Abstract
It has been reported that actin polymerization is regulated by protein tyrosine phosphorylation in smooth muscle on contractile stimulation. The role of protein serine/threonine phosphorylation in modulating actin dynamics is underinvestigated. SLK (Ste20-like kinase) is a serine/threonine protein kinase that plays a role in apoptosis, cell cycle, proliferation, and migration. The function of SLK in smooth muscle is mostly unknown. Here, SLK knockdown (KD) inhibited acetylcholine (ACh)-induced actin polymerization and contraction without affecting myosin light chain phosphorylation at Ser-19 in human airway smooth muscle. Stimulation with ACh induced paxillin phosphorylation at Ser-272, which was reduced in SLK KD cells. However, SLK did not catalyze paxillin Ser-272 phosphorylation in vitro. But, SLK KD attenuated Plk1 (polo-like kinase 1) phosphorylation at Thr-210. Plk1 mediated paxillin phosphorylation at Ser-272 in vitro. Expression of the nonphosphorylatable paxillin mutant S272A (substitution of alanine at Ser-272) attenuated the agonist-enhanced F-actin/G-actin ratios without affecting myosin light chain phosphorylation. Because N-WASP (neuronal Wiskott-Aldrich Syndrome Protein) phosphorylation at Tyr-256 (an indication of its activation) promotes actin polymerization, we also assessed the role of paxillin phosphorylation in N-WASP activation. S272A paxillin inhibited the ACh-enhanced N-WASP phosphorylation at Tyr-256. Together, these results suggest that SLK regulates paxillin phosphorylation at Ser-272 via Plk1, which modulates N-WASP activation and actin polymerization in smooth muscle. SLK-mediated actin cytoskeletal reorganization may facilitate force transmission between the contractile units and the extracellular matrix.
Keywords: smooth muscle, actin cytoskeleton, contraction, phosphorylation, signal transduction
Clinical Relevance
We discovered a novel mechanism that controls actin dynamics and contraction in smooth muscle. This finding provides a fundamental knowledge to investigate smooth muscle diseases such as asthma.
Smooth muscle contraction can be viewed as “moving of a car.” Myosin may serve as an “engine” for smooth muscle contraction, whereas the actin cytoskeleton may act as a “transmission system” in smooth muscle. Both myosin activation and actin cytoskeletal reorganization are necessary for smooth muscle contraction (1–7). Increased actin filament assembly may strengthen the connection between the actin cytoskeleton and the integrin-associated complex, which promotes force transmission from the contractile units to the extracellular matrix (1, 2, 6, 8). Actin polymerization may also enhance the number of contractile units and promote smooth muscle contraction (1, 9, 10).
It has been reported that actin polymerization is regulated by protein tyrosine phosphorylation in various cell types, including smooth muscle (1, 2, 6). For instance, contractile stimulation activates protein tyrosine kinases (e.g., c-Abl, focal adhesion kinase), which subsequently catalyze tyrosine phosphorylation of actin-regulatory proteins (e.g., cortactin, p130CAS, Abi1, paxillin). Tyrosine-phosphorylated proteins in turn activate N-WASP (neuronal Wiskott-Aldrich Syndrome Protein; a master regulator of actin polymerization) and actin polymerization in smooth muscle (1, 2, 6). However, the knowledge regarding the role of protein serine/threonine phosphorylation in actin dynamics is limited.
SLK (Ste20-like kinase) is a serine/threonine protein kinase that plays a role in apoptosis, cell cycle, proliferation, and migration (11–14). During G2/M transition, SLK phosphorylates and activates Plk1 (polo-like kinase 1) (15), which regulates centrosome formation, spindle assembly, and cytokinesis (16). Our previous studies have shown that Plk1 plays a role in regulating human airway smooth muscle contraction (17). This raises the possibility that SLK may be involved in smooth muscle contraction.
Paxillin is an adapter protein that has been implicated in actin dynamics, focal adhesion assembly, adhesion, cell migration, and smooth muscle contraction (2, 18–20). Paxillin undergoes phosphorylation at Ser-273 (equivalent to human paxillin Ser-272, NCBI Accession number, NP_002850.2) during migration of Chinese hamster ovary (CHO)-K1 cells (21) and canine airway smooth muscle on contractile activation (22). Paxillin phosphorylation at this residue regulates cell migration and smooth muscle contraction (21, 22).
In this study, we find that SLK regulates paxillin phosphorylation at Ser-272 via Plk1, which modulates actin polymerization and the contraction in smooth muscle. Thus, SLK is an upstream regulator for the actin cytoskeleton in smooth muscle.
Methods
Cell Culture
Human airway smooth muscle (HASM) cells were prepared from human bronchi and adjacent tracheas obtained from the International Institute for Advanced Medicine as previously described (3–5, 23, 24). Human tissues were nontransplantable and consented by donors for research. This study was approved by the Albany Medical College Committee on Research Involving Human Subjects. Smooth muscle cells within passage 5 were used for the studies. Primary cells from three donors were used for most experiments. Characteristics of donors are summarized in Table 1. In some cases, duplicate or triplicate experiments from cells of one donor were used for analysis.
Table 1.
Characteristics of Lung Donors
| Donor | No Asthma | Age (yr) | Race and Ethnicity | Sex | Cause of Death |
|---|---|---|---|---|---|
| 1 | Yes | 25 | Hispanic | M | Blunt injury |
| 2 | Yes | 32 | White | F | Asphyxiation |
| 3 | Yes | 56 | White | M | Head trauma |
Immunoblot Analysis
Western blotting of cell lysis was performed using the experimental procedures as previously described (17, 23, 25–27). Anti-SLK (1:250) was purchased from Santa Cruz Biotechnology (#sc-79068, L/N B2410). Total Plk1 antibody (1:1,000) was purchased from EMD Millipore (#05-844, L/N 2477015). Anti-SLK and anti-Plk1 were validated by using corresponding knockdown (KD) cells. GAPDH antibody (1:10,000) was purchased from Ambion (AM4300, L/N 1311029) and validated by assessing molecular weight of detected bands. Anti–p-paxillin (S272) (SAB4301321, L/N 461211762; Sigma) and anti-paxillin (#610052/ L/N 7208686; BD Biosciences) were validated by assessing molecular weight of detected bands. Phospho-Plk1 (T210) antibody (1:500) was purchased from Cell Signaling (#9062S, L/N 1) and validated by assessing molecular weight of detected bands and higher intensity in stimulated cells. Antibody against phospho-myosin light chain (Ser-19, 1:250) was purchased from Santa Cruz Biotechnology (sc-19849-R, L/N B2411) and validated by assessing molecular weight of detected bands and higher intensity in stimulated cells. Anti–myosin light chain (1:2,000) was a gift of Dr. Gunst (28, 29) and validated by assessing molecular weight of detected bands. Anti–phospho-N-WASP (Y256) (#AB1966, L/N 27954912838736; EMD Millipore) and anti–N-WASP (#sc-10121, L/N G2211; Santa Cruz Biotechnology) were validated by assessing molecular weight of detected bands and higher intensity in stimulated cells. Finally, vendors have provided data sheets to show that antibodies were validated by positive controls.
The levels of proteins were quantified by scanning densitometry of immunoblots (Fuji Multigauge Software or GE IQTL software). The luminescent signals from all immunoblots were within the linear range.
Virus-mediated RNA Interference
For Plk1 KD, lentiviruses encoding Plk1 shRNA (sc-36277-V) or control shRNA (sc-108080) were purchased from Santa Cruz Biotechnology. Stable HASM Plk1 KD cells were generated using the methods as previously described (17). Plk1 KD cells and cells expressing control shRNA were stable at least five passages after initial infection.
Analysis of F-Actin/G-Actin Ratios
The ratios of F-actin and G-actin in smooth muscle were evaluated using the fractionation assay as previously described (4, 5, 23, 30).
Co-IP Analysis
Co-IP analysis was performed using the experimental procedures as previously described (3–5, 23, 25).
In Vitro Kinase Assay
Purified SLK (#14-652, L/N D7HN034U-B) and Plk1 (#14-777) were purchased from EMD Millipore. Purified paxillin was purchased from Raybiotech (#P49023-2, L/N 11G1113W). Active Plk1 or SLK (40 ng) and 1 μg paxillin were placed in 20 μl kinase buffer containing 20 mM HEPES (pH7.5), 60 mM NaCl, 2 mM MgCl2, 5 mM EGTA, and 100 μM ATP. Kinase reaction mix was incubated at 30°C for 30 minutes and stopped by the addition of the SDS sample buffer (30, 31). The samples were boiled for 5 minutes and separated by SDS-PAGE followed by membrane transfer. The membrane was probed with phospho-paxillin antibody, stripped, and reprobed with paxillin antibody.
Mutagenesis, Plasmid Purification, and Cell Transfection
S272A paxillin (alanine substitution at Ser-272) was generated by using Quick change II XL site-directed mutagenesis kit (Agilent Technologies). The template plasmid pmcherry-paxillin (Addgene plasmid #50526) was a gift of Kenneth Yamada of National Institute of Dental and Craniofacial Research. Sequence of the 5′-primer was 5′-GGA CGA GCT GAT GGC TGC GCT GTC GGA TTT CAA G-3′. The 3′ primer was 5′-CTT GAA ATC CGA CAG CGC AGC CAT CAG CTC GTC C-3′. The primers were synthesized by ThermoFisher. The PCR product was subcloned into pcDNA3 3 × Flag and was transformed into XL10-Gold Ultracompetent cells (Agilent Technologies). Plasmids were purified by using the Pureklink Quick Plasmid Miniprep kit (Invitrogen). DNA sequencing was performed by Genewiz.
For SLK KD, SLK siRNA (sc-76514) and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. Cell transfection was performed using the siRNA transfection reagent (sc-29528; Santa Cruz).
Measurement of Human Bronchial Ring Contraction
The study was approved by Albany Medical College Institutional Review Board. Bronchial rings (diameter, 5 mm) were prepared from human lungs obtained from the International Institutes for Advanced Medicine (see above). Bronchial rings were placed in physiological saline solution at 37°C in a 25-ml organ bath and attached to a Grass force transducer that had been connected to a computer with A/D converter (Grass). The thin epithelium layer of human bronchial rings was removed by using forceps. We used reversible permeabilization (28–30) to introduce SLK or control siRNA, the constructs of wild-type (WT) or mutant paxillin, into human bronchi as previously described (5, 17, 23). Contraction on acetylcholine (ACh) (100 μM, 10 min) activation was compared before and after transduction. For biochemical analysis, human tissues were frozen using liquid nitrogen and pulverized as previously described (5, 32, 33).
Statistical Analysis
All statistical analysis was performed using Prism software (GraphPad Software). Differences between pairs of groups were analyzed by Student’s t test. Comparison among multiple groups was performed by one-way or two-way ANOVA followed by a post hoc test (Tukey’s multiple comparisons). Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
Results
SLK Regulates Actin Polymerization without Affecting Myosin Light Chain Phosphorylation
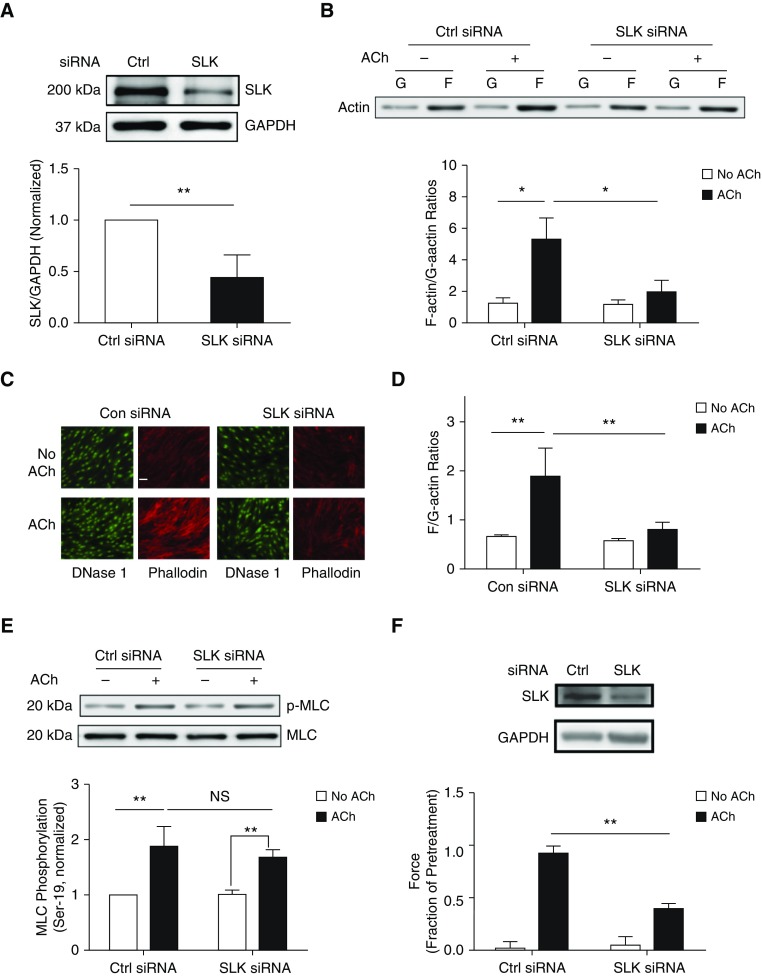
To assess the role of SLK in smooth muscle, we silenced SLK protein expression in HASM cells by using siRNA. Immunoblot analysis showed that treatment with SLK siRNA reduced SLK protein expression by 55% (Figure 1A). Because SLK is involved in cell proliferation (14), complete KD of SLK impairs cell viability. Thus, we used the experimental condition, in which SLK was partially downregulated, for the following studies.
Figure 1.
Ste20-like kinase (SLK) regulates actin polymerization and smooth muscle contraction without affecting myosin light chain phosphorylation at Ser-19. (A) Human airway smooth muscle (HASM) cells were treated with control (Ctrl or Con) or SLK siRNA for 2 days. Protein expression in these cells was evaluated by IB. Data are mean values of experiments using five batches of cell culture from three donors. Error bars indicate SD. (B) HASM cells treated with Ctrl or SLK siRNA were stimulated with 10−4 M acetylcholine (ACh) for 5 minutes or left unstimulated. F-actin/G-actin ratios in cells were evaluated using the fractionation assay. Data are mean values from six batches of cell culture from three donors. Error bars indicate SD. (C and D) Fluorescent images illustrating the effects of SLK knockdown (KD) on F/G-actin ratios in HASM cells. Phalloidin was used to detect F-actin, whereas DNase I was used to detect G-actin. SLK KD attenuates F/G-actin ratios on stimulation with ACh (10−4 M, 5 min). Data are means ± SD (n = 23–30 images from three independent experiments). Scale bar: 30 μm. (E) Myosin light chain (MLC) phosphorylation (p) at Ser-19 in cells treated with Ctrl or SLK siRNA was assessed by immunoblot analysis. Basal and ACh (10−4 M, 5 min) -induced myosin phosphorylation was similar in cells expressing Ctrl siRNA or SLK siRNA. NS = not significant. Data are mean values from four batches of cell culture from three donors. Error bars indicate SD. (F) Immunoblots showing the effects of SLK siRNA on SLK expression in human bronchial tissues. Blots are representative of three identical experiments. Contraction of human bronchial rings to ACh was evaluated, after which siRNA was introduced into tissues by reversible permeabilization. Contractile responses (ACh, 10−4 M, 5 min) were compared before and after incubation. Passive tension is normalized to the ACh-induced contractile response before incubation. Data are mean values of six samples from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Student’s t-test was used for statistical analysis of A and F. Two-way ANOVA was used for statistical analysis of B, D, and E.
To assess the role of SLK in actin dynamics, we evaluated F-actin/G-actin ratios in SLK KD cells by using the actin fractionation assay and by using labeled phalloidin and deoxyribonuclease I. SLK KD significantly reduced the increase in F-actin/G-actin ratios 5 minutes after ACh stimulation (Figures 1B–1D). However, myosin light chain phosphorylation at Ser-19 was not affected by SLK KD (Figure 1E). Furthermore, we used reversible permeabilization to introduce SLK siRNA into human bronchial rings. Contractile response was reduced by SLK KD (Figure 1F). In addition, SLK KD did not dramatically affect passive tension (Figure 1F).
SLK KD Diminishes Paxillin Phosphorylation at Ser-272 in Smooth Muscle on Contractile Stimulation
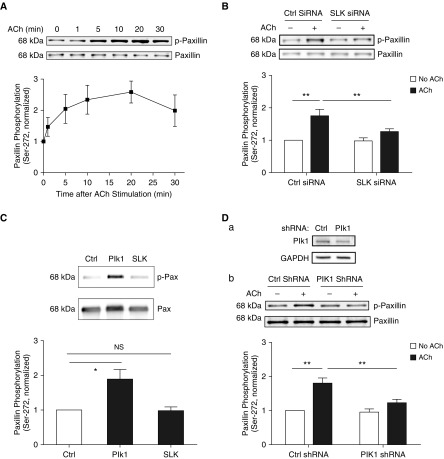
Because rodent paxillin phosphorylation at Ser-273 (equivalent to human paxillin Ser-272) has been implicated in CHO-K1 cell migration, a process associated with actin dynamics (21), we hypothesized that SLK may modulate actin dynamics by affecting paxillin phosphorylation at Ser-272. First, we determined whether paxillin gets phosphorylated at this residue on contractile stimulation. Immunoblot analysis showed that ACh stimulation induced paxillin phosphorylation at Ser-272 in smooth muscle cells, which significantly increased after 5 minutes (Figure 2A). We then determined whether SLK KD affects paxillin phosphorylation 5 minutes after ACh stimulation. We chose the time point because ACh stimulation for 5 minutes significantly increases F/G-actin ratios, myosin light chain phosphorylation, and contractile force in HASM cells/tissues (4, 5, 23) besides paxillin phosphorylation at Ser-272. SLK silencing reduced the ACh-stimulated paxillin phosphorylation at Ser-272 (Figure 2B). The results suggest that SLK regulates paxillin phosphorylation at Ser-272 in smooth muscle during agonist activation.
Figure 2.
Differential roles of SLK and Plk1 (polo-like kinase 1) in regulating paxillin phosphorylation at Ser-272. (A) ACh stimulation enhances paxillin Ser-272 phosphorylation. HASM cells were exposed to 10−4 M ACh for different time points. Paxillin phosphorylation at Ser-272 was assessed by immunoblot analysis. Data are mean values of experiments from five batches of cell culture from three donors. Error bars indicate SD. (B) Smooth muscle cells transfected with Ctrl or SLK siRNA were stimulated with 10−4 M ACh for 5 minutes, or unstimulated. Paxillin phosphorylation at this residue was evaluated by IB. SLK KD inhibits the ACh-induced paxillin phosphorylation in cells. Data are mean values of experiments from five batches of cell culture from three donors. Error bars represent SD. (C) In vitro kinase assay shows that Plk1, but not SLK, catalyzes paxillin phosphorylation at Ser-272. Data are mean values of four independent in vitro kinase reactions. Error bars indicate SD. (D) Stable Plk1 KD in HASM cells were verified by immunoblot analysis. (a) Blots are representative of four identical experiments. (b) Cells expressing Ctrl or Plk1 shRNA were stimulated with 10−4 M ACh for 5 minutes, or unstimulated. Paxillin phosphorylation was evaluated by immunoblot analysis. Data are mean values of experiments from five batches of cell culture from three donors. Error bars represent SD. *P < 0.05 and **P < 0.01. Student’s t-test was used for statistical analysis of A. One-way ANOVA was used for statistical analysis of C. Two-way ANOVA was used for statistical analysis of B and D.
Paxillin Phosphorylation at Ser-272 Is Not Catalyzed by SLK In Vitro
Next, we determined whether SLK directly catalyzes paxillin phosphorylation at this residue. Unexpectedly, the addition of SLK in the buffer containing purified human paxillin did not promote paxillin phosphorylation at this residue (Figure 2C). The results indicate that SLK indirectly mediates paxillin phosphorylation at Ser-272 during contractile activation.
Plk1 Regulates Paxillin Phosphorylation at Ser-272 in Smooth Muscle on Contractile Stimulation
Because Plk1 is a serine/threonine protein kinase that has a role in smooth muscle contraction (17, 34), we evaluated whether paxillin phosphorylation at this position is regulated by Plk1. Intriguingly, the in vitro kinase assay showed that the addition of Plk1 led to paxillin phosphorylation at Ser-272 (Figure 2C), suggesting a direct role of Plk1 in paxillin phosphorylation at this position. More importantly, Plk1 KD attenuated paxillin phosphorylation at Ser-272 in smooth muscle in response to ACh stimulation (Figure 2D).
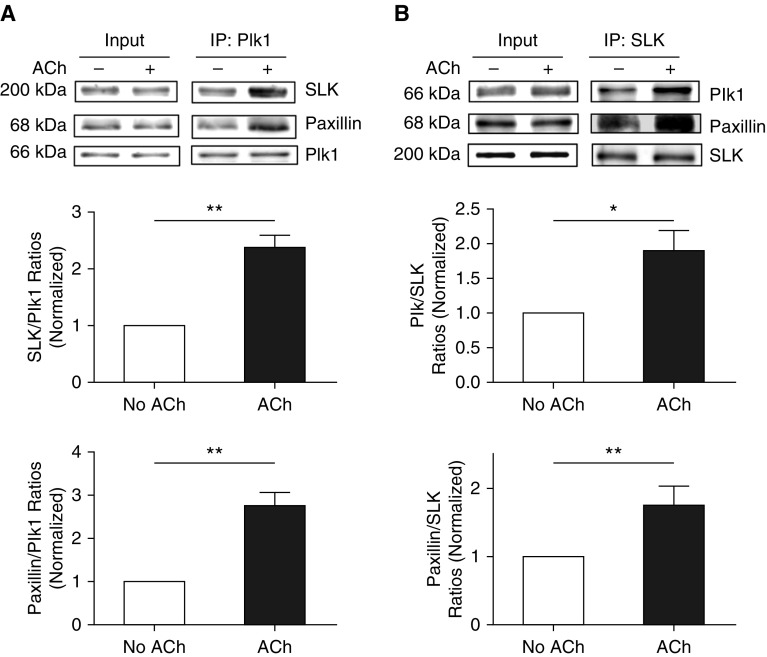
ACh Activation Enhances Formation of a Multiprotein Complex Including SLK, Plk1, and Paxillin in Smooth Muscle
To further evaluate the roles of SLK and Plk1 in paxillin, we determined whether contractile activation increases their interactions in smooth muscle by using co-IP analysis. The amount of SLK precipitated with Plk1 in ACh-stimulated cells was higher than unstimulated cells (Figure 3A). The ratios of SLK over Plk1 were increased in cells treated with ACh compared with unstimulated cells (Figure 3A). Similarly, contractile stimulation enhanced the interaction of paxillin with Plk1 in smooth muscle (Figure 3A). Moreover, reverse co-IP analysis verified the formation of the multiprotein complex (Figure 3B). The results suggest that SLK complexes with Plk1 and paxillin in unstimulated cells, and contractile stimulation enhances the assembly of the multiprotein complex including SLK, Plk1, and paxillin in smooth muscle.
Figure 3.
Activation with ACh enhances formation of the multiprotein complex including SLK, Plk1, and paxillin. (A) Extracts of unstimulated and ACh (10−4 M, 5 min)-stimulated HASM cells were immunoprecipitated with Plk1 antibody. Blots of the immunoprecipitates and input were probed with antibodies against SLK, Plk1, and paxillin. Protein ratios of SLK/Plk1 and paxillin/SLK in stimulated cells are normalized to the ratios obtained from unstimulated cells. Data are mean values of experiments from four batches of cell culture from three donors. Error bars represent SD. (B) Reverse co-IP analysis also demonstrates that ACh stimulation increases the assembly of the multiprotien complex. Protein ratios of paxillin/SLK and Plk1/SLK in ACh-treated cells are normalized to untreated cells. Data are mean values of experiments from four batches of cell culture from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Student’s t-test was used for statistical analysis.
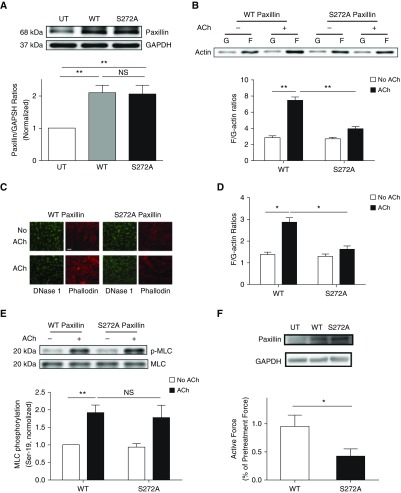
Paxillin Phosphorylation at Ser-272 Modulates Actin Polymerization
Next, we assessed whether paxillin phosphorylation at this residue affects actin dynamics in smooth muscle by introducing WT or S272A paxillin mutant into smooth muscle cells. Immunoblot analysis showed the expression of WT and S272A paxillin in smooth muscle cells (Figure 4A). Compared with cells expressing WT paxillin, S272A paxillin reduced the ACh-induced enhancement of F-actin/G-actin ratios as evidenced by the fractionation assay (Figure 4B) and fluorescent microscopy (Figures 4C and 4D). However, the expression of S272A paxillin did not affect myosin light chain phosphorylation at Ser-19 (Figure 4E). Moreover, we used reversible permeabilization to introduce WT or S272A paxillin into human bronchial rings. Introduction of S272A paxillin reduced contractile response as compared with the rings treated with WT paxillin (Figure 4F).
Figure 4.
Paxillin phosphorylation at Ser-272 modulates actin polymerization, but not myosin light chain phosphorylation, at Ser-19 in smooth muscle. (A) Extracts of untransfected (UT) cells and cells expressing wild-type (WT) or S272A paxillin were evaluated by immunoblot analysis. Data are mean values of experiments from four batches of cell culture from three donors. Error bars represent SD. (B) HASM cells expressing WT or S272A paxillin were stimulated with ACh (10−4 M, 5 min), or left unstimulated. F-actin/G-actin ratios were evaluated using the fractionation assay. Data are mean values of four batches of cell culture from three donors. Error bars indicate SD. (C and D) Fluorescent images illustrating the effects of S272A paxillin on F/G-actin ratios in HASM cells. Expression of S272A paxillin attenuates the ACh (10−4 M, 5 min) -induced F/G-actin ratios. Data are means ± SD (n = 24–26 images from three independent experiments). Scale bar: 30 μm. (E) Smooth muscle cells expressing recombinant paxillin were stimulated with ACh (10−4 M, 5 min) or left unstimulated. MLC phosphorylation at Ser-19 was determined by IB. Data are mean values of four batches of cell culture from three donors. Error bars represent SD. (F) Immunoblots showing the expression of WT or S272A paxillin in human bronchial tissues. Blots are representative of three identical experiments. Contractile response of human bronchial rings was evaluated, followed by reversible permeabilization to introduce constructs for WT or S272A paxillin. Active force (ACh, 10−4 M, 5 min) was compared before and after the introduction. Data are mean values of six samples from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. One-way ANOVA was used for statistical analysis of A. Two-way ANOVA was used for statistical analysis of B, D, and E. Student’s t test was used for statistical analysis of F.
N-WASP Phosphorylation at Tyr-256 Is Regulated by Paxillin Phosphorylation, Plk1, and SLK
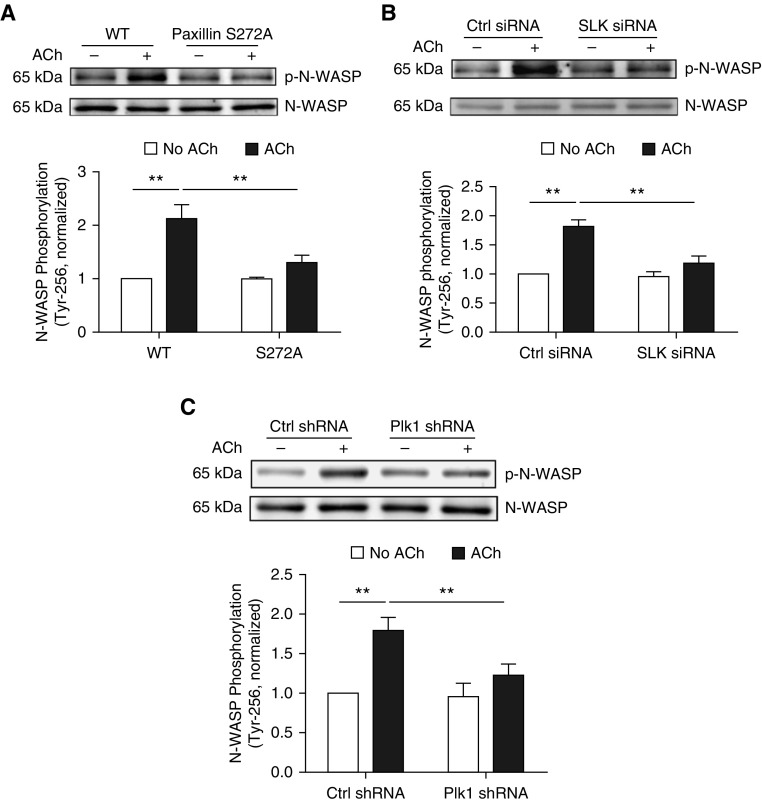
Previous studies have shown that N-WASP is a key actin polymerization activator in smooth muscle and other cell types (1, 2, 35). To determine whether paxillin serine/threonine phosphorylation affects N-WASP, we evaluated the effects of S272A paxillin on N-WASP phosphorylation at Tyr-256 (an indication of N-WASP activation) (35). Compared with cells expressing WT paxillin, ACh-induced N-WASP phosphorylation was diminished in cells treated with S272A paxillin (Figure 5A).
Figure 5.
N-WASP phosphorylation at Tyr-256 is regulated by paxillin phosphorylation at Ser-272, SLK, and Plk1 in smooth muscle. (A) Smooth muscle cells expressing WT or S272A paxillin were stimulated with ACh (10−4 M, 5 min) or left unstimulated. N-WASP phosphorylation at Tyr-256 was evaluated by immunoblot analysis. Data are mean values of four batches of cell culture from three donors. Error bars indicate SD. (B) Cells treated with Ctrl or SLK siRNA were stimulated with 10−4 M ACh for 5 minutes, or unstimulated. N-WASP phosphorylation at Tyr-256 was evaluated by IB. Data are mean values of experiments from four batches of cell culture from three donors. Error bars represent SD. (C) Cells expressing Ctrl or Plk1 shRNA were stimulated with 10−4 M ACh for 5 minutes, or unstimulated, followed by immunoblot analysis. Data are mean values of experiments from four batches of cell culture from three donors. Error bars indicate SD. **P < 0.01. Two-way ANOVA was used for statistical analysis.
Because SLK and Plk1 mediate paxillin phosphorylation at Ser-272, we also evaluated whether they affect N-WASP phosphorylation. KD of SLK or Plk1 attenuated the ACh-induced phosphorylation of N-WASP at Tyr-256 (Figures 5B and 5C).
SLK Regulates Phosphorylation of Plk1 in Smooth Muscle in Response to Agonist Activation
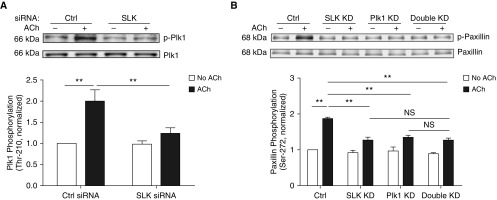
Contractile activation increases Plk1 phosphorylation at Thr-210 in smooth muscle (17). We evaluated whether SLK regulates Plk1 activation in smooth muscle by determining the effects of SLK KD on Plk1 phosphorylation at Thr-210. SLK silencing reduced the ACh-induced Plk1 phosphorylation at this position (Figure 6A).
Figure 6.
SLK mediates Plk1 phosphorylation at Thr-210 in smooth muscle. (A) Extracts of unstimulated or stimulated HASM cells treated with Ctrl or SLK siRNA were immunoblotted with antibodies against phospho-Plk1 (Thr-210) and total Plk1. Data are mean values of experiments from five batches of cell culture from three donors. Error bars indicate SD. (B) Paxillin phosphorylation at Ser-272 on ACh stimulation is similar in single KD cells and double KD cells. Control cells, SLK KD cells, Plk1 KD cells, and double KD cells were stimulated with ACh (10−4 M, 5 min) or left unstimulated. Paxillin phosphorylation at Ser-272 was evaluated by immunoblot analysis. Data are mean values of experiments from three batches of cell culture from three donors. Error bars indicate SD. **P < 0.01. Two-way ANOVA was used for statistical analysis.
Effects of Double KD of SLK and Plk1 on Paxillin Phosphorylation at Ser-272
To further assess the role of SLK and Plk1 in paxillin phosphorylation, we performed the double KD experiments by using SLK siRNA and Plk1 shRNA. The ACh-induced paxillin phosphorylation at this residue in double KD cells was similar to the phosphorylation levels in either SLK KD cells or Plk1 KD cells (Figure 6B). These results suggest that the effects of SLK and Plk1 on paxillin phosphorylation are not additive.
SLK Regulates the Plk1-Paxillin Pathway in Airway Smooth Muscle in Response to Stimulation with Histamine and 5-Hydroxtryptamine
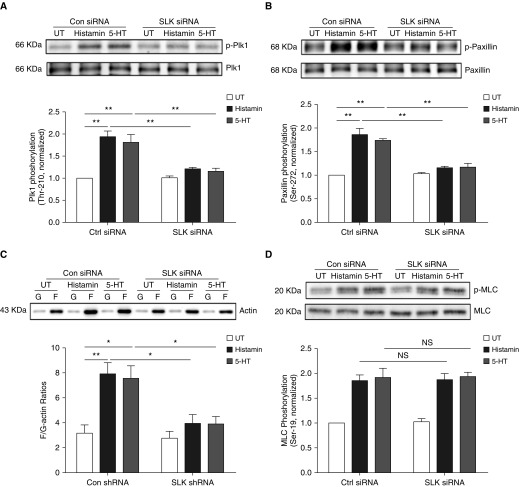
Thus far, we predominantly used ACh as a contractile agonist in our protocol. To evaluate the role of SLK in smooth muscle on other contractile stimuli, we evaluated the effects of SLK KD on the Plk1-paxillin cascade and myosin activation on stimulation with histamine or 5-hydroxtryptamine (5-HT). SLK KD attenuated Plk1 phosphorylation at Thr-210, paxillin phosphorylation at Ser-272, and F/G-actin ratios on stimulation with histamine and 5-HT without affecting myosin light chain phosphorylation in these cells (Figure 7).
Figure 7.
Role of SLK in the Plk1-paxillin pathway on stimulation with histamine and 5-HT. (A) Extracts of unstimulated or stimulated HASM cells treated with Ctrl or SLK siRNA were immunoblotted with antibodies against phospho-Plk1 (Thr-210) and total Plk1. Data are mean values of experiments from four batches of cell culture from three donors. Error bars indicate SD. (B and C) Paxillin phosphorylation at Ser-272 and F/G-actin ratios on stimulation with histamine or 5-HT are reduced by SLK KD. Data are mean values of experiments from four batches of cell culture from three donors. Error bars indicate SD. (D) Histamine- or 5-HT–stimulated myosin light chain phosphorylation at Ser-19 is not affected by SLK KD. Data are mean values of experiments from four batches of cell culture from three donors. Error bars indicate SD. *P < 0.05 and **P < 0.01. Two-way ANOVA was used for statistical analysis.
Discussion
Actin filament polymerization plays an important role in regulating smooth muscle contraction by promoting force transmission between the contractile units and the extracellular matrix and increasing the contractile units (1, 6, 10). There is a wealth of evidence to suggest that actin dynamics are regulated by protein tyrosine phosphorylation in cells, particularly in smooth muscle (1, 2, 6). Our current results show that the serine/threonine protein kinase SLK regulates actin dynamics and smooth muscle contraction without affecting myosin activation. To the best of our knowledge, this is the first evidence to suggest that SLK participates in the modulation of actin dynamics in smooth muscle on contractile stimulation.
Interestingly, activation of angiotensin II type 2 receptor induced SLK-mediated RhoA Ser188 phosphorylation, which inactivated RhoA and induced vasodilation (36). However, another study suggests that Plk1 activates RhoA (an activator of actin polymerization) (37), which promotes vascular smooth muscle contraction (34). Furthermore, our current results demonstrate that SLK can activate Plk1 (Figure 6A), which is supported by previous studies by others (14, 15). Thus, it is not surprising that SLK KD inhibits actin polymerization and smooth muscle contraction in this study. The discrepancy between our results and the previous study (36) may stem from the difference between airway smooth muscle versus vascular smooth muscle. It has been shown that the cellular process in airway smooth muscle may not be the exact same as in vascular smooth muscle. For instance, RhoA activation promotes vascular smooth muscle contraction by inhibiting myosin phosphatase and increasing myosin light chain phosphorylation (38). In contrast, RhoA activation stimulates airway smooth muscle contraction by enhancing assembly of the adhesome at the membrane, and not by affecting myosin light chain phosphorylation (37).
SLK regulates actin dynamics probably by controlling paxillin Ser-272 phosphorylation in human smooth muscle cells (Figures 2A and 2B). Interestingly, SLK did not catalyze paxillin phosphorylation at this residue in vitro (Figure 2C). In contrast, Plk1 mediated paxillin Ser-272 phosphorylation in vitro (Figure 2C) and in smooth muscle on contractile activation (Figure 2D). Plk1 has been shown to be involved in the regulation of smooth muscle contraction (17, 34). Contractile activation promoted the formation of the multiprotein complex including SLK, Plk1, and paxillin in smooth muscle (Figure 3). Moreover, SLK regulated Plk1 activation in smooth muscle (Figure 6A). Furthermore, the effects of SLK and Plk1 on paxillin phosphorylation were not additive (Figure 6B). These results suggest that contractile activation facilitates the formation of the multiprotein complex containing SLK, Plk1, and paxillin by which SLK activates Plk1 and promotes paxillin Ser-272 phosphorylation. In canine airway smooth muscle, paxillin phosphorylation at an analogous position is regulated by p21-activated kinase (PAK) (22). Therefore, paxillin Ser-272 phosphorylation can be regulated by both the SLK-Plk1 pathway and PAK in smooth muscle during contractile stimulation.
We found that contractile activation enhanced paxillin phosphorylation at Ser-272 in smooth muscle. Paxillin phosphorylation at this position may regulate actin polymerization via N-WASP activation (Figures 4B and 5A). In motile CHO-K1 cells, paxillin serine phosphorylation at an analogous position recruits a multiprotein complex including PAK, which promotes adhesion turnover (21). Studies from our laboratory and others have shown that PAK can activate p130 Crk-associated substrate (p130CAS), which subsequently stimulates c-Abl tyrosine kinase, N-WASP, the Arp2/3 complex, and actin dynamics in smooth muscle cells (1, 5, 19, 30, 32, 39). In canine airway smooth muscle, paxillin serine phosphorylation at this position recruited a protein complex containing GIT1 (G-protein–coupled receptor kinase-interacting protein) and the Cdc42 guanine exchange factor βPIX (Pak interactive exchange factor). Assembly of the PAK-GIT1-βPIX-paxillin complex was necessary for Cdc42 and N-WASP activation, actin polymerization, and contraction (22). Thus, it is possible that paxillin phosphorylation at Ser-272 may regulate actin polymerization through the two pathways.
In this study, we used ACh, histamine, and 5-HT as contractile agonists, because these agonists play a role in the pathogenesis of airway hyperresponsiveness (1, 40). In addition, M3 receptor couples with Gq and G12/13 in smooth muscle (41). H1 receptor couples with Gq and G11, whereas 5-HT2A receptor couples with Gq and G12/13 (42–44). Future studies are needed to assess which G protein subunit predominantly mediates the agonist-induced response.
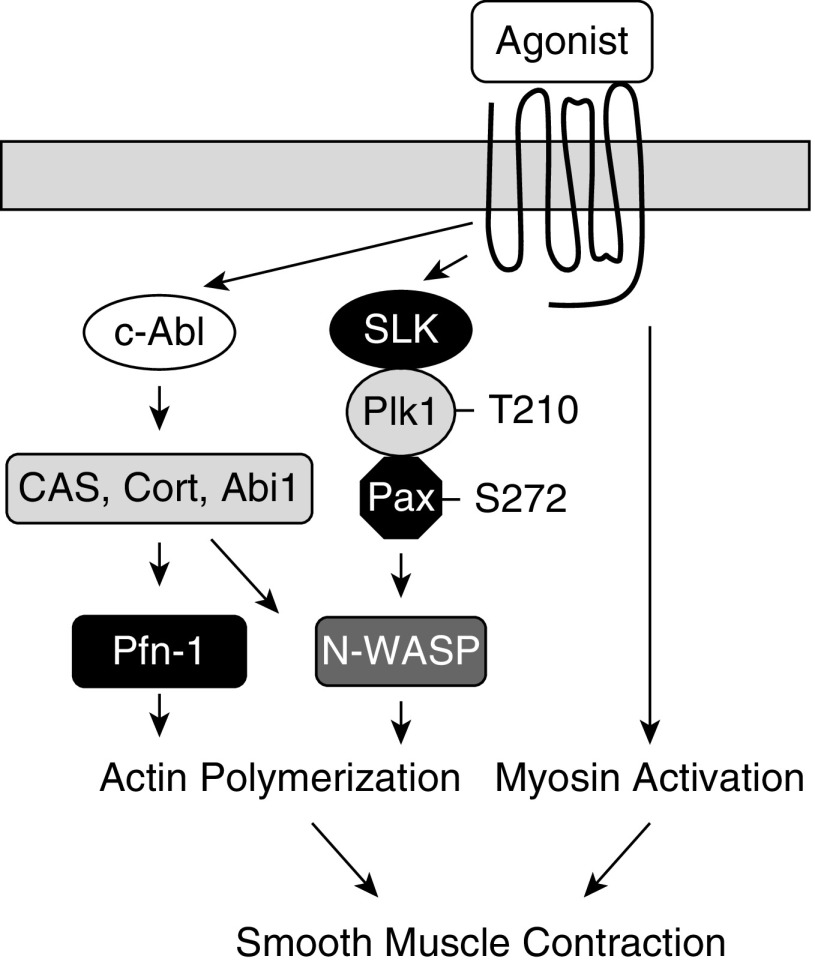
Our current study unveils a novel cellular process that regulates actin dynamics in smooth muscle. In addition to myosin light chain phosphorylation at Ser-19, contractile agonists (e.g., ACh) promote the formation of the multiprotein complex including SLK, Plk1, and paxillin, which activates Plk1 by catalyzing phosphorylation at Thr-210. Activated Plk1 mediates paxillin phosphorylation at Ser-272 and promotes N-WASP activation and actin polymerization. Both actin polymerization and myosin activation are necessary for smooth muscle contraction (Figure 8).
Figure 8.
Mechanism of human airway smooth muscle contraction. Contractile activation induces c-Abl–mediated actin polymerization and myosin light chain phosphorylation in human airway smooth muscle (1, 2, 4, 5). In this study, we discovered that agonist stimulation promotes the assembly of the multiprotein complex including SLK, Plk1, and paxillin, which phosphorylates Plk1 at Thr-201 and activates Plk1. Activated Plk1 mediates paxillin phosphorylation at Ser-272, promoting N-WASP activation and actin polymerization. Abi1 = Abl interactor 1; CAS = p130Crk-associated substrate; Cort = cortactin; Pax = paxillin.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Jia Li, Brennan Gerlach, Alyssa C. Rezey, and Guoning Liao for technical assistance.
Footnotes
Supported by National Heart, Lung, and Blood Institute grants HL-110951, HL-130304, and HL-145392 from the National Institutes of Health (D.D.T.).
Author Contributions: Y.W. performed physiological and biochemical experiments and drafted the manuscript. R.W. carried out cellular and biochemical studies. D.D.T. conceived the research direction, coordinated the study, and revised the paper. All authors reviewed the results and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0310OC on January 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tang DD. The dynamic actin cytoskeleton in smooth muscle. Adv Pharmacol. 2018;81:1–38. doi: 10.1016/bs.apha.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Tang DD. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir Res. 2015;16:134. doi: 10.1186/s12931-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T, Cleary RA, Wang R, Tang DD. Glia maturation factor-γ phosphorylation at Tyr-104 regulates actin dynamics and contraction in human airway smooth muscle. Am J Respir Cell Mol Biol. 2014;51:652–659. doi: 10.1165/rcmb.2014-0125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, Cleary RA, Wang T, Li J, Tang DD. The association of cortactin with profilin-1 is critical for smooth muscle contraction. J Biol Chem. 2014;289:14157–14169. doi: 10.1074/jbc.M114.548099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Cleary RA, Wang R, Tang DD. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem. 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamin R, Morgan KG. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J Physiol. 2012;590:4145–4154. doi: 10.1113/jphysiol.2012.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HR, Graceffa P, Ferron F, Gallant C, Boczkowska M, Dominguez R, et al. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am J Physiol Cell Physiol. 2010;298:C559–C571. doi: 10.1152/ajpcell.00431.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002;16:72–76. doi: 10.1096/cj.01-0104hyp. [DOI] [PubMed] [Google Scholar]
- 10.Herrera AM, Martinez EC, Seow CY. Electron microscopic study of actin polymerization in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1161–L1168. doi: 10.1152/ajplung.00298.2003. [DOI] [PubMed] [Google Scholar]
- 11.Machicoane M, de Frutos CA, Fink J, Rocancourt M, Lombardi Y, Garel S, et al. SLK-dependent activation of ERMs controls LGN-NuMA localization and spindle orientation. J Cell Biol. 2014;205:791–799. doi: 10.1083/jcb.201401049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabourin LA, Rudnicki MA. Induction of apoptosis by SLK, a Ste20-related kinase. Oncogene. 1999;18:7566–7575. doi: 10.1038/sj.onc.1203119. [DOI] [PubMed] [Google Scholar]
- 13.Fokin AI, Klementeva TS, Nadezhdina ES, Burakov AV. SLK/LOSK kinase regulates cell motility independently of microtubule organization and Golgi polarization. Cytoskeleton (Hoboken) 2016;73:83–92. doi: 10.1002/cm.21276. [DOI] [PubMed] [Google Scholar]
- 14.Al-Zahrani KN, Baron KD, Sabourin LA. Ste20-like kinase SLK, at the crossroads: a matter of life and death. Cell Adhes Migr. 2013;7:1–10. doi: 10.4161/cam.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellinger-Ziegelbauer H, Karasuyama H, Yamada E, Tsujikawa K, Todokoro K, Nishida E. Ste20-like kinase (SLK), a regulatory kinase for polo-like kinase (Plk) during the G2/M transition in somatic cells. Genes Cells. 2000;5:491–498. doi: 10.1046/j.1365-2443.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 16.Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–440. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Wang R, Gannon OJ, Rezey AC, Jiang S, Gerlach BD, et al. Polo-like kinase 1 regulates vimentin phosphorylation at ser-56 and contraction in smooth muscle. J Biol Chem. 2016;291:23693–23703. doi: 10.1074/jbc.M116.749341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci. 2008;121:2435–2444. doi: 10.1242/jcs.018044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang DD, Gerlach BD. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir Res. 2017;18:54. doi: 10.1186/s12931-017-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rembold CM, Tejani AD, Ripley ML, Han S. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol. 2007;293:C993–C1002. doi: 10.1152/ajpcell.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol. 2006;173:587–589. doi: 10.1083/jcb.200509075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Huang Y, Gunst SJ. p21-Activated kinase (Pak) regulates airway smooth muscle contraction by regulating paxillin complexes that mediate actin polymerization. J Physiol. 2016;594:4879–4900. doi: 10.1113/JP272132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Wang R, Cleary RA, Gannon OJ, Tang DD. Recruitment of β-catenin to N-cadherin is necessary for smooth muscle contraction. J Biol Chem. 2015;290:8913–8924. doi: 10.1074/jbc.M114.621003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Mercaitis OP, Jia L, Panettieri RA, Tang DD. Raf-1, actin dynamics, and abelson tyrosine kinase in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2013;48:172–178. doi: 10.1165/rcmb.2012-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Rezey AC, Wang R, Tang DD. Role and regulation of Abelson tyrosine kinase in Crk-associated substrate/profilin-1 interaction and airway smooth muscle contraction. Respir Res. 2018;19:4. doi: 10.1186/s12931-017-0709-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao G, Wang R, Rezey AC, Gerlach BD, Tang DD. Microrna mir-509 regulates erk1/2, the vimentin network, and focal adhesions by targeting plk1. Sci Rep. 2018;8:12635. doi: 10.1038/s41598-018-30895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long J, Liao G, Wang Y, Tang DD. Specific protein 1, c-Abl and ERK1/2 form a regulatory loop. J Cell Sci. 2019;132:jcs222380. doi: 10.1242/jcs.222380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y, Gunst SJ. Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism. J Biol Chem. 2015;290:11403–11416. doi: 10.1074/jbc.M115.645788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Zhang W, Gunst SJ. Activation of vinculin induced by cholinergic stimulation regulates contraction of tracheal smooth muscle tissue. J Biol Chem. 2011;286:3630–3644. doi: 10.1074/jbc.M110.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281:34716–34724. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L240–L248. doi: 10.1152/ajplung.00199.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Li Q, Tang DD. Role of vimentin in smooth muscle force development. Am J Physiol Cell Physiol. 2006;291:C483–C489. doi: 10.1152/ajpcell.00097.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Cárcer G, Wachowicz P, Martínez-Martínez S, Oller J, Méndez-Barbero N, Escobar B, et al. Plk1 regulates contraction of postmitotic smooth muscle cells and is required for vascular homeostasis. Nat Med. 2017;23:964–974. doi: 10.1038/nm.4364. [DOI] [PubMed] [Google Scholar]
- 35.Bompard G, Caron E. Regulation of WASP/WAVE proteins: making a long story short. J Cell Biol. 2004;166:957–962. doi: 10.1083/jcb.200403127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilluy C, Rolli-Derkinderen M, Loufrani L, Bourgé A, Henrion D, Sabourin L, et al. Ste20-related kinase SLK phosphorylates Ser188 of RhoA to induce vasodilation in response to angiotensin II Type 2 receptor activation. Circ Res. 2008;102:1265–1274. doi: 10.1161/CIRCRESAHA.107.164764. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W, Huang Y, Wu Y, Gunst SJ. A novel role for RhoA GTPase in the regulation of airway smooth muscle contraction. Can J Physiol Pharmacol. 2015;93:129–136. doi: 10.1139/cjpp-2014-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somlyo AV, Khromov AS, Webb MR, Ferenczi MA, Trentham DR, He ZH, et al. Smooth muscle myosin: regulation and properties. Philos Trans R Soc Lond B Biol Sci. 2004;359:1921–1930. doi: 10.1098/rstb.2004.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett A, Pellet-Many C, Zachary IC, Evans IM, Frankel P. p130Cas: a key signalling node in health and disease. Cell Signal. 2013;25:766–777. doi: 10.1016/j.cellsig.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Amrani Y, Panettieri RA. Airway smooth muscle: contraction and beyond. Int J Biochem Cell Biol. 2003;35:272–276. doi: 10.1016/s1357-2725(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 41.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849–G853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- 42.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31:187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 43.Mak JC, Roffel AF, Katsunuma T, Elzinga CR, Zaagsma J, Barnes PJ. Up-regulation of airway smooth muscle histamine H(1) receptor mRNA, protein, and function by beta(2)-adrenoceptor activation. Mol Pharmacol. 2000;57:857–864. [PubMed] [Google Scholar]
- 44.Arrang JM. Pharmacological properties of histamine receptor subtypes. Cell Mol Biol. 1994;40:275–281. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.