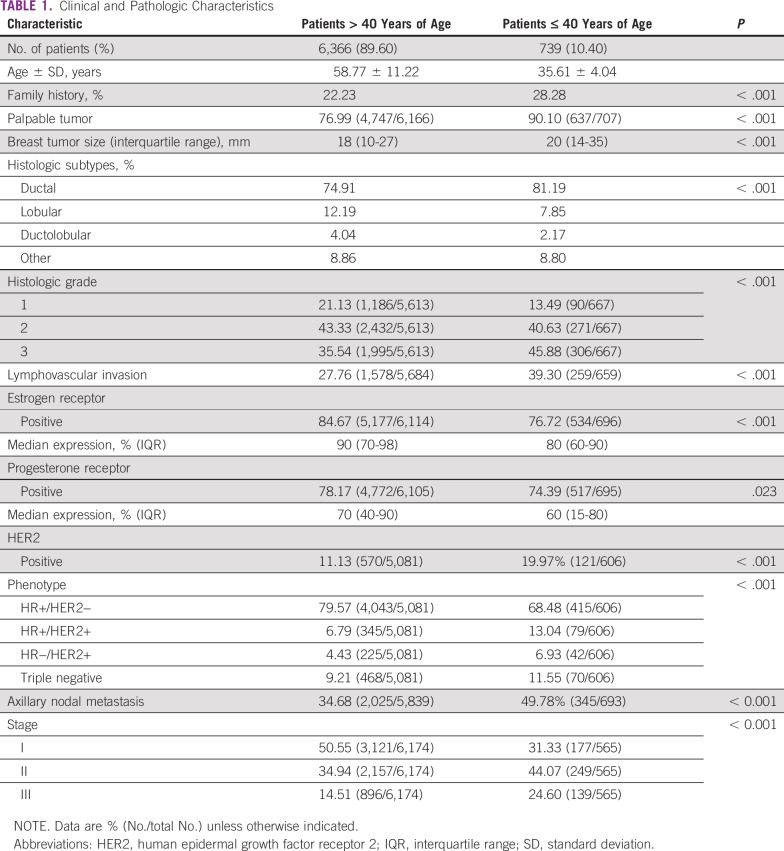
Abstract
PURPOSE
Multiple studies have reported that breast cancer in young patients is associated with aggressive characteristics, and it is suggested that prognosis is worse independently of pathologic variables.
PATIENTS AND METHODS
We performed a retrospective analysis of the Breast Cancer Registry of the Argentinian Society of Mastology, including public and private centers. Patients ≤ 40 years of age at diagnosis were classified as “young,” and patients ≤ 35 years of age at diagnosis were classified as “very young.” Univariate and multivariate analyses were performed to detect differences between groups.
RESULTS
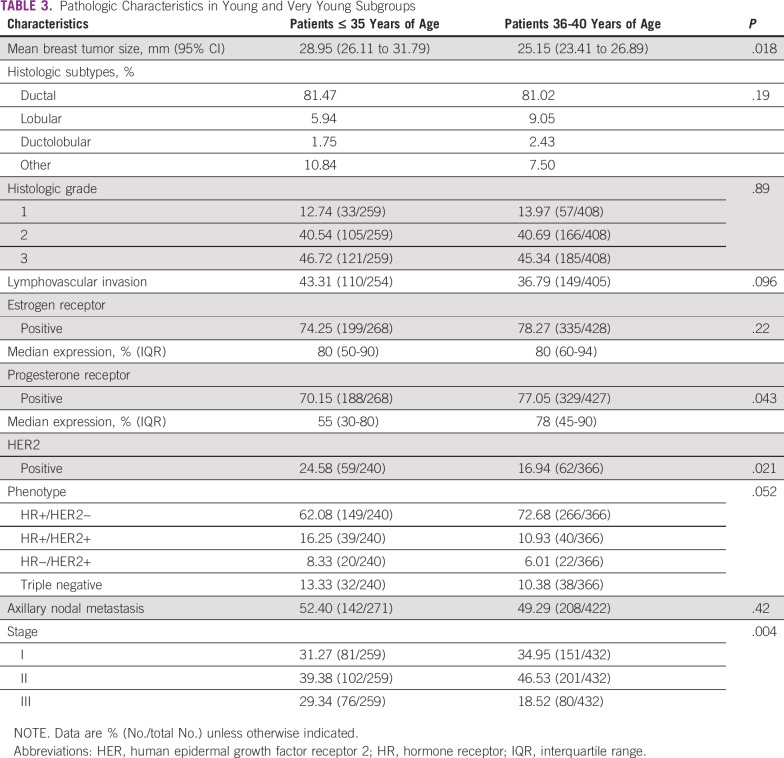
Patients ≤ 40 years of age comprised 10.40% (739/7,105) of the participants, with an average age of 35.61 ± 4.04 years. Multivariate analysis showed that human epidermal growth factor receptor 2 (HER2)-positive tumor phenotype (odds ratio [OR], 1.82), nodal involvement (OR, 1.69), histologic grade (grade 3 OR, 1.41), and tumor size (T2 OR, 1.37; T3-T4, 1.47) were independently associated with younger age at diagnosis. Patients ≤ 35 years of age (n = 286), compared with patients 36 to 40 years of age, had a higher proportion of HER2 tumors (24.58% v 16.94%; P = .021), absence of progesterone receptor expression (29.85% v 22.95%; P = .043), and stage 3 cancer (29.34% v 18.52%; P < .001). Fewer breast-conserving surgeries (75.37% v 62.89%; P < .001) and more adjuvant chemotherapy (59.04% v 36.66%; P < 0.001) were reported in patients ≤ 40 years of age.
CONCLUSION
In the population studied, breast cancer in young women was associated with aggressive pathologic features and locally advanced disease at the time of diagnosis. Moreover, tumor characteristics in very young patients with breast cancer nested in the population ≤ 40 years of age showed differences in important prognostic factors. More high-quality evidence is needed to improve treatment strategies in these patients.
INTRODUCTION
Breast cancer is the second most frequent form of cancer globally and the most common cancer in women. In Argentina, 19,000 new patients are diagnosed each year, and the incidence rate is 71 per 100,000.1 According to WHO, 146,000 new cases of breast cancer are detected in women < 40 years of age worldwide annually.1a Although considerable variation is evidenced in different reports, it is estimated that among patients with breast cancer, the proportion of women < 40 years of age is 7%.2 Multiple studies have reported that breast cancer in younger patients generally presents with more aggressive characteristics, such as lymphovascular invasion, high tumor grade, human epidermal growth factor receptor 2 (HER2) overexpression, or absence of hormone receptor expression.3-9 Furthermore, it is suggested that young age is an independent poor prognostic factor.10-12
For these reasons, the scientific community is starting to consider breast cancer in this population as a different biologic entity. In this scenario, evidence and knowledge in relation to epidemiology, biologic behavior, and optimal treatment strategies are scarce. The objective of this analysis was to identify epidemiologic and pathologic characteristics and treatment decisions in patients < 40 years of age, comparing these aspects with older patients. Moreover, as a secondary objective, we intended to detect the existence of unique epidemiologic characteristics in the subgroup of patients ≤ 35 years of age within the young age group.
CONTEXT
Key Objective
There is a scarcity of real-world, high-quality data on breast cancer in young women, especially in South America. Addressing the current status in Argentina should help clinicians, researchers, and decision makers better understand unmet needs.
Knowledge Generated
This large cohort of patients with breast cancer has shown that young patients present with larger tumors, a higher prevalence of aggressive characteristics, more advanced stages, and HER2 overexpression. Very young patients nested in the ≤ 40 years of age population manifest this characteristics even more deeply.
Relevance
Findings displayed in the present study reflect the characteristics of breast cancer in different age groups in Argentina and should help interpret the disease landscape for prognosis understanding and additional improvement of patient care.
PATIENTS AND METHODS
A retrospective analysis of the Breast Cancer Registry (Registro de Cáncer de Mama [RCM]) of the Argentinian Society of Mastology was performed. This prospective-retrospective electronic database was initiated in 2010, and it includes the collaboration of public and private centers in Buenos Aires, La Plata, Córdoba, and Tucumán. RCM is a breast surgery–based registry, including patients with early and locally advanced disease (resectable) only. Patients were included prospectively from 2010, and previously treated patients could also be retrospectively added. This analysis was conducted between January 2000 and January 2017. All patients included in the RCM database signed informed consent forms allowing sharing of their data. The majority of the Argentinian population is white. Information was entered independently by each participating center, according to pathologic and medical records. Patients were included in the analysis if they were diagnosed with invasive breast cancer and excluded if no information regarding age was entered. Patients were defined as “young” if age at breast cancer diagnosis was ≤ 40 years, and a subgroup of “very young” was considered within this group if the age at diagnosis was ≤ 35 years. Patients were considered to have a positive family history if they described breast cancer events in first- and second-degree relatives. Tumors were classified as hormone receptor–positive (HR+)/HER2−, HR+/HER2+, HR−/HER2+, or HR−/HER2− according to hormone receptors and HER2 expression. If any of these variables were missing, the patient was excluded from phenotype classification. HR was considered positive if immunohistochemical analysis (IHC) was greater than 1% for estrogen and/or progesterone receptors (PRs). HER2 was considered positive if it presented overexpression (3+) by IHC. If IHC was 2+, tumors were analyzed by fluorescence in situ hybridization, and they were considered positive if their ratio was > 2.2 or HER2 gene copy was > 6.0.13 Neither patients with surgical procedures before 2005 nor patients with microinvasive breast carcinoma, defined as invasive carcinoma of the breast with no invasive focus measuring more than 1 mm, had routine HER2 assessment in our country.
This research complied with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Inclusion of patients in the database was approved by the local institutional review boards of all participating institutions.
Statistical Analysis
Categorical variables were expressed as absolute numbers and percentages. Continuous variables were described in terms of means and standard deviations if normally distributed or medians and interquartile ranges otherwise. Comparisons among groups were conducted using the Student t test and Wilcoxon rank sum test depending on distribution for continuous variables, and χ2 test and Fisher exact test for categorical variables. Logistic regression analysis was performed to evaluate the independent association between age and clinical and pathologic characteristics. A P value < .05 was considered statistically significant. Statistical analysis was performed with STATA 14 (STATA, College Station, TX).
RESULTS
Patients’ Characteristics
Young patients comprised 10.40% of the included patients with invasive breast cancer (739/7,105). Clinical and pathologic characteristics are presented in Table 1. The average age in this group was 35.61 ± 4.04 years. As expected, a higher proportion of family history of breast cancer was observed in this population (28.28% [209/739] v 22.23% [1,415/6,366]; P < .001). Moreover, tumors were more likely to be clinically detectable on physical examination in young women (90.10%; 637/707) than in patients > 40 years of age (76.99%; 4,747/6,166; P < .001).
TABLE 1.
Clinical and Pathologic Characteristics
Differences between the two groups were also observed in tumor size, histologic subtypes, tumor grade, and lymphovascular invasion. In 606 of the 739 young patients, it was possible to determine the tumor phenotype, with 68.48% being classified as HR+/HER2−, 13.04% classified as HR+/HER2+, 6.93% classified as HR−/HER2+, and 11.55% classified as triple negative. A higher proportion of the more aggressive phenotypes was evidenced in young patients compared with patients > 40 years of age (HR+/HER2+, 6.79%; HR−/HER2+, 4.43%; and triple negative, 9.21%; P < .001). In relation to axillary involvement, 49.78% of the young patients presented with lymph node metastases, with a median of three positive nodes (IQR, 1-7), demonstrating more frequent nodal metastasis than in the older subgroup.
Multivariate Analysis of Clinical and Pathologic Characteristics
Multivariate analysis showed that characteristics independently associated with age ≤ 40 years were HER2 phenotype, nodal involvement, histologic grade, and tumor size (Table 2).
TABLE 2.
Multivariate Analysis of Pathologic Characteristics Associated With Age

Very Young Patients With Breast Cancer
By comparing the subgroup of patients ≤ 35 years of age (n = 286) with patients between 36 and 40 years of age, specific differences were detected (Table 3). First, a higher proportion of HER2+ tumors was observed (24.58% v 16.94%; P = .021; Fig 1A). Second, tumors were more frequently negative for PR expression (29.85% v 22.95%; P = .043; Fig 1B). Average tumor size was 28.95 mm (95% CI, 26.11 to 31.79 mm) v 25.15 mm (95% CI, 23.41 to 26.89 mm; P = .018) in patients between 36 and 40 years of age. When disease stage was defined, a higher proportion of stage 3 tumors was observed in very young patients (29.34% v 18.52%; P < .001; Fig 1C).
TABLE 3.
Pathologic Characteristics in Young and Very Young Subgroups
FIG 1.
Clinical characteristics presenting statistically significant differences in patients ≤ 35 years of age in relation to the patients between 36 and 40 years of age. (A) Proportion of human epidermal growth factor receptor 2 (HER2) tumors (P = .02). (B) Proportion of tumors with negative progesterone receptor (PR) expression (P = .043). (C) Stage classification (P < .001).
Treatment Strategies
Breast-conserving surgery was performed in 62.89% of the younger patients, representing a substantially lower proportion than the rate for older patients (75.37%; P < .001). In patients ≤ 40 years of age, 59.04% underwent treatment with adjuvant chemotherapy, whereas only 36.66% of the patients > 40 years of age received this treatment (P < .001). Neoadjuvant chemotherapy was the treatment of choice in 17.59% and 8.37% of the younger and older groups, respectively (P < .001). In the adjuvant setting, a higher proportion of young patients received anthracyclines (79.49% v 72.35%; P = .005) and taxanes (50.28% v 39.20%; P < .001). In contrast, a lower proportion of cyclophosphamide, methotrexate, and fluorouracil chemotherapy regimen was reported (15.73% v 22.40%; P = .005). Patients with HER2+ tumors received trastuzumab in a similar proportion in both age groups (98.53% v 95.60%; P = .26). No differences were observed in the use of anthracyclines in the neoadjuvant setting (92.31% v 88.18%; P = .18). Regarding hormonal therapy, 24.46% of the HR+ young patients received luteinizing hormone-releasing hormone (LHRH) analogs. The initial treatment of choice included tamoxifen in 97.52% of patients. In the group of patients with breast-conserving surgery, no differences were observed between age groups in relation to radiotherapy administration (P = .26).
Treatment strategies in the group of patients ≤ 35 years of age were similar to those in patients between 36 and 40 years regarding surgical procedure (P = .80), chemotherapy administration (P = .26), radiotherapy (P = .50), and hormonal therapy (P = .39). Nevertheless, a greater proportion of patients ≤ 35 years of age with HR+ tumors received LHRH analogs as adjuvant treatment (25.00% v 13.61%; P = .002).
DISCUSSION
In this study, we evaluated a large and representative Argentine database. We identified substantial clinicopathologic differences between young (≤ 40 years of age) and very young (≤ 35 years of age) patients with breast cancer. Moreover, differences in characteristics within the young group of patients, with more aggressive pathologic features, were shown. HER2 phenotype, high histologic grade, tumor size, and nodal metastasis were independently associated with young age in multivariate analysis.
The definition of young age is controversial, and studies have variously defined young age as age at diagnosis < 35, 40, 45, or even 50 years. All these classifications are arbitrary because there are no data to determine this distinction. European School of Oncology (ESO)–European Society for Medical Oncology (ESMO) 3rd international consensus guidelines for breast cancer in young women defines young women as those < 40 years of age.14 The rate of breast cancer in this group has been stable for the last 20 years.15 Approximately 6.6% of all patients with breast cancer are diagnosed in women < 40 years of age, 2.4% in women < 35 years of age, and 0.65% in women < 30 years of age,12,16 as the cumulative incidence of breast cancer follows an exponential function below the age of 40, after which it increases linearly.17 In the RCM database, 10.4% of the 7,105 patients were ≤ 40 years of age. This result is higher compared with US databases2 and akin to Asian registries.18,19 In relation to patients ≤ 35, our analysis showed a prevalence similar to studies addressing this population specifically.12,20 Clinical characteristics in this cohort of patients were in line with data reported in past studies worldwide, indicating that these patients present with larger tumors, a higher prevalence of aggressive characteristics, more advanced stages, and HER2 overexpression.4-7,9,12,19-26
It should be noted that in our retrospective analysis, almost 50% of young Argentinean patients had lymph node involvement at the time of diagnosis. This clinical presentation, as well as the presence of palpable tumors, could be explained by the lack of screening mammography recommendations for women < 40 years of age. In Argentina, according to our National Cancer Institute (Instituto Nacional del Cancer) guidelines, screening mammography should be offered to women between 50 and 70 years of age.
Many studies have confirmed that compared with older women, young women have higher proportions of triple-negative and HER2+ cancers.3,11,22,27 The phenotype distribution observed in our population is similar to that described by cited authors in relation to HER2+ but not triple-negative tumors. Other Latin American authors have reported on the increased incidence of basal-like or triple-negative carcinomas among young women. Carvalho et al28 found that 25.8% of women < 35 years of age had a basal-like phenotype, and Alvarado-Cabrero et al29 described 26% of women with triple-negative phenotype. The greater percentage of tumors with HER2 overexpression in younger patients observed in our study is supported by other studies.30,31
It is partially accepted that young women diagnosed with breast cancer have inferior clinical outcomes, and it is natural to wonder whether the inferior outcome is attributable to an over-representation of adverse pathologic features or whether age is an independent risk factor. The biologic variability described previously is likely to be the main contributing factor responsible for the mortality disparities observed. To further explore age-specific differences in breast cancer biology, several groups have evaluated the role of differential tumor gene expression comparing patients of different age groups.11,22,32 Authors have concluded that age alone does not appear to provide an additional layer of biologic complexity above that of breast cancer subtype and grade; therefore, when considering treatment programs, decisions should be driven by subtype biology and performance status, and much less influenced by age.
Evidence in relation to tumor characteristics in very young patients with breast cancer nested in the ≤ 40-years-of-age–population is still contradictory. We found a higher proportion of HER2-enriched subtypes, lower PR expression, and a higher rate of patients with advanced disease at diagnosis, in relation to tumor size and clinical stage. Similarly, Kollias et al,5 documented that patients diagnosed with breast cancer at < 35 years of age were more likely to have high-grade tumors and vascular invasion. However, tumor size and lymph node involvement were similar between the included age groups. An analysis based on premenopausal patients referred to the European Institute of Oncology showed that among the very young group (< 35 years of age), there was a higher prevalence of high-grade tumors, tumors classified as HR negative, and HER2 overexpression.33 Analysis of very young patients has not shown any differences in some cohorts, contrary to our results.3,10 Breast cancer in women < 40 years of age should prompt one to consider familial breast cancer syndromes and genetic testing because this situation may have clinical consequences. Regrettably, our database does not contain information on germinal mutations.
With respect to surgery, young women are generally treated similarly to their older counterparts, because factors guiding surgical decisions are the same. However, younger women have higher local recurrence rates than older women when treated with breast-conserving surgery.34-37 In this setting, some authors have questioned whether breast-conserving therapy among young women represents optimal therapy.38-40 Nevertheless, no studies have demonstrated that conservative surgery in young women has a negative impact on survival. In our sample, breast-conserving surgery was performed more frequently in older patients. Series such as the ones published by Bharat et al6 or Fredholm et al,12 showed that young women were more likely to undergo mastectomy rather than breast-conservation therapy. It is difficult to ascertain whether these differences could be attributed to preference, tumor characteristics, or increased rates of germline mutations that demand more aggressive treatment, because other studies show that breast conservation rates are similar in both age groups.10
Adjuvant chemotherapy was administered to almost 60% of the younger patients compared with 36.66% of the older patients. Different authors reported that chemotherapy was more commonly given to young women.10,12,20 Similar to other studies, a high proportion of patients had undergone chemotherapy regimens including anthracyclines, which are often recommended for patients with a high risk of disease recurrence.33
Multiple questions still arise while addressing the topic of breast cancer in young patients. This analysis has shown considerable clinicopathologic differences between age groups in Argentina. The strengths of our study reside in its large, population-based sample and thorough analysis of its database. To our knowledge, this is the first study analyzing this unique population with breast cancer in our country. Unfortunately, missing information in the database was one of the principal limitations, in addition to the lack of outcome data because the health system in Argentina has limited follow-up access. The poor prognosis of young women with breast cancer raises critical questions, and treatment decisions in this age group are complex. We are in need of more high-quality evidence to enlighten our knowledge and to optimize strategies to help our patients.
ACKNOWLEDGMENT
Centers collaborating with the Registro de Cáncer de Mama: Breast Clínica de la Mama (La Plata, Buenos Aires); Hospital Fernandez (Ciudad Autónoma de Buenos Aires [CABA]); Instituto Alexander Fleming (CABA); Hospital General de Agudo “P. Piñero” (CABA); Hospital Penna (CABA); Instituto de Oncología “Angel Roffo” (CABA); Hospital Rivadavia (CABA); Hospital Marie Curie (CABA); Hospital Nacional de Clínicas (Córdoba); Sanatorio Modelo de Caseros (Buenos Aires); F. Gutierrez Zigaran, MD, Hospital Posadas (Buenos Aires); Hospital de la Madre y el Niño; Hospital Aeronáutico Central (CABA); Hospital Centro de Salud (Tucumán); Instituto Modelo de Ginecologia y Obstetricia (Córdoba); Corporación Médica San Martín (Buenos Aires); and Laura Ruiz Díaz, MD, Sanatorio Adventista del Plata (Entre Ríos).
AUTHOR CONTRIBUTIONS
Conception and design: Verónica Fabiano, Pablo Mandó, Manglio Rizzo, Carolina Ponce, Federico Coló, Martín Loza, Jose Loza, María Victoria Costanzo, Adrián Nervo, Jorge Nadal, Reinaldo Chacón
Administrative support: Mora Amat, Florencia Perazzo
Provision of study materials or patients: Verónica Fabiano, Federico Coló, Martín Loza, Jose Loza, Daniel Mysler, Adrián Nervo
Collection and assembly of data: Verónica Fabiano, Pablo Mandó, Federico Coló, María Victoria Costanzo, Jorge Nadal
Data analysis and interpretation: Pablo Mandó, Manglio Rizzo, Carolina Ponce, Mora Amat, Daniel Mysler, María Victoria Costanzo, Jorge Nadal, Florencia Perazzo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jgo/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Pablo Mandó
Travel, Accommodations, Expenses: AstraZeneca
Manglio Rizzo
Consulting or Advisory Role: Takeda
Travel, Accommodations, Expenses: Takeda
Carolina Ponce
Travel, Accommodations, Expenses: Roche
Federico Coló
Speakers' Bureau: Roche
Jorge Nadal
Consulting or Advisory Role: Novartis, Roche, Pfizer, Lilly
Speakers' Bureau: Novartis, Roche, Pfizer, Varifarma
Travel, Accommodations, Expenses: Roche, Pfizer, Gador
Florencia Perazzo
Consulting or Advisory Role: AstraZeneca, Pfizer, Novartis, MSD Oncology
Speakers' Bureau: AstraZeneca, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cancer INd : El cáncer de mama en cifras en. Argentina, 2019. https://www.argentina.gob.ar/salud/instituto-nacional-del-cancer/estadisticas/incidencia
- 1a.Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(suppl 1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton LA, Sherman ME, Carreon JD, et al. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–1648. doi: 10.1093/jnci/djn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins LC, Marotti JD, Gelber S, et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 4.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 5.Kollias J, Elston CW, Ellis IO, et al. Early-onset breast cancer—Histopathological and prognostic considerations. Br J Cancer. 1997;75:1318–1323. doi: 10.1038/bjc.1997.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat A, Aft RL, Gao F, et al. Patient and tumor characteristics associated with increased mortality in young women (< or =40 years) with breast cancer. J Surg Oncol. 2009;100:248–251. doi: 10.1002/jso.21268. [DOI] [PubMed] [Google Scholar]
- 7.Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78:1838–1843. doi: 10.1002/(sici)1097-0142(19961015)78:8<1838::aid-cncr27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: Features of disease at presentation. Ann Oncol. 2002;13:273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 9.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208:341–347. doi: 10.1016/j.jamcollsurg.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3:65–72. doi: 10.3816/CBC.2002.n.013. [DOI] [PubMed] [Google Scholar]
- 11. Azim H, Michiels S, Bedard PL, et al: Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res 18:1341-1351, 2012. [DOI] [PubMed]
- 12.Fredholm H, Eaker S, Frisell J, et al. Breast cancer in young women: Poor survival despite intensive treatment. PLoS One. 2009;4:e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 14.Paluch-Shimon S, Pagani O, Partridge AH, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast. 2017;35:203–217. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. 2012;9:460–470. doi: 10.1038/nrclinonc.2012.102. [DOI] [PubMed] [Google Scholar]
- 16. Anders CK, Johnson R, Litton J, et al: Breast cancer before age 40 years. Semin Oncol 36:237-249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assi HA, Khoury KE, Dbouk H, et al. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(suppl 1):S2–S8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E-K, Noh WC, Han W, et al. Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: A nationwide registry-based study. World J Surg. 2011;35:1244–1253. doi: 10.1007/s00268-011-1071-1. [DOI] [PubMed] [Google Scholar]
- 19.Ahn SH, Son BH, Kim SW, et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: Nationwide survival data in Korea--a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 20.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albain KS, Allred DC, Clark GM: Breast cancer outcome and predictors of outcome: Are there age differentials? J Natl Cancer Inst Monogr 16:35-42, 1994. [PubMed] [Google Scholar]
- 22.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 23.Shannon C, Smith IE. Breast cancer in adolescents and young women. Eur J Cancer. 2003;39:2632–2642. doi: 10.1016/s0959-8049(03)00669-5. [DOI] [PubMed] [Google Scholar]
- 24.Caldarella A, Crocetti E, Bianchi S, et al. Female breast cancer status according to ER, PR and HER2 expression: A population based analysis. Pathol Oncol Res. 2011;17:753–758. doi: 10.1007/s12253-011-9381-z. [DOI] [PubMed] [Google Scholar]
- 25.Gajdos C, Tartter PI, Bleiweiss IJ, et al. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523–529. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 26.Kroman N, Jensen MB, Wohlfahrt J, et al. Factors influencing the effect of age on prognosis in breast cancer: Population based study. BMJ. 2000;320:474–478. doi: 10.1136/bmj.320.7233.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund MJ, Butler EN, Hair BY, et al. Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: A population-based study and first report. Cancer. 2010;116:2549–2559. doi: 10.1002/cncr.25016. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho FM, Bacchi LM, Santos PP, et al. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics (São Paulo) 2010;65:1033–1036. doi: 10.1590/S1807-59322010001000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarado-Cabrero I, Valencia-Cedillo R, Barroso-Bravo S. Breast cancer in Mexican women younger than age 45 years: A clinicopathologic study of 1,320 cases. Mod Pathol. 2011;24(suppl 1s):24–74. www.nature.com/articles/modpathol201115 [Google Scholar]
- 30.Agrup M, Stål O, Olsen K, et al. C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat. 2000;63:23–29. doi: 10.1023/a:1006498721508. [DOI] [PubMed] [Google Scholar]
- 31.Hartley MC, McKinley BP, Rogers EA, et al. Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: A case-control study. Am Surg. 2006;72:1189–1194. [PubMed] [Google Scholar]
- 32.Anders CK, Fan C, Parker JS, et al. Breast carcinomas arising at a young age: Unique biology or a surrogate for aggressive intrinsic subtypes? J Clin Oncol. 2011;29:e18–e20. doi: 10.1200/JCO.2010.28.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancello G, Maisonneuve P, Rotmensz N, et al. Prognosis and adjuvant treatment effects in selected breast cancer subtypes of very young women (<35 years) with operable breast cancer. Ann Oncol. 2010;21:1974–1981. doi: 10.1093/annonc/mdq072. [DOI] [PubMed] [Google Scholar]
- 34.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 35.Kurtz JM, Jacquemier J, Amalric R, et al. Why are local recurrences after breast-conserving therapy more frequent in younger patients? J Clin Oncol. 1990;8:591–598. doi: 10.1200/JCO.1990.8.4.591. [DOI] [PubMed] [Google Scholar]
- 36.Freedman GM, Hanlon AL, Fowble BL, et al. Recursive partitioning identifies patients at high and low risk for ipsilateral tumor recurrence after breast-conserving surgery and radiation. J Clin Oncol. 2002;20:4015–4021. doi: 10.1200/JCO.2002.03.155. [DOI] [PubMed] [Google Scholar]
- 37.Arvold ND, Taghian AG, Niemierko A, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coulombe G, Tyldesley S, Speers C, et al: Is mastectomy superior to breast-conserving treatment for young women? Int J Radiat Oncol Biol Phys 67:1282-1290, 2007. [DOI] [PubMed]
- 39. doi: 10.1002/cncr.2820741331. McCormick B: Selection criteria for breast conservation. The impact of young and old age and collagen vascular disease. Cancer 74, 430-435, 1994 (1; suppl) [DOI] [PubMed] [Google Scholar]
- 40.White JR, Halberg FE, Rabinovitch R, et al. American College of Radiology appropriateness criteria on conservative surgery and radiation: Stages I and II breast carcinoma. J Am Coll Radiol. 2008;5:701–713. doi: 10.1016/j.jacr.2008.02.026. [DOI] [PubMed] [Google Scholar]