Abstract
Introduction
The contralateral prophylactic mastectomy (CPM) rate in the U.S. has been steadily increasing. This is of particular concern because many women who undergo this procedure are candidates for breast-conserving surgery.
Areas covered
CPM’s medical benefit is related to the risk of contralateral cancer development and whether CPM provides a survival benefit. Contralateral cancer rates have decreased, and CPM does not provide a survival benefit. Other potential benefits of the procedure may be improved quality of life; these data are reviewed. Research efforts have been undertaken to better understand the decision-making process of patients who consider, and ultimately undergo, this procedure.
Expert opinion
Decisional traits, personal values, the desire for peace of mind, and the desire to obtain breast symmetry are important factors that drive a woman’s decision to undergo CPM. Additionally, many patients lack knowledge on how different types of breast surgery impact outcomes. To improve the shared decision-making process, a stepwise approach to address possible misconceptions, and clarify the real risks/benefits of this procedure should be utilized. A clear recommendation (for/against) should be made for every patient with newly diagnosed breast cancer who considers CPM. Communication tools to assist patients and surgeons in this process are sorely needed.
Keywords: Contralateral prophylactic mastectomy, harms, benefits, shared decision making, patient education
1.0. Introduction
Although the majority of women with unilateral breast cancer (BC) will obtain no oncologic benefit from contralateral prophylactic mastectomy (CPM), its use in the United States for both invasive and in situ disease has increased markedly over the last two decades (Figure 1) [1–9]. The proportion of women with stage I-III BC who underwent CPM increased from 1.8% in 1998 to 12.7% in 2012 [8,9]. This trend, although most pronounced among young women (where the rate of CPM is as high as 35%) [3,9–12], is seen across all age ranges [8,9] and has not yet reached a plateau [8,13]. Geographic variations in both rates of CPM and the magnitude of increase in its use have been observed [14]. The role of providers and patients in the choice of CPM is complex. Although initially thought to be a provider-driven decision, recent studies have shown that the providers’ contribution is marginal, accounting for only 20% of the variability in CPM rates [15], and this is largely a patient-driven decision [16–19]. The reasons why women opt to undergo more radical surgery are multifactorial. Desire to reduce the risk of contralateral breast cancer (CBC), obtain peace of mind, and improve survival are among the most commonly reported reasons for undergoing CPM [16,17,19–22]. New approaches to patient education about the real risk of developing CBC, the risks associated with CPM, and the lack of survival benefit are needed to help reverse this trend. This review will focus on patients with sporadic BC without a history of prior mantle radiation (i.e., patients who do not have an increased risk of developing CBC). We will discuss the potential benefits and harms of CPM, the psychosocial factors that drive the decision to undergo CPM, and the shared decision-making process necessary to help women make the best decision regarding this surgical procedure.
Figure 1.
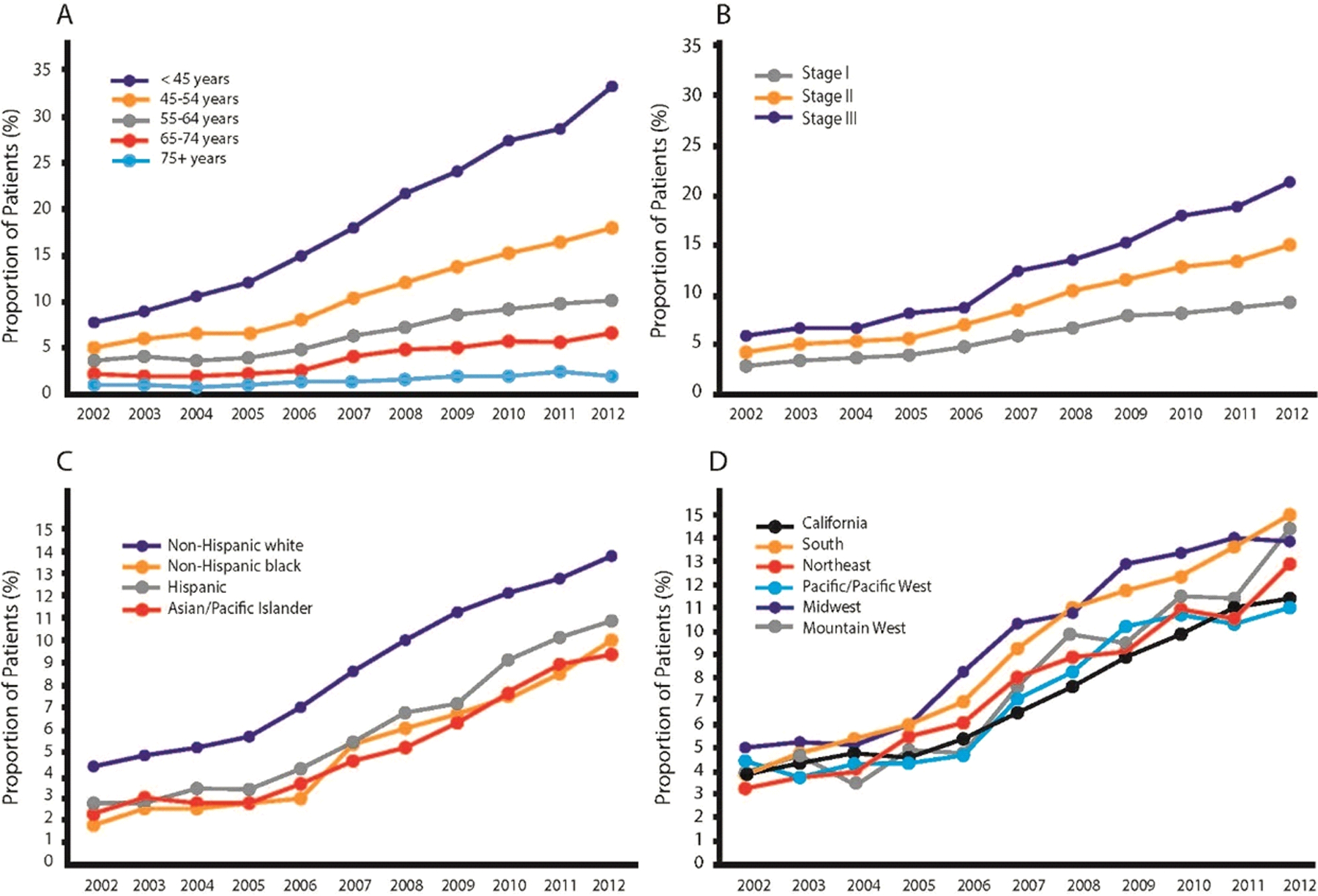
Annual trends (2002–2012) in type of surgery for women diagnosed with stage I-III breast cancer according to: A) age at diagnosis; B) American Joint Committee on Cancer stage; C) racial group; D) geographic (Surveillance, Epidemiology, and End Results) region.
(Adapted with permission from Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg. 2017;265(3):581–589.).
Abbreviations: AJCC: American Joint Committee on Cancer; SEER: Surveillance, Epidemiology, and End Results
2.0. Potential Benefits and Harms of Contralateral Prophylactic Mastectomy
2.1. Contralateral breast cancer
After unilateral sporadic BC, the risk of developing a new malignancy in the contralateral breast varies based on tumor biology, age at diagnosis, and family history [23]. Due to the widespread use of adjuvant endocrine therapy for early stage BC [24], the rate of CBC in the United States has been decreasing since 1985 [25]. Currently, after primary BC, the risk of developing CBC ranges from 0.25–0.4%/year for patients with estrogen receptor (ER) positive tumors treated with tamoxifen, and between 0.45–0.6%/year for ER negative tumors [24,26]. The reduction in CBC with endocrine therapy is seen across all age groups, so that even among women in their early 30s at diagnosis, the absolute risk of CBC at 10 years ranges between 3–8 % according to the ER status of the first tumor [24]. Cytotoxic chemotherapy has been shown to reduce the risk of CBC by 30% [27], and newer targeted therapies such as anti-HER2 treatment also appear to lower the incidence of CBC [28]. A population-based study from the Netherlands reported a 43% CBC risk reduction with the combination of trastuzumab and polychemotherapy compared to no adjuvant therapy [27]. Although CPM is extremely effective at reducing the risk of CBC, with a relative risk reduction of 96% [29–31], in a population with a low risk of developing CBC, the absolute benefit is very low (e.g., for women with an ER positive tumor and a 20-year risk of CBC of 5–8%, CPM would avoid the development of 2–3 CBCs per 1000 treated) [30].
2.2. Survival benefit
In order to have a survival benefit from CPM, patients would need to survive their index cancer, and then develop and succumb to CBC [32]. Retrospective studies have shown contradictory results in terms of survival [3,33–36]. However, in the studies suggesting a survival benefit for CBC, the magnitude of the survival benefit was greater than the incidence of CBC in the control group, indicating the selection of healthier, lower-risk women for the procedure [37]. For example, Bedrosian et al. found a 4.8% absolute reduction in BC mortality with CPM in women 18–49 years of age with stage I-II ER negative cancer, even though the incidence of CBC in the control group was only 0.9% [38]. To eliminate potential selection bias, Portschy et al. developed a Markov model to estimate the survival benefit of CPM. Among women with stage I and II sporadic BC, the absolute 20-year survival benefit from CPM was < 1% among all age, ER status, and cancer stage groups [39], and a Cochrane Review concluded that, currently, there is insufficient evidence that CPM improves survival [40].
2.3. Psychosocial outcomes
Parker et al. reported psychological outcomes among 288 women who underwent surgery for non-hereditary unilateral BC. Before surgery, those who underwent CPM (n = 50), had greater cancer worry, more cancer distress, and more body image concerns than those who underwent unilateral mastectomy (UM). At 6, 12, and 18 months after surgery, CPM recipients still had higher cancer distress and body image concerns than their counterparts, and also had lower quality of life (QoL) [41].
Cancer worry is a well-known psychological factor that influences the decision to undergo CPM [18,22,42]. Parker et al. found that cancer worry level decreased over time among CPM recipients and became similar to that of those who did not have CPM, indicating that CPM is effective in reducing cancer worry [41]. However, women who received CPM had greater body image concerns that persisted 1.5 years after surgery [41], and studies with longer follow-up have shown that body image concerns after CPM persist over time [43].
In the study of Parker et al., women who underwent CPM had decreased physical, social, emotional, and functional well-being [41]. In contrast, in a large cross-sectional study that evaluated QoL with the BREAST-Q questionnaire at a median of 4.6 years after surgery, Hwang et al. found that women who had CPM with reconstruction had higher satisfaction with their breasts and higher psychological well-being than women who had UM [44]. However, the differences between the groups were small and likely not clinically significant [44,45]. Koslow et al. compared BREAST-Q data at a median of 51.9 months postoperatively between women who had CPM and UM, (both with implant-based reconstruction) and also found that women with CPM had higher breast satisfaction, but no differences in other QoL domains were found [46]. Other studies have reported an adverse effect of CPM on feelings of femininity [43,47–49]. Frost et al. found that with long-term follow-up, approximately one-quarter of CPM recipients had difficulty looking at themselves naked and felt physically less attractive [43]. The degree of impact on sexuality and body image varies with age. Rosenberg et al. reported that among a cohort of young (age < 40 years) BC survivors who underwent CPM, 42% had a worse sense of sexuality than they expected, and 31% had more self-consciousness about their appearance than they expected [22]. Interestingly, a prospective study conducted in Sweden reported high rates of dissatisfaction with body appearance and femininity, but no impact on sexuality[50]. This study, however, had no control group. Although CPM with reconstruction increases symmetry and breast satisfaction, its impact on QoL is not fully understood, and patients should be informed of this.
2.4. Decisional satisfaction
Many studies have shown that women who choose to undergo CPM tend to be satisfied and have no decisional regret [19,21,22,51,52]. Frost et al. reported that among 583 women who had CPM (72% with reconstruction), at a mean time after surgery of 10.3 years, 83% were satisfied and would have chosen CPM again [48]. At 20.2 years after surgery, satisfaction increased (90%) as did the proportion of women who would have chosen CPM again (92%) [43]. Diminished sexual relationships and feelings of femininity, lack of information at the time of surgery, and increased level of stress after CPM, are factors associated with decreased satisfaction and with being less likely to choose CPM again [43,48,52].
2.5. Surgical complications
In a series of 600 patients undergoing surgery for unilateral BC, Miller at al. found that after adjusting for covariates (including reconstruction), patients undergoing CPM were 2.7 times more likely to have major complications compared with patients who underwent UM [53]. These findings were confirmed in a large study by the American College of Surgeons National Surgery Quality Improvement Program (ACS NSQIP) that found higher rates of wound, infectious, and overall complications among patients who received CPM compared to UM (5.8%, 2.2%, and 7.6% versus 2.9%, 0.8%, and 4.2% respectively) [54]. In the study of Rosenberg and colleagues, 33% of patients reported that the number of surgeries/procedures needed was higher than they initially thought, 25% stated that pain at the surgical site was worse than expected, and 28% reported that numbness and tingling in the chest was also worse than expected [22]. Because more surgery is associated with more complications, patients should be clearly informed about the increased operative risks of CPM.
2.6. Delay in treatment
Using data from the National Cancer Database, Sharpe et al. have demonstrated that CPM is associated with longer time to surgery compared to UM (median number of days from diagnosis to surgery: 40 versus 33, respectively) irrespective of reconstruction. If reconstruction was performed, patients were twice as likely to experience delay. Time to adjuvant chemotherapy was also longer (69 days versus 66 days). Notably, both delays increased from 2003 to 2010 [55]. Although, individually, these delays are of no clinical consequence, major surgical complications following CPM could result in further treatment delay that could impact oncologic outcomes, especially in high-risk patients [56].
2.7. Financial hardship
Greenup at al. investigated the financial harm after breast cancer surgery among 607 women with stage 0-III BC and found that 35% of participants reported financial hardship as a result of their cancer treatment. In multivariable analysis, the receipt of bilateral mastectomy (with or without reconstruction) was associated with a greater likelihood of reporting financial burden compared to breast conservation (OR 1.89, 95% CI 1.07–3.33) [57]. This is explained by the cost of bilateral mastectomy with reconstruction being $6’400 to $27’000 higher than the cost of lumpectomy with whole breast irradiation, due to both the cost of intervention and cost of complications [58].
3.0. Sociodemographic, Clinical, and Psychological Predictors of Contralateral Prophylactic Mastectomy
The sociodemographic and clinical predictors of CPM are well known and include younger age [6,10,12,59,60], white race [2,20,59], higher education [19,20,59], private insurance [59,61], positive family history [20,62], in situ disease [4,6], genetic testing (with a positive or negative result) [20], preoperative MRI [20], and availability of reconstructive surgery [10,19,63].
The decision-making process and personal values associated with the consideration of CPM have been investigated in a large (n = 2362) population-based survey. Women who preferred to make their own treatment decision (versus those who preferred the physician tell them what to do), and those who valued the possibility of avoiding radiation exposure and reducing cancer worry were more likely to strongly consider CPM [64]. Two recent, prospective (with pre- and post- surgical assessment) studies have further explored psychosocial factors associated with CPM uptake. Parker et al. reported that among 117 women diagnosed with early BC, less BC knowledge and greater cancer worry were associated with interest in CPM before the surgical visit, but only cancer worry predicted CPM uptake [18]. Metcalfe at al. evaluated 506 women diagnosed with unilateral BC for level of anxiety, depression, and psychological and sexual well-being, and found no differences between women who opted for CPM and those who did not. In their study, the only presurgical psychological factors associated with choosing CPM were lower breast satisfaction and lower level of optimism [60]. These findings are consistent with other retrospective studies that found the most common reasons for selecting CPM were cancer worry (fear of the cancer coming back and fear of harboring an occult malignancy), wanting peace of mind, and the desire for better cosmetic outcomes [19,20,22,59,65].
4.0. Patient Education
4.1. Giving recommendations on contralateral prophylactic mastectomy as part of the decision-making process
The proportion of patients reporting a recommendation from their surgeon against CPM is low: approximately 30% [59,66]. This may be due to many factors, such as the fear of generating misunderstanding, further complicating the treatment decision process, and a willingness to respect patient autonomy and values [66–68]. A population-based study demonstrated that a surgeon recommendation against CPM did not substantively increase patient dissatisfaction, use of second opinion, or loss of patient to a second surgeon [66]. Physicians should therefore feel comfortable in both addressing CPM and actively recommending against it if not clinically indicated. This is of major importance because women who do not receive recommendations (for or against) are more likely to undergo the procedure. This was clearly shown in a population-based study that analyzed the influence of surgeons’ recommendation on treatment receipt, finding that CPM rates were 10 times higher among women who did not receive a recommendation versus those who received one against it (1.9% versus 19%, respectively) [59].
4.2. Addressing possible misconceptions
Patients with newly diagnosed BC tend to overestimate their risk of CBC [69]. Additionally, Jagsi et al. recently reported that of patients who consider CPM, only 38.1% are aware that CPM does not improve survival in all BC patients, and that only 43.5% have adequate knowledge about the effect of CPM on BC recurrence. Additionally, 37.3% of CPM recipients in this study believed that CPM improves survival for all BC patients [59]. These data underline the importance of addressing misconceptions that patients may have about potential outcome benefits of CPM [59], and of clearly and simply stating what the procedure will and will not accomplish. Surgeons should also point out that other therapeutic interventions such as endocrine therapy do improve survival, and decrease the risk of recurrence and new primary BCs [26,70]. Although satisfaction rates among CPM recipients are high, poor information at the time of surgery is a predictor of increased regret. Therefore, enhancing the decision-making process may decrease regret after surgery [18,52].
4.3. Addressing potential benefits, harms, and psychological issues
Figure 2 shows an algorithm for a stepwise informed discussion with patients with a pre-existing desire for CPM. Possible harms, benefits, and psychological factors associated with cancer diagnosis, such as cancer worry, should be properly addressed [18]. Communication with patients about CPM is particularly challenging because the motivation behind wanting more extensive surgery is based on complex reactions. It is important to reassure patients that the majority of women with unilateral BC do not undergo CPM since the internet is rife with advice that this is the “safest” approach. The fact that the contralateral breast will be carefully screened and any abnormalities promptly evaluated should also be discussed. Although not a comfortable conversation for many physicians, the risk of death from the index cancer is often higher than the risk of developing a second BC, and emphasizing the need to avoid unnecessary surgery which may delay prompt and appropriate treatment of the index cancer may also be helpful. Many authors have suggested the development of a dynamic decision aid to guide the shared decision-making process for practitioners and patients [71]. Results from a pilot study have shown that in-visit decision aids are effective at improving knowledge about how surgical procedures affect outcomes [72]. An online decision support tool is currently being evaluated in a multi-institutional randomized trial; the primary outcomes of the study are acceptability, changes in patient knowledge, and reductions in decisional conflicts about CPM [73]. The questions and concerns of family and friends should be thoroughly addressed since their opinions have been shown to influence patient surgical choices [19,74].
Figure 2.
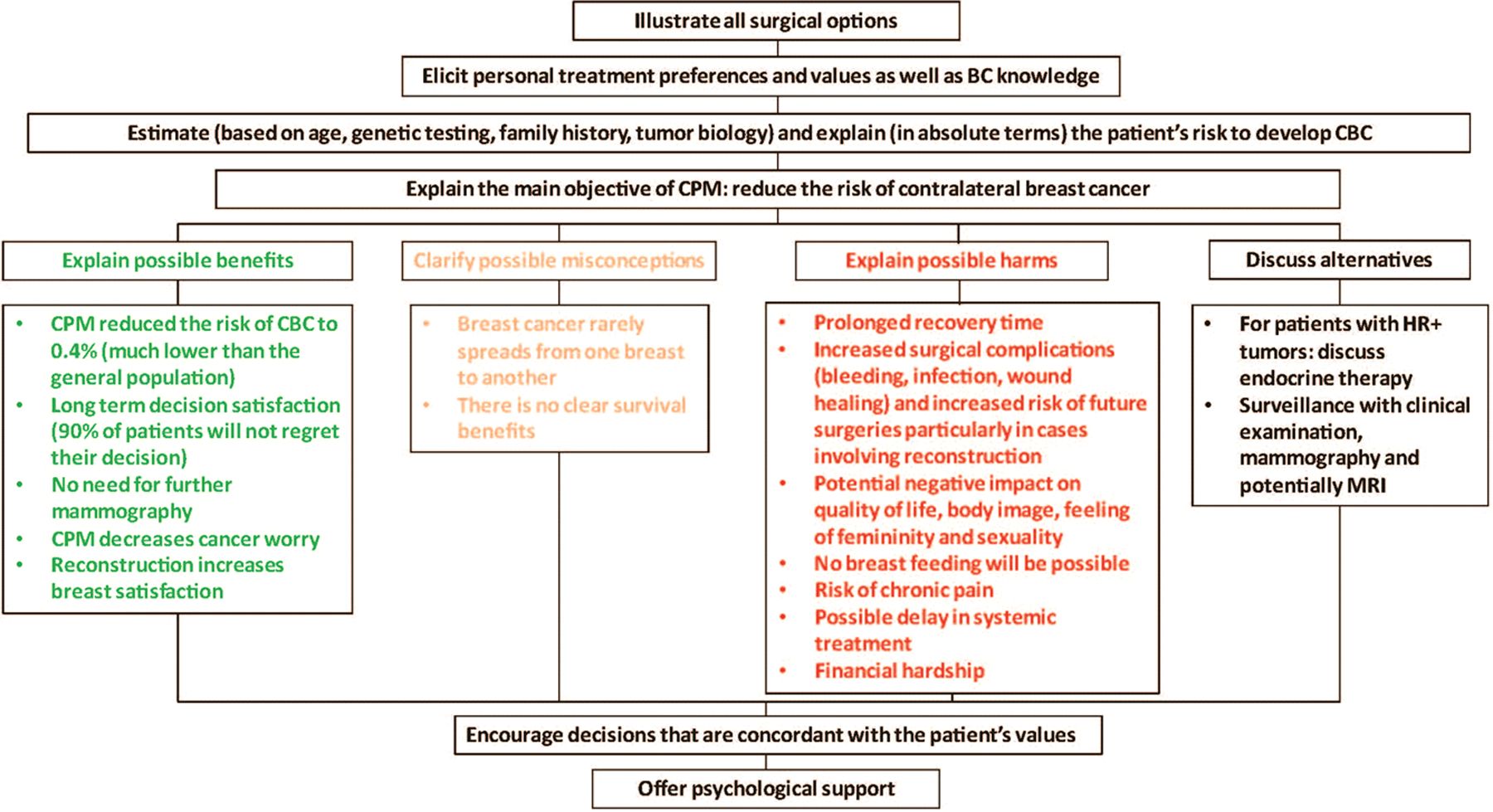
Framework for a stepwise informed discussion with patients with sporadic unilateral breast cancer who are considering CPM.
Abbreviations: BC: breast cancer, CBC: contralateral breast cancer, CPM: contralateral prophylactic mastectomy
5.0. Expert opinion
Physicians should actively advise patients against extensive surgical procedures that do not impact oncological outcomes [75,76]. When surgeons provide a recommendation, patients are likely to follow it [15,59,77]. Yet, only 30% of patients at average risk of CBC receive a recommendation regarding CPM from a surgeon [59]. This likely reflects the current emphasis on shared decision making and incorporation of patient values into treatment decisions, but unlike the choice between breast conservation and mastectomy in a cancer patient, CPM is a medically unnecessary procedure. Since 2005 there has been a decrease in the rate of breast conservation, with a concomitant 14% annual increase in the rate of CPM and stable rates of UM [63]. Katz et al. have shown that the estimated rate of CPM for surgeons who favored BCS the most and who were reluctant to perform CPM was only 4% versus 34% for those surgeons who favored BCS the least and were the least reluctant to perform CPM [15]. Additionally, recent surveys of surgeons performing breast surgery have found great variability in their knowledge of CBC risk, and current rates of local recurrence after BCS and mastectomy [78,79]. This means that patients with similar CBC risk may receive different information and undergo different procedures based on their surgeon’s preferences or misinformation.
Great effort was expended by the surgical community to show that decreasing BC surgery was oncologically safe [80–83], but, paradoxically, surgical trends have reversed in the last 15 years [84–86]. This is particularly troublesome, since we now understand that local recurrence is usually a function of tumor biology, and that bigger surgery does not cure bad biology. The findings discussed in this article indicate a clear need for better education of both patients and surgeons to decrease the use of CPM in women who are unlikely to benefit from the procedure.
Considering improvements in breast reconstruction—particularly the enthusiasm for nipple-sparing mastectomy [87]—and the lack of effective strategies to address the emotional aspects of patient CPM choice, we believe that CPM rates will continue to rise over the next five years. Nevertheless, educational tools will help surgeons enhance the shared CPM decision-making process based on data while incorporating patient wants. Research in this area will help determine if improved knowledge of CPM outcomes will affect treatment decisions. If proven effective, the use of such tools should be endorsed by national guidelines. Since surgeon beliefs have been shown to influence the variation in CPM use, understanding the reasons for different attitudes between surgeons should also be the focus of future research.
Article highlights/Main findings.
Contralateral prophylactic mastectomy does not improve cancer outcomes, and it increases the rate of surgical complications
It is unclear if contralateral prophylactic mastectomy improves quality of life
Knowledge of the possible harms and benefits of contralateral prophylactic mastectomy among breast cancer patients is low
Addressing possible misconceptions is crucial to helping patients make the best decision
Surgeon recommendations against contralateral prophylactic mastectomy are effective in decreasing its rate, but are only reported by a small percentage of patients
Educational aids may enhance the shared decision-making process
Funding
The preparation of this manuscript was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Declaration of interest
Dr. Monica Morrow has received speaking honoraria from Genomic Health and Roche. Dr. Giacomo Montagna is supported by the Ticino Cancer League, the Hanne Liebermann Foundation, the Fondation Ancrage, and the HEMMI-Stiftung. These funding bodies had no role in the writing of this manuscript
References
- 1.Yao K, Stewart AK, Winchester DJ, Winchester DP. Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol. October 2010;17(10):2554–2562. [DOI] [PubMed] [Google Scholar]
- 2.Grimmer L, Liederbach E, Velasco J, Pesce C, Wang CH, Yao K. Variation in Contralateral Prophylactic Mastectomy Rates According to Racial Groups in Young Women with Breast Cancer, 1998 to 2011: A Report from the National Cancer Data Base. J Am Coll Surg. July 2015;221(1):187–196. [DOI] [PubMed] [Google Scholar]
- 3.Kurian AW, Lichtensztajn DY, Keegan TH, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA. September 3 2014;312(9):902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. March 20 2009;27(9):1362–1367. [DOI] [PubMed] [Google Scholar]
- 5.Worni M, Akushevich I, Greenup R, et al. Trends in Treatment Patterns and Outcomes for Ductal Carcinoma In Situ. J Natl Cancer Inst. December 2015;107(12):djv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Coopey SB, Gadd MA, Hughes KS, Chang DC, Oseni TO. Trends in Unilateral and Contralateral Prophylactic Mastectomy Use in Ductal Carcinoma In Situ of the Breast: Patterns and Predictors. Ann Surg Oncol. November 2019;26(12):3863–3873. [DOI] [PubMed] [Google Scholar]
- 7.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. Jan 2015;150(1):9–16. [DOI] [PubMed] [Google Scholar]
- **8.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg. Mar 2017;265(3):581–589. [DOI] [PubMed] [Google Scholar]; This study of considerable interest shows how CPM has increased over time.
- 9.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. Nov 20 2007;25(33):5203–5209. [DOI] [PubMed] [Google Scholar]
- 10.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. June 1 2011;29(16):2158–2164. [DOI] [PubMed] [Google Scholar]
- 11.Christian N, Zabor EC, Cassidy M, Flynn J, Morrow M, Gemignani ML. Contralateral Prophylactic Mastectomy Use After Neoadjuvant Chemotherapy. Ann Surg Oncol. November 15 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazow SP, Riba L, Alapati A, James TA. Comparison of breast-conserving therapy vs mastectomy in women under age 40: National trends and potential survival implications. Breast J. July 2019;25(4):578–584. [DOI] [PubMed] [Google Scholar]
- 13.Marmor S, Altman AM, Mayleben WT, et al. The use of contralateral prophylactic mastectomy among elderly patients in the United States. Breast Cancer Res Treat. August 2019;177(1):175–183. [DOI] [PubMed] [Google Scholar]
- 14.Nash R, Goodman M, Lin CC, et al. State Variation in the Receipt of a Contralateral Prophylactic Mastectomy Among Women Who Received a Diagnosis of Invasive Unilateral Early-Stage Breast Cancer in the United States, 2004–2012. JAMA Surg. July 1 2017;152(7):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Katz SJ, Hawley ST, Hamilton AS, et al. Surgeon Influence on Variation in Receipt of Contralateral Prophylactic Mastectomy for Women With Breast Cancer. JAMA Surg. Jan 1 2018;153(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of considerable interest evaluates surgeon influence on variation in CPM uptake.
- 16.Covelli AM, Baxter NN, Fitch MI, McCready DR, Wright FC. ‘Taking control of cancer’: understanding women’s choice for mastectomy. Ann Surg Oncol. February 2015;22(2):383–391. [DOI] [PubMed] [Google Scholar]
- 17.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol. October 2012;19(10):3246–3250. [DOI] [PubMed] [Google Scholar]
- 18.Parker PA, Peterson SK, Bedrosian I, et al. Prospective Study of Surgical Decision-making Processes for Contralateral Prophylactic Mastectomy in Women With Breast Cancer. Ann Surg. January 2016;263(1):178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soran A, Ibrahim A, Kanbour M, et al. Decision making and factors influencing long-term satisfaction with prophylactic mastectomy in women with breast cancer. Am J Clin Oncol. April 2015;38(2):179–183. [DOI] [PubMed] [Google Scholar]
- 20.Hawley ST, Jagsi R, Morrow M, et al. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg. June 2014;149(6):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Chagpar A. Factors associated with decision to undergo contralateral prophylactic mastectomy versus unilateral mastectomy. Am J Surg. July 2019;218(1):170–174. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SM, Tracy MS, Meyer ME, et al. Perceptions, knowledge, and satisfaction with contralateral prophylactic mastectomy among young women with breast cancer: a cross-sectional survey. Ann Intern Med. September 17 2013;159(6):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiner AS, John EM, Brooks JD, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women’s Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. February 1 2013;31(4):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Nichols HB, Berrington de Gonzalez A, Lacey JV Jr., Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. April 20 2011;29(12):1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of interest illustrates CBC risk stratified by age and ER receptor status.
- 25.Mariotto A, Feuer EJ, Harlan LC, Wun LM, Johnson KA, Abrams J. Trends in use of adjuvant multi-agent chemotherapy and tamoxifen for breast cancer in the United States: 1975–1999. J Natl Cancer Inst. November 6 2002;94(21):1626–1634. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists’ Collaborative G, Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. August 27 2011;378(9793):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Kramer I, Schaapveld M, Oldenburg HSA, et al. The influence of adjuvant systemic regimens on contralateral breast cancer risk and receptor subtype. J Natl Cancer Inst. January 30 2019. [DOI] [PubMed] [Google Scholar]; This study of interest evaluates the impact of modern adjuvant treatments on CBC risk reduction.
- 28.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. October 20 2005;353(16):1673–1684. [DOI] [PubMed] [Google Scholar]
- 29.McDonnell SK, Schaid DJ, Myers JL, et al. Efficacy of contralateral prophylactic mastectomy in women with a personal and family history of breast cancer. J Clin Oncol. October 1 2001;19(19):3938–3943. [DOI] [PubMed] [Google Scholar]
- 30.Geiger AM, Yu O, Herrinton LJ, et al. A population-based study of bilateral prophylactic mastectomy efficacy in women at elevated risk for breast cancer in community practices. Arch Intern Med. March 14 2005;165(5):516–520. [DOI] [PubMed] [Google Scholar]
- 31.Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg. December 2014;260(6):1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narod SA. The impact of contralateral mastectomy on mortality in BRCA1 and BRCA2 mutation carriers with breast cancer. Breast Cancer Res Treat. July 2011;128(2):581–583. [DOI] [PubMed] [Google Scholar]
- 33.Pesce C, Liederbach E, Wang C, Lapin B, Winchester DJ, Yao K. Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol. October 2014;21(10):3231–3239. [DOI] [PubMed] [Google Scholar]
- 34.Yao K, Winchester DJ, Czechura T, Huo D. Contralateral prophylactic mastectomy and survival: report from the National Cancer Data Base, 1998–2002. Breast Cancer Res Treat. December 2013;142(3):465–476. [DOI] [PubMed] [Google Scholar]
- 35.Boughey JC, Hoskin TL, Degnim AC, et al. Contralateral prophylactic mastectomy is associated with a survival advantage in high-risk women with a personal history of breast cancer. Ann Surg Oncol. October 2010;17(10):2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrinton LJ, Barlow WE, Yu O, et al. Efficacy of prophylactic mastectomy in women with unilateral breast cancer: a cancer research network project. J Clin Oncol. July 1 2005;23(19):4275–4286. [DOI] [PubMed] [Google Scholar]
- 37.Kruper L, Kauffmann RM, Smith DD, Nelson RA. Survival analysis of contralateral prophylactic mastectomy: a question of selection bias. Ann Surg Oncol. October 2014;21(11):3448–3456. [DOI] [PubMed] [Google Scholar]
- 38.Bedrosian I, Hu CY, Chang GJ. Population-based study of contralateral prophylactic mastectomy and survival outcomes of breast cancer patients. J Natl Cancer Inst. March 17 2010;102(6):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Portschy PR, Kuntz KM, Tuttle TM. Survival outcomes after contralateral prophylactic mastectomy: a decision analysis. J Natl Cancer Inst. August 2014;106(8). [DOI] [PubMed] [Google Scholar]
- 40.Carbine NE, Lostumbo L, Wallace J, Ko H. Risk-reducing mastectomy for the prevention of primary breast cancer. Cochrane Database Syst Rev. April 5 2018;4:CD002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **41.Parker PA, Peterson SK, Shen Y, et al. Prospective Study of Psychosocial Outcomes of Having Contralateral Prophylactic Mastectomy Among Women With Nonhereditary Breast Cancer. J Clin Oncol. September 1 2018;36(25):2630–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of considerable interest evaluates the impact of CPM on cancer worry levels and patient quality of life.
- 42.Beesley H, Holcombe C, Brown SL, Salmon P. Risk, worry and cosmesis in decision-making for contralateral risk-reducing mastectomy: analysis of 60 consecutive cases in a specialist breast unit. Breast. April 2013;22(2):179–184. [DOI] [PubMed] [Google Scholar]
- 43.Frost MH, Hoskin TL, Hartmann LC, Degnim AC, Johnson JL, Boughey JC. Contralateral prophylactic mastectomy: long-term consistency of satisfaction and adverse effects and the significance of informed decision-making, quality of life, and personality traits. Ann Surg Oncol. October 2011;18(11):3110–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang ES, Locklear TD, Rushing CN, et al. Patient-Reported Outcomes After Choice for Contralateral Prophylactic Mastectomy. J Clin Oncol. May 1 2016;34(13):1518–1527. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg SM, King TA. Contralateral prophylactic mastectomy and quality of life: answering the unanswered questions? Gland Surg. June 2016;5(3):261–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koslow S, Pharmer LA, Scott AM, et al. Long-term patient-reported satisfaction after contralateral prophylactic mastectomy and implant reconstruction. Ann Surg Oncol. October 2013;20(11):3422–3429. [DOI] [PubMed] [Google Scholar]
- 47.Bloom DL, Chapman BM, Wheeler SB, et al. Reframing the conversation about contralateral prophylactic mastectomy: Preparing women for postsurgical realities. Psychooncology. February 2019;28(2):394–400. [DOI] [PubMed] [Google Scholar]
- 48.Frost MH, Slezak JM, Tran NV, et al. Satisfaction after contralateral prophylactic mastectomy: the significance of mastectomy type, reconstructive complications, and body appearance. J Clin Oncol. November 1 2005;23(31):7849–7856. [DOI] [PubMed] [Google Scholar]
- 49.Brandberg Y, Sandelin K, Erikson S, et al. Psychological reactions, quality of life, and body image after bilateral prophylactic mastectomy in women at high risk for breast cancer: a prospective 1-year follow-up study. J Clin Oncol. August 20 2008;26(24):3943–3949. [DOI] [PubMed] [Google Scholar]
- 50.Unukovych D, Sandelin K, Liljegren A, et al. Contralateral prophylactic mastectomy in breast cancer patients with a family history: a prospective 2-years follow-up study of health related quality of life, sexuality and body image. Eur J Cancer. November 2012;48(17):3150–3156. [DOI] [PubMed] [Google Scholar]
- 51.Altschuler A, Nekhlyudov L, Rolnick SJ, et al. Positive, negative, and disparate--women’s differing long-term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast J. Jan-Feb 2008;14(1):25–32. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery LL, Tran KN, Heelan MC, et al. Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol. September 1999;6(6):546–552. [DOI] [PubMed] [Google Scholar]
- 53.Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. December 2013;20(13):4113–4120. [DOI] [PubMed] [Google Scholar]
- 54.Osman F, Saleh F, Jackson TD, Corrigan MA, Cil T. Increased postoperative complications in bilateral mastectomy patients compared to unilateral mastectomy: an analysis of the NSQIP database. Ann Surg Oncol. October 2013;20(10):3212–3217. [DOI] [PubMed] [Google Scholar]
- 55.Sharpe SM, Liederbach E, Czechura T, Pesce C, Winchester DJ, Yao K. Impact of bilateral versus unilateral mastectomy on short term outcomes and adjuvant therapy, 2003–2010: a report from the National Cancer Data Base. Ann Surg Oncol. September 2014;21(9):2920–2927. [DOI] [PubMed] [Google Scholar]
- 56.Gagliato Dde M, Gonzalez-Angulo AM, Lei X, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. March 10 2014;32(8):735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenup RA, Rushing CN, Fish LJ, et al. Perspectives on the Costs of Cancer Care: A Survey of the American Society of Breast Surgeons. Ann Surg Oncol. October 2019;26(10):3141–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith BD, Jiang J, Shih YC, et al. Cost and Complications of Local Therapies for Early-Stage Breast Cancer. J Natl Cancer Inst. January 2017;109(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- **59.Jagsi R, Hawley ST, Griffith KA, et al. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg. March 1 2017;152(3):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study of considerable interest evaluates the impact of surgeon recommendations regarding CPM.
- 60.Metcalfe KA, Retrouvey H, Kerrebijn I, et al. Predictors of uptake of contralateral prophylactic mastectomy in women with nonhereditary breast cancer. Cancer. August 22 2019. [DOI] [PubMed] [Google Scholar]
- 61.Ward EP, Unkart JT, Bryant A, Murphy J, Blair SL. Influence of Distance to Hospital and Insurance Status on the Rates of Contralateral Prophylactic Mastectomy, a National Cancer Data Base study. Ann Surg Oncol. October 2017;24(10):3038–3047. [DOI] [PubMed] [Google Scholar]
- 62.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol. October 2009;16(10):2697–2704. [DOI] [PubMed] [Google Scholar]
- 63.Albornoz CR, Matros E, Lee CN, et al. Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer: The Role of Breast Reconstruction. Plast Reconstr Surg. June 2015;135(6):1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawley ST, Griffith KA, Hamilton AS, et al. The association between patient attitudes and values and the strength of consideration for contralateral prophylactic mastectomy in a population-based sample of breast cancer patients. Cancer. December 1 2017;123(23):4547–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.King TA, Gurevich I, Sakr R, Patil S, Stempel M, Morrow M. Occult malignancy in patients undergoing contralateral prophylactic mastectomy. Ann Surg. July 2011;254(1):2–7. [DOI] [PubMed] [Google Scholar]
- 66.Katz SJ, Janz NK, Abrahamse P, et al. Patient Reactions to Surgeon Recommendations About Contralateral Prophylactic Mastectomy for Treatment of Breast Cancer. JAMA Surg. July 1 2017;152(7):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angelos P, Bedrosian I, Euhus DM, Herrmann VM, Katz SJ, Pusic A. Contralateral Prophylactic Mastectomy: Challenging Considerations for the Surgeon. Ann Surg Oncol. October 2015;22(10):3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fayanju OM, Hwang ES. Contralateral Prophylactic Mastectomy: Aligning Patient Preferences and Provider Recommendations. JAMA Surg. March 1 2017;152(3):282–283. [DOI] [PubMed] [Google Scholar]
- 69.Abbott A, Rueth N, Pappas-Varco S, Kuntz K, Kerr E, Tuttle T. Perceptions of contralateral breast cancer: an overestimation of risk. Ann Surg Oncol. October 2011;18(11):3129–3136. [DOI] [PubMed] [Google Scholar]
- 70.Early Breast Cancer Trialists’ Collaborative G. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. October 3 2015;386(10001):1341–1352. [DOI] [PubMed] [Google Scholar]
- 71.Schellenberg AE, Stypulkowski A, Cordeiro E, Holloway CMB, Eisen A, Scheer AS. Practitioner Opinion on Contralateral Prophylactic Mastectomy: How Do We Steer a Patient-Driven Discussion? Ann Surg Oncol. October 2019;26(11):3489–3494. [DOI] [PubMed] [Google Scholar]
- 72.Yao K, Belkora J, Bedrosian I, et al. Impact of an In-visit Decision Aid on Patient Knowledge about Contralateral Prophylactic Mastectomy: A Pilot Study. Ann Surg Oncol. January 2017;24(1):91–99. [DOI] [PubMed] [Google Scholar]
- 73.https://clinicaltrials.gov/ct2/show/study/NCT02918474. Accessed 10/31/2019.
- 74.Huang J, Chagpar A. Effect of decision-making resources on satisfaction with decision to undergo contralateral prophylactic mastectomy (CPM). Am J Surg. September 21 2019. [DOI] [PubMed] [Google Scholar]
- 75.Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic Mastectomy Consensus Statement from the American Society of Breast Surgeons: Additional Considerations and a Framework for Shared Decision Making. Ann Surg Oncol. October 2016;23(10):3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk-Reducing) Mastectomy. Ann Surg Oncol. February 2017;24(2):375–397. [DOI] [PubMed] [Google Scholar]
- 77.Huang J, Chagpar A. Active Participation in Decision-Making in Contralateral Prophylactic Mastectomy for Patients With Breast Cancer. J Surg Res. October 2019;242:129–135. [DOI] [PubMed] [Google Scholar]
- 78.Yao K, Belkora J, Sisco M, et al. Survey of the Deficits in Surgeons’ Knowledge of Contralateral Prophylactic Mastectomy. JAMA Surg. April 2016;151(4):391–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kantor O, Chang C, Bleicher RJ, et al. Physician Knowledge of Breast Cancer Recurrence and Contralateral Breast Cancer Risk is Associated with Increased Recommendations for Contralateral Prophylactic Mastectomy: a Survey of Physicians at NAPBC-Accredited Centers. Ann Surg Oncol. October 2019;26(10):3080–3088. [DOI] [PubMed] [Google Scholar]
- 80.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. October 17 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 81.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. October 17 2002;347(16):1227–1232. [DOI] [PubMed] [Google Scholar]
- 82.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated by breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. May 20 2009;27(15):2466–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zumsteg ZS, Morrow M, Arnold B, et al. Breast-conserving therapy achieves locoregional outcomes comparable to mastectomy in women with T1–2N0 triple-negative breast cancer. Ann Surg Oncol. October 2013;20(11):3469–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dragun AE, Huang B, Tucker TC, Spanos WJ. Increasing mastectomy rates among all age groups for early stage breast cancer: a 10-year study of surgical choice. Breast J. Jul-Aug 2012;18(4):318–325. [DOI] [PubMed] [Google Scholar]
- 85.Lucas DJ, Sabino J, Shriver CD, Pawlik TM, Singh DP, Vertrees AE. Doing more: trends in breast cancer surgery, 2005 to 2011. Am Surg. January 2015;81(1):74–80. [PubMed] [Google Scholar]
- 86.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. October 2009;16(10):2682–2690. [DOI] [PubMed] [Google Scholar]
- 87.Wong SM, Chun YS, Sagara Y, Golshan M, Erdmann-Sager J. National Patterns of Breast Reconstruction and Nipple-Sparing Mastectomy for Breast Cancer, 2005–2015. Ann Surg Oncol. October 2019;26(10):3194–3203. [DOI] [PubMed] [Google Scholar]


