Abstract
Background
Acne is an inflammatory disorder with a high global burden. It is common in adolescents and primarily affects sebaceous gland‐rich areas. The clinical benefit of the topical acne treatments azelaic acid, salicylic acid, nicotinamide, sulphur, zinc, and alpha‐hydroxy acid is unclear.
Objectives
To assess the effects of topical treatments (azelaic acid, salicylic acid, nicotinamide, zinc, alpha‐hydroxy acid, and sulphur) for acne.
Search methods
We searched the following databases up to May 2019: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers.
Selection criteria
Clinical randomised controlled trials of the six topical treatments compared with other topical treatments, placebo, or no treatment in people with acne.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Key outcomes included participants' global self‐assessment of acne improvement (PGA), withdrawal for any reason, minor adverse events (assessed as total number of participants who experienced at least one minor adverse event), and quality of life.
Main results
We included 49 trials (3880 reported participants) set in clinics, hospitals, research centres, and university settings in Europe, Asia, and the USA.
The vast majority of participants had mild to moderate acne, were aged between 12 to 30 years (range: 10 to 45 years), and were female. Treatment lasted over eight weeks in 59% of the studies. Study duration ranged from three months to three years.
We assessed 26 studies as being at high risk of bias in at least one domain, but most domains were at low or unclear risk of bias.
We grouped outcome assessment into short‐term (less than or equal to 4 weeks), medium‐term (from 5 to 8 weeks), and long‐term treatment (more than 8 weeks). The following results were measured at the end of treatment, which was mainly long‐term for the PGA outcome and mixed length (medium‐term mainly) for minor adverse events.
Azelaic acid
In terms of treatment response (PGA), azelaic acid is probably less effective than benzoyl peroxide (risk ratio (RR) 0.82, 95% confidence interval (CI) 0.72 to 0.95; 1 study, 351 participants), but there is probably little or no difference when comparing azelaic acid to tretinoin (RR 0.94, 95% CI 0.78 to 1.14; 1 study, 289 participants) (both moderate‐quality evidence). There may be little or no difference in PGA when comparing azelaic acid to clindamycin (RR 1.13, 95% CI 0.92 to 1.38; 1 study, 229 participants; low‐quality evidence), but we are uncertain whether there is a difference between azelaic acid and adapalene (1 study, 55 participants; very low‐quality evidence).
Low‐quality evidence indicates there may be no differences in rates of withdrawal for any reason when comparing azelaic acid with benzoyl peroxide (RR 0.88, 95% CI 0.60 to 1.29; 1 study, 351 participants), clindamycin (RR 1.30, 95% CI 0.48 to 3.56; 2 studies, 329 participants), or tretinoin (RR 0.66, 95% CI 0.29 to 1.47; 2 studies, 309 participants), but we are uncertain whether there is a difference between azelaic acid and adapalene (1 study, 55 participants; very low‐quality evidence).
In terms of total minor adverse events, we are uncertain if there is a difference between azelaic acid compared to adapalene (1 study; 55 participants) or benzoyl peroxide (1 study, 30 participants) (both very low‐quality evidence). There may be no difference when comparing azelaic acid to clindamycin (RR 1.50, 95% CI 0.67 to 3.35; 1 study, 100 participants; low‐quality evidence). Total minor adverse events were not reported in the comparison of azelaic acid versus tretinoin, but individual application site reactions were reported, such as scaling.
Salicylic acid
For PGA, there may be little or no difference between salicylic acid and tretinoin (RR 1.00, 95% CI 0.92 to 1.09; 1 study, 46 participants; low‐quality evidence); we are not certain whether there is a difference between salicylic acid and pyruvic acid (1 study, 86 participants; very low‐quality evidence); and PGA was not measured in the comparison of salicylic acid versus benzoyl peroxide.
There may be no difference between groups in withdrawals when comparing salicylic acid and pyruvic acid (RR 0.89, 95% CI 0.53 to 1.50; 1 study, 86 participants); when salicylic acid was compared to tretinoin, neither group had withdrawals (both based on low‐quality evidence (2 studies, 74 participants)). We are uncertain whether there is a difference in withdrawals between salicylic acid and benzoyl peroxide (1 study, 41 participants; very low‐quality evidence).
For total minor adverse events, we are uncertain if there is any difference between salicylic acid and benzoyl peroxide (1 study, 41 participants) or tretinoin (2 studies, 74 participants) (both very low‐quality evidence). This outcome was not reported for salicylic acid versus pyruvic acid, but individual application site reactions were reported, such as scaling and redness.
Nicotinamide
Four studies evaluated nicotinamide against clindamycin or erythromycin, but none measured PGA. Low‐quality evidence showed there may be no difference in withdrawals between nicotinamide and clindamycin (RR 1.12, 95% CI 0.49 to 2.60; 3 studies, 216 participants) or erythromycin (RR 1.40, 95% CI 0.46 to 4.22; 1 study, 158 participants), or in total minor adverse events between nicotinamide and clindamycin (RR 1.20, 95% CI 0.73 to 1.99; 3 studies, 216 participants; low‐quality evidence). Total minor adverse events were not reported in the nicotinamide versus erythromycin comparison.
Alpha‐hydroxy (fruit) acid
There may be no difference in PGA when comparing glycolic acid peel to salicylic‐mandelic acid peel (RR 1.06, 95% CI 0.88 to 1.26; 1 study, 40 participants; low‐quality evidence), and we are uncertain if there is a difference in total minor adverse events due to very low‐quality evidence (1 study, 44 participants). Neither group had withdrawals (2 studies, 84 participants; low‐quality evidence).
Authors' conclusions
Compared to benzoyl peroxide, azelaic acid probably leads to a worse treatment response, measured using PGA. When compared to tretinoin, azelaic acid probably makes little or no difference to treatment response. For other comparisons and outcomes the quality of evidence was low or very low.
Risk of bias and imprecision limit our confidence in the evidence. We encourage the comparison of more methodologically robust head‐to‐head trials against commonly used active drugs.
Keywords: Adolescent, Adult, Child, Female, Humans, Male, Young Adult, Acne Vulgaris, Acne Vulgaris/drug therapy, Adapalene, Adapalene/adverse effects, Adapalene/therapeutic use, Anti-Bacterial Agents, Anti-Bacterial Agents/therapeutic use, Benzoyl Peroxide, Benzoyl Peroxide/therapeutic use, Bias, Clindamycin, Clindamycin/adverse effects, Clindamycin/therapeutic use, Dermatologic Agents, Dermatologic Agents/adverse effects, Dermatologic Agents/therapeutic use, Dicarboxylic Acids, Dicarboxylic Acids/adverse effects, Dicarboxylic Acids/therapeutic use, Erythromycin, Erythromycin/adverse effects, Erythromycin/therapeutic use, Glycolates, Glycolates/therapeutic use, Keratolytic Agents, Keratolytic Agents/therapeutic use, Mandelic Acids, Mandelic Acids/therapeutic use, Niacinamide, Niacinamide/adverse effects, Niacinamide/therapeutic use, Patient Dropouts, Patient Dropouts/statistics & numerical data, Pyruvic Acid, Pyruvic Acid/adverse effects, Pyruvic Acid/therapeutic use, Quality of Life, Salicylic Acid, Salicylic Acid/therapeutic use, Sulfur, Sulfur/therapeutic use, Tretinoin, Tretinoin/therapeutic use, Zinc, Zinc/therapeutic use
Plain language summary
Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc, and fruit acid (alpha‐hydroxy acid) for acne
Background
Acne vulgaris ('acne') is a costly and common skin disorder in which hair follicles become blocked. Acne affects up to 85% of adolescents and young adults. Topical retinoids (treatment derived from vitamin A) and antimicrobials (treatment that kills micro‐organisms such as bacteria) are common treatments. Other topical medications are also used, but there are concerns about their efficacy and safety.
Review question
This Cochrane Review aimed to assess the effects of six topical treatments (azelaic acid, salicylic acid, nicotinamide, sulphur, zinc, and alpha‐hydroxy acid (organic acids found in food, sometimes known as fruit acid) on people with acne when compared with an inactive substance (placebo), no treatment, or other topical treatments. The evidence is current to May 2019.
Study characteristics
We included 49 trials (3880 reported participants). At least one study assessed each eligible treatment.
Most trial participants were female, aged between 12 and 30 years, with mild to moderate acne. Nearly 60% of the trials treated participants for longer than eight weeks. Study duration ranged from three months to three years.
Nine trials reported pharmaceutical support. The studies were mainly conducted in Europe, Asia, and the USA, in clinics, hospitals, research centres, and universities.
Key results
The following results were measured at the end of treatment, which was mainly long term (more than 8 weeks) for the outcome 'Participants' global self‐assessment of acne improvement' (PGA) and mixed in length, but mainly medium term (from 5 to 8 weeks), for 'Total number of participants who experienced at least one minor side effect'.
Azelaic acid probably leads to worse PGA when compared to benzoyl peroxide, but when compared to tretinoin, there is probably little or no difference (both moderate‐quality evidence). When comparing azelaic acid to clindamycin, there may be little or no difference in PGA (low‐quality evidence), but we are uncertain whether azelaic acid reduces PGA compared to adapalene (very low‐quality evidence).
In terms of participant withdrawal (for any reason), there may be no difference when azelaic acid is compared with benzoyl peroxide, clindamycin, and tretinoin (all low‐quality evidence). We are uncertain whether azelaic acid reduces withdrawals when compared to adapalene (very low‐quality evidence).
We are uncertain whether azelaic acid has fewer total minor adverse events when compared to adapalene or benzoyl peroxide (very‐low quality evidence). When comparing azelaic acid to clindamycin, there may be no difference in total adverse events (low‐quality evidence). The studies that compared azelaic acid with tretinoin only reported individual side effects (e.g. scaling).
We are uncertain if there is a difference between salicylic acid and pyruvic acid on PGA score (very low‐quality evidence). There may be little or no difference between salicylic acid and tretinoin in PGA (low‐quality evidence). No study comparing salicylic acid with benzoyl peroxide assessed PGA. There may be no difference in withdrawals when comparing salicylic acid and pyruvic acid; there were no withdrawals when salicylic acid was compared to tretinoin (both low‐quality evidence). We are uncertain if there is a difference in withdrawals between salicylic acid and benzoyl peroxide (very low‐quality evidence).
We are uncertain whether salicylic acid reduces total minor adverse events when compared to benzoyl peroxide or tretinoin (very low‐quality evidence). For salicylic acid compared with pyruvic acid only individual application site reactions were reported (e.g. scaling and redness).
None of the four studies assessing nicotinamide (compared to clindamycin or erythromycin) assessed PGA. Nicotinamide may make no difference to withdrawals when compared to clindamycin or erythromycin, and may make no difference to total minor adverse events when compared to clindamycin (both low‐quality evidence); however, no studies comparing nicotinamide with erythromycin looked at total minor adverse events.
Glycolic acid peels may make no difference to PGA when compared to salicylic‐mandelic acid peels (low‐quality evidence), we are uncertain of the effect on total minor adverse events (very low‐quality evidence), and there were no withdrawals (low‐quality evidence).
Quality of the evidence
Our evidence quality was mixed for the PGA outcome (very low to moderate), mainly low quality for withdrawals, and very low quality for total minor side effects. We had some concerns with the small size of the studies and how they were conducted.
Summary of findings
Summary of findings 1. Azelaic acid compared to adapalene.
| Azelaic acid compared to adapalene for acne | ||||||
| Patient or population: participants with acne Settings: industry‐sponsored, single‐site study in Germany (1 study) Intervention: topical azelaic acid Comparison: topical adapalene | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical adapalene | Topical azelaic acid | |||||
|
Participants' global self‐assessment of acne improvement Improved to very much improved (long term: treatment duration > 8 weeks) |
842 per 1000 | 749 per 1000 (573 to 985) | RR 0.89 (0.68 to 1.17) | 55 (1 study) | ⊕⊝⊝⊝ Very lowa | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
53 per 1000 | 139 per 1000 (17 to 1000) | RR 2.64 (0.33 to 20.99) | 55 (1 study) | ⊕⊝⊝⊝ Very lowb | ‐ |
|
Total number of participants who experienced at least one minor adverse event (medium term: treatment duration from 5 to 8 weeks) |
263 per 1000 | 305 per 1000 (124 to 750) | RR 1.16 (0.47 to 2.85) | 55 (1 study) | ⊕⊝⊝⊝ Very lowc | The authors reported no "significant difference" in the incidence of erythema, dryness, and itching between treatment groups. |
|
Quality of life Dermatology Life Quality Index (long term: treatment duration > 8 weeks) |
The authors reported that there was no "statistically significant" difference (P = 0.549) between azelaic acid and adapalene. | 55 (1 study) |
⊕⊝⊝⊝ Very lowd |
Skewed data reported. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included, and study had unclear allocation concealment and high risk of performance bias. Two levels for imprecision: wide CI and optimal sample size not met. bDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included, with unclear allocation concealment and high risk of performance bias. Two levels for imprecision: very wide CI and optimal sample size not met. cDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included, with high risk of performance bias and unclear allocation concealment and blinding of outcome assessment. Two levels for imprecision: wide CI and optimal sample size not met. dDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear allocation concealment and high risk of performance bias. Two levels for imprecision: very small population size. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 2. Azelaic acid compared to benzoyl peroxide.
| Azelaic acid compared to benzoyl peroxide for acne | ||||||
| Patient or population: participants with acne Settings: multicentres, recruitment in Germany, Netherlands, Norway, and Greece (1 study); not described (1 study) Intervention: topical azelaic acid Comparison: topical benzoyl peroxide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical benzoyl peroxide | Topical azelaic acid | |||||
|
Participants' global self‐assessment of acne improvement Good or very good improvement (long term: treatment duration > 8 weeks) |
771 per 1000 | 633 per 1000 (555 to 733) | RR 0.82 (0.72 to 0.95) | 351 (1 study) | ⊕⊕⊕⊝ Moderatea | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
246 per 1000 | 216 per 1000 (147 to 317) | RR 0.88 (0.60 to 1.29) | 351 (1 studies) | ⊕⊕⊝⊝ Lowb | ‐ |
|
Total number of participants who experienced at least one minor adverse event (short term: treatment duration ≤ 4 weeks) |
133 per 1000 | 67 per 1000 (7 to 659) | RR 0.50 (0.05 to 4.94) | 30 (1 study) | ⊕⊝⊝⊝ Very lowc | The authors reported that people in the azelaic acid group experienced less dryness and desquamation, but more itching when compared to those in the benzoyl peroxide group. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level to moderate quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance bias and other bias, and with high risk of attrition and reporting bias. bDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance bias and other bias, and with high risk of attrition and reporting bias. One level for imprecision: wide CI. cDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with high risk of detection bias and unclear risk of selection, performance, attrition bias.Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 3. Azelaic acid compared to clindamycin.
| Azelaic acid compared to clindamycin for acne | ||||||
| Patient or population: participants with acne Settings: multicentres, recruitment in Germany, Netherlands, Norway, and Greece (1 study); three clinics in Tehran (1 study) Intervention: topical azelaic acid Comparison: topical clindamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical clindamycin | Topical azelaic acid | |||||
|
Participants' global self‐assessment of acne improvement Good or very good improvement (long term: treatment duration > 8 weeks) |
591 per 1000 | 668 per 1000 (544 to 816) | RR 1.13 (0.92 to 1.38) | 229 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
103 per 1000 | 134 per 1000 (49 to 367) | RR 1.30 (0.48 to 3.56) | 329 (2 studies) | ⊕⊕⊝⊝ Lowb | ‐ |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
160 per 1000 | 240 per 1000 | RR 1.5 (0.67 to 3.35) | 100 (1 study) |
⊕⊕⊝⊝ Lowc | There was no difference in minor adverse events (such as scaling and dry skin) between azelaic acid 5% gel and clindamycin 2% gel. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance, and other bias, and with high risk of attrition and reporting bias. One level for imprecision: wide CI and optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: both studies had unclear risk of selection and performance bias, and one study had a high risk of attrition and reporting bias. One level for imprecision: wide CI and optimal sample size not met. cDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance and detection bias. One level for imprecision: CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 4. Azelaic acid compared to tretinoin.
| Azelaic acid compared to tretinoin for acne | ||||||
| Patient or population: participants with acne Settings: multicentres in one study; not described (1 study) Intervention: topical azelaic acid Comparison: topical tretinoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical tretinoin | Topical azelaic acid | |||||
|
Participants' global self‐assessment of acne improvement Good to excellent improvement (long term: treatment duration > 8 weeks) |
623 per 1000 | 586 per 1000 (486 to 711) | RR 0.94 (0.78 to 1.14) | 289 (1 study) | ⊕⊕⊕⊝ Moderatea | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
90 per 1000 | 59 per 1000 (26 to 132) | RR 0.66 (0.29 to 1.47) | 309 (2 studies) | ⊕⊕⊝⊝ Lowb | ‐ |
| Total number of participants who experienced at least one minor adverse event | See comment | See comment | See comment | See comment | See comment | Total number of participants who experienced at least one adverse event not reported. The rate of erythema and scaling was considerably higher in the tretinoin group than that in the azelaic acid group. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by one level to moderate quality evidence. One level for risk of bias: only one study included with a high risk of attrition bias and unclear risk of selection and performance bias. bDowngraded by two levels to low quality evidence. One level for risk of bias: both studies with unclear risk of selection and performance bias, one study with high risk of attrition bias and the other with high risk of reporting bias. One level for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 5. Salicylic acid compared to benzoyl peroxide.
| Salicylic acid compared to benzoyl peroxide for acne | ||||||
| Patient or population: participants with acne Settings: not described Intervention: topical salicylic acid Comparison: topical benzoyl peroxide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical benzoyl peroxide | Topical salicylic acid | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
See comment | See comment | Not estimable | 41 (1 study) | ⊕⊝⊝⊝ Very lowa | Neither treatment group had any withdrawals. |
|
Total number of participants who experienced at least one minor adverse event (medium term: treatment duration from 5 to 8 weeks) |
95 per 1000 | 20 per 1000 (1 to 391) | RR 0.21 (0.01 to 4.11) | 41 (1 study) | ⊕⊝⊝⊝ Very lowb | The authors reported that zero out of 20 people in the 2% salicylic acid microgel group versus two out of 21 people in the benzoyl peroxide 10% cream group experienced minor adverse events. |
|
Quality of life ARQL (medium term: treatment duration from 5 to 8 weeks) |
The authors stated that subjects treated with salicylic acid microgel experienced better improvement when compared to 10% benzoyl peroxide. | 41 (1 study) | ⊕⊝⊝⊝ Very lowc |
No numerical data reported. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ARQL: acne‐related quality of life; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance, detection, and reporting bias. Two levels for imprecision: very small total sample size. bDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear selection, performance, and reporting bias. Two levels for imprecision: wide CI and optimal sample size not met. cDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance, detection, and reporting bias. Two levels for imprecision: very small total sample size. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 6. Salicylic acid compared to pyruvic acid.
| Salicylic acid compared to pyruvic acid for acne | ||||||
| Patient or population: participants with acne Settings: Al‑Zahra Hospital Dermatology Clinic and Isfahan Skin Research Centre Intervention: topical salicylic acid Comparison: topical pyruvic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical pyruvic acid | Topical salicylic acid | |||||
|
Participants' global self‐assessment of acne improvement Good to excellent improvement (medium term: treatment duration from 5 to 8 weeks) |
395 per 1000 | 443 per 1000 (269 to 727) | RR 1.12 (0.68 to 1.84) | 86 (1 study) | ⊕⊝⊝⊝ Very lowa | ‐ |
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
419 per 1000 | 373 per 1000 (222 to 628) | RR 0.89 (0.53 to 1.50) | 86 (1 study) | ⊕⊕⊝⊝ Lowb | ‐ |
| Total number of participants who experienced at least one minor adverse event | See comment | See comment | See comment | See comment | See comment | Total number of participants who experienced at least one adverse event not reported. Although the authors did report no "significant difference" in minor adverse events (scaling in the first to fourth sessions, redness, burning, and itching) between the two peeling (30% salicylic acid and 50% pyruvic acid). |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by three levels to very low quality evidence. Two levels for risk of bias: only one study included with high risk of attrition and other bias and unclear risk of selection and performance bias. One level for imprecision: wide CI and optimal sample size is not met. bDowngraded by two levels to low quality evidence. One level for imprecision: wide CI and optimal sample size not met. One level for risk of bias: only one study included with high risk of attrition and other bias, and unclear risk of selection and performance bias. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 7. Salicylic acid compared to tretinoin.
| Salicylic acid compared to tretinoin for acne | ||||||
| Patient or population: participants with acne Settings: Skin Disease and Leishmaniasis Research Center and Isfahan University of Medical Sciences clinics (1 study); not described (1 study) Intervention: topical salicylic acid Comparison: topical tretinoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical tretinoin | Topical salicylic acid | |||||
|
Participants' global self‐assessment of acne improvement
Moderate to excellent improvement (long term: treatment duration > 8 weeks) |
1000 per 1000 | 1000 per 1000 (920 to 1000) | RR 1.00 (0.92 to 1.09) | 46 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
See comment | See comment | Not estimable | 74 (2 studies) | ⊕⊕⊝⊝ Lowb | Neither study had any withdrawals. |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
541 per 1000 | 741 per 1000 (357 to 1000) | RR 1.37 (0.66 to 2.87) | 74 (2 studies) | ⊕⊝⊝⊝ Very lowc | The authors in one study reported no "statistically significant" differences in the incidence of dryness, peeling, erythema, burning and itching between treatment groups at any study week. All side effects reported in the two studies were of mild to moderate intensity and transient. |
|
Quality of life AQOL (long term: treatment duration > 8 weeks) |
The authors reported no "significant differences" in AQOL between salicylic acid group (end of study: 0.95 ± 1.9) and tretinoin group (end of study: 0.91 ± 1.64) at baseline and at the end of the study | 46 (1 study) | ⊕⊝⊝⊝ Very lowd |
Skewed data reported. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AQOL: acne quality of life; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with unclear risk of random sequence generation, allocation concealment, and blinding of participants and personnel. One level for imprecision: optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: both studies with unclear risk of selection bias, one with unclear risk of performance bias and the other with high risk of performance and unclear risk of reporting bias. One level for imprecision: small total sample size. cDowngraded by three levels to very low quality evidence. One level for risk of bias: two studies with unclear risk of selection bias and high risk of detection bias. Two levels for imprecision: wide CI and optimal sample size not met. dDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear risk of random sequence generation, allocation concealment, and blinding of participants and personnel. Two levels for imprecision: very small population size and wide CI. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 8. Nicotinamide compared to clindamycin.
| Nicotinamide compared to clindamycin for acne | ||||||
| Patient or population: participants with acne Settings: multicentres in USA (1 study); a teaching clinic of dermatology in Iran (1 study); St‐Alzahra hospital, Isfahan University of Medical Sciences, Isfahan, Iran (1 study) Intervention: topical nicotinamide Comparison: topical clindamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical clindamycin | Topical nicotinamide | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
74 per 1000 | 83 per 1000 (36 to 193) | RR 1.12 (0.49 to 2.60) | 216 (3 studies) | ⊕⊕⊝⊝ Lowa | Two trials had no withdrawals. |
|
Total number of participants who experienced at least one minor adverse event (medium term: treatment duration from 5 to 8 weeks) |
185 per 1000 | 222 per 1000 (135 to 369) | RR 1.20 (0.73 to 1.99) | 216 (3 studies) | ⊕⊕⊝⊝ Lowb | Local application site reactions (e.g. itching, burning, crusting) were reported in two studies. In the third study, the authors reported no side effects during the treatment. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: three studies included and all with unclear risk of bias, two with unclear risk of performance bias, one with high risk of attrition bias. One level for imprecision: wide CI and optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: all three studies with unclear risk of selection and detection bias, two out of three studies with unclear risk of performance bias. One level for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 9. Nicotinamide compared to erythromycin.
| Nicotinamide compared to erythromycin for acne | ||||||
| Patient or population: participants with acne Settings: Laboratoire Dermscan (Villeurbanne) Intervention: topical nicotinamide Comparison: topical erythromycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical erythromycin | Topical nicotinamide | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
63 per 1000 | 89 per 1000 (29 to 267) | RR 1.40 (0.46 to 4.22) | 158 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
| Total number of participants who experienced at least one minor adverse event | See comment | See comment | See comment | See comment | See comment | Total number of participants who experienced at least one adverse event not reported. There was "no difference" in occurrence of pertinent clinical signs and functional or physical signs between treatment groups. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with unclear risk of selection and performance bias. One level for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Summary of findings 10. Glycolic acid (alpha‐hydroxy acid) compared to salicylic‐mandelic acid.
| Glycolic acid (alpha‐hydroxy acid) compared to salicylic‐mandelic acid for acne | ||||||
| Patient or population: participants with acne Settings: Dermatology and Andrology Department of Beha University hospital, Egyptian patients (only study); recruitment in India (1 study) Intervention: topical glycolic acid (alpha‐hydroxy acid) Comparison: topical salicylic‐mandelic acid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical salicylic‐mandelic acid | Topical glycolic acid | |||||
|
Participants' global self‐assessment of acne improvement Fair to good improvement (long term: treatment duration > 8 weeks) |
900 per 1000 | 954 per 1000 (792 to 1000) | RR 1.06 (0.88 to 1.26) | 40 (1 studies) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
See comment | See comment | Not estimable | 84 (2 studies) |
⊕⊕⊝⊝ Lowb | Neither study had any withdrawals. |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
227 per 1000 | 409 per 1000 (164 to 1000) | RR 1.80 (0.72 to 4.52) | 44 (1 study) | ⊕⊝⊝⊝ Very lowc | Four (20%) participants in salicylic‐mandelic acid peel experienced a burning or stinging sensation against two (10%) in glycolic acid peel. Sixteen participants (80%) in salicylic‐mandelic acid peel developed visible desquamation against eight (40%) in glycolic acid peel (P = 0.025). |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. Two levels for imprecision: wide CI and optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: two studies included, one with unclear risk of selection, performance and reporting bias, the other with unclear risk of performance bias. One level for imprecision: small total sample size. cDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with high risk of detection bias and unclear risk of selection, performance bias. Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Background
Please see the glossary in Table 11 for an explanation of medical terms used throughout the text.
1. Glossary of medical terms.
| Medical term | Explanation |
| Acne vulgaris | A common chronic skin disorder of sebaceous follicles, mainly affecting the face, chest, and backa |
| Chemokine | A group of small cytokines that act as chemical messengers to induce chemotaxis in leukocytesc |
| Comedone | A clogged hair follicle in the skin. It can present as a blackhead or whiteheada |
| Cytokine | A small protein released by cells that function as molecular messengers between cellsc |
| Erythema | Redness of the skin, caused by vascular congestion or increased perfusionb |
| Hyperkeratosis | Thickening of the outer layer of skin often associated with a quantitative abnormality of keratinb |
| Keratinocytes | The predominant cell type in the epidermis, forming a touch protective layera |
| Microcomedones | Early and small plugging of the follicle with excess keratin and sebumb |
| Nodule | A solid mass in the skin, more than 0.5 cm in diameterb |
| Papule | A circumscribed palpable elevation, less than 0.5 cm in diameterb |
| Pilosebaceous unit | A structure consisting of a hair follicle, sebaceous gland, and an arrector pili muscleb |
|
Propionibacterium acnes (Cutibacterium acnes) |
Gram‐positive bacterium related to acne developmentb |
| Pustule | A visible accumulation of free pusb |
| Scar | Skin areas of fibrous tissue replacing normal skin after injuryb |
| Sebum | The oily, waxy substance produced by sebaceous glandsb |
| Stratum corneum | The outermost layer of the epidermis, where cells have lost nuclei and cytoplasmic organellesb |
| Toll‐like receptor | A class of proteins that recognise conserved products unique to microbial metabolism in immune responsec |
aAndrews' Diseases of the Skin: Clinical Dermatology, 11th Edition, 2011, Elsevier Inc. bRook's Textbook of Dermatology, Eighth Edition, 2010, Blackwell Publishing Ltd. cImmunology, Sixth Edition, 2001, Harcourt Asia Pte Ltd.
Description of the condition
Acne is a common inflammatory disorder of pilosebaceous units (Landow 1997). It results in non‐inflammatory lesions known as comedones (whiteheads or blackheads) and inflammatory lesions including papules, pustules, or nodules (Ramli 2012). Acne primarily affects sebaceous gland‐rich areas, such as the face, shoulders, back, and upper chest (Katsambas 2008).
Acne comprises acne vulgaris, acne variants, and acneiform eruptions in clinical practice (Table 12). Acne vulgaris is the most common type of acne, which mainly affects adolescents and young adults. Prevalence in young people aged 12 to 24 years is as high as 85% (Bhate 2013). Acne severity in boys correlates with pubertal maturation. One study of healthy Danish boys showed that the mean age of onset of puberty has fallen from 11.92 between 1991 and 1993 to 11.66 between 2006 and 2008 (Sorensen 2010). Previous studies showed that 50% of boys aged 10 or 11 years had more than 10 comedones (Lucky 1991), and 78% of girls aged eight to 12 years had acne (Lucky 1997). Acne often begins in the early teens and it can persist through the third decade or even later, but the intensity and duration varies for each individual (Bhate 2013). Recently, several reports have suggested increased prevalence of an adult form of acne vulgaris (Khunger 2012; Rademaker 2014). Adult acne mainly affects women and the prevalence in adult women is estimated to be 14% (Williams 2006). In addition, although uncommon, physicians can come across people with childhood acne classified according to the age of onset (neonatal, infantile, mid‐childhood, and prepubertal) (Antoniou 2009; Krakowski 2007).
2. Clinical classification of acnea.
| Acne vulgaris | |
| Acne variants | Neonatal acne |
| Infantile acne | |
| Acne conglobata | |
| Acne fulminans | |
| SAPHO syndrome | |
| PAPA syndrome | |
| Acne excoriee des jeunes filles | |
| Acne mechanica | |
| Acne with solid facial oedema | |
| Acne with associated endocrinology abnormalities | |
| Acneiform eruptions | Steroid folliculitis |
| Drug‐induced acne | |
| Epidermal growth factor receptor inhibitor associated eruption | |
| Occupational acne and chloracne | |
| Gram‐negative folliculitis | |
| Radiation acne | |
| Tropical acne | |
| Acne aestivalis | |
| Pseudoacne of the nasal crease | |
| Apert syndrome |
aFitzpatrick's Dermatology in General Medicine, Eighth edition, 2012, The McGraw‐Hill Companies, Inc.
To date, there are various grading systems for severity assessment, but with no consensus (Lehmann 2002; Ramli 2012). Moreover, there are no grading systems that fulfil all essential criteria required for an ideal acne grading scale (Tan 2012; Tan 2013). Acne experts suggest that scales served as investigator global assessment grading measures may be helpful to establish an ideal scale (Tan 2013). There are various grading systems used in clinical practice. According to the predominant types of lesions, study authors can classify acne vulgaris as comedonal, papulopustular, and nodular acne (Ramli 2012), or classify acne vulgaris as mild, moderate, severe, and cystic acne (Dayal 2017). However, when the predominant lesion type is difficult to determine, physicians may consider it as polymorphic acne (Kharfi 2001). Study authors may also classify acne vulgaris as mild, moderate, and severe based on the acne grading of the face, back and chest (O'Brien 1998). When conducting a clinical trial, study authors may classify acne based on different grading systems or scales such as the Allen‐Smith Scale (Aksakal 1997), Cunliffe grading system (Bae 2013), investigator's static global assessment score (Schaller 2016), and Michaelson acne severity index (Kar 2013). However, all the current acne grading systems have shortcomings and a consistently applied standard for grading acne severity is urgently needed (Tan 2013).
The mechanism that causes the disease is unknown, but it is widely accepted that increased sebum excretion induced by androgens, follicular hyperkeratinisation, Cutibacterium acnes (C acnes, formerly Propionibacterium acnes) (Dreno 2018), and bacterial hypercolonisation, as well as inflammation, are the major pathogenetic factors for acne (Friedlander 2010). A keratinous plug forms at the follicular infundibulum resulting from hyperkeratosis in the follicle, initiating the formation of microcomedones (Cunliffe 2000). Within these microcomedones is an anaerobic lipid‐rich environment suitable for the growth of C acnes (Brown 1998). The C acnes then hydrolyse triglycerides into glycerol and free fatty acids, which can initiate the inflammatory response (Dessinioti 2010; Thiboutot 2016). The cell surface toll‐like receptors, which play critical roles in the immune response against micro‐organisms, are involved in this bacteria‐mediated inflammatory response by triggering the release of pro‐inflammatory cytokines (Kim 2005).
Although acne vulgaris is not life‐threatening and usually regresses in the third decade (Thiboutot 2016), it may cause serious psychological distress, as well as pain, and may considerably compromise the quality of life of the individual. Embarrassment, shame, and lack of confidence are important consequences resulting from acne vulgaris. Furthermore, scarring and embarrassment from acne begins at approximately the same age that adolescents are undergoing significant emotional and physical changes which, if combined, can be devastating. Indeed, there have been reports suggesting that severe acne can result in permanent physical scarring and even suicidal ideation (Dunn 2011; Misery 2011).
Description of the intervention
Treatment options for acne are often targeted at the factors implicated in acne development, such as sebaceous hypersecretion, abnormal keratinisation, C acnes bacteria colonisation, and the inflammation process (Titus 2012). The choice of treatments depends on the type and extent of acne (Gollnick 2003). Topical therapy is the preferred choice of treatment for mild acne and is also useful for moderate to severe acne (Akhavan 2003). The current mainstay of topical therapy for acne vulgaris includes retinoids (such as adapalene and tretinoin) and antimicrobials, such as benzoyl peroxide and antibiotics (Akhavan 2003; Titus 2012; Well 2013). However, other topical medications such as azelaic acid, salicylic acid, topical nicotinamide, sulphur, zinc, and alpha‐hydroxy acid (such as glycolic acid and mandelic acid) are also effective for acne treatment (Akhavan 2003; ElRefaei 2015; Habbema 1989; Shahmoradi 2013; Sharad 2013).
How the intervention might work
Topical azelaic acid
As an ingredient found in many whole grain cereals and animal products, azelaic acid is a well‐known aliphatic dicarboxylic acid, and it is useful in acne treatment due to its antimicrobial and anticomedonal properties (Akhavan 2003). Twice‐daily application of 20% cream formation (Azelex) (Titus 2012), approved by the US Food and Drug Administration (FDA) for acne, can lead to an improvement of conditions within four weeks of initiation of therapy (Akhavan 2003; Cunliffe 1989). Compared to Azelex, the 15% gel (Finacea) has better bioavailability (Frampton 2004; Titus 2012). Azelaic acid 20% cream monotherapy or in combination therapy with glycolic acid (Graupe 1996; Spellman 1998), azelaic acid 20% (Iraji 2007) or 15% gel (Thiboutot 2008), azelaic acid 5% gel in combination with clindamycin 2% (Pazoki‐Toroudi 2011), or erythromycin 2% (Pazoki‐Toroudi 2010), are all effective treatments for acne. Azelaic acid 20% cream can reduce the number of both non‐inflammatory and inflammatory lesions and has an efficacy comparable to the other approved standard treatments, including benzoyl peroxide and erythromycin, as well as tretinoin, but it is better tolerated by people with fewer side effects (Simonart 2012; Spellman 1998).
Azelaic acid is able to competitively antagonise the activity of mitochondrial oxidoreductases and 5‐alpha‐reductase (Passi 1989; Stamatiadis 1988). The mechanism of action of azelaic acid in acne treatment may relate to its inhibitory effects on mitochondrial oxidoreductase and DNA synthesis (Fitton 1991). It has a predominant antibacterial activity on C acnes by inhibiting protein synthesis (Bojar 1991), and has a modest comedolytic effect by inhibiting the proliferation and differentiation of human keratinocytes, as well as an anti‐inflammatory action by inhibiting the generation of pro‐inflammatory oxygen derivatives in neutrophils (Akamatsu 1991; Sieber 2014). It can also reduce sebum production on the forehead, chin, and cheek through its inhibitory effect on the conversion from testosterone to 5‐dehydrotestosterone (Passi 1989).
Adverse effects of azelaic acid are mild and transient. About 5% to 10% of people report a burning or stinging sensation, tightness of the skin, and erythema in the treated area, but this usually only lasts for a few weeks (Graupe 1996). Azelaic acid can cause hypopigmentation, so physicians should monitor its use in people with dark skin (Akhavan 2003). Azelaic acid is a US FDA pregnancy category B drug. It has minimal systemic absorption when used topically. Use in pregnancy and lactation should not be a cause for concern (Bozzo 2011), although the excretion of azelaic acid into milk has been demonstrated, and caution is advised in nursing mothers (Akhavan 2003).
Topical salicylic acid
Salicylic acid is often incorrectly recognised as a beta‐hydroxy acid but it is actually an O‐hydroxybenzoic acid (Kempiak 2008), and it is useful in the treatment of acne vulgaris due to its keratolytic and comedolytic effects (Akarsu 2012; Akhavan 2003). Salicylic acid is a component of most over‐the‐counter acne preparations (Simonart 2012). Its concentration varies from 0.5% to 3.0% (Babayeva 2011; Zander 1992), and it is available in washes (Choi 2010), creams (Zheng 2013), and lotions (Babayeva 2011). Chemical peel of salicylic acid at a concentration of 20% to 30% is also available and useful in acne treatment (Bae 2013). Salicylic acid monotherapy (Strauss 2007), or combination therapy with benzoyl peroxide (Akarsu 2012; Seidler 2010), or clindamycin phosphate (NilFroushzadeh 2009; Touitou 2008) can improve acne lesions. Salicylic acid 20% or 30% peels (Kempiak 2008), or salicylic 20%/mandelic acid 10% peels (Garg 2009) are also effective for the treatment of acne vulgaris. Previous studies have shown that topical salicylic acid has mild to moderate activity against both non‐inflammatory lesions and inflammatory lesions in acne vulgaris (Akarsu 2012; Degitz 2008; Thiboutot 2009). It is approved for use in children with acne (Akhavan 2003).
Salicylic acid can break down the follicular keratotic plugs through dissolving the intercellular cement holding the stratum corneum cells and promoting the desquamation of follicular epithelium (Akarsu 2012; Akhavan 2003). It also has anti‐inflammatory capabilities, affecting the arachidonic acid cascade (Lee 2003).
When used at concentrations of 2% or higher, salicylic acid can cause local skin peeling and discomfort to some degree (Akarsu 2012; Boutli 2003). Salicylic acid is a FDA pregnancy category C drug (Kempiak 2008). There are no studies conducted in lactating women on topical use of salicylic acid and little is known about the excretion of salicylic acid in breast milk. Therefore, physicians advise women during lactation to avoid the use of salicylate (Akhavan 2003; Bozzo 2011).
Topical nicotinamide
Nicotinamide serves as the active form of niacin, having anti‐inflammatory effects in acne (Shalita 1995). Twice‐daily application of 4% or 5% nicotinamide gel for eight weeks can lead to significant improvement of acne conditions (Khodaeiani 2013; Shalita 1995a). Researchers published the first study that assessed nicotinamide in 1995 and the data suggest that 4% nicotinamide gel has comparable efficacy to 1% clindamycin gel in the treatment of inflammatory acne vulgaris (Shalita 1995). Another study also supports the comparable efficacy of 4% nicotinamide gel to 1% clindamycin gel in moderate inflammatory acne vulgaris (Khodaeiani 2013). When used at a concentration of 5%, nicotinamide gel is as effective as clindamycin 2% gel for the treatment of mild to moderate acne vulgaris (Shahmoradi 2013). Nicotinamide 4% linoleic acid‐rich phosphatidylcholine produced global clinical improvements in acne (Morganti 2011).
The mechanisms of action are mainly due to its potent anti‐inflammatory effect (Shalita 1995a), and inhibition of sebum production (Draelos 2006a). Nicotinamide exerts its anti‐inflammatory effects by inhibiting C acnes‐induced chemokine IL‐8 production in keratinocytes through interfering with NF‐kappa B by inhibiting PARP‐1 and mitogen‐activated protein kinases (MAPK) pathways (Grange 2009).
Only mild stinging or burning at the application site is reported during topical use of nicotinamide (Shalita 1995a). It is safe for women in pregnancy, although the FDA pregnancy category rating of topical nicotinamide is not available (Rolfe 2014). Nicotinamide is excreted in breast milk, but no data regarding topical nicotinamide use in women who are pregnant or lactating are available (Rolfe 2014; Stockton 1990).
Topical sulphur
Sulphur is a yellow non‐metallic chemical element with antifungal, antibacterial, and keratolytic properties (Gupta 2004). The topical sulphur‐containing preparations at concentrations of 1% to 10% are helpful for acne treatment (Akhavan 2003), even though they may be both comedonal and comedolytic (Mills 1972). Sulphur is available in the form of lotions, foam, creams, ointments, and soaps. When used together with benzoyl peroxide or sodium sulphacetamide, sulphur shows a better therapeutic effect on acne vulgaris. For example, sodium sulphacetamide 10% with sulphur 5% emollient foam (Del Rosso 2009), sodium sulphacetamide with sulphur lotion (Breneman 1993), and benzoyl peroxide 10% plus sulphur in the range 2% to 5% cream (Danto 1966; Wilkinson 1966) are all effective acne treatments.
The mechanism of action may be due to sulphur's keratolytic action and consequent inhibitory effect on the proliferation of C acnes (Gupta 2004). It is thought that sulphur interacts with cysteine in keratinocytes resulting in the production of hydrogen sulphide, which has a keratolytic effect by rupturing the disulphide bonds of cysteine molecules in keratin (Pace 1965).
Adverse events are rare during topical use of sulphur. Commonly reported adverse effects include dryness and itching of the skin (Breneman 1993; Gupta 2004; Tarimci 1997). Sulphur is a FDA pregnancy category C drug (Akhavan 2003). Little is known about the excretion of sulphur in breast milk. Therefore, caution should be used by breastfeeding mothers (Akhavan 2003).
Topical zinc
Zinc is known as an essential trace element (Sharquie 2008). It has antimicrobial as well as anti‐inflammatory actions and it is useful for many dermatological problems (Habbema 1989; Sharquie 2007; Sharquie 2008). Physicians often use zinc plus antibiotic combination products for acne treatment (Cunliffe 2005; Habbema 1989). For example, researchers used the form of erythromycin (4%) plus zinc (1.2%) and clindamycin (1%) plus zinc (0.52%), which can be applied twice‐daily for 12 weeks or more (Cunliffe 2005; Habbema 1989). Previous studies have documented that the combination of zinc with antibiotics is more advantageous to acne patients than antibiotics alone (Cunliffe 2005a; Habbema 1989). Some reports suggest that zinc acetate contributes most to the antimicrobial action of an erythromycin/zinc combination (Fluhr 1999). When used alone, topical zinc is also useful for acne patients. Recently, zinc sulphate solution has been showed to be effective in the treatment of acne vulgaris, though it may be less effective than tea lotion (Sharquie 2008).
The mechanism of action may be due to zinc's antimicrobial, anti‐inflammatory, and other actions (Fluhr 1999; Sharquie 2008). Several reports have documented the anti‐propionibacterial activity of zinc in vitro (Bojar 1994; Fluhr 1999). The efficacy on inflammatory lesions by zinc suggests the importance of its anti‐inflammatory actions on acne treatment (Dreno 1989).
There are no important adverse effects reported during topical use of zinc (Cunliffe 2005; Sharquie 2008). The adverse effects include burning sensation and itching, but they are always mild and transient (Sharquie 2008). Although the FDA pregnancy category rating of topical zinc is not available, oral zinc sulphate is a pregnancy category C drug (Chien 2016).
Topical fruit acid (alpha‐hydroxy acid)
Alpha‐hydroxy acid (or fruit acid) refers to a special group of organic acids that can be found in natural foods (Hunt 1992). It is useful in a variety of dermatological conditions with abnormal keratinisation (Hunt 1992; Sharad 2013). Glycolic acid belongs to alpha‐hydroxy acids, which can be used for chemical peeling at concentrations ranging from 20% to 70% (Sharad 2013). Glycolic acid peel is the most common fruit peel (Sharad 2013). In Asian acne patients, the use of 50% glycolic acid peels once in three weeks for 10 weeks can result in significant resolution of comedones, papules, and pustules (Wang 1997). Another study also suggests the efficacy of glycolic acid peels (20% to 70%) in the reduction of both non‐inflamed and inflamed lesions when applied twice every four weeks for six months (Ilknur 2010). Moreover, they are also useful in nodule‐cystic acne and acne scars (Atzori 1999; Wang 1997). Therefore, glycolic acid peel is a useful alternative treatment for acne (Sharad 2013). In addition to glycolic acid, gluconolactone (14%), another alpha‐hydroxy acid, has showed a significant therapeutic effect in reducing acne lesions (Hunt 1992).
The mechanism of action may be due to the modification of keratinisation by alpha‐hydroxy acids, and the anti‐inflammatory activity of alpha‐hydroxy acids may also play a role in acne improvement (Hunt 1992).
The adverse effects are always minimal during topical use of alpha‐hydroxy acids (Hunt 1992; Ilknur 2010), and patient tolerance is reported to be good (Sharad 2013). Glycolic acid is a pregnancy category N drug (rating is not available) but there are no published reports demonstrating any adverse effects during pregnancy (Chien 2016).
Why it is important to do this review
The Global Burden of Disease (GBD) 2010 and 2013 projects measured disease burden using disability‐adjusted life year (DALY) metrics (Hay 2014; Karimkhani 2017); of the 15 dermatologic conditions, acne vulgaris was the skin disease with the second highest percentage of total DALYs either in the GBD 2010 or GBD 2013 study (Hay 2014; Karimkhani 2017). Thus, the global burden of acne is very high (Hay 2014; Karimkhani 2017). A recent report, however, has demonstrated that the limited number of reviews and protocols published in the Cochrane Database of Systematic Reviews (CDSR) does not reflect disease disability estimates for acne and that this topic is underrepresented (Karimkhani 2014). Cochrane Reviews on oral treatments including minocycline (Garner 2012) and contraceptive pills (Arowojolu 2012) for acne have been conducted. Topical treatments including retinoids, benzoyl peroxide (Yang 2014), and antibiotics for acne are (or will be) dealt with in other Cochrane Reviews.
We know of several reviews on some of these topical treatments for acne (Gamble 2012; Haider 2004; Lehmann 2001; Purdy 2011; Seidler 2010). Three of these reviews demonstrated that use of topical azelaic acid shows benefits for mild and moderate acne and is comparable to topical retinoid or benzoyl peroxide (Gamble 2012; Haider 2004; Purdy 2011). However, there is only limited evidence to demonstrate that topical salicylic acid (Gamble 2012), nicotinamide (Purdy 2011), sulphur (Gamble 2012; Lehmann 2001), zinc (Gamble 2012), and alpha‐hydroxy acid (Sharad 2013) may be beneficial for acne treatment. A review on salicylic acid did not include adequate intervention arms and did not assess side effects of the treatments (Seidler 2010). In summary, most of the up to date evidence on these medications is from summary reviews (Gamble 2012; Haider 2004; Purdy 2011; Sharad 2013), and the only two systematic reviews identified are either out of date (Lehmann 2001), or without clear assessment of the quality of evidence (Seidler 2010).
Given the various limitations of previous reviews and the new evidence from recent studies on the use of azelaic and salicylic acids, nicotinamide, sulphur, zinc, and alpha‐hydroxy acid, we feel it is important to systematically assess their benefits and harms for the treatment of acne vulgaris using Cochrane methodology.
The plans for this review were published as a protocol with a slightly different title, 'Topical azelaic acid, salicylic acid, nicotinamide, and sulphur for acne' (Liu 2014).
Objectives
To assess the effects of topical treatments (azelaic acid, salicylic acid, nicotinamide, zinc, alpha‐hydroxy acid, and sulphur) for acne.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Randomised trials with a cross‐over design were eligible. We excluded cluster‐RCTs and quasi‐RCTs trials (e.g. trials that allocate by using date of birth, case record number, or alternation).
Types of participants
We included participants with acne vulgaris who have been diagnosed based on clinical definition, regardless of age, gender, acne severity, and previous treatments. Studies were also eligible where participants were diagnosed as having papulopustular, inflammatory, juvenile, or polymorphic acne.
We excluded trials in which participants had a diagnosis of other forms of acne variants or acneiform eruptions, as listed in Table 12.
Types of interventions
Topical azelaic acid, topical salicylic acid, topical nicotinamide, topical sulphur, topical zinc, and topical fruit acid (alpha‐hydroxy acid) with any treatment regimen, duration, dose, and delivery mode, compared with:
other topical treatments;
placebo;
no treatment.
The trials that compared the intervention treatments with each other were eligible for inclusion. The concomitant use of other topical or oral medications for acne vulgaris had to be the same in both intervention arms.
Types of outcome measures
Primary outcomes
Participants' global self‐assessment of acne improvement (e.g. measured by a 4‐point scale: excellent, good, fair, and poor)
Withdrawal for any reason
Secondary outcomes
Change in lesion counts (total, or inflamed and non‐inflamed separately)
Physicians' global evaluation of acne improvement
Minor adverse events (assessed as the total number of participants who experienced at least 1 minor adverse event)
Quality of life
Timing
We assessed treatment efficacy by grouping the outcomes into short‐term treatment (less than or equal to 4 weeks), medium‐term treatment (from 5 to 8 weeks) and long‐term treatment (more than 8 weeks). Where there was more than one follow‐up point within the same time period, we used the longest one.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist searched the following databases up to 1 May 2019.
Cochrane Skin Group Specialised Register using the search strategy in Appendix 1
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 5) in the Cochrane Library using the search strategy in Appendix 2.
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3.
Embase via OVID (from 1974) using the strategy in Appendix 4.
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trials registers
We (HL and HY) searched the following trials registers up to 1 May 2019 using the search terms (azelaic acid, salicylic acid, o‐hydroxybenzoic acid, nicotinamide, niacinamide, sulphur, sulfur, zinc, fruit acid, alpha‐hydroxy acid, and glycolic acid) combined with health condition 'acne'.
ISRCTN registry (www.isrctn.com).
ClinicalTrials.gov (www.clinicaltrials.gov).
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch).
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching other resources
References from included studies
We checked the bibliographies of included studies for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse events of the target interventions. However, we examined data on adverse effects from the included studies we identified if present.
Data collection and analysis
Selection of studies
Two review authors (HL and HY) independently inspected the titles and abstracts of all studies identified for eligibility. For studies that appeared to be eligible, we retrieved the full text of reports for reassessment to see whether they met the inclusion criteria. We resolved discrepancies by discussion between review authors (HL and HY) and, if necessary, input by a third review author (JX or HS).
Data extraction and management
For data collection, we used a data extraction form adapted from a standard one and the form was piloted followed by minor revisions. We extracted data on an intention‐to‐treat (ITT) basis and used Review Manager 5 for the analysis of data (Review Manager 2014).
Two review authors (HL and HY) independently extracted data from eligible studies using an ITT approach and one review author (FP) extracted data from studies published in German. We collected both qualitative and quantitative information according to Table 7.3.a, 'Checklist of items to consider in data collection or data extraction', in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we collected characteristics of the included studies in sufficient detail to populate a table of 'Characteristics of included studies'. Where further information was required, we contacted the authors for clarification. We resolved any disagreements by discussion and, if necessary, involved a third review author (JX or HS). In the case of data displayed only in graphs or figures, if we were unable to contact study authors, we extracted the data manually using a ruler but only included the data if two review authors independently collected the same results.
The review authors were not blinded to journals, authors, or their academic affiliations. HL, HS and HY entered the data into the Review Manager 5 software (Review Manager 2014).
Assessment of risk of bias in included studies
Two authors (HL and HS) independently assessed the methodological quality of eligible studies using the 'Risk of bias' tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion and, if necessary, involved a third review author (JX or GL). We assessed the following domains for bias.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We categorised the risk of bias in each domain as either 'low', 'high', or 'unclear'. Where two or more out of seven domains within a trial were rated as 'high' risk of bias, we considered including the trial in a sensitivity analysis.
Measures of treatment effect
Interpretation
If possible, we compared the pooled estimates with the minimally important difference (MID) values for both primary and secondary outcomes to aid interpretation. We used the suggested MID from the literature, such as MID estimates for acne lesion counts (Gerlinger 2011), and Acne‐Specific Quality of Life Questionnaire (Acne‐QoL) outcomes (McLeod 2003).
Dichotomous data
For binary outcomes, we calculated the risk ratio (RR) and its 95% confidence interval (CI) to summarise estimates of treatment effect, because the RR was more intuitive than the odds ratio (OR) (Boissel 1999), which was often misinterpreted as RR by clinicians (Deeks 2002).
Continuous data
Summary statistic
For continuous outcomes, we calculated the mean difference (MD) and its 95% CI to summarise data, and used standardised mean difference (SMD) with 95% CI where different measurement scales had been used across studies.
Skewed data
Data from continuous outcomes were often not normally distributed and statistics to summarise average (medians) and spread of data (quartiles, minimum and maximum, and ranges) were used in this case. We summarised such variables using the summary statistics for skewed data in additional tables rather than in the main analysis, and we did not analyse the treatment effect sizes to avoid applying parametric tests to data with skewed distribution. We classified data as skewed when the mean was less than twice the standard deviation (SD), but only when the data were from a scale or outcome measure that had positive values with a minimum value of zero (Altman 1996). Sometimes trials used means to summarise skewed data from very large trials. In this situation, we entered the data into analysis but a sensitivity analysis was necessary.
Ordinal data
Results of participant and doctor evaluations may be presented as short ordinal data. In this situation, we converted this type of data into dichotomous data (e.g. 'improved' or 'not improved'), and we conducted a sensitivity analysis using different cut off points (e.g. 'greatly improved' or 'not greatly improved'). We treated long ordinal data as continuous data.
Unit of analysis issues
We considered the individual participant to be our unit of analysis. For trials with a cross‐over design, we extracted data from a paired t‐test and approximated a paired analysis using the generic inverse variance method. We pooled the randomised cross‐over trials separately from parallel trials.
Where a trial had more than two intervention arms, we identified the interventions relevant to our review and combined arms to create a single pair‐wise comparison. If the combination of groups was impossible, we directly included the correlated or eligible comparisons and addressed them in different meta‐analyses as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For 'split‐face' design trials, in which different body parts were randomised to different interventions, we treated them as specific forms of cross‐over trials, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In this case, we incorporated these trials to approximate a paired analysis using the generic inverse variance method and we conducted a sensitivity analysis. We conducted meta‐analyses of these trials separately from parallel trials.
Finally, trials in which randomisation occurred at a per person level, but multiple body parts received the same intervention and a separate outcome measure was made for each body part, were similar to cluster‐randomised trials except that each participant was a cluster. We excluded these trials, as previously stated.
Dealing with missing data
Overall loss of credibility
Data lose their credibility after a certain degree of loss to follow‐up (Xia 2007; Xia 2009). Where more than 50% of participants withdrew before the end of the trial, we excluded these data from the analysis (with the exception of outcome of 'withdrawal for any reason' and 'minor adverse events').
Binary data
Where there was attrition between 0% and 50% for a binary outcome, we managed data based on the ITT principle. We made 'lost to follow‐up' the worst outcome, that was to say, we imputed participants reported as 'lost to follow‐up' as treatment failures for analysis. This assumption was also applicable to negative outcomes such as adverse events.
Continuous data
Where there was attrition between 0% and 50% for a continuous outcome, we reproduced the completer‐only data and used them within the analysis. In many cases, we could not extract the measures of variance for continuous data directly from the report. We calculated the SD from available data (e.g. 95% CI, standard error, exact P value, and t statistic) according to the method described in Section 7.7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We contacted the trial authors for further information. If these methods were unsuccessful, we used the mean SDs from other studies within the same analysis.
Assessment of heterogeneity
We assessed heterogeneity across studies using the Chi² test and the I² statistic. If the I² statistic was equal to or greater than 50%, significant heterogeneity was present, and we investigated the included studies for their clinical, methodological, and statistical similarities. When necessary, we also employed prespecified subgroup analyses to explore any unexplained heterogeneity. If the I² statistic was equal to or greater than 80%, we presented the data in a forest plot but did not calculate a pooled estimate.
Assessment of reporting biases
Funnel plots are useful to assess reporting biases but have limited power in the case of small‐study effects (Higgins 2011). We had planned to assess reporting bias by using funnel plots. However, as none of the meta‐analyses had 10 or more studies on primary outcomes for a test intervention, we were not able to produce funnel plots.
Data synthesis
We employed a random‐effect model for all pooled analyses. Where results were estimated for individual studies with low numbers of events (fewer than 10 in total), or where the total sample size was fewer than 30 participants and a RR was used, we reported the proportion of outcomes in each treatment group together with a P value from Fisher's Exact test.
Subgroup analysis and investigation of heterogeneity
We set up subgroup analyses according to the different comparators used in the control group and the different time periods of treatment duration for the test interventions. However, we did not conduct a test for subgroup differences due to a lack of adequate numbers of studies per group. In future updates, we plan to conduct subgroup analyses if we find substantial heterogeneity in a meta‐analysis with at least 10 trials on primary outcomes. We will consider the forms, concentrations, and dosing regimens of the interventions for subgroup analyses.
Sensitivity analysis
Where possible, we employed sensitivity analyses by excluding low methodological quality trials from the meta‐analysis: those with 'high' risk of bias for two or more of the seven domains as defined in the 'Risk of bias' tables. Where inclusion or exclusion of these low methodological quality trials did not make significant changes to treatment efficacy, we retained these trials in the final meta‐analysis.
If trials were reported with randomisation and balanced baseline demographic characteristics in each group, we included the trials and entered them into a sensitivity analysis.
'Summary of findings' tables and GRADE assessments
We created 'Summary of findings' tables in our review, in which we summarised the primary outcomes (participants' global self‐assessment of acne improvement, and withdrawal for any reason), and secondary outcomes (minor adverse events and quality of life) for the most important comparisons. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for these four outcomes, and documented all the assessments of the body of evidence using the GRADEpro GDT software (Higgins 2011).
Results
Description of studies
Results of the search
The Electronic searches identified 386 records. Our screening of the bibliographies of included studies identified five studies. Our search in the trial registers identified 40 studies. Therefore, we had a total of 431 records. After removing duplicates, we had 429 records. We excluded 295 records based on titles and abstracts. We attempted to obtain the full texts or abstracts of the remaining 134 records. We excluded 55 studies reported in 60 references that did not meet the inclusion criteria (see Characteristics of excluded studies). We added 12 records to Studies awaiting classification and classified three studies as Ongoing studies. We included 49 studies reported in the remaining 59 references (see Characteristics of included studies). Please see the study flow diagram for a further description of our screening process (Figure 1).
1.
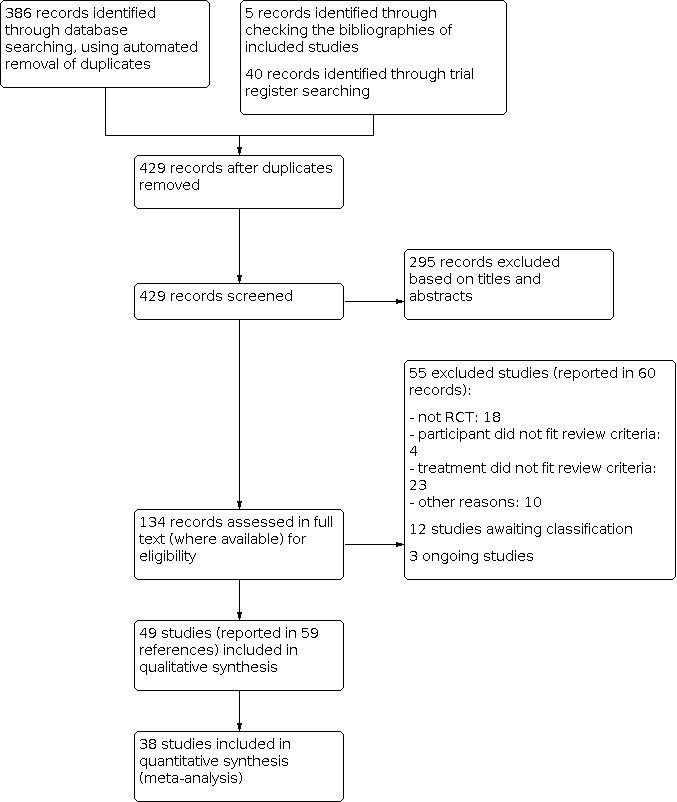
Study flow diagram
Included studies
We included a total number of 49 studies. Of these studies, 47 included 3880 participants, whereas the other two studies did not report the sample size (Chantalat 2007; Chen 2007). See 'Characteristics of included studies' tables for detailed descriptions of each included study. We attempted to contact eight authors in order to obtain additional data. Only two authors provided data, four authors did not respond to our enquiries, and the other two authors responded with insufficient information.
Trial size
The trial size ranged from n = 13 to n = 351. In 19 studies, all participants completed the trial (n = 871), while 23 studies had various numbers of participants leaving the study early (between 2% to 39.5%). Seven studies did not describe dropout information (Cavicchini 1989; Chantalat 2005; Chantalat 2007; Chen 2007; Dunlap 1997; Hayashi 2012; Techapichetvanich 2011).
Unit of allocation
For unit of allocation, 42 studies randomised individual participants into parallel groups. Six studies with 175 participants were split‐face studies (Bae 2013; Hayashi 2012; Ilknur 2010; Kessler 2008; Kim 1999; Levesque 2011). One study (n = 30) was a cross‐over randomised trial (Shalita 1989).
Gollnick 2004a and Gollnick 2004b were two studies described in one report. Likewise, Katsambas 1989a and Katsambas 1989b were two studies reported in the same paper.
Participants
Forty‐seven trials included 3880 participants, and the other two trials did not report the sample size (Chantalat 2007; Chen 2007). The authors in Chantalat 2007 did not report the severity of acne and authors in Chen 2007 included participants with mild to moderate acne. Of the 3880 participants from 47 trials, 2939 participants (75.7%) from 33 trials included mild to moderate acne vulgaris graded by various acne severity grading systems or scales. The severity of the remaining participants were as follows.
140 participants from three studies had moderate to severe acne (Aksakal 1997; ElRefaei 2015; Kar 2013).
20 participants from one study had mild to moderately severe acne (Kessler 2008).
188 participants from three studies had unknown severity illness (Hayashi 2012; Picosse 2015; Vasarinsh 1969).
399 participants from five studies probably had mild to moderate acne (Barbareschi 1991; Cavicchini 1989; Ilknur 2010; Katsambas 1989b; Levesque 2011).
150 participants from one study probably had moderate to severe acne (Dunlap 1997).
44 participants from one study probably had moderate to severe/cystic acne (Garg 2009).
With respect to seven studies with no acne severity grading, we presented a possible grading by using a simple system (mild, moderate, severe, cystic) based on the predominant lesions present as reported in Dayal 2017. For a detailed description, please see Table 13.
3. Acne severity for all studies.
| Studies | Acne severity | Notes |
| Akarsu 2012 | Mild to moderate | Defined as "10‐50 inflamed lesions and 10‐100 non‐inflamed lesions above the mandibular line, no cystic or nodular lesions." |
| Aksakal 1997 | Moderate to severe | Graded by using the Allen‐Smith Scale (grades of 4 to 8) |
| Babayeva 2011 | Mild to moderate | Defined as "10‐50 inflamed lesions and 10‐100 non‐inflamed lesions above the mandibular line, no cystic or nodular lesions." |
| Bae 2013 | Mild to moderate | Graded using the Burke and Cunliffe Scale (Leeds technique) (grades of 0.25 to 3.0) |
| Barbareschi 1991 | Probably mild to moderatea | Participants with comedonic acne included, no further details |
| Bojar 1994 | Mild to moderate | Graded using the Burke and Cunliffe Scale (Leeds technique) (grades of 0.5 to 3.0) |
| Cavicchini 1989 | Probably mild to moderatea | Participants with papulopustular acne included, median number of inflamed lesions was less than 20 |
| Chantalat 2005 | Mild to moderate | Acne severity grading method not reported, this study was published as an abstract |
| Chantalat 2006 | Mild to moderate | Acne severity grading method not reported, this study was published as an abstract |
| Chantalat 2007 | Not reported | Acne severity grading method not reported, this study was published as an abstract |
| Chen 2007 | Mild to moderate | Acne severity grading method not reported, this study was published as an abstract |
| Cunliffe 1989 | Mild to moderate | Acne severity grading method not reported, only mentioned "the trial was for the treatment of mild to moderate acne" in the Discussion section, no details |
| Cunliffe 2005 | Mild to moderate | Graded using the Leeds Revised Acne Grading Scale (grades of 2 to 7) |
| Dayal 2017 | Mild to moderate | Graded using a simple system (based on the predominant lesions present: mild, moderate, severe, cystic) |
| Draelos 2016 | Mild to moderate | Acne severity grading method not reported, a minimum of 10 non‐inflamed lesions and a minimum of 10 inflamed lesions |
| Dunlap 1997 | Probably moderate to severea | Grade Ⅱ or Ⅲ, Pillsbury classification system |
| Eady 1996 | Mild to moderate | Graded using the Leeds technique, no details |
| ElRefaei 2015 | Moderate to severe | Graded according to the Hayashi classification system (mild, moderate, severe, or very severe) |
| Garg 2009 | Probably moderate to severe/cystica | Mean baseline Michaelsson acne severity index score > 80, the improvement of comedones, papules, pustules, nodules and cysts was assessed |
| Gollnick 2004a | Mild to moderate | Acne severity grading method not reported |
| Gollnick 2004b | Mild to moderate | Acne severity grading method not reported |
| Hayashi 2012 | Not reported | Total lesion counts > 30, no further details, this study was published as an abstract |
| Hunt 1992 | Mild to moderate | Graded using the Burke and Cunliffe Scale (Leeds technique), no further details |
| Ilknur 2010 | Probably mild to moderatea | Graded using the Burke and Cunliffe Scale (Leeds technique), grades of 0.25 to 2.0, participants with non‐inflamed lesions and superficial inflamed lesions |
| Iraji 2007 | Mild to moderate | Graded using the Burke and Cunliffe Scale (Leeds technique), no further details |
| Jaffary 2016 | Mild to moderate | Defined as "no more than five pustules forms and no cysts, nodules, and colloidal deep scar" |
| Kar 2013 | Moderate to severe | Average baseline Michaelsson acne severity index in the two treatment arms was 64.1 ± 4.4 and 63.0 ± 5.1 |
| Katsambas 1989a | Moderate inflammatory acne | Degree Ⅱ or Ⅲ, Plewig‐Kligmann classification system, participants with papulopustular acne were included |
| Katsambas 1989b | Probably mild to moderatea | Participants with comedonal acne, no further details |
| Kessler 2008 | Mild to moderately severe | Acne severity grading method not reported, a minimum of 10 papules and/or pustules |
| Khodaeiani 2013 | Moderate inflammatory acne | Grade Ⅲ, the Leeds technique |
| Kim 1999 | Mild to moderate | Graded using the Leeds technique, grades of 0.25 to 2.0 |
| Levesque 2011 | Probably mild to moderatea | Subjects with comedonal acne (at least 5 non‐inflamed lesions on each side of the face and < 30 inflamed lesions on entire face) |
| NilFroushzadeh 2009 | Mild to moderate | Acne severity grading method not reported |
| Ozkan 2000 | Mild to moderate | Graded using the Leeds technique, ≤ 3.0 |
| Pazoki‐Toroudi 2010 | Mild to moderate | Defined as "at least 10 inflammatory lesions on the face and with a maximum of three nodules" |
| Pazoki‐Toroudi 2011 | Mild to moderate | A clinical diagnosis of mild to moderate acne, ≥ 10 facial lesions |
| Picosse 2015 | Not reported | No details, this study was published as an abstract |
| Schaller 2016 | Mild to moderate | Investigators' static global assessment score of mild or moderate |
| Shahmoradi 2013 | Mild to moderate | Self‐defined grading system (mild acne: the presence of non‐inflammatory lesions, and the number of the papules, and pustules to be < 10 without any nodules or cysts; moderate acne: the presence of non‐inflammatory lesions and the number of the papules and pustules to be < 20 without any nodules or cysts) |
| Shalita 1981 | Mild to moderate | Grade Ⅰ or Ⅱ, Pillsbury classification system |
| Shalita 1989 | Mild to moderate | Self‐defined grading system (the presence of at least 10 papulopustular lesions on the face accompanied by a minimum of 5 comedones, as well as a grade of 4 to 6 on the Allen‐Smith Acne Severity Scale) |
| Shalita 1995 | Moderate inflammatory acne | Self‐defined grading system (the presence of at least 15 papules and/or pustules on the face) |
| Sharquie 2008 | Mild to moderate | Mild acne: the count of pustules < 20 and the count of papules < 10; moderate acne: the count of pustules ranged between 20 and 40 and the count of papules ranged between 10 and 30 |
| Stinco 2007 | Mild to moderate | Participants with mild or moderate comedonic or papulopustular acne, a minimum of 20 facial non‐inflammatory lesions and 10 inflamed lesions |
| Techapichetvanich 2011 | Mild to moderate | Acne severity grading method not reported, this study was published as an abstract |
| Thielitz 2015 | Mild to moderate | Graded using a modified Investigators' Static Global assessment (grades of 2 to 4) and the Leeds Revised Acne Grading Scale (grades of 2 to 7) |
| Vasarinsh 1969 | Not reported | Not reported |
| Weltert 2004 | Moderate inflammatory acne | Participants with moderate inflammatory ance on face (≥ 5 inflammatory elements, papules or pustules) |
aPossible acne severity, graded by using a simple system based on the predominant lesions present (Dayal 2017), grade 1 (mild): comedones, occasional papules; grade 2 (moderate): papules, comedones, few pustules; grade 3 (severe): predominant pustules, nodules, abscesses; grade 4 (cystic): mainly cysts, abscesses, widespread scarring.
Forty‐one studies described the age of participants (ranging from 10 years to 45 years old). One study included participants ≥ 16 years old (range unclear) (Hayashi 2012), and one study reported the means and standard deviations (SDs) for age (range unclear) (Dayal 2017). Six studies did not report this information. Most people were aged between 12 and 30 years old. As for sex distribution, 38 studies with 3154 participants described this information, of which 1295 were males and 1842 were females (there was no gender information about 17 withdrawals from Bojar 1994, Ilknur 2010 and Schaller 2016). Eleven studies did not report the exact number of male and female participants.
Settings
Twenty‐two studies did not provide information on setting. Seven studies were multiple‐centre clinical trials (Cunliffe 2005: 8 centres in the UK, 1 in France, and 1 in Germany; Gollnick 2004a and Gollnick 2004b: centres in Germany, Netherlands, Norway, and Greece; Hayashi 2012: centres in Japan; Katsambas 1989b: no details; Schaller 2016: 11 centres in Germany; Shalita 1995: centres in the USA).
Twenty studies were set in: clinics in Iran (Khodaeiani 2013; Pazoki‐Toroudi 2010; Pazoki‐Toroudi 2011), hospitals in India (Garg 2009; Kar 2013) and Iraq (Sharquie 2008), research centres in Iran (Jaffary 2016), university settings (Bae 2013; Kim 1999 in Korea; Dayal 2017 in India; ElRefaei 2015 in Egypt; Kessler 2008 in USA; Ozkan 2000 in Turkey; Shahmoradi 2013 in Iran; Thielitz 2015 in Germany; Vasarinsh 1969 in USA), or other (Draelos 2016; Levesque 2011 in USA; NilFroushzadeh 2009 in Iran; Weltert 2004 in France).
Interventions
For the treatment group, 18 studies assessed salicylic acid in the form of lotion (concentrations of 2% or 3%, e.g. Babayeva 2011), chemical peel (concentrations of 20% or 30%, e.g. Bae 2013), gel (concentration of 2%, e.g. Draelos 2016), microgel (concentrations of 0.5% or 2%, e.g. Chantalat 2006), and solution (concentration of 0.5%, e.g. Shalita 1981), and 18 studies addressed azelaic acid in the form of cream (concentration of 20%, e.g. Schaller 2016) and gel (concentrations of 5%, 15%, or 20%, e.g. Gollnick 2004a); four studies investigated nicotinamide in the form of gel (concentration of 4% or 5%, Khodaeiani 2013; Shahmoradi 2013; Shalita 1995; Weltert 2004), while three studies addressed zinc in the form of gel (concentration of 1%, Cunliffe 2005), lotion (concentration of 1.2%, Bojar 1994), and solution (concentration of 5%; Sharquie 2008); one study assessed sulphur in the form of lotion (concentration of 2%, Vasarinsh 1969); five studies tested the efficacy and safety of glycolic acid (alpha‐hydroxy acid) in the form of chemical peel (concentration ranging from 20% to 70%, ElRefaei 2015; Garg 2009; Ilknur 2010; Kessler 2008; Kim 1999) and one study investigated gluconolactone (alpha‐hydroxy acid) in the form of lotion (concentration of 14%, Hunt 1992). In the Kessler 2008 study, the authors compared salicylic acid with alpha‐hydroxy acid (glycolic acid).
Seven studies used topical clindamycin, erythromycin, or benzoyl peroxide as co‐interventions (Babayeva 2011; Bojar 1994; Cunliffe 2005; NilFroushzadeh 2009; Pazoki‐Toroudi 2010; Pazoki‐Toroudi 2011; Vasarinsh 1969). Two studies included a co‐intervention of topical clindamycin plus benzoyl peroxide (Akarsu 2012; Techapichetvanich 2011). One study used oral isotretinoin as co‐intervention (Kar 2013). The remaining 39 studies did not include co‐interventions.
The treatment period of the interventions ranged from five days to 12 months. We grouped three studies into short‐term (less than or equal to 4 weeks) treatment duration (Bae 2013; Draelos 2016; Shalita 1989), 15 studies into medium‐term (from 5 to 8 weeks) treatment duration, and 29 studies into long‐term (more than 8 weeks) treatment duration. Two studies did not report treatment duration (Chantalat 2005; Chantalat 2007).
Three studies had a post‐treatment follow‐up period ranging from eight weeks to 12 weeks (ElRefaei 2015; Garg 2009; Kessler 2008). In Thielitz 2015, one treatment arm had a post‐treatment follow‐up period of 24 weeks. Forty‐three studies did not report the post‐treatment follow‐up period. Two studies did not mention the treatment period of the interventions and the post‐treatment follow‐up period (Chantalat 2005; Chantalat 2007).
None of the studies reported the precise duration of the trial (from recruitment to last follow‐up). Nine studies reported the study time period ranging from three months to three years (ElRefaei 2015; Gollnick 2004a; Gollnick 2004b; Kar 2013; Shahmoradi 2013; Shalita 1981; Sharquie 2008; Thielitz 2015; Vasarinsh 1969). Cunliffe 2005 ran through autumn, winter and early spring. Seven studies reported recruitment time periods ranging from six months to one year (Jaffary 2016; Khodaeiani 2013; Levesque 2011; NilFroushzadeh 2009; Pazoki‐Toroudi 2010; Pazoki‐Toroudi 2011; Schaller 2016).
Comparators
For the control group, 31 studies used active treatments, such as clindamycin, erythromycin, tretinoin, Jessner's solution, benzoyl peroxide, and so on. Fourteen studies had placebo/vehicle control or no treatment; and four studies had multiple treatment groups with both active therapy and placebo control (Barbareschi 1991; Draelos 2016; Hunt 1992; Vasarinsh 1969). In five included studies (Cunliffe 1989; Hunt 1992; Iraji 2007; Katsambas 1989a; Shalita 1981), study authors considered the term 'vehicle' to be the same as 'placebo'. In one study (Iraji 2007), the excipients used in the vehicle did not contain antimicrobial agents which may have some therapeutic effects. In another two studies (Eady 1996; Vasarinsh 1969), the study authors used a lotion base as placebo. Thus, 'vehicle' was equal to 'placebo' in this review and we pooled the two groups together in the same comparison.
Outcomes
Nineteen studies reported global self‐assessment of acne improvement (assessed by the participants). Of the 19 studies, 16 used Likert‐type or Likert‐like scales, one used a visual analogue scale (Cunliffe 2005), one used a questionnaire with known contents (Kessler 2008), and one used a preference test (Kim 1999). Thirty studies did not report this primary outcome. Forty‐two studies reported the withdrawal information (19 studies reported no withdrawals), while seven studies did not report this outcome (Cavicchini 1989; Chantalat 2005; Chantalat 2007; Chen 2007; Dunlap 1997; Hayashi 2012; Techapichetvanich 2011).
For secondary outcomes, 44 studies reported the change in lesion counts (assessed by the investigators or physicians), while five studies did not report this outcome (Draelos 2016; Kim 1999; Ozkan 2000; Picosse 2015; Shahmoradi 2013). Seventeen studies described the physicians' global evaluation of acne improvement (assessed by the physicians) (15 studies used Likert‐like scales, two studies used visual analogue scales (Cunliffe 2005; ElRefaei 2015); the remaining 32 did not report this outcome.
Forty‐five studies reported minor adverse events (assessed as total number of participants who experienced at least 1 minor adverse event), but the number of participants who experienced adverse events was not always reported. Of the 45 studies, four reported no adverse events during the study (Chen 2007; Draelos 2016; Shahmoradi 2013; Shalita 1981), eight used four‐point Likert scales or similar scales (Akarsu 2012; Babayeva 2011; Cunliffe 1989; Cunliffe 2005; Hunt 1992; Schaller 2016; Stinco 2007; Thielitz 2015), and one used a visual analogue scale to assess the severity of adverse events (Levesque 2011). Four trials did not report this outcome (Barbareschi 1991; Bojar 1994; Dunlap 1997; Shalita 1989).
Six studies reported quality of life assessed by the participants (Akarsu 2012; Babayeva 2011; Chantalat 2006; Kim 1999; Schaller 2016; Thielitz 2015). Of these, three studies used the Acne‐Specific Quality of Life Questionnaire (Acne‐QoL) (Akarsu 2012; Babayeva 2011; Chantalat 2006), two studies used the Dermatology Life Quality Index (DLQI) Questionnaire (Schaller 2016; Thielitz 2015), and one study used preference test questions (Kim 1999). The rest of the studies (43) did not report quality of life. Of the 49 included studies, 24 studies reported other outcomes like skin barrier functions and acne severity Index.
Funding
Thirty‐one trials did not describe the study funding sources. Authors from three trials were employees of a pharmaceutical company (Gollnick 2004a; Gollnick 2004b; Thielitz 2015). Nine trials received support from a pharmaceutical company or corporation (Bojar 1994; Chen 2007; Cunliffe 2005; Draelos 2016; Hunt 1992; Kar 2013; Schaller 2016; Shalita 1989; Shalita 1995). The posters from two trials were funded by Johnson & Johnson Consumer and Personal Products Worldwide (Chantalat 2006; Chantalat 2007). One trial received support from two persons with unknown positions (Eady 1996). One trial received funding from a university (Pazoki‐Toroudi 2011), and one trial received support from the National Institute of Health, US Public Health Service and The Detroit General Hospital Research Corporation (Vasarinsh 1969). Only one trial clearly stated that there was no funding or financial source in support of the work (Khodaeiani 2013).
Excluded studies
We excluded 55 studies: 18 studies were not RCTs; four studies focused on healthy people or participants with rosacea; 23 presented ineligible interventions or comparisons; and in 10 studies we could not contact authors for clarification of randomisation. See 'Characteristics of excluded studies' tables for detailed descriptions of each excluded study.
Studies awaiting assessment
We added 12 records to studies awaiting classification (see the 'Characteristics of studies awaiting classification' tables). Reasons include: unable to obtain the full text (Bartosova 1978; Cavicchini 1989a; Draelos 2015; Giannotti 1989; Pisani 1991; Ponzio 1994, Zheng 2019), the study was listed as completed on a trial registry but no results could be obtained (IRCT201010094269N3; NCT00031096; NCT02755545; TCTR20190118001), and only a conference abstract was identified and it was not clear if the study met the inclusion criteria of the review (Kern 2019).
Ongoing studies
We identified three studies as ongoing and added details to the 'Characteristics of ongoing studies' tables. Comparisons included salicylic acid peel versus glycolic acid peel (ChiCTR1800018343), 35% glycolic acid peels versus 20% salicylic acid peels (CTRI/2018/06/014615), and salicylic acid plus Epiduo 0.1% to 2.5% topical gel versus moisturiser plus Epiduo 0.1% to 2.5% topical gel (NCT03832647).
Risk of bias in included studies
Please refer to Figure 2 and Figure 3 for a graphical overview of the risk of bias of included studies. See the 'Characteristics of included studies' tables for detailed assessment of risk of bias of each included study.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Only eight studies (16.3%) mentioned appropriate randomisation methods and we judged them at low risk of bias. Of this group, the authors performed randomisation using the FORTRAN 77 RANDT program (Cunliffe 1989), computerized randomisation (Dayal 2017), sealed envelope (ElRefaei 2015), drawing (Ilknur 2010), random number table (Kar 2013), random permuted block (Kim 1999), computer‐generated schedule (Schaller 2016), and minimisation method (Thielitz 2015). We judged the majority of included studies (41 studies) at unclear risk of bias for randomisation because these studies mentioned randomisation but provided inadequate description of randomisation methods.
Allocation concealment
Only one (2%) study reported the method used for allocation concealment and we rated it at low risk of bias (ElRefaei 2015; Figure 3). The remaining 48 studies did not address this issue or did not provide sufficient information to permit judgement.
Blinding
Of the 49 included studies, 21 had a double‐blind design, 18 had a single‐blind design, and the remaining 10 did not state this issue. Of the 21 studies (42.9%) with a double‐blind design, only two clearly stated their method to ensure blinding of participants and assessors (Hunt 1992; Iraji 2007). We rated the two studies to be at low risk of bias for both domains. Eleven studies with a 'double‐blind' design did not present sufficient information about method to ensure blinding of participants/personnel and assessors, we therefore judged them at unclear risk of bias for both domains in these studies (Bojar 1994; Chantalat 2005; Chantalat 2006; Draelos 2016; Gollnick 2004a; Gollnick 2004b; Pazoki‐Toroudi 2010; Pazoki‐Toroudi 2011; Shahmoradi 2013; Shalita 1995; Vasarinsh 1969). We judged the other seven of 21 double‐blinded studies to be at low risk of performance bias and unclear risk of detection bias (Chantalat 2007; Chen 2007; Cunliffe 1989; Eady 1996; Katsambas 1989a; Khodaeiani 2013; Techapichetvanich 2011). In addition, we judged one of 21 double‐blinded studies to be at unclear risk of performance bias and low risk of detection bias (Kessler 2008).
Of the 18 single‐blind studies, eight followed an assessor or observer masked design. Of the eight studies, five did not provide sufficient information on method to ensure blinding of assessors throughout the study, therefore we judged the risk of performance bias as high and detection bias as unclear (Akarsu 2012; Bae 2013; Ilknur 2010; Kim 1999; Cunliffe 2005). We judged two of the eight studies to be at unclear risk of performance bias and at low risk of detection bias (Dayal 2017; ElRefaei 2015), as the two studies clearly stated how blinding of assessors was ensured. Moreover, we judged one of the eight studies to be at high risk of performance bias and low risk of detection bias (Schaller 2016), as the participants and personnel were open to interventions.
Five of the 18 single‐blind studies were investigator blinded. Of the five studies, three provided sufficient information on method to ensure blinding of investigators, we judged one to be at high risk of performance bias and at low risk of detection bias (Kar 2013), and one to be at unclear risk of performance bias and at low risk of detection bias (Levesque 2011), and one at high risk of performance bias and unclear risk of detection bias (Thielitz 2015). Two out of the five studies did not provide sufficient information on method to ensure blinding of investigators, we judged them to be at unclear risk of performance bias, and one to be at high risk (Dunlap 1997), and the other one to be at low risk of detection bias (Hayashi 2012).
Two of the 18 single‐blind studies did not provide sufficient information on how blinding was ensured, and we judged them to be at unclear risk of bias for both domains (Jaffary 2016; Sharquie 2008). Another two of the 18 studies were participant‐blinded, we judged them to be at high risk of detection bias, and one to be at unclear risk of performance bias due to the use of identical tubes (Cavicchini 1989), and the other one to be at high risk of performance bias due to probably insufficient blinding of personnel (NilFroushzadeh 2009). One of the 18 studies was open to assessor, and we judged it to be at unclear risk of performance bias and at high risk of detection bias (Babayeva 2011).
Ten studies did not mention blinding. For this reason, we judged nine of the 10 studies to be at unclear risk of bias for both domains (Aksakal 1997; Barbareschi 1991; Katsambas 1989b; Ozkan 2000; Picosse 2015; Shalita 1981; Shalita 1989; Stinco 2007; Weltert 2004). We judged one study to be at unclear risk of performance bias and at high risk of detection bias since the assessor was not blinded (Garg 2009).
Incomplete outcome data
We judged a total of 31 studies (63.3%) to be at low risk of attrition bias as they ensured the completeness of outcome data. Of this group, 19 studies had no dropouts, five studies provided data based on intention‐to‐treat (ITT) population, and in seven studies the dropout rate (< 10% per group) was not considered enough to introduce bias.
Of the 11 studies (22.4%) that did not provide complete outcome data, we judged at high risk of attrition bias. Of these, 10 studies had a dropout rate of more than 10% per group and one did not state which dropouts belonged to which treatment group (Bojar 1994).
It remained unclear if the outcome data were sufficiently addressed in seven trials (14.3%), we judged them to be at unclear risk of attrition bias.
Selective reporting
Twenty‐three studies (46.9%) reported results for all prespecified outcomes mentioned in the study protocol or their methods section (if the protocol was not available), and we judged them to be at low risk of reporting bias. We judged four studies (8.2%) to be at high risk of reporting bias due to no results reported for prespecified outcomes or additionally reported outcomes that were not mentioned in the methods section. In the remaining 22 studies (44.9%), we rated them to be at unclear risk of reporting bias. Of this group, 14 studies did not provide data in a detailed manner and eight studies were published as abstract or poster only.
Other potential sources of bias
In 42 studies (85.7%), we identified no other potential sources of bias and we judged them to be at low risk of bias.
We judged three studies (6.1%) to be at high risk of other potential sources of bias. In one trial, there was baseline imbalance in total lesion counts across groups and the author used a Student's t‐test with no post hoc analysis to compare the mean lesions of three treatment groups (Hunt 1992). In one study, there was a suspicion of fraudulent data reporting (Jaffary 2016). In a trial with a cross‐over design, there was no washout period between the first and second phases of the study (Shalita 1989).
In four studies (8.2%) it was unclear whether the identified problems would introduce bias. One study reported in the statistical approach that they used non‐inferiority borders of 15% (Gollnick 2004a); however, it was not fully clear whether they described an equivalence trial or an inferiority trial. In addition, the total number of participants in table one of the publication was incorrectly reported. In another study (Gollnick 2004b), the authors reported an advantage for azelaic acid, although the difference was not significant, moreover, the duration of therapy differed between treatment groups. The local adverse events were compared in an indirect fashion and the authors were employees of a pharmaceutical company that marketed azelaic acid in both studies (Gollnick 2004a and Gollnick 2004b). In another study (Draelos 2016), one author was an employee of GlaxoSmithKline Consumer Healthcare Ltd at the time the study was conducted and the sponsor reviewed the final manuscript before submission. Finally in one trial (Schaller 2016), three authors were employees of GlaxoSmithKline Consumer Healthcare Ltd and held stocks/shares in this company.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
In this review, we assessed treatment efficacy of all six test interventions (topical azelaic acid, topical salicylic acid, topical nicotinamide, topical sulphur, topical zinc, and topical fruit acid (alpha‐hydroxy acid) by grouping the outcomes into short‐term treatment (less than or equal to 4 weeks), medium‐term treatment (from 5 to 8 weeks) and long‐term treatment (more than 8 weeks). Particularly, we specified the treatment duration for long‐term outcomes. The included studies measured their outcomes at the end of treatment unless otherwise specified. We presented outcome data collected in the post‐treatment follow‐up period in a narrative way.
With respect to the outcome, change in lesion counts, 'percentage reduction from baseline' referred to the difference between the mean lesion counts at the beginning and end of treatment divided by the mean lesion counts at the beginning of treatment, and 'number of lesions post‐intervention' referred to the lesion counts after therapy.
Comparison 1: topical azelaic acid versus other topical treatments
Participants' global self‐assessment of acne improvement
The single Thielitz 2015 trial compared azelaic acid 15% gel with adapalene 0.1% gel, and the authors assessed participants' global self‐assessment of acne improvement using a one to seven grading system (7 grades between 'very much improved' (1) and 'very much worse' (7)). We found that there were no significant differences between the two groups in 'improved' to 'very much improved' in the medium term (risk ratio (RR) 0.74, 95% confidence interval (CI) 0.52 to 1.06; 1 study, 55 participants) and long term (3 months after start of treatment) (RR 0.89, 95% CI 0.68 to 1.17; 1 study, 55 participants; very low‐quality evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 1: Participants' global self‐assessment of acne improvement
The single Gollnick 2004a trial compared azelaic acid 15% gel with benzoyl peroxide 5% gel, and the authors assessed the outcome using a four‐point Likert‐type rating scale (very good, good, moderate, poor). Results supported that benzoyl peroxide group had a statistically significant improvement (good or very good improvement) compared to azelaic acid in the long term (4 months after start of treatment) (RR 0.82, 95% CI 0.72 to 0.95; 1 study, 351 participants; moderate‐quality evidence; Analysis 1.1; Table 2).
The single Pazoki‐Toroudi 2011 trial compared azelaic acid 5% gel with clindamycin 2% gel, and the authors assessed this outcome using a five‐point Likert‐type scale (very satisfied, satisfied, moderately satisfied, unsatisfied, very unsatisfied). We found no significant difference in moderately to very satisfied improvement in the long term (3 months after start of treatment) (RR 0.95, 95% CI 0.81 to 1.12; 1 study, 100 participants; Analysis 1.1). Another single trial, Gollnick 2004b, compared azelaic acid 15% gel with clindamycin 1% gel, and the authors assessed the outcome using a four‐point Likert‐type rating scale (very good, good, moderate, poor). We found that there was no significant difference in good or very good improvement in the long term (4 months after start of treatment) (RR 1.13, 95% CI 0.92 to 1.38; 1 study, 229 participants; low‐quality evidence; Analysis 1.1; Table 3).
The single Pazoki‐Toroudi 2010 trial compared azelaic acid 20% gel with erythromycin 2% gel, and the authors assessed this outcome using a five‐point Likert‐type scale (very satisfied, satisfied, moderately satisfied, unsatisfied, very unsatisfied). We found no significant difference in moderately to very satisfied improvement in the long term (3 months after start of treatment) (RR 1.06, 95% CI 0.85 to 1.32; 1 study, 66 participants; Analysis 1.1).
The single Katsambas 1989b trial compared 20% azelaic acid cream with 0.05% tretinoin cream, and the authors reported the outcome using a four‐point Likert‐type rating scale (excellent, good, moderate, poor). Results showed that there was no significant difference in good to excellent improvement in the long term (6 months after start of treatment) (RR 0.94, 95% CI 0.78 to 1.14; 1 study, 289 participants; moderate‐quality evidence; Analysis 1.1; Table 4).
The single Schaller 2016 trial compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel, and the authors reported this outcome using a 7‐point scale (0 = very much improved, 1 = much improved, 2 = minimally improved, 3 = no change, 4 = minimally worse, 5 = much worse, 6 = very much worse) at all terms (4, 8, and 12 weeks after start of treatment). The study authors compared the proportion of participants with ratings of 'much improved/very much improved' and there was a clear difference between groups in favour of benzoyl peroxide 3% + clindamycin 1% gel in the medium and long term. Short term: RR 0.74 (95% CI 0.54 to 1.02; 1 study, 221 participants); medium term: RR 0.72 (95% CI 0.55 to 0.95; 1 study, 221 participants); and long term: RR 0.75 (95% CI 0.57 to 0.99; 1 study, 221 participants) (Analysis 1.1). The quality of evidence was low (Table 14).
4. Azelaic acid compared to benzoyl peroxide/clindamycin.
| Azelaic acid compared to benzoyl peroxide/clindamycin for acne | ||||||
| Patient or population: participants with acne Settings: 11 study centres in Germany Intervention: topical azelaic acid Comparison: topical benzoyl peroxide/clindamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical benzoyl peroxide/clindamycin | Topical azelaic acid | |||||
|
Participants' global self‐assessment of acne improvement
Much to very much improved (long term: treatment duration > 8 weeks) |
559 per 1000 | 419 per 1000 (318 to 553) | RR 0.75 (0.57 to 0.99) | 221 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
63 per 1000 | 73 per 1000 | RR 1.15 (0.43 to 3.07) |
221 (1 study) | ⊕⊕⊝⊝ Lowb | ‐ |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
559 per 1000 | 693 per 1000 (564 to 849) | RR 1.24 (1.01 to 1.52) | 221 (1 study) | ⊕⊕⊝⊝ Lowb | The common application site reactions include pruritus, pain, erythema and dryness. |
|
Quality of life CDLQI (long term: treatment duration > 8 weeks) |
The authors reported that a greater mean (SD) change in CDLQI was noted with benzoyl peroxide 3% + clindamycin 1% gel (‐60.5% ± 70.6, n = 107) versus azelaic acid 20% cream (‐36.8% ± 74.8, n = 108). | 215 (1 study) | ⊕⊕⊝⊝ Lowc |
Skewed data reported. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CDLQI: Children's Dermatology Life Quality Index; CI: confidence interval; RR: risk ratio; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and with unclear risk of allocation concealment and other bias. One level for imprecision: optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and with unclear allocation concealment and other bias. One level for imprecision: wide CI and optimal sample size not met. cDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of allocation concealment and other bias. One level for imprecision: total population size is less than 400 and wide CI. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
We rated the findings measured in the long term as very low‐ to moderate‐quality evidence due to risk of bias and/or imprecision (Table 1; Table 14; Table 2; Table 3; Table 4).
Withdrawal for any reason
For this outcome, two studies compared azelaic acid with adapalene (Stinco 2007; Thielitz 2015), and there was no clear difference in the short term (RR 0.80, 95% CI 0.05 to 12.01; 1 study, 45 participants) and long term (12 weeks after start of treatment) (RR 2.64, 95% CI 0.33 to 20.99; 1 study, 55 participants) (Analysis 1.2). The quality of evidence was very low (Table 1). When azelaic acid was compared with benzoyl peroxide (Gollnick 2004a; Stinco 2007), there was also no clear difference in the short term (RR 0.40, 95% CI 0.04 to 4.10; 1 study, 45 participants) and long term (16 weeks after start of treatment) (RR 0.88, 95% CI 0.60 to 1.29; 1 study, 351 participants) (Analysis 1.2). The quality of evidence was low (Table 2). When azelaic acid was compared with clindamycin, one study reported no withdrawals in the medium term (Ozkan 2000). Another two studies compared azelaic acid with clindamycin in the long term (treatment duration over 12 weeks) (Gollnick 2004b; Pazoki‐Toroudi 2011), there was no clear difference (RR 1.30, 95% CI 0.48 to 3.56; 2 studies, 329 participants; low‐quality evidence; Analysis 1.2; Table 3). When azelaic acid was compared with metronidazole, one study reported no withdrawals in the long term (12 weeks after start of treatment) (Analysis 1.2). When 20% azelaic acid cream was compared with 0.05% tretinoin cream (Barbareschi 1991; Katsambas 1989b), there was no statistically significant difference in the long term (treatment duration over 12 weeks) (RR 0.66, 95% CI 0.29 to 1.47; 2 studies, 309 participants; low‐quality evidence; Analysis 1.2; Table 4). When azelaic acid 20% cream was compared with benzoyl peroxide 3% + clindamycin 1% gel (Schaller 2016), there was also no clear difference in the long term (12 weeks after start of treatment) (RR 1.15, 95% CI 0.43 to 3.07; 1 study, 221 participants; low‐quality evidence; Analysis 1.2; Table 14).
1.2. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 2: Withdrawal for any reason
We rated the findings measured in the long term as very low‐ to low‐quality of evidence due to risk of bias and imprecision (Table 1; Table 14; Table 2; Table 3; Table 4).
Change in lesion counts
Total (percentage reduction from baseline)
One study comparing azelaic acid 5% gel with clindamycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2011). Clindamycin had an advantage over azelaic acid in reducing total lesion counts in the short term (mean difference (MD) ‐12.03, 95% CI ‐13.01 to ‐11.05; 1 study, 100 participants; Analysis 1.3), medium term (MD ‐14.41, 95% CI ‐15.47 to ‐13.35; 1 study, 96 participants; Analysis 1.3), and long term (12 weeks after start of treatment) (MD ‐11.95, 95% CI ‐13.28 to ‐10.62; 1 study, 88 participants; Analysis 1.3). The differences were statistically significant.
1.3. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 3: Change in lesion counts ‐ total (percentage reduction from baseline)
Another trial compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel (Schaller 2016). There was a clear difference for all treatment terms (4, 8 and 12 weeks after start of treatment) in favour of benzoyl peroxide 3% + clindamycin 1% gel. Short term: MD ‐13.00 (95% CI ‐19.23 to ‐6.77; 1 study, 212 participants); medium term: MD ‐15.10 (95% CI ‐23.01 to ‐7.19; 1 study, 206 participants); and long term: MD ‐18.50 (95% CI ‐26.46 to ‐10.54; 1 study, 211 participants) (Analysis 1.3).
Total
Only Thielitz 2015 compared azelaic acid 15% gel with adapalene 0.1% gel reported the data in the long term (3 months after start of treatment); however, the data were skewed and we could therefore not present the data in a forest plot and instead presented it in a table (see table in Analysis 1.4). There was no statistically significant difference between the azelaic acid and adapalene group in percentage reduction from baseline (P = 0.396).
1.4. Analysis.
Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 4: Change in lesion counts ‐ total
| Change in lesion counts ‐ total | ||||
| Study | Time points | Azelaic acid | Other topical treatments | P value |
| long term | ||||
| Thielitz 2015 | Long term: three months after start of treatment (percent reduction of total lesions) (n, mean ± SD) | AzA9M: n =17, 33.54 ± 39.96 AzA3M: n = 19, 38.75 ± 29.24 |
Adapalene: n = 19, 48.87 ± 26.39 | P = 0.396 (AzA9M + AzA3M versus Adapalene) |
In one study with no usable outcome data (Ozkan 2000), the authors stated that azelaic acid (form and concentration unknown) was more effective in reducing acne grade, assessed using the Leeds' technique, when compared to clindamycin phosphate (concentration unknown).
Inflamed (percentage reduction from baseline)
The single Schaller 2016 trial compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel. There was a clear difference between groups at all treatment terms (4, 8 and 12 weeks after start of treatment) in favour of benzoyl peroxide 3% + clindamycin 1% gel. Short term: MD ‐14.10 (95% CI ‐22.02 to ‐6.16; 1 study, 212 participants); medium term: MD ‐15.90 (95% CI ‐23.74 to ‐8.06; 1 study, 2016 participants); and long term: MD ‐17.30 (95% CI ‐24.73 to ‐9.87; 1 study, 211 participants) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 5: Change in lesion counts ‐ inflamed (percentage reduction from baseline)
Inflamed (number of lesions post‐intervention)
The single Stinco 2007 trial compared azelaic acid (form and concentration unknown) with adapalene and benzoyl peroxide. There was no clear difference between azelaic acid and adapalene (MD 0.00, 95% CI ‐2.47 to 2.47; 1 study, 43 participants; Analysis 1.6) or benzoyl peroxide (MD ‐0.10, 95% CI ‐2.54 to 2.34; 1 study, 42 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 6: Change in lesion counts ‐ inflamed (number of lesions post‐intervention)
Inflamed
Five studies reported data for this outcome; however, the data were not available for meta‐analysis, so we presented the data in Analysis 1.7. Aksakal 1997 only reported the P value; the authors reported that azelaic acid 20% cream was more effective in reducing inflamed lesions when compared to metronidazole 1% cream (P < 0.001). Results from Dunlap 1997 demonstrated the significant advantage of 3% erythromycin/5% benzoyl peroxide over 20% azelaic acid in reducing inflammatory lesions. The authors from Gollnick 2004a reported that the median percentage reduction of inflamed lesions was 70% in the azelaic acid 15% gel group and 77% in the benzoyl peroxide 5% gel group (P > 0.05). Authors from Gollnick 2004b reported that the median percentage reduction of inflamed lesions was 71% in the azelaic acid 15% gel group and 63% in the clindamycin 1% gel group (P > 0.05). Data from Thielitz 2015 were skewed and the authors compared azelaic acid 15% gel with adapalene 0.1% gel; there was no significant difference between groups in percentage change of inflamed lesions in the long term (3 months after start of treatment) (P = 0.816).
1.7. Analysis.
Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 7: Change in lesion counts ‐ inflamed
| Change in lesion counts ‐ inflamed | ||||
| Study | Time points | Azelaic acid | Topical treatments (Comparator) | P value |
| Aksakal 1997 | Unclear | Unclear, not reported | Unclear, not reported (metronidazole) | P < 0.001 (statistical method not reported), Quote: "The results of this study showed that the AZA cream is more effective than the metronidazole cream in reducing counts of inflamed and non‐inflamed lesions of acne" |
| Dunlap 1997 | Medium term (number of lesion) | Unclear, not reported | Unclear, not reported (3% erythromycin/5% benzoyl peroxide) | Unclear, not reported. Quote: "The result of the study demonstrated significant differences favoring 3% erythromycin/5% benzoyl peroxide over 20% azelaic acid for the following parameters: 1) reduction in inflammatory lesion..." |
| Gollnick 2004a | Long term: four months after start of treatment (Median of percent reduction) | 70% | 77% (benzoyl peroxide) | P > 0.05 |
| Gollnick 2004b | Long term: four months after start of treatment (Median of percent reduction) | 71% | 63% (clindamycin) | P > 0.05 |
| Thielitz 2015 | Long term: three months after start of treatment (percent change) (n, mean ± SD) | AzA3M: n = 19, ‐32.31 ± 38.85 | n = 19, ‐37.99 ± 37.63 (Adapalene) | P = 0.816 |
Papules (percentage reduction from baseline)
Only one study that compared azelaic acid 5% gel with clindamycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2011). Clindamycin 2% gel had an advantage over azelaic acid 5% gel in reducing the papular lesion count in the short term (MD ‐23.74, 95% CI ‐24.54 to ‐22.94; 1 study, 100 participants; Analysis 1.8), medium term (MD ‐34.25, 95% CI ‐35.51 to ‐32.99; 1 study, 96 participants; Analysis 1.8) and long term (12 weeks after start of treatment) (MD ‐25.03, 95% CI ‐26.38 to ‐23.68; 1 study, 88 participants; Analysis 1.8). The differences were statistically significant.
1.8. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 8: Change in lesion counts ‐ papules (percentage reduction from baseline)
Papules (number of lesions post‐intervention)
Only one study that compared azelaic acid 20% gel with erythromycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2010). Participants in the erythromycin 2% gel group had less papules post‐intervention than those in the azelaic acid 20% gel group in the short term (MD 3.40, 95% CI 2.99 to 3.81; 1 study, 66 participants; Analysis 1.9), and medium term (MD 2.05, 95% CI 1.60 to 2.50; 1 study, 66 participants; Analysis 1.9). The difference was statistically significant. There was no clear difference between topical azelaic acid 20% gel and erythromycin 2% gel in the long term (12 weeks after start of treatment) (MD ‐0.17, 95% CI ‐0.36 to 0.02; 1 study, 66 participants; Analysis 1.9).
1.9. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 9: Change in lesion counts ‐ papules (number of lesions post‐intervention)
Pustules (percentage reduction from baseline)
Only one study that compared azelaic acid 5% gel with clindamycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2011). Clindamycin 2% gel was better than azelaic acid 5% gel in reducing the pustular lesion count in the short term (MD ‐6.53, 95% CI ‐7.45 to ‐5.61; 1 study, 100 participants; Analysis 1.10), medium term (MD ‐9.07, 95% CI ‐10.42 to ‐7.72; 1 study, 96 participants; Analysis 1.10), and long term (12 weeks after start of treatment) (MD ‐9.71, 95% CI ‐11.33 to ‐8.09; 1 study, 88 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 10: Change in lesion counts ‐ pustules (percentage reduction from baseline)
Pustules (number of lesions post‐intervention)
Only one study that compared azelaic acid 20% gel with erythromycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2010). There was a difference between topical azelaic acid 20% gel and erythromycin 2% gel in the short term (MD ‐2.31, 95% CI ‐2.64 to ‐1.98; 1 study, 66 participants; Analysis 1.11), medium term (MD ‐2.95, 95% CI ‐3.24 to ‐2.66; 1 study, 66 participants; Analysis 1.11), and long term (12 weeks after start of treatment) (MD ‐1.80, 95% CI ‐1.97 to ‐1.63; 1 study, 66 participants; Analysis 1.11), which were all in favour of azelaic acid 20% gel.
1.11. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 11: Change in lesion counts ‐ pustules (number of lesions post‐intervention)
Non‐inflamed
We found five relevant trials that collected data for this outcome; however, the data were not available for meta‐analysis, so we presented the data in a table (see table in Analysis 1.12). Aksakal 1997 only reported the P value; the authors reported that azelaic acid 20% cream was more effective in reducing non‐inflamed lesions when compared to metronidazole 1% cream (P < 0.001). Barbareschi 1991 compared 20% azelaic acid cream with 0.05% retinoic acid cream; the authors did not report the P value and MDs. Results from Dunlap 1997 demonstrated significant advantage of 3% erythromycin/5% benzoyl peroxide over 20% azelaic acid in reducing comedones. The authors from Gollnick 2004a reported that the median percentage reduction of non‐inflamed lesions was 60% in the azelaic acid 15% gel group and 71% in the benzoyl peroxide 5% gel group (P > 0.05). Authors from Gollnick 2004b reported that the median percentage reduction of non‐inflamed lesions was 57% in the azelaic acid 15% gel group and 45% in the clindamycin 1% gel group (P < 0.05). Data from Thielitz 2015 were skewed and results also showed that there was no significant difference in percentage reduction of non‐inflamed lesions (P = 0.063) or microcomedones (P = 0.25) in the long term (3 months after start of treatment) between azelaic acid 15% gel and adapalene 0.1% gel.
1.12. Analysis.
Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 12: Change in lesion counts ‐ non‐inflamed
| Change in lesion counts ‐ non‐inflamed | ||||
| Study | Time points | Azelaic acid | Topical treatments | P value |
| Aksakal 1997 | Unclear (number of lesions post intervention) | Less counts | More counts (metronidazole) | P < 0.001 |
| Barbareschi 1991 | Long term: four months after start of treatment, reduction in number of lesions (mean) | 23 | 35 (Retinoic acid) | Unclear, the author did not test the difference between groups. |
| Dunlap 1997 | Medium term: reduction in number of comedones | Unclear, not reported | Unclear, not reported (3% erythromycin/5% benzoyl peroxide) | Unclear, not reported. Quote: " The result of the study demonstrated significant differences favoring 3% erythromycin/5% benzoyl peroxide over 20% azelaic acid for the following parameters: 2) reduction in comedones..." |
| Gollnick 2004a | Long term: four months after start of treatment (Median of percent reduction) | 60% | 71% (benzoyl peroxide) | P > 0.05 |
| Gollnick 2004b | Long term: four months after start of treatment (Median of percent reduction) | 57% | 45% (clindamycin) | P < 0.05 |
| Thielitz 2015 | Long term: three months after start of treatment (percent reduction of non‐inflamed lesions) (n, mean ± SD) | AzA9M: n =17, 26.36 ± 57.64 AzA3M: n = 19, 41.25 ± 32.92 |
Adapalene: n = 19, 55.21 ± 29.75 | P = 0.063 (AzA9M + AzA3M versus Adapalene) |
| Thielitz 2015 | Long term: three months after start of treatment, percent reduction of microcomedones (n, mean ± SD) | AzA9M: n =17, 10.61 ± 44.32 AzA3M: n = 19, 18.63 ± 54.70 |
Adapalene: n = 19, 27.06 ± 50.15 | P = 0.25 (AzA9M + AzA3M versus Adapalene) |
Non‐inflamed (percentage reduction from baseline)
One study that compared azelaic acid 5% gel with clindamycin 2% gel contributed data for this outcome (Pazoki‐Toroudi 2011). Clindamycin 2% gel was better than azelaic acid 5% gel in reducing the non‐inflamed lesion (comedones) count in the short term (MD ‐5.81, 95% CI ‐6.80 to ‐4.82; 1 study, 100 participants; Analysis 1.13); however, this clear advantage was not observed in the medium term (MD 0.11, 95% CI ‐1.24 to 1.46; 1 study, 96 participants; Analysis 1.13) and long term (12 weeks after start of treatment) (MD ‐1.11, 95% CI ‐2.91 to 0.69; 1 study, 88 participants; Analysis 1.13).
1.13. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 13: Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline)
Schaller 2016 compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel. There was a clear difference between groups for all treatment terms (4, 8 and 12 weeks after start of treatment) in favour of benzoyl peroxide 3% + clindamycin 1% gel. Short term: MD ‐11.10 (95% CI ‐18.64 to ‐3.56; 1 study, 212 participants); medium term: MD ‐13.00 (95% CI ‐22.76 to ‐3.24; 1 study, 206 participants); and long term: MD ‐18.50 (95% CI ‐28.33 to ‐8.67; 1 study, 211 participants) (Analysis 1.13).
Non‐inflamed (number of lesions post‐intervention)
One study compared azelaic acid 20% gel with erythromycin 2% gel (Pazoki‐Toroudi 2010). There was a difference between topical azelaic acid 20% gel and erythromycin 2% gel in the short term (MD ‐2.68, 95% CI ‐2.89 to ‐2.47; 1 study, 66 participants; Analysis 1.14), medium term (MD ‐1.80, 95% CI ‐2.10 to ‐1.50; 1 study, 66 participants; Analysis 1.14) and long term (12 weeks after start of treatment) (MD ‐2.07, 95% CI ‐2.25 to ‐1.89; 1 study, 66 participants; Analysis 1.14), which were all in favour of azelaic acid 20% gel.
1.14. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 14: Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention)
Another study compared azelaic acid (form and concentration unknown) with benzoyl peroxide and adapalene (Stinco 2007). There was no clear difference between azelaic acid and adapalene in the medium term (MD ‐3.00, 95% CI ‐8.07 to 2.07; 1 study, 43 participants; Analysis 1.14). In addition, there was also no clear difference between azelaic acid and benzoyl peroxide in the medium term (MD ‐4.40, 95% CI ‐10.77 to 1.97; 1 study, 42 participants; Analysis 1.14).
Physicians' global evaluation of acne improvement
For this outcome, no data reported in the short and medium term. Gollnick 2004a assessed the outcome using a four‐point Likert‐type rating scale (very good, good, moderate, poor). Study authors compared azelaic acid 15% gel with benzoyl peroxide 5% gel, and the benzoyl peroxide group demonstrated a statistically significant good or very good improvement in the long term (4 months after start of treatment) (RR 0.84, 95% CI 0.74 to 0.96; 1 study, 351 participants; Analysis 1.15).
1.15. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 15: Physicians' global evaluation of acne improvement
Gollnick 2004b assessed the outcome using a four‐point Likert‐type rating scale (very good, good, moderate, poor). Study authors compared azelaic acid 15% gel with clindamycin 1% gel in good or very good improvement in the long term (4 months after start of treatment), and there was no significant difference (RR 0.92, 95% CI 0.78 to 1.10; 1 study, 229 participants; Analysis 1.15). Only Katsambas 1989b compared azelaic acid 20% cream with tretinoin 0.05% cream in good to excellent improvement in the long term (6 months after start of treatment), and there was no significant difference (RR 0.94, 95% CI 0.80 to 1.11; 1 study, 289 participants; Analysis 1.15).
Schaller 2016 compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel and the authors reported this outcome using a six‐point scale (6 points from clear (0) to very clear (5)) for all treatment terms (4, 8 and 12 weeks after start of treatment). There was a clear significant difference in the medium term (RR 0.54, 95% CI 0.31 to 0.95; 1 study, 221 participants) and long term (RR 0.53, 95% CI 0.33 to 0.87; 1 study, 221 participants), which was in favour of benzoyl peroxide 3% + clindamycin 1% gel group. But there was no clear difference in the short term (RR 0.58, 95% CI 0.29 to 1.17; 1 study, 221 participants) (Analysis 1.15).
Dunlap 1997 had no usable outcome data and the authors stated that azelaic acid 20% cream was less effective in physician global evaluation when compared to 3% erythromycin/5% benzoyl peroxide gel. The authors also stated that azelaic acid 20% cream was inferior to 3% erythromycin/5% benzoyl peroxide gel in overall acne condition improvement and reduction of inflammatory lesions and comedones.
Minor adverse events
Total events ‐ azelaic acid versus adapalene
Thielitz 2015 reported no "significant difference" between topical azelaic acid 15% gel and adapalene 1% gel (RR 1.16, 95% CI 0.47 to 2.85; 1 study, 55 participants; Analysis 1.16). We rated this finding as very low‐quality evidence due to risk of bias and imprecision (Table 1).
1.16. Analysis.

Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 16: Minor adverse events
Total events ‐ azelaic acid versus benzoyl peroxide
Cavicchini 1989 reported that one out of 15 people in the azelaic acid 20% cream group versus two out of 15 people in the benzoyl peroxide 5% gel group experienced minor adverse events (P = 1.00, Fisher's Exact test). There was no clear difference between topical azelaic acid 20% cream and benzoyl peroxide 5% gel (RR 0.50, 95% CI 0.05 to 4.94; 1 study, 30 participants; Analysis 1.16). We rated this finding as very low‐quality evidence due to risk of bias and imprecision (Table 2).
Total events ‐ azelaic acid versus benzoyl + clindamycin
Schaller 2016 compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel. The participants who received benzoyl peroxide 3% + clindamycin 1% gel experienced fewer total number of events, though there was no clear significant difference (RR 1.24, 95% CI 1.01 to 1.52; 1 study, 221 participants; Analysis 1.16). We rated this finding as low‐quality evidence due to risk of bias and imprecision (Table 14).
Total events ‐ azelaic acid versus clindamycin
Pazoki‐Toroudi 2011 compared azelaic acid 5% gel with clindamycin 2% gel. There was no clear difference between treatment groups (RR 1.50, 95% CI 0.67 to 3.35; 1 study, 100 participants; Analysis 1.16). We rated this finding as low‐quality evidence due to risk of bias and imprecision (Table 3).
Total events ‐ azelaic acid versus erythromycin
Pazoki‐Toroudi 2010 reported that 16 out of 35 people in the azelaic acid 20% gel group versus 17 out of 31 people in the erythromycin 2% gel group experienced total events. There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 0.83, 95% CI 0.51 to 1.35; 1 study, 66 participants; Analysis 1.16).
Application site pain ‐ azelaic acid versus benzoyl + clindamycin
Schaller 2016 compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel. There was a significant difference between groups showing azelaic acid caused more application site pain (RR 3.17, 95% CI 1.41 to 7.12; 1 study, 221 participants; Analysis 1.16).
Burning ‐ azelaic acid versus benzoyl peroxide
Gollnick 2004a showed there was no significant difference between topical azelaic acid 15% gel and benzoyl peroxide 5% gel (RR 1.10, 95% CI 0.61 to 1.97; 1 study, 351 participants; Analysis 1.16).
Burning ‐ azelaic acid versus clindamycin
Gollnick 2004b reported burning in 12/114 participants in the azelaic acid 15% gel treatment group compared to 0/115 participants in the clindamycin 1% gel group. There was a significant difference between groups, showing azelaic acid caused more burning (RR 25.22, 95% CI 1.51 to 420.92; 1 study, 229 participants; Analysis 1.16), but the confidence interval was very wide.
Burning ‐ azelaic acid versus tretinoin
Katsambas 1989b reported no significant difference between topical azelaic acid 20% cream and tretinoin 0.05% cream (RR 0.80, 95% CI 0.38 to 1.71; 1 study, 289 participants; Analysis 1.16).
Scaling ‐ azelaic acid versus clindamycin
Pazoki‐Toroudi 2011 reported that four out of 50 people in the azelaic acid 5% gel group versus six out of 50 people in the clindamycin 2% gel group experienced scaling (P = 0.741, Fisher's Exact test). There was no significant difference between topical azelaic acid 5% gel and clindamycin 2% gel (RR 0.67, 95% CI 0.20 to 2.22; 1 study, 100 participants; Analysis 1.16).
Scaling ‐ azelaic acid versus erythromycin
Pazoki‐Toroudi 2010 reported that four out of 35 people in the azelaic acid 20% gel group versus two out of 31 people in the erythromycin 2% gel group experienced scaling (P = 0.68, Fisher's Exact test). There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 1.77, 95% CI 0.35 to 9.01; 1 study, 66 participants; Analysis 1.16).
Scaling ‐ azelaic acid versus tretinoin
Katsambas 1989b reported that topical azelaic acid 20% cream had lower risk of scaling than tretinoin 0.05% cream (RR 0.58, 95% CI 0.37 to 0.91; 1 study, 289 participants; Analysis 1.16). This difference was statistically different.
Erythema ‐ azelaic acid versus adapalene
Stinco 2007 reported no significant difference between topical azelaic acid (form and concentration unknown) and adapalene (RR 0.80, 95% CI 0.30 to 2.10; 1 study, 45 participants; Analysis 1.16).
Erythema ‐ azelaic acid versus benzoyl peroxide
Stinco 2007 reported no significant difference between topical azelaic acid (form and concentration unknown) and benzoyl peroxide (RR 0.48, 95% CI 0.21 to 1.09; 1 study, 45 participants; Analysis 1.16).
Erythema ‐ azelaic acid versus benzoyl peroxide/clindamycin
Schaller 2016 reported no significant difference between topical azelaic acid 20% cream and benzoyl peroxide 3% + clindamycin 1% gel (RR 1.68, 95% CI 0.41 to 6.87; 1 study, 221 participants; Analysis 1.16).
Erythema ‐ azelaic acid versus clindamycin
Pazoki‐Toroudi 2011 reported that three out of 50 people in the azelaic acid 5% gel group versus four out of 50 people in the clindamycin 2% gel group experienced erythema (P = 1.00, Fisher's Exact test). There was no significant difference between topical azelaic acid 5% gel and clindamycin 2% gel (RR 0.75, 95% CI 0.18 to 3.18; 1 study, 100 participants; Analysis 1.16).
Erythema ‐ azelaic acid versus erythromycin
Pazoki‐Toroudi 2010 reported that three out of 35 people in the azelaic acid 20% gel group versus four out of 31 people in the erythromycin 2% gel group experienced erythema (P = 0.70, Fisher's Exact test). There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 0.66, 95% CI 0.16 to 2.74; 1 study, 66 participants; Analysis 1.16).
Erythema ‐ azelaic acid versus tretinoin
Katsambas 1989b reported that topical azelaic acid 20% cream had lower risk of erythema than tretinoin 0.05% cream (RR 0.64, 95% CI 0.41 to 0.99; 1 study, 289 participants; Analysis 1.16), and this difference was statistically significant.
Dryness ‐ azelaic acid versus adapalene
Stinco 2007 reported no significant difference between topical azelaic acid (form and concentration unknown) and adapalene (RR 0.80, 95% CI 0.51 to 1.26; 1 study, 45 participants; Analysis 1.16).
Dryness ‐ azelaic acid versus benzoyl peroxide
Gollnick 2004a and Stinco 2007 reported no significant difference between topical azelaic acid and benzoyl peroxide (RR 0.56, 95% CI 0.27 to 1.16; 2 studies, 396 participants; Analysis 1.16).
Dryness ‐ azelaic acid versus benzoyl peroxide/clindamycin
Schaller 2016 reported no significant difference between topical azelaic acid 20% cream and benzoyl peroxide 3% + clindamycin 1% gel (RR 1.51, 95% CI 0.26 to 8.88; 1 study, 221 participants; Analysis 1.16).
Dryness ‐ azelaic acid versus clindamycin
Gollnick 2004b and Pazoki‐Toroudi 2011 reported no significant difference between topical azelaic acid and clindamycin (RR 2.44, 95% CI 0.96 to 6.19; 2 studies, 329 participants; Analysis 1.16).
Dryness ‐ azelaic acid versus erythromycin
Pazoki‐Toroudi 2010 reported that two out of 35 people in the azelaic acid 20% gel group versus four out of 31 people in the erythromycin 2% gel group experienced dryness (P = 0.41, Fisher's Exact test). There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 0.44, 95% CI 0.09 to 2.25; 1 study, 66 participants; Analysis 1.16).
Oiliness ‐ azelaic acid versus clindamycin
Pazoki‐Toroudi 2011 reported that five out of 50 people in the azelaic acid 5% gel group versus four out of 50 people in the clindamycin 2% gel group experienced oiliness (P = 1.00, Fisher's Exact test). There was no significant difference between topical azelaic acid 5% gel and clindamycin 2% gel (RR 1.25, 95% CI 0.36 to 4.38; 1 study, 100 participants; Analysis 1.16).
Oiliness ‐ azelaic acid versus erythromycin
Pazoki‐Toroudi 2010 reported that three out of 35 people in the azelaic acid 20% gel group versus three out of 31 people in the erythromycin 2% gel group experienced oiliness (P = 1.00, Fisher's Exact test). There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 0.89, 95% CI 0.19 to 4.07; 1 study, 66 participants; Analysis 1.16).
Itching ‐ azelaic acid versus adapalene
Stinco 2007 reported no significant difference between topical azelaic acid (form and concentration unknown) and adapalene (RR 1.23, 95% CI 0.84 to 1.79; 1 study, 45 participants; Analysis 1.16).
Itching ‐ azelaic acid versus benzoyl peroxide
Gollnick 2004a and Stinco 2007 reported itching was higher in the azelaic acid group compared with the benzoyl peroxide group, but the RR was uncertain due to the wide CI spanning 1 (RR 3.29, 95% CI 0.24 to 45.29; 2 studies, 396 participants; Analysis 1.16).
Itching ‐ azelaic acid versus benzoyl peroxide/clindamycin
Schaller 2016 reported a significant difference between groups, showing azelaic acid 20% cream caused more itching compared with benzoyl peroxide 3% + clindamycin 1% gel (RR 3.15, 95% CI 1.49 to 6.68; 1 study, 221 participants; Analysis 1.16).
Itching ‐ azelaic acid versus clindamycin
Gollnick 2004b and Pazoki‐Toroudi 2011 reported more itching in the azelaic acid group compared with the clindamycin group, but the RR was uncertain due to the wide CI spanning 1 (RR 2.56, 95% CI 0.68 to 9.57; 2 studies, 329 participants; Analysis 1.16).
Itching ‐ azelaic acid versus erythromycin
Some studies, for example Pazoki‐Toroudi 2010, reported this outcome as 'pruritus'. We contacted authors to confirm whether 'pruritus' was equal to 'itching'; authors from Schaller 2016 replied they were the same thing. Therefore, we considered 'pruritus' and 'itching' to be the same thing and we used 'itching' throughout the text.
Pazoki‐Toroudi 2010 reported that four out of 35 people in the azelaic acid 20% gel group versus three out of 31 people in the erythromycin 2% gel group experienced itching (P = 1.00, Fisher's Exact test). There was no significant difference between topical azelaic acid 20% gel and erythromycin 2% gel (RR 1.18, 95% CI 0.29 to 4.87; 1 study, 66 participants; Analysis 1.16).
Red skin ‐ azelaic acid versus benzoyl peroxide
Gollnick 2004a reported no significant difference between topical azelaic acid 15% gel and benzoyl peroxide 5% gel (RR 0.68, 95% CI 0.36 to 1.26; 1 study, 351 participants; Analysis 1.16).
Red skin ‐ azelaic acid versus clindamycin
Gollnick 2004b reported that topical azelaic acid 15% gel had a higher risk of red skin than clindamycin 1% gel (RR 6.05, 95% CI 1.39 to 26.44; 1 study, 229 participants; Analysis 1.16), and the difference was statistically significant.
Desquamation ‐ azelaic acid versus benzoyl peroxide
Gollnick 2004a reported a statistically significant difference between topical azelaic acid 15% gel and benzoyl peroxide 5% gel (RR 0.25, 95% CI 0.08 to 0.73; 1 study, 351 participants; Analysis 1.16), which indicated a lower risk of desquamation after using azelaic acid.
Eczema ‐ azelaic acid versus clindamycin
Gollnick 2004b reported that zero out of 114 people in the azelaic acid 15% gel group versus four out of 115 people in the clindamycin 1% gel group experienced eczema (P = 0.12, Fisher's Exact test). There was no clear difference between topical azelaic acid 15% gel and clindamycin 1% gel (RR 0.11, 95% CI 0.01 to 2.06; 1 study, 229 participants; Analysis 1.16).
Quality of life
Schaller 2016 compared azelaic acid 20% cream with benzoyl peroxide 3% + clindamycin 1% gel (12 weeks after start of treatment) using the 10‐question Children's Dermatology Life Quality Index (CDLQI; 0 = not at all, 1 = a little, 2 = a lot, 3 = very much, low = well) to assess how much the skin disease affected participants' lives. The percentage change from baseline (skewed data) was in favour of benzoyl peroxide 3% + clindamycin 1% gel (‐36.8% ± 74.8 in azelaic acid group versus ‐60.5% ± 70.6 in benzoyl peroxide + clindamycin group). The quality of evidence was low (Table 14).
Thielitz 2015 compared azelaic acid 15% gel with adapalene 0.1% gel; we were unable to present the skewed data from this study in a forest plot, so we presented the data in a table (see table in Analysis 1.17). There was no "statistically significant" difference (P = 0.549) in absolute change of Dermatology Life Quality Index in the long term (3 months after start of treatment) between azelaic acid and adapalene (‐1.88 ± 3.35 and ‐2.74 ± 2.90 in azelaic acid group versus ‐2.58 ± 4.68 in adapalene group). The quality of evidence was very low (Table 1).
1.17. Analysis.
Comparison 1: Topical azelaic acid versus other topical treatments, Outcome 17: Quality of life
| Quality of life | ||||
| Study | Time points | Azelaic acid | Topical treatments | P value |
| Schaller 2016 | Long term: 12 weeks after start of treatment ‐ Children's Dermatology Life Quality Index (percent change, mean ± SD) | ‐36.8% ± 74.8, n = 108 | ‐60.5% ± 70.6, n = 107 | unclear |
| Thielitz 2015 | Long term: three months after start of treatment ‐ Dermatology Life Quality Index questionnaire (absolute change, n mean ± SD) | AzA9M: n =17, ‐1.88 ± 3.35 AzA3M: n = 19, ‐2.74 ± 2.90 |
Adapalene: n = 19, ‐2.58 ± 4.68 | P = 0.549 (adapalene vs AzA9M + 3M) |
Comparison 2: topical azelaic acid versus placebo
Participants' global self‐assessment of acne improvement
No study collected data for this outcome.
Withdrawal for any reason
One trial compared azelaic acid 20% gel with placebo (Iraji 2007), neither treatment group experienced withdrawals in the medium term (Analysis 2.1). Three relevant trials reported data in the long term (Barbareschi 1991; Cunliffe 1989; Katsambas 1989a). There was no clear difference between topical azelaic acid 20% cream and placebo cream in the long term (treatment duration more than 8 weeks) (RR 1.60, 95% CI 0.55 to 4.66; 3 studies, 152 participants; low‐quality evidence; Analysis 2.1; Table 15), and two of the three studies reporting zero events.
2.1. Analysis.

Comparison 2: Topical azelaic acid versus placebo, Outcome 1: Withdrawal for any reason
5. Azelaic acid compared to placebo.
| Azelaic acid compared to placebo for acne | ||||||
| Patient or population: participants with acne Settings: not described (4 studies) Intervention: topical azelaic acid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Topical azelaic acid | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
63 per 1000 | 101 per 1000 (35 to 295) | RR 1.60 (0.55 to 4.66) | 152 (3 studies) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Total number of participants who experienced at least one minor adverse event (medium term: treatment duration from 5 to 8 weeks) |
See comment | See comment | RR 19.00 (1.16 to 312.42) | 60 (1 study) | ⊕⊝⊝⊝ Very lowb | 9/30 versus 0/30 experienced minor adverse events. In the other studies in this comparison, events such as scaling, dry skin, erythema, oiliness, and pruritus were reported, but the number of participants with these events were low and similar across groups. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: three studies included, two with unclear risk of selection bias and one with unclear risk of allocation concealment, one study with high risk of reporting bias and one with high risk of attrition bias. One level for imprecision: wide CI and optimal sample size not met. bDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included, and study with unclear random sequence generation and allocation concealment. Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Change in lesion counts
> 50% inflamed reduction
For this outcome, we only found one relevant trial that reported data in the long term (3 months after start of treatment) (Cunliffe 1989). The study stated that 10 participants using azelaic acid 20% cream demonstrated a reduction of at least 50% in inflamed lesions, compared to only one participant using placebo cream by the end of study (RR 10.00, 95% CI 1.41 to 70.99; 1 study, 40 participants; Analysis 2.2). However, the result had serious imprecision due to a very small sample size.
2.2. Analysis.

Comparison 2: Topical azelaic acid versus placebo, Outcome 2: Change in lesion counts ‐ > 50% inflamed reduction
Inflamed (percentage reduction from baseline)
Three studies reported data for this outcome in the short, medium and long term (Cunliffe 1989; Hayashi 2012; Katsambas 1989a). Cunliffe 1989 and Katsambas 1989a were parallel trials and Hayashi 2012 was a split‐face trial. As there were no means ± standard deviations (SDs) or SDs presented, we described the data in a table (see table in Analysis 2.3). All three studies concluded that compared to placebo, azelaic acid showed a greater percentage reduction in inflamed lesions in the short, medium, and long term (3 months after start of treatment).
2.3. Analysis.
Comparison 2: Topical azelaic acid versus placebo, Outcome 3: Change in lesion counts ‐ inflamed (percentage reduction from baseline)
| Change in lesion counts ‐ inflamed (percentage reduction from baseline) | |||
| Study | Azelaic acid (mean) | Placebo (mean) | P value |
| short term | |||
| Cunliffe 1989 | Unclear, not reported | Unclear, not reported | P < 0.001, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| medium term | |||
| Cunliffe 1989 | Unclear, not reported | Unclear, not reported | P < 0.0025, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| Katsambas 1989a | Unclear, not reported | Unclear, not reported | P < 0.05, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| long term | |||
| Cunliffe 1989 | Long term: three months after start of treatment, unclear, not reported | Unclear, not reported | P < 0.001, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| Katsambas 1989a | Long term: three months after start of treatment, 72% | 47% | P < 0.05, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| long term (split‐face trials) | |||
| Hayashi 2012 | Long term: 12 weeks after start of treatment, 68.7% | 54.5% | unclear, not reported |
> 50% non‐inflamed reduction
We only found one relevant trial and it reported data in the long term (3 months after start of treatment) (Cunliffe 1989). By the end of study, a higher rate of > 50% non‐inflamed reduction was observed in the azelaic acid group 20% cream (11/20) than in the placebo cream group (4/20) (RR 2.75, 95% CI 1.05 to 7.20; 1 study, 40 participants; Analysis 2.4). However, the result had serious imprecision due to a very small sample size.
2.4. Analysis.

Comparison 2: Topical azelaic acid versus placebo, Outcome 4: Change in lesion counts ‐ > 50% non‐inflamed reduction
Non‐inflamed (percentage reduction from baseline)
Cunliffe 1989 reported data over the medium and long term (3 months after start of treatment); however, the authors only reported the P value with no means and SDs, so we presented the data in a table (see table in Analysis 2.5). When compared to placebo cream, the azelaic acid 20% cream showed a greater percentage reduction in non‐inflamed lesions in the medium (P < 0.027) and long term (P < 0.027). The other split‐face study only reported the percentage reduction with no SDs and P value (Hayashi 2012; Analysis 2.5).
2.5. Analysis.
Comparison 2: Topical azelaic acid versus placebo, Outcome 5: Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline)
| Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | |||
| Study | Azelaic acid (mean) | Placebo (mean) | P value |
| medium term | |||
| Cunliffe 1989 | Unclear, not reported | Unclear, not reported | P < 0.027, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| long term | |||
| Cunliffe 1989 | Long term: three months after start of treatment, unclear, not reported | Unclear, not reported | P < 0.027, the results demonstrated significant difference favouring azelaic acid over placebo cream. |
| long term (split‐face trials) | |||
| Hayashi 2012 | Long term: 12 weeks after start of treatment, 59.0% | 46.5% | Unclear, not reported |
Various types of acne (percentage reduction from baseline)
Three studies reported data for medium and long term outcomes (Hayashi 2012; Iraji 2007; Katsambas 1989a); however, as the SDs were missing, we presented the data in a table (see table in Analysis 2.6). Apart from pustule number (the percentage reduction was greater in the azelaic acid group, P = 0.08), azelaic acid 20% gel showed a better effect in percentage reduction of total lesion numbers (P = 0.002), comedone numbers (P = 0.001), papule numbers (P = 0.003), and acne severity index (P = 0.001), when compared to placebo in the medium term (Iraji 2007). There was also a significant difference between azelaic acid 20% cream and placebo cream in comedone percentage reduction in the long term (3 months after start of treatment) (55.6% for azelaic acid, 0% for placebo) in favour of azelaic acid 20% cream (no exact P value reported) (Katsambas 1989a). Another split‐face study (Hayashi 2012), also suggested the advantage of topical azelaic acid 20% cream over placebo in total lesion reduction in the long term (12 weeks after start of treatment) (P < 0.001).
2.6. Analysis.
Comparison 2: Topical azelaic acid versus placebo, Outcome 6: Change in lesion counts (percentage reduction from baseline)
| Change in lesion counts (percentage reduction from baseline) | ||||
| Study | Subgroup | Topical azelaic acid | Placebo | P value |
| medium term | ||||
| Iraji 2007 | Papules number | 51.2% | 19.3% | 0.003 |
| Iraji 2007 | Total lesion counts | 60.6% | 19.9% | 0.002 |
| Iraji 2007 | Acne severity index | 65.2% | 21.3% | 0.001 |
| Iraji 2007 | Comedones numbers | 87.3% | 23.2% | 0.001 |
| Iraji 2007 | Pustules number | 42.1% | 17.8% | 0.08 |
| long term | ||||
| Katsambas 1989a | Long term: three months after start of treatment, comedones numbers | 55.6% | 0% | significant difference |
| long term (split‐face trials) | ||||
| Hayashi 2012 | Long term: 12 weeks after start of treatment, total lesion counts | 59.6% | 45.6% | <0.001 |
Various types of acne (number of lesions post‐intervention)
Pazoki‐Toroudi 2010 reported short‐term data. However, the number of participants in the azelaic acid group was missing, so we presented the data in a table (see table in Analysis 2.7). The azelaic acid 20% gel group showed lower papule numbers (P < 0.001), pustule numbers (P < 0.001), and comedone numbers (P < 0.001) when compared with placebo after treatment.
2.7. Analysis.
Comparison 2: Topical azelaic acid versus placebo, Outcome 7: Change in lesion counts (number of lesions post‐intervention)
| Change in lesion counts (number of lesions post‐intervention) | ||||
| Study | Subgroup | Topical azelaic acid | Placebo | P value |
| short term | ||||
| Pazoki‐Toroudi 2010 | comedones | mean ± SD: 6.12 ± 0.39 | mean ± SD: 30.02 ± 3.0, n = 20 | < 0.001 |
| Pazoki‐Toroudi 2010 | papules | mean ± SD: 14.02 ± 0.78 | mean ± SD: 22.22 ± 1.64, n = 20 | < 0.001 |
| Pazoki‐Toroudi 2010 | pustules | mean ± SD: 5.9 ± 0.61 | mean ± SD: 12.22 ± 1.04, n = 20 | < 0.001 |
Comedones (reduction in number of lesions post‐intervention)
Only Barbareschi 1991 reported data for this outcome and SDs were missing. The azelaic acid 20% cream reduced more lesion counts than placebo treatment in the long term (4 months after start of treatment); however, the authors did not test whether the difference was significant (Analysis 2.8). In addition, azelaic acid 20% cream showed better effect in reducing comedones (measured by scanning electron microscopy) than placebo in the long term (4 months after start of treatment) (Analysis 2.8).
2.8. Analysis.
Comparison 2: Topical azelaic acid versus placebo, Outcome 8: Change in lesion counts ‐ comedones (reduction in number of lesions post‐intervention)
| Change in lesion counts ‐ comedones (reduction in number of lesions post‐intervention) | |||
| Study | Azelaic acid | Placebo | P value |
| long term | |||
| Barbareschi 1991 | Long term: four months after start of treatment, 23 | 3 | Unclear, the author did not test the difference between groups. |
| Barbareschi 1991 | Four months after start of treatment, number of lesions reduction post intervention, scanning electron microscopy measured comedones: 9.4±6.93 | 0.1±3.14 | 0.05 |
Physicians' global evaluation of acne improvement
Katsambas 1989a reported data in the long term (3 months after start of treatment). The authors assessed this outcome using a four‐point system (75% to 100% reduction of the initial total lesion count: excellent; 50% to 75% reduction: good; 25% to 50% reduction: moderate; less than 25%: poor response). There was a statistically significant difference between topical azelaic acid 20% cream and placebo cream in good to excellent improvement (RR 1.64, 95% CI 1.00 to 2.67; 1 study, 92 participants; Analysis 2.9), which was in favour of azelaic acid 20% cream. However, the estimated result was fairly imprecise due to the small sample size.
2.9. Analysis.

Comparison 2: Topical azelaic acid versus placebo, Outcome 9: Physicians' global evaluation of acne improvement
Minor adverse events
Burning
Katsambas 1989a reported that four out of 43 people in the azelaic acid 20% cream group versus one out of 49 people in the placebo cream group experienced burning (P = 0.18, Fisher's Exact test). Burning was reported more in the topical azelaic acid 20% cream group compared with the placebo cream but the RR was imprecise due to the uncertainty from the wide CI (RR 4.56, 95% CI 0.53 to 39.24; 1 study, 92 participants; Analysis 2.10).
2.10. Analysis.

Comparison 2: Topical azelaic acid versus placebo, Outcome 10: Minor adverse events
Scaling
Katsambas 1989a and Pazoki‐Toroudi 2010 reported that five out of 78 people in the azelaic acid group versus two out of 69 people in the placebo group experienced scaling (P = 0.448, Fisher's Exact test). There was no clear difference between topical azelaic acid and placebo due to the wide CIs surrounding the effect size (RR 1.49, 95% CI 0.16 to 13.48; 2 studies, 147 participants; Analysis 2.10).
Erythema
Katsambas 1989a and Pazoki‐Toroudi 2010 reported that five out of 78 people in the azelaic acid group versus two out of 69 people in the placebo group experienced erythema (P = 0.448, Fisher's Exact test). There was no clear difference between topical azelaic acid and placebo (RR 1.96, 95% CI 0.39 to 9.78; 2 studies, 147 participants; Analysis 2.10).
Dryness
Pazoki‐Toroudi 2010 reported that two out of 35 people in the azelaic acid 20% gel group versus zero out of 20 people in the placebo group experienced dryness (P = 0.529, Fisher's Exact test). There was no significant difference between treatment groups (RR 2.92, 95% CI 0.15 to 57.90; 1 study, 55 participants; Analysis 2.10)
Oiliness
Pazoki‐Toroudi 2010 reported that three out of 35 people in the azelaic acid 20% gel group versus zero out of 20 people in the placebo group experienced oiliness (P = 0.293, Fisher's Exact test). There was no difference between treatment groups (RR 4.08, 95% CI 0.22 to 75.25; 1 study, 55 participants; Analysis 2.10)
Itching
Katsambas 1989a and Pazoki‐Toroudi 2010 reported that six out of 78 people in the azelaic acid group versus zero out of 69 people in the placebo group experienced itching (P = 0.03, Fisher's Exact test). There was no clear difference between treatment groups (RR 5.45, 95% CI 0.68 to 43.53; 2 studies, 147 participants; Analysis 2.10).
Total events
Iraji 2007 reported that nine out of 30 people in the azelaic acid 20% gel group versus zero out of 30 people in the placebo group experienced minor adverse events (P = 0.002, Fisher's Exact test). Result of meta‐analysis showed that topical azelaic acid 20% gel had a higher rate of adverse events than placebo (RR 19.00, 95% CI 1.16 to 312.42; 1 study, 60 participants; Analysis 2.10). However, the result was imprecise as the estimated CI was very wide. We assessed the evidence as very low quality due to risk of bias and imprecision (Table 15).
Quality of life
No study collected data for this outcome.
Comparison 3: topical azelaic acid versus no treatment (including studies with a co‐intervention in both arms)
Participants' global self‐assessment of acne improvement
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 assessed this outcome using a five‐point Likert‐type scale (very satisfied, satisfied, moderately satisfied, unsatisfied, very unsatisfied). We found no significant difference in moderately to very satisfied improvement between treatment groups in the long term (3 months after start of treatment) (RR 1.08, 95% CI 0.94 to 1.24; 2 studies, 171 participants; Analysis 3.1).
3.1. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 1: Participants' global self‐assessment of acne improvement
Withdrawal for any reason
Pazoki‐Toroudi 2011 and Picosse 2015 found no statistically significant difference between topical azelaic acid and no treatment in the long term (treatment duration over 8 weeks) (RR 0.67, 95% CI 0.37 to 1.22; 2 studies, 150 participants; Analysis 3.2).
3.2. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 2: Withdrawal for any reason
Change in lesion counts
Total (percentage reduction from baseline)
Pazoki‐Toroudi 2011 compared azelaic acid 5% and clindamycin 2% combination gel with clindamycin 2% gel alone. Results showed that the combination gel was superior to clindamycin 2% gel alone in percentage reduction of total lesions. There was a clear difference between treatment groups in the short term (MD 7.62, 95% CI 6.24 to 9.00; 1 study, 100 participants; Analysis 3.3), medium term (MD 12.48, 95% CI 11.12 to 13.84; 1 study, 97 participants; Analysis 3.3), and long term (3 months after start of treatment) (MD 16.08, 95% CI 14.56 to 17.60; 1 study, 87 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 3: Change in lesion counts ‐ total (percentage reduction from baseline)
Non‐inflamed (percentage reduction from baseline)
Pazoki‐Toroudi 2011 compared azelaic acid 5% and clindamycin 2% combination gel with clindamycin 2% gel alone. Results showed that the combination gel was superior to clindamycin 2% gel alone in percentage reduction of non‐inflamed lesions. There was a clear difference between treatment groups in the short term (MD 4.30, 95% CI 3.05 to 5.55; 1 study, 100 participants; Analysis 3.4), medium term (MD 14.63, 95% CI 12.89 to 16.37; 1 study, 97 participants; Analysis 3.4), and long term (3 months after start of treatment) (MD 13.67, 95% CI 11.59 to 15.75; 1 study, 87 participants; Analysis 3.4).
3.4. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 4: Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline)
Papules (percentage reduction from baseline)
Pazoki‐Toroudi 2011 compared azelaic acid 5% and clindamycin 2% combination gel with clindamycin 2% gel alone. Results showed that the combination gel was superior to clindamycin 2% gel alone in percentage reduction of papules. There was a clear difference between treatment groups in the short term (MD 6.59, 95% CI 5.40 to 7.78; 1 study, 100 participants; Analysis 3.5), medium term (MD 8.08, 95% CI 6.71 to 9.45; 1 study, 97 participants; Analysis 3.5), and long term (3 months after start of treatment) (MD 14.51, 95% CI 12.95 to 16.07; 1 study, 87 participants; Analysis 3.5).
3.5. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 5: Change in lesion counts ‐ papules (percentage reduction from baseline)
Pustules (percentage reduction from baseline)
Pazoki‐Toroudi 2011 compared azelaic acid 5% and clindamycin 2% combination gel with clindamycin 2% gel alone. Results showed that the combination gel was superior to clindamycin 2% gel alone in percentage reduction of pustules. There was a clear difference between treatment groups in the short term (MD 9.89, 95% CI 8.66 to 11.12; 1 study, 100 participants; Analysis 3.6), medium term (MD 14.73, 95% CI 13.03 to 16.43; 1 study, 97 participants; Analysis 3.6), and long term (3 months after start of treatment) (MD 20.05, 95% CI 17.96 to 22.14; 1 study, 87 participants; Analysis 3.6).
3.6. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 6: Change in lesion counts ‐ pustules (percentage reduction from baseline)
Inflamed (number of lesions post‐intervention)
Pazoki‐Toroudi 2010 reported data for this outcome, but the number of participants was missing. When compared to erythromycin 2% gel alone, the azelaic acid 5% and erythromycin 2% combination gel group showed lower papule numbers and pustule numbers after treatment in the short, medium, and long term (3 months after start of treatment) (P < 0.01) (Analysis 3.7).
3.7. Analysis.
Comparison 3: Topical azelaic acid versus no treatment, Outcome 7: Change in lesion counts ‐ inflamed (number of lesions post‐intervention)
| Change in lesion counts ‐ inflamed (number of lesions post‐intervention) | ||||
| Study | Subgroup | Topical azelaic acid | No treatment | P value |
| short term | ||||
| Pazoki‐Toroudi 2010 | pustules | mean ± SD: 5.72 ± 0.66 | mean ± SD: 8.21 ± 0.74 | < 0.01 |
| Pazoki‐Toroudi 2010 | papules | mean ± SD: 8.53 ± 0.62 | mean ± SD: 10.62 ± 0.92 | < 0.01 |
| medium term | ||||
| Pazoki‐Toroudi 2010 | pustules | mean ± SD: 4.2 ± 0.39 | mean ± SD: 8.06 ± 0.51 | < 0.01 |
| Pazoki‐Toroudi 2010 | papules | mean ± SD: 6.01 ± 0.23 | mean ± SD: 8.16 ± 0.31 | < 0.01 |
| long term | ||||
| Pazoki‐Toroudi 2010 | pustules | mean ± SD: 4.22 ± 0.3 | mean ± SD: 7.12 ± 0.27 | < 0.01 |
| Pazoki‐Toroudi 2010 | papules | mean ± SD: 6.27 ± 0.41 | mean ± SD: 11.04 ± 0.54 | < 0.01 |
Comedones (number of lesions post‐intervention)
Pazoki‐Toroudi 2010 reported data for this outcome, but the number of participants was missing. When compared to erythromycin 2% gel alone, the azelaic acid 5% and erythromycin 2% combination gel group showed lower comedone numbers after treatment in the short, medium, and long term (3 months after start of treatment) (P < 0.01) (Analysis 3.8).
3.8. Analysis.
Comparison 3: Topical azelaic acid versus no treatment, Outcome 8: Change in lesion counts ‐ comedones (number of lesions post‐intervention)
| Change in lesion counts ‐ comedones (number of lesions post‐intervention) | ||||
| Study | Subgroup | Topical azelaic acid | No treatment | P value |
| short term | ||||
| Pazoki‐Toroudi 2010 | comedones | mean ± SD: 5.24 ± 0.61 | mean ± SD: 8.8 ± 0.46 | < 0.01 |
| medium term | ||||
| Pazoki‐Toroudi 2010 | comedones | mean ± SD: 2.22 ± 0.21 | mean ± SD: 7.32 ± 0.51 | < 0.01 |
| long term | ||||
| Pazoki‐Toroudi 2010 | comedones | mean ± SD: 2.28 ± 0.09 | mean ± SD: 5.36 ± 0.31 | < 0.01 |
Physicians' global evaluation of acne improvement
No study collected data for this outcome.
Minor adverse events
Scaling
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that four out of 90 people in the azelaic acid group versus eight out of 81 people in the no treatment group experienced scaling. There was no clear difference between topical azelaic acid and no treatment (RR 0.47, 95% CI 0.15 to 1.50; 2 studies, 171 participants; Analysis 3.9).
3.9. Analysis.

Comparison 3: Topical azelaic acid versus no treatment, Outcome 9: Minor adverse events
Erythema
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that four out of 90 people in the azelaic acid group versus nine out of 81 people in the no treatment group experienced erythema. There was no clear difference between topical azelaic acid and no treatment (RR 0.39, 95% CI 0.12 to 1.21; 2 studies, 171 participants; Analysis 3.9).
Dryness
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that five out of 90 people in the azelaic acid group versus seven out of 81 people in the no treatment group experienced dryness. There was no clear difference between topical azelaic acid and no treatment (RR 0.61, 95% CI 0.20 to 1.85; 2 studies, 171 participants; Analysis 3.9).
Oiliness
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that six out of 90 people in the azelaic acid group versus seven out of 81 people in the no treatment group experienced oiliness. There was no clear difference between topical azelaic acid and no treatment (RR 0.78, 95% CI 0.27 to 2.24; 2 studies, 171 participants; Analysis 3.9).
Itching
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that five out of 90 people in the azelaic acid group versus six out of 81 people in the no treatment group experienced itching (RR 0.73, 95% CI 0.23 to 2.29; 2 studies, 171 participants; Analysis 3.9).
Total events
Pazoki‐Toroudi 2010 and Pazoki‐Toroudi 2011 reported that 18 out of 90 people in the azelaic acid group versus 25 out of 81 people in the no treatment group experienced total events (RR 0.59, 95% CI 0.36 to 0.97; 2 studies, 171 participants; Analysis 3.9).
Quality of life
No study collected data for this outcome.
Comparison 4: topical salicylic acid versus other topical treatments
Participants' global self‐assessment of acne improvement
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion (n = 46) and the authors assessed this outcome using a five‐point scale (0: worsening or unchanged, 1: mild improvement, 2: moderate improvement, 3: good improvement and 4: excellent improvement). Both treatments demonstrated significant moderate to excellent improvement, and there was no difference between groups in the long term (12 weeks after start of treatment) (RR 1.00, 95% CI 0.92 to 1.09; 1 study, 46 participants; Analysis 4.1). We assessed the evidence as low quality due to risk of bias and imprecision (Table 7).
4.1. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 1: Participants' global self‐assessment of acne improvement
Jaffary 2016 compared salicylic acid 30% peels with pyruvic acid 50% peels and the authors assessed this outcome using a four‐point scale (excellent, good, fair, poor) in the medium term (8 weeks after start of treatment). Both treatments demonstrated significant good to excellent improvement, and there was no clear difference between groups (RR 1.12, 95% CI 0.68 to 1.84; 1 study, 86 participants; Analysis 4.1). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 6).
Participants' global self‐assessment of acne improvement (%) ‐ split‐face designs
Bae 2013 and Kessler 2008, using a split‐face design, reported data for this outcome; however, the means and SDs or SDs alone were missing, so we presented the data in a table (see table in Analysis 4.2). Bae 2013 assessed this outcome using a four‐point scale (3 = good improvement, 2 = moderate improvement, 1 = mild improvement, 0 = no improvement or worsening) showed that the percentage of participants with good to mild improvement in the short term was 92.3% in the 30% salicylic acid group and 84.6% in Jessner's solution group. Another study used patient self‐questionnaires to assess this outcome at two months post‐treatment (treatment duration of 10 weeks, measured at the post‐treatment follow‐up period) (Kessler 2008). The percentage of participants with 'more improved' was 35% in the 30% salicylic acid peel group and 41% in the 30% glycolic acid peel group.
4.2. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 2: Participants' global self‐assessment of acne improvement
| Participants' global self‐assessment of acne improvement | |||||
| Study | Duration | Items | Salicylic acid | Topical treatments (comparator) | P value |
| split‐face trials | |||||
| Bae 2013 | short term | mild to good improvement | 12 (92.3%) | 11 (84.6%) (Jessner's Solution) | P value was not reported; Quote" "In terms of subject global assessment...there is no significant differences between the groups..." |
| Kessler 2008 | treatment duration of 10 weeks, measured at two months post‐treatment | patient self‐assessment questionnaire | 35% | 41%(glycolic acid peel) | P value was not reported. |
| parallel trial | |||||
| Chantalat 2006 | medium term | acne parameters and broad skin benefits | Unclear, not reported | Unclear, not reported (10% benzoyl peroxide, BPO) | Unclear, not reported. Quote: "Subject self assessment results show that subjects treated with the microgel complex reported a greater improvement...compared to 10% BPO." |
Withdrawal for any reason
Jaffary 2016 compared salicylic acid 30% peels with pyruvic acid 50% peels in the medium term, and there was no clear difference between groups (RR 0.89, 95% CI 0.53 to 1.50; 1 study, 86 participants; Analysis 4.3). We assessed the evidence as low quality due to risk of bias and imprecision (Table 6).
4.3. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 3: Withdrawal for any reason
When salicylic acid was compared with benzoyl peroxide, two trials reported no withdrawals in the short term (Draelos 2016; Shalita 1989), and Chantalat 2006 also reported no withdrawals in the medium term (Analysis 4.3). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 5).
Babayeva 2011 and NilFroushzadeh 2009 compared salicylic acid with tretinoin; neither treatment group had any withdrawals in the long term (12 weeks after start of treatment) (Analysis 4.3). We assessed the evidence as low quality due to risk of bias and imprecision (Table 7).
One trial compared salicylic acid 30% peels with Jessner's solution (Dayal 2017); neither treatment group had any withdrawals in the long term (12 weeks after start of treatment) (Analysis 4.3).
Change in lesion counts
Total lesion (number of lesions post‐intervention)
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. the tretinoin group had a lower total lesion count than topical salicylic acid after intervention. The difference was significant in the short term (MD 7.70, 95% CI 1.89 to 13.51; 1 study, 46 participants; Analysis 4.4). However, the estimated RR had a very wide 95% CI which led to an imprecise result. The difference between topical salicylic acid and tretinoin in the medium term was uncertain due to the wide CI (MD 2.80, 95% CI ‐3.31 to 8.91; 1 study, 46 participants; Analysis 4.4), but showed greater certainty in the long term (12 weeks after start of treatment) (MD 3.60, 95% CI ‐0.06 to 7.26; 1 study, 46 participants; Analysis 4.4).
4.4. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 4: Change in lesion counts ‐ total (number of lesions post‐intervention)
Draelos 2016 had no usable outcome data; the authors compared the short‐term efficacy of salicylic acid 2% gel, benzoyl peroxide 3% gel and vehicle gel. The authors demonstrated the overall improvement in target lesion parameters of swelling, diameter and erythema from baseline, but there was no significant difference among these groups. In Chantalat 2007 (also with no usable outcome data), the authors stated that 0.5% salicylic acid in mild foaming cleaner formulations could significantly inhibit emerging acne lesions when compared to its vehicle. In Chen 2007 (again with no usable outcome data), the authors showed that salicylic acid in a microgel complex was superior to its vehicle in reducing total lesion counts and global acne severity.
Inflamed (number of lesions post‐intervention)
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. The tretinoin group had a lower inflamed lesion count than topical salicylic acid after intervention. The difference was significant in the short term (MD 4.30, 95% CI 0.50 to 8.10; 1 study, 46 participants; Analysis 4.5). There was no clear difference between topical salicylic acid and tretinoin in the medium term (MD 2.70, 95% CI ‐0.47 to 5.87; 1 study, 46 participants; Analysis 4.5), and long term (12 weeks after start of treatment) (MD 1.10, 95% CI ‐1.03 to 3.23; 1 study, 46 participants; Analysis 4.5).
4.5. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 5: Change in lesion counts ‐ inflamed (number of lesions post‐intervention)
Inflamed (mean counts or %)
Three studies reported data for this outcome; however, the SDs were missing, so we presented the data in a table (see table in Analysis 4.6). Babayeva 2011 reported the percentage reduction of inflamed lesions in the long term (12 weeks after start of treatment) was 77.6% in the salicylic acid 3% lotion group and 81.5% in the tretinoin 0.05 cream group; however, this difference was not significant. In one trial with a split‐face design (Bae 2013), the author reported no difference in reduction of inflamed lesions between the salicylic acid 30% peel group and Jessner's solution group. Another split‐face trial reported there no difference in mean inflamed lesion counts post‐intervention in the long term (12 weeks after start of treatment) between the salicylic acid 20% or 30% peel group and the lipohydroxy acid 5% or 10% peel group (P = 0.111) (Levesque 2011). Chantalat 2005, with no usable outcome data, compared a proprietary 2% salicylic acid microgel with 10% benzoyl peroxide cream, and the authors found that the clinical resolution of target inflammatory lesions was more rapid with the novel 2% salicylic acid microgel than 10% benzoyl peroxide cream.
4.6. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 6: Change in lesion counts ‐ inflamed (mean counts or %)
| Change in lesion counts ‐ inflamed (mean counts or %) | ||||
| Study | Outcome | Salicylic acid | Topical treatments (comparator) | P value |
| parallel trials | ||||
| Babayeva 2011 | Percent reduction ‐inflamed (long term) | 77.6% | 81.5% | P value was not reported. Quote: "...these differences were not statistically significant..." |
| split‐face trials | ||||
| Bae 2013 | Average reduction of inflamed lesion (medium term) | 4.4 | 5.7 (Jessner's solution peels) | P value was not reported; Quote" Inflammatory acne lesion counts did not differ significantly between salicylic acid and Jessner's solution peels" |
| Levesque 2011 | Mean inflamed lesion counts post intervention (long term: 12 weeks after start of treatment) | 3.9 | 3.7 (lipohydroxyacid peels) | P = 0.111. Quote:"The mean number of inflammatory lesions was 6.1 and 6.6 at Day 14 (baseline) and 3.7 and 3.9 at Day 98 on the sides that received the LHA and salicylic acid peels..." |
Papules (number of lesions post‐intervention)
Jaffary 2016 reported no clear difference between salicylic acid 30% peels and pyruvic acid 50% peels in the short and medium term (Analysis 4.7). Short term: MD 0.87 (95% CI ‐2.48 to 4.22; 1 study, 52 participants) and medium term: MD 1.12 (95% CI ‐1.55 to 3.79; 1 study, 52 participants).
4.7. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 7: Change in lesion counts ‐ papules (number of lesions post‐intervention)
Pustules (number of lesions post‐intervention)
Jaffary 2016 reported no clear difference between salicylic acid 30% peels and pyruvic acid 50% peels in the short and medium term (Analysis 4.8). Short term: MD ‐0.08 (95% CI ‐0.85 to 0.69; 1 study, 52 participants) and medium term: MD 0.31 (95% CI ‐0.53 to 1.15; 1 study, 52 participants).
4.8. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 8: Change in lesion count ‐ pustules (number of lesions post‐intervention)
Non‐inflamed (number of lesions post‐intervention)
Only one study that compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion contributed data for this outcome (Babayeva 2011). There was no statistically significant difference between topical salicylic acid and tretinoin in the short term (MD 3.90, 95% CI ‐0.03 to 7.83; 1 study, 46 participants; Analysis 4.9) and medium term (MD 0.30, 95% CI ‐3.55 to 4.15; 1 study, 46 participants; Analysis 4.9). However, there was a clear difference between topical salicylic acid and tretinoin in the long term (12 weeks after start of treatment) (MD 2.50, 95% CI 0.11 to 4.89; 1 study, 46 participants; Analysis 4.9), with less non‐inflamed lesion counts in the tretinoin group.
4.9. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 9: Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention)
Jaffary 2016 compared salicylic acid 30% peels with pyruvic acid 50% peels; participants receiving pyruvic acid 50% peels had fewer non‐inflamed lesions, though there was no clear difference between groups in the short (MD 19.89, 95% CI ‐7.65 to 47.43; 1 study, 52 participants) and medium terms (MD 17.48, 95% CI ‐6.45 to 41.41; 1 study, 52 participants) (Analysis 4.9).
Non‐inflamed (counts or percentage)
Three studies reported data for this outcome; however, the SDs were missing, so we presented the data in a table (see table in Analysis 4.10). Babayeva 2011 reported no difference in the long term (12 weeks after start of treatment) between the salicylic acid 3% lotion group and tretinoin 0.05% cream group (81.5% versus 87.2%). The split‐face design trial of Bae 2013 reported a medium‐term significant number reduction of non‐inflamed lesion counts in the salicylic acid 30% peel group compared to the Jessner's solution group (average number reduction of non‐inflamed counts 8 versus 4.3). In another split‐face trial (Levesque 2011), there was no difference in percentage reduction of non‐inflamed lesion counts in the long term (12 weeks after start of treatment) between the salicylic acid 20% or 30% peel group and the lipohydroxy acid 5% or 10% peel group (P = 0.878).
4.10. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 10: Change in lesion counts ‐ non‐inflamed (counts or %)
| Change in lesion counts ‐ non‐inflamed (counts or %) | ||||
| Study | Time points | Salicylic acid | Topical treatments (comparator) | P value |
| parallel trials | ||||
| Babayeva 2011 | Percent reduction ‐ long term | 81.5% | 87.2% | P value was not reported. Quote: "...these differences were not statistically significant..." |
| split‐face trials | ||||
| Bae 2013 | Average number reduction of non‐inflamed ‐ medium term | 8 | 4.3 | 0.04 |
| Levesque 2011 | Percent reduction ‐ long term: 12 weeks after start of treatment | 48.5% | 55.6% (lipohydroxyacid peels) | 0.878 |
Various types of acne lesions (counts or percentage)
Four studies reported data for this outcome; however, the SDs were missing, so we presented the data in a table (see table in Analysis 4.11). In a parallel trial (NilFroushzadeh 2009), 2% salicylic acid plus 1% clindamycin lotion showed a greater percentage reduction in closed comedones (P = 0.011), papules (P = 0.031), and total lesions (P = 0.039) in the long term (12 weeks after start of treatment) when compared to 0.025% tretinoin plus 1% clindamycin lotion. In a split‐face trial that compared salicylic acid 30% peel with glycolic acid 30% peel (Kessler 2008), there was no statistically significant difference between groups in percentage reduction of total lesions at one month post‐treatment (P > 0.05) (treatment duration of 10 weeks, measured at the post‐treatment follow‐up period). In Shalita 1989, with a cross‐over design that compared salicylic acid 2% cleaner with 10% benzoyl peroxide wash, there was no statistically significant difference between groups in number of comedones post‐intervention.
4.11. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 11: Change in lesion counts ‐ various types of acne lesions (counts or %)
| Change in lesion counts ‐ various types of acne lesions (counts or %) | ||||
| Study | Time points | Salicylic acid | Topical treatments (comparator) | P value |
| parallel trials | ||||
| Babayeva 2011 | Percent reduction ‐ total lesions (long term: 12 weeks after start of treatment) | 80.2% | 85.6% (Tretinoin) | P value was not reported. Quote: "...these differences were not statistically significant..." |
| NilFroushzadeh 2009 | Percent reduction ‐ open comedones (long term: 12 weeks after start of treatment) | 64.26% | 67% (Tretinoin) | >0.05 |
| NilFroushzadeh 2009 | Percent reduction ‐ papule (long term: 12 weeks after start of treatment) | 84.5% | 71.67% (Tretinoin) | 0.031 |
| NilFroushzadeh 2009 | Percent reduction ‐ total lesions (long term: 12 weeks after start of treatment) | 77.91% | 72.20% (Tretinoin) | 0.039 |
| NilFroushzadeh 2009 | Percent reduction ‐ pustules (long term: 12 weeks after start of treatment) | 90% | 76.19% (Tretinoin) | >0.05 |
| NilFroushzadeh 2009 | Percent reduction ‐ closed comedones (long term: 12 weeks after start of treatment) | 87.05% | 60.94% (Tretinoin) | 0.011 |
| split‐face trials | ||||
| Kessler 2008 | Percent reduction ‐ total lesion(treatment duration of 10 weeks, measured at one month post‐treatment) | 47% | 43% (30% glycolic acid peel) | > 0.05 |
| cross‐over trials | ||||
| Shalita 1989 | number of lesions post intervention ‐ comedo counts (short term) | 10.9 | 14.9 (10% benzoyl peroxide wash) | No difference |
Physicians' global evaluation of acne improvement
Babayeva 2011 compared salicylic acid 3% gel with tretinoin 0.05% cream and reported long term data (12 weeks after start of treatment) (n = 46) and the authors assessed this outcome using a five‐point scale (0: worsening or unchanged, 1: mild improvement, 2: moderate improvement, 3: good improvement and 4: excellent improvement). There was no difference between topical salicylic acid and tretinoin in moderate to excellent improvement (RR 1.00, 95% CI 0.92 to 1.09; 1 study, 46 participants; Analysis 4.12). Dayal 2017 reported data collected in the long term (12 weeks after start of treatment) (n = 40) and the authors assessed this outcome using percentage decrease in Michaelsson acne scores (MAS) (good improvement: > 50% decrease in MAS; fair improvement: 21% to 50% decrease in MAS; poor improvement: 11% to 20% decrease in MAS; no change: 0% to 10% decrease in MAS), and there was no clear difference between 30% salicylic acid peels and Jessner's solution peels (RR 1.11, 95% CI 0.93 to 1.31; 1 study, 40 participants; Analysis 4.12). Jaffary 2016 compared salicylic acid 30% peels with pyruvic acid 50% peels and the authors assessed this outcome using percentage decrease of acne severity index (ASI) (excellent = improved more than 75%, good = improved 50% to 75%, moderate = improved 25% to 50%, poor = improved < 25%), and there was no clear difference between salicylic acid 30% peels and pyruvic acid 50% peels (RR 1.20, 95% CI 0.70 to 2.06; 1 study, 86 participants; Analysis 4.12).
4.12. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 12: Physicians' global evaluation of acne improvement
Physicians' global evaluation of acne improvement ‐ split‐face trials
In one split‐face trial (Kessler 2008), the authors assessed this outcome using a five‐point system (good: more than 50% improvement, fair: 21% to 50% improvement, poor: 10% to 20% improvement, no change, or worse) but did not report whether there was a statistical difference between the salicylic acid 30% peel group and glycolic acid 30% peel group at two months post‐treatment (treatment duration of 10 weeks, measured at the post‐treatment follow‐up period). The SDs were missing, so we presented the data in a table (see table in Analysis 4.13).
4.13. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 13: Physicians' global evaluation of acne improvement (%)
| Physicians' global evaluation of acne improvement (%) | ||||
| Study | Time points | Salicylic acid | Topical treatments (comparator) | P value |
| split‐face trials | ||||
| Kessler 2008 | Good to fair improvement (treatment duration of 10 weeks, measured at two months post‐treatment) | 81% | 75% (30% glycolic acid) | Unclear, not reported. The author did not report whether there is any statistical difference between groups. |
In another study with a split‐face design that included a total of 20 participants suffering from comedonal acne (Levesque 2011), each participant received salicylic acid peels (20% or 30%) on one side of the face and lipohydroxy acid peels (5% or 10%) on the other side of the face. The authors assessed this outcome using a three‐point scoring system (1 = worse, 2 = stable, 3 = improved). There was a significant difference between groups in the short term (MD ‐0.40, 95% CI ‐0.67 to ‐0.13; 1 study; Analysis 4.14), which was in favour of lipohydroxy acid (5% or 10%) peel. However, there was no significant difference between groups in the medium term (MD 0.10, 95% CI ‐0.17 to 0.37; 1 study; Analysis 4.14) and long term (12 weeks after start of treatment) (MD 0.00, 95% CI ‐0.24 to 0.24; 1 study; Analysis 4.14).
4.14. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 14: Physicians' global evaluation of acne improvement
Minor adverse events
Dryness ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. There was no significant difference between topical salicylic acid and tretinoin (RR 1.17, 95% CI 0.70 to 1.94; 1 study, 46 participants; Analysis 4.15).
4.15. Analysis.

Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 15: Minor adverse events
Peeling ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. There was no significant difference between topical salicylic acid and tretinoin (RR 0.71, 95% CI 0.40 to 1.26; 1 study, 46 participants; Analysis 4.15).
Erythema ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. There was no significant difference between topical salicylic acid and tretinoin (RR 0.88, 95% CI 0.38 to 2.01; 1 study, 46 participants; Analysis 4.15).
Burning ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. There was no significant difference between topical salicylic acid and tretinoin (RR 1.14, 95% CI 0.50 to 2.63; 1 study, 46 participants; Analysis 4.15).
Itching ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. The authors reported that three out of 23 people in the 3% alcohol‐based salicylic acid plus clindamycin 1% lotion group versus five out of 23 people in the tretinoin 0.05% cream plus clindamycin 1% lotion group experienced itching (P = 0.70, Fisher's Exact test). There was no significant difference between treatment groups (RR 0.60, 95% CI 0.16 to 2.22; 1 study, 46 participants; Analysis 4.15).
Postpeel burning and stinging ‐ salicylic acid versus Jessner's solution
Dayal 2017 compared salicylic acid 30% peel with Jessner's solution peel, and we found no clear difference between salicylic acid and Jessner's solution (RR 1.44, 95% CI 0.81 to 2.58; 1 study, 40 participants; Analysis 4.15).
Postpeel erythema ‐ salicylic acid versus Jessner's solution
Dayal 2017 compared salicylic acid 30% peel with Jessner's solution peel, and we found no clear difference between salicylic acid and Jessner's solution (RR 1.50, 95% CI 0.50 to 4.52; 1 study, 40 participants; Analysis 4.15).
Postpeel hyperpigmentation ‐ salicylic acid versus Jessner's solution
Dayal 2017 compared salicylic acid 30% peel with Jessner's solution peel. The authors reported that one out of 20 people in the salicylic acid 30% peel group versus three out of 20 people in the Jessner's solution peel group experienced postpeel hyperpigmentation (P = 0.61, Fisher's Exact test). There was no clear difference between salicylic acid and Jessner's solution (RR 0.33, 95% CI 0.04 to 2.94; 1 study, 40 participants; Analysis 4.15).
Total events ‐ salicylic acid versus benzoyl peroxide
In one study with 90 people (Draelos 2016), the author compared the short‐term efficacy of salicylic acid 2% gel, benzoyl peroxide 3% gel and vehicle gel. They reported that no adverse events were observed during this five‐day study (30 participants in each treatment group) (Analysis 4.15).
Chantalat 2006 reported this outcome in the medium term. The authors reported that zero out of 20 people in the 2% salicylic acid microgel group versus two out of 21 people in the benzoyl peroxide 10% cream group experienced minor adverse events (P = 0.49, Fisher's Exact test). There was no clear difference between the 2% salicylic acid microgel group and benzoyl peroxide 10% cream group due to the wide CI (RR 0.21, 95% CI 0.01 to 4.11; 1 study, 41 participants; Analysis 4.15). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 5).
Total events ‐ salicylic acid versus tretinoin
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion and NilFroushzadeh 2009 compared 2% salicylic acid plus 1% clindamycin lotion with 0.025% tretinoin plus 1% clindamycin lotion. There was no clear difference between treatment groups due to the wide CI (RR 1.37, 95% CI 0.66 to 2.87; 2 studies, 74 participants; Analysis 4.15). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 7).
Total events ‐ salicylic acid versus lipohydroxy acid
Levesque 2011 was a split‐face design that compared salicylic acid peel (20% or 30% ) with lipohydroxy acid peel (5% or 10%), the authors did not report total number of participants who experienced at least one minor adverse event but evaluated minor adverse events using a 10 cm visual analogue scale. There was no difference for itching (P = 0.412), tightness (P = 0.108), and erythema (P = 0.103). Salicylic acid peels induced more desquamation (P = 0.007) and dryness (P < 0.05) but less stinging (P = 0.017) and burning (P = 0.021) when compared to lipohydroxy acid peels.
Quality of life ‐ AQOL (score, post‐intervention)
Babayeva 2011 compared 3% alcohol‐based salicylic acid plus clindamycin 1% lotion with tretinoin 0.05% cream plus clindamycin 1% lotion. The study reported no statistically difference between groups for this outcome in the long term (12 weeks after start of treatment); however, the data were skewed, so we therefore could only present the data in a table (see table in Analysis 4.16). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 7). Chantalat 2006 did not provide means ± SDs, and the study compared salicylic acid 2% microgel with 10% benzoyl peroxide cream and found a significant improvement in quality of life with salicylic acid 2% microgel (Analysis 4.16). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 5).
4.16. Analysis.
Comparison 4: Topical salicylic acid versus other topical treatments, Outcome 16: Quality of life (QoL) ‐ AQOL (score, post‐intervention)
| Quality of life (QoL) ‐ AQOL (score, post‐intervention) | ||||
| Study | Time points | Salicylic acid: mean ± sd | Topical treatments: mean ± sd (comparator) | P value |
| Babayeva 2011 | Long term: 12 weeks after start of treatment | 0.95 ± 1.9 | 0.91 ± 1.64 (tretinoin) | P value was not reported. Quote: "there were no significant differences in AQOL between treatment groups at baseline and at the end of the study" |
| Chantalat 2006 | Short term and medium term | Unclear, not reported | Unclear, not reported | P value was not reported. Quote: "ARQL results show that subjects treated with novel microgel complex experienced a significant improvement in ARQL starting 2 weeks after baseline and continuing through the 6‐week study." |
Comparison 5: topical salicylic acid versus placebo
Participants' global self‐assessment of acne improvement
Participants' global self‐assessment of acne improvement (score, high = well)
Only one study contributed data for this outcome assessed by a seven‐point interval rating scale (Eady 1996). There was no significant difference between topical salicylic acid 2% lotion and placebo lotion in the short term (MD 0.30, 95% CI ‐0.12 to 0.72; 1 study, 106 participants; Analysis 5.1) or long term (12 weeks after start of treatment) (MD 0.40, 95% CI ‐0.05 to 0.85; 1 study, 99 participants; Analysis 5.1). However, in the medium term, participants in the salicylic acid group recorded a higher score for acne improvement than those in the placebo group, the difference was clearly significant (MD 0.50, 95% CI 0.23 to 0.77; 1 study, 102 participants; Analysis 5.1).
5.1. Analysis.

Comparison 5: Topical salicylic acid versus placebo, Outcome 1: Participants' global self‐assessment of acne improvement (score, high=well)
Withdrawal for any reason
One trial compared salicylic acid 2% gel with vehicle gel (Draelos 2016), and neither treatment group had any withdrawals in the short term (Analysis 5.2). Another two trials reported data in the long term (12 weeks after start of treatment) (Eady 1996; Shalita 1981), and there was no clear difference between salicylic acid and placebo due to the wide CI (RR 2.07, 95% CI 0.76 to 5.68; 2 studies, 163 participants; Analysis 5.2), but one study reported no events.
5.2. Analysis.

Comparison 5: Topical salicylic acid versus placebo, Outcome 2: Withdrawal for any reason
Change in lesion counts
Mean counts or percentage reduction
Three studies reported data for this outcome; however, the authors did not provide mean and/or SDs. Eady 1996 only reported the P values, and results showed that salicylic acid 2% lotion treatment had better efficacy in whitehead (long term, P < 0.002), papules (long term, P = 0.022), and total lesion (medium term, P < 0.043; and long term, P < 0.001) number reduction when compared to placebo. In Techapichetvanich 2011, the percentage reduction of non‐inflamed lesions in the long term (10 weeks after start of treatment) was 84.0% for the salicylic acid (20% or 30%) peel group and 36.0% for the vehicle peel group (P = 0.001), and the percentage reduction of total lesions in the long term was 84.0% for the salicylic acid (20% or 30%) peel group and 26.0% for the vehicle peel group (P = 0.001), suggesting the benefits of salicylic acid treatment. The Shalita 1981 study also suggested the advantage of salicylic acid 0.5% solution over placebo in percentage reduction in total lesions in the long term, although the author did not state whether the difference was of statistical significant (Analysis 5.3).
5.3. Analysis.
Comparison 5: Topical salicylic acid versus placebo, Outcome 3: Change in lesion counts (counts or %)
| Change in lesion counts (counts or %) | ||||
| Study | Subgroup | Salicylic acid | Placebo | P value |
| Eady 1996 | Number of whitehead reduction (short, medium and long term) | Unclear, not reported | Unclear, not reported | no difference; no difference; < 0.002 |
| Eady 1996 | Number of total counts reduction (short, medium and long term) | Unclear, not reported | Unclear, not reported | no difference; < 0.043; < 0.001 |
| Eady 1996 | Number of papules reduction (short, medium and long term) | Unclear, not reported | Unclear, not reported | no difference; no difference; 0.022 |
| Shalita 1981 | Percent reduction ‐total (long term: 12 weeks after start of treatment) | 38.4% | 24.6% | not report and the author did not state whether this difference is significant |
| Techapichetvanich 2011 | Percent reduction ‐ noninflamed (long term: 10 weeks after start of treatment) | 83.77% | 25.83% | 0.001 |
| Techapichetvanich 2011 | Percent reduction ‐ total counts (long term: 10 weeks after start of treatment) | 83.97% | 35.94% | 0.001 |
Inflamed (counts or percentage)
Two studies reported data for this outcome; however, the authors did not provide mean and/or SDs, so we presented the data in a table (Analysis 5.4). Eady 1996 showed a statistically significant difference between topical salicylic acid 2% lotion and placebo lotion in the long term (12 weeks after start of treatment) (P < 0.022) in favour of salicylic acid, but no difference in the short and medium term for reduction in number of inflamed lesions. Shalita 1981 did not report the P value, but the data suggested the advantage of salicylic acid 0.5% solution over placebo for percentage reduction of inflamed lesions in the long term (12 weeks after start of treatment).
5.4. Analysis.
Comparison 5: Topical salicylic acid versus placebo, Outcome 4: Change in lesion counts ‐ inflamed (counts or %)
| Change in lesion counts ‐ inflamed (counts or %) | ||||
| Study | Subgroup | Salicylic acid | Placebo | P value |
| Eady 1996 | Number reduction ‐ short, medium and long term (12 weeks after start of treatment) | Unclear, not reported | Unclear, not reported | no difference; no difference; < 0.022 |
| Shalita 1981 | percent reduction ‐ short term | 29.5% | 20% | not report and the author did not state whether this difference is significant |
| Shalita 1981 | percent reduction ‐ medium term | 44.6% | 23% | not report and the author did not state whether this difference is significant |
| Shalita 1981 | percent reduction ‐ long term: 12 weeks after start of treatment | 54% | 29% | not report and the author did not state whether this difference is significant |
Non‐inflamed (counts or percentage)
Two studies reported data for this outcome; however, the authors did not provide mean and/or SDs (Analysis 5.5). Eady 1996 showed that topical salicylic acid 2% lotion demonstrated a significant reduction in number of non‐inflamed lesions in the medium (P = 0.047) and long term (12 weeks after start of treatment) (P < 0.001) compared to placebo lotion, but no difference in the short term (no exact P value reported). Shalita 1981 showed no significant difference between groups for percentage reduction of closed comedones in the long term (12 weeks after start of treatment) (no exact P value reported); however, the percentage reduction of open comedones was significant in the salicylic acid 0.5% solution group compared to placebo (no exact P value reported).
5.5. Analysis.
Comparison 5: Topical salicylic acid versus placebo, Outcome 5: Change in lesion counts ‐ non‐inflamed (counts or %)
| Change in lesion counts ‐ non‐inflamed (counts or %) | ||||
| Study | Subgroup | Salicylic acid | Placebo | P value |
| Eady 1996 | Number reduction ‐ short, medium and long term (12 weeks after start of treatment) | Unclear, not reported | Unclear, not reported | no difference; 0.047; < 0.001 |
| Shalita 1981 | Percent reduction ‐ open comedones (long term: 12 weeks after start of treatment) | 39% | 28% | not reported, but the author stated the difference is significant. |
| Shalita 1981 | Percent reduction ‐ closed comedones (long term: 12 weeks after start of treatment) | 21% | 21% | No difference |
Physicians' global evaluation of acne improvement
Shalita 1981 compared salicylic acid 0.5% solution with placebo and reported long term data (12 weeks after start of treatment) (n = 49). The authors assessed this outcome using a four‐point Likert‐type scale (excellent, good, fair, poor). There were more participants rated as good or excellent improvement in the salicylic acid 0.5% solution group than participants in the placebo group (RR 2.16, 95% CI 1.17 to 4.0; 1 study, 49 participants; Analysis 5.6).
5.6. Analysis.

Comparison 5: Topical salicylic acid versus placebo, Outcome 6: Physicians' global evaluation of acne improvement
Minor adverse events
Total adverse events
Draelos 2016 included 90 participants and compared the short‐term efficacy of salicylic acid 2% gel, benzoyl peroxide 3% gel and vehicle gel. They reported that no adverse events were observed during this five‐day study (30 participants in each treatment group) (Analysis 5.7).
5.7. Analysis.

Comparison 5: Topical salicylic acid versus placebo, Outcome 7: Minor adverse events
Shalita 1981 included 49 participants and compared 0.5% salicylic acid solution with placebo solution in the long term. However, there were no adverse events reported in either treatment group (Analysis 5.7); the authors only stated that side effects were minimal.
Quality of life
No study collected data for this outcome.
Comparison 6: topical salicylic acid versus no treatment (including studies with a co‐intervention in both arms)
Participants' global self‐assessment of acne improvement
For this outcome, there were no data reported in the short and medium term. Akarsu 2012 reported data in the long term (12 weeks after start of treatment) and the authors assessed this outcome using a five‐point scale (0 = worsening or unchanged, 1 = mild improvement, 2 = moderate improvement, 3 = good improvement, 4 = excellent improvement). This trial compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel in moderate to excellent improvement. There was no significant difference between treatment groups (RR 0.96, 95% CI 0.86 to 1.07; 1 study, 50 participants; Analysis 6.1). We assessed the evidence as low quality due to risk of bias and imprecision (Table 16).
6.1. Analysis.

Comparison 6: Topical salicylic acid versus no treatment, Outcome 1: Participants' global self‐assessment of acne improvement
6. Salicylic acid compared to no treatment.
| Salicylic acid compared to no treatment for acne | ||||||
| Patient or population: participants with acne Settings: Skin Disease and Leishmaniasis Research Center and Isfahan University of Medical Sciences clinics (1 study); a tertiary care hospital of Eastern India (1 study); not described (1 study) Intervention: topical salicylic acid Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Topical salicylic acid | |||||
|
Participants' global self‐assessment of acne improvement
Moderate to excellent improvement (long term: treatment duration > 8 weeks) |
1000 per 1000 | 960 per 1000 (860 to 1000) | RR 0.96 (0.86 to 1.07) | 50 (1 study) | ⊕⊕⊝⊝ Lowa | Clindamycin/benzoyl peroxide was a co‐intervention given in both arms. |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
‐ | ‐ | RR 3.0 (0.13 to 70.30) | 138 (3 studies) | ⊕⊝⊝⊝ Verylowb | Two studies had no withdrawals. |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
436 per 1000 |
1000 per 1000 (61 to 1000) |
RR 3.43 (0.14 to 82) | 78 (2 studies) | ⊕⊝⊝⊝ Verylowc | All side effects reported in the study were of mild to moderate intensity and transient. |
|
Quality of life AQOL (long term: treatment duration > 8 weeks) |
The authors reported no "significant differences" in AQOL between treatment groups (salicylic acid/clindamycin/benzoyl peroxide group versus clindamycin/benzoyl peroxide group) at baseline and the end of the study. | 50 (1 study) | ⊕⊝⊝⊝ Very lowd |
Median and 95% CI reported. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AQOL: acne quality of life; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of selection bias. One level for imprecision: optimal sample size not met. bDowngraded by three levels to very low quality evidence. One level for risk of bias: three studies included and all with unclear risk of allocation concealment and high risk of performance bias, two studies with unclear risk of random sequence generation. Two levels for imprecision: very wide CI and optimal sample size not met. cDowngraded by three levels to very low quality evidence. One level for risk of bias: two studies included and both with unclear risk of selection and high risk of performance bias, one with unclear risk of reporting bias. Two levels for imprecision: very wide CI and optimal sample size not met. dDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of selection bias. One level for imprecision: very small total sample size. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Withdrawal for any reason
Three relevant trials reported data for this outcome in the long term (treatment duration over 12 weeks) (Akarsu 2012; Kar 2013; NilFroushzadeh 2009). Two trials had no withdrawals during treatment (Kar 2013; NilFroushzadeh 2009). There was no clear difference between treatment groups due to the wide CI (RR 3.00, 95% CI 0.13 to 70.30; 3 studies, 138 participants, two of which reported no events, Analysis 6.2). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 16).
6.2. Analysis.

Comparison 6: Topical salicylic acid versus no treatment, Outcome 2: Withdrawal for any reason
Change in lesion counts
Total (percentage reduction from baseline)
Three studies reported data for this outcome (Akarsu 2012; Kar 2013; NilFroushzadeh 2009; however, the authors did not provide mean and/or SDs. Two studies reported higher percentage reduction of lesion counts in the salicylic acid group than that in the no treatment group (Akarsu 2012; NilFroushzadeh 2009), and authors stated the difference was statistically significant in the long term (12 weeks after start of treatment). Kar 2013 also reported higher percentage reduction of total lesion counts in the salicylic acid group in the long term (16 weeks after start of treatment), although the authors did not state whether the difference was statistically significant. (Analysis 6.3).
6.3. Analysis.
Comparison 6: Topical salicylic acid versus no treatment, Outcome 3: Change in lesion counts ‐ total (percentage reduction from baseline)
| Change in lesion counts ‐ total (percentage reduction from baseline) | ||||
| Study | Subgroup | Salicylic acid | No treatment | P value |
| Akarsu 2012 | Percent reduction ‐ total (long term: 12 weeks after start of treatment) | 94.3% | 79.2% | Quote:"The mean percent reductions in NIL, IL and TL counts were significantly higher for patients in group 1 as opposed to the patients in group 2 at week 12". |
| Kar 2013 | Percent reduction ‐ total (long term: 16 weeks after start of treatment) | 92.5% | 73.4% | not report and the author did not state whether this difference is significant |
| NilFroushzadeh 2009 | Percent reduction ‐ total lesions (long term: 12 weeks after start of treatment) | 77.91% | 55.95% | 0.039 |
Inflamed (percentage reduction from baseline)
Two studies reported data for this outcome; however, the authors did not provide mean and/or SDs, so we presented the data in a table (see table in Analysis 6.4). Study authors compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. The study reported higher percentage reduction of lesion counts in the salicylic acid group than in the no treatment group (98.2% versus 73.8%); the authors stated the difference was statistically significant in the long term (12 weeks after start of treatment) (Akarsu 2012). Another study also reported higher percentage reduction of papules and pustules in the salicylic acid group in the long term (12 weeks after start of treatment) (NilFroushzadeh 2009).
6.4. Analysis.
Comparison 6: Topical salicylic acid versus no treatment, Outcome 4: Change in lesion counts ‐ inflamed (percentage reduction from baseline)
| Change in lesion counts ‐ inflamed (percentage reduction from baseline) | ||||
| Study | Subgroup | Salicylic acid | No treatment | P value |
| Akarsu 2012 | long term: 12 weeks after start of treatment | 98.2% | 73.8% | Quote:"The mean percent reductions in NIL, IL and TL counts were significantly higher for patients in group 1 as opposed to the patients in group 2 at week 12". |
| NilFroushzadeh 2009 | long term: 12 weeks after start of treatment | papules: 84.5% pustules: 90% |
papules: 26.63% pustules: 80% |
papules: P = 0.031; pustules: P > 0.05 |
Non‐inflamed (percentage reduction from baseline)
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel; however, the authors did not provide SDs. In Akarsu 2012 the percentage reduction of non‐inflamed lesions in the long term (12 weeks after start of treatment) was significantly greater in participants receiving salicylic acid treatment (94.7%) than no treatment (81.1%), although the P value was unclear. Authors NilFroushzadeh 2009 compared 2% salicylic acid + 1% clindamycin lotion with 1% clindamycin lotion and results showed higher percentage reduction of comedones in the salicylic acid group (Analysis 6.5).
6.5. Analysis.
Comparison 6: Topical salicylic acid versus no treatment, Outcome 5: Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline)
| Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | ||||
| Study | Subgroup | Salicylic acid | No treatment | P value |
| Akarsu 2012 | long term: 12 weeks after start of treatment | 94.7% | 81.1% | Quote:"The mean percent reductions in NIL, IL and TL counts were significantly higher for patients in group 1 as opposed to the patients in group 2 at week 12". |
| NilFroushzadeh 2009 | long term: 12 weeks after start of treatment | open comedones:64.26% closed comedones:87.05% |
open comedones:58.33% closed comedones:31.28% |
open comedones: P > 0.05 closed comedones: P = 0.011 |
Physicians' global evaluation of acne improvement
Akarsu 2012 reported data collected in the long term (12 weeks after start of treatment). This trial compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel and assessed this outcome using a 5‐point scale (0 = worsening or unchanged, 1 = mild improvement, 2 = moderate improvement, 3 = good improvement, 4 = excellent improvement). There was no significant difference between treatment groups in the moderate to excellent improvement categories (RR 0.96, 95% CI 0.86 to 1.07; 1 study, 50 participants; Analysis 6.6).
6.6. Analysis.

Comparison 6: Topical salicylic acid versus no treatment, Outcome 6: Physicians' global evaluation of acne improvement
Minor adverse events
Dryness
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. Results showed a higher risk of dryness in the salicylic acid/clindamycin/benzoyl peroxide group than control (16/25 versus 6/25). The difference was significant (RR 2.67, 95% CI 1.25 to 5.68; 1 study, 50 participants; Analysis 6.7).
6.7. Analysis.

Comparison 6: Topical salicylic acid versus no treatment, Outcome 7: Minor adverse events
Peeling
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. There was no clear difference between treatment groups (RR 1.50, 95% CI 0.74 to 3.03; 1 study, 50 participants; Analysis 6.7).
Erythema
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. The estimated RR had a very wide 95% CI resulting in imprecision (RR 4.00, 95% CI 0.94 to 17.00; 1 study, 50 participants; Analysis 6.7),
Burning
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. There was no clear difference between treatment groups (RR 1.67, 95% CI 0.71 to 3.89; 1 study, 50 participants; Analysis 6.7).
Itching
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel. The authors reported that five out of 25 people in the salicylic acid/clindamycin/benzoyl peroxide group versus three out of 25 people in the clindamycin/benzoyl peroxide group experienced itching (P = 0.70, Fisher's Exact test). There was no significant difference between treatment groups (RR 1.67, 95% CI 0.45 to 6.24; 1 study, 50 participants; Analysis 6.7).
Total events
Akarsu 2012 and NilFroushzadeh 2009 compared salicylic acid with no treatment in the long term (12 weeks after start of treatment). There was no clear difference between treatment groups due to a wide CI (RR 3.43, 95% CI 0.14 to 82.00; 2 studies, 78 participants; Analysis 6.7). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 16).
Quality of life ‐ AQOL (score, post‐intervention)
Akarsu 2012 compared 3% salicylic acid to 1% clindamycin lotion and 5% benzoyl peroxide gel with 1% clindamycin lotion and 5% benzoyl peroxide gel in the long term (12 weeks after start of treatment); however, the data were not available for meta‐analysis (Analysis 6.8). At the end of the study, the median and 95% CI of the AQOL score was 0.5 (0.6 to 2.1) in the salicylic acid/clindamycin/benzoyl peroxide treatment group and 1 (1.5 to 4.3) in the clindamycin/benzoyl peroxide group, the study authors may have reported the wrong data as the median was not included in the 95% CI. The authors reported no "statistically significant" difference between treatment groups. We assessed the evidence as very low quality (Table 16).
6.8. Analysis.
Comparison 6: Topical salicylic acid versus no treatment, Outcome 8: Quality of life (QoL) ‐ AQOL (score, post‐intervention)
| Quality of life (QoL) ‐ AQOL (score, post‐intervention) | ||||
| Study | Time points | Salicylic acid (median, 95%CI) | No treatment (median, 95%CI) | P value |
| Akarsu 2012 | Long term: 12 weeks after start of treatment | 0.5 (0.6‐2.1) | 1 (1.5‐4.3) | Quote: "there were no significant differences in AQOL between treatment groups at baseline and at the end of the study". |
Comparison 7: topical nicotinamide versus other topical treatments
Participants' global self‐assessment of acne improvement
No study collected data for this outcome.
Withdrawal for any reason
We found three trials that compared nicotinamide with clindamycin (Khodaeiani 2013; Shahmoradi 2013; Shalita 1995), and there was no significant difference between the treatment groups in the medium term, two of which reported zero events, (RR 1.13, 95% CI 0.49 to 2.60; 3 studies, 216 participants; Analysis 7.1). We rated this as low‐quality evidence due to risk of bias and imprecision (Table 8).
7.1. Analysis.

Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 1: Withdrawal for any reason
Only Weltert 2004 compared topical nicotinamide 4% gel with erythromycin 4% gel, and there was no significant difference between the treatment groups (RR 1.40, 95% CI 0.46 to 4.22; 1 study, 158 participants; Analysis 7.1). We rated this as low‐quality evidence due to risk of bias and imprecision (Table 9).
Change in lesion counts
Inflamed (number of lesions post‐intervention)
There were no long term data for this outcome. Khodaeiani 2013 reported short and medium term data (n = 80). There was no clear difference between topical nicotinamide 4% gel and clindamycin 1% gel in the short term (MD 0.97, 95% CI ‐0.92 to 2.86; 1 study, 80 participants; Analysis 7.2), or the medium term (MD 0.92, 95% CI ‐1.25 to 3.09; 1 study, 80 participants; Analysis 7.2).
7.2. Analysis.

Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 2: Change in lesion counts ‐ inflamed (number of lesions post‐intervention)
Inflamed counts (counts or percentage)
Two studies reported data for this outcome; however, the data were not available for meta‐analysis; they are presented in a table (see table in Analysis 7.3). Shalita 1995 compared nicotinamide 4% gel with clindamycin 1% gel, there was no difference in percentage reduction of inflamed lesions in the short (P = 0.06, no means ± SDs provided) and medium term (P = 0.17, skewed data). Weltert 2004 compared nicotinamide 4% gel with erythromycin 4% gel, but the study authors did not report the P value and the data were skewed.
7.3. Analysis.
Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 3: Change in lesion counts ‐ inflamed counts (counts or %)
| Change in lesion counts ‐ inflamed counts (counts or %) | ||||
| Study | Subgroup | Nicotinamide (mean, sd) | Topical treatments (mean, sd) | P value |
| short term | ||||
| Shalita 1995 | Percent reduction ‐ inflamed | Unclear | Unclear | 0.06 |
| medium term | ||||
| Shalita 1995 | Percent reduction ‐ inflamed | 59.5±41.2 | 42.7±41.3 | 0.17 |
| Weltert 2004 | Number of lesions post intervention ‐ inflamed lesion counts | 4±8 | 5±5 | not report, the author did not state whether the difference is statistical significant. |
Comedones (number of lesions post‐intervention)
Weltert 2004 reported data collected in the medium term (n = 158). There was no clear difference between topical nicotinamide 4% gel and erythromycin 4% gel (MD ‐1.00, 95% CI ‐2.10 to 0.10; 1 study, 158 participants; Analysis 7.4).
7.4. Analysis.

Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 4: Change in lesion counts ‐ comedones (number of lesions post‐intervention)
Physicians' global evaluation of acne improvement
Shalita 1995 reported data collected in the short and medium term. The authors assessed this outcome using a 5‐point scoring system (+3 = much better, +2 = moderately better, +1 = slightly better, 0 = no change, ‐1 = worse). There was no significant difference between topical nicotinamide 4% gel and clindamycin 1% gel in the moderately better or much better categories in the short term (RR 0.93, 95% CI 0.53 to 1.66; 1 study, 76 participants) or medium term (RR 0.95, 95% CI 0.60 to 1.50; 1 study, 76 participants; Analysis 7.5).
7.5. Analysis.

Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 5: Physicians' global evaluation of acne improvement
Shalita 1995 also reported the P value for this outcome (Analysis 7.6). There was no statistically significant difference between topical nicotinamide 4% gel and clindamycin 1% gel in the percentage of participants in the moderately better or much better categories in the short term (P = 0.36) or medium term (P = 0.19) for physicians' global evaluation of acne improvement.
7.6. Analysis.
Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 6: Physicians' global evaluation of acne improvement
| Physicians' global evaluation of acne improvement | ||||
| Study | Items | Nicotinamide (%) | Clindamycin (%) | P value |
| short term | ||||
| Shalita 1995 | moderately or much better improvement | 36% | 40% | 0.36 |
| medium term | ||||
| Shalita 1995 | moderately or much better improvement | 86% | 68% | 0.19 |
Minor adverse events
Itching ‐ nicotinamide versus clindamycin
Khodaeiani 2013 reported that four out of 40 people in the nicotinamide 4% gel group versus three out of 40 people in the clindamycin 1% gel group experienced itching (P = 1.00, Fisher's Exact test). There was no significant difference between topical nicotinamide 4% gel and clindamycin 1% gel (RR 1.33, 95% CI 0.32 to 5.58; 1 study, 80 participants; Analysis 7.7).
7.7. Analysis.

Comparison 7: Topical nicotinamide versus other topical treatments, Outcome 7: Minor adverse events
Burning ‐ nicotinamide versus clindamycin
Khodaeiani 2013 reported that seven out of 40 people in the nicotinamide 4% gel group versus two out of 40 people in the clindamycin 1% gel group experienced burning (P = 0.15, Fisher's Exact test). There was no clear difference between topical nicotinamide 4% gel and clindamycin 1% gel as the CI was wide (RR 3.50, 95% CI 0.77 to 15.83; 1 study, 80 participants; Analysis 7.7).
Crusting ‐ nicotinamide versus clindamycin
Khodaeiani 2013 reported that two out of 40 people in the nicotinamide 4% gel group versus three out of 40 people in the clindamycin 1% gel group experienced crusting (P = 1.00, Fisher's Exact test). There was no significant difference between nicotinamide 4% gel and clindamycin 1% gel (RR 0.67, 95% CI 0.12 to 3.78; 1 study, 80 participants; Analysis 7.7).
Greasy skin ‐ nicotinamide versus clindamycin
Khodaeiani 2013 reported that zero out of 40 people in the nicotinamide 4% gel group versus three out of 40 people in the clindamycin 1% gel group experienced greasy skin (P = 0.24, Fisher's Exact test). There was no clear difference between nicotinamide 4% gel and clindamycin 1% gel (RR 0.14, 95% CI 0.01 to 2.68; 1 study, 80 participants; Analysis 7.7).
Dermatitis ‐ nicotinamide versus clindamycin
Khodaeiani 2013 reported that one out of 40 people in the nicotinamide 4% gel group versus zero out of 40 people in the clindamycin 1% gel group experienced dermatitis (P = 1.00, Fisher's Exact test). There was no clear difference between nicotinamide 4% gel and clindamycin 1% gel (RR 3.00, 95% CI 0.13 to 71.51; 1 study, 80 participants; Analysis 7.7), as the estimated RR had a very wide 95% CI resulting in imprecision.
Total events ‐ nicotinamide versus clindamycin
Three trials compared nicotinamide with clindamycin (Khodaeiani 2013; Shahmoradi 2013; Shalita 1995). There was no significant difference between topical nicotinamide and clindamycin (RR 1.20, 95% CI 0.73 to 1.99; 3 studies, 216 participants; Analysis 7.7). We rated this as low‐quality evidence due to risk of bias and imprecision (Table 8).
Pertinent clinical signs ‐ nicotinamide versus erythromycin (short term)
Weltert 2004 found "no significant difference" between topical nicotinamide 4% gel and erythromycin 4% gel (RR 1.33, 95% CI 0.60 to 2.99; 1 study, 158 participants; Analysis 7.7).
Pertinent clinical signs ‐ nicotinamide versus erythromycin (medium term)
Weltert 2004 found "no significant difference" between topical nicotinamide 4% gel and erythromycin 4% gel (RR 1.10, 95% CI 0.50 to 2.44; 1 study, 158 participants; Analysis 7.7).
Funtional or physical signs ‐ nicotinamide versus erythromycin (short term)
Weltert 2004 found "no significant difference" between topical nicotinamide 4% gel and erythromycin 4% gel (RR 1.05, 95% CI 0.61 to 1.82; 1 study, 158 participants; Analysis 7.7).
Funtional or physical signs ‐ nicotinamide versus erythromycin (medium term)
Weltert 2004 found "no significant difference" between topical nicotinamide 4% gel and erythromycin 4% gel (RR 0.75, 95% CI 0.38 to 1.48; 1 study, 158 participants; Analysis 7.7).
Quality of life
No study collected data for this outcome.
Comparison 8: topical sulphur versus other topical treatments
Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 assessed this outcome using a numerical point scoring system (greatly improved +2, somewhat improved +1, no change 0, worse ‐1); however, the SDs were missing (Analysis 8.1). The study authors compared topical sulphur 2% lotion to topical benzoyl peroxide 5% lotion, the average score (high = well) was 0.75 in the sulphur group and 0.66 in the benzoyl peroxide group. The authors did not state whether the difference was statistically significant. We assessed the evidence as very low‐quality due to risk of bias and imprecision (Table 17).
8.1. Analysis.
Comparison 8: Topical sulphur versus other topical treatments, Outcome 1: Participants' global self assessment of acne improvement (numerical point system defined by investigator, high = well)
| Participants' global self assessment of acne improvement (numerical point system defined by investigator, high = well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.15 | 0.66 | 0.75 | 0.53 | not report, the author did not state whether the difference is statistical significant. |
7. Sulphur compared to benzoyl peroxide.
| Sulphur compared to benzoyl peroxide for acne | ||||||
| Patient or population: participants with acne Settings: Wayne State University Health Service Intervention: topical sulphur Comparison: topical benzoyl peroxide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical benzoyl peroxide | Topical sulphur | |||||
|
Participants' global self‐assessment of acne improvement Numerical point system defined by investigator, high = well (medium term: treatment duration from 5 to 8 weeks) |
The score (high = well) was 0.75 in sulphur group and 0.66 in benzoyl peroxide group. | 34 (1 study) | ⊕⊝⊝⊝ Very lowa | SDs were missing. | ||
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
125 per 1000 | 334 per 1000 (78 to 1000) | RR 2.67 (0.62 to 11.39) | 34 (1 study) | ⊕⊝⊝⊝ Very lowb | ‐ |
| Total number of participants who experienced at least one minor adverse event | See comment | See comment | See comment | See comment | See comment | Total number of participants who experienced at least one adverse event not reported. But the authors reported that five patients in the benzoyl peroxide group (5/16) versus zero in sulphur group (0/18) developed erythema and drying. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by four levels to very low quality evidence. Two levels for risk of bias: only one study included with high risk of attrition bias and unclear risk of selection, performance, detection, and reporting bias. Two levels for imprecision: very small sample size. bDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with high risk of attrition bias and unclear risk of selection, performance, detection, and reporting bias. Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Withdrawal for any reason
Vasarinsh 1969 reported that six out of 18 people in the topical sulphur 2% lotion group versus two out of 16 people in the benzoyl peroxide 5% lotion group withdrew from the trial (P = 0.23, Fisher's Exact test). There was no clear difference between the topical sulphur 2% lotion group and the topical benzoyl peroxide 5% lotion group in the medium term (RR 2.67, 95% CI 0.62 to 11.39; 1 study, 34 participants; Analysis 8.2). We assessed the evidence as very low‐quality due to risk of bias and imprecision (Table 17).
8.2. Analysis.

Comparison 8: Topical sulphur versus other topical treatments, Outcome 2: Withdrawal for any reason
Change in lesion counts (scores, high = well)
Vasarinsh 1969 compared topical sulphur 2% lotion with topical benzoyl peroxide 5% lotion; however, the SDs were missing, so we presented the data in a table (see table in Analysis 8.3). The average scores (high = well) of comedone‐pustule and papule‐cyst in participants receiving sulphur treatment were lower than in the benzoyl peroxide group. The authors did not state whether the difference was statistically significant.
8.3. Analysis.
Comparison 8: Topical sulphur versus other topical treatments, Outcome 3: Change in lesion counts (scores, high = well)
| Change in lesion counts (scores, high = well) | ||||||
| Study | Time points | Sulphur‐benzoyl peroxide | Benzoyl peroxide | Sulphur | Placebo | P value |
| Vasarinsh 1969 | Numerical point system defined by investigator (high=well): comedone‐pustule (medium term) | 0.81 | 0.55 | ‐0.70 | 0.00 | not reported, the author did not state whether the difference is statistical significant. |
| Numerical point system defined by investigator (high=well): papule‐cyst (medium term) | 0.91 | 0.69 | 0.30 | 0.53 | not reported, the author did not state whether the difference is statistical significant. | |
Physicians' global evaluation of acne improvement
Vasarinsh 1969 compared topical sulphur 2% lotion with topical benzoyl peroxide 5% lotion. Trial authors assessed this outcome by using a scoring system defined by investigators (unchanged or worse: ‐4 to 0, minimal improvement: 0.1 to 3.99, moderate improvement: 4 to 5.99, good improvement: 6 to 8). There was no significant difference between treatment groups in the moderate to good improvement categories in the medium term (RR 1.24, 95% CI 0.49 to 3.15; 1 study, 34 participants; Analysis 8.4).
8.4. Analysis.

Comparison 8: Topical sulphur versus other topical treatments, Outcome 4: Physicians' global evaluation of acne improvement
Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 compared topical sulphur 2% lotion with topical benzoyl peroxide 5% lotion; however, the SDs were missing, so we presented the data in a table (see table in Analysis 8.5). Trial authors assessed this outcome using a numerical point scoring system (complete improvement +3, moderate improvement +2, slight improvement +1, questionable 0, no change 0, worse ‐1). The average score (high = well) was 0.50 in the sulphur treatment group and 1.07 in the benzoyl peroxide group. However, the authors did not state whether the difference was statistically significant.
8.5. Analysis.
Comparison 8: Topical sulphur versus other topical treatments, Outcome 5: Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well)
| Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.53 | 1.07 | 0.50 | 0.94 | not report, the author did not state whether the difference is statistical significant. |
Minor adverse events
Erythema and drying ‐ sulphur versus benzoyl peroxide
Vasarinsh 1969 reported that zero out of 18 people in the topical sulphur 2% lotion group versus five out of 16 people in the benzoyl peroxide 5% lotion group experienced erythema and drying (P = 0.02, Fisher's Exact test). We did not present the RR and 95% CI because the presence of zero events in one arm led to discordant results (Analysis 8.6).
8.6. Analysis.

Comparison 8: Topical sulphur versus other topical treatments, Outcome 6: Minor adverse events
Quality of life
No study collected data for this outcome.
Comparison 9: topical sulphur versus placebo
Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 compared sulphur 2% lotion with placebo lotion using a numerical point scoring system (greatly improved +2, somewhat improved +1, no change 0, worse ‐1); however, the SDs were missing (Analysis 9.1). The average score (high = well) was 0.75 in participants receiving sulphur treatment and 0.53 in the placebo group. The authors did not state whether the difference was statistically significant. We assessed the evidence as very low‐quality due to risk of bias and imprecision (Table 18).
9.1. Analysis.
Comparison 9: Topical sulphur versus placebo, Outcome 1: Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high=well)
| Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high=well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.15 | 0.66 | 0.75 | 0.53 | not report, the author did not state whether the difference is statistical significant. |
8. Sulphur compared to placebo.
| Sulphur compared to placebo for acne | ||||||
| Patient or population: participants with acne Settings: Wayne State University Health Service Intervention: topical sulphur Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Topical sulphur | |||||
|
Participants' global self‐assessment of acne improvement Numerical point system defined by investigator, high = well (medium term: treatment duration from 5 to 8 weeks) |
The score (high = well) was 0.75 in participants receiving sulphur treatment and 0.53 in placebo group. | 37 (1 study) | ⊕⊝⊝⊝ Very lowa | SDs were missing. | ||
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
211 per 1000 | 333 per 1000 (112 to 989) | RR 1.58 (0.53 to 4.70) | 37 (1 study) | ⊕⊝⊝⊝ Very lowb | ‐ |
| Total number of participants who experienced at least one minor adverse event | See comment | See comment | See comment | See comment | See comment | Total number of participants who experienced at least one adverse event not reported. Two participants in the placebo group (2/19) versus zero in sulphur group (0/18) developed erythema and drying. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by four levels to very low quality evidence. Two levels for risk of bias: only one study included with high risk of attrition bias and unclear risk of selection, performance, and reporting bias. Two levels for imprecision: very small sample size. bDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with high risk of attrition bias and unclear risk of selection, performance, and reporting bias. Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Withdrawal for any reason
Vasarinsh 1969 reported that six out of 18 people in the topical sulphur 2% lotion group versus four out of 19 people in the placebo group withdrew from the trial. There was no clear difference between topical sulphur 2% lotion and placebo in the medium term (RR 1.58, 95% CI 0.53 to 4.70; 1 study, 37 participants; Analysis 9.2). We assessed the evidence as very low‐quality due to risk of bias and imprecision (Table 18).
9.2. Analysis.

Comparison 9: Topical sulphur versus placebo, Outcome 2: Withdrawal for any reason
Change in lesion counts (scores, high=well)
Only one study reported data for this outcome; however, the SDs were missing (Analysis 9.3). Vasarinsh 1969 compared sulphur 2% lotion with placebo lotion, the score (high = well) of comedone‐pustule was ‐ 0.70 in participants receiving sulphur treatment and 0.00 in the placebo group; the authors did not state whether this difference was statistically significant. In addition, the score (high = well) of papule‐cyst was 0.30 in participants receiving sulphur treatment and 0.53 in the placebo group; the authors did not state whether this difference was statistically significant.
9.3. Analysis.
Comparison 9: Topical sulphur versus placebo, Outcome 3: Change in lesion counts (scores, high = well)
| Change in lesion counts (scores, high = well) | ||||||
| Study | Time points | Sulphur‐benzoyl peroxide | Benzoyl peroxide | Sulphur | Placebo | P value |
| Vasarinsh 1969 | Numerical point system defined by investigator (high=well): comedone‐pustule (medium term) | 0.81 | 0.55 | ‐0.70 | 0.00 | not reported, the author did not state whether the difference is statistical significant. |
| Numerical point system defined by investigator (high=well): papule‐cyst (medium term) | 0.91 | 0.69 | 0.30 | 0.53 | not reported, the author did not state whether the difference is statistical significant. | |
Physicians' global evaluation of acne improvement
Vasarinsh 1969 compared topical sulphur 2% lotion with placebo using a scoring system defined by investigators (unchanged or worse: ‐4 to 0, minimal improvement: 0.1 to 3.99, moderate improvement: 4 to 5.99, good improvement: 6 to 8). There was no significant difference between treatment groups in the moderate to good improvement categories in the medium term (RR 1.48, 95% CI 0.57 to 3.82; 1 study, 37 participants; Analysis 9.4).
9.4. Analysis.

Comparison 9: Topical sulphur versus placebo, Outcome 4: Physicians' global evaluation of acne improvement
Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 compared sulphur 2% lotion with placebo lotion; however, the SDs were missing (Analysis 9.5). Trial authors assessed this outcome using a numerical point scoring system (complete improvement +3, moderate improvement +2, slight improvement +1, questionable 0, no change 0, worse ‐1). The average score (high = well) was 0.50 in participants receiving sulphur treatment and 0.94 in the placebo group. The authors did not state whether this difference was statistically significant.
9.5. Analysis.
Comparison 9: Topical sulphur versus placebo, Outcome 5: Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well)
| Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.53 | 1.07 | 0.50 | 0.94 | not report, the author did not state whether the difference is statistical significant. |
Minor adverse events
Erythema and drying
Vasarinsh 1969 compared sulphur 2% lotion with placebo lotion. The authors reported that zero out of 18 people in the topical sulphur 2% lotion group versus two out of 19 people experienced erythema and drying (P = 0.49, Fisher's Exact test). There was no clear difference between treatment groups (RR 0.21, 95% CI 0.01 to 4.11; 1 study, 37 participants; Analysis 9.6).
9.6. Analysis.

Comparison 9: Topical sulphur versus placebo, Outcome 6: Minor adverse events ‐ erythema and drying
Quality of life
No study collected data for this outcome.
Comparison 10: topical sulphur versus no treatment (including studies with a co‐intervention in both arms)
Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 compared sulphur 2% + benzoyl peroxide 5% lotion with benzoyl peroxide 5% lotion using a numerical point scoring system (greatly improved +2, somewhat improved +1, no change 0, worse ‐1); however, the SDs were missing (Analysis 10.1). The average score (high = well) was 1.15 in participants receiving sulphur + benzoyl peroxide treatment and 0.66 in the benzoyl peroxide group. The authors did not state whether the difference was statistically significant.
10.1. Analysis.
Comparison 10: Topical sulphur versus no treatment, Outcome 1: Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well)
| Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.15 | 0.66 | 0.75 | 0.53 | not report, the author did not state whether the difference is statistical significant. |
Withdrawal for any reason
Vasarinsh 1969 reported that six out of 19 people in the topical sulphur 2% + benzoyl peroxide 5% lotion group versus two out of 16 people in the benzoyl peroxide 5% lotion group withdrew from the trial (P = 0.24, Fisher's Exact test). There was no clear difference between topical sulphur 2% + benzoyl peroxide 5% lotion and benzoyl peroxide 5% lotion in the medium term (RR 2.53, 95% CI 0.59 to 10.83; 1 study, 35 participants; Analysis 10.2).
10.2. Analysis.

Comparison 10: Topical sulphur versus no treatment, Outcome 2: Withdrawal for any reason
Change in lesion counts (scores, high = well)
Only one study reported data for this outcome; however, the SDs were missing (Analysis 10.3). Vasarinsh 1969 compared sulphur 2% + benzoyl peroxide 5% lotion with benzoyl peroxide 5% lotion, the score (high = well) of comedone‐pustule was 0.81 in participants receiving sulphur 2% + benzoyl peroxide 5% lotion and 0.55 in the benzoyl peroxide 5% lotion group, the score (high = well) of papule‐cyst was 0.91 in participants receiving sulphur 2% + benzoyl peroxide 5% lotion treatment and 0.69 in the benzoyl peroxide 5% lotion group. The authors did not state whether this difference was statistically significant.
10.3. Analysis.
Comparison 10: Topical sulphur versus no treatment, Outcome 3: Change in lesion counts (scores, high = well)
| Change in lesion counts (scores, high = well) | ||||||
| Study | Time points | Sulphur‐benzoyl peroxide | Benzoyl peroxide | Sulphur | Placebo | P value |
| Vasarinsh 1969 | Numerical point system defined by investigator (high=well): comedone‐pustule (medium term) | 0.81 | 0.55 | ‐0.70 | 0.00 | not reported, the author did not state whether the difference is statistical significant. |
| Numerical point system defined by investigator (high=well): papule‐cyst (medium term) | 0.91 | 0.69 | 0.30 | 0.53 | not reported, the author did not state whether the difference is statistical significant. | |
Physicians' global evaluation of acne improvement
Vasarinsh 1969 compared topical sulphur 2% + benzoyl peroxide 5% lotion with benzoyl peroxide 5% lotion using a scoring system defined by investigators (unchanged or worse: ‐4 to 0, minimal improvement: 0.1 to 3.99, moderate improvement: 4 to 5.99, good improvement: 6 to 8). There was no significant difference between treatment groups in the moderate to good improvement categories in the medium term (RR 1.52, 95% CI 0.64 to 3.61; 1 study, 35 participants; Analysis 10.4).
10.4. Analysis.

Comparison 10: Topical sulphur versus no treatment, Outcome 4: Physicians' global evaluation of acne improvement
Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well)
Vasarinsh 1969 compared topical sulphur 2% + benzoyl peroxide 5% lotion with benzoyl peroxide 5% lotion; however, the SDs were missing (Analysis 10.5). Trial authors assessed this outcome by using a numerical point scoring system (complete improvement +3, moderate improvement +2, slight improvement +1, questionable 0, no change 0, worse ‐1). The average score (high = well) was 1.53 in participants receiving sulphur 2% + benzoyl peroxide 5% treatment and 1.07 in the benzoyl peroxide 5% group. The authors did not state whether this difference was statistically significant.
10.5. Analysis.
Comparison 10: Topical sulphur versus no treatment, Outcome 5: Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high=well)
| Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high=well) | |||||
| Study | Sulfur‐benzoyl peroxide | Benzoyl peroxide | Sulfur | Placebo | P value |
| medium term | |||||
| Vasarinsh 1969 | 1.53 | 1.07 | 0.50 | 0.94 | not report, the author did not state whether the difference is statistical significant. |
Minor adverse events
Erythema and drying
Vasarinsh 1969 reported that four out of 19 people in the topical sulphur 2% + benzoyl peroxide 5% lotion group versus five out of 16 people in the benzoyl peroxide 5% lotion group experienced erythema and drying (P = 0.70, Fisher's Exact test). There was no clear difference between treatment groups (RR 0.22, 95% CI 0.22 to 2.09; 1 study, 35 participants; Analysis 10.6).
10.6. Analysis.

Comparison 10: Topical sulphur versus no treatment, Outcome 6: Minor adverse events ‐ erythema and drying
Quality of life
No study collected data for this outcome.
Comparison 11: topical zinc versus other topical treatments
Participants' global self‐assessment of acne improvement
No study collected data for this outcome.
Withdrawal for any reason
Sharquie 2008 reported that three out of 23 people in the 5% zinc sulphate solution group versus four out of 24 people in the 2% tea lotion group withdrew from the trial (P = 1.00, Fisher's Exact test). There was no significant difference between the 5% zinc sulphate solution group and the 2% tea lotion group in the medium term (RR 0.78, 95% CI 0.20 to 3.12; 1 study, 47 participants; Analysis 11.1). We rated the quality of evidence as very low due to risk of bias and imprecision (Table 19).
11.1. Analysis.

Comparison 11: Topical zinc versus other topical treatments, Outcome 1: Withdrawal for any reason
9. Zinc compared to tea.
| Zinc compared to tea for acne | ||||||
| Patient or population: participants with acne Settings: Department of Dermatology and Venereology, Baghdad Teaching Hospital, Baghdad, Iraq Intervention: topical zinc Comparison: topical tea | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical tea | Topical zinc | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (medium term: treatment duration from 5 to 8 weeks) |
167 per 1000 | 130 per 1000 (33 to 520) | RR 0.78 (0.20 to 3.12) | 47 (1 study) | ⊕⊝⊝⊝ Very lowa | ‐ |
|
Total number of participants who experienced at least one minor adverse event (medium term: treatment duration from 5 to 8 weeks) |
208 per 1000 | 304 per 1000 (113 to 823) | RR 1.46 (0.54 to 3.95) | 47 (1 study) | ⊕⊝⊝⊝ Very lowb | Five people experienced burning and two experienced itching in the zinc sulphate treatment group, in contrast, five people had mild itching in the tea lotion treatment group. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by three levels to very low quality evidence. One level for risk of bias: only one study included with unclear risk of selection, performance, and reporting bias. Two levels for imprecision: wide CI and optimal sample size not met. bDowngraded by four levels to very low quality evidence. Two levels for risk of bias: only one study included with high risk of attrition bias and with unclear risk of selection, performance, detection, and reporting bias. Two levels for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Change in lesion counts
Papules (number of lesions post‐intervention)
Sharquie 2008 reported data collected in the medium term. There was no clear difference between 5% zinc sulphate solution and 2% tea lotion (MD ‐2.44, 95% CI ‐7.80 to 2.92; 1 study, 40 participants; Analysis 11.2).
11.2. Analysis.

Comparison 11: Topical zinc versus other topical treatments, Outcome 2: Change in lesion counts ‐ papules (number of lesions post‐intervention)
Pustules (number of lesions post‐intervention)
Sharquie 2008 reported data collected in the medium term. There was no significant difference between 5% zinc sulphate solution and 2% tea lotion (MD ‐0.70, 95% CI ‐6.97 to 5.57; 1 study, 40 participants; Analysis 11.3).
11.3. Analysis.

Comparison 11: Topical zinc versus other topical treatments, Outcome 3: Change in lesion counts ‐ pustules (number of lesions post‐intervention)
Physicians' global evaluation of acne improvement
For this outcome, there were no data reported in the short and long term; Sharquie 2008 reported data collected in the medium term and assessed this outcome using a three‐point system defined by the trial authors (reduction of more than 50% inflamed lesion count: good; 10% to 50% reduction: moderate; less than 10% reduction: no response). There was no significant difference between 5% zinc sulphate solution and 2% tea lotion in the good or moderate response categories (RR 0.80, 95% CI 0.51 to 1.24; 1 study, 47 participants; Analysis 11.4).
11.4. Analysis.

Comparison 11: Topical zinc versus other topical treatments, Outcome 4: Physicians' global evaluation of acne improvement
Minor adverse events ‐ total events
Zinc versus tea
Sharquie 2008 reported no significant difference between 5% zinc sulphate solution and 2% tea lotion (RR 1.46, 95% CI 0.54 to 3.95; 1 study, 47 participants; Analysis 11.5). We rated the quality of evidence as very low due to risk of bias and imprecision (Table 19).
11.5. Analysis.

Comparison 11: Topical zinc versus other topical treatments, Outcome 5: Minor adverse events ‐ total events
Quality of life
No study collected data for this outcome.
Comparison 12: topical zinc versus no treatment (including studies with a co‐intervention in both arms)
Participants' global self‐assessment of acne improvement (visual analogue scale)
Only one study that compared zinc/clindamycin 1% gel with clindamycin 1% gel reported data for this outcome (Cunliffe 2005); however, the data were not available for meta‐analysis due to no reporting of means ± SDs (Analysis 12.1). The study authors only reported that there was no significant difference between treatment groups for this outcome in the long term (16 weeks after start of treatment) but they did not report the visual analogue scale or P values. We assessed the evidence as low quality due to risk of bias and imprecision (Table 20).
12.1. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 1: Participants' global self‐assessment of acne improvement (visual analogue scale)
| Participants' global self‐assessment of acne improvement (visual analogue scale) | |||
| Study | Topical zinc (mean, SD) | No treatment (mean, SD) | P value |
| long term | |||
| Cunliffe 2005 | Long term: 16 weeks after start of treatment, unclear, not reported | Unclear, not reported | P value was not reported. Quote:" There were no significant difference." |
10. Zinc compared to no treatment.
| Zinc compared to no treatment for acne | ||||||
| Patient or population: participants with acne Settings: eight centres in the UK, one in France and one in Germany Intervention: topical zinc plus clindamycin 1% gel Comparison: no treatment plus clindamycin 1% gel | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Topical zinc | |||||
|
Participants' global self‐assessment of acne improvement Visual analogue scale (long term: treatment duration > 8 weeks) |
The study authors only reported no significant difference between treatment groups. | 163 (1 study) | ⊕⊕⊝⊝ Lowa | Clindamycin 1% gel was a co‐intervention given in both arms. No numerical data provided. | ||
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
72 per 1000 | 87 per 1000 (31 to 249) | RR 1.21 (0.43 to 3.45) | 163 (1 study) | ⊕⊕⊝⊝ Lowb | Clindamycin 1% gel was a co‐intervention given in both arms. |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
See comment | See comment | Not estimable | 163 (1 study) | ⊕⊕⊝⊝ Lowc | Clindamycin 1% gel was a co‐intervention given in both arms. The authors only report number of adverse events, not number of participants ‐ 91 adverse events in 80 zinc/clindamycin participants and 117 adverse events in 83 clindamycin participants. |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of selection bias. One level for imprecision: small sample size. bDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of selection bias. One level for imprecision: wide CI and optimal sample size not met. cDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of performance bias and unclear risk of selection bias. One level for imprecision: small sample size. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Withdrawal for any reason
Cunliffe 2005 compared zinc/clindamycin 1% gel with clindamycin 1% gel. There was no significant difference between treatment groups in the long term (16 weeks after start of treatment) (RR 1.21, 95% CI 0.43 to 3.45; 1 study, 163 participants; Analysis 12.2). We assessed the evidence as low quality due to risk of bias and imprecision (Table 20).
12.2. Analysis.

Comparison 12: Topical zinc versus no treatment, Outcome 2: Withdrawal for any reason
Change in lesion counts
Total (lesion counts reduction)
Only one study that compared zinc/clindamycin 1% gel with clindamycin 1% gel reported data for this outcome (Cunliffe 2005); however, the data were not available for meta‐analysis due to no reporting of means and SDs; instead we presented the data in a table (see table in Analysis 12.3). There was no significant difference between treatment groups in the medium and long term (16 weeks after start of treatment) (P = 0.707), but the authors did not report the data in each group.
12.3. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 3: Change in lesion counts ‐ total (lesion counts reduction)
| Change in lesion counts ‐ total (lesion counts reduction) | |||
| Study | Topical zinc (mean, SD) | No treatment (mean, SD) | P value |
| medium term | |||
| Cunliffe 2005 | Unclear, not reported | Unclear, not reported | 0.707 |
| long term | |||
| Cunliffe 2005 | Long term: 16 weeks after start of treatment, unclear, not reported | Unclear, not reported | 0.707 |
Inflamed (lesion counts reduction)
Two studies reported data for this outcome; however, the data were not available for meta‐analysis due to no reporting of means and SDs Analysis 12.4. Bojar 1994 compared 1.2% zinc/4% erythromycin with 4% erythromycin in the long term (12 weeks after start of treatment) and reported an improvement in both groups after intervention; however, they did not state whether there was a difference between groups. In another trial that compared zinc/clindamycin 1% gel with clindamycin 1% gel (Cunliffe 2005), the results demonstrated no difference in the long term (16 weeks after start of treatment) between groups (P = 0.626); the authors also did not report the values of reduction in inflamed lesion counts.
12.4. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 4: Change in lesion counts ‐ inflamed (lesion counts reduction)
| Change in lesion counts ‐ inflamed (lesion counts reduction) | |||
| Study | Topical zinc (mean, SD) | No treatment (mean, SD) | P value |
| short term | |||
| Bojar 1994 | Unclear | Unclear | Unclear |
| medium term | |||
| Bojar 1994 | Unclear | Unclear | Unclear |
| long term | |||
| Bojar 1994 | 12 weeks after start of treatment, Unclear | Unclear | Unclear |
| Cunliffe 2005 | 16 weeks after start of treatment, Unclear, not reported | Unclear, not reported | 0.626 |
Non‐inflamed (lesion count reduction)
Two studies reported data for this outcome; however, the data were not available for meta‐analysis due to no reporting of means and SDs; we presented the data in a table instead (see table in Analysis 12.5). Bojar 1994 compared 1.2% zinc/4% erythromycin with 4% erythromycin in the long term (12 weeks after start of treatment), the study authors did not report the values of reduction in non‐inflamed lesion counts or the P value. In another trial that compared zinc/clindamycin 1% gel with clindamycin 1% gel (Cunliffe 2005), the results demonstrated no difference in the medium and long term (16 weeks after start of treatment) between groups (P = 0.769); the authors also did not report the values of reduction in non‐inflamed lesion counts.
12.5. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 5: Change in lesion counts ‐ non‐inflamed (lesion counts reduction)
| Change in lesion counts ‐ non‐inflamed (lesion counts reduction) | |||
| Study | Topical zinc (mean, SD) | No treatment (mean, SD) | P value |
| short term | |||
| Bojar 1994 | Unclear | Unclear | Unclear |
| medium term | |||
| Bojar 1994 | Unclear | Unclear | Unclear |
| Cunliffe 2005 | Unclear | Unclear | 0.769 |
| long term | |||
| Bojar 1994 | 12 weeks after start of treatment, Unclear | Unclear | Unclear |
| Cunliffe 2005 | 16 weeks after start of treatment, Unclear | Unclear | 0.769 |
Physicians' global evaluation of acne improvement (visual analogue scale)
Cunliffe 2005 compared zinc/clindamycin 1% gel with clindamycin 1% gel; however, the data were not available for meta‐analysis due to no reporting of means or SDs; we presented the data in a table (see table in Analysis 12.6). There was no significant difference between groups in the long term (16 weeks after start of treatment) but the study authors did not report the visual analogue scale or P values.
12.6. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 6: Physicians' global evaluation of acne improvement (visual analogue scale)
| Physicians' global evaluation of acne improvement (visual analogue scale) | |||
| Study | Topical zinc (mean, SD) | No treatment (mean, SD) | P value |
| long term | |||
| Cunliffe 2005 | 16 weeks after start of treatment, Unclear | Unclear | No difference, no exact P value reported |
Minor adverse events
We did not find any studies that reported total number of participants experiencing at least one minor adverse event. Cunliffe 2005 compared zinc/clindamycin 1% gel with clindamycin 1% gel, but the study authors did not report the P value and showed results as count data (Analysis 12.7). The total number of adverse events was 91 in 80 participants receiving zinc/clindamycin 1% gel and 117 in 83 participants receiving clindamycin 1% gel. We assessed the evidence as low quality due to risk of bias and imprecision (Table 20).
12.7. Analysis.
Comparison 12: Topical zinc versus no treatment, Outcome 7: Minor adverse events
| Minor adverse events | ||||
| Study | Time points | Topical zinc (counts/n) | No treatment (counts/n) | P value |
| Cunliffe 2005 | long term (12 weeks after start of treatment) | 91/80 | 117/83 | Unclear |
Quality of life
No study collected data for this outcome.
Comparison 13: topical alpha‐hydroxy acid versus other topical treatments
Participants' global self‐assessment of acne improvement
ElRefaei 2015 and Garg 2009 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels. ElRefaei 2015 used a visual analogue scale (poor < 30% improvement; fair 30% to 60% improvement; and good > 60% improvement); Garg 2009 also used a visual analogue scale (good > 60%; fair 31% to 60%; poor < 30%; no change, worse). Both treatments demonstrated significant fair to good improvement, and there was no clear difference between groups in the long term (12 weeks after start of treatment) (RR 1.06, 95% CI 0.88 to 1.26; 1 study, 40 participants; Analysis 13.1). We assessed the evidence as low quality due to imprecision (Table 10). In Garg 2009, the authors collected data in the post‐treatment follow‐up period and reported that 20 out of 22 people in the 35% glycolic acid peel group versus 20 out of 22 people in the 20% salicylic ‐ 10% mandelic acid peel group demonstrated fair to good improvement at three months post‐treatment (treatment duration of 12 weeks, measured in the post‐treatment follow‐up period).
13.1. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 1: Participants' global self‐assessment of acne improvement
Withdrawal for any reason
Hunt 1992 compared topical alpha‐hydroxy acid (gluconolactone 14% in solution) and benzoyl peroxide 5% lotion. There was no significant difference between treatment groups in the long term (12 weeks after start of treatment) (RR 0.83, 95% CI 0.27 to 2.55; 1 study, 100 participants; Analysis 13.2). We assessed the evidence as low quality due to risk of bias and imprecision (Table 21).
13.2. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 2: Withdrawal for any reason
11. Gluconolactone (alpha‐hydroxy acid) compared to benzoyl peroxide.
| Gluconolactone (alpha‐hydroxy acid) compared to benzoyl peroxide for acne | ||||||
| Patient or population: participants with acne Settings: not described Intervention: topical gluconolactone (alpha‐hydroxy acid) Comparison: topical benzoyl peroxide | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Topical benzoyl peroxide | Topical gluconolactone | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
120 per 1000 | 100 per 1000 (32 to 306) | RR 0.83 (0.27 to 2.55) | 100 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
500 per 1000 | 240 per 1000 (135 to 425) | RR 0.48 (0.27 to 0.85) | 100 (1 study) | ⊕⊕⊝⊝ Lowb | Dryness was the most commonly reported problem in treatment groups |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of other bias, and with unclear risk of selection bias and reporting bias. One level for imprecision: wide CI and optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of other bias and unclear risk of selection and reporting bias. One level for imprecision: optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
ElRefaei 2015 and Garg 2009 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels; neither treatment group had any withdrawals in the long term (12 weeks after start of treatment) (Analysis 13.2). We assessed the evidence as low quality due to risk of bias and imprecision (Table 10).
Change in lesion counts
Non‐inflamed (number of lesions post‐intervention)
ElRefaei 2015 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels in the short and medium term; there was a clear significant difference between groups at both treatment terms in favour of 20% salicylic ‐ 10% mandelic acid peels. Short term: MD 10.00 (95% CI 4.41 to 15.59; 1 study, 40 participants) and medium term: MD 11.90 (95% CI 7.17 to 16.63; 1 study, 40 participants) (Analysis 13.3). The authors also reported data collected at two months post‐treatment (treatment duration of 12 weeks, measured at the post‐treatment follow‐up period); the 20% salicylic ‐ 10% mandelic acid peels was favoured (1.8 ± 1.99 in 20% salicylic ‐ 10% mandelic acid peels group versus 14.3 ± 10.03 in the 35% glycolic acid peels group).
13.3. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 3: Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention)
Papules (number of lesions post‐intervention)
ElRefaei 2015 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels in the long term; there was a clear significant difference between groups in favour of 20% salicylic ‐ 10% mandelic acid peels (MD 1.25, 95% CI 0.36 to 2.14; 1 study, 40 participants) (Analysis 13.4). The authors also reported data collected at two months post‐treatment (treatment duration of 12 weeks, measured at the post‐treatment follow‐up period); the 20% salicylic ‐ 10% mandelic acid peels group was favoured (2.45 ± 1.28 in 20% salicylic ‐ 10% mandelic acid peels group versus 3.4 ± 1.57 in the 35% glycolic acid peels group).
13.4. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 4: Change in lesion counts ‐ papules (number of lesions post‐intervention)
Pustules (number of lesions post‐intervention)
ElRefaei 2015 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels in the long term; there was a clear significant difference between groups in favour of 20% salicylic ‐ 10% mandelic acid peels (MD 1.20, 95% CI 0.44 to 1.96; 1 study, 40 participants) (Analysis 13.5). The authors also reported data collected at two months post‐treatment (treatment duration of 12 weeks, measured at the post‐treatment follow‐up period); the 20% salicylic ‐ 10% mandelic acid peels group was favoured (2.2 ± 1.47 in 20% salicylic ‐ 10% mandelic acid peels group versus 3.75 ± 1.997 in the 35% glycolic acid peels group).
13.5. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 5: Change in lesion counts ‐ pustules (number of lesions post‐intervention)
Total (counts or percentage)
Hunt 1992, a parallel study, reported data for this outcome; however, the data were not available for meta‐analysis due to no reporting of means and SDs (Analysis 13.6). The study authors only stated there was no significant difference in total lesion count reduction between alpha‐hydroxy acid (gluconolactone 14% in solution) and benzoyl peroxide 5% lotion in the short, medium, and long term (12 weeks after start of treatment), but they did not report data in each group.
13.6. Analysis.
Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 6: Change in lesion counts ‐ total (counts or %)
| Change in lesion counts ‐ total (counts or %) | ||||
| Study | Time points | Alpha‐hydroxy acid, mean ± SD or % | Topical treatments, mean ± SD or % | P vaule |
| parallel trials | ||||
| Hunt 1992 | Short term ‐ lesion counts reduction | gluconolactone 14% in solution, not reported | benzoyl peroxide 5% lotion, not reported | No difference |
| Hunt 1992 | Long term: 12 weeks after start of treatment ‐ lesion counts reduction | gluconolactone 14% in solution, not reported | benzoyl peroxide 5% lotion, not reported | No difference |
| Hunt 1992 | Medium term ‐ lesion counts reduction | gluconolactone 14% in solution, not reported | benzoyl peroxide 5% lotion, not reported | No difference |
| split‐face trials | ||||
| Kessler 2008 | Percent reduction ‐ total lesion(treatment duration of 10 weeks, measured at one month post‐treatment) | 30% glycolic acid peel, 43% | 30% salicylic acid peel, 47% | > 0.05 |
Kessler 2008, a split‐face design, also reported data for this outcome. The study authors compared 30% glycolic acid peels with 30% salicylic acid peels; there was no statistically significant difference between groups in percentage reduction of total lesions at one month post‐treatment (P > 0.05) (treatment duration of 10 weeks, measured at the post‐treatment follow‐up period) (Analysis 13.6).
Inflamed (counts)
Two studies reported data for this outcome. Hunt 1992 did not provide means and SDs, and in Ilknur 2010, data were skewed, so we could only present the data in a table (see table in Analysis 13.7). In the parallel Hunt 1992 trail that compared alpha‐hydroxy acid (gluconolactone 14% in solution) with benzoyl peroxide 5% lotion, there was significant difference in the medium (P < 0.05) and long term (12 weeks after start of treatment) (P < 0.05), indicating that benzoyl peroxide can lead to a greater reduction in lesion counts, but no difference was observed in the short term. In the split‐face Ilknur 2010 trial that compared alpha‐hydroxy acid (glycolic acid) 20% to 70% peels with amino fruit acid 20% to 60% peels, there was no significant difference between groups in number of inflamed lesions in the short, medium, and long term (6 months after start of treatment) (P > 0.05). The mean number of inflamed lesions post‐intervention in the alpha‐hydroxy acid (glycolic acid) peel group was close to that in the amino fruit acid peels group for the same time period, but the data were skewed (e.g. mean ± SD: 10.08 ± 5.72 in the alpha‐hydroxy acid group; 8.67 ± 4.48 in the amino fruit acid peels group).
13.7. Analysis.
Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 7: Change in lesion counts ‐ inflamed (counts)
| Change in lesion counts ‐ inflamed (counts) | ||||
| Study | Time points | Alpha‐hydroxy acid (mean, SD) | Benzoyl peroxide (mean, SD) | P value |
| parallel trials | ||||
| Hunt 1992 | Short term ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | No difference |
| Hunt 1992 | Medium term ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | Benzoyl peroxide was significantly better than gluconolactone at eight and twelve weeks (P < 0.05) |
| Hunt 1992 | Long term: 12 weeks after start of treatment ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | Benzoyl peroxide was significantly better than gluconolactone at eight and twelve weeks (P< 0.05) |
| split‐face trials | ||||
| Ilknur 2010 | Long term: six months after start of treatment (number of lesions post intervention) | 6.88±5.18 | 7.00±7.26 | >0.05 |
| Ilknur 2010 | Short term (number of lesions post intervention) | 10.08±5.72 | 8.67±4.48 | >0.05 |
| Ilknur 2010 | Medium term (number of lesions post intervention) | 8.29±4.50 | 8.88±4.81 | >0.05 |
Non‐inflamed (counts)
Two studies reported data for this outcome; however, Hunt 1992 did not provide means and SDs and in Ilknur 2010, data were skewed (Analysis 13.8). In the parallel Hunt 1992 trial that compared alpha‐hydroxy acid (gluconolactone 14% in solution) with benzoyl peroxide 5% lotion, there was no significant difference between groups in lesion count reduction in the short, medium, and long term (12 weeks after start of treatment) (no exact P value reported). In the split‐face Ilknur 2010 trial that compared alpha‐hydroxy acid (glycolic acid) 20% to 70% peels with amino fruit acid 20% to 60% peels, there was no significant difference between groups in number of non‐inflamed lesions in the short, medium, and long term (6 months after start of treatment) (P > 0.05). The mean number of non‐inflamed lesions post‐intervention in the alpha‐hydroxy acid (glycolic acid) peel group was close to that in the amino fruit acid peels group for the same time period, but the data were skewed (e.g. mean ± SD: 36.29 ± 37.37 in the alpha‐hydroxy acid group; 36.00 ± 40.42 in the amino fruit acid peels group).
13.8. Analysis.
Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 8: Change in lesion counts ‐ non‐inflamed (counts)
| Change in lesion counts ‐ non‐inflamed (counts) | ||||
| Study | Time points | Alpha‐hydroxy acid (mean, SD) | Benzoyl peroxide (mean, SD) | P value |
| parallel trials | ||||
| Hunt 1992 | Medium term ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | No difference |
| Hunt 1992 | Long term: 12 weeks after start of treatment ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | No difference |
| Hunt 1992 | Short term ‐ lesion counts reduction | Unclear, not reported | Unclear, not reported | No difference |
| split‐face trials | ||||
| Ilknur 2010 | Long term: six months after start of treatment (number of lesions post intervention) | 18.29±12.93 | 17.13±14.22 | >0.05 |
| Ilknur 2010 | Medium term (number of lesions post intervention) | 36.29±37.37 | 36.00±40.42 | >0.05 |
| Ilknur 2010 | Short term (number of lesions post intervention) | 42.67±50.36 | 43.17±50.38 | >0.05 |
Physicians' global evaluation of acne improvement
ElRefaei 2015 and Garg 2009 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels using the same five‐point visual analogue scale (worse; no change; poor: < 30% improvement; fair: 31% to 60% improvement; good: > 60% improvement). ElRefaei 2015 reported that two out of 20 people in the 35% glycolic acid peels group versus five out of 20 people in the 20% salicylic ‐ 10% mandelic acid peels group demonstrated fair to good improvement in the short term (P = 0.41, Fisher's Exact test). The 35% glycolic acid peels group showed fewer numbers of participants with fair to good improvement for all treatment terms; the difference was statistically significant in the medium (RR 0.29, 95% CI 0.11 to 0.72; 1 study, 40 participants) but not in the short term (RR 0.40, 95% CI 0.09 to 1.83; 1 study, 40 participants) or long term (RR 0.95, 95% CI 0.79 to 1.13; 1 study, 40 participants) (Analysis 13.9). In ElRefaei 2015, the authors also collected data at the post‐treatment follow‐up period and reported that 16 out of 20 people in the 35% glycolic acid peels group versus 19 out of 20 people in the 20% salicylic ‐ 10% mandelic acid peels group demonstrated fair to good improvement at two months post‐treatment (treatment duration of 12 weeks, measured at the post‐treatment follow‐up period). Garg 2009 reported that 20 out of 22 people in the 35% glycolic acid peels group versus 21 out of 22 people in the 20% salicylic ‐ 10% mandelic acid peels group demonstrated fair to good improvement at three months post‐treatment (treatment duration of 12 weeks, measured at the post‐treatment follow‐up period).
13.9. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 9: Physicians' global evaluation of acne improvement
Physicians' global evaluation of acne improvement (percentage) ‐ split‐face design
Only one study reported data for this outcome at two months post‐treatment (treatment duration of 10 weeks, measured at the post‐treatment follow‐up period); however, the SDs were missing, so we presented the data in a table (see table in Analysis 13.10). The trial authors assessed this outcome using a five‐point system (good: more than 50% improvement; fair: 21% to 50% improvement; poor: 10% to 20% improvement; no change; or worse). In this split‐face trial (Kessler 2008), the authors did not report whether there was a difference between the alpha‐hydroxy acid (glycolic acid 30% peel) group and the salicylic acid 30% peel group.
13.10. Analysis.
Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 10: Physicians' global evaluation of acne improvement (%)
| Physicians' global evaluation of acne improvement (%) | ||||
| Study | Time points | Alpha‐hydroxy acid, % | Comparator, % | P value |
| split‐face trials | ||||
| Kessler 2008 | Good to fair improvement (treatment duration of 10 weeks, measured at two months post‐treatment) | 30% glycolic acid peels, 75% | 30% salicylic acid peels, 81% | Unclear, not reported. The author did not report whether there is any statistical difference between groups. |
Minor adverse events
Total events ‐ gluconolactone (alpha‐hydroxy acid) versus benzoyl peroxide
Hunt 1992 compared alpha‐hydroxy acid (gluconolactone 14% in solution) with benzoyl peroxide 5% lotion and showed that alpha‐hydroxy acid had a lower risk of minor adverse events than benzoyl peroxide (12/50 versus 25/50). There was a significant difference (RR 0.48, 95% CI 0.27 to 0.85; 1 study, 100 participants; Analysis 13.11). We assessed the evidence as low quality due to risk of bias and imprecision (Table 21).
13.11. Analysis.

Comparison 13: Topical alpha‐hydroxy acid versus other topical treatments, Outcome 11: Minor adverse events
Total events ‐ glycolic acid versus salicylic ‐ mandelic acid
Garg 2009 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels; there was no difference between groups (RR 1.80, 95% CI 0.72 to 4.52; 1 study, 44 participants; Analysis 13.11). We assessed the evidence as very low quality due to risk of bias and imprecision (Table 10).
In one split‐face study with no usable outcome data (Kim 1999), the authors compared 70% glycolic acid peels with Jessner's solution peels. The authors demonstrated the equal treatment effect and lesser degree of exfoliation in the glycolic acid arm.
Burning or sensation
ElRefaei 2015 reported that two out of 20 people in the 35% glycolic acid peels group versus four out of 20 people in the 20% salicylic ‐ 10% mandelic acid peels group experienced burning or sensation (P = 0.66, Fisher's Exact test). There was no difference between groups (RR 0.50, 95% CI 0.10 to 2.43; 1 study, 40 participants; Analysis 13.11).
Desquamation
ElRefaei 2015 and Garg 2009 compared 35% glycolic acid peels with 20% salicylic ‐ 10% mandelic acid peels; there was no difference between groups (RR 1.03, 95% CI 0.11 to 9.60; 2 studies, 84 participants; Analysis 13.11).
Dryness
ElRefaei 2015 reported that two out of 20 people in the 35% glycolic acid peels group versus three out of 20 people in the 20% salicylic ‐ 10% mandelic acid peels group experienced dryness (P = 1.00, Fisher's Exact test); there was no difference between groups (RR 0.67, 95% CI 0.12 to 3.57; 1 study, 40 participants; Analysis 13.11).
Hunt 1992 compared alpha‐hydroxy acid (gluconolactone 14% in solution) with benzoyl peroxide 5% lotion but with no numerical data. The authors only reported that the number of participants who experienced dryness in the benzoyl peroxide group was significantly greater than that in the alpha‐hydroxy acid (gluconolactone 14% in solution) group (P < 0.02).
Acne flare
ElRefaei 2015 and Garg 2009 reported that three out of 42 people in the 35% glycolic acid peels group versus three out of 42 people in the 20% salicylic ‐ 10% mandelic acid peels group experienced acne flare (P = 1.00, Fisher's Exact test); there was no difference between groups (RR 1.00, 95% CI 0.22 to 4.63; 2 studies, 84 participants; Analysis 13.11).
Quality of life
No study collected data for this outcome.
Comparison 14: topical alpha‐hydroxy acid versus placebo
Participants' global self‐assessment of acne improvement
No study collected data for this outcome.
Withdrawal for any reason
For this outcome, we only found one relevant trial that compared alpha‐hydroxy acid (gluconolactone 14% in solution) with placebo. Hunt 1992 reported data in the long term (12 weeks after start of treatment). The authors reported that five out of 50 people in the gluconolactone 14% solution group versus four out of 50 people in the placebo group withdrew from the trial (P = 1.00, Fisher's Exact test). There was no significant difference between topical alpha‐hydroxy acid and placebo (RR 1.25, 95% CI 0.36 to 4.38; 1 study, 100 participants; Analysis 14.1). We assessed the evidence as low quality due to risk of bias and imprecision (Table 22).
14.1. Analysis.

Comparison 14: Topical alpha‐hydroxy acid versus placebo, Outcome 1: Withdrawal for any reason
12. Gluconolactone (alpha‐hydroxy acid) compared to placebo.
| Gluconolactone (alpha‐hydroxy acid) compared to placebo for acne | ||||||
| Patient or population: participants with acne Settings: not described Intervention: topical gluconolactone (alpha‐hydroxy acid) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo/no treatment | Topical gluconolactone | |||||
| Participants' global self‐assessment of acne improvement | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
|
Withdrawal for any reason (long term: treatment duration > 8 weeks) |
80 per 1000 | 100 per 1000 (29 to 350) | RR 1.25 (0.36 to 4.38) | 100 (1 study) | ⊕⊕⊝⊝ Lowa | ‐ |
|
Total number of participants who experienced at least one minor adverse event (long term: treatment duration > 8 weeks) |
100 per 1000 | 240 per 1000 (91 to 631) | RR 2.40 (0.91 to 6.31) | 100 (1 study) | ⊕⊕⊝⊝ Lowb | Participants in gluconolactone group reported more erythema, burning and stinging, pruritus and scaling than those in the placebo group, but these differences were not "significant". |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of other bias and unclear risk of selection and reporting bias. One level for imprecision: wide CI and optimal sample size not met. bDowngraded by two levels to low quality evidence. One level for risk of bias: only one study included with high risk of other bias and unclear risk of selection and reporting bias. One level for imprecision: wide CI and optimal sample size not met. *We choose a mean baseline risk from the studies included in meta‐analysis, calculated as number of participants in the control groups with event divided by total number of participants in control groups (study population) as assumed risk.
Change in lesion counts
No study collected data for this outcome.
Physicians' global evaluation of acne improvement
No study collected data for this outcome.
Minor adverse events ‐ total events
For this outcome, there were no data reported in the short term and medium term. We only found one relevant trial that compared alpha‐hydroxy acid (gluconolactone 14% in solution) with its placebo. Hunt 1992 reported data collected in the long term (12 weeks after start of treatment). There was no "significant" difference between topical alpha‐hydroxy acid and placebo (RR 2.40, 95% CI 0.91 to 6.31; 1 study, 100 participants; Analysis 14.2). We assessed the evidence as low quality due to risk of bias and imprecision (Table 22).
14.2. Analysis.

Comparison 14: Topical alpha‐hydroxy acid versus placebo, Outcome 2: Minor adverse events ‐ total events
Quality of life
No study collected data for this outcome.
Assessment of reporting bias
We did not conduct funnel plots for primary outcomes, as the number of included studies in each forest plot was fewer than 10.
Sensitivity analysis
We were unable to perform sensitivity analyses due to the small numbers of studies.
Discussion
Summary of main results
We evaluated six test interventions in this review (topical azelaic acid, topical salicylic acid, topical nicotinamide, topical sulphur, topical zinc, and topical fruit acid (alpha‐hydroxy acid). The most‐assessed treatments were salicylic acid and azelaic acid (assessed by 73.5% of the included studies). The least assessed treatment was sulphur (1 study). With regard to the primary outcomes, 38.8% of the studies measured 'participants global self‐assessment of acne improvement', and 85.7% measured 'withdrawal for any reason'. Minor side effects were well‐reported, but the total number was not always reported in each study; some studies only reported the number of individual side effects. Quality of life was least reported, with only 12.2% of the studies measuring this outcome.
Although certain outcomes and interventions were well‐assessed, evidence quality ranged from very low to moderate, with most of the evidence deemed very low or low quality, meaning we cannot draw definitive conclusions about the treatments in question.
The following results were measured at the end of treatment, which was long‐term (except for 2 studies where it was medium‐term) for the outcome 'participants' global self‐assessment of acne improvement’ and mixed length (medium‐term mainly) for minor adverse events. We assessed minor adverse events as total number of participants who experienced at least one minor adverse event.
Azelaic acid
For participants' global self‐assessment of acne improvement, there is probably little or no difference between treatments when azelaic acid is compared to tretinoin (moderate‐quality evidence; Table 4), and there may be little or no difference between treatments when azelaic acid is compared to clindamycin (low‐quality evidence; Table 3). We are uncertain if there is a difference between azelaic acid and adapalene (very low‐quality evidence; Table 1). Azelaic acid is probably less effective than benzoyl peroxide (moderate‐quality evidence; Table 2).
When assessing our other primary outcome, withdrawal (for any reason), low‐quality evidence showed there may be no difference between azelaic acid versus benzoyl peroxide, clindamycin, or tretinoin (Table 2; Table 3; Table 4). When comparing azelaic acid to adapalene, we are uncertain of the effect on number of withdrawals due to very low‐quality evidence (Table 1).
Most adverse events reported were mild in nature and were limited to the application sites. Based on results of single small studies, we are not certain of total minor adverse events when comparing azelaic acid with adapalene or benzoyl peroxide (very low‐quality evidence; Table 1; Table 2). There may be no difference between groups in total minor adverse events when comparing azelaic acid to clindamycin (low‐quality evidence; Table 3). In the studies comparing azelaic acid to tretinoin, the total number of minor adverse events were not reported, but the study authors did report individual application site reactions, such as scaling (Table 4).
Quality of life was not well‐assessed by the studies evaluating azelaic acid, and we are uncertain of the effect of azelaic acid compared to adapalene due to very low‐quality evidence (Table 1).
Salicylic acid
For participants' global self‐assessment of acne improvement, results may be similar with salicylic acid compared to pyruvic acid tretinoin (low‐quality evidence; Table 7). We are not certain of the effect of salicylic acid compared to pyruvic acid (very low‐quality evidence; Table 6). This outcome was not assessed in the study comparing salicylic acid to benzoyl peroxide (Table 5).
When comparing salicylic acid to benzoyl peroxide, we are uncertain of the effect on number of withdrawals due to very low‐quality evidence (Table 5). There may be no difference between groups in the number of withdrawals when comparing salicylic acid and pyruvic acid (low‐quality evidence; Table 6). There were no withdrawals when salicylic acid was compared to tretinoin (low‐quality evidence; Table 7).
All side effects reported were of mild to moderate intensity and transient. The incidence of total minor adverse events when comparing salicylic acid with benzoyl peroxide or tretinoin was uncertain due to very low‐quality evidence (Table 5; Table 7). Total minor adverse events were not reported in the trial comparing salicylic acid to pyruvic acid (Table 6), but individual application site reactions were reported, such as scaling and redness.
Quality of life was not well‐assessed by the studies evaluating salicylic acid, and we are uncertain of the effect of salicylic acid compared to benzoyl peroxide or tretinoin due to very low‐quality evidence (Table 5; Table 7).
Nicotinamide
Out of the four studies which assessed nicotinamide against clindamycin or erythromycin, none reported data for participants' global self‐assessment of acne improvement. There may be no differences in rate of withdrawals when comparing nicotinamide to clindamycin or erythromycin, based on low‐quality evidence (Table 8; Table 9). Most adverse events reported were local application site reactions. Based on the results of three studies, there may be no difference in the incidence of total minor adverse events when comparing nicotinamide with clindamycin (low‐quality evidence; Table 8). The total number of minor adverse events was not reported for nicotinamide versus erythromycin. No studies collected data for quality of life.
Alpha‐hydroxy (fruit) acid
Glycolic acid peels may make no difference to participants' global self‐assessment of acne improvement when compared with salicylic‐mandelic acid peels, and there were no withdrawals in this comparison (both low‐quality evidence; Table 10). We are uncertain if there is a difference between the two groups in total minor adverse events due to very low‐quality evidence (Table 10). Adverse events associated with alpha‐hydroxy acid treatment were always mild in nature. This comparison did not assess quality of life.
Overall completeness and applicability of evidence
The eligible evidence included in this review is not sufficient to address all of our prespecified objectives. The limited number of randomised controlled trials (RCTs) concerning the use of the review's topical treatments of interest make it hard to evaluate their efficacy, and combining study results for meta‐analysis was challenging due to the variability in conducting and reporting of trials.
Of the 49 included studies, 47 studies had a total of 3880 participants, and two studies did not report the sample size (Chantalat 2007; Chen 2007). All eligible trials investigated participants with different forms or severity of acne vulgaris. Of the total included participants in this review, 75.7% (2939 participants from 33 trials) had mild to moderate acne, 10.3% (399 participants from 5 trials) probably also had mild to moderate acne, 4.8% (188 participants from 3 trials) had unknown acne severity, and only a part of the remaining 9.1% (354 participants from 6 trials) had a severe form of acne.
The age of participants from 41 studies ranged from 10 years old to 45 years old; the other eight studies did not report these data. Based on what the trials reported, most participants were aged 12 to 30 years, and there were more female than male participants, which is reflective of the age group and sex in which acne is most prevalent.
Treatment was overwhelmingly of medium‐ to long‐term duration. Medium‐term treatment was classed as treatment applied for four to eight weeks, and long‐term treatment was deemed as any intervention applied for more than eight weeks. The length of treatment duration in some studies (e.g. a 5‐day treatment duration in Draelos 2016) could have been too short to detect a significant difference between treatment groups.
We identified RCTs for all of the six test interventions. Most studies assessed salicylic acid and azelaic acid; four studies investigated nicotinamide; three studies tested the efficacy and safety of zinc; and six studies investigated the efficacy and safety of alpha‐hydroxy acid. Sulphur was the least‐assessed intervention, with only one study testing its efficacy and safety. However, the least‐assessed treatments (sulphur, zinc, and gluconolactone) are no longer used in common current clinical practice. Thirty‐one studies compared the above interventions with active treatments, such as clindamycin, erythromycin, tretinoin, and benzoyl peroxide. Fourteen studies compared the interventions with placebo control or no treatment. Four studies compared the interventions with both active treatments and placebo. This means that for some of the core comparisons we found no evidence. For example, we did not identify any studies comparing azelaic acid with placebo that assessed the outcome of participants' global self‐assessment of acne improvement; neither did we identify any studies comparing nicotinamide with placebo or no treatment that assessed any of the outcomes.
Less than half of the trials assessed the primary outcome 'participants' global self‐assessment of acne improvement' (19/49). In terms of safety, 42/50 trials measured 'withdrawal for any reason', and although the total number of minor adverse events was not always reported, individual side effects were. The least‐reported outcome was quality of life, which was measured by six studies.
Quality of the evidence
Limitations in study design and implementation
Our assessments of risk of bias revealed considerable variations in study design and implementation among the included studies. Only eight studies clearly addressed how the randomisation was performed (Cunliffe 1989; Dayal 2017; ElRefaei 2015; Ilknur 2010; Kar 2013; Kim 1999; Schaller 2016; Thielitz 2015), and only one study author stated how to conceal the allocation (ElRefaei 2015). Thirty‐nine studies had a double‐ or single‐blind design, but more than half of the studies did not report sufficient information about their methods to confirm blinding of participants/personnel and assessors. The remaining 10 studies did not mention blinding information. We rated 11 of the included trials to be at high risk of attrition bias. It should be noted that a significant proportion of the outcome data were of skewed distribution, and we presented them in additional tables in the Data and analyses section. In addition, the study authors frequently did not report SDs and the continuous data could not be analysed in meta‐analysis in such instances. For most of the comparisons, it was only possible to get pooled estimates with limited number of trials. Therefore, we downgraded all of the outcomes assessed via GRADE by at least one level for study limitations due to high/unclear risk of bias (we downgraded a small minority of outcomes by two levels for very serious study limitations).
Indirectness of the evidence
The included studies in our review assessed representative populations, though no studies included participants with neonatal and infantile acne. In our review, we included both active‐ and placebo‐controlled trials, rendering it applicable to assess the efficacy of most of the interventions. However, we failed to include some trials that compared nicotinamide to placebo/no treatment. The evidence provided by the included head‐to‐head trials was both relevant and direct. We did not downgrade the 'indirectness' domain in all GRADE assessments.
Inconsistency of the results
We failed to find high levels of heterogeneity in all cases, mainly because the evidence for many comparisons/outcomes was based on a single study. Therefore, we did not downgrade for 'inconsistency' in any of our GRADE assessments.
Imprecision of the results
The very limited number of included studies examining six test interventions did not allow us to substantively evaluate the degree of precision of the effect estimates. In most instances, there was only a single study in each comparison, and wide confidence intervals (CIs), small sample sizes (optimal sample size is not met) or total number of events (< 300) were responsible for the imprecision. Therefore, we downgraded the majority of outcomes assessed via GRADE by one level for serious imprecision.
Publication bias
We were unable to assess publication bias because none of the comparisons had more than 10 studies. Therefore, it is meaningless to create funnel plots. We did not downgrade the 'Publication bias' domain in any GRADE assessments.
Potential biases in the review process
As shown in Electronic searches, we conducted a systematic electronic search of databases. In addition, we also checked the bibliographies of included studies for further references to relevant trials. However, the fact that 15 potentially eligible studies have not yet been incorporated may be a source of potential bias. We were unable to contact some study authors for further data due to the lack of a correspondence email address. We contacted study authors in order to obtain additional data, but only a few replied and not all provided us with the requested data.
Although there were some departures from the protocol (see Differences between protocol and review), these changes are unlikely to be a potential source of bias. We did not make any a posteriori decisions about the analysis or investigation of heterogeneity after seeing the data.
Our inclusion of studies investigating a synergistic salicylic acid microgel complex could be questioned (Chantalat 2005; Chantalat 2006; Chantalat 2007; Chen 2007). These four studies were published as abstracts with no contact emails; thus, based on this insufficient information, we did not know the components and the drug delivery system of the intervention.
Agreements and disagreements with other studies or reviews
There have been no reviews published which evaluated these topical treatments in a systematic way. Our review substantially updates and improves the previous work in this area. The findings of this review generally agree with the findings in previous summary reviews (Gamble 2012; Haider 2004; Purdy 2011).
Authors' conclusions
Implications for practice.
Presently, clinicians often choose topical retinoids and antimicrobials as the first choice of treatment for mild and moderate acne (Akhavan 2003; Titus 2012; Well 2013). The data in this review show there is no high‐quality evidence to determine the effects of the topical treatments azelaic acid, salicylic acid, nicotinamide, sulphur, zinc, and alpha‐hydroxy acid over the commonly used topical drugs. In some cases, the comparative studies suggest no difference between these topical treatments and commonly used retinoids or antimicrobials, but we cannot draw definitive conclusions due to very low‐ to low‐quality evidence. The limited number of trials and other issues (e.g. inadequate reporting) make it hard to obtain high‐quality evidence.
We cannot draw conclusions about the effect of the following comparisons on the outcome 'participants' global self‐assessment of acne improvement', as the quality of evidence is very low or the outcome was not reported.
Azelaic acid compared to adapalene
Salicylic acid compared to pyruvic acid
Salicylic acid compared to benzoyl peroxide
Nicotinamide compared to clindamycin
Nicotinamide compared to erythromycin
In terms of treatment response (participants' global self‐assessment of acne improvement; PGA), azelaic acid is probably less effective than benzoyl peroxide (moderate‐quality evidence), and there may be little or no difference in PGA when comparing azelaic acid to clindamycin (low‐quality evidence). There is probably little or no difference when comparing azelaic acid to tretinoin (moderate‐quality evidence). There may be little or no difference in PGA between salicylic acid and tretinoin (low‐quality evidence). There may be no difference in PGA when comparing glycolic acid peel to salicylic‐mandelic acid peel (low‐quality evidence).
We cannot draw conclusions about the effect of the following comparisons on the outcome 'withdrawal for any reason', as the quality of evidence is very low or the outcome was not reported.
Azelaic acid compared to adapalene
Salicylic acid compared to benzoyl peroxide
Based on low‐quality evidence, there may be no differences in rates of withdrawal for any reason when comparing the following.
Azelaic acid with benzoyl peroxide, clindamycin, or tretinoin
Salicylic acid with pyruvic acid
Nicotinamide with clindamycin or erythromycin
There were no withdrawals in the comparisons of salicylic acid versus tretinoin and glycolic acid versus salicylic‐mandelic acid.
We cannot draw conclusions about the effect of the following comparisons on minor adverse events, assessed as total number of participants who experienced at least one minor adverse event, as the quality of evidence is very low or the outcome was not reported.
Azelaic acid compared to adapalene
Azelaic acid compared to benzoyl peroxide
Azelaic acid compared to tretinoin
Salicylic acid compared to benzoyl peroxide
Salicylic acid with pyruvic acid
Salicylic acid was compared to tretinoin
Nicotinamide compared to erythromycin
Glycolic acid (alpha‐hydroxy acid) compared to salicylic‐mandelic acid (peel)
There may be no difference in minor adverse events when comparing azelaic acid to clindamycin.
The adverse events caused by these treatments included mainly application site reactions such as erythema, scaling, dry skin, burning, peeling, and itching, and the risk of specific adverse events (e.g. erythema) was mostly similar between treatment groups.
We do not have sufficient evidence to determine the efficacy and safety of sulphur, zinc, and gluconolactone, which are no longer used in clinical practice.
In the absence of high‐quality evidence for these treatments, clinical decisions may continue to be guided by clinical experiences and patients' preferences.
Implications for research.
There is a need for further head‐to‐head comparisons of the topical treatments azelaic acid, salicylic acid, nicotinamide, and glycolic acid with commonly used active drugs (topical retinoids and antimicrobials). Moreover, trials comparing these topical treatments with vehicle/placebo or no treatment are also required. This will confirm their efficacy for treating mild to moderate acne.
Randomised trials with a parallel or cross‐over design are necessary. With respect to cross‐over trials, study authors should report the outcome data as previously suggested (Elbourne 2002). Study authors should clearly report the severity of illness. Participants irrespective of age, severity, or gender need to be included. Study authors should clearly report the co‐interventions in each treatment arm. A long‐term treatment duration (over 8 weeks) for future trials is suggested. We do not recommend trials authors measure the drug efficacy in the post‐treatment follow‐up period as these topical medications most probably have no long‐lasting effect after withdrawal of therapy.
The variability in conducting and reporting of trials significantly hampered combining study results for meta‐analysis. We recommend standardisation of outcome reporting in future trials. The trial authors should use a standardised scale (e.g. measured by a four‐point scale: excellent, good, fair, and poor) to measure participants' global self‐assessment of acne improvement, and the authors should provide a clear description of how the outcome was measured. Study authors should also report the number of withdrawals from the trial and the reasons for withdrawals. In addition, development of a standardised scale for physicians' global evaluation of acne improvement is necessary, and the report of number of participants would be better. We recommend study authors report the total number of participants who experienced at least one adverse event, but not report adverse events as count data. It would also be useful if study authors presented the total number of participants who experienced individual side effects, e.g. redness. Assessment of quality of life using a validated instrument (e.g. Acne‐Specific Quality of Life Questionnaire; Acne‐QoL) is highly desirable. Adherence to recommendations from the Cochrane Skin ‐ Core Outcome Set Initiative would improve and standardise outcome measurement (CS‐COUSIN 2019).
Unfortunately, the study authors often presented inadequate data or information, for example, randomisation was not clearly described, allocation concealment was not reported, it was unclear who was blinded, results were presented in figures with no raw data, standard deviations (SDs) were not mentioned and could not be obtained in any way, and exact P values were not reported. Furthermore, 11/49 studies had high attrition bias; therefore, efforts should be made to ensure participants remain in the study. We have to acknowledge that many included studies in this review predate the CONSORT recommendations (Begg 1996; Moher 2001), but future studies should ensure they adhere to the CONSORT recommendations on trials to guarantee the full availability of all data. Many of our analyses were limited to single study data. These studies had small sample sizes; hence, we downgraded the majority of our evidence for imprecision. Future studies should ensure a sample size calculation is used. We could not assess publication bias because of the limited number of studies in each comparison.
Feedback
Intention to treat analysis, 21 July 2020
Summary
A comment was received from Sarah King who has conducted a systematic review on AA for acne, querying whether the authors conducted ITT analysis. She cites an example: in Katsambas 1989a, they have used the full data set (i.e. n = 92 in their Analysis 2.9), but it does not appear that the primary study authors conducted ITT analysis (n = 80 as there were drop‐outs reported). Did the authors use the full numbers in other analyses as well or use numbers analysed by study authors? If they used ITT analysis, how did they impute the data ‐ if they did this?
Reply
As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; 16.2.2 Intention‐to‐treat issues for dichotomous data), for dichotomous data, there are two options for this issue: 1) available case analysis; 2) ITT analysis using imputation, based on analysis of the total number of randomized participants, irrespective of how the original study authors analysed the data. We used the second one. This is to say, we extracted data on ITT basis (once‐randomised‐always‐analyse) as stated in our protocol. Thus, we used the 'full data set' in our review for dichotomous data. We assumed that all the missing participants 'did not experience the event'. For example, in Analysis 2.9, all the drop‐outs did not experience 'Good to excellent improvement'. There is no consensus on the best way to handle these missing participants in an analysis. The choice of imputation methods should be based on clinical judgement.
Contributors
Lead author Haibo Liu and Cochrane Skin feedback editor Urbà González.
What's new
| Date | Event | Description |
|---|---|---|
| 8 December 2020 | Amended | Feedback received 21 July 2020; response published. |
History
Protocol first published: Issue 11, 2014 Review first published: Issue 5, 2020
Acknowledgements
The authors would like to thank Cochrane Skin editorial base for their support in the completion of this review.
The authors would like to thank Carolina Freitas (research assistant and handsearcher, Brazilian Cochrane Centre) for the translation of non‐English reports in the review process.
The authors would also like to thank Sai Zhao (Systematic Review Solutions. Ltd) for the coaching on the methodology and reporting standards of this review.
The Cochrane Skin editorial base wishes to thank Robert Dellavalle, Cochrane Dermatology Editor for this review; Matthew Grainge, Statistical Editor; Ching‐Chi Chi, Methods Editor; the clinical referee, Jerry Tan; the consumer referee, who wishes to remain anonymous; and Clare Dooley, who copy‐edited the review.
Appendices
Appendix 1. Skin Group Specialised Register/CRS search strategy
acne and (azelaic or azeleic or salicylic or niacinamide or nicotinamide or sulfur or sulphur or ascorbic or fruit or zinc)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Acne Vulgaris] explode all trees #2 acne:ti,ab,kw #3 #1 or #2 #4 MeSH descriptor: [Dicarboxylic Acids] explode all trees #5 ((azelaic or azeleic) and acid*):ti,ab,kw #6 MeSH descriptor: [Salicylic Acid] explode all trees #7 salicylic acid*:ti,ab,kw #8 o‐hydroxybenzoic acid*:ti,ab,kw #9 MeSH descriptor: [Niacinamide] explode all trees #10 niacinamide:ti,ab,kw #11 nicotinamide:ti,ab,kw #12 MeSH descriptor: [Sulfur] explode all trees #13 sulphur:ti,ab,kw #14 sulfur:ti,ab,kw #15 MeSH descriptor: [Ascorbic Acid] explode all trees #16 ascorbic acid*:ti,ab,kw #17 fruit acid*:ti,ab,kw #18 MeSH descriptor: [Fruit] explode all trees #19 (topical and zinc):ti,ab,kw #20 MeSH descriptor: [Zinc] explode all trees #21 {or #4‐#20} #22 #3 and #21
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Acne Vulgaris/ 2. acne.ti,ab. 3. 1 or 2 4. exp Dicarboxylic Acids/ 5. azelaic acid$.ti,ab. 6. azeleic acid$.ti,ab. 7. exp Salicylic Acid/ 8. salicylic acid$.ti,ab. 9. o‐hydroxybenzoic acid$.ti,ab. 10. exp Niacinamide/ 11. niacinamide.ti,ab. 12. nicotinamide.ti,ab. 13. Sulfur/ 14. sulphur.ti,ab. 15. sulfur.ti,ab. 16. exp Ascorbic Acid/ 17. ascorbic acid$.ti,ab. 18. fruit acid$.ti,ab. 19. Fruit/ 20. Zinc/ and topical.ti,ab. 21. (topical and zinc).ti,ab. 22. or/4‐21 23. randomized controlled trial.pt. 24. controlled clinical trial.pt. 25. randomized.ab. 26. placebo.ab. 27. clinical trials as topic.sh. 28. randomly.ab. 29. trial.ti. 30. 23 or 24 or 25 or 26 or 27 or 28 or 29 31. exp animals/ not humans.sh. 32. 30 not 31 33. 3 and 22 and 32
[Lines 23‐32: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. exp acne vulgaris/ 2. acne.mp. 3. 1 or 2 4. exp dicarboxylic acid/ 5. azelaic acid$.ti,ab. 6. azeleic acid$.ti,ab. 7. azelaic acid/ 8. salicylic acid/ 9. salicylic acid$.ti,ab. 10. o‐hydroxybenzoic acid$.ti,ab. 11. exp nicotinamide/ 12. niacinamide.ti,ab. 13. nicotinamide.ti,ab. 14. sulfur/ 15. sulphur.ti,ab. 16. sulfur.ti,ab. 17. exp ascorbic acid/ 18. fruit acid$.ti,ab. 19. exp fruit/ 20. (topical and zinc).ti,ab. 21. zinc/ and topical.ti,ab. 22. ascorbic acid$.ti,ab. 23. or/4‐22 24. crossover procedure.sh. 25. double‐blind procedure.sh. 26. single‐blind procedure.sh. 27. (crossover$ or cross over$).tw. 28. placebo$.tw. 29. (doubl$ adj blind$).tw. 30. allocat$.tw. 31. trial.ti. 32. randomized controlled trial.sh. 33. random$.tw. 34. or/24‐33 35. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 36. human/ or normal human/ 37. 35 and 36 38. 35 not 37 39. 34 not 38 40. 3 and 23 and 39
Appendix 5. LILACS search strategy
acne and (azelaic or azeleic or azelaico or salicilico or salicylic or niacinamide or nicotinamide or sulfur or sulphur or azufre or ascorbic or ascorbico or fruit or zinc or cinc)
These terms combined with the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1. Topical azelaic acid versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Participants' global self‐assessment of acne improvement | 7 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 azelaic acid versus adapalene ‐ improved to very much improved (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.2 azelaic acid versus adapalene ‐ improved to very much improved (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.3 azelaic acid versus benzoyl peroxide ‐ good or very good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.4 azelaic acid versus clindamycin ‐ good or very good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.5 azelaic acid versus clindamycin ‐ moderately satisfied to very satisfied improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.6 azelaic acid versus erythromycin ‐ moderately satisfied to very satisfied improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.7 azelaic acid versus tretinoin ‐ good to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.8 azelaic acid versus benzoyl peroxide/clindamycin ‐ much to very much improved (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.9 azelaic acid versus benzoyl peroxide/clindamycin ‐ much to very much improved (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.10 azelaic acid versus benzoyl peroxide/clindamycin ‐ much to very much improved (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Withdrawal for any reason | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 azelaic acid versus adapalene (short term) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.05, 12.01] |
| 1.2.2 azelaic acid versus adapalene (long term) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [0.33, 20.99] |
| 1.2.3 azelaic acid versus benzoyl peroxide (short term) | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.04, 4.10] |
| 1.2.4 azelaic acid versus benzoyl peroxide (long term) | 1 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.29] |
| 1.2.5 azelaic acid versus clindamycin (medium term) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2.6 azelaic acid versus clindamycin (long term) | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.48, 3.56] |
| 1.2.7 azelaic acid versus metronidazole (long term) | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2.8 azelaic acid versus tretinoin (long term) | 2 | 309 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.29, 1.47] |
| 1.2.9 azelaic acid versus benzoyl peroxide/clindamycin (long term) | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.43, 3.07] |
| 1.3 Change in lesion counts ‐ total (percentage reduction from baseline) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.1 azelaic acid versus clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.2 azelaic acid versus clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.3 azelaic acid versus clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.4 azelaic acid versus benzoyl peroxide/clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.5 azelaic acid versus benzoyl peroxide/clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3.6 azelaic acid versus benzoyl peroxide/clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.4 Change in lesion counts ‐ total | 1 | Other data | No numeric data | |
| 1.4.1 long term | 1 | Other data | No numeric data | |
| 1.5 Change in lesion counts ‐ inflamed (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.1 azelaic acid versus benzoyl peroxide/clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.2 azelaic acid versus benzoyl peroxide/clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.5.3 azelaic acid versus benzoyl peroxide/clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6 Change in lesion counts ‐ inflamed (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.1 azelaic acid versus adapalene (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.6.2 azelaic acid versus benzoyl peroxide (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.7 Change in lesion counts ‐ inflamed | 5 | Other data | No numeric data | |
| 1.8 Change in lesion counts ‐ papules (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.1 azelaic acid versus clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.2 azelaic acid versus clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.8.3 azelaic acid versus clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9 Change in lesion counts ‐ papules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.1 azelaic acid versus erythromycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.2 azelaic acid versus erythromycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.9.3 azelaic acid versus erythromycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10 Change in lesion counts ‐ pustules (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10.1 azelaic acid versus clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10.2 azelaic acid versus clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.10.3 azelaic acid versus clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11 Change in lesion counts ‐ pustules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11.1 azelaic acid versus erythromycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11.2 azelaic acid versus erythromycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.11.3 azelaic acid versus erythromycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.12 Change in lesion counts ‐ non‐inflamed | 6 | Other data | No numeric data | |
| 1.13 Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.1 azelaic acid versus clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.2 azelaic acid versus clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.3 azelaic acid versus clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.4 azelaic acid versus benzoyl peroxide/clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.5 azelaic acid versus benzoyl peroxide/clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.13.6 azelaic acid versus benzoyl peroxide/clindamycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14 Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14.1 azelaic acid versus erythromycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14.2 azelaic acid versus erythromycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14.3 azelaic acid versus erythromycin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14.4 azelaic acid versus adapalene (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.14.5 azelaic acid versus benzoyl peroxide (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.15 Physicians' global evaluation of acne improvement | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.1 azelaic acid versus benzoyl peroxide ‐ good or very good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.2 azelaic acid versus clindamycin ‐ good or very good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.3 azelaic acid versus tretinoin ‐ good to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.4 azelaic acid versus benzoyl peroxide/clindamycin ‐ clear to almost clear (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.5 azelaic acid versus benzoyl peroxide/clindamycin ‐ clear to almost clear (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.15.6 azelaic acid versus benzoyl peroxide/clindamycin ‐ clear to almost clear (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.16 Minor adverse events | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.16.1 total events ‐ azelaic acid versus adapalene (medium term) | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.47, 2.85] |
| 1.16.2 total events ‐ azelaic acid versus benzoyl peroxide (short term) | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.05, 4.94] |
| 1.16.3 total events ‐ azelaic acid versus benzoyl peroxide/clindamycin (long term) | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.01, 1.52] |
| 1.16.4 total events ‐ azelaic acid versus clindamycin (long term) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.67, 3.35] |
| 1.16.5 total events ‐ azelaic acid versus erythromycin (long term) | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.35] |
| 1.16.6 application site pain ‐ azelaic acid versus benzoyl peroxide/clindamycin | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.17 [1.41, 7.12] |
| 1.16.7 burning ‐ azelaic acid versus benzoyl peroxide | 1 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.61, 1.97] |
| 1.16.8 burning ‐ azelaic acid versus clindamycin | 1 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 25.22 [1.51, 420.92] |
| 1.16.9 burning ‐ azelaic acid versus tretinoin | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.71] |
| 1.16.10 scaling ‐ azelaic acid versus clindamycin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.20, 2.22] |
| 1.16.11 scaling ‐ azelaic acid versus erythromycin | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.35, 9.01] |
| 1.16.12 scaling ‐ azelaic acid versus tretinoin | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.37, 0.91] |
| 1.16.13 erythema ‐ azelaic acid versus adapalene | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.30, 2.10] |
| 1.16.14 erythema ‐ azelaic acid versus benzoyl peroxide | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.09] |
| 1.16.15 erythema ‐ azelaic acid versus benzoyl peroxide/clindamycin | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.41, 6.87] |
| 1.16.16 erythema ‐ azelaic acid versus clindamycin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.18] |
| 1.16.17 erythema ‐ azelaic acid versus erythromycin | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.16, 2.74] |
| 1.16.18 erythema ‐ azelaic acid versus tretinoin | 1 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.41, 0.99] |
| 1.16.19 dryness ‐ azelaic acid versus adapalene | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.51, 1.26] |
| 1.16.20 dryness ‐ azelaic acid versus benzoyl peroxide | 2 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.27, 1.16] |
| 1.16.21 dryness ‐ azelaic acid versus benzoyl peroxide/clindamycin | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.26, 8.88] |
| 1.16.22 dryness ‐ azelaic acid versus clindamycin | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 2.44 [0.96, 6.19] |
| 1.16.23 dryness ‐ azelaic acid versus erythromycin | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.25] |
| 1.16.24 oiliness ‐ azelaic acid versus clindamycin | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.36, 4.38] |
| 1.16.25 oiliness ‐ azelaic acid versus erythromycin | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.19, 4.07] |
| 1.16.26 itching ‐ azelaic acid versus adapalene | 1 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.79] |
| 1.16.27 itching ‐ azelaic acid versus benzoyl peroxide | 2 | 396 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [0.24, 45.29] |
| 1.16.28 itching ‐ azelaic acid versus benzoyl peroxide/clindamycin | 1 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [1.49, 6.68] |
| 1.16.29 itching ‐ azelaic acid versus clindamycin | 2 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 2.56 [0.68, 9.57] |
| 1.16.30 itching ‐ azelaic acid versus erythromycin | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.29, 4.87] |
| 1.16.31 red skin ‐ azelaic acid versus benzoyl peroxide | 1 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.36, 1.26] |
| 1.16.32 red skin ‐ azelaic acid versus clindamycin | 1 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 6.05 [1.39, 26.44] |
| 1.16.33 desquamation ‐ azelaic acid versus benzoyl peroxide | 1 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.73] |
| 1.16.34 eczema ‐ azelaic acid versus clindamycin | 1 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.06] |
| 1.17 Quality of life | 2 | Other data | No numeric data |
Comparison 2. Topical azelaic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Withdrawal for any reason | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1.1 medium term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1.2 long term | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2 Change in lesion counts ‐ > 50% inflamed reduction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 long term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Change in lesion counts ‐ inflamed (percentage reduction from baseline) | 3 | Other data | No numeric data | |
| 2.3.1 short term | 1 | Other data | No numeric data | |
| 2.3.2 medium term | 2 | Other data | No numeric data | |
| 2.3.3 long term | 2 | Other data | No numeric data | |
| 2.3.4 long term (split‐face trials) | 1 | Other data | No numeric data | |
| 2.4 Change in lesion counts ‐ > 50% non‐inflamed reduction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.4.1 long term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.5 Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | 2 | Other data | No numeric data | |
| 2.5.1 medium term | 1 | Other data | No numeric data | |
| 2.5.2 long term | 1 | Other data | No numeric data | |
| 2.5.3 long term (split‐face trials) | 1 | Other data | No numeric data | |
| 2.6 Change in lesion counts (percentage reduction from baseline) | 3 | Other data | No numeric data | |
| 2.6.1 medium term | 1 | Other data | No numeric data | |
| 2.6.2 long term | 1 | Other data | No numeric data | |
| 2.6.3 long term (split‐face trials) | 1 | Other data | No numeric data | |
| 2.7 Change in lesion counts (number of lesions post‐intervention) | 1 | Other data | No numeric data | |
| 2.7.1 short term | 1 | Other data | No numeric data | |
| 2.8 Change in lesion counts ‐ comedones (reduction in number of lesions post‐intervention) | 1 | Other data | No numeric data | |
| 2.8.1 long term | 1 | Other data | No numeric data | |
| 2.9 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.9.1 Good to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.10 Minor adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.10.1 burning | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [0.53, 39.24] |
| 2.10.2 scaling | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.16, 13.48] |
| 2.10.3 erythema | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [0.39, 9.78] |
| 2.10.4 dryness | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 2.92 [0.15, 57.90] |
| 2.10.5 oiliness | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 4.08 [0.22, 75.25] |
| 2.10.6 itching | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 5.45 [0.68, 43.53] |
| 2.10.7 total events (medium term) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 19.00 [1.16, 312.42] |
Comparison 3. Topical azelaic acid versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Participants' global self‐assessment of acne improvement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1.1 moderately satisfied to very satisfied improvement (long term) | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.94, 1.24] |
| 3.2 Withdrawal for any reason | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 long term | 2 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.37, 1.22] |
| 3.3 Change in lesion counts ‐ total (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.1 short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.2 medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.3.3 long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4 Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 short term | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 medium term | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.3 long term | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 Change in lesion counts ‐ papules (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.5.1 short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.5.2 medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.5.3 long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.6 Change in lesion counts ‐ pustules (percentage reduction from baseline) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.6.1 short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.6.2 medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.6.3 long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.7 Change in lesion counts ‐ inflamed (number of lesions post‐intervention) | 1 | Other data | No numeric data | |
| 3.7.1 short term | 1 | Other data | No numeric data | |
| 3.7.2 medium term | 1 | Other data | No numeric data | |
| 3.7.3 long term | 1 | Other data | No numeric data | |
| 3.8 Change in lesion counts ‐ comedones (number of lesions post‐intervention) | 1 | Other data | No numeric data | |
| 3.8.1 short term | 1 | Other data | No numeric data | |
| 3.8.2 medium term | 1 | Other data | No numeric data | |
| 3.8.3 long term | 1 | Other data | No numeric data | |
| 3.9 Minor adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.9.1 scaling | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.15, 1.50] |
| 3.9.2 erythema | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.21] |
| 3.9.3 dryness | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.20, 1.85] |
| 3.9.4 oiliness | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.24] |
| 3.9.5 itching | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.23, 2.29] |
| 3.9.6 total events (long term) | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.36, 0.97] |
Comparison 4. Topical salicylic acid versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Participants' global self‐assessment of acne improvement | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.1 salicylic acid versus tretinoin ‐ moderate to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1.2 salicylic acid versus pyruvic acid ‐ good to excellent improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2 Participants' global self‐assessment of acne improvement | 3 | Other data | No numeric data | |
| 4.2.1 split‐face trials | 2 | Other data | No numeric data | |
| 4.2.2 parallel trial | 1 | Other data | No numeric data | |
| 4.3 Withdrawal for any reason | 7 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3.1 salicylic acid versus pyruvic acid (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3.2 salicylic acid versus benzoyl peroxide (short term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3.3 salicylic acid versus benzoyl peroxide (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3.4 salicylic acid versus tretinoin (long term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3.5 salicylic acid versus Jessner's solution (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.4 Change in lesion counts ‐ total (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.1 salicylic acid versus tretinoin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.2 salicylic acid versus tretinoin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.4.3 salicylic acid versus tretinoin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5 Change in lesion counts ‐ inflamed (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.1 salicylic acid versus tretinoin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.2 salicylic acid versus tretinoin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.5.3 salicylic acid versus tretinoin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.6 Change in lesion counts ‐ inflamed (mean counts or %) | 3 | Other data | No numeric data | |
| 4.6.1 parallel trials | 1 | Other data | No numeric data | |
| 4.6.2 split‐face trials | 2 | Other data | No numeric data | |
| 4.7 Change in lesion counts ‐ papules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7.1 salicylic acid versus pyruvic acid (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.7.2 salicylic acid versus pyruvic acid (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8 Change in lesion count ‐ pustules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8.1 salicylic acid versus pyruvic acid (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.8.2 salicylic acid versus pyruvic acid (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9 Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.1 salicylic acid versus tretinoin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.2 salicylic acid versus tretinoin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.3 salicylic acid versus tretinoin (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.4 salicylic acid versus pyruvic acid (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.9.5 salicylic acid versus pyruvic acid (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.10 Change in lesion counts ‐ non‐inflamed (counts or %) | 3 | Other data | No numeric data | |
| 4.10.1 parallel trials | 1 | Other data | No numeric data | |
| 4.10.2 split‐face trials | 2 | Other data | No numeric data | |
| 4.11 Change in lesion counts ‐ various types of acne lesions (counts or %) | 4 | Other data | No numeric data | |
| 4.11.1 parallel trials | 2 | Other data | No numeric data | |
| 4.11.2 split‐face trials | 1 | Other data | No numeric data | |
| 4.11.3 cross‐over trials | 1 | Other data | No numeric data | |
| 4.12 Physicians' global evaluation of acne improvement | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.12.1 salicylic acid versus tretinoin ‐ moderate to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.12.2 salicylic acid versus Jessner's solution ‐ fair to good improvement (long term)) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.12.3 salicylic acid versus pyruvic acid ‐ good to excellent improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.13 Physicians' global evaluation of acne improvement (%) | 1 | Other data | No numeric data | |
| 4.13.1 split‐face trials | 1 | Other data | No numeric data | |
| 4.14 Physicians' global evaluation of acne improvement | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.1 salicylic acid versus lipohydroxy acid ‐ 3‐point scale defined by investigator, high = well (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.2 salicylic acid versus lipohydroxy acid ‐ 3‐point scale defined by investigator, high = well (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.14.3 salicylic acid versus lipohydroxy acid ‐ 3‐point scale defined by investigator, high = well (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.15 Minor adverse events | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.15.1 dryness ‐ salicylic acid versus tretinoin | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.94] |
| 4.15.2 peeling ‐ salicylic acid versus tretinoin | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.26] |
| 4.15.3 erythema ‐ salicylic acid versus tretinoin | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.38, 2.01] |
| 4.15.4 burning ‐ salicylic acid versus tretinoin | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.50, 2.63] |
| 4.15.5 itching ‐ salicylic acid versus tretinoin | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.16, 2.22] |
| 4.15.6 postpeel burning and stinging ‐ salicylic acid versus Jessner's solution | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.81, 2.58] |
| 4.15.7 postpeel erythema ‐ salicylic acid versus Jessner's solution | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.50, 4.52] |
| 4.15.8 postpeel hyperpigmentation ‐ salicylic acid versus Jessner's solution | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.94] |
| 4.15.9 total events ‐ salicylic acid versus benzoyl peroxide (short term) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 4.15.10 total events ‐ salicylic acid versus benzoyl peroxide (medium term) | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.01, 4.11] |
| 4.15.11 total events ‐ salicylic acid versus tretinoin (long term) | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.66, 2.87] |
| 4.16 Quality of life (QoL) ‐ AQOL (score, post‐intervention) | 2 | Other data | No numeric data |
Comparison 5. Topical salicylic acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Participants' global self‐assessment of acne improvement (score, high=well) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.1 short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.2 medium term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1.3 long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Withdrawal for any reason | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2.1 short term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2.2 long term | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Change in lesion counts (counts or %) | 3 | Other data | No numeric data | |
| 5.4 Change in lesion counts ‐ inflamed (counts or %) | 2 | Other data | No numeric data | |
| 5.5 Change in lesion counts ‐ non‐inflamed (counts or %) | 2 | Other data | No numeric data | |
| 5.6 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.6.1 good or excellent improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7 Minor adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7.1 total events (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7.2 total events (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Topical salicylic acid versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Participants' global self‐assessment of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1.1 moderate to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Withdrawal for any reason | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2.1 long term | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3 Change in lesion counts ‐ total (percentage reduction from baseline) | 3 | Other data | No numeric data | |
| 6.4 Change in lesion counts ‐ inflamed (percentage reduction from baseline) | 2 | Other data | No numeric data | |
| 6.5 Change in lesion counts ‐ non‐inflamed (percentage reduction from baseline) | 2 | Other data | No numeric data | |
| 6.6 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.6.1 moderate to excellent improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.7 Minor adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.7.1 dryness | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.25, 5.68] |
| 6.7.2 peeling | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.74, 3.03] |
| 6.7.3 erythema | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 4.00 [0.94, 17.00] |
| 6.7.4 burning | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.71, 3.89] |
| 6.7.5 itching | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.45, 6.24] |
| 6.7.6 total events (long term) | 2 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 3.43 [0.14, 82.00] |
| 6.8 Quality of life (QoL) ‐ AQOL (score, post‐intervention) | 1 | Other data | No numeric data |
Comparison 7. Topical nicotinamide versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Withdrawal for any reason | 4 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.1 nicotinamide versus clindamycin (medium term) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.1.2 nicotinamide versus erythromycin (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 Change in lesion counts ‐ inflamed (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2.1 nicotinamide versus clindamycin (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.2.2 nicotinamide versus clindamycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.3 Change in lesion counts ‐ inflamed counts (counts or %) | 2 | Other data | No numeric data | |
| 7.3.1 short term | 1 | Other data | No numeric data | |
| 7.3.2 medium term | 2 | Other data | No numeric data | |
| 7.4 Change in lesion counts ‐ comedones (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.4.1 nicotinamide versus erythromycin (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.5 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5.1 nicotinamide versus clindamycin ‐ moderately or much better improvement (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.5.2 nicotinamide versus clindamycin ‐ moderately or much better improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.6 Physicians' global evaluation of acne improvement | 1 | Other data | No numeric data | |
| 7.6.1 short term | 1 | Other data | No numeric data | |
| 7.6.2 medium term | 1 | Other data | No numeric data | |
| 7.7 Minor adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.7.1 itching ‐ nicotinamide versus clindamycin | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.32, 5.58] |
| 7.7.2 burning ‐ nicotinamide versus clindamycin | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [0.77, 15.83] |
| 7.7.3 crusting ‐ nicotinamide versus clindamycin | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.78] |
| 7.7.4 greasy skin ‐ nicotinamide versus clindamycin | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.68] |
| 7.7.5 dermatitis ‐ nicotinamide versus clindamycin | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.51] |
| 7.7.6 total events ‐ nicotinamide versus clindamycin (medium term) | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.73, 1.99] |
| 7.7.7 pertinent clinical signs ‐ nicotinamide versus erythromycin (short term) | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.60, 2.99] |
| 7.7.8 pertinent clinical signs ‐ nicotinamide versus erythromycin (medium term) | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.44] |
| 7.7.9 functional or physical signs ‐ nicotinamide versus erythromycin (short term) | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.82] |
| 7.7.10 functional or physical signs ‐ nicotinamide versus erythromycin (medium term) | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.38, 1.48] |
Comparison 8. Topical sulphur versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Participants' global self assessment of acne improvement (numerical point system defined by investigator, high = well) | 1 | Other data | No numeric data | |
| 8.1.1 medium term | 1 | Other data | No numeric data | |
| 8.2 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.2.1 sulphur versus benzoyl peroxide (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.3 Change in lesion counts (scores, high = well) | 1 | Other data | No numeric data | |
| 8.4 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.4.1 sulphur versus benzoyl peroxide ‐ moderate to good improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.5 Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well) | 1 | Other data | No numeric data | |
| 8.5.1 medium term | 1 | Other data | No numeric data | |
| 8.6 Minor adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.6.1 erythema and drying ‐ sulphur versus benzoyl peroxide | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Topical sulphur versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high=well) | 1 | Other data | No numeric data | |
| 9.1.1 medium term | 1 | Other data | No numeric data | |
| 9.2 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.2.1 medium term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.3 Change in lesion counts (scores, high = well) | 1 | Other data | No numeric data | |
| 9.4 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.4.1 moderate to good improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.5 Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high = well) | 1 | Other data | No numeric data | |
| 9.5.1 medium term | 1 | Other data | No numeric data | |
| 9.6 Minor adverse events ‐ erythema and drying | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Topical sulphur versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Participants' global self‐assessment of acne improvement (numerical point system defined by investigator, high = well) | 1 | Other data | No numeric data | |
| 10.1.1 medium term | 1 | Other data | No numeric data | |
| 10.2 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.2.1 medium term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.3 Change in lesion counts (scores, high = well) | 1 | Other data | No numeric data | |
| 10.4 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.4.1 moderate to good improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10.5 Physicians' global evaluation of acne improvement (numerical point system defined by investigator, high=well) | 1 | Other data | No numeric data | |
| 10.5.1 medium term | 1 | Other data | No numeric data | |
| 10.6 Minor adverse events ‐ erythema and drying | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Topical zinc versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.1.1 zinc versus tea (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.2 Change in lesion counts ‐ papules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.2.1 zinc versus tea (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.3 Change in lesion counts ‐ pustules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.3.1 zinc versus tea (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.4 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.4.1 zinc versus tea ‐ moderate or good response (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.5 Minor adverse events ‐ total events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.5.1 zinc versus tea (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Topical zinc versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Participants' global self‐assessment of acne improvement (visual analogue scale) | 1 | Other data | No numeric data | |
| 12.1.1 long term | 1 | Other data | No numeric data | |
| 12.2 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.2.1 long term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 12.3 Change in lesion counts ‐ total (lesion counts reduction) | 1 | Other data | No numeric data | |
| 12.3.1 medium term | 1 | Other data | No numeric data | |
| 12.3.2 long term | 1 | Other data | No numeric data | |
| 12.4 Change in lesion counts ‐ inflamed (lesion counts reduction) | 2 | Other data | No numeric data | |
| 12.4.1 short term | 1 | Other data | No numeric data | |
| 12.4.2 medium term | 1 | Other data | No numeric data | |
| 12.4.3 long term | 2 | Other data | No numeric data | |
| 12.5 Change in lesion counts ‐ non‐inflamed (lesion counts reduction) | 2 | Other data | No numeric data | |
| 12.5.1 short term | 1 | Other data | No numeric data | |
| 12.5.2 medium term | 2 | Other data | No numeric data | |
| 12.5.3 long term | 2 | Other data | No numeric data | |
| 12.6 Physicians' global evaluation of acne improvement (visual analogue scale) | 1 | Other data | No numeric data | |
| 12.6.1 long term | 1 | Other data | No numeric data | |
| 12.7 Minor adverse events | 1 | Other data | No numeric data |
Comparison 13. Topical alpha‐hydroxy acid versus other topical treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Participants' global self‐assessment of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.1.1 glycolic acid versus salicylic‐mandelic acid ‐ fair to good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.2 Withdrawal for any reason | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.2.1 gluconolactone versus benzoyl peroxide (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.2.2 glycolic acid versus salicylic‐mandelic acid (long term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.3 Change in lesion counts ‐ non‐inflamed (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.3.1 glycolic acid versus salicylic‐mandelic acid (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.3.2 glycolic acid versus salicylic‐mandelic acid (medium term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.4 Change in lesion counts ‐ papules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.4.1 glycolic acid versus salicylic‐mandelic acid (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.5 Change in lesion counts ‐ pustules (number of lesions post‐intervention) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.5.1 glycolic acid versus salicylic‐mandelic acid (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13.6 Change in lesion counts ‐ total (counts or %) | 2 | Other data | No numeric data | |
| 13.6.1 parallel trials | 1 | Other data | No numeric data | |
| 13.6.2 split‐face trials | 1 | Other data | No numeric data | |
| 13.7 Change in lesion counts ‐ inflamed (counts) | 2 | Other data | No numeric data | |
| 13.7.1 parallel trials | 1 | Other data | No numeric data | |
| 13.7.2 split‐face trials | 1 | Other data | No numeric data | |
| 13.8 Change in lesion counts ‐ non‐inflamed (counts) | 2 | Other data | No numeric data | |
| 13.8.1 parallel trials | 1 | Other data | No numeric data | |
| 13.8.2 split‐face trials | 1 | Other data | No numeric data | |
| 13.9 Physicians' global evaluation of acne improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.9.1 glycolic acid versus salicylic‐mandelic acid ‐ fair to good improvement (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.9.2 glycolic acid versus salicylic‐mandelic acid ‐ fair to good improvement (medium term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.9.3 glycolic acid versus salicylic‐mandelic acid ‐ fair to good improvement (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 13.10 Physicians' global evaluation of acne improvement (%) | 1 | Other data | No numeric data | |
| 13.10.1 split‐face trials | 1 | Other data | No numeric data | |
| 13.11 Minor adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.11.1 total events ‐ gluconolactone versus benzoyl peroxide (long term) | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 13.11.2 total events ‐ glycolic acid versus salicylic ‐ mandelic acid (long term) | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.72, 4.52] |
| 13.11.3 burning or sensation ‐ glycolic acid versus salicylic ‐ mandelic acid | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.10, 2.43] |
| 13.11.4 desquamation ‐ glycolic acid versus salicylic ‐ mandelic acid | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.11, 9.60] |
| 13.11.5 dryness ‐ glycolic acid versus salicylic ‐ mandelic acid | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 13.11.6 acne flare ‐ glycolic acid versus salicylic ‐ mandelic acid | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.22, 4.63] |
Comparison 14. Topical alpha‐hydroxy acid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Withdrawal for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.1.1 gluconolactone versus placebo (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.2 Minor adverse events ‐ total events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 14.2.1 gluconolactone versus placebo (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akarsu 2012.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the efficacy and safety of the addition of 3% SA in 70% alcohol treatment to 1% CDP lotion and 5% BPO gel and compare it with the addition of only 70% alcohol to 1% CDP lotion and 5% BPO gel in the treatment of mild to moderate facial AV Design: parallel Unit of allocation: individuals Allocation: randomisation; no details of sequence generation methods Blinding: only single‐blind (assessors as following) was used Duration of trial (from recruitment to last follow‐up): not described Dropouts: one withdrawal because of change of city |
|
| Participants |
Population description: mild to moderate facial AV Setting: not described Randomised number: 50 Age (years): 18 to 28 in treatment group; 18 to 29 in control group Sex (M/F): 7/17 in treatment group; 6/19 in control group Severity of illness: mild to moderate Total lesion counts: 80.50 (72.83 to 94.84) in treatment group; and 77.00 (76.06 to 95.14) in control group; Inflammatory lesion counts: 25.50 (21.01 to 29.24) in treatment group; and 28.00 (21.64 to 29.40) in control group; Non‐inflammatory lesion counts: 60.00 (49.39 to 68.02) in treatment group; and 59.00 (50.43 to 67.33) in control group |
|
| Interventions |
Name of treatment group: SA and CDP + BPO group n = 25 Description: the addition of 3% SA in 70% alcohol treatment to 1% CDP lotion and 5% BPO gel Treatment period: 12 weeks Timing: twice‐daily (morning and evening) Name of treatment group: CDP + BPO group n = 25 Description: combination of CDP and BPO Treatment period: 12 weeks Timing: twice‐daily (morning and evening) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to receive topical treatments for AV with one of the two treatment protocols...". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment were described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This 12‐week study was designed as a single‐blind, randomised, 1:1 parallel group comparative investigation...". Comment: only single‐blind (assessors as following) was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Evaluations were performed by a blinded investigator to avoid subjective bias at baseline and after 2, 4, 8 and 12 weeks of treatment." Comment: insufficient information about method to ensure blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient voluntarily withdrew from the study after the first visit because of change of city." Comment: 4% of dropouts happened in the intervention group. Although no ITT analysis was used and imbalance rates of dropouts presented in the study, this withdrawal was unlikely to influence the effect. |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Aksakal 1997.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy and skin tolerance of metronidazole 1% cream and AZA 20% cream in the treatment of moderate to severe acne Design: parallel Unit of allocation: individuals Allocation: randomisation; no details of sequence generation methods Blinding: unclear Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: moderate to severe AV Setting: not described Randomised number: 40 Age (years): average 19.2 (range 14 to 27) Sex (M/F): 2/18 in treatment group; 14/6 in control group Severity of illness: moderate to severe acne |
|
| Interventions |
Name of treatment group: AZA n = 20 Description: AZA 20% Treatment period: 12 weeks Timing: twice‐daily Name of treatment group: metronidazole n = 20 Description: metronidazole 1% Treatment period: 12 weeks Timing: twice‐daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Authors did not report this outcome |
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Forty patients with only moderate to severe acne participated in this randomised, comparative study". Comment: no details of random methods was described |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment were described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Forty patients...participated in...", "the study was completed with 40 patients". Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient baseline data reported |
| Other bias | Low risk | No other potential bias identified |
Babayeva 2011.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the efficacy and safety of combination therapy with all‐TRA 0.05% cream plus CDP 1% lotion (all‐TRA + CDP group) in comparison with the combination of 3% alcohol‐based SA plus CDP 1% lotion (SA + CDP group) in the treatment of mild to moderate facial AV Design: parallel Unit of allocation: patients Allocation: randomised; no details of random methods were provided Blinding: single‐blinding; open label for assessors Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: mild to moderate facial AV Setting: not described Randomised number: 46 Age: 18 to 31 in treatment group; 18 to 26 in control group Sex (M/F): 5/18 in treatment group; 5/18 in control group Severity of illness: mild to moderate Total lesion counts: 66.52 ± 8.04 in treatment group; and 66.52 ± 8.04 in control group; Inflammatory lesion counts: 21.95 ± 7.18 in treatment group; and 20.65 ± 7.73 in control group; Non‐inflammatory lesion counts: 44.78 ± 6.12 in treatment group; and 45.43 ± 6.38 in control group |
|
| Interventions |
Name of treatment group: SA + CDP group n = 23 Description: combination of 3% alcohol‐based SA plus CDP 1% lotion Treatment period: 12 weeks Timing: twice‐daily Name of treatment group: all‐TRA + CDP group n = 23 Description: all‐TRA 0.05% cream plus CDP 1% lotion Treatment period: 12 week Timing: twice‐daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This 12‐week study was designed as a single‐blind, randomised, 1:1 parallel group and comparative investigation..."; Quote: "Patients were randomised to receive topical treatments for AV with one of two topical agent combinations..." Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | No detail of concealment approach was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "This 12‐week study was designed as a single‐blind, randomised, 1:1 parallel group and comparative investigation...", Comment: unclear which side was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Evaluations were performed by an investigator aware of the treatment allocation at baseline and after 2, 4, 8 and 12 weeks of treatment." Comment: outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The 12‐week treatment periods were completed by all subjects." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Bae 2013.
| Study characteristics | ||
| Methods |
Aim of study: to compare the effectiveness and side effects of SA peels and Jessner's solution peels in the treatment of acne using a split‐face model Design: within subjects, split‐face design Unit of allocation: split‐face Allocation: randomised; no details of random sequence generation methods Blinding: evaluator blinded only Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: mild to moderate acne according to the Cunliffe grading system Setting: university setting in Korea Randomised number: 13 Age: mean: 22.6; range: 20 to 28 Sex (M/F): 13/0 Severity of illness: mild to moderate Non‐inflammatory lesion counts: 18.6 ± 20.9 in one side of face versus 22.7 ± 26.2 in other side; Inflammatory lesion counts: 14.2 ± 6.0 versus 12.5 ± 7.8 |
|
| Interventions |
Name of treatment group: 30% SA n = 13 faces Description: 30% SA Treatment period: 4 weeks Timing: three times every 2 weeks Name of treatment group: Jessner's solution n = 13 faces Description: 14 g of resorcinol, 14 g of SA, 14 mL of lactic acid, and ethanol quantum satis 100 mL Treatment period: 4 weeks Timing: three times every 2 weeks |
|
| Outcomes |
Primary outcomes (see notes)
Secondary outcomes
|
|
| Notes |
Funding: not described This was a 'split‐face'. According to the protocol of this review, only summary statistics were used to conduct analysis using the generic inverse variance method and it was separate from parallel trials. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Split‐face, within‐subjects design study. No randomisation method was described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Double blinding was impossible because the two chemical peels showed different acute responses" Comment: blinding probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "the evaluator blinding method was used" Comment: insufficient information about method to ensure blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the 13 participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding subject global assessment |
| Other bias | Low risk | No other potential bias identified |
Barbareschi 1991.
| Study characteristics | ||
| Methods |
Aim of study: to investigate the activity against comedones of 20% AZA cream compared with 0.05% RA cream and placebo cream using clinical and scanning electron microscopy evaluation Design: parallel Unit of allocation: individuals Allocation: randomised; authors did not describe their sequence generation methods Blinding: unclear Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: comedonal acne Setting: not described Randomised number: 30 Age: AZA group: 15 to 27; RA group: 16 to 25; Placebo group: 15 to 28 Sex (M/F): 5/5 (AZA group); 6/4 (RA group); 3/7 (placebo group) Severity of illness: comedonal acne |
|
| Interventions |
Name of treatment group 1: 20% AZA cream n = 10 Description: 20% AZA cream (Skinoren, Schering) Treatment period: 4 months Timing: twice‐daily Name of control group 2: 0.05% RA n = 10 Description: 0.05% RA Treatment period: 4 months Timing: twice‐daily Name of control group 3: placebo cream n = 10 Description: placebo cream Treatment period: 4 months Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly allocated to three groups...". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No information of allocation concealment was described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The same observer made the clinical assessment..." Comment: insufficient detail reported about method used to ensure blinding of outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the 30 participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | High risk | "Side effects" not reported |
| Other bias | Low risk | No other potential bias identified |
Bojar 1994.
| Study characteristics | ||
| Methods |
Aim of study: This double‐blind study was carried out to assess the ability of 4% w/v erythromycin with and without 1.2% w/v zinc acetate to reduce the numbers of erythromycin‐resistant propionibacterium in vivo, and also to monitor the acquisition of resistant strains de novo during therapy Design: parallel, active‐control Unit of allocation: individuals Allocation: randomised; no further detail Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: 7 in total |
|
| Participants |
Population description: mild to moderate AV (grades 0.5 to 3.0 on the Burke and Cunliffe Scale) Setting: not described Randomised number: 52 Age (years): treatment group: 17.9 years, 13 to 27 years; control group: 20.4 years, 15 to 37 years Sex (M/F): 30/15 in both groups Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: erythromycin with zinc acetate n = 20 (number of participants completed the study, number of randomised participants was unknown) Description: topical 4% w/v erythromycin with 1.2% w/v zinc acetate, in a base consisting of 26% w/v di‐isopropyl sebacate and 57% w/v ethanol. Applied with a plain soap for skin cleansing Treatment period: 12 weeks Timing: twice daily Name of treatment group: erythromycin n = 25 (number of participants completed the study, number of randomised participants was unknown) Description: topical 4% w/v erythromycin, in a base consisting of 26% w/v di‐isopropyl sebacate and 57% w/v ethanol. Applied with a plain soap for skin cleansing Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes |
Funding Brocades Pharma for financial support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to treatment with either...". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Seven of the original 52 patients failed to attend one or more follow‐up appointments, and have been excluded from the data analysis". Comment: the author did not report which of the seven participants belonged to which group, number of missing data considered enough to introduce bias |
| Selective reporting (reporting bias) | High risk | "Side effects" not reported |
| Other bias | Low risk | No other potential bias identified |
Cavicchini 1989.
| Study characteristics | ||
| Methods |
Aim of study: to compare the activity of topical AZA with that of BPO in participants suffering from papulopustular acne Design: parallel Unit of allocation: patients Allocation: unclear Blinding: single‐blinding for participants Duration of trial (from recruitment to last follow‐up): not described Dropouts: not described |
|
| Participants |
Population description: papulopustular acne Setting: not described Randomised number: 30 Age: not described Sex: either sex Severity of illness: papulopustular acne |
|
| Interventions |
Name of treatment group: AZA group n = unclear (see notes) Description: topical 20% AZA cream Treatment period: 6 months Timing: initially nightly (firstly 2 weeks) and subsequently twice a day Name of treatment group: BPO group n = unclear (see notes) Description: 5% BPO gel Treatment period: 6 months Timing: initially nightly (firstly 2 weeks) and subsequently twice a day |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The study authors did not report the number of participants allocated to each treatment group. Funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A group of 30 patients...were randomly assigned to treatment with.." Comment: no detail of random sequence generation was provided |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote "AZA cream and BPO gel were stored in identical tubes." Comment: participants were blinded to treatment group, blinding of personnel unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Single blinding for participants was used rather than outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No reporting baseline characteristics |
| Other bias | Low risk | No other potential bias identified |
Chantalat 2005.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the efficacy of twice daily application of the novel 2% SA acne treatment compared to twice daily application of 10% BPO treatment or untreated (control) Design: parallel Unit of allocation: patients Allocation: unclear Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: not described |
|
| Participants |
Population description: mild to moderate acne Setting: not described Randomised number: unclear Age: not described Sex: either sex Severity of illness: mild to moderate acne |
|
| Interventions |
Name of treatment group: 2% SA group n = unclear (see notes) Description: novel 2% SA acne treatment Treatment period: unclear Timing: twice daily Name of treatment group: BPO group n = unclear (see notes) Description: 10% BPO gel Treatment period: unclear Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The study authors did not report the number of participants allocated to each treatment group. Funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors only reported the number of participants who completed trial but did not report total number of randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Chantalat 2006.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the clinical efficacy of a formulation containing the microgel complex with 2% SA (n = 20) versus a 10% BPO cream (n = 21) Design: parallel Unit of allocation: individuals Allocation: randomised without further details Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: no withdrawal |
|
| Participants |
Population description: mild to moderate facial AV Setting: not described Randomised number: 41 Age (years): 12 to 30 years Sex (M/F): not reported Severity of illness: mild to moderate acne |
|
| Interventions |
Name of treatment group: 2% SA n = 20 Description: a novel treatment containing a synergistic microgel complex was developed to have sebum solubilising properties, enhanced delivery of SA, and skin moisturisation and conditioning properties Treatment period: 6 weeks Timing: twice daily Name of treatment group: 10% BPO cream n = 21 Description: 10% BPO is a widely used topical agent to treat inflammatory acne Treatment period: 6 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: 100% of this poster funded by Johnson & Johnson Consumer and Personal Products Worldwide | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A 6‐week, double‐blind, randomised, controlled clinical study was conducted..." Comment: but no details of random sequence were reported |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Chantalat 2007.
| Study characteristics | ||
| Methods |
Aim of study: a second cleanser (cleanser B) containing the microgel complex with 0.5% SA was evaluated Design: double‐blind, randomised, vehicle controlled design Unit of allocation: patients Allocation: unclear Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: not described |
|
| Participants |
Population description: acne participants Setting: not described Randomised number: unclear Age: not described Sex: unclear Severity of illness: not described |
|
| Interventions |
Name of treatment group: 0.5% SA group n = unclear (see notes) Description: a cleanser containing the microgel complex with 0.5% SA Treatment period: unclear Timing: unclear Name of treatment group: vehicle group n = unclear (see notes) Description: vehicle group Treatment period: unclear Timing: unclear |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The study authors did not report the number of participants allocated to each treatment group. Funding: 100% is sponsored by Johnson & Johnson Consumer & Personal Products Worldwide |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was 'double‐blinded' and vehicle controlled, blinding probably sufficient. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was 'double‐blinded' and vehicle controlled. Insufficient information about how blinding of outcome assessor was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not report the total number of randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Chen 2007.
| Study characteristics | ||
| Methods |
Aim of study: the acne treatment benefits of this oil‐free cleanser (SA microgel complex) were evaluated Design: double‐blind, randomised, vehicle controlled design Unit of allocation: patients Allocation: unclear Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: not described |
|
| Participants |
Population description: subjects of Fitzpatrick skin types I‐V Setting: not described Randomised number: unclear Age: 12 through 35 years Sex: either sex Severity of illness: mild to moderate acne |
|
| Interventions |
Name of treatment group: SA group n = unclear Description: the cleanser containing the SA microgel complex Treatment period: unclear Timing: unclear Name of treatment group: vehicle group n = unclear Description: vehicle group (no detailed information provided) Treatment period: unclear Timing: unclear |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: supported by Neutrogena Corporation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was 'double‐blinded' and vehicle controlled, blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was 'double‐blinded' and vehicle controlled. Insufficient information about how blinding of outcome assessor was ensured |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not report total number of randomised participants. |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Cunliffe 1989.
| Study characteristics | ||
| Methods |
Aim of study: the present investigation was concerned with, for the first time, comparing AZA with placebo therapy for acne Design: parallel Unit of allocation: individuals Allocation: allocation was randomised (FORTRAN 77 RANDT program) Blinding: double‐blinding; this is a placebo control trial Duration of trial (from recruitment to last follow‐up): not described Dropouts: no |
|
| Participants |
Population description: AV Setting: not described Randomised number: 40 Age (years): unclear Sex (M/F): 26/14 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA n = 20 Description: AZA 20% Treatment period: 12 weeks Timing: twice daily Name of treatment group: placebo n = 20 Description: placebo Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was randomised (FORTRAN 77 RANDT program) |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was 'double‐blinded' and placebo‐controlled, blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was 'double‐blinded' and placebo‐controlled. Insufficient information about how blinding of outcome assessor was ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients returned for clinical assessment..." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No baseline data for each group reported. Insufficient reporting "adverse events" |
| Other bias | Low risk | No other potential bias identified |
Cunliffe 2005.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy and safety of a 1% clindamycin/zinc gel when applied to the face once daily or twice daily with a 1% clindamycin lotion applied twice daily for 16 weeks in participants with mild to moderate AV Design: parallel Unit of allocation: individuals Allocation: unclear Blinding: observer‐blind Duration of trial (from recruitment to last follow‐up): the study ran through autumn, winter and early spring Dropouts: 10/83 for clindamycin/zinc gel qd; 7/80 for clindamycin/zinc gel bid; 6/83 for clindamycin lotion bid |
|
| Participants |
Population description: mild to moderate acne Setting: 8 centres in the UK, 1 in France and 1 in Germany Randomised number: 163 Age: 12 to 40 years Sex: either sex Severity of illness: mild to moderate acne graded between 2 and 7 with at least 15 inflammatory and 10 non‐inflammatory lesions, but fewer than 75 lesions of either type |
|
| Interventions |
Name of treatment group: clindamycin/zinc gel n = 80 Description: a topical acne treatment in which CDP equivalent to 1% clindamycin ('clindamycin/zinc gel') presented in a gel formulation has received marketing authorisations in a number of EU and non‐EU countries Treatment period: 16 weeks Timing: twice daily Name of treatment group: clindamycin lotion bid n = 83 Description: 1% clindamycin lotion Treatment period: 16 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes |
Funding: this study was sponsored by Strakan Pharmaceuticals Ltd. For interventions: authors also used a 'clindamycin/zinc gel qd [three times/day]' group, in which timing was different from control group (twice/day). Therefore, reviewers did not consider it in the analysis. Regarding outcomes: all primary and secondary efficacy variables at 16 weeks had conclusions based upon an analysis of the last observation carried forward (LOCF) for the ITT population only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "An observer‐blind design was used due to the difficulty in blinding the two different topical formulations". Comment: blinding probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The investigator and assessors of all clinical variables were blinded to treatment allocation to avoid bias" Comment: insufficient information about blinding method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐three (9%) patients failed to complete the study, withdrawal rates were similar across the treatment groups..." "All primary and secondary efficacy variables at 16 weeks had conclusions based upon an analysis of the last observation carried forward (LOCF) for the intention‐to‐treat (ITT) population". Comment: ITT analysis. similar withdrawal rates across treatment groups, and missing data sufficiently addressed |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Dayal 2017.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy and safety of 30% SA versus JS peels in treatment of mild to moderate facial acne in Indian participants Design: parallel Unit of allocation: individuals Allocation: computerised randomisation was used Blinding: observer‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: no dropouts |
|
| Participants |
Population description: mild to moderate acne Setting: Department of dermatology, Pt. BD Sharma, Universitiy of Health Sciences, Rohtak, India Randomised number: 40 Age: SA: 17.8 ± 1.88; Jessner's solution: 16.8 ± 2.09 Sex (M/F): SA (12/8); Jessner's solution (14/6) Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: SA n = 20 Description: SA 30% peel Treatment period: 12 weeks Timing: peels were performed 2 weeks apart with total of six peels Name of treatment group: Jessner's solution n = 20 Description: a combination of SA (14%), resorcinol (14%), and lactic acid (14%) in 95% ethanol Treatment period: 12 weeks Timing: peels were performed 2 weeks apart with total of six peels |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients with grade I and II acne vulgaris were randomly divided into two groups of 20 each, based on computerized randomisation". Comment: computerized randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A certified dermatologist, who was kept blinded throughout the study, evaluated…” Comment: outcome assessor was kept blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Draelos 2016.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the short‐term effect of a BPO 3% gel on acne lesions Design: parallel Unit of allocation: individuals Allocation: no details of concealment was described Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: no dropouts |
|
| Participants |
Population description: mild to moderate acne Setting: single centre in USA Randomised number: 90 Age: 12 to 45 years; BPO 22.1 ± 9.90; SA 17.8 ± 6.22; vehicle 20.3 ± 7.47 Sex (M/F): BPO (12/18); SA (18/12); vehicle (13/17) Severity of illness: participants with mild to moderate acne |
|
| Interventions |
Name of treatment group: SA n = 30 Description: SA 2% gel Treatment period: four days Timing: once daily Name of treatment group: BPO n = 30 Description: BPO 3% gel Treatment period: four days Timing: once daily Name of treatment group: vehicle n = 30 Description: vehicle Treatment period: four days Timing: once daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: this study was funded by GlaxoSmithKline Consumer Healthcare Ltd; Zoe Diana Draelos MD and Dror Rom PhD received remuneration for consultancy services, and Keith Ertel PhD was an employee of GSK at the time the study was conducted. The sponsor reviewed the final manuscript before submission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " Eligible subjects were randomised to use one of three test articles…". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "A 5‐day, double‐blind, randomised clinical trial was conducted..." Comment: although 'double‐blind' was mentioned, no details were reported for its identification |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A 5‐day, double‐blind, randomised clinical trial was conducted..." Comment: although 'double‐blind' was mentioned, no details were reported for its identification |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Ninety subjects were enrolled and all completed the study". Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the study protocol. |
| Other bias | Unclear risk | Quote: "This study was funded by GlaxoSmithKline Consumer Healthcare Ltd; Zoe Diana Draelos MD and Dror Rom PhD received remuneration for consultancy services, and Keith Ertel PhD was an employee of GSK at the time the study was conducted. The sponsor reviewed the final manuscript before submission". Comment: unclear whether an important risk of bias exists |
Dunlap 1997.
| Study characteristics | ||
| Methods |
Aim of study: to compare a 3% erythromycin/5% BPO combination in gel versus 20% AZA cream in the treatment of AV Design: parallel Unit of allocation: individuals Allocation: unclear Blinding: investigator‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: not reported |
|
| Participants |
Population description: people with acne Setting: not described Randomised number: 150 Age: 13 to 30 years Sex: either sex Severity of illness: grade II or III, Pillsbury classification |
|
| Interventions |
Name of treatment group: AZA n = unclear (see notes) Description: 20% AZA cream Treatment period: 8 weeks Timing: twice daily Name of treatment group: erythromycin/BPO n = unclear (see notes) Description: 3% erythromycin/5% BPO Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Authors did not report this outcome |
|
| Notes | The study authors did not report the number of participants allocated to each group. Funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was with a "investigator‐blind" design, blinding of participants probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was with a "investigator‐blind" design, we were not sure whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants randomised to each group was not reported |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Eady 1996.
| Study characteristics | ||
| Methods |
Aim of study: not described Design: parallel Unit of allocation: patients Allocation: randomisation; no details Blinding: placebo‐control, double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: 15 |
|
| Participants |
Population description: mild‐moderate facial acne Setting: not described Randomised number: 114 Age: 16 to 25 years Sex (M/F): 86/28 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: SA group n = 56 Description: A 2% SA and 10% hydroalcoholic lotion Treatment period: 12 weeks Timing: twice daily Name of treatment group: placebo group n = 58 Description: 10% hydroalcoholic lotion Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: this study is supported by Proctor and Gamble | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The study was a stratified, randomised, double‐blind parallel‐group clinical study" Comment: but no details of random sequence were reported |
| Allocation concealment (selection bias) | Unclear risk | No details of concealments were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This was a 'double‐blind', 'placebo‐controlled' trial, binding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This was a 'double‐blind', 'placebo‐controlled' trial. Insufficient information about how blinding of outcome assessor was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 15 (13%) patients dropped out during the course of study." Comment: no ITT analysis, but number of missing data not considered to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | No baseline data for each group reported. Insufficient reporting "adverse events" |
| Other bias | Low risk | No other potential bias identified |
ElRefaei 2015.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the efficacy and the tolerability of a combination of 20% salicylic–10% mandelic acid peel against a 35% glycolic acid peel in the treatment of active AV Design: parallel Unit of allocation: individuals Allocation: sealed envelope method was used Blinding: assessor‐blind Duration of trial (from recruitment to last follow‐up): conducted from March 2012 to March 2013 Dropouts: no dropouts |
|
| Participants |
Population description: participants with facial AV Setting: Dermatology and Andrology Department of Beha University hospital, Egypt Randomised number: 40 Age: 14 to 29 years; 35% glycolic acid peel: 19.55 ± 4.19; 20% salicylic–10% mandelic acid peel: 19.8 ± 4.02 Sex (M/F): 35% glycolic acid peel (5/15); 20% salicylic–10% mandelic acid peel (3/17) Severity of illness: moderate to severe, 20 participants in 20% salicylic–10% mandelic acid peel had moderate acne compared with 19 participants with moderate acne and one with severe acne in GAP |
|
| Interventions |
Name of treatment group: glycolic acid n = 20 Description: 35% glycolic acid peel Treatment period: 12 weeks Timing: seven peeling sessions were conducted for each group every two weeks Name of treatment group: 20% salicylic–10% mandelic acid peel n = 20 Description: 20% salicylic–10% mandelic acid peel Treatment period: 12 weeks Timing: seven peeling sessions were conducted for each group every two weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " This randomised clinical trial was carried out on 40 patients who were divided randomly, by the sealed envelope method…". Comment: sealed envelope method was used |
| Allocation concealment (selection bias) | Low risk | Quote: " This randomised clinical trial was carried out on 40 patients who were divided randomly, by the sealed envelope method…". Comment: sealed envelope method was used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This was not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two uninvolved dermatologists made a subjective assessment…". Comment: blinding probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of them (32 females and eight males) completed the study." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Garg 2009.
| Study characteristics | ||
| Methods |
Aim of study: to compare the therapeutic efficacy and tolerability of 35% glycolic acid peels and 20% salicylic–10% mandelic acid peels in active acne Design: parallel Unit of allocation: individuals Allocation: no details Blinding: not described Duration of trial (from recruitment to last follow‐up): not described Dropouts: no dropouts |
|
| Participants |
Population description: Indian participants with Fitzpatrick skin types IV to VI with AV Setting: hospital in India, all participants were Indian Randomised number: 44 Age: mean: 22 ± 3.0; range 16 to 27 Sex (M/F): 11/33 Severity of illness: participants with AV and post‐acne scarring and hyperpigmentation not responding to conventional treatment for 3 or more months |
|
| Interventions |
Name of treatment group: glycolic acid peels n = 22 Description: 35% glycolic peels Treatment period: 12 weeks Timing: fortnightly intervals for six sessions Name of treatment group: 20% salicylic–10% mandelic acid peels n = 22 Description: 20% salicylic–10% mandelic acid peels Treatment period: 12 weeks Timing: fortnightly intervals for six sessions |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: " The treating physician made an objective assessment of..." Comment: blinding probably insufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 44 patients (33 women and 11 men) completed the study." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No baseline data for each group reported. Insufficient data regarding "subjective assessment of response". |
| Other bias | Low risk | No other potential bias identified |
Gollnick 2004a.
| Study characteristics | ||
| Methods |
Aim of study: to clinically test 15% AZA gel against 5% BPO in participants with mild to moderate AV Design: parallel, double‐blind, randomised, multicentre, phase III trial Unit of allocation: patients Allocation: randomisation; no details Blinding: double‐blinded study; no details Duration of trial (from recruitment to last follow‐up): 1997 to 2000 Dropouts: 5 participants in the AZA group and 10 participants in the BPO group discontinued the study due to adverse events. 38 participants in the AZA group and 43 participants in the BPO group did not complete the scheduled 4 months treatment. |
|
| Participants |
Population description: mild to moderate AV Setting: multicentres, recruitment in Germany, Netherlands, Norway, and Greece Randomised number: 351; 176 to AZA group and 175 to BPO group Age, median years (range): AZA group: 20 (13 to 45); BPO group: 19 (11 to 42) Sex, numbers male/female (% male): AZA group: 68/108 (63%); BPO group: 67/108 (62%) Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA group, N = 176 Description: 15% AZA in hydro gel (brand: Skinoren 15% gel) topical application 2 times per day for 4 months Treatment period: 4 months Timing: 2 times per day Name of control group: BPO group, N = 175 Description: 5% BPO in hydro gel (brand: Scherogel) topical application 2 times per day for 4 months Treatment period: 4 months Timing: 2 times per day |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not reported; two authors were employees of the pharmaceutical company Schering | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants continuously dropped out, which is shown in Table 2 (of the paper) for the time points 1, 2, 3, ≥ 4 months from 100% down to 75%. 15 participants dropped out because of minor local adverse events. Number of missing data considered enough to introduce bias. |
| Selective reporting (reporting bias) | High risk | Additionally reported outcome (subjective global evaluations) that were not mentioned in the Method section |
| Other bias | Unclear risk | The study reported on the statistical approach that they used non‐inferiority borders of 15%. The reporting appears not fully clear whether they describe an equivalence trial or an inferiority trial. Local adverse events were compared between Gollnick 2004a (BPO) and Gollnick 2004b (Clinda) in an indirect fashion. In Table 1, the total number of participants with acne of the face was incorrectly reported 351, but should be 251. Conflict of interest: authors are employees of a pharmaceutical company that marketed AZA |
Gollnick 2004b.
| Study characteristics | ||
| Methods |
Aim of study: to clinically test 15% AZA gel against 1% clindamycin gel in participants with mild to moderate AV Design: parallel, double‐blind, randomised, multicentre, phase III trial Unit of allocation: patients Allocation: randomisation; no details Blinding: double‐blinded study; no details Duration of trial (from recruitment to last follow‐up): 1997 to 2000 Dropouts: 5 participants in the AZA group and 1 participant in the clindamycin group discontinued the study due to adverse events. 20 participants in the AZA group and 10 participants in the clindamycin group did not complete the scheduled 4 months treatment. |
|
| Participants |
Population description: mild to moderate AV Setting: multicentres, recruitment in Germany, Netherlands, Norway, and Greece Randomised number: 229; 114 to azelaic group and 115 to clindamycin group Age, median years (range): AZA group: 19 (14 to 50); clindamycin group: 19 (13 to 38) Sex, numbers male/female (% male): AZA group: 47/67 (41%); clindamycin group: 55/60 (48%) Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA group, N = 114 Description: 15% AZA in hydro gel (brand: Skinoren 15% Gel) topical application 2 times per day for 4 months Treatment period: 4 months Timing: 2 times per day Name of control group: clindamycin group, N = 115 Description: 1% clindamycin in hydro gel (brand: Basocin Acne Gel) topical application 2 times per day for 4 months Treatment period: 4 months Timing: 2 times per day |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not reported; two authors were employees of the pharmaceutical company Schering | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants continuously dropped out, which is shown in Table 2 for the time points 1, 2, 3, ≥ 4 months from 100% down to 83%. Six participants dropped out possibly because of minor local adverse events. Number of missing data considered enough to introduce bias |
| Selective reporting (reporting bias) | High risk | Additionally reported outcome (Subjective global evaluations) that were not mentioned in the Method section |
| Other bias | Unclear risk | Minor local adverse events were compared between Gollnick 2004a and Gollnick 2004b in an indirect fashion. In study 2, the duration of therapy differed between test and control arm. An advantage was reported for AZA, although the difference was not significant. Conflict of interest: authors are employees of a pharmaceutical company that marketed AZA |
Hayashi 2012.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate AZA 20% cream efficacy in Japanese participants with AV Design: randomised placebo‐controlled investigator‐blinded split‐face study Unit of allocation: face Allocation: randomisation; no details Blinding: blinding for investigators Duration of trial (from recruitment to last follow‐up): not described Dropouts: not described |
|
| Participants |
Population description: Japanese participants with AV Setting: multicentres in Japan, all participants were Japanese Randomised number: 66 Age: ≥ 16 years old Sex: unclear Severity of illness: AV with more than 30 total lesion counts |
|
| Interventions |
Name of treatment group: 20% AZA cream n = unclear (see notes) Description: 20% AZA cream Treatment period: 12 weeks Timing: twice daily Name of treatment group: placebo n = unclear (see notes) Description: placebo group Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | The study authors did not report the number of participants allocated to each group. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was an 'investigator‐blinded', 'placebo‐controlled' study. Blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was an 'investigator‐blinded', 'placebo‐controlled' study. Insufficient information about how blinding of outcome assessor was ensured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This was not stated. |
| Selective reporting (reporting bias) | Unclear risk | Study published as poster only |
| Other bias | Low risk | No other potential bias identified |
Hunt 1992.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy and skin tolerance of a topical alpha hydroxy acid preparation gluconolactone 14% in solution, with its vehicle alone (placebo) and 5% BPO lotion in treatment of mild to moderate acne Design: parallel Unit of allocation: patients Allocation: randomisation; no details Blinding: blinding for participants, investigators and assessors Duration of trial (from recruitment to last follow‐up): not described Dropouts: 15 dropouts with reasons; 4 discontinued due to irritation of the skin |
|
| Participants |
Population description: mild to moderate acne Setting: not described Randomised number: 150 Age: 20.1 (13 to 36) years Sex: 76/74 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: alpha‐hydroxy acid group. n = 50 Description: alpha hydroxy acid preparation gluconolactone 14% in aqueous solution (formulation developed by Narhex Australia Pty Ltd) Treatment period: not described Timing: not described Name of control group 1: placebo group. n = 50 Description: the vehicle of treatment group Treatment period: not described Timing: not described Name of control group 2: BPO group. n = 50 Description: 5% BPO water‐based lotion Treatment period: not described Timing: not described |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: Narhex Australia Pty Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomised into three treatment groups..." Comment: but no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | Although "identical numbered packages" were used here, no more details for enough concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All treatments were supplied in 100 ml aliquots, pre‐packed in identical numbered packages...both doctor and patients were blinded". Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...and patients were instructed not to describe to the assessing doctor any characteristics of the product such as colour, smell or consistency" Comment: blinding probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 15 (10%) dropouts with reasons; number of missing data not considered enough to introduce bias significantly |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding "adverse events" |
| Other bias | High risk | Suspicious baseline imbalance in total lesion counts among groups (76.8 ± 7.5 in gluconolactone group; 94.7 ± 11.1 in placebo group; 76.5 ± 7.0 in BPO group); the use of a Student's t‐test with no posthoc analysis to compare the means of lesions of three treatment groups |
Ilknur 2010.
| Study characteristics | ||
| Methods |
Aim of study: to compare the therapeutic effects of glycolic acid and amino fruit acids peels in participants with AV Design: single‐blind, randomised, right ‐ left comparison study Unit of allocation: split‐face Allocation: randomisation; drawing Blinding: single blinding; assessors Duration of trial (from recruitment to last follow‐up): not described Dropouts: 6 |
|
| Participants |
Population description: 0.25 to 2 grades AV Setting: not described Randomised number: 30 Age: 18.96 (13 to 30) years Sex: 7/17 (completed data) Severity of illness: grades of 0.25 to 2, Leeds technique |
|
| Interventions |
Name of treatment group: glycolic acid n = 24 sides of faces Description: GA solution was applied (glycolic acid peels; Neostrata, Princeton, NJ, USA) at concentrations from the lowest to the highest (20%, 35%, 50%, 70%). It is a type of fruit acid. Treatment period: 6 months Timing: 2 to 6 minutes/peel; entire period is 6 months Name of treatment group: amino fruit acids gel group; n = 24 sides of faces Description: amino fruit acid gel was applied (amino fruit acid peels; exCel Cosmeceuticals, Bloomfield Hills, MI, USA) at concentrations from the lowest to the highest (20%, 30%, 40%, 50%, 60%) Treatment period: 6 months Timing: 2 to 6 minutes/peel; entire period is 6 months |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "In order to determine which therapy would be performed on which side of the face, randomisation was conducted by drawing." Comment: drawing is reliable for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study is single‐blind which was applied to the outcome investigators and method to ensure blinding was not described. Blinding of participants probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "In the clinical assessment, each side of the face was evaluated by a doctor blinded to the study..." Comment: the method to ensure blinding of outcome assessor throughout the study was not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 dropouts (20%) with reasons; no ITT analysis was used. Number of missing data considered enough to introduce bias significantly |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Iraji 2007.
| Study characteristics | ||
| Methods |
Aim of study: our objective in this study was to evaluate the efficacy of 20% AZA gel in the treatment of mild to moderate AV. Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: double‐blind; participants and investigators Duration of trial: not described Dropouts: no |
|
| Participants |
Population description: mild to moderate acne Setting: not described Randomised number: 60 Age: average 18.3 in AZA group; 16.93 in placebo group Sex: 10/20 in AZA group; 13/17 in placebo group Severity of illness: mild to moderate acne |
|
| Interventions |
Name of treatment group: AZA group; n = 30 Description: 20% AZA gel Treatment period: 6 weeks Timing: twice daily Name of treatment group: vehicle gel; n = 30 Description: composed of carbopol 934 (1%), glycerin (5%) and triethanolamine (0.2% to 0.5%) Treatment period: 6 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both physicians and patients were blinded to the type of treatment". Comment: this study was 'vehicle‐controlled', 'double‐blinded', blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both physicians and patients were blinded to the type of treatment. The same dermatologist counted the number of comedones, papules and pustules on the face at each visit." Comment: this study was 'vehicle‐controlled', 'double‐blinded', blinding probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 60 patients completed the study." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Jaffary 2016.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of pyruvic acid 50% and SA 30% peeling in the treatment of mild to moderate acne Design: parallel Unit of allocation: individuals Allocation: no details of concealment Blinding: one‐blind Duration of trial (from recruitment to last follow‐up): recruited within the second 6 months of 2010 Dropouts: SA group: 16; pyruvic acid group: 18 |
|
| Participants |
Population description: mild to moderate acne Setting: Al‑Zahra Hospital Dermatology Clinic and Isfahan Skin Research Centre, Iran Randomised number: 86 Age: 15 to 40 years; SA group: 23.05 ± 5.7; pyruvic acid group: 25.07 ± 6 Sex (M/F): SA group (4/39); pyruvic acid group (3/40) Severity of illness: participants with mild to moderate acne |
|
| Interventions |
Name of treatment group: SA n = 43 Description: SA 30% peels Treatment period: eight weeks Timing: applied every two weeks Name of treatment group: pyruvic acid n = 43 Description: pyruvic acid 50% peels Treatment period: eight weeks Timing: applied every two weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: " The patients randomly allocated in one of the two groups under study". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: " In a prospective one‑blinded clinical trial..." Comment: unclear which side was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: " In a prospective one‑blinded clinical trial..." Comment: unclear which side was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'As‐treated' analysis was used with substantial withdrawals in treatment groups. |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section. |
| Other bias | High risk | Suspicion of fraudulent data reporting (20 participants in group one completed treatment period reported in the text, but 25 reported in Figure and 27 reported in Table) |
Kar 2013.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of oral isotretinoin and oral isotretinoin with 20% SA peels in participants with moderate to severe acne Design: parallel Unit of allocation: patients Allocation: unclear Blinding: investigator‐blinded Duration of trial (from recruitment to last follow‐up): carried out between April 2012 and March 2013 Dropouts: no |
|
| Participants |
Population description: moderate to severe acne Setting: Department of Skin and VD of a Tertiary Care Hospital of Eastern India Randomised number: 60 Age: range 18 to 25 years; mean ± SD 20 ± 1.9 years in combination group; 20.6 ± 1.9 years in isotretinoin group Sex: 16/14 in combination group; 13/17 in isotretinoin group Severity of illness: moderate to severe MAS score 64.1 ± 4.4 in combination group; 63 ± 5.1 in isotretinoin group |
|
| Interventions |
Name of treatment group: salicyclic acid and isotretinoin group; n = 30 Description: 20 mg oral isotretinoin once daily along with 20% SA peels every two weeks Treatment period: 16 weeks Timing: 20 mg oral isotretinoin once daily along with 20% SA peels every two weeks Name of treatment group: Isotretinoin group; n = 30 Description: 20 mg oral isotretinoin Treatment period: 16 weeks Timing: once daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: SA peel (Vedasol ‐ 20 gel from Vedaderm Inc. Chicago) used in this study was supplied by Percos India. Isotretinoin (Cap Tretiva 20 mg) was supplied by Intas Pharmaceuticals bearing lot number S12B018 and expiry date January 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised using a random number table." Comment: random number table is reliable for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Investigator 1 (1st author) did the group allocation of the patients using a random number table. Investigator 2 (2nd author) performed the chemical peeling on patients in the second group using 20% SA." Comment: blinding of participants probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The 3rd investigator (3rd author) was blinded from the group allocation and treatment modalities. She did the MASI scoring and evaluation of all patients at all the visits." Comment: binding of investigator probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Katsambas 1989a.
| Study characteristics | ||
| Methods |
Aim of study: to test whether AZA is an active drug in acne therapy (i.e. is a 20% AZA cream clinically superior to its vehicle?) Design: parallel Unit of allocation: patients Allocation: randomisation; no details Blinding: placebo design, double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: 12 (13%) of dropouts with reasons |
|
| Participants |
Population description: papulo‐pustular acne (degree 2 or 3 of Plewig‐Kligmann) Setting: not described Randomised number: 92 Age: 19 (13 to 27) years in treatment group and 19 (14 to 34) years Sex: 17/26 in treatment group and 10/39 in control group Severity of illness: moderate inflammatory acne |
|
| Interventions |
Name of treatment group: AZA group; n = 43 Description: 20% AZA cream provided by Schering AG, West Berlin Treatment period: 3 months Timing: twice daily Name of treatment group: placebo group; n = 49 Description: cream base of AZA group Treatment period: 3 months Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Allocation to treatment with 20% azelaic acid cream or with the cream base was random." Comment: no details of randomisation were described |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "92 patients with papulo‐pustular acne were enrolled in a 3‐month, double‐blind study". Comment: this was a 'double‐blind', 'vehicle‐controlled' study, blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "92 patients with papulo‐pustular acne were enrolled in a 3‐month, double‐blind study". Comment: this was a 'double‐blind', 'vehicle‐controlled' study, insufficient information about how blinding of outcome assessor was ensured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 10% dropout rates in each group with reasons; loss to follow‐up is not balanced between groups |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Katsambas 1989b.
| Study characteristics | ||
| Methods |
Aim of study: to test how effective AZA cream is against comedonal acne (e.g. as compared with topical tretinoin) Design: parallel Unit of allocation: patients Allocation: unclear Blinding: unclear Duration of trial (from recruitment to last follow‐up): not described Dropouts: 23 withdrawals due to adverse events; 84 withdrawals (29%) without reasons |
|
| Participants |
Population description: comedonal acne Setting: multicentres, no further details Randomised number: 289 Age: 18 (12 to 38) years in treatment group and 17 (11 to 47) years Sex: 71/72 in treatment group and 66/80 in control group Severity of illness: comedonal acne |
|
| Interventions |
Name of treatment group: AZA group; n = 143 Description: 20% AZA cream Treatment period: 6 months Timing: once a day for the first 2 weeks then twice daily Name of treatment group: tretinoin group; n = 146 Description: 0.05% tretinoin cream Treatment period: 6 months Timing: once a day for the first 2 weeks then twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomly assigned to treatments..." Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 84 dropouts (29%) without reasons; all participants analysed but no imputation method reported |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Kessler 2008.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of alpha‐ and beta‐hydroxy acid chemical peels in the treatment of mild to moderately severe facial AV Design: split‐face, double‐blind, randomised, controlled study Unit of allocation: faces Allocation: randomisation; no details Blinding: double‐blind; but only assessors were blinded clearly Duration of trial (from recruitment to last follow‐up): not described Dropouts: 3 |
|
| Participants |
Population description: mild to moderately severe facial AV Setting: University School of Medicine, USA Randomised number: 20 Age: 24 (13 to 38) years Sex: 7/13 Severity of illness: mild to moderately severe |
|
| Interventions |
Name of treatment group: glycolic acid; n = 20 faces Description: 30% glycolic acid (Glyderm, Valeant Pharmaceuticals Inc. Costa Mesa, CA, formerly ICN Pharmaceuticals Inc.) Treatment period: 10 weeks Timing: every 2 weeks; 4 to 5 minutes each treatment Name of treatment group: SA; n = 20 faces Description: 30% SA (B‐LIFTx, Bradley Pharmaceuticals, Inc., Fairfield, NJ, formerly Bioglan Pharmaceuticals) Treatment period: 10 weeks Timing: every 2 weeks; 4 to 5 minutes each treatment |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Treatment sites were randomly assigned before the first treatment visit by assigning one side of the face to receive the 30% glycolic acid...". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although 'double‐blind' was mentioned, no details were reported for its identification. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded evaluator performed the quantitative and clinical assessment from baseline through the 2‐month follow‐up." Comment: blinding probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts (15%) with reasons and ITT analysis was used |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding investigator and patient assessment of acne improvement |
| Other bias | Low risk | No other potential bias identified |
Khodaeiani 2013.
| Study characteristics | ||
| Methods |
Aim of study: to compare efficacy of the topical 4% nicotinamide and 1% clindamycin gels in participants with AV Design: parallel, randomised, double‐blind clinical trial Unit of allocation: patients Allocation: unclear Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): recruited from July 2010 through July 2011 Dropouts: none |
|
| Participants |
Population description: moderate inflammatory facial AV (grade III) Setting: teaching clinic of dermatology in Iran Randomised number: 80 Age: 23.88 ± 3.67 in nicotinamide group; 23.25 ± 3.77 in clindamycin group Sex: 15/25 in nicotinamide group; 13/27 in clindamycin group Severity of illness: moderate inflammatory acne Duration of disease (years): 2.65 ± 0.98 in nicotinamide group; 2.38 ± 0.98 in clindamycin group |
|
| Interventions |
Name of treatment group: nicotinamide group; n = 40 Description: topical 4% nicotinamide carbomer as a gelling agent, water with methyl alcohol and propyl paraben, glycerin and polyethylene glycol, as well as 4% nicotinamide Treatment period: 8 weeks Timing: twice daily Name of treatment group: clindamycin group; n = 40 Description: carbomer as a gelling agent, water with methyl alcohol and propyl paraben, glycerin and polyethylene glycol, as well as 1% clindamycin; triethanolamine as an extra‐agent Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: there was no funding or financial source in support of the present work | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomised in two equal groups to receive either topical 1% clindamycin gel, or topical 4% nicotinamide gel twice daily for eight consecutive weeks." Comment: no details of randomisation method was provided |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both preparations were provided in similar 80 mg tubes marked A or B, known only to the trial coordinator and pharmacy staff." Comment: blinding probably sufficient |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Kim 1999.
| Study characteristics | ||
| Methods |
Aim of study: to compare the effectiveness of treatment and side effects in the treatment of facial acne by two agents, 70% glycolic acid and Jessner's solution Design: split‐face Unit of allocation: body part Allocation: random permuted block method was used Blinding: assessor ‐ blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: participants with acne grades of 0.25 to 2.0 (mild to moderate acne by Dr. Cunliffe's grading system) Setting: Department of Dermatology, Seoul National University Hospital (Seoul, Korea) Randomised number: 26 Age: 16 to 27 years old; median age: 19 Sex (M/F): 4/22 Severity of illness: participants with acne grades of 0.25 to 2.0 (mild to moderate acne by Dr. Cunliffe's grading system) |
|
| Interventions |
Name of treatment group: glycolic acid n = 26 Description: 70% glycolic acid peel Treatment period: six weeks Timing: the procedures were repeated 3 times every 2 weeks Name of treatment group: Jessner's solution n = 26 Description: Jessner's solution (resorcinol, SA, lactic acid in ethanol; Delasco, Council Bluffs, IA) Treatment period: six weeks Timing: the procedures were repeated 3 times every 2 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " For randomisation, we used the random permuted block method to make random allocation of two treatment methods." Comment: random permuted block method was used |
| Allocation concealment (selection bias) | Unclear risk | Quote: " For randomisation, we used the random permuted block method to make random allocation of two treatment methods." Comment: the authors did not report this issue |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "…the evaluator binding method was used for our study". Comment: insufficient information about how blinding of outcome assessor was ensured throughout the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Twenty‐six patients (22 females and 4 males) aged from 16 to 27 years old, completed the clinical trial." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Levesque 2011.
| Study characteristics | ||
| Methods |
Aim of study: the goal of this study was to compare the safety and efficacy of peels with LHA (lipohydroxy acid) and peels with SA in subjects with comedonal acne Design: split‐face Unit of allocation: body parts Allocation: sequentially numbered envelops Blinding: investigator‐blind Duration of trial (from recruitment to last follow‐up): recruited between August 2007 and January 2008 Dropouts: three |
|
| Participants |
Population description: adult subjects with at least five non‐inflammatory acne lesions on each side of the face and < 30 inflammatory lesions on the entire face Setting: Hamzavi Dermatology, USA Randomised number: 20 Age: 29.0 Sex: 1/19 Severity of illness: comedonal acne non‐inflammatory lesions per hemi‐face at Day 14 (baseline) 13.3 ± 7.7 (range 5 to 33) |
|
| Interventions |
Name of treatment group: SA peel; n = 20 sites Description: 20% or 30%, Biomedic Micropeel Plus; LRP Treatment period: 12 weeks (every other week for a total of 6 peels) Timing: every other week for a total of six peels Name of treatment group: LHA peel; n = 20 sites Description: LHA: 2‐hydroxy 5‐octanoyl benzoic acid; 5% or 10%, Biomedic LHA‐PEEL, La Roche‐Posay Pharmaceutical Laboratories (LRP, Asnieres, France) Treatment period: 12 weeks (every other week for a total of 6 peels) Timing: every other week for a total of six peels |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation list was prepared manually by the sponsor". Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were assigned to treatments with sequentially numbered envelopes". Comment: it did not describe whether "sealed" method was used |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Board‐certified dermatologists who enrolled the subjects and performed the efficacy and tolerance evaluation were kept blinded throughout the study". Comment: whether participants were blinded to treatment was unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Board‐certified dermatologists who enrolled the subjects and performed the efficacy and tolerance evaluation were kept blinded throughout the study". Comment: blinding probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three dropouts with reasons, missing data have been imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
NilFroushzadeh 2009.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of combination treatment of clindamycin + SA, versus clindamycin + tretinoin versus Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: single‐blind Duration of trial (from recruitment to last follow‐up): recruited from September 06 to August 07 Dropouts: none |
|
| Participants |
Population description: mild‐to moderate AV Setting: Skin Disease and Leishmaniasis Research Center and Isfahan University of Medical Sciences clinics, Iran Randomised number: 42 Age: 15 to 25 years Sex: (M/F): 0/42 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: 1% clindamycin + 2% SA lotion; n = 14 Description: 1% clindamycin + 2% SA lotion Treatment period: 12 weeks Timing: twice daily Name of control group 1: 1% clindamycin + 0.025% tretinoin; n = 14 Description: 1% clindamycin + 0.025% tretinoin Treatment period: 12 weeks Timing: once nightly Name of control group 2: 1% clindamycin lotion; n = 14 Description: 1% clindamycin Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was a single‐blinded clinical trial, no details of randomisation methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were blinded to the type of treatment". Comment: It did not describe how "blinding" method was used. This study was 'single‐blinded', blinding of personnel probably insufficient |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was a 'single‐blinded' (patient‐blinded) trial, blinding of investigator probably insufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the study." Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No baseline data for each group reported |
| Other bias | Low risk | No other potential bias identified |
Ozkan 2000.
| Study characteristics | ||
| Methods |
Aim of study: we investigated the emergence of resistant CNS after 8 weeks of topical therapy with AZA and CDP, and we compared their clinical efficacy Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: no details Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: participants with acne Setting: Hospital of Medical Faculty of Osmangazi University, Turkey Randomised number: 40 Age: 20.85 ± 3.0 in AZA group; 21.75±2.6 in clindamycin group Sex (M/F): 5/15 in AZA group; 6/14 in clindamycin group Severity of illness: having an acne grade ≤ 3.0 according to the Leeds' acne assessment technique |
|
| Interventions |
Name of treatment group: azelaic acid group; n = 20 Description: AZA Treatment period: 8 weeks Timing: twice daily Name of treatment group: clindamycin; n = 20 Description: CDP Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was designed as a randomised and controlled trial". Comment: no details of random methods were described |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Pazoki‐Toroudi 2010.
| Study characteristics | ||
| Methods |
Aim of study: we evaluated the effects of a combination of AA 5% and erythromycin 2% (AzE) on mild to moderate AV Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): recruited between March 2008 and February 2009 Dropouts: 21 dropouts |
|
| Participants |
Population description: mild to moderate facial AV Setting: three dermatology clinics in Tehran, Iran Randomised number: 147 Age: placebo group, AA 20% group, erythromycin 2% group and the AzE group was 20.75 ±1.83, 19.24 ± 2.45, 22.1 ± 1.89 and 20.33 ± 2.43 Sex (M/F): 86/61 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA; n = 35 Description: 20% AA Treatment period: 12 weeks. Timing: twice daily Name of treatment group: Erythromycin; n = 31 Description: erythromycin 2% Treatment period: 12 weeks Timing: twice daily Name of treatment group: AZA + erythromycin (AzE); n = 40 Description: AZA 5% + erythromycin 2% Treatment period: 12 weeks Timing: twice daily Name of treatment group: placebo; n = 20 Description: hydroxypropyl cellulose, propylene glycol, ethyl alcohol, and deionised water Treatment period: for 4 weeks and then returned to routine treatment determined by the dermatologist Timing: unclear |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to four treatment groups". Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Both the patients and their dermatologists blinded about the type of treatment". Comment: it did not describe how "blinding" method was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Both the patients and their dermatologists blinded about the type of treatment". Comment: it did not describe how "blinding" method was used. Blinding of outcome assessor unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 21 (14%) dropouts. No ITT analysis. Sufficient reporting of attrition and the number of missing outcome data not considered enough to introduce bias |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Pazoki‐Toroudi 2011.
| Study characteristics | ||
| Methods |
Aim of study: we evaluated the effect of a combination of AA 5% and clindamycin 2% (AA‐Clin) on mild to moderate AV Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): recruited from April 2009 to November 2009 Dropouts: 6 participants did not refer to the centre in week 8 (3 from AA 5%, 1 from clindamycin 2%, and 2 from AA‐Clin group), and 18 participants did not refer to the centre in week 12 (5 from AA 5%, 7 from clindamycin 2%, and 6 from AA‐Clin group). For participants who did not refer to the centre at weeks 8 or 12, data for patient's satisfaction were collected from them by calling or inviting for a final evaluation. Two of these participants did not refer to the centre because of the lack of effect (AA 5% group), and the rest of them for other reasons. |
|
| Participants |
Population description: mild to‐moderate facial AV Setting: three clinics in Tehran, Iran Randomised number: 150 Age: clindamycin 2%, AA 5%, and AA‐Clin: 23.39 ± 2.69, 22.48 ± 2.50, and 22.1 ± 1.89 Sex (M/F): unclear Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA‐clindamycin gel (AA‐Clin); n = 50 Description: AZA 5% and clindamycin 2% Treatment period: 12 weeks Timing: twice daily Name of control group 1: topical AZA; n = 50 Description: AZA 5% Treatment period: 12 weeks Timing: twice daily Name of control group 2: topical Clin; n = 50 Description: Clin 2% Treatment period: 12 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes |
Funding: all parts of present work was funded by Tehran University of medical Sciences. Comparison of AZA versus Clin was analysed in 'Topical AZA versus any topical treatment'; comparison of AZA‐clindamycin gel (AZA‐Clin) versus clindamycin was presented in 'Topical AZA versus no treatment' |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were assigned randomly to one of the three treatment groups". Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Both patients and their dermatologists were blinded regarding the type of treatment". Comment: no details of blinding methods were provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Both patients and their dermatologists were blinded regarding the type of treatment". Comment: no details of blinding methods were provided. Blinding of outcome assessor unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used. Missing data have been imputed using appropriate methods. |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Picosse 2015.
| Study characteristics | ||
| Methods |
Aim of study: evaluate the efficacy of AZA 15% gel for maintenance treatment for 1 year after oral isotretinoin Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: unclear Duration of trial (from recruitment to last follow‐up): not described Dropouts: 7 in AZA group; 12 in control group |
|
| Participants |
Population description: participants with AV who were about to complete the treatment of isotretinoin Setting: not described Randomised number: 50 Age: 14 to 35 (mean 20.2) Sex: not reported Severity of illness: not described |
|
| Interventions |
Name of treatment group: AZA; n = 25 Description: AZA 15% gel Treatment period: 12 months Timing: twice daily Name of control group: control group; n = 25 Description: control group Treatment period: 12 months Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient reporting of attrition, and the number of missing data considered enough to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Schaller 2016.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy, tolerability and safety of a combination of BPO 3% and clindamycin 1% (BPO + CLN) with AZA 20% for the topical treatment of mild to moderate AV Design: parallel Unit of allocation: individuals Allocation: computer‐generated schedule was used Blinding: assessor‐blind Duration of trial (from recruitment to last follow‐up): recruited between 21 February 2014 and 2 June 2014 Dropouts: 7 in AZA group; 4 in BPO + CLN group |
|
| Participants |
Population description: mild to moderate acne Setting: 11 study centres in Germany Randomised number: 221 Age: 12 to 45 years; AZA 20.0 ± 6.9; BPO + CLN 20.1 ± 7.1 Sex (M/F): AZA (51/58); BPO + CLN (47/61) Severity of illness: participants with mild to moderate acne |
|
| Interventions |
Name of treatment group: AZA n = 109 Description: AZA 20% cream Treatment period: 12 weeks Timing: twice daily Name of treatment group: BPO + clindamycin n = 30 Description: BPO 3% + clindamycin 1% gel Treatment period: 12 weeks Timing: once daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: the study (GSK200398) was funded by Stiefel, a GSK company. MSch has been a member of the advisory board of Bayer Healthcare and Galderma for the past 2 years and has received lecture fees from AbbVie, Bayer Healthcare, Galderma and La Roche‐Posay. MSeb has been an advisor or investigator for AbbVie, Novartis, Janssen‐Cilag, Lilly, Amgen, Celgene, Galderma, Leo Pharma, GSK, and Pfizer. Three authors are employees of GSK and hold stocks/shares in GSK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation (1: 1) to treatments was performed using a computer‐generated schedule". Comment: computer‐generated schedule method was described |
| Allocation concealment (selection bias) | Unclear risk | We judged an unclear risk of bias because the authors did not report this issue. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients, site staff responsible for distribution and drug accountability and individuals involved in the conduct, analysis and reporting of clinical study data were not blinded to treatment assignment." Comment: we judged a high risk of bias because the authors reported that participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Acne lesion assessors were blinded to treatment. Patients were instructed not to use the study treatments in the presence of the acne assessor". Comment: blinding of outcome assessor probably sufficient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The drop‐out rate was low, with only 15 out of 221 (6.8%) patients leaving the study post‐randomisation". Comment: we judged a low risk of bias because the attrition rate was low and similar in both treatment arms. And because the authors performed an ITT analysis including the data of all but four of the randomised participants. |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the study protocol |
| Other bias | Unclear risk | Comment: three authors are employees of GSK and hold stocks/shares in GSK. Unclear whether an important risk of bias exists |
Shahmoradi 2013.
| Study characteristics | ||
| Methods |
Aim of study: this randomised clinical trial evaluated the efficacy of 5% nicotinamide gel versus 2% clindamycin gel in the treatment of mild‐moderate AV Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): performed 2009 to 2010 Dropouts: none |
|
| Participants |
Population description: mild or moderate AV Setting: St‐Alzahra hospital, Isfahan University of Medical Sciences, Isfahan, Iran Randomised number: 60 Age: nicotinamide gel and clindamycin gel: 20.83 ± 3.34 years and 21.17 ± 3.53 years Sex (M/F): 0/60 Severity of illness: mild or moderate |
|
| Interventions |
Name of treatment group: 5% nicotinamide; n = 30 Description: 5% nicotinamide gel provided by Isfahan Pharmacy School in the same containers Treatment period: 8 weeks Timing: twice daily Name of treatment group: 2% clindamycin; n = 30 Description: 2% clindamycin provided by Isfahan Pharmacy School in the same containers Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This was a randomised controlled clinical trial. No details of randomisation methods were provided. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Both physicians and patients were blinded to the type of treatment" Comment: the study author did not report blinding method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No one was excluded from the study and all of the participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Shalita 1981.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the treatment of mild and moderate AV with SA in an alcohol‐detergent vehicle Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: not described Duration of trial (from recruitment to last follow‐up): conducted from early March to 6 June 1980 Dropouts: none |
|
| Participants |
Population description: mild to moderate acne Setting: not described Randomised number: 49 Age: 12 to 20 Sex (M/F): 17/32 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: SA; n = 25 Description: 0.5 % SA in an alcoholic detergent solution (Stri‐Dex Medicated pads) Treatment period: 12 weeks Timing: not reported Name of treatment group: placebo; n = 24 Description: pads soaked in buffered water Treatment period: 12 weeks Timing: not reported |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: none known | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The test products were randomised and coded by the sponsor of the study". Comment: no details of random methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It reported all the 45 participants' results, no missing outcome data |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Shalita 1989.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of a widely used BPO wash with a product incorporating 2% SA in a detergent‐based vehicle system Design: cross‐over Unit of allocation: individuals Allocation: randomisation; no details Blinding: not described Duration of trial (from recruitment to last follow‐up): not described Dropouts: none |
|
| Participants |
Population description: mild to moderate acne Setting: not mentioned Randomised number: 30 Age: 13 to 31 years Sex (M/F): 15/15 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: SA; n = 15 Description: SA 2% Treatment period: 2 weeks Timing: once or twice daily Name of treatment group: BPO; n = 15 Description: 10% BPO wash Treatment period: 2 weeks Timing: once or twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: this research was supported by a grant from GenDerm Corporation, Northbrook, Illinois | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of random sequence generation were described |
| Allocation concealment (selection bias) | Unclear risk | No details of allocation concealment were described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed study, no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting data regarding change in inflammatory lesions |
| Other bias | High risk | No washout period between the first and second phases of the study |
Shalita 1995.
| Study characteristics | ||
| Methods |
Aim of study: to determine the efficacy and safety of topically applied 4% nicotinamide gel compared to 1% clindamycin gel, in treating inflammatory AV Design: parallel, active‐control Unit of allocation: individuals Allocation: randomisation; no details Blinding: double‐blinding Duration of trial (from recruitment to last follow‐up): not described Dropouts: 9 in nicotinamide group; 8 in clindamycin group |
|
| Participants |
Population description: moderate inflammatory AV Setting: multicentres in USA Randomised number: 76 Age: 13 to 35 years (mean age 21.3 years) Sex (M/F): 23/53 Severity of illness: moderate inflammatory acne; defined by the presence of at least 15 papules and/or pustules on the face |
|
| Interventions |
Name of treatment group: 4% nicotinamide gel; n = 38 Description: 4% nicotinamide gel Treatment period: 8 weeks Timing: twice daily Name of treatment group: 1% clindamycin gel; n = 38 Description: 1% clindamycin gel Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: supported in part by Genderm Corporation, Lincolnshire, IL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who met...in a double‐blind, randomised manner" Comment: but no details of random sequence generation were described |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients who met...in a double‐blind, randomised manner" Comment: but no details of blinding method were described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Patients who met...in a double‐blind, randomised manner" Comment: but no details of blinding method were described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Reasons for premature withdrawal included adverse experience (nicotinamide 2), lost to follow‐up (nicotinamide 7, clindamycin 5), non‐medical reasons (clindamycin 5) or condition unchanged/worsened from baseline (clindamycin 1)". Comment: no ITT analysis with substantial withdrawals, number of missing data considered enough to introduce bias |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the methods section |
| Other bias | Low risk | No other potential bias identified |
Sharquie 2008.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the effectiveness of 2% tea lotion in comparison with 5% zinc sulphate solution in the treatment of AV Design: parallel Unit of allocation: individuals Allocation: randomisation; no details Blinding: single‐blind Duration of trial (from recruitment to last follow‐up): conducted from June 2006 to December 2007 Dropouts: 7 |
|
| Participants |
Population description: mild to moderate facial AV Setting: Department of Dermatology and Venereology, Baghdad Teaching Hospital, Baghdad, Iraq Randomised number: 47 Age (years): both groups 13 to 27 years, mean ± SD 19.5 ± 3.5 years Sex (M/F): 14/33 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: zinc sulphate; n = 23 Description: 5% zinc sulphate solution was prepared by dissolving 5 grams of zinc sulphate crystals in 95 mL of distilled water preservative Treatment period: 8 weeks Timing: twice daily Name of treatment group: tea; n = 24 Description: the tea leaves (Apple brand mark) were extracted with distilled water (35 gm of tea was mixed with 100 mL boiling hot distilled water for 30 min), then we allowed the tea extract to cool down, and took 100 mL of tea extract and 100 mL of distilled water, and it was weighed. The 2% tea lotion (100 mL) was prepared by adding 75 mL of the tea extract to 25 mL of ethanol, which was used as a preservative. Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were divided randomly into 2 groups. Group A: 24 participants were treated with 2% tea lotion. Group B used 5% zinc sulphate solution. No details of randomisation methods were provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This is a 'single‐blind' trial. Unclear which side was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is a 'single‐blind' trial. Unclear which side was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 participants completed the course of treatment. No ITT analysis, but per protocol analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding change in inflamed lesion counts |
| Other bias | Low risk | No other potential bias identified |
Stinco 2007.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the sebostatic effect of three anti‐acneic ingredients AZA, adapalene and BPO) conveyed in cream and to determine whether there is a correlation with the therapeutic results Design: parallel Unit of allocation: individuals Allocation: randomisation; not reported Blinding: not reported Duration of trial (from recruitment to last follow‐up): not described Dropouts: four |
|
| Participants |
Population description: mild or moderate comedonal or papulopustular acne Setting: not described Randomised number: 65 Age: 12 to 24 years Sex: 35/50 Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: AZA; n = 25 Description: not reported Treatment period: not reported Timing: once daily Name of treatment group: BPO; n = 20 Description: not reported Treatment period: not reported Timing: once daily Name of treatment group: adapalene; n = 20 Description: not reported Treatment period: not reported Timing: once daily Name of treatment group: volunteers; n = 20 Description: the same mild detergent Treatment period: not reported Timing: once daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes |
Funding: not reported Data from the volunteers group was excluded from the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated to one of three groups of treatment". Comment: no details of randomisation methods were provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sixty‐one participants and 16 volunteers completed the study, reasons for attrition reported, the dropouts not considered enough to introduce bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding change in non‐inflamed and inflamed lesion counts |
| Other bias | Low risk | No other potential bias identified |
Techapichetvanich 2011.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate efficacy and tolerability of a combination of serial SA peels and topical standard regimen consisting of 5% BPO and 1% clindamycin lotion comparing with topical regimen alone in the treatment of mild to moderately severe facial AV Design: parallel, randomised, placebo controlled study Unit of allocation: individuals Allocation: randomisation; not reported Blinding: double‐blind Duration of trial (from recruitment to last follow‐up): not described Dropouts: unclear |
|
| Participants |
Population description: participants with mild or moderate acne Setting: not described Randomised number: 37 Age: not described Sex: not described Severity of illness: mild to moderate |
|
| Interventions |
Name of treatment group: SA group; n = unclear Description: 20% or 30% SA peels Treatment period: six weeks Timing: once a week Name of treatment group: vehicle group; n = unclear Description: vehicle group Treatment period: six weeks Timing: once a week |
|
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is a 'double‐blind', 'placebo‐controlled' trial, blinding probably sufficient. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is a 'double‐blind', 'placebo‐controlled' trial, insufficient information about how blinding of assessor was ensured throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This was not stated |
| Selective reporting (reporting bias) | Unclear risk | Study published as abstract only |
| Other bias | Low risk | No other potential bias identified |
Thielitz 2015.
| Study characteristics | ||
| Methods |
Aim of study: to evaluate the efficacy of AZA 15% gel versus no treatment during maintenance therapy of female adult acne and to compare its efficacy and safety versus adapalene 0.1% gel (AD) during a 9‐month period (3‐month treatment and 6‐month maintenance treatment) Design: parallel Unit of allocation: individuals Allocation: randomisation; not reported Blinding: investigator‐blind Duration of trial (from recruitment to last follow‐up): study period was between August 2011 and October 2012 Dropouts at the end of treatment phase: AZA9M (AZA gel twice/day for 9 months): n = 3; AZA3M (AZA gel twice/day for 3 months followed by a 6‐month observational phase): n = 2; AD9M (adapalene 0.1% gel once daily for 9 months): n = 1 |
|
| Participants |
Population description: adult female participants with mild to moderate acne Setting: industry‐sponsored single‐site study in university, Germany Randomised number: 55 Age: AZA9M: 30.58 ± 9.28; AZA3M: 28.14 ± 4.56; AD9M: 28.94 ± 6.71 Sex: all subjects are females of European origin Severity of illness: mild to moderate acne |
|
| Interventions |
Name of treatment group: AZA9M; n = 17 Description: AZA 15% gel twice daily for 9 months Treatment period: 36 weeks Timing: twice daily Name of treatment group: AZA3M; n = 19 Description: AZA gel for three months followed by a six‐month observational phase Treatment period: 12 weeks Timing: twice daily Name of treatment group: AD9M; n = 19 Description: adapalene 0.1% gel once daily for nine months Treatment period: 36 weeks Timing: once daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: Intendis GmbH, Max‐Dohrn‐Str. 10, 10589 Berlin, Germany. This was an investigator‐initiated trial. The funder was not involved in the development of the study protocol, the data collection or analysis and the preparation of the manuscript. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The three arms were randomised in the ratio 1 : 1: 1, using the minimization method of Pocock and Simon and a stratification for age (18–29 years; 30–45 years) and severity classification at study entry..." Comment: minimisation method is reliable for random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The study materials were dispensed by a designated person other than the investigator and the subjects were instructed not to discuss the study materials, treatment schedule and potential side‐effects with the investigator". Comment: the subjects are not blinded and the investigators seemed blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The study materials were dispensed by a designated person other than the investigator and the subjects were instructed not to discuss the study materials, treatment schedule and potential side‐effects with the investigator". Comment: unclear whether outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The author performed both ITT and per‐protocol analysis, reasons for attrition reported |
| Selective reporting (reporting bias) | Low risk | Results reported for all prespecified outcomes in the study protocol |
| Other bias | Low risk | No other potential bias identified |
Vasarinsh 1969.
| Study characteristics | ||
| Methods |
Aim of study: in order to avoid a number of the pitfalls that detract from the value of previous published reports, the present controlled study utilised a variety of criteria, in an attempt to assess several major aspects of a multifaceted condition Design: parallel Unit of allocation: individuals Allocation: randomisation; not reported Blinding: double‐blind, no details Duration of trial (from recruitment to last follow‐up): conducted from September 1966 to May 1967 Dropouts: 18 dropouts |
|
| Participants |
Population description: consecutive participants applying for acne therapy Setting: Wayne State University Health Service, USA Randomised number: 72 Age: not reported Sex (M/F): 30/42 Severity of illness: not reported |
|
| Interventions |
Name of treatment group: sulfur + BPO; n = 19 Description: sulfur2% and BPO 5% Treatment period: minimum four weeks, maximum 14 weeks, average 6.2 weeks Timing: overnight or twice daily Name of treatment group: BPO; n = 16 Description: 5% BPO Treatment period: minimum four weeks, maximum 14 weeks, average 6.2 weeks Timing: overnight or twice daily Name of treatment group: sulfur; n = 18 Description: 2% sulfur Treatment period: minimum four weeks, maximum 14 weeks, average 6.2 weeks Timing: overnight or twice daily Name of treatment group: placebo; n = 19 Description: not reported Treatment period: minimum four weeks, maximum 14 weeks, average 6.2 weeks Timing: overnight or twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes |
Funding: supported in part by Research Grant AM‐07194 and training Grant AM‐05267‐08 from the National Institutes of Health, U.S Public Health Service, and The Detroit General Hospital Research Corporation. In this review, two comparisons were included: sulfur + BPO versus BPO and Sulfur versus BPO. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Medications were assigned in a double‐blind randomised manner with..." Comment: but the authors did not mention details on randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Medications were assigned in a double‐blind randomised manner with..." Comment: but no details on how and who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Medications were assigned in a double‐blind randomised manner with..." Comment: but no details on how and who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 72 participants were accepted for study and 54 participants completed the required observation period. No ITT analysis performed, the number of missing outcome data considered enough to introduce bias significantly |
| Selective reporting (reporting bias) | Unclear risk | Insufficient reporting about baseline data |
| Other bias | Low risk | No other potential bias identified |
Weltert 2004.
| Study characteristics | ||
| Methods |
Aim of study: to compare the efficacy of nicotinamide to a reference comparative product in the local treatment of moderate acne with a predominant inflammatory component, i.e. erythromycin Design: parallel, active control Unit of allocation: individuals Allocation: randomisation; no details Blinding: unclear Duration of trial (from recruitment to last follow‐up): not described Dropouts: 7 in treatment; 5 in control |
|
| Participants |
Population description: acne with inflammatory predominance Setting: Laboratoire Dermscan (Villeurbanne), France, all subjects were Caucasian. Randomised number: 158 Age: 19.0 +/‐ 2.7 in treatment; 19.3 +/‐ 2.9 in control Sex (M/F): 29/50 in treatment; 29/50 in control Severity of illness: moderate inflammatory acne on face (≥ 5 inflammatory elements, papules or pustules) |
|
| Interventions |
Name of treatment group: 4% nicotinamide gel; n = 79 Description: product: Exfoliac NC Gel (Merck Medication Familiale, France) Galenic form: gel Formula: active ingredient: niacinamide 4% Excipients: aqua, alcohol denat., laureth‐12, magnesium aluminium silicate, hydroxypropyl methyl cellulose, citric acid Treatment period: 8 weeks Timing: twice daily Name of treatment group: erythromycin; n = 79 Description: erythromycin, titre 1000 UI/mg Galenic form: liquid gel for local application Formula: active ingredient: erythromycin 4% Excipients: 96% ethyl alcohol, hydroxypropyl cellulose Treatment period: 8 weeks Timing: twice daily |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes that were not analysed in this review
|
|
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although 'randomised' was mentioned, no details were reported for random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 and 5 dropouts in treatment and control group. No imputation method reported, but number of missing data not considered as likely to introduce bias significantly |
| Selective reporting (reporting bias) | Unclear risk | Insufficient data regarding "Safety" assessed through clinical scoring |
| Other bias | Low risk | No other potential bias identified |
all‐TRA: all‐trans retinoic acid AQOL: Acne quality of life AV: acne vulgaris AZA: azelaic acid BPO: benzoyl peroxide CDP: clindamycin phosphate CLN: clindamycin ITT: intention‐to‐treat MAS: Michaelsson acne severity RA: retinoic acid SA: salicylic acid SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel 2015 | It was reported that "All participants underwent facial chemical peeling: 25% TCA on the right half of their face and 30% salicylic acid on the left half every 2 weeks for 2 months." The whole randomisation remains doubtful: individuals were not randomised because all persons received the same treatment. The side of the face was not randomised because all persons received the test intervention on the right side and the control intervention on the left side. Finally, there was no obvious and no described randomisation. |
| Anonymous 1996 | This report was not a RCT (summary review). |
| Barak‐Shinar 2017 | Comparison is salicylic acid + botanicals plus soap versus soap. Botanicals are only given in the treatment group; hence, we cannot determine the efficacy of salicylic acid in the comparison. |
| Barkovic 2012 | The study was published as an abstract. No randomisation was reported or implied. The study author could not be contacted to obtain clarification. |
| Bissonnette 2009 | The type of intervention (lipo hydroxy acid) was ineligible for inclusion. |
| Breno 2002 | The type of intervention (lipo hydroxy acid) was not eligible for inclusion. |
| Capitanio 2012 | The type of interventions was not eligible for inclusion (zinc‐oligosaccharide versus vehicle control). |
| Chantalat 2006a | No randomisation was reported or implied and the study author could not be contacted to obtain clarification. |
| Chassard 2006 | This was a pharmacokinetic study. |
| Chu 1997 | The type of interventions was not eligible for inclusion. |
| Cochran 1985 | The study author could not be contacted and we were unable to obtain clarification. No wording that might connected to a RCT could be found (no randomisation, no concealment, no "accidental" assignment, no generation of randomisation numbers, no central management of allocation). |
| Coret 2006 | The study author could not be contacted and we were unable to obtain clarification. No randomisation was reported or implied. |
| Cotterill 1980 | This report was not a clinical trial (summary review). |
| Cunliffe 1992 | This report was not a RCT (summary review). |
| Danto 1966 | The type of interventions were not eligible for inclusion (5% sulfur‐10% benzoyl versus 5% sulphur) and sulphur was the concomitant topical medications for acne vulgaris. |
| De Bersaques 1972 | The type of interventions (topical vitamin A acid) was not eligible for inclusion. |
| Dos 2003 | The study author could not be contacted and we were unable to obtain clarification. No wording that might connected to a RCT could be found (no randomisation, no concealment, no "accidental" assignment, no generation of randomisation numbers, no central management of allocation). |
| Draelos 2006 | There were two studies in this report (Japanese and USA study) and the participants in the two studies were healthy subjects. |
| Elstein 1981 | The type of intervention (sulfurated lime) was ineligible for inclusion. |
| Fang 2001 | Not a RCT |
| Fu 2003 | The type of intervention (lipohydroxyacid) was ineligible for inclusion. |
| Gebicki 2003 | The type of intervention (1‐methylnicotinamide) was ineligible for inclusion. |
| Gollnick 1989 | This report was published as a summary review. |
| Gollnick 1997 | This was a non‐systematic review (this report was published in German and Frank Peinemann provided the information). |
| Green 2013 | The type of interventions (MaxClarity, Proactiv, Murad) were not eligible for inclusion. |
| Gupta 2004 | This report was published as a review. |
| Habbema 1989 | The type of interventions was not eligible for inclusion. |
| Hjorth 1989 | The report published two studies. The type of comparisons in the two studies were not eligible for inclusion (20% azelaic acid cream versus oral tetracycline). |
| Khodaeinai 2014 | The type of interventions was not eligible for inclusion (10% azelaic acid gel with hydro‐alcoholic base versus alcohol‐free base). |
| Kirton 1967 | The study design was not eligible for inclusion (non‐randomised). |
| Kreysel 1967 | The type of interventions (aknichthol versus aknichthol dexa) were not eligible for inclusion. (This report was published in German and Frank Peinemann provided the information). |
| Lee 2003 | The study design was not eligible for inclusion (not RCT). |
| Leyden 1997 | The participants were not eligible for inclusion (healthy subjects). |
| Linss 1981 | The type of interventions (oral medications) were not eligible for inclusion. (This report was published in German and Frank Peinemann provided the information). |
| MacDonald 1976 | The type of intervention (actinac) was not eligible for inclusion. |
| Miller 2005 | The study was published as an abstract. No randomisation was reported or implied and the study author could not be contacted. |
| NCT00848744 | The trial compared formulations from the same treatment. |
| Norris 1987 | This study was published as a summary of a poster. No randomisation was reported or implied and the study author could not be contacted. |
| Pastuszka 2012 | This paper was published as a review. |
| Pereira 1994 | The study was not described as randomised. All included patients received the same treatment/intervention: salicylic acid and sulphur lotion for topical use. (This study was published in Portuguese and Carolina Freitas provided the information). |
| Pierard‐Franchimont 1995 | The participants were not eligible for inclusion (healthy subjects). |
| Plewig 1969 | The type of interventions (vitamin A acid versus sulphur‐resorcinol versus benzoyl peroxide) were not eligible for inclusion. |
| Rougier 2002 | The type of intervention (lipohydroxyacid) was ineligible for inclusion. |
| Sardesai 2003 | The study author could not be contacted and we were unable to obtain clarification. No wording that might connect to a RCT could be found (no randomisation, no concealment, no "accidental" assignment, no generation of randomisation numbers, no central management of allocation). |
| Schachner 1990 | The type of interventions were not eligible for inclusion (erythromycin‐zinc versus vehicle). |
| Shemer 2002 | All participants received same intervention (non‐randomised). |
| Souza 2005 | The participants were not eligible for inclusion as patients with rosacea were included. |
| Tarimci 1997 | The study design was not eligible for inclusion (non‐randomised). |
| Thomas 1951 | The study design was not eligible for inclusion (not RCT). |
| Touitou 2008 | The type of interventions was not eligible for inclusion (clindamycin‐salicylic acid versus placebo). |
| van Steenbergen 1968 | The study author could not be contacted and we were unable to obtain clarification. There was no wording reported that might be connected to a RCT. No randomisation, no concealment, no "accidental" assignment, no generation of randomisation numbers, no central management of allocation were founded. (This study was published in German and Frank Peinemann provided the information). |
| Wang 1997 | The study was not randomised due to the patients being divided according to the degree of greasiness of their facial skin. |
| Wilkinson 1966 | The study design was not eligible for inclusion (not RCT). |
| Wilson 2007 | The type of intervention was ineligible for inclusion. |
| Woodruff 2013 | The study was published as an abstract. No randomisation was reported or implied and the study author could not be contacted. |
RCT: randomised controlled trial TCA: trichloroacetic acid
Characteristics of studies awaiting classification [ordered by study ID]
Bartosova 1978.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: 5% benzoyl peroxide B: 3% salicylic acid C: 5% resorcin |
| Outcomes |
|
| Notes | We had no access to this full report. |
Cavicchini 1989a.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | A: 20% azelaic acid B: unknown |
| Outcomes |
|
| Notes | We had no access to this full report. |
Draelos 2015.
| Methods | Randomised trial |
| Participants | 80 female/male subjects 12+ years with mild to moderate acne (at least 10 inflammatory and 10 non‐inflammatory lesions) |
| Interventions | A: unclear B: benzoyl peroxide |
| Outcomes |
|
| Notes | We had no access to this full report. |
Giannotti 1989.
| Methods | Unknown, this report had two studies |
| Participants | Participants with moderate inflammatory acne; participants with comedo acne |
| Interventions | A: 20% azelaic acid cream B: vehicle C: 0.05% tretinoin cream |
| Outcomes |
|
| Notes | We had no access to this full report. |
IRCT201010094269N3.
| Methods | This is a double‐blinded randomised clinical trial |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions | Intervention group: combination of erythromycin 4 g and salicylic acid in 10 cc propylene glycol and alcohol 70 degrees to an overall volume of 100 cc applied to the face with cotton applicator twice a day for 3 months. Control group: combination of erythromycin 4 g in 10 cc propylene glycol and alcohol 70 degrees to an overall volume of 100 cc applied to the face with cotton applicator twice a day for 3 months. Total number of participants enrolled: 50 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
|
| Notes | Study completed, no results. The email we sent to the study authors had been rejected for unknown reason. |
Kern 2019.
| Methods | Unknown, this report had two studies. First study ‐ randomised split‐face design |
| Participants | First study: 40 subjects, ages 16 to 25 Second study: 30 subjects, ages 18 to 45 |
| Interventions | First study: cleansing device and salicylic acid cleaner Second study: unknown |
| Outcomes |
|
| Notes | Conference abstract only with very limited information. No contact details of the author. Unable to judge whether the study meets all of our inclusion criteria, including diagnosis of acne |
NCT00031096.
| Methods | This is a randomised, double‐blind, multicentre study |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions | A: Azelaic Acid 15% gel (SH H 655 BA) applied topically two times per day for 12 weeks (number of participants unclear) B: Vehicle gel (SH H 655 PBA) applied topically two times per day for 12 weeks (number of participants unclear) Total estimated number of participants enrolled: 879 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
|
| Notes | The study is sponsored by Bayer company. Completed, but no results posted. |
NCT02755545.
| Methods | This is a randomised, double‐blind, multicentre trial |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions | A: adapalene applied topically to the entire face or other affected area of the skin once daily (number of participants unclear) B: salicylic acid applied topically to affected area of the skin one to three times daily (number of participants unclear) Total estimated number of participants enrolled: 127 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
|
| Notes | This study is sponsored by Galderma Laboratories, LP. Completed but no results posted. |
Pisani 1991.
| Methods | Unknown |
| Participants | Unknown |
| Interventions | Unknown |
| Outcomes | Unknown |
| Notes | We had no access to this full report. |
Ponzio 1994.
| Methods | Randomised trial |
| Participants | Seventy volunteers, aged 12 to 17, with initial forms of acne |
| Interventions | Triclosan and salicylic acid |
| Outcomes |
|
| Notes | We had no access to this full report. |
TCTR20190118001.
| Methods | A randomised, double‐blinded, split‐face, controlled trial |
| Participants |
Inclusion criteria of the trial Gender: both Age limit: minimum 18 Years: maximum 99 Years
Exclusion criteria of the trial
|
| Interventions | A: Jessner's solution B: Salicylic acid 30% Total number of participants enrolled: 35 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
|
| Notes | Recruitment status: completed (no results provided) Source(s) of monetary or material support: Dermatological Society of Malaysia |
Zheng 2019.
| Methods | A randomised, split‐face, open‐label, single‐centre study |
| Participants | Subjects with mild to moderate acne |
| Interventions | A: 2% supramolecular salicylic acid B: 0.01% adapalene plus 5% benzoyl peroxide |
| Outcomes |
|
| Notes | Cannot access full‐text journal article |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800018343.
| Study name | Comparative study for salicylic acid vs glycolic acid in the treatment of mild‐to‐moderate acne vulgaris |
| Methods | Randomised parallel controlled trial |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
|
| Interventions | A: Salicylic acid peel B: Glycolic acid peel Total estimated number of participants enrolled: 60 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
Definition: not provided. Visits: not provided. |
| Starting date | November 2018 |
| Contact information | Name: Jiang Xian Address: 37 Guoxue Xiang, Wuhou District, Chengdu China, 610041 Telephone: +86 18980601693 Email: jennyxianj@163.com Affiliation: West China Hospital, Sichuan University Name: Li Xiaoxue Address: 37 Guoxue Xiang, Wuhou District, Chengdu China, 610041 Telephone: +86 15528260238 Email: li‐xiaoxue@foxmail.com Affiliation: West China Hospital, Sichuan University Apps.who.int‐ID: ChiCTR1800018343 |
| Notes | Recruiting, Self‐raised trial |
CTRI/2018/06/014615.
| Study name | Comparative efficacy of glycolic acid 35% vs salicylic acid 20% peel in acne vulgaris |
| Methods | Randomised, parallel group trial |
| Participants |
Inclusion criteria of the trial
Mild acne (grade 1): comedones < 30; predominance of comedones papules < 10; No scarring Moderate acne (grade 2): comedones any number; predominance of papules > 10; nodules < 3; scarring +/‐ Severe acne (grade 3): comedones any number; many nodules; papules any number; nodules/cysts > 3; scarring + Exclusion criteria of the trial
Age minimum: not provided Age maximum: not provided Gender: not provided |
| Interventions | Group 1 participants will be given 35% glycolic acid (GA) peels which will be applied for a period of 3 minutes or until appearance of erythema, whichever is earlier. The applied GA shall be neutralised with 10% sodium bicarbonate solution and distilled water soaked gauze pads. Group 2 participants will be given 20% salicylic acid (SA) peels which will be applied until there is uniform light white coat of pseudo frost. After pseudo frosting, the peel will be washed away with distilled water. Total estimated number of participants enrolled: 60 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
Definition: not provided Visits: see above |
| Starting date | July 2018 |
| Contact information | Name: Dr Gurvinder Pal Thami Address: Department of Dermatology, Government Medical College and Hospital, Sector 32, Chandigarh 160030 Chandigarh, CHANDIGARH India Telephone: not provided Email: thamigp@gmail.com Affiliation: Government Medical College and Hospital, Chandigarh Name: Dr Purva Pande Address: Department Of Dermatology, Government Medical College and Hospital, Sector 32, Chandigarh 160030 Chandigarh, India Telephone: not provided Email: thamigp@gmail.com Affiliation: Government Medical College and Hospital, Chandigarh Apps.who.int‐ID: CTRI/2018/06/014615 |
| Notes | Not yet recruiting |
NCT03832647.
| Study name | Anti‐acne efficacy of a dermo‐cosmetic product associated with the fixed combination adapalene 0.1%/benzoyl peroxide 2.5% treatment versus this treatment associated with a standard moisturizer in male and female subjects presenting with mild to moderate acne |
| Methods | A randomised, double‐blinded trial |
| Participants |
Inclusion criteria of the trial
Exclusion criteria of the trial
Age: 16 to 35 years Gender: both |
| Interventions | Experimental group: Salicylic acid and Epiduo 0.1% to 2.5% topical gel. Salicylic acid once‐a‐day in the morning during 12 weeks and Epiduo gel once‐a‐day in the evening (before bedtime) over 12 weeks. Placebo comparator group: moisturiser and Epiduo 0.1% to 2.5% topical gel moisturiser: once‐a‐day, in the morning, over 12 weeks. Epiduo gel: once‐a‐day, in the evening (before bedtime) over 12 weeks. Total estimated number of participants enrolled: 200 |
| Outcomes |
Primary outcome of the trial
Secondary outcomes of the trial
Definition: see above Visits: see above |
| Starting date | February 2019 |
| Contact information | Karine Duhamelle +33153684684 karine.duhamelle@intertek.com Caroline Derome +33153684688 caroline.derome@intertek.com |
| Notes | Not yet recruiting |
Differences between protocol and review
Types of interventions/Objectives: we added topical zinc and fruit acid (alpha‐hydroxy acid) as included interventions, as the aim of this review was to include all topical treatments other than antimicrobials/retinoids.
Types of outcome measures/Secondary outcomes: we assessed 'minor adverse events' as 'total number of participants who experienced at least one minor adverse event' which would be more clear and precise.
Types of outcome measures: we clarified the timing definitions.
Measures of treatment effect: in the protocol, we planned to conduct a sensitivity analysis using different cut‐off points (e.g. 'greatly improved' or 'not greatly improved'). We did not do this because of the limited number of trials included in each comparison.
Several trials compared one of the six topical treatments plus drug X to drug X alone. In this case, we considered drug X as the concomitant medication in both treatment arms and we deemed this kind of comparison to be: one of six topical treatments versus no treatment. We extracted and analysed these data in the comparisons of 'topical treatments versus no treatment'.
Data synthesis: in the protocol, we planned to employ a fixed‐effect model for pooled analyses unless the I² statistic measure of heterogeneity was equal to or greater than 30%, in which case we used the random‐effects model. However, we used the random‐effect model throughout all analyses, as suggested by the reviewer, as it would be likely there would be clinical and methodological heterogeneity between any pooled studies.
Data collection and analysis: in the protocol, we planned to create 'Summary of findings' tables for primary outcomes. We also summarised the secondary outcome of 'minor adverse events ‐ total events' and 'quality of life'.
We edited the title so that the scientific term for 'fruit acid' was included, to enable visibility of the review in search results.
We excluded trials in which participants had a diagnosis of neonatal and infantile acne.
Contributions of authors
HS was the contact person with the editorial base. HL, HS, JX, and FP co‐ordinated contributions from the co‐authors and wrote the final draft of the review. HL and HY screened papers against eligibility criteria. HY obtained data on ongoing and unpublished studies. HL and HS appraised the quality of papers. HL, HY, and FP extracted data for the review and sought additional information about papers. HL and HY entered data into RevMan. HL and HS analysed and interpreted data. HL, JX and FP worked on the methods sections. HL and HS drafted the clinical sections of the background and responded to the clinical comments of the referees. GL responded to the methodology and statistics comments of the referees. LL was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. HL is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Sources of support
Internal sources
Jinling Hospital, Nanjing, China
Zhejiang University, Hangzhou, China
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Haibo Liu: nothing to declare Haiyan Yu: nothing to declare Jun Xia: nothing to declare Ling Liu: nothing to declare Guan J Liu: nothing to declare Hong Sang: nothing to declare Frank Peinemann: nothing to declare Jerry Tan, clinical referee: advisor, consultant, and/or investigator for Allergan, Almirall, Cipher, Galderma, and Valeant
Edited (no change to conclusions)
References
References to studies included in this review
Akarsu 2012 {published data only}
- Akarsu S, Fetil E, Yücel F, Gül E, Güne AT. Efficacy of the addition of salicylic acid to clindamycin and benzoyl peroxide combination for acne vulgaris. Journal of Dermatology 2012;39(5):433-8. [CENTRAL: CN-00882159] [PMID: ] [DOI] [PubMed] [Google Scholar]
Aksakal 1997 {published data only}
- Aksakal AB, Koruyucu M, Onder M, Oztas MO, Gurer MA. A comparative study of metronidazole 1% cream versus azelaic acid 20% cream in the treatment of acne. Gazi Medical Journal 1997;8(4):144-7. [CENTRAL: CN-00195442] [Google Scholar]
Babayeva 2011 {published data only}
- Babayeva L, Akarsu S, Fetil E, Güne AT. Comparison of tretinoin 0.05% cream and 3% alcohol-based salicylic acid preparation in the treatment of acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2011;25(3):328-33. [CENTRAL: CN-00787953] [DOI] [PubMed] [Google Scholar]
Bae 2013 {published data only}
- Bae BG, Park CO, Shin H, Lee SH, Lee YS, Lee SJ, et al. Salicylic acid peels versus Jessner's solution for acne vulgaris: a comparative study. Dermatologic Surgery 2013;39(2):248-53. [CENTRAL: CN-00878368] [DOI] [PubMed] [Google Scholar]
Barbareschi 1991 {published data only}
- Barbareschi M, Hendricks I, Angius A, Cattaneo M, Monti M. The anticomedonic activity of azelaic acid investigated by means of scanning electron microscopy on horny layer biopsy. Journal of Dermatological Treatment 1991;2(2):55-7. [CENTRAL: CN-00191948] [Google Scholar]
Bojar 1994 {published data only}
- Bojar RA, Eady EA, Jones CE, Cunliffe WJ, Holland KT. Inhibition of erythromycin-resistant propionibacteria on the skin of acne patients by topical erythromycin with and without zinc. British Journal of Dermatology 1994;130(3):329-36. [CENTRAL: CN-00100271] [DOI] [PubMed] [Google Scholar]
Cavicchini 1989 {published data only}
- Cavicchini S, Caputo R. Long-term treatment of acne with 20% azelaic acid cream. Acta Dermato-venereologica. Supplementum 1989;69(143):40-4. [CENTRAL: CN-00351262] [PubMed] [Google Scholar]
Chantalat 2005 {published data only}
- Chantalat J, Stamatas G, Kollias N, Liu JC. Comparative efficacy of target acne lesion resolution using a novel 2% salicylic acid composition versus 10% benzoyl peroxide. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):37. [CENTRAL: CN-00602613] [Google Scholar]
Chantalat 2006 {published data only}
- *.Chantalat J, Liu JC. Six-week safety and efficacy evaluation of a synergistic microgel complex versus 10% benzoyl peroxide in the treatment of mild to moderate acne. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB14. [CENTRAL: CN-00602658] [Google Scholar]
- Chantalat J, Luedtke K, Wiegand B, Liu JC. Significant improvement in acne quality of life by a topical treatment containing a synergistic microgel complex and 2% salicylic acid. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB21. [CENTRAL: CN-00602548] [Google Scholar]
- Chantalat J, Stamatas G, Liu JC, Chen T. Treating emerging acne. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB21. [CENTRAL: CN-00602574] [Google Scholar]
Chantalat 2007 {published data only}
- Chantalat J, Chen T, Stephens TJ, Pappert AS. Salicylic acid microgel complex provides significant acne benefits in foaming cleanser formulations. Journal of the American Academy of Dermatology 2007;56(2):AB24. [CENTRAL: CN-00615939] [Google Scholar]
Chen 2007 {published data only}
- Chen T, Herndon JH, Stephens TJ, Appa Y. Rapid and lasting acne reduction by an oil free cleanser containing salicylic acid microgel complex. Journal of the American Academy of Dermatology 2007;56(2):AB20. [CENTRAL: CN-00616006] [Google Scholar]
Cunliffe 1989 {published data only}
- Cunliffe WJ, Holland KT. Clinical and laboratory studies on treatment with 20% azelaic acid cream for acne. Acta Dermato-venereologica. Supplementum 1989;69(Suppl 143):31-4. [CENTRAL: CN-00062182] [PubMed] [Google Scholar]
Cunliffe 2005 {published data only}
- Cunliffe WJ, Bojar R, Kanis R, Fernandez C. An observer-blind, randomized, multicentre study of a topical zinc-clindamycin gel (Zindaclin) or clindamycin lotion (Dalacin T) applied twice daily in patients with acne. British Journal of Dermatology 2002;147(Suppl 62):46. [CENTRAL: CN-00487814] [Google Scholar]
- *.Cunliffe WJ, Fernandez C, Bojar R, Kanis R, West F, Zindaclin Clinical Study Group. An observer-blind parallel-group, randomized, multicentre clinical and microbiological study of a topical clindamycin/zinc gel and a topical clindamycin lotion in patients with mild/moderate acne. Journal of Dermatological Treatment 2005;16(4):213-8. [CENTRAL: CN-00553078] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dayal 2017 {published data only}
- Dayal S, Amrani A, Sahu P, Jain VK. Jessner's solution vs. 30% salicylic acid peels: a comparative study of the efficacy and safety in mild-to-moderate acne vulgaris. Journal of Cosmetic Dermatology 2017;16(1):43-51. [CENTRAL: CN-01367383] [DOI] [PubMed] [Google Scholar]
Draelos 2016 {published data only}
- *.Draelos ZD, Ertel K, Rom D. Five-day study to judge the short-term effect of a benzoyl peroxide 3% gel on acne lesions. Journal of Cosmetic Dermatology 2016;15(4):350-7. [CENTRAL: CN-01300011] [DOI] [PubMed] [Google Scholar]
- NCT02052752. A five day clinical study to examine the effects of a benzoyl peroxide treatment on facial acne lesions. clinicaltrials.gov/show/nct02052752 (first received 3 February 2014).
Dunlap 1997 {published data only}
- Dunlap FE. An investigator blinded randomized study comparing a 3% eryhtromycin / 5% benzoyl peroxide combination in gel versus 20% azelaic acid cream in the treatment of acne vulgaris. Journal of Investigative Dermatology 1997;108(3):392. [CENTRAL: CN-00355137] [Google Scholar]
Eady 1996 {published data only}
- Eady EA, Burke BM, Pulling K, Cunliffe WJ. The benefit of 2% salicylic acid lotion in acne - a placebo-controlled study. Journal of Dermatological Treatment 1996;7(2):93-6. [CENTRAL: CN-00169967] [Google Scholar]
ElRefaei 2015 {published data only}
- ElRefaei AM, Abdel Salam HA, Sorour NE. Salicylic-mandelic acid versus glycolic acid peels in Egyptian patients with acne vulgaris. Journal of the Egyptian Women's Dermatologic Society 2015;12(3):196-202. [CENTRAL: CN-01306696] [Google Scholar]
Garg 2009 {published data only}
- Garg VK, Sinha S, Sarkar R. Glycolic acid peels versus salicylic-mandelic acid peels in active acne vulgaris and post-acne scarring and hyperpigmentation: a comparative study. Dermatologic Surgery 2009;35(1):59-65. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gollnick 2004a {published data only}
- Gollnick HP, Graupe K, Zaumseil RP. Azelaic acid 15 % gel in the treatment of acne vulgaris. Combined results of two double-blind clinical comparative studies. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2004;2(10):841-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gollnick 2004b {published data only}
- Gollnick HP, Graupe K, Zaumseil RP. Azelaic acid 15 % gel in the treatment of acne vulgaris. Combined results of two double-blind clinical comparative studies. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2004;2(10):841-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hayashi 2012 {published data only}
- Hayashi N, Koyanagi E, Nogita T, Fujiyama M, Kawashima M. A randomized placebo-controlled investigator-blinded face split study of 20% azelaic acid cream to evaluate the efficacy and safety in Japanese patients with acne vulgaris. Journal of Dermatology 2012;39(Suppl 1):249-50. [CENTRAL: CN-01032268] [Google Scholar]
Hunt 1992 {published data only}
- Hunt MJ, Barnetson RS. A comparative study of gluconolactone versus benzoyl peroxide in the treatment of acne. Australasian Journal of Dermatology 1992;33(3):131-4. [CENTRAL: CN-00093199] [DOI] [PubMed] [Google Scholar]
Ilknur 2010 {published data only}
- Ilknur T, Demirtasoglu M, Biçak MU, Ozkan S. Glycolic acid peels versus amino fruit acid peels for acne. Journal of Cosmetic and Laser Therapy 2010;12(5):242-5. [CENTRAL: CN-00769352] [DOI] [PubMed] [Google Scholar]
Iraji 2007 {published data only}
- *.Iraji F, Sadeghinia A, Shahmoradi Z, Siadat AH, Jooya A. Efficacy of topical azelaic acid gel in the treatment of mild-moderate acne vulgaris. Indian Journal of Dermatology, Venereology and Leprology 2007;73(2):94-6. [CENTRAL: CN-01093647] [DOI] [PubMed] [Google Scholar]
- Iraji F. Efficacy of topical azelaic acid gel (20%) for acne. In: 7th Asian Congress of Dermatology incorporating the 5th Regional Conference of Paediatric Dermatology Kuala Lumpur, Malaysia; 2005 September 28-October 1. 2005:329. [CENTRAL: CN-00602212]
Jaffary 2016 {published data only}
- Jaffary F, Faghihi G, Saraeian S, Hosseini SM. Comparison the effectiveness of pyruvic acid 50% and salicylic acid 30% in the treatment of acne. Journal of Research in Medical Sciences 2016;21(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kar 2013 {published data only}
- Kar BR, Tripathy S, Panda M. Comparative study of oral isotretinoin versus oral isotretinoin + 20% salicylic acid peel in the treatment of active acne. Journal of Cutaneous & Aestheic Surgery 2013;6(4):204-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katsambas 1989a {published data only}
- Katsambas A, Graupe K, Stratigos J. Clinical studies of 20% azelaic acid cream in the treatment of acne vulgaris. Comparison with vehicle and topical tretinoin. Acta Dermato-Venereologica. Supplementum 1989;69(Suppl 143):35-9. [CENTRAL: CN-00062183] [PubMed] [Google Scholar]
Katsambas 1989b {published data only}
- Katsambas A, Graupe K, Stratigos J. Clinical studies of 20% azelaic acid cream in the treatment of acne vulgaris. Comparison with vehicle and topical tretinoin. Acta Dermato-Venereologica. Supplementum 1989;69(Suppl 143):35-9. [CENTRAL: CN-00062183] [PubMed] [Google Scholar]
Kessler 2008 {published data only}
- Kessler E, Flanagan K, Chia C, Rogers C, Glaser DA. Comparison of alpha- and beta-hydroxy acid chemical peels in the treatment of mild to moderately severe facial acne vulgaris. Dermatologic Surgery 2008;34(1):45-50; discussion 51. [CENTRAL: CN-00621503] [DOI] [PubMed] [Google Scholar]
Khodaeiani 2013 {published data only}
- Khodaeiani E, Fouladi RF, Amirnia M, Saeidi M, Karimi ER. Topical 4% nicotinamide vs. 1% clindamycin in moderate inflammatory acne vulgaris. International Journal of Dermatology 2013;52(8):999-1004. [CENTRAL: CN-00919313] [DOI] [PubMed] [Google Scholar]
Kim 1999 {published data only}
- Kim SW, Moon SE, Kim JA, Eun HC. Glycolic acid versus Jessner's solution: which is better for facial acne patients? A randomized prospective clinical trial of split-face model therapy. Dermatologic Surgery 1999;25(4):270-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Levesque 2011 {published data only}
- Levesque A, Hamzavi I, Seite S, Rougier A, Bissonnette R. Randomized trial comparing a chemical peel containing a lipophilic hydroxy acid derivative of salicylic acid with a salicylic acid peel in subjects with comedonal acne. Journal of Cosmetic Dermatology 2011;10(3):174-8. [CENTRAL: CN-00806039] [DOI] [PubMed] [Google Scholar]
NilFroushzadeh 2009 {published data only}
- NilFroushzadeh MA, Siadat AH, Baradaran EH, Moradi S. Clindamycin lotion alone versus combination lotion of clindamycin phosphate plus tretinoin versus combination lotion of clindamycin phosphate plus salicylic acid in the topical treatment of mild to moderate acne vulgaris: a randomized control trial. Indian Journal of Dermatology, Venereology and Leprology 2009;75(3):279-82. [CENTRAL: CN-00729040] [DOI] [PubMed] [Google Scholar]
Ozkan 2000 {published data only}
- Ozkan M, Durmaz G, Sabuncu I, Saracoglu N, Akgun Y, Urer SM. Clinical efficacy of topical clindamycin phosphate and azelaic acid on acne vulgaris and emergence of resistant coagulase-negative staphylococci. Turkish Journal of Medical Sciences 2000;30(5):483-7. [CENTRAL: CN-00424538] [Google Scholar]
Pazoki‐Toroudi 2010 {published data only}
- Pazoki-Toroudi H, Nassiri-Kashani M, Tabatabaie H, Ajami M, Habibey R, Shizarpour M, et al. Combination of azelaic acid 5% and erythromycin 2% in the treatment of acne vulgaris. Journal of Dermatological Treatment 2010;21(3):212-6. [CENTRAL: CN-00748446] [DOI] [PubMed] [Google Scholar]
Pazoki‐Toroudi 2011 {published data only}
- Pazoki-Toroudi H, Nilforoushzadeh MA, Ajami M, Jaffary F, Aboutaleb N, Nassiri-Kashani M, et al. Combination of azelaic acid 5% and clindamycin 2% for the treatment of acne vulgaris. Cutaneous and Ocular Toxicology 2011;30(4):286-91. [CENTRAL: CN-00882826] [DOI] [PubMed] [Google Scholar]
Picosse 2015 {published data only}
- Picosse FR, Dos Santos Guadanhim LR, Nascimento LS, Paiva JM, Bagatin E. Azelaic acid as an option for maintenance treatment after oral isotretinoin: a comparative and longitudinal study-preliminary results. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB5. [EMBASE: 71894787] [Google Scholar]
Schaller 2016 {published data only}
- EUCTR2013-004158-81-Outside-EU/EEA. A multi-centre, single-blind, parallel group, clinical evaluation of the efficacy and safety of clindamycin 1% / benzoyl peroxide 3% and azelaic acid 20% in the topical treatment of mild to moderate acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-004158-81-Outside-EU/EEA (first received 21 September 2017).
- NCT02058628. Comparison of the efficacy and safety of clindamycin + benzoyl peroxide formulation with azelaic acid formulation in the treatment of acne vulgaris. clinicaltrials.gov/show/nct02058628 (first received 10 February 2014).
- *.Schaller M, Sebastian M, Ress C, Seidel D, Hennig M. A multicentre, randomized, single-blind, parallel-group study comparing the efficacy and tolerability of benzoyl peroxide 3%/clindamycin 1% with azelaic acid 20% in the topical treatment of mild-to-moderate acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2016;30(6):966-73. [CENTRAL: CN-01369030] [DOI] [PubMed] [Google Scholar]
Shahmoradi 2013 {published data only}
- Shahmoradi Z, Iraji F, Siadat AH, Ghorbaini A, Nilforoushzadeh MA. Comparison of topical 5% nicotinamid and 2% clindamycin gels in the treatment of the mild to moderate acne vulgaris: A double-blinded randomized clinical trial. Journal of Isfahan Medical School 2015;32(316):2279-85. [CENTRAL: CN-01070524] [PMC free article] [PubMed] [Google Scholar]
- *.Shahmoradi Z, Iraji F, Siadat AH, Ghorbaini A. Comparison of topical 5% nicotinamid gel versus 2% clindamycin gel in the treatment of the mild-moderate acne vulgaris: A double-blinded randomized clinical trial. Journal of Research in Medical Sciences 2013;18(2):115-7. [CENTRAL: CN-00908552] [PMC free article] [PubMed] [Google Scholar]
Shalita 1981 {published data only}
- Shalita AR. Treatment of mild and moderate acne vulgaris with salicylic acid in an alcohol-detergent vehicle. Cutis; Cutaneous Medicine for the Practitioner 1981;28(5):556-8, 561. [CENTRAL: CN-00269323] [PubMed] [Google Scholar]
Shalita 1989 {published data only}
- *.Shalita AR. Comparison of a salicylic acid cleanser and a benzoyl peroxide wash in the treatment of acne vulgaris. Clinical Therapeutics 1989;11(2):264-7. [CENTRAL: CN-00185376] [PubMed] [Google Scholar]
- Shalita AR. Comparison of a salicylic acid cleanser and a benzoyl peroxide wash in the treatment of acne vulgaris. Revista Brasileira de Medicina 1998;55(3):143-5. [CENTRAL: CN-00200583] [PubMed] [Google Scholar]
Shalita 1995 {published data only}
- Shalita AR, Chalker DK, Parish LC, Bernstein JE, Evans CS. The effects of topical nicotinamide on acne vulgaris. Journal of Investigative Dermatology 1992;98(4):604. [CENTRAL: CN-00324761] [Google Scholar]
- *.Shalita AR, Smith JG, Parish LC, Sofman MS, Chalker DK. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. International Journal of Dermatology 1995;34(6):434-7. [CENTRAL: CN-00117614] [DOI] [PubMed] [Google Scholar]
Sharquie 2008 {published data only}
- Sharquie KE, Noaimi AA, Al-Salih MM. Topical therapy of acne vulgaris using 2% tea lotion in comparison with 5% zinc sulphate solution. Saudi Medical Journal 2008;29(12):1757-61. [CENTRAL: CN-00686729] [PubMed] [Google Scholar]
Stinco 2007 {published data only}
- Stinco G, Bragadin G, Trotter D, Pillon B, Patrone P. Relationship between sebostatic activity, tolerability and efficacy of three topical drugs to treat mild to moderate acne. Journal of the European Academy of Dermatology and Venereology 2007;21(3):320-5. [CENTRAL: CN-00586948] [DOI] [PubMed] [Google Scholar]
Techapichetvanich 2011 {published data only}
- Techapichetvanich T, Rajatanavin N, Sahawatwong S. The combination of salicylic acid peel with 5% benzoyl peroxide and 1% clindamycin lotion in a treatment of acne vulgaris: a randomized, doubleblind, placebo controlled study. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB13. [CENTRAL: CN-00843979] [Google Scholar]
Thielitz 2015 {published data only}
- DRKS00003170. An evaluator-blind controlled parallel-group study to assess efficacy and safety of Skinoren® 15% gel and Differin® 0.1% gel for the treatment and maintenance treatment of facial acne vulgaris and late-type acne in females. apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00003170 (first received 20 July 2011).
- NCT01387048. Study for long-term treatment of acne vulgaris with Skinoren versus Differin. clinicaltrials.gov/show/nct01387048 (first received 4 July 2011).
- *.Thielitz A, Lux A, Wiede A, Kropf S, Papakonstantinou E, Gollnick H. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. Journal of the European Academy of Dermatology and Venereology 2015;29(4):789-96. [CENTRAL: CN-01254690] [DOI] [PubMed] [Google Scholar]
Vasarinsh 1969 {published data only}
- Vasarinsh P. Benzoyl peroxide- sulfur lotions in acne vulgaris- a controlled study. Cutis; cutaneous medicine for the practitioner 1969;5(1):65-9. [CENTRAL: CN-00269401] [Google Scholar]
Weltert 2004 {published data only}
- Weltert Y, Chartier S, Gibaud C, Courau S, Pechenart P, Sirvent A, et al. Double-blind clinical assessment of the efficacy of a 4% nicotinamide gel (Exfoliac NC Gel) versus a 4% erythromycin gel in the treatment of moderate acne with a predominant inflammatory component. Nouvelles Dermatologiques 2004;23(7 I):385-94. [CENTRAL: CN-00516675] [Google Scholar]
References to studies excluded from this review
Abdel 2015 {published data only}
- Abdel Meguid AM, Abd Elaziz Ahmed Attallah D, Omar H. Trichloroacetic acid versus salicylic acid in the treatment of acne vulgaris in dark-skinned patients. Dermatologic Surgery 2015;41(12):1398-404. [CENTRAL: CN-01169046] [DOI] [PubMed] [Google Scholar]
Anonymous 1996 {published data only}
- Anonymous. Azelaic acid-a new topical drug for acne. Medical Letter on Drugs & Therapeutics 1996;38(976):52-3. [CENTRAL: 8637482] [PubMed] [Google Scholar]
Barak‐Shinar 2017 {published data only}
- Barak-Shinar D, Draelos Z. A randomized controlled study of a novel botanical acne spot treatment. Journal of Drugs in Dermatology 2017;16(6):599-603. [CENTRAL: CN-01416572] [PubMed] [Google Scholar]
Barkovic 2012 {published data only}
- Barkovic S, Sigler M, Stephens T, Appa Y. A double-blind placebo-controlled evaluation of a 0.5% salicylic acid moisturizer for rapid and continuous acne improvement. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB13. [CENTRAL: CN-01034436] [Google Scholar]
Bissonnette 2009 {published data only}
- *.Bissonnette R, Bolduc C, Seité S, Nigen S, Provost N, Maari C, et al. Randomized study comparing the efficacy and tolerance of a lipophillic hydroxy acid derivative of salicylic acid and 5% benzoyl peroxide in the treatment of facial acne vulgaris. Journal of Cosmetic Dermatology 2009;8(1):19-23. [CENTRAL: CN-00698297] [DOI] [PubMed] [Google Scholar]
- NCT00624676. Efficacy and tolerance of a derivative of salicylic acid and 5% benzoyl peroxide in facial acne vulgaris. clinicaltrials.gov/show/nct00624676 (first received 27 February 2008).
Breno 2002 {published data only}
- *.Bréno B, Khammari A, Richard A, Rougier A. Interest of a new salicylic acid derivative in the prevention of acne relapses. European Journal of Dermatology 2002;12(4):LI-LIII. [CENTRAL: CN-00409133] [PubMed] [Google Scholar]
- Dreno B, Khammari A, Richard A, Rougier A. Interest of a new salicylic acid derivative in the prevention of acne relapses. Annales de Dermatologie et de Venereologie 2002;129:P0016. [CENTRAL: CN-00454201] [PubMed] [Google Scholar]
Capitanio 2012 {published data only}
- Capitanio B, Sinagra JL, Weller RB, Brown C, Berardesca E. Randomized controlled study of a cosmetic treatment for mild acne. Clinical and Experimental Dermatology 2012;37(4):346-49. [CENTRAL: CN-00841318] [DOI] [PubMed] [Google Scholar]
Chantalat 2006a {published data only}
- Chantalat J, Liu JC. A novel acne cleanser containing a synergistic microgel complex with 2% salicylic acid for the treatment of mild to moderate acne. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB14. [CENTRAL: CN-00602280] [Google Scholar]
Chassard 2006 {published data only}
- Chassard D, Kanis R, Namour F, Evene E, Ntssikoussalabongui B, Schmitz V. A single centre, open-label, cross-over study of pharmacokinetics comparing topical zinc/clindamycin gel (Zindaclin) and topical clindamycin lotion (Dalacin T) in subjects with mild to moderate acne. Journal of Dermatological Treatment 2006;17(3):154-7. [CENTRAL: CN-00566630] [DOI] [PubMed] [Google Scholar]
Chu 1997 {published data only}
- *.Chu A, Huber FJ, Plott RT. The comparative efficacy of benzoyl peroxide 5%/erythromycin 3% gel and erythromycin 4%/zinc 1.2% solution in the treatment of acne vulgaris. British Journal of Dermatology 1997;136(2):235-8. [CENTRAL: CN-00137636] [PubMed] [Google Scholar]
- Chu A, Huber FJ, Plott RT. The comparative safety and efficacy of benzoyl peroxide 5% erythromycin 3% gel and erythromycin 4% zinc 1% solution in the treatment of acne vulgaris. Journal of Investigative Dermatology 1997;108(3):392. [CENTRAL: CN-00352565] [PubMed] [Google Scholar]
- Chu A. The comparative safety and efficacy of benzoyl peroxide 5% erythromycin 3% gel and erythromycin 4% zinc 1.2% solution in the treatment of acne vulgaris. British Journal of Dermatology 1996;135(Suppl 47):30. [CENTRAL: CN-00415429] [Google Scholar]
Cochran 1985 {published data only}
- Cochran RJ, Tucker SB, Flannigan SA. Topical zinc therapy for acne vulgaris. International Journal of Dermatology 1985;24(3):188-90. [CENTRAL: CN-00038131] [DOI] [PubMed] [Google Scholar]
Coret 2006 {published data only}
- Coret C, Chantalat J, Miller D, Kurtz E. Fast-acting treatment of mild to moderate acne lesions by a novel 2% salicylic acid microgel complex. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB18. [CENTRAL: CN-00602686] [Google Scholar]
Cotterill 1980 {published data only}
- Cotterill JA. Acne vulgaris and its management. Physiotherapy 1980;66(2):41-3. [PMID: ] [PubMed] [Google Scholar]
Cunliffe 1992 {published data only}
- Cunliffe WJ. Strategy of treating acne vulgaris. Journal of the European Academy of Dermatology and Venereology 1992;1(1):43-52. [CENTRAL: CN-00454175] [Google Scholar]
Danto 1966 {published data only}
- Danto JL, Maddin WS, Stewart WD, Nelson AJ. A controlled trial of benzoyl peroxide and precipitated sulfur cream in acne vulgaris. Applied Therapeutics 1966;8(7):624-5. [CENTRAL: CN-00000721] [PubMed] [Google Scholar]
De Bersaques 1972 {published data only}
- De Bersaques J. Topical vitamin A acid. Review of the literature and results of a clinical trial in acne. Archives Belges de Dermatologie 1972;28(4):315-34. [CENTRAL: 4274476] [PubMed] [Google Scholar]
Dos 2003 {published data only}
- Dos SK, Barbhuiya JN, Jana S, Dey SK. Comparative evaluation of clindamycin phosphate 1% and clindamycin phosphate 1% with nicotinamide gel 4% in the treatment of acne vulgaris. Indian Journal of Dermatology, Venereology and Leprology 2003;69(1):8-9. [PMID: ] [PubMed] [Google Scholar]
Draelos 2006 {published data only}
- Draelos ZD, Matsubara A, Smiles K. The effect of 2% niacinamide on facial sebum production. Journal of Cosmetic and Laser Therapy 2006;8(2):96-101. [CENTRAL: CN-00565803] [DOI] [PubMed] [Google Scholar]
Elstein 1981 {published data only}
- Elstein W. Topical deodorized polysulfides. Broadscope acne therapy. Cutis 1981;28(4):468-72. [CENTRAL: CN-00026529] [PubMed] [Google Scholar]
Fang 2001 {published data only}
- Fang H, Mo JR, Ruan LM, Lu LZ. A clinical controlled trial of 0.1% adpalene gel versus sulfur camphor compound cream in the treatment of acne vulgaris. Journal of Clinical Dermatology 2001;30(4):249-50. [CENTRAL: CN-00454222] [Google Scholar]
Fu 2003 {published data only}
- Fu WW, Li M, Liu JH, Gu J, Wang XM, Alain R, et al. Efficacy and safety of a new salicylic acid derivative (lHA) alone or as a complement of tretinoin in acne treatment. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):171. [CENTRAL: CN-00478540] [Google Scholar]
Gebicki 2003 {published data only}
- Gebicki J, Sysa-Jedrzejowska A, Adamus J, Wozniacka A, Rybak M, Zielonka J. 1-Methylnicotinamide: A potent anti-inflammatory agent of vitamin origin. Polish Journal of Pharmacology 2003;55(1):109-12. [PMID: ] [PubMed] [Google Scholar]
Gollnick 1989 {published data only}
- Gollnick H, Graupe K. Azelaic acid for the treatment of acne: Comparative trials. Journal of Dermatological Treatment 1989;1(Suppl 1):27-30. [EMBASE: 1990381168] [Google Scholar]
Gollnick 1997 {published data only}
- Gollnick H, Melzer A. Erythromycin and an erythromycin/zinc combination in the topical treatment of acne [Erythromycin und eine Erythromycin-Zink-Kombination in der topischen Aknetherapie]. Zeitschrift fur Dermatologie 1997;183(1):8-18. [CENTRAL: 1997176115] [Google Scholar]
Green 2013 {published data only}
- Green L, Kircik LH, Gwazdauskas J. Randomized, controlled, evaluator-blinded studies conducted to compare the efficacy and tolerability of 3 over-the-counter acne regimens in subjects with mild or moderate acne. Journal of Drugs in Dermatology 2013;12(2):180-5. [CENTRAL: CN-01164713] [PubMed] [Google Scholar]
Gupta 2004 {published data only}
- Gupta AK, Nicol K. The use of sulfur in dermatology. Journal of Drugs in Dermatology 2004;3(4):427-31. [PubMed] [Google Scholar]
Habbema 1989 {published data only}
- Habbema L, Koopmans B, Menke HE, Doornweerd S, De Boulle K. A 4% erythromycin and zinc combination (Zineryt) versus 2% erythromycin (Eryderm) in acne vulgaris: a randomized, double-blind comparative study. British Journal of Dermatology 1989;121(4):497-502. [CENTRAL: CN-00194960] [DOI] [PubMed] [Google Scholar]
Hjorth 1989 {published data only}
- Hjorth N, Graupe K. Azelaic acid for the treatment of acne. A clinical comparison with oral tetracycline. Acta Dermato-Venereologica. Supplementum 1989;69(Suppl 143):45-8. [CENTRAL: CN-00062184] [PMID: ] [PubMed] [Google Scholar]
Khodaeinai 2014 {published data only}
- Khodaeinai E, Babaeinejad S, Amirnia M, Shokry J, Karimi LR, Fouladi DF, et al. Efficacy of 10% azelaic acid gel with hydro-alcoholic or alcohol-free bases in mild to moderate acne vulgaris; the first clinical trial. Journal of Medical Sciences (Faisalabad) 2014;14(2):87-91. [CENTRAL: CN-00978045] [Google Scholar]
Kirton 1967 {published data only}
- Kirton V. Evaluation of new preparation for the treatment of acne vulgaris. British Journal of Clinical Practice 1967;21(3):127-8. [CENTRAL: CN-00001370] [PubMed] [Google Scholar]
Kreysel 1967 {published data only}
- Kreysel HW. On the clinical aspects and therapy of acne vulgaris. Trial report of treatment with Acnichtol and Acnichtol Dexa. Munchener medizinische Wochenschrift (1950) 1967;109(50):2658-62. [PMID: ] [PubMed] [Google Scholar]
Lee 2003 {published data only}
- Lee HS, Kim IH, Monheit GD. Salicylic acid peels for the treatment of acne vulgaris in Asian patients. Dermatologic Surgery 2003;29(12):1196-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leyden 1997 {published data only}
- Leyden JJ, Gans EH. Evaluation of the antimicrobial effects in vivo of Triaz (TM) Gel (benzoyl peroxide special gel), Cleocin-T(TM) Lotion (clindamycin phosphate lotion), and Azelex (TM) Cream (azelaic acid cream) in humans. Journal of Dermatological Treatment 1997;8(Suppl 2):S7-S10. [CENTRAL: CN-00446359] [Google Scholar]
Linss 1981 {published data only}
- Linss G, Schuster G, Freier B, Schielinsky C, Bach W, Lenski K. Investigation of the bioelements zinc, copper and magnesium in acne-patients. A therapeutic study with zinc sulfate in a double-blind trial. Dermatologische Monatschrift 1981;167(4):228-33. [CENTRAL: CN-00025681] [PubMed] [Google Scholar]
MacDonald 1976 {published data only}
- MacDonald RH, McGrath H, Ray SK. Clinical trial of Actinac in acne. British Journal of Clinical Practice 1976;30(10):194-6. [CENTRAL: CN-00015161] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller D, Smith G, Kurtz ES. Improvement in mild to moderate inflammatory acne lesions with a novel salicylic acid topical acne treatment. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):25-6. [CENTRAL: CN-00602638] [Google Scholar]
NCT00848744 {published data only}
- NCT00848744. Comparison of two salicylic acid formulations. clinicaltrials.gov/ct2/show/study/NCT00848744 (first received 20 February 2009).
Norris 1987 {published data only}
- Norris J, Cunliffe WJ, Burke B. Azelaic acid really does work in acne- a double blind national and international study. British Journal of Dermatology 1987;117(Suppl 32):34-5. [CENTRAL: CN-00402109] [DOI: 10.1111/j.1365-2133.1987.tb12024.x] [DOI] [Google Scholar]
Pastuszka 2012 {published data only}
- Pastuszka M, Kaszuba A. Status of combination drugs with betamethasone dipropionate and salicylic acid in the treatment of skin diseases. Postepy Dermatologii i Alergologii 2012;29(3):196-204. [EMBASE: 2012473003] [Google Scholar]
Pereira 1994 {published data only}
- Pereira CA, Carvalho MT, Tristao OC, Valle AF, Barbosa MC, Oliveira MA, et al. Clinical trial of an association of salicylic acid and sulphur in the treatment of acne vulgaris. Folha Medica 1994;108(1-2):25-7. [EMBASE: 1994106999] [Google Scholar]
Pierard‐Franchimont 1995 {published data only}
- Piérard-Franchimont C, Goffin V, Visser JN, Jacoby H, Piérard GE. A double-blind controlled evaluation of the sebosuppressive activity of topical erythromycin-zinc complex. European Journal of Clinical Pharmacology 1995;49(1-2):57-60. [CENTRAL: CN-00126824] [DOI] [PubMed] [Google Scholar]
Plewig 1969 {published data only}
- Plewig G. Vitamin A acid. Topical treatment in acne vulgaris. Pennsylvania Medicine 1969;72(12):33-5. [PMID: ] [PubMed] [Google Scholar]
Rougier 2002 {published data only}
- Rougier A, Richard A. Efficacy and safety of a new salicylic acid derivative as a complement of vitamin A acid in acne treatment. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl s1):119. [CENTRAL: CN-00478741] [PubMed] [Google Scholar]
Sardesai 2003 {published data only}
- Sardesai VR, Kambli VM. Comparison of efficacy of topical clindamycin and nicotinamide combination with plain clindamycin for the treatment of acne vulgaris and acne resistant to topical antibiotics. Indian Journal of Dermatology, Venereology and Leprology 2003;69(2):138-9. [CENTRAL: CN-00663081] [PubMed] [Google Scholar]
Schachner 1990 {published data only}
- Schachner L, Eaglstein W, Kittles C, Mertz P. Topical erythromycin and zinc therapy for acne. Journal of the American Academy of Dermatology 1990;22(2 Pt 1):253-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shemer 2002 {published data only}
- Shemer A, Weiss G, Amichai B, Kaplan B, Trau H. Azelaic acid (20%) cream in the treatment of acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2002;16(2):178-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Souza 2005 {published data only}
- De Souza A, Christiansen C, Jamie S. The use of salicylic acid in a new delivery system as a co-adjuvant topical treatment for acne vulgaris. Aesthetic Surgery Journal 2005;25(1):40-3. [CENTRAL: CN-00575589] [DOI] [PubMed] [Google Scholar]
Tarimci 1997 {published data only}
- Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. Journal of Clinical Pharmacy and Therapeutics 1997;22(4):301. [CENTRAL: CN-00149589] [DOI] [PubMed] [Google Scholar]
Thomas 1951 {published data only}
- Thomas CC, Hardy MK. Acne vulgaris; clinical trial of a sulfur-resorcinol cream. Journal of the American Medical Womens Association 1951;6(5):176-8. [PMID: ] [PubMed] [Google Scholar]
Touitou 2008 {published data only}
- NCT00361322. Efficacy and safety of a preparation containing an antibiotic and anti-Inflammatory agent in acne vulgaris. clinicaltrials.gov/show/nct00361322 (first received 8 August 2006).
- *.Touitou E, Godin B, Shumilov M, Bishouty N, Ainbinder D, Shouval R, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2008;22(5):629-31. [CENTRAL: CN-00631273] [DOI] [PubMed] [Google Scholar]
van Steenbergen 1968 {published data only}
- Steenbergen EP, Jonge H, Smit F. Topical treatment of acne vulgaris. Statement on a comparative study of the action of topically applied corticosteroids with the action of sulfur and resorcin as well as a placebo [Ortliche Behandlung bei Acne vulgaris. Angaben uber eine vergleichende Untersuchung der Wirkung ortlich applizierter Kortikosteroide gegenuber der Wirkung von Schwefel und Resorzin sowie eines Placebos]. Dermatologica 1968;137(3):139-49. [CENTRAL: CN-00002653] [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang CM, Huang CL, Hu CT, Chan HL. The effect of glycolic acid on the treatment of acne in Asian skin. Dermatologic Surgery 1997;23(1):23-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wilkinson 1966 {published data only}
- Wilkinson RD, Adam JE, Murray JJ, Craig GE. Benzoyl peroxide and sulfur: foundation for acne management. Canadian Medical Association Journal 1966;95(1):28-9. [PMID: 4223133 ] [PMC free article] [PubMed] [Google Scholar]
Wilson 2007 {published data only}
- Wilson DC, Meadows KP. A comparison of a novel benzoyl peroxide system with a combination benzoyl peroxide and clindamycin product: A 2-week split face study of effectiveness and tolerability. Journal of the American Academy of Dermatology 2007;56(2 Supp 2):AB24. [CENTRAL: CN-00615964] [Google Scholar]
Woodruff 2013 {published data only}
- Woodruff J, Appa Y. A double-blind, placebo-controlled evaluation of a 2% salicylic acid cleanser for improvement of acne vulgaris. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB12. [CENTRAL: CN-01008293] [Google Scholar]
References to studies awaiting assessment
Bartosova 1978 {published data only}
- Bartosova L, Melichar M, Sternbersky J. External therapy of acne vulgaris in a controlled clinical trial. I. Comparison of the effectiveness of treatment after isolated time intervals of comedo therapy. Cesko-Slovenska Dermatologie 1978;53(3):157-63. [PMID: ] [PubMed] [Google Scholar]
Cavicchini 1989a {published data only}
- Cavicchini S, Caputo R. National and international experiences with azelaic acid cream in the treatment of papulo-pustular acne [Esperienze cliniche nazionali ed internazionali con una crema a base di acido azelaico nel trattamento dell'acne papulo-pustolosa]. Giornale Italiano di Dermatologia e Venereologia 1989;124(10):465-70. [PMID: ] [PubMed] [Google Scholar]
Draelos 2015 {published data only}
- Draelos ZD. Assessing the value of botanical anti-inflammatory agents in an OTC acne treatment regimen. Journal of Drugs in Dermatology 2015;14(12):1418-21. [CENTRAL: CN-01200536] [PubMed] [Google Scholar]
Giannotti 1989 {published data only}
- Giannotti B. National and international clinical experiences with azelaic acid cream in the treatment of comedo acne [Esperienze cliniche nazionali ed internazionali con una crema a base di acido azelaico nel trattamento dell'acne comedonica]. Giornale Italiano di Dermatologia e Venereologia 1989;124(10):471-7. [PMID: ] [PubMed] [Google Scholar]
IRCT201010094269N3 {published data only}
- IRCT201010094269N3. Effect of combined topical solution 2% salicylic acid and erythromycin 4% with erythromycin 4% topical solution in the treatment of mild to moderate acne vulgaris. en.irct.ir/trial/4534 (first received 19 October 2011).
Kern 2019 {published data only}
- Kern D, Namkoong J, Riggs M, Holly K, Knaggs H. A novel facial treatment cleansing device improves acne skin. Journal of Investigative Dermatology 2019;139(5 Suppl):S166. [EMBASE: 2001808174] [Google Scholar]
NCT00031096 {published data only}
- NCT00031096. Comparison of efficacy and safety of azelaic acid 15% gel with its vehicle in subjects with mild to moderate acne. clinicaltrials.gov/ct2/show/study/NCT00031096 (first received 22 February 2002).
NCT02755545 {published data only}
- NCT02755545. A clinical trial to evaluate the efficacy of two acne treatments. clinicaltrials.gov/ct2/show/study/NCT02755545 (first received 29 April 2016).
Pisani 1991 {published data only}
- Pisani M, Guerrera V, Grimaldi FF. Treatment of acne vulgaris with an ointment containing azelaic acid (12%), L-carnitine (2%), enoxolone (1%): Double-blind study versus placebo. Chronica Dermatologica 1991;1(3):339-44. [CENTRAL: CN-00185158] [Google Scholar]
Ponzio 1994 {published data only}
- Ponzio HA, Bier RT, Bozko MP. Clinical evaluation of a line of products for the control of acne in teenagers. Anais Brasileiros de Dermatologia 1994;69(6):461-5. [CENTRAL: CN-00179147] [Google Scholar]
TCTR20190118001 {published data only}
- TCTR20190118001. A study comparing the efficacy and safety of Jessner's solution and salicylic acid 30% Chemical Peel and in the management of acne vulgaris and post acne hyperpigmentation. www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=4304 (first received 1 January 2019).
Zheng 2019 {published data only}
- Zheng Y, Yin S, Xia Y, Chen J, Ye C, Zeng Q, et al. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: a randomized, split-face, open-label, single-center study. Cutaneous and ocular toxicology 2019;38(1):48-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR1800018343 {published data only}
- ChiCTR1800018343. Comparative study for salicylic acid vs glycolic acid in the treatment of mild-to-moderate acne vulgaris. www.chictr.org.cn/showproj.aspx?proj=19551 (first received 12 September 2018).
CTRI/2018/06/014615 {published data only}
- CTRI/2018/06/014615. Comparison of use of glycolic acid and salicylic acid peel in acne (pimples). www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=26734 (first received 26 June 2018).
NCT03832647 {published data only}
- NCT03832647. Anti-acne efficacy of a dermo-cosmetic product associated with the fixed combination adapalene 0.1%/ benzoyl peroxide 2.5% treatment versus this treatment associated with a standard moisturizer in male and female subjects presenting with mild to moderate acne. clinicaltrials.gov/ct2/show/study/nct03832647 (first received 6 February 2019).
Additional references
Akamatsu 1991
- Akamatsu H, Komura J, Asada Y, Miyachi Y, Niwa Y. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Archives of Dermatological Research 1991;283(3):162-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Akhavan 2003
- Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. American Journal of Clinical Dermatology 2003;4(7):473-92. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Antoniou 2009
- Antoniou C, Dessinioti C, Stratigos AJ, Katsambas AD. Clinical and therapeutic approach to childhood acne: an update. Pediatric Dermatology 2009;26(4):373-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Arowojolu 2012
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA. Combined oral contraceptive pills for treatment of acne. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No: CD004425. [DOI: 10.1002/14651858.CD004425.pub6] [DOI] [Google Scholar]
Atzori 1999
- Atzori L, Brundu MA, Orru A, Biggio P. Glycolic acid peeling in the treatment of acne. Journal of the European Academy of Dermatology and Venereology 1999;12(2):119-22. [PMID: ] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bhate 2013
- Bhate K, Williams HC. Epidemiology of acne vulgaris. British Journal of Dermatology 2013;168(3):474-85. [DOI] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie 1999;54(4):405-11. [PubMed] [Google Scholar]
Bojar 1991
- Bojar RA, Holland KT, Cunliffe WJ. The in-vitro antimicrobial effects of azelaic acid upon Propionibacterium acnes strain P37. Journal of Antimicrobial Chemotherapy 1991;28(6):843-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Boutli 2003
- Boutli F, Zioga M, Koussidou T, Ioannides D, Mourellou O. Comparison of chloroxylenol 0.5% plus salicylic acid 2% cream and benzoyl peroxide 5% gel in the treatment of acne vulgaris: a randomized double-blind study. Drugs Under Experimental & Clinical Research 2003;29(3):101-5. [PubMed] [Google Scholar]
Bozzo 2011
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Canadian Family Physician 2011;57(6):665-7. [PMC free article] [PubMed] [Google Scholar]
Breneman 1993
- Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. International Journal of Dermatology 1993;32(5):365-7. [DOI] [PubMed] [Google Scholar]
Brown 1998
- Brown SK, Shalita AR. Acne vulgaris. Lancet 1998;351(9119):1871-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chien 2016
- Chien AL, Qi J, Rainer B, Sachs DL, Helfrich YR. Treatment of acne in pregnancy. Journal of the American Board of Family Medicine 2016;29(2):254-62. [PMID: ] [DOI] [PubMed] [Google Scholar]
Choi 2010
- Choi YS, Suh HS, Yoon MY, Min SU, Kim JS, Jung JY, et al. A study of the efficacy of cleansers for acne vulgaris. Journal of Dermatological Treatment 2010;21(3):201-5. [DOI] [PubMed] [Google Scholar]
CS‐COUSIN 2019
- Cochrane Skin - Core Outcome Set Initiative (CS-COUSIN). cs-cousin.org/ (accessed prior to 22 February 2019).
Cunliffe 2000
- Cunliffe WJ, Holland DB, Clark SM, Stables GI. Comedogenesis: some new aetiological, clinical and therapeutic strategies. British Journal of Dermatology 2000;142(6):1084-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cunliffe 2005a
- Cunliffe WJ, Fernandez C, Bojar R, Kanis R, West F. An observer-blind parallel-group, randomized, multicentre clinical and microbiological study of a topical clindamycin/zinc gel and a topical clindamycin lotion in patients with mild/moderate acne. Journal of Dermatological Treatment 2005;16(4):213-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575-600. [DOI] [PubMed] [Google Scholar]
Degitz 2008
- Degitz K, Ochsendorf F. Pharmacotherapy of acne. Expert Opinion on Pharmacotherapy 2008;9(6):955-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Rosso 2009
- Del Rosso JQ. The use of sodium sulfacetamide 10%-sulfur 5% emollient foam in the treatment of acne vulgaris. Journal of Clinical & Aesthetic Dermatology 2009;2(8):26-9. [PMC free article] [PubMed] [Google Scholar]
Dessinioti 2010
- Dessinioti C, Katsambas AD. The role of Propionibacterium acnes in acne pathogenesis: facts and controversies. Clinics in Dermatology 2010;28(1):2-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Draelos 2006a
- Draelos ZD. The effect of a daily facial cleanser for normal to oily skin on the skin barrier of subjects with acne. Cutis 2006;78(1 Suppl):34-40. [PMID: ] [PubMed] [Google Scholar]
Dreno 1989
- Dreno B, Amblard P, Agache P, Sirot S, Litoux P. Low doses of zinc gluconate for inflammatory acne. Acta Dermato-Venereologica 1989;69(6):541-3. [PMID: ] [PubMed] [Google Scholar]
Dreno 2018
- Dreno B, Pecastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. Journal of the European Academy of Dermatology and Venereology 2018;32 Suppl 2:5-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunn 2011
- Dunn LK, O'Neill JL, Feldman SR. Acne in adolescents: quality of life, self-esteem, mood, and psychological disorders. Dermatology Online Journal 2011;17(1):1. [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fitton 1991
- Fitton A, Goa KL. Azelaic acid. A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs 1991;41(5):780-98. [DOI] [PubMed] [Google Scholar]
Fluhr 1999
- Fluhr JW, Bosch B, Gloor M, Hoffler U. In-vitro and in-vivo efficacy of zinc acetate against propionibacteria alone and in combination with erythromycin. Zentralblatt fur Bakteriologie 1999;289(4):445-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
Frampton 2004
- Frampton JE, Wagstaff AJ. Azelaic acid 15% gel: in the treatment of papulopustular rosacea. American Journal of Clinical Dermatology 2004;5(1):57-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Friedlander 2010
- Friedlander SF, Eichenfield LF, Fowler JF Jr, Fried RG, Levy ML, Webster GF. Acne epidemiology and pathophysiology. Seminars in Cutaneous Medicine & Surgery 2010;29(2 Suppl 1):2-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gamble 2012
- Gamble R, Dunn J, Dawson A, Petersen B, McLaughlin L, Small A, et al. Topical antimicrobial treatment of acne vulgaris: an evidence-based review. American Journal of Clinical Dermatology 2012;13(3):141-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garner 2012
- Garner SE, Eady A, Bennett C, Newton JN, Thomas K, Popescu CM. Minocycline for acne vulgaris: efficacy and safety. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD002086. [DOI: 10.1002/14651858.CD002086.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerlinger 2011
- Gerlinger C, Schmelter T. Determining the non-inferiority margin for patient reported outcomes. Pharmaceutical Statistics 2011;10(5):410-3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gollnick 2003
- Gollnick HP, Krautheim A. Topical treatment in acne: current status and future aspects. Dermatology 2003;206(1):29-36. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 22 February 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Grange 2009
- Grange PA, Raingeaud J, Calvez V, Dupin N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. Journal of Dermatological Science 2009;56(2):106-12. [DOI] [PubMed] [Google Scholar]
Graupe 1996
- Graupe K, Cunliffe WJ, Gollnick HP, Zaumseil RP. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis 1996;57(1 Suppl):20-35. [PubMed] [Google Scholar]
Haider 2004
- Haider A, Shaw JC. Treatment of acne vulgaris. JAMA 2004;292(6):726-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hay 2014
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. Journal of Investigative Dermatology 2014;134(6):1527-34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Karimkhani 2014
- Karimkhani C, Boyers LN, Prescott L, Welch V, Delamere FM, Nasser M, et al. Global burden of skin disease as reflected in Cochrane Database of Systematic Reviews. JAMA Dermatology 2014;150(9):945-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karimkhani 2017
- Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatology 2017;153(5):406-12. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Katsambas 2008
- Katsambas A, Dessinioti C. New and emerging treatments in dermatology: acne. Dermatologic Therapy 2008;21(2):86-95. [DOI] [PubMed] [Google Scholar]
Kempiak 2008
- Kempiak SJ, Uebelhoer N. Superficial chemical peels and microdermabrasion for acne vulgaris. Seminars in Cutaneous Medicine & Surgery 2008;27(3):212-20. [DOI] [PubMed] [Google Scholar]
Kharfi 2001
- Kharfi M, Tekaya N, Zeglaoui F, Ezzine N, Mokhtar I, Kamoun F, et al. Comparative study of the efficacy and tolerance of 12% glycolic acid cream and 0.05% retinoic acid cream for polymorphic acne [Etude comparative de l'efficacite et de la tolerance d'une creme a 12% d'acide glycolyque et d'une creme a 0.05% d'acide retinoique dans l'acne polymorphe]. La Tunisie Medicale 2001;79(6-7):374-7. [PMID: ] [PubMed] [Google Scholar]
Khunger 2012
- Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian Journal of Dermatology, Venereology & Leprology 2012;78(3):335-41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology 2005;211(3):193-8. [DOI] [PubMed] [Google Scholar]
Krakowski 2007
- Krakowski AC, Eichenfield LF. Pediatric acne: clinical presentations, evaluation, and management. Journal of Drugs in Dermatology 2007;6(6):589-93. [MEDLINE: ] [PubMed] [Google Scholar]
Landow 1997
- Landow K. Dispelling myths about acne. Postgraduate Medicine 1997;102(2):94-9, 103-4, 110-2. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lehmann 2001
- Lehmann HP, Andrews JS, Robinson KA, Holloway VL, Goodman SN. Management of acne. Summary, Evidence Report/Technology Assessment: Number 17. AHRQ Publication No. 01-E018, March 2001. Agency for Healthcare Research and Quality, Rockville, MD.
Lehmann 2002
- Lehmann HP, Robinson KA, Andrews JS, Holloway V, Goodman SN. Acne therapy: a methodologic review. Journal of the American Academy of Dermatology 2002;47(2):231-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lucky 1991
- Lucky AW, Biro FM, Huster GA, Morrison JA, Elder N. Acne vulgaris in early adolescent boys. Correlations with pubertal maturation and age. Archives of Dermatology 1991;127(2):210-6. [PMID: ] [PubMed] [Google Scholar]
Lucky 1997
- Lucky AW, Biro FM, Simbartl LA, Morrison JA, Sorg NW. Predictors of severity of acne vulgaris in young adolescent girls: results of a five-year longitudinal study. The Journal of Pediatrics 1997;130(1):30-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
McLeod 2003
- McLeod LD, Fehnel SE, Brandman J, Symonds T. Evaluating minimal clinically important differences for the acne-specific quality of life questionnaire. Pharmacoeconomics 2003;21(15):1069-79. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mills 1972
- Mills OH Jr, Kligman AM. Is sulphur helpful or harmful in acne vulgaris? British Journal of Dermatology 1972;86(6):620-7. [DOI] [PubMed] [Google Scholar]
Misery 2011
- Misery L. Consequences of psychological distress in adolescents with acne. Journal of Investigative Dermatology 2011;131(2):290-2. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285(15):1987-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
Morganti 2011
- Morganti P, Berardesca E, Guarneri B, Guarneri F, Fabrizi G, Palombo P, et al. Topical clindamycin 1% vs. linoleic acid-rich phosphatidylcholine and nicotinamide 4% in the treatment of acne: a multicentre-randomized trial. International Journal of Cosmetic Science 2011;33(5):467-76. [DOI] [PubMed] [Google Scholar]
O'Brien 1998
- O'Brien SC, Lewis JB, Cunliffe WJ. The Leeds Revised Acne Grading System. Journal of Dermatological Treatments 1998;9(4):215-20. [Google Scholar]
Pace 1965
- Pace WE. A benzoyl peroxide-sulfur cream for acne vulgaris. Canadian Medical Association Journal 1965;93:252-4. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Passi 1989
- Passi S, Picardo M, De Luca C, Nazzaro-Porro M. Mechanism of azelaic acid action in acne. Giornale Italiano di Dermatologia e Venereologia 1989;124(10):455-63. [PubMed] [Google Scholar]
Purdy 2011
- Purdy S, Berker D. Acne vulgaris. Clinical Evidence 2011;2011:1714. [MEDLINE: ] [Google Scholar]
Rademaker 2014
- Rademaker M, Wishart JM, Birchall NM. Isotretinoin 5 mg daily for low-grade adult acne vulgaris--a placebo-controlled, randomized double-blind study. Journal of the European Academy of Dermatology & Venereology 2014;28(6):747-54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ramli 2012
- Ramli R, Malik AS, Hani AF, Jamil A. Acne analysis, grading and computational assessment methods: an overview. Skin Research & Technology 2012;18(1):1-14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolfe 2014
- Rolfe HM. A review of nicotinamide: treatment of skin diseases and potential side effects. Journal of Cosmetic Dermatology 2014;13(4):324-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Seidler 2010
- Seidler EM, Kimball AB. Meta-analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in acne. Journal of the American Academy of Dermatology 2010;63(1):52-62. [DOI] [PubMed] [Google Scholar]
Shalita 1995a
- Shalita AR, Smith JG, Parish LC, Sofman MS, Chalker DK. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. International Journal of Dermatology 1995;34(6):434-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sharad 2013
- Sharad J. Glycolic acid peel therapy - a current review. Clinical, Cosmetic and Investigational Dermatology 2013;6:281-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharquie 2007
- Sharquie KE, Khorsheed AA, Al-Nuaimy AA. Topical zinc sulphate solution for treatment of viral warts. Saudi Medical Journal 2007;28(9):1418-21. [PMID: ] [PubMed] [Google Scholar]
Sieber 2014
- Sieber MA, Hegel JK. Azelaic acid: Properties and mode of action. Skin Pharmacology and Physiology 2014;27(Suppl 1):9-17. [PMID: ] [DOI] [PubMed] [Google Scholar]
Simonart 2012
- Simonart T. Newer approaches to the treatment of acne vulgaris. American Journal of Clinical Dermatology 2012;13(6):357-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sorensen 2010
- Sorensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. Journal of Clinical Endocrinology and Metabolism 2010;95(1):263-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Spellman 1998
- Spellman MC, Pincus SH. Efficacy and safety of azelaic acid and glycolic acid combination therapy compared with tretinoin therapy for acne. Clinical Therapeutics 1998;20(4):711-21. [DOI] [PubMed] [Google Scholar]
Stamatiadis 1988
- Stamatiadis D, Bulteau-Portois MC, Mowszowicz I. Inhibition of 5 alpha-reductase activity in human skin by zinc and azelaic acid. British Journal of Dermatology 1988;119(5):627-32. [PMID: ] [DOI] [PubMed] [Google Scholar]
Stockton 1990
- Stockton DL, Paller AS. Drug administration to the pregnant or lactating woman: a reference guide for dermatologists. Journal of the American Academy of Dermatology 1990;23(1):87-103. [PMID: ] [DOI] [PubMed] [Google Scholar]
Strauss 2007
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, et al. Guidelines of care for acne vulgaris management. Journal of the American Academy of Dermatology 2007;56(4):651-63. [DOI] [PubMed] [Google Scholar]
Tan 2012
- Tan J, Wolfe B, Weiss J, Stein-Gold L, Bikowski J, Del Rosso J, et al. Acne severity grading: determining essential clinical components and features using a Delphi consensus. Journal of the American Academy of Dermatology 2012;67(2):187-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tan 2013
- Tan JK, Jones E, Allen E, Pripotnev S, Raza A, Wolfe B. Evaluation of essential clinical components and features of current acne global grading scales. Journal of the American Academy of Dermatology 2013;69(5):754-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thiboutot 2008
- Thiboutot D. Versatility of azelaic acid 15% gel in treatment of inflammatory acne vulgaris. Journal of Drugs in Dermatology 2008;7(1):13-6. [MEDLINE: ] [PubMed] [Google Scholar]
Thiboutot 2009
- Thiboutot D, Gollnick H, Bettoli V, Dreno B, Kang S, Leyden JJ, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. Journal of the American Academy of Dermatology 2009;60(5 Suppl):S1-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thiboutot 2016
- Thiboutot D, Zaenglein A. Pathogenesis, clinical manifestations, and diagnosis of acne vulgaris. www.uptodate.com/contents/pathogenesis-clinical-manifestations-and-diagnosis-of-acne-vulgaris (accessed 2 June 2016).
Titus 2012
- Titus S, Hodge J. Diagnosis and treatment of acne. American Family Physician 2012;86(8):734-40. [MEDLINE: ] [PubMed] [Google Scholar]
Well 2013
- Well D. Acne vulgaris: A review of causes and treatment options. Nurse Practitioner 2013;38(10):22-31; quiz 32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams C, Layton AM. Persistent acne in women: implications for the patient and for therapy. American Journal of Clinical Dermatology 2006;7(5):281-90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Xia 2007
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. The Leeds Outcomes Stakeholders Survey (LOSS) Study. In: Proceedings of the 15th Cochrane Colloquium; 2007 Oct 23-27. Sao Paulo: The Cochrane Collaboration, 2007.
Xia 2009
- Xia J, Adams C, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Losing participants before the trial ends erodes credibility of findings. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Yang 2014
- Yang Z, Zhang Y, Lazic ME, Li H, Hu J, Zhang Y, et al. Topical benzoyl peroxide for acne. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD011154. [DOI: 10.1002/14651858.CD011154] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zander 1992
- Zander E, Weisman S. Treatment of acne vulgaris with salicylic acid pads. Clinical Therapeutics 1992;14(2):247-53. [PubMed] [Google Scholar]
Zheng 2013
- Zheng Y, Wan M, Chen H, Ye C, Zhao Y, Yi J, et al. Clinical evidence on the efficacy and safety of an antioxidant optimized 1.5% salicylic acid (SA) cream in the treatment of facial acne: an open, baseline-controlled clinical study. Skin Research & Technology 2013;19(2):125-30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Liu 2014
- Liu H, Yu H, Xia J, Liu L, Liu GJ, Sang H. Topical azelaic acid, salicylic acid, nicotinamide, and sulphur for acne. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD011368. [DOI: 10.1002/14651858.CD011368] [DOI] [PMC free article] [PubMed] [Google Scholar]


