥ 18 years of age, and they lived in the United States.
Trial Design
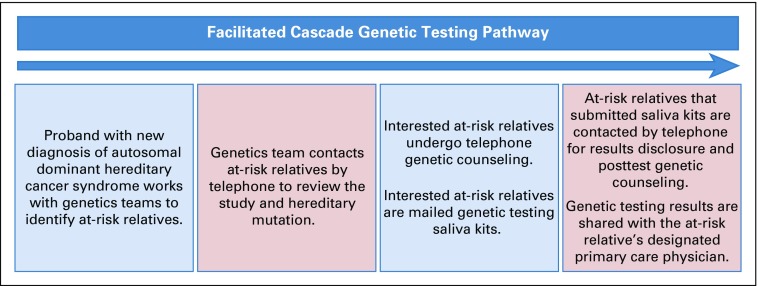
This prospective study was approved by the institutional review board. The facilitated cascade testing pathway bundled the following 6 separate components: (1) The genetics team facilitated identification of ARRs. The proband met with a genetics physician and genetics team navigator to review the pathogenic variant and family history through creation of a pedigree. The genetics team educated the proband on which family members were at risk for carrying the pathogenic variant. The proband consented to contact of the ARR and provided ARR contact information. (2) Direct telephone contact of ARRs was made by the genetics team. The proband could elect to contact the ARR first to inform him or her of the study or could give permission for the genetics team to directly contact the ARR. (3) Telephone genetic counseling was offered to ARRs. The genetics physician, using a script, described the study protocol and offered the ARR participation. ARRs interested in participating completed informed consent. ARRs were then offered telephone genetic counseling. (4) Mailed saliva kit genetic testing was provided to ARRs. After genetic counseling, ARRs interested in genetic testing were mailed saliva kit tests; single-site genetic testing for the pathogenic variant of interest was offered free of charge. (5) Telephone disclosure of the genetic testing results and posttest telephone genetic counseling were provided. For ARRs completing genetic testing, the genetics physician contacted the ARRs by telephone to disclose testing results. The ARR was also telephoned by a certified genetic counselor to undergo thorough posttest counseling. (6) The genetics team facilitated sharing of genetic results and clinical recommendations. The genetic testing results and guideline-based recommendations for genetically targeted primary disease prevention were sent to the ARR’s designated primary care physician with the ARR’s consent. The ARR was contacted 6 months later to evaluate utilization of targeted primary disease prevention (Fig 1).
FIG 1.
Facilitated cascade genetic testing pathway.
End Points
The primary outcome was feasibility of this facilitated cascade testing pathway defined by completion of genetic testing among ARRs. Secondary outcomes included uptake of genetic counseling; rationale for declining counseling and testing; testing results; uptake of cancer surveillance and risk-reducing surgery; and patient-reported satisfaction, anxiety, depression, distress, and uncertainty at the time of genetic testing and at the 6-month follow-up. The validated quality-of-life (QOL) survey instruments included the Hospital Anxiety and Depression Scale,18 Satisfaction With Decision Scale (SDS),19 and the Multidimensional Impact of Cancer Risk Assessment questionnaire.20
Statistical Analysis
The distribution of continuous variables was tested for normality via the Kolmogorov-Smirnov test. Univariable tests were applied on the basis of whether the variable of interest was distributed normally (ie, t test, analysis of variance) or not normally (ie, Mann-Whitney U test, Kruskal-Wallis test, Friedman test). Associations between categorical variables were evaluated using the χ2 test or Fisher’s exact test, as appropriate for category size. For assessment of QOL, validated surveys were scored per the survey protocol. For each patient, scores were compared between the initial surveys and 6-month follow-up surveys using the Wilcoxon signed rank test for paired data. All P values are 2-sided with statistical significance evaluated at the P = .05 level. All analyses were performed in SPSS Version 24 (IBM SPSS Statistics for Windows, Version 24.0; IBM, Armonk, NY).
RESULTS
Probands and ARRs
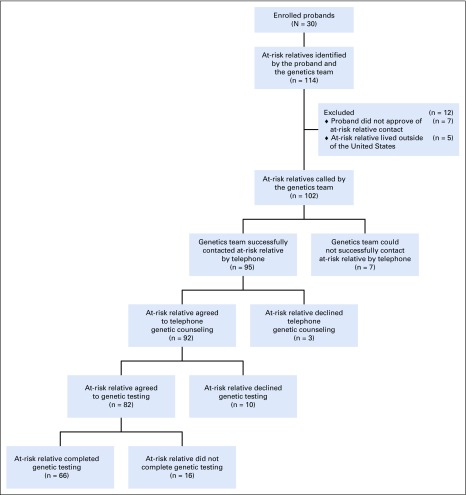
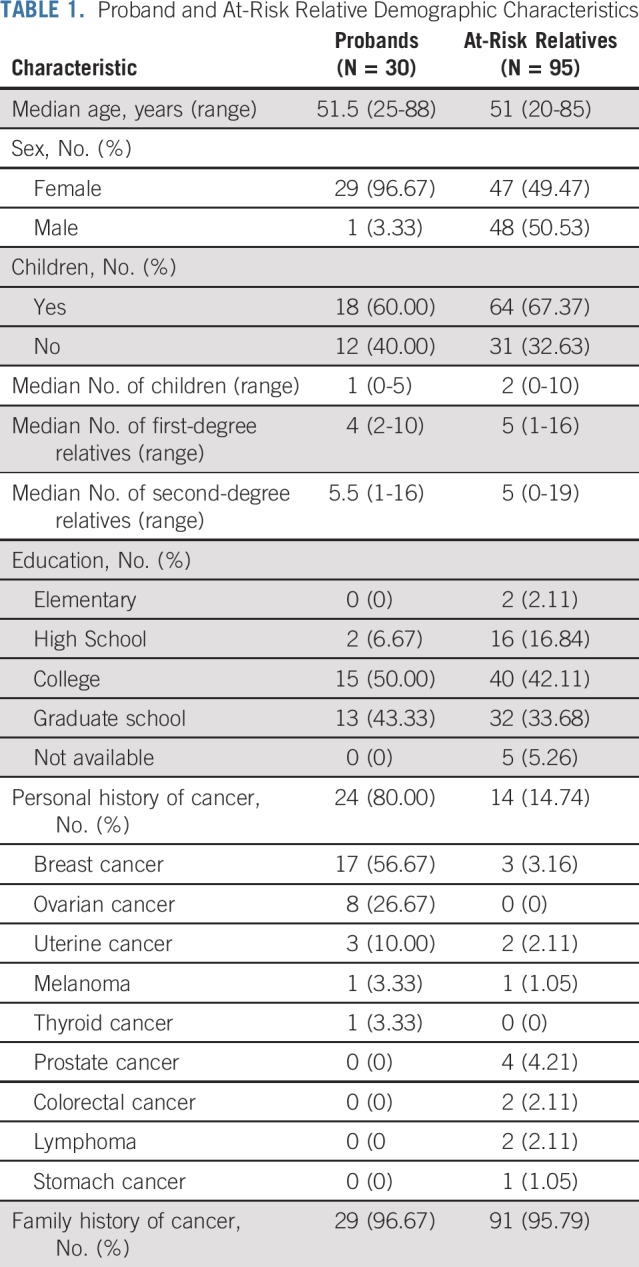
Thirty probands were enrolled in this feasibility trial. After meeting with the genetics team, probands identified 114 ARRs, with a median of 2 ARRs designated per proband (range, 0-18 ARRs). Five ARRs were ineligible because of residence outside of the United States. There were 7 ARRs for whom the genetics team recommended genetic counseling and testing but the proband did not consent to contact for the following reasons: decision not to disclose results to children younger than age 25 years (n = 2), concern that ARR was too ill from other medical issues (n = 1), strained family relationship (n = 1), or no reason provided (n = 3). The genetics team attempted to contact the remaining 102 ARRs by telephone. Three telephone attempts were made for each ARR; if these attempts were not successful, the ARR was deemed to be uncontacted. Telephone contact was established with 95 ARRs (83% of all designated ARRs; Appendix Fig A1, online only; Table 1).
TABLE 1.
Proband and At-Risk Relative Demographic Characteristics
Uptake of Genetic Testing
Among the 95 ARRs successfully contacted by the genetics team, 100% agreed to participate in the study. Among the 95 ARRs successfully contacted, 92 (97%) agreed to telephone genetic counseling regarding the familial pathogenic variant, 82 (86%) agreed to genetic testing, and 66 (70%) completed genetic testing. Among all 114 identified ARRs, 81% underwent telephone genetic counseling, 72% agreed to genetic testing, and 58% completed genetic testing. The rate of cascade testing uptake among ARRs was significantly higher than rates reported in the current literature (58% [95% CI, 49% to 67%] v 30%, respectively; P < .001).7-11
Rationale for Declining Genetics Services
Three ARRs agreed to participate but, after learning about the cancer predisposition syndrome identified in their relative and the available cascade testing pathway, declined telephone genetic counseling. Reasons for declining genetic counseling included the following: “I am concerned about genetic discrimination,” “I already have a BRCA2 diagnosis,” and “I am not interested.” Among the 92 ARRs who completed genetic counseling, 10 declined genetic testing. Reasons for declining testing after counseling included the following: already had undergone genetic testing (n = 2), concern about the cost of genetic testing (n = 2), concern about the effect of positive test results on insurance coverage (n = 2), no reason provided (n = 2), genetic testing saliva kit was submitted without an identifier (n = 1), and preferred to await mother’s genetic testing results (n = 1).
Predictors of Uptake of Genetic Testing
ARRs with living children were more likely to complete genetic testing compared with those without children (77% v 55%, respectively; P = .036). The median age of ARRs who completed genetic testing was significantly older than those who did not (57 years [range, 24-85 years] v 44 years [range, 20-78], respectively; P = .008). ARRs reporting a family history of cancer had a trend toward higher uptake of genetic testing than those without a reported family history (93% v 67%, respectively; P = .06). Sex, number of first- or second-degree relatives, education, and personal cancer history were not associated with uptake of genetic counseling or testing.
Results of Cascade Genetic Testing
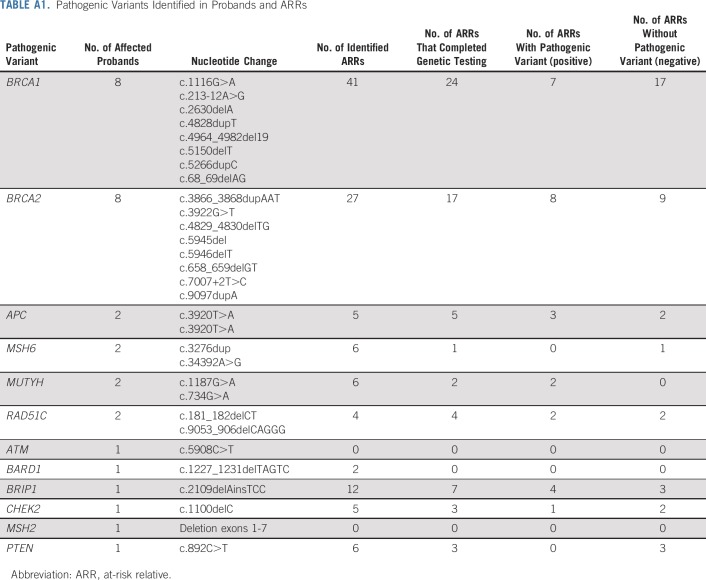
Among the 66 ARRs who completed genetic testing, 27 (41%) were found to carry the familial pathogenic variant. Thirty-eight ARRs had a negative testing result. One ARR submitted 2 saliva samples; however, neither sample had sufficient DNA content for testing. See Appendix Table A1 (online only) for a description of pathogenic variants identified in probands and ARRs.
Genetically Targeted Primary Disease Prevention
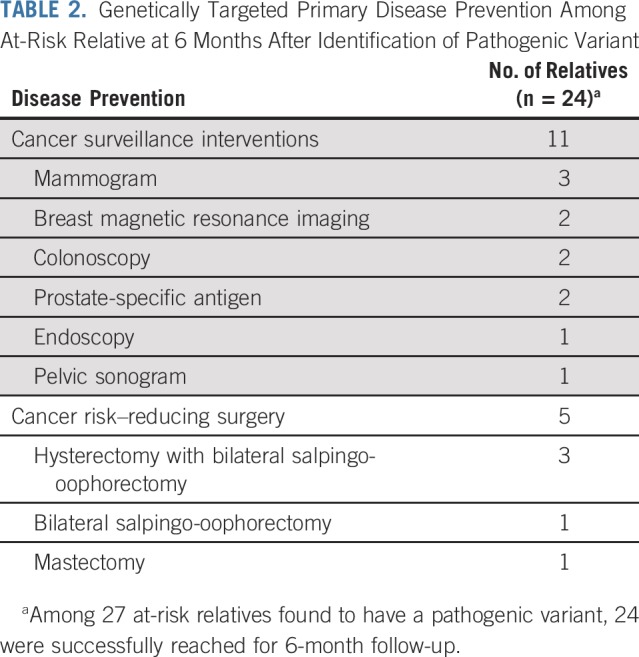
ARRs were contacted 6 months after disclosure of genetic testing results to evaluate downstream implications of cascade testing. Among 92 ARRs who agreed to genetic counseling, 86 (94%) were reached for follow-up. Four ARRs reported a total of 6 additional relatives in their family who underwent genetic testing, representing a cascade of the original cascade. Among 27 ARRs found to harbor pathogenic variants, 24 (89%) were successfully reached for 6-month follow-up. Seven of these ARRs had undergone a total of 11 cancer surveillance interventions as a result of the cascade testing. Four ARRs reported undergoing a total of 5 cancer risk–reducing surgeries as a result of the cascade testing (Table 2).
TABLE 2.
Genetically Targeted Primary Disease Prevention Among At-Risk Relative at 6 Months After Identification of Pathogenic Variant
QOL Measures
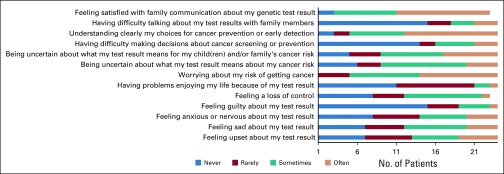
Among the 30 probands, 24 (80%) completed QOL surveys at time of cascade testing and at the 6-month follow-up. Probands reported the highest possible score for satisfaction with their decision to participate in cascade testing at the time of cascade testing and at the 6-month follow-up. Probands reported normal levels of anxiety and depression at time of cascade testing and 6-month follow-up (Table 3). Fifteen probands (63% of those completing the survey) reported being uncertain about what the test results mean for their own cancer risk and the cancer risk of their children or other family members. Five probands (21%) reported never or rarely understanding clearly their choices for cancer prevention and early detection. Six probands (25%) reported sometimes or often having difficulty talking about test results with family (Fig 2).
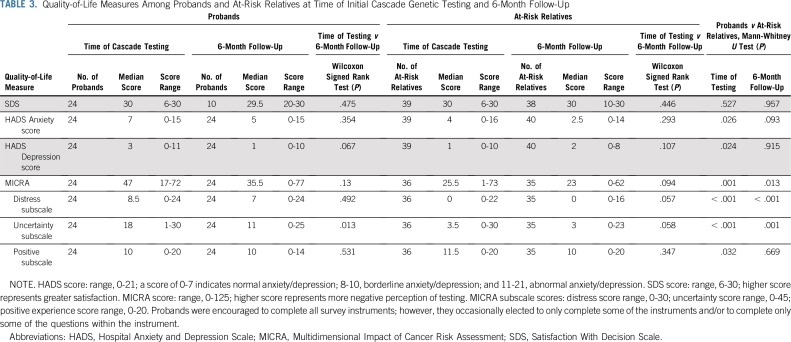
TABLE 3.
Quality-of-Life Measures Among Probands and At-Risk Relatives at Time of Initial Cascade Genetic Testing and 6-Month Follow-Up
FIG 2.
Results from selected responses to the Multidimensional Impact of Cancer Risk Assessment questionnaire among probands at time of cascade genetic testing (n = 24). Probands were encouraged to complete all questions within the survey instrument; however, they occasionally elected to only complete a portion of the survey.
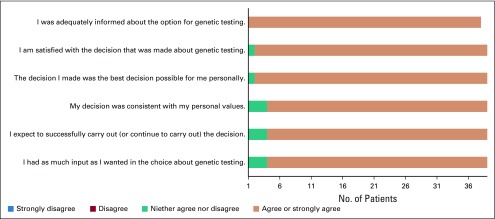
Among the 95 ARRs successfully contacted by telephone, 39 (41%) completed QOL surveys at the time of genetic counseling or results disclosure for those who underwent testing, and 40 (42%) completed QOL surveys at the 6-month follow-up. Similar to probands, ARRs reported the highest possible score for satisfaction with the decision to participate in cascade testing at both time points. ARRs demonstrated normal levels of anxiety and depression at the time of genetic counseling and at the 6-month follow-up. ARRs did not report a negative perception of the impact of their cancer risk assessment experience with low scores on the distress, uncertainty, and positive subscales (Table 3). On the basis of the SDS survey, 100% of responding ARRs reported that they were adequately informed about the option for genetic testing. Ninety-five percent of ARRs reported feeling satisfied with their decision regarding genetic testing (Fig 3).
FIG 3.
Results of the Satisfaction With Decision Scale among at-risk relatives at time of genetic counseling or testing (n = 39). At-risk relatives were encouraged to complete all questions within the survey instrument; however, they occasionally elected to only complete a portion of the survey.
There were significant differences in the QOL measures when comparing probands to ARRs. ARRs reported significantly lower levels of anxiety and depression at time of cascade testing compared with probands. Probands reported a more negative perception of their cancer risk assessment experience at the time of cascade testing and the 6-month follow-up. Probands also reported greater distress and uncertainty with their cancer risk assessment at the time of cascade testing and the 6-month follow-up (Table 3).
DISCUSSION
To fully realize the benefits of cancer genetic testing and genetically targeted disease prevention, these services must be extended to disease-free relatives with cascade testing.21 However, there are many barriers to cascade testing, including the burden on the proband to inform, shortage of adequately trained genetics specialists, and existing reimbursement and insurance policies, resulting in testing uptake rates of < 30% among ARRs.7-11 Prior studies demonstrate that cascade testing is most effective when relatives are contacted directly by the medical team and testing convenience is prioritized.15-17 In a large study of cascade testing for hereditary cancer syndromes, Caswell-Jin et al22 report that among 1,101 probands, 2,280 first-degree relatives were identified and 47.5% completed genetic testing through a low-cost online initiative. With the barriers and prior successful strategies in mind, we aimed to improve access to counseling and testing for ARRs by bundling multiple interventions into a facilitated cascade testing pathway. Among the 114 identified ARRs, 81% underwent telephone genetic counseling and 58% completed genetic testing. Among the 95 ARRs successfully contacted by telephone, 97% underwent genetic counseling and 70% completed genetic testing. Among the 66 ARRs who underwent genetic testing, 41% were found to have the familial pathogenic variant.
The most common reasons provided for declining genetic testing were concern about future access to insurance, genetic discrimination, and cost. Currently, many genetic testing laboratories offer free single-site genetic testing for relatives of an affected individual. For individuals at high risk who do not qualify for such family-based programs, testing is often covered by insurance or self-pay options for under $250. Despite our counseling that testing on this protocol was without charge, cost was cited as a reason for declining testing. This likely reflects a lingering misconception that genetic testing is largely uninsurable and unaffordable and emphasizes the importance of educating patients and physicians about updated testing costs and access to free single-site testing for some ARRs.
Twenty-four ARRs with positive genetic testing provided information on utilization of strategies for cancer prevention or early detection 6 months after results disclosure. Among these 24 ARRs, 7 (29%) underwent at least 1 cancer surveillance study and 4 (17%) underwent a cancer risk–reducing surgery. These results offer preliminary confirmation that ARRs act on and potentially benefit from the information gained through cascade testing. Follow-up was performed at a short interval after testing, and greater utilization of cancer preventative strategies may be revealed on future evaluation.
Although professional organizations and guidelines call for the implementation of cascade testing, debate remains about the conflict between the proband’s privacy and the rights of ARRs to be notified about genetic information.23 This study required proband consent for all ARR contact, and QOL measurements demonstrated no adverse sequelae. QOL measurements did reveal that individuals with newly diagnosed cancer-associated pathogenic variants have uncertainty about the implications of these results for themselves and their relatives and difficulty communicating test results with relatives. This was unexpected because all probands underwent in-person genetic counseling with a trained genetics provider before study enrollment. These results further emphasize potential advantages of direct contact of ARRs by the medical team. Patients with a new diagnosis of a cancer-associated pathogenic variant often are simultaneously coping with a new cancer diagnosis and may be further burdened by requests by providers to inform ARRs of the need for counseling. Our data suggest that even after formal genetic counseling, probands often do not fully understand the implications of or available cancer prevention strategies for the pathogenic variant, which may result in the sharing of inaccurate or incomplete information. Our findings support the rationale for a paradigm shift whereby the medical team takes on the effort to inform ARRs, thus addressing uncertainty among probands and ensuring communication of accurate information.
ARRs reported significantly lower levels of anxiety and depression and a more positive experience at the time of cascade testing compared with probands. This is not surprising given that most ARRs did not have a new cancer diagnosis and many had negative genetic testing results. It was reassuring that ARRs reported high levels of satisfaction with genetic testing. Recent literature supports both patient and provider satisfaction with telephone-based genetic counseling and suggests that telephone genetic counseling is associated with lower cost and similar clinical outcomes, including knowledge, decision conflict, and QOL.24,25 Currently, resources for and access to in-person genetic medicine consultation services are limited both for newly diagnosed patients with cancer and ARRs. Cascade genetic testing offers a favorable context for telegenetic services; DNA testing via mailed saliva kits provides access to genetic services for ARRs who may be geographically remote from their affected relative.
An important limitation of this study is that it was a small feasibility trial without a control arm. Future studies can evaluate the facilitated cascade pathway compared with standard of care or other strategies relying on ARR contact by the proband. ARRs enrolling in this study were offered testing only for the known familial pathogenic variant (single-site testing) and not extensive panel testing. Prior studies of cascade genetic testing demonstrate that up to 5% of first-degree relatives undergoing cascade genetic testing may have an unexpected pathogenic variant that the proband did not share.22 It is likely that this incremental yield reflects what would be detected in population-based screening; however, future studies can evaluate single-site versus expansive panel testing in the setting of familial cascade genetic assessment. We sought to evaluate the feasibility of the testing strategy and included probands with any autosomal dominant hereditary cancer syndrome. As a result, our population included pathogenic variants with varying levels of penetrance and clinical actionability, which may have contributed to differences in testing uptake among ARRs. This study did not address the cost associated with cascade testing. Although many genetic testing laboratories offer free testing for relatives of an affected individual, to demonstrate sustainability, future work is needed to evaluate the cost effectiveness associated with genetic counseling, sequencing, and cancer preventative services per additional quality-adjusted life-year. Finally, those ARRs who avoided contact or declined counseling or testing did not complete the QOL instruments, so our reported satisfaction may be overstated. Similarly, we cannot assess any perceptions of intrusion or violation of privacy, as the ARRs feeling this way did not provide follow-up data.
Most individuals in the United States carrying a cancer susceptibility gene remain unaware of their pathogenic variant and cancer risk. At least 50% of these individuals would be missed if genetics referrals relied on family history–based clinical guidelines alone.26 Cascade testing presents an opportunity to identify these at-risk individuals before a cancer diagnosis. As an example, Offit et al27 propose that a cascade of genetic testing initiated by testing at time of cancer diagnosis could identify all at-risk individuals in the United States in less than a decade. However, the success of any model will rely on an effective and feasible pathway for implementing cascade testing. We propose a strategy of facilitation by the medical team combined with convenient at-home counseling and testing for ARRs to significantly reduce the burden of cancer as a result of familial pathogenic variants.
APPENDIX
FIG A1.
CONSORT flow diagram.
TABLE A1.
Pathogenic Variants Identified in Probands and ARRs
Footnotes
This study has been presented at the Society of Gynecologic Oncology Annual Winter Meeting, Lake Tahoe, CA, January 17-19, 2019.
Supported by the Weill Cornell Medicine Clinical and Translational Science Center, the Matthew Bell Foundation, and Invitae.
See accompanying Editorial on page 1371
AUTHOR CONTRIBUTIONS
Conception and design: Melissa K. Frey, Francesca Tubito, Kevin Holcomb
Financial support: Paul Christos
Administrative support: Francesca Tubito
Provision of study materials or patients: Francesca Tubito
Collection and assembly of data: Melissa K. Frey, Ryan M. Kahn, Eloise Chapman-Davis, Maira Pires, Samantha Anderson, Bailey Jordan, Thomas A. Caputo
Data analysis and interpretation: Melissa K. Frey, Ryan M. Kahn, Paul Christos, Samantha Anderson, Semanti Mukherjee, Bailey Jordan, Stephanie V. Blank, Ravi N. Sharaf, Kenneth Offit, Kevin Holcomb, Steven Lipkin
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prospective Feasibility Trial of a Novel Strategy of Facilitated Cascade Genetic Testing Using Telephone Counseling
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Melissa K. Frey
Research Funding: Invitae (Inst)
Semanti Mukherjee
Stock and Other Ownership Interests: Regeneron
Stephanie V. Blank
Stock and Other Ownership Interests: Johnson & Johnson
Research Funding: Roche (Inst), Tesaro (Inst), Merck (Inst)
Uncompensated Relationships: AstraZeneca
Kevin Holcomb
Research Funding: Fujirebio Diagnostics
Expert Testimony: Johnson & Johnson
No other potential conflicts of interest were reported.
REFERENCES
- 1.Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800–3806. doi: 10.1200/JCO.2017.73.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on Gynecologic Practice ACOG Committee Opinion No. 727: Cascade testing: Testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:e31–e34. doi: 10.1097/AOG.0000000000002457. [DOI] [PubMed] [Google Scholar]
- 3.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology white paper. Gynecol Oncol. 2017;146:217–224. doi: 10.1016/j.ygyno.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Offit K. The future of clinical cancer genomics. Semin Oncol. 2016;43:615–622. doi: 10.1053/j.seminoncol.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547–1553. doi: 10.1200/JCO.2013.53.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: Do the ends justify the means? Cancer Prev Res (Phila) 2011;4:1–5. doi: 10.1158/1940-6207.CAPR-10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blandy C, Chabal F, Stoppa-Lyonnet D, et al. Testing participation in BRCA1/2-positive families: Initiator role of index cases. Genet Test. 2003;7:225–233. doi: 10.1089/109065703322537241. [DOI] [PubMed] [Google Scholar]
- 8.Fehniger J, Lin F, Beattie MS, et al. Family communication of BRCA1/2 results and family uptake of BRCA1/2 testing in a diverse population of BRCA1/2 carriers. J Genet Couns. 2013;22:603–612. doi: 10.1007/s10897-013-9592-4. [DOI] [PubMed] [Google Scholar]
- 9.Katapodi MC, Viassolo V, Caiata-Zufferey M, et al. Cancer predisposition cascade screening for hereditary breast/ovarian cancer and Lynch syndromes in Switzerland: Study protocol. JMIR Res Protoc. 2017;6:e184. doi: 10.2196/resprot.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthers GK, Armstrong J, McCormack J, et al. Letting the family know: Balancing ethics and effectiveness when notifying relatives about genetic testing for a familial disorder. J Med Genet. 2006;43:665–670. doi: 10.1136/jmg.2005.039172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: A systematic review. Clin Gastroenterol Hepatol. 2013;11:1093–1100. doi: 10.1016/j.cgh.2013.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Newson AJ, Humphries SE. Cascade testing in familial hypercholesterolaemia: How should family members be contacted? Eur J Hum Genet. 2005;13:401–408. doi: 10.1038/sj.ejhg.5201360. [DOI] [PubMed] [Google Scholar]
- 13.Sermijn E, Delesie L, Deschepper E, et al. The impact of an interventional counselling procedure in families with a BRCA1/2 gene mutation: Efficacy and safety. Fam Cancer. 2016;15:155–162. doi: 10.1007/s10689-015-9854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest LE, Burke J, Bacic S, et al. Increased genetic counseling support improves communication of genetic information in families. Genet Med. 2008;10:167–172. doi: 10.1097/GIM.0b013e318164540b. [DOI] [PubMed] [Google Scholar]
- 15.Evans DG, Binchy A, Shenton A, et al. Comparison of proactive and usual approaches to offering predictive testing for BRCA1/2 mutations in unaffected relatives. Clin Genet. 2009;75:124–132. doi: 10.1111/j.1399-0004.2008.01146.x. [DOI] [PubMed] [Google Scholar]
- 16.Hadfield SG, Humphries SE. Implementation of cascade testing for the detection of familial hypercholesterolaemia. Curr Opin Lipidol. 2005;16:428–433. doi: 10.1097/01.mol.0000174152.76554.d6. [DOI] [PubMed] [Google Scholar]
- 17.Katz SJ, Kurian AW, Morrow M. Treatment decision making and genetic testing for breast cancer: Mainstreaming mutations. JAMA. 2015;314:997–998. doi: 10.1001/jama.2015.8088. [DOI] [PubMed] [Google Scholar]
- 18.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.Holmes-Rovner M, Kroll J, Schmitt N, et al. Patient satisfaction with health care decisions: The satisfaction with decision scale. Med Decis Making. 1996;16:58–64. doi: 10.1177/0272989X9601600114. [DOI] [PubMed] [Google Scholar]
- 20.Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21:564–572. [PubMed] [Google Scholar]
- 21.Samimi G, Bernardini MQ, Brody LC, et al. Traceback: A proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329–2337. doi: 10.1200/JCO.2016.70.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caswell-Jin JL, Zimmer AD, Stedden W, et al. Cascade genetic testing of relatives for hereditary cancer risk: Results of an online initiative. J Natl Cancer Inst. 2019 doi: 10.1093/jnci/djy147. 111:95-98, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke W, Press N. Ethical obligations and counseling challenges in cancer genetics. J Natl Compr Canc Netw. 2006;4:185–191. doi: 10.6004/jnccn.2006.0018. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs AS, Schwartz MD, Valdimarsdottir H, et al. Patient and genetic counselor perceptions of in-person versus telephone genetic counseling for hereditary breast/ovarian cancer. Fam Cancer. 2016;15:529–539. doi: 10.1007/s10689-016-9900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;32:618–626. doi: 10.1200/JCO.2013.51.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandelker D, Zhang L, Kemel Y, et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA. 2017;318:825–835. doi: 10.1001/jama.2017.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening. J Clin Oncol. 2020;38:1398–1408. doi: 10.1200/JCO.19.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]