Abstract
Enhanced expression of the cellular antioxidant glutathione peroxidase (GPX)-1 prevents cigarette smoke–induced lung inflammation and tissue destruction. Subjects with chronic obstructive pulmonary disease (COPD), however, have decreased airway GPX-1 levels, rendering them more susceptible to disease onset and progression. The mechanisms that downregulate GPX-1 in the airway epithelium in COPD remain unknown. To ascertain these factors, analyses were conducted using human airway epithelial cells isolated from healthy subjects and human subjects with COPD and lung tissue from control and cigarette smoke–exposed A/J mice. Tyrosine phosphorylation modifies GPX-1 expression and cigarette smoke activates the tyrosine kinase c-Src. Therefore, studies were conducted to evaluate the role of c-Src on GPX-1 levels in COPD. These studies identified accelerated GPX-1 mRNA decay in COPD airway epithelial cells. Targeting the tyrosine kinase c-Src with siRNA inhibited GPX-1 mRNA degradation and restored GPX-1 protein levels in human airway epithelial cells. In contrast, silencing the tyrosine kinase c-Abl, or the transcriptional activator Nrf2, had no effect on GPX-1 mRNA stability. The chemical inhibitors for c-Src (saracatinib and dasanitib) restored GPX-1 mRNA levels and GPX-1 activity in COPD airway cells in vitro. Similarly, saracatinib prevented the loss of lung Gpx-1 expression in response to chronic smoke exposure in vivo. Thus, this study establishes that the decreased GPX-1 expression that occurs in COPD lungs is at least partially due to accelerated mRNA decay. Furthermore, these findings show that targeting c-Src represents a potential therapeutic approach to augment GPX-1 responses and counter smoke-induced lung disease.
Keywords: chronic obstructive pulmonary disease, glutathione peroxidase-1, cellular Src kinase, mRNA degradation, cigarette smoke
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States, and the age-adjusted mortality for this disease is increasing (1), which underscores the need for better therapies. Our research group demonstrated that overexpressing antioxidants, such as glutathione peroxidase (GPX)-1 and superoxide dismutase (SOD)-1 prevents cigarette smoke–induced emphysema in mice (2, 3) illustrating the therapeutic potential of antioxidants in COPD. However, pharmacokinetic factors limit the bioavailability of orally administered antioxidants as a treatment for COPD (4). As an example, researchers found that n-acetyl cysteine (NAC) administration (600 mg daily) only transiently increased lung lavage glutathione levels (5), and a subsequent study showed no effect on lung tissue or plasma glutathione levels with even higher NAC doses (6). This likely explains why the BRONCUS study, one of the largest antioxidant treatment studies in COPD, found that NAC at a dose of 600 mg daily did not improve lung function or prevent exacerbations in this disease (7). Attaining enhanced antioxidant defenses in the lung will require a better understanding of the mechanisms that regulate endogenous lung antioxidant expression. Once this is achieved, these processes could be targeted to effectively augment lung antioxidant activity.
GPXs are a family of selenium-dependent and-independent antioxidant enzymes that reduce lipid hydroperoxides to their corresponding alcohols and convert hydrogen peroxide into water (8). Subjects with COPD are deficient in selenium (9), which could contribute to the reduced GPX-1 activity observed in the disease. GPX-1 is critical in the lung and Gpx-1−/− mice have enhanced susceptibility to cigarette smoke–induced oxidative injury and emphysema (3, 10). We identified decreased GPX-1 expression in the airway epithelium of patients with COPD (3). GPX-1 has multiple subcellular localizations, and this gives it the potential to affect diverse processes, such as cell signaling, mitochondrial function, and gene expression (11). Despite its essential function, the factors responsible for the reduced GPX-1 levels in the airway epithelium in COPD remain unknown.
In contrast to other enzymatic antioxidants, GPX-1 mRNA is highly unstable and degrades rapidly in HepG2 (liver hepatocellular carcinoma cells) and CaCo-2 (colorectal adenocarcinoma) cells (12). This instability is attributed to differences in the 3′ untranslated region (UTR) of the mRNA, because GPX-2 and GPX-4 mRNA become unstable when the 3′UTR of GPX-1 is fused to their mRNA (13). Post-transcriptional regulation of mRNA plays a critical role in tightly controlling gene expression (14). Given its inherent instability, we examined if accelerated mRNA decay could account for the decreased GPX-1 levels in COPD airway epithelial cells.
Tyrosine kinases, such as c-Src, can modulate endonuclease-mediated mRNA decay (15), and c-Src is activated in COPD airway epithelial cells (16). Moreover, tyrosine kinases regulate GPX-1 activity (17), so we hypothesized that c-Src could modulate GPX-1 expression in the airway epithelium. Our group previously demonstrated that c-Src inhibition protected against cigarette smoke–induced emphysema in mice (16). Here, we investigate how c-Src influences GPX-1 expression and mRNA half-life in primary human airway epithelial cells isolated from subjects with COPD compared with cells from healthy nonsmokers (NS). Similarly, we also explore the impact of cigarette smoke and c-Src inhibition on Gpx-1 expression in mice.
Methods
Details and expanded methodology are included in the data supplement.
Cell Cultures
Monolayers of human small airway epithelial (SAE) cells (Lonza) from healthy subjects and from patients with physician-diagnosed COPD, as established by GOLD criteria, were submerged, cultured, and treated with 5% cigarette smoke extract (CSE) or PBS, as previously described (18). Cells were only used for experiments at passages 3–6 and at a confluency of approximately 70%. SAE cells were transfected by administering siRNA for control, c-Abl-, c-Src-, or Nrf2-specific siRNAs (all from Qiagen). Cells were treated with 0, 0.5, 1, and 5 nM dasatinib and 0 and 0.5 μM saracatinib (LC Laboratories) for 24 hours. SAE cells were also exposed to 100 ng/ml actinomycin D in media. Lactate dehydrogenase (LDH) release assays assessed cell viability after treatments.
Animal Models
The male and female A/J mice (The Jackson Laboratory) used for these experiments were at least 8 weeks old to ensure that normal lung development was complete. Each experimental group had at least six animals per group and studies were timed to ensure that the air-exposed controls and smoke-exposed mice were the same age. A/J mice were administered 10 mg/kg saracatinib (LC Laboratories) in the vehicle (0.5% hydroxypropylmethylcellulose, 0.1% polysorbate) or vehicle alone daily by gavage 2 hours before smoke exposure for 2 months. Mice were exposed to cigarette smoke in a chamber (Teague Enterprises) for 4 hours per day, 5 days per week, at an average total particulate matter concentration of 80–120 mg/m3. University of Kentucky reference research cigarettes 3R4F were used to generate cigarette smoke. All animal experiments were performed with approval from St. Luke’s Roosevelt’s and State University of New York Downstate’s Institutional Animal Care and Use Committee. New analyses were conducted on lung tissue samples from a previously published study (16). This overall study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and Institutional Animal Care and Use Committee guidelines.
Forced Oscillation Measurements
Using the FlexiVent system (SCIREQ Inc.), lung compliance was measured in mice as previously described (19).
Histology
The lungs underwent pressure fixation and morphometric analysis following our previously published protocol (20, 21) and in accordance with the American Thoracic Society/European Respiratory Society statement on quantitative assessment of lung structure (22). Fixed sections (4 μm) of paraffin-embedded lungs were hematoxylin and eosin stained.
Protein Assays
Immunoblots for p-Src, GPX-1, c-Abl, c-Src, Nrf2, and β-Actin, as well as c-Src and GPX-1 activity assays, were conducted on the lung tissue and cell lysates per established laboratory protocols (3, 16).
Quantitative PCR Analysis
Gene expression was determined by quantitative PCR (qPCR) using Taqman probes (Life Technologies/Applied Biosystems).
Statistical Analyses
Data are expressed as dot plots with the means (±SEM) highlighted. Student’s t test (two tailed) compared differences between two groups. Experiments with more than two groups were analyzed by ANOVA with Tukey’s post hoc test analysis. P values for significance were set at 0.05, and all significant changes are noted with asterisks. All analyses were performed using GraphPad Prism Software (Version 6.0 h for Mac OS X).
Results
COPD Airway Epithelial Cells Have Reduced GPX-1 Expression
GPX-1 responses were investigated in primary human SAE cells isolated from NS and subjects with COPD. We focused on this cell type because dysfunction of the small airways is central to the pathogenesis of this disease (23). First, the gene expression of SOD1 and GPX genes 1–4 was examined in SAE cells isolated from NS and subjects with COPD, with gene expression relative to each other gene. In SAE cells, GPX-1 was the highest expressed antioxidant gene tested (Figure 1A). SAE cells from subjects with COPD express significantly less GPX-1. Moreover, these other antioxidants were not significantly altered in the COPD disease state, and none compensated for the loss of GPX-1 in this disease (Figure 1A). In contrast to the heightened GPX-2 and GPX-3 expression present in lung samples of subjects with COPD (24), significantly reduced GPX-1 gene expression was noted in SAE cells from subjects with COPD (Figure 1B). CSE administration enhanced GPX-1 expression in SAE cells from nonsmokers, but not from SAE cells isolated from subjects with COPD (Figure 1C). This blunting of GPX-1 mRNA expression corresponded with GPX-1 activity (Figure 1D) and total GPX-1 protein levels (Figure 1E). Thus, a defect in GPX-1 responsiveness and overall GPX1 antioxidant capacity occurs within the small airways of subjects with COPD.
Figure 1.
Small airway epithelial (SAE) cells from chronic obstructive pulmonary disease (COPD) organ donors have reduced glutathione peroxidase (GPX)-1 responses. (A) Superoxide dismutase (SOD)-1 and GPX genes were quantified in SAE cells isolated from nonsmoker healthy subjects (NS) and subjects with COPD by quantitative PCR (qPCR), and are shown as relative mRNA levels for each antioxidant gene compared with nonsmoker GPX-1 mRNA levels. (B) SAE cells isolated from nonsmokers and patients with COPD were cultured and GPX-1 mRNA expression determined by qPCR. (C) Cells were treated with vehicle or 5% cigarette smoke extract (CSE) for 24 hours before determining GPX-1 mRNA expression determined by qPCR. (D) Protein lysates were examined for GPX-1 activity by enzymatic assays. (E) Total GPX-1 levels were determined in Western blots for GPX-1. β-actin was used as a loading control. Optical intensity quantification and calculated ratios of total GPX-1 to β-actin were determined, and are presented as densitometry units (DU). Graphs are represented as mean ± SEM. *P < 0.05, when comparing both treatments connected by a line. All analysis had an n ≥ 3 per group. The comparisons between two individual groups were determined by Student’s t test or greater than two groups by one-way ANOVA and Tukey’s post hoc tests. RQ = relative quantification.
Chronic Smoke Exposure Inhibits GPX-1 Expression in Mice
To further determine the relationship between smoke exposure, GPX-1 expression, and activity, A/J mice were exposed to cigarette smoke for multiple time points up to 2 months—2 months was chosen because we previously found that this mouse strain develops emphysematous changes within this time period (25). Acute exposure to cigarette smoke (up to 8 d) enhanced Gpx-1 expression (Figure 2A), but these changes were transient, and Gpx-1 returned to baseline after subacute exposure (1 mo). In contrast, a chronic 2-month smoke exposure led to a loss of lung Gpx-1 mRNA expression, as determined by qPCR (Figure 2A) and enzymatic activity (Figure 2B). Reduced total lung GPX activity was also observed in smoke-exposed animals (Figure 2B). Immunoblots confirmed that Gpx-1 lung levels are reduced after 2-month exposure to cigarette smoke in A/J mice (Figure 2C). These findings are consistent with previous evidence demonstrating that GPX-1 activity is decreased after chronic smoke exposure (3). Of note, overall Gpx-1 activity was also decreased (Figure 2B) in these mice, indicating that other Gpx species do not compensate for the loss of Gpx-1.
Figure 2.
Assessing smoke exposure duration on lung Gpx-1 expression in mice. (A) Time-dependent changes of lung Gpx-1 expression to smoke exposure in mice were evaluated by qPCR. (B) Total Gpx-1 activity and Gpx-1 activity and (C) total protein expression for Gpx-1 were measured by enzymatic assays and Western blots, respectively. β-actin was used as a loading control and protein levels were quantified by densitometric analysis. (D) Lung compliance was measured in mice exposed to cigarette smoke at different time points up to 2 months. Mean linear intercept (MLI) analysis, an estimator of the volume-to-surface ratio of the acinar airspace complex, was also measured in the same mice. Graphs are represented as mean ± SEM of three measurements. *P < 0.05, when comparing both treatments connected by a line determined by one-way ANOVA and Tukey’s post hoc tests. All analysis had n ≥ 6 per group.
We analyzed lung compliance and mean linear intercept in mice exposed to cigarette smoke for 2 months to examine how Gpx-1 expression correlated with smoke-mediated changes in lung function and morphometry. A/J mice exposed to cigarette smoke for 2 months showed changes in both parameters, consistent with the development of emphysema (Figure 2D). Importantly, these physiologic and emphysematous changes occurred concurrently with reduced Gpx-1 expression and activity. Therefore, chronic smoke exposure was associated with reduced GPX-1 expression levels, both in SAE cells and in mouse lungs. This allows us to use these in vitro and in vivo models to investigate the factors that downregulate GPX-1 expression in COPD.
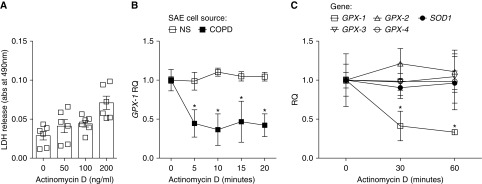
GPX-1 mRNA Degradation Is Accelerated in the Epithelium of Subjects with COPD
To evaluate whether the reduced expression of GPX-1 in COPD airway epithelium was due to mRNA stability, we investigated GPX-1 mRNA expression and rate of decay in SAE cells collected from NS and subjects with COPD. To determine the mRNA decay rate, we quantified the amount of GPX-1 mRNA remaining at various times after actinomycin D treatment. LDH assays were conducted to establish an actinomycin D concentration that did not induce toxicity. At 100 ng/ml, actinomycin D induced no significant release of LDH from SAE cells (Figure 3A), and this concentration was used in subsequent experiments. Upon actinomycin D treatment, GPX-1 mRNA degraded faster in cells from patients with COPD compared with cells from NS (Figure 3B). A significant decrease in GPX-1 mRNA stability was observed within 5 minutes of actinomycin D treatment in cells from subjects with COPD. This contrasted with other antioxidant genes, GPX-2, -3, and -4 and SOD1, which were all stable over 1 hour after actinomycin D treatment in NS cells (Figure 3C). This indicates that a decreased mRNA half-life is at least partially responsible for the reduced levels of GPX-1 mRNA in airway epithelial cells from subjects with COPD.
Figure 3.
Enhanced GPX-1 mRNA degradation in COPD epithelium samples. (A) Lactate dehydrogenase (LDH) release assays were performed in SAE cells from NS donors treated with actinomycin D. (B) GPX-1 expression was measured by qPCR in SAE cells from NS and patient with COPD cells treated with 100 ng/ml actinomycin D and collected at various times after treatment. RQ was expressed compared with time zero of actinomycin D treatment for NS and COPD samples. (C) Gene expression of GPX-1, GPX-2, GPX-3, GPX-4, and SOD1 were determined using qPCR in actinomycin D–treated SAE cells from NS. Graphs are represented as mean ± SEM of three measurements. *P < 0.05, when comparing both treatments at the same time point. All analysis had an n = 6 per group. The comparisons between two individual groups were determined by Student’s t test or greater than two groups by one-way ANOVA and Tukey’s post hoc tests.
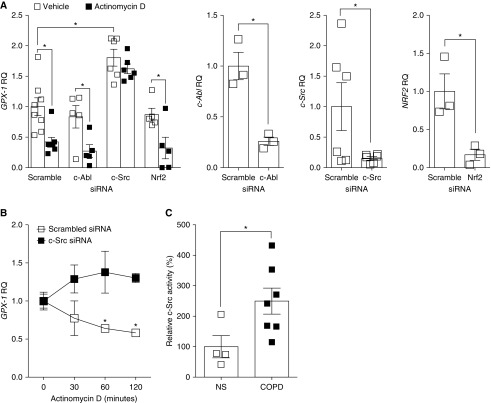
GPX-1 mRNA Instability Is Mediated by c-Src Activation
Regulation of GPX-1 was previously reported to be controlled by c-Abl and Arg tyrosine kinases (17). We and others also observed that the tyrosine kinase, c-Src, is phosphorylated and activated by smoke exposure (16). Therefore, we investigated whether c-Abl and c-Src could mediate GPX-1 gene expression, and mRNA degradation. Nrf2, c-Abl, and c-Src expression were silenced using siRNA. Nrf2 was investigated, as it is a major regulator of antioxidant responses (26). At 30 minutes after treatment with c-Src siRNA and actinomycin D, increased GPX-1 was observed in SAE cells from NS (Figure 4A). In contrast, c-Abl or Nrf2 silencing had no effect on GPX-1 expression (Figure 4A). We confirmed c-Abl, c-Src, and Nrf2 gene silencing by qPCR (Figure 4A). Silencing c-Src stabilized GPX-1 mRNA for over 2 hours after actinomycin D administration (Figure 4B). SAE cells from subjects with COPD have heightened c-Src kinase activity compared with SAE cells from NS SAE cells (Figure 4C). Therefore, these findings establish that c-Src regulates GPX-1 levels in the airway epithelium in COPD. Targeting the heightened c-Src activity that occurs in COPD could restore GPX-1 levels to counter injurious inflammatory and proteolytic responses in human airway epithelial cells.
Figure 4.
The tyrosine kinase c-Src regulates GPX-1 mRNA degradation in COPD epithelia. (A) SAE cells from NS were transfected with siRNA specific for c-Abl, c-Src, Nrf2, or scrambled control and 48 hours later treated with 100 ng/ml actinomycin D for 30 minutes. Gene expression of GPX-1 was determined using qPCR. Representative qPCR shows knockdown of each target and GPX-1 levels. β-actin was used as a loading control. (B) GPX-1 expression was also examined in c-Src–silenced cells up to 2 hours after 100 ng/ml actinomycin D treatment. (C) Relative c-Src activity was determined in SAE cells from NS and subjects with COPD. Graphs are represented as mean ± SEM of three measurements. *P < 0.05, when comparing both treatments connected by a line. All analysis had an n ≥ 4 per group for qPCR and activity assays. The comparisons between two individual groups were determined by Student’s t test.
Chemical Inhibition of Src Activity Enhances GPX-1 Levels
Because c-Src silencing enhanced GPX-1 responses, the effect of two Src kinase inhibitors, dasatinib and saracatinib, was investigated in SAE cells. Saracatinib inhibits Src and Bcr-Abl tyrosine kinase activity, and dasatinib blocks Src and Bcr-Abl tyrosine kinases, c-KIT, EPHA2 (Ephrin type A receptor 2), and PDGFRβ (platelet derived growth factor beta). Immunoblot analysis determined that dasatinib at concentrations greater than or equal to 1 nM induced expression of GPX-1 and blocked phosphorylation of Src at tyrosine site 416 (Figure 5A). Phosphorylation of Src kinases at the tyrosine 416 site induces tyrosine kinase activity (27). Equally, treatment of SAE cells with 0.5 μM saracatinib induced GPX-1 expression and inhibited Src phosphorylation and c-Src activity (Figure 5B). LDH release assays were undertaken to test cell viability after dasatinib and saracatinib treatment, and showed no toxicity at the concentrations used (Figures 5A and 5B). SAE cells treated with saracatinib had enhanced GPX-2 gene expression (Figure 5C). Therefore, inhibition of c-Src by dasatinib and saracatinib enhances GPX responses in the airway epithelium.
Figure 5.
Inhibition of the tyrosine kinase c-Src enhances GPX-1 mRNA in epithelia. Media for SAE cells isolated from NS were supplemented with (A) dasatinib or (B) saracatinib for 24 hours and immunoblots for p-Src (Tyr416), GPX-1, and β-actin were performed. Densitometry for the ratio of GPX-1 to β-actin was assessed. LDH release assays were performed for the media of dasatinib- or saracatinib-treated cells to determine toxicity. (B) Relative c-Src activity was determined in SAE cells from NS treated with saracatinib. (C) SOD1 and GPX genes were quantified in SAE cells isolated from subjects with COPD after treatment with saracatinib for 24 hours, by qPCR. Graphs are presented as mean ± SEM of three measurements. *P < 0.05, when comparing both treatments connected by a line. All analysis had a n ≥ 4 per group for qPCR and activity assays. The comparisons between two individual groups were determined by Student’s t test or multiple groups with ANOVA with Tukey’s post hoc test analysis. p = phosphate.
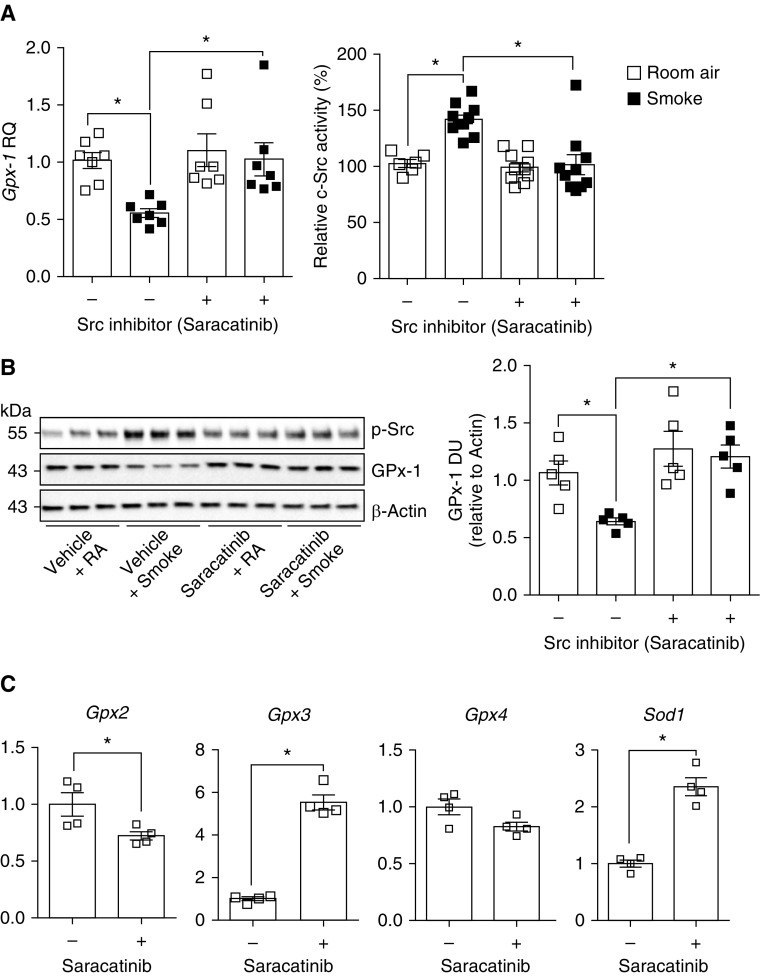
Because Src kinase inhibition enhances GPX-1 levels in vitro, we investigated the therapeutic effects of saracatinib on smoke-induced c-Src activity and GPX-1 suppression in vivo. Our group previously demonstrated that saracatinib could reduce cigarette smoke–induced emphysema in mice (16). The results of these studies now define a targetable mechanism for this protective effect. Daily saracatinib administration prevented the reduction in lung Gpx-1 expression mediated by 2 months of cigarette smoke exposure (Figure 6A). As expected, saracatinib treatment inhibited c-Src activation in smoke-exposed mice (Figure 6A). Gpx-1 immunoblots confirmed that saracatinib treatment maintained lung Gpx-1 protein levels of smoke-exposed animals (Figure 6B). Interestingly, saracatinib treatment induced expression of Gpx-1, Gpx-3, and Sod1, while partially reducing GPX-2 expression (Figure 6C). Therefore, elevated c-Src signaling is, in part, responsible for the reduced expression of GPX-1 in the airways of subjects with COPD. Moreover, this shows, for the first time, that inhibiting c-Src could preserve GPX-1 levels after chronic cigarette smoke exposure in the lung.
Figure 6.
Saracatinib treatment prevents cigarette smoke–induced inhibition of Gpx-1 expression in mice. A/J mice were exposed to cigarette smoke daily for 2 months in combination with daily oral administration of vehicle or saracatinib (AZD-0530). (A) Gpx-1 gene expression and c-Src activity were determined by qPCR and enzymatic activity assays, respectively. (B) Immunoblots for p-Src (Tyr416), GPX-1, and β-actin were performed. Densitometry for the ratio of GPX-1 to β-actin was assessed. (C) SOD1 and GPX genes were quantified in vehicle- and saracatinib-treated animals, by qPCR. Graphs are represented as mean ± SEM of n ≥ 5 animals per group. *P < 0.05, when comparing both treatments connected by a line. RA = room air.
Discussion
The outcomes from these studies establish that enhanced c-Src signaling diminishes GPX-1 expression via regulation of RNA stability/degradation in the airway epithelium of subjects with COPD. Furthermore, our findings indicate that the loss of GPX-1 in the airway epithelium could increase the susceptibility to cigarette smoke–induced injury and likely accelerates disease progression in subjects with COPD. Silencing the tyrosine kinase, c-Src, stabilized GPX-1 mRNA and the chemical inhibitors of c-Src, reversed the loss of GPX-1 expression that occurs after chronic cigarette smoke exposure, both in SAE cells in vitro and in mouse lungs in vivo. Thus, these results demonstrate that c-Src activity in the airway epithelium of subjects with COPD could be manipulated to enhance protective GPX-1 antioxidant responses in this critical cell type in this disease.
Before this study, our group determined that GPX-1 expression and activity were downregulated in COPD airways, which subsequently exacerbated endoplasmic reticulum stress and proteolytic and inflammation responses (3, 28). GPX-1 tempers inflammation by activating phosphatases like protein tyrosine phosphatase 1B (PTP1B) and protein phosphatase 2A (PP2A) (3). This is important in COPD, as PTP1B (29) and PP2A (30) inactivation are linked to airway epithelial cell inflammation and the loss of lung function. Moreover, it was recently shown that activating PP2A prevents cigarette smoke–induced COPD in mice (30). Our research group previously demonstrated that GPX-1 regulates smoke-induced AP-1 (activator protein), NF-κB, KC (keratinocyte chemoattractant), IL-17, MMP-3 (matrix metalloproteinase), MMP-9 and MMP-12 responses (3). Not surprisingly, GPX-1 expression is linked to many other oxidant stress and antioxidant defense responses. Indeed, evidence indicates that the accelerated GPX-1 mRNA decay in the airway epithelium could affect apoptosis, mitochondrial function, and other key pathogenic responses in COPD (31).
GPX-1 is primarily known for its role in converting H2O2 to H2O (32). GPX-1 plays a particularly important role, because it detoxifies H2O2, which can be converted to the highly toxic hydroxyl radical in the presence of reduced transition metals (33). Antioxidant expression increases in the mitochondria in response to enhanced oxidative stress. However, deficiencies in antioxidant defenses occur in certain disease states, thereby disrupting normal redox balance (3). In ischemia–reperfusion injury in the heart, the absence of GPX-1 expression increases mitochondrial reactive oxygen species and decreases mitochondrial membrane potential and ATP production (34). Furthermore, the complete absence of GPX-1 impaired cellular proliferation and enhanced the susceptibility to oxidant-mediated death (35). Therefore, tight control of GPX-1 plays a central role in regulating redox balance to maintain normal mitochondrial responses and function. The loss of GPX-1 antioxidant activity that occurs in SAE cells in COPD (3) could account for the mitochondrial dysfunction observed in these cells in this disease (36).
Nrf2 is a key transcriptional factor that promotes lung antioxidant expression (37). Nrf2 regulates glutathione levels, largely via GPX-2 (37), glutathione s-transferase (38), and glutamate-cysteine ligase modifier (39), but it has less effect on GPX1 expression (40). These studies showed that Nrf2 silencing did not alter GPX-1 levels in human airway epithelial cells. This suggests that other factors, such as post-transcriptional regulation, could determine expression of this key lung antioxidant. As mentioned previously (12) and here in our study, GPX-1 mRNA is highly unstable and degrades rapidly in HepG2 and CaCo-2 cells. This instability is attributed to differences in the 3′UTR of the mRNA (13). Therefore, mRNA-binding proteins, which target this 3′ region, likely influence GPX-1 mRNA levels. Cigarette smoke exposure inactivates the mRNA-destabilizing protein, tristetraprolin (TTP) (41), which controls the transcription of proinflammatory cytokines and chemokines (42). PP2A dephosphorylates and activates TTP to destabilize the mRNA of proinflammatory cytokines and chemokines to resolve inflammation (43), but whether TTP acts upon GPX-1 mRNA is unknown. Alternatively, multiple microRNA, such as microRNA-185-5p, are predicted to regulate GPX genes, including GPX-1 (44). c-Src activates endonuclease-mediated mRNA decay by modulating the mRNA endonuclease, PMR1 (45), and we found that c-Src destabilizes GPX-1 mRNA. However, the intermediary factors through which c-Src regulates GPX-1 mRNA stability and expression levels in COPD require further investigation.
Given the role of oxidative stress in the onset and progression of COPD, researchers have sought to enhance nonenzymatic antioxidant defenses through dietary or pharmacological means. As discussed previously, pharmacokinetic factors limit the efficacy of this approach in COPD. Plant-derived antioxidants, such as β-carotene and α-tocopherol, are hydrophobic, and their intestinal absorption requires a high concentration of lipids (46). Even when absorbed, phase II modifications by the liver, such as glucuronidation, sulfation, and methylation, rapidly inactivate phytochemical antioxidants (47). Lastly, multidrug resistance–related proteins 1 and 2 limit their biological effects in the lung by actively pumping these antioxidants out of the intracellular compartment (48). Instead of dietary supplementation, a more effective approach may be to focus on the processes that impair endogenous antioxidant responses in this disease. Here, we show, for the first time, that targeting Src signaling blocks GPX-1 mRNA decay and effectively enhances the intracellular levels and enzymatic activity of this key lung antioxidant. Furthermore, this increase in GPX-1 levels triggered by Src inhibition was associated with protection against smoke-mediated compliance and emphysematous changes in vivo. Inhibitors for c-Src have been investigated for lung cancer (49) and idiopathic pulmonary fibrosis (50), but not for COPD. Our findings show that Src inhibition could overcome the limitations of exogenously administered antioxidants by directly countering the processes that accelerate GPX-1 mRNA degradation in the lung.
In conclusion, c-Src activity diminishes GPX-1 gene expression by enhancing mRNA instability. How c-Src alters GPX-1 mRNA stability remains to be determined, and it is conceivable that factors downstream of c-Src could potentially be targeted to further boost GPX-1 responses in the lung. This is important, because enhancing lung GPX-1 activity counters reactive oxygen species, ER stress, inflammation, and proteolytic responses that promote disease progression.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Pulmonary Division of the State University of New York Downstate Medical Center for their support.
Footnotes
Supported by Flight Attendant Medical Research Institute grant CIA160005 and the Alpha-1 Foundation grant 493373 (P.G.), Flight Attendant Medical Research Institute grant CIA160028 (R.F.F.), and National Institutes of Health grant 7R01HL116597-06 (S.M.M.).
Author Contributions: Performed experiments—A.J.D., W.E., A.E.W., J.M., C.R., A.L., and P.G. Conception and design—P.G. and R.F.F. Analysis and interpretation—A.J.D., S.M.M., P.G., and R.F.F. Drafting the manuscript for important intellectual content—A.J.D., S.M.M., P.G., and R.F.F.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0177OC on December 4, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tonnessen BH, Severson SR, Hurt RD, Miller VM. Modulation of nitric-oxide synthase by nicotine. J Pharmacol Exp Ther. 2000;295:601–606. [PubMed] [Google Scholar]
- 2.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, et al. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geraghty P, Hardigan AA, Wallace AM, Mirochnitchenko O, Thankachen J, Arellanos L, et al. The glutathione peroxidase 1-protein tyrosine phosphatase 1B-protein phosphatase 2A axis: a key determinant of airway inflammation and alveolar destruction. Am J Respir Cell Mol Biol. 2013;49:721–730. doi: 10.1165/rcmb.2013-0026OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foronjy R, Wallace A, D’Armiento J. The pharmokinetic limitations of antioxidant treatment for COPD. Pulm Pharmacol Ther. 2008;21:370–379. doi: 10.1016/j.pupt.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgeman MM, Marsden M, MacNee W, Flenley DC, Ryle AP. Cysteine and glutathione concentrations in plasma and bronchoalveolar lavage fluid after treatment with N-acetylcysteine. Thorax. 1991;46:39–42. doi: 10.1136/thx.46.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridgeman MM, Marsden M, Selby C, Morrison D, MacNee W. Effect of N-acetyl cysteine on the concentrations of thiols in plasma, bronchoalveolar lavage fluid, and lung tissue. Thorax. 1994;49:670–675. doi: 10.1136/thx.49.7.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decramer M, Rutten-van Mölken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 8.Flohé L. Glutathione peroxidase. Basic Life Sci. 1988;49:663–668. doi: 10.1007/978-1-4684-5568-7_104. [DOI] [PubMed] [Google Scholar]
- 9.Santos MC, Oliveira AL, Viegas-Crespo AM, Vicente L, Barreiros A, Monteiro P, et al. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers. 2004;9:461–469. doi: 10.1080/13547500400024768. [DOI] [PubMed] [Google Scholar]
- 10.Duong C, Seow HJ, Bozinovski S, Crack PJ, Anderson GP, Vlahos R. Glutathione peroxidase-1 protects against cigarette smoke–induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2010;299:L425–L433. doi: 10.1152/ajplung.00038.2010. [DOI] [PubMed] [Google Scholar]
- 11.Lubos E, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, et al. Glutathione peroxidase-1 deficiency augments proinflammatory cytokine-induced redox signaling and human endothelial cell activation. J Biol Chem. 2011;286:35407–35417. doi: 10.1074/jbc.M110.205708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingler K, Böcher M, Flohé L, Kollmus H, Brigelius-Flohé R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem. 1999;259:149–157. doi: 10.1046/j.1432-1327.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 13.Müller C, Wingler K, Brigelius-Flohé R. 3′UTRs of glutathione peroxidases differentially affect selenium-dependent mRNA stability and selenocysteine incorporation efficiency. Biol Chem. 2003;384:11–18. doi: 10.1515/BC.2003.002. [DOI] [PubMed] [Google Scholar]
- 14.Elton TS, Martin MM. Angiotensin II type 1 receptor gene regulation: transcriptional and posttranscriptional mechanisms. Hypertension. 2007;49:953–961. doi: 10.1161/HYPERTENSIONAHA.106.070565. [DOI] [PubMed] [Google Scholar]
- 15.Ernst J, Ghanem L, Bar-Joseph Z, McNamara M, Brown J, Steinman RA. IL-3 and oncogenic Abl regulate the myeloblast transcriptome by altering mRNA stability. PLoS One. 2009;4:e7469. doi: 10.1371/journal.pone.0007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geraghty P, Hardigan A, Foronjy RF. Cigarette smoke activates the proto-oncogene c-src to promote airway inflammation and lung tissue destruction. Am J Respir Cell Mol Biol. 2014;50:559–570. doi: 10.1165/rcmb.2013-0258OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem. 2003;278:39609–39614. doi: 10.1074/jbc.M305770200. [DOI] [PubMed] [Google Scholar]
- 18.Mercer BA, Kolesnikova N, Sonett J, D’Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem. 2004;279:17690–17696. doi: 10.1074/jbc.M313842200. [DOI] [PubMed] [Google Scholar]
- 19.Shalaby KH, Gold LG, Schuessler TF, Martin JG, Robichaud A. Combined forced oscillation and forced expiration measurements in mice for the assessment of airway hyperresponsiveness. Respir Res. 2010;11:82. doi: 10.1186/1465-9921-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foronjy RF, Dabo AJ, Taggart CC, Weldon S, Geraghty P. Respiratory syncytial virus infections enhance cigarette smoke induced COPD in mice. PLoS One. 2014;9:e90567. doi: 10.1371/journal.pone.0090567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, et al. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1149–L1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 22.Hsia CC, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hackett NR, Heguy A, Harvey BG, O’Connor TP, Luettich K, Flieder DB, et al. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol. 2003;29:331–343. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- 25.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D’Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res. 2005;31:547–562. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- 26.Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohé R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter T. A tail of two src’s: mutatis mutandis. Cell. 1987;49:1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- 28.Geraghty P, Baumlin N, Salathe MA, Foronjy RF, D’Armiento JM. Glutathione peroxidase-1 suppresses the unfolded protein response upon cigarette smoke exposure. Mediators Inflamm. 2016;2016:9461289. doi: 10.1155/2016/9461289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foronjy RF, Ochieng PO, Salathe MA, Dabo AJ, Eden E, Baumlin N, et al. Protein tyrosine phosphatase 1B negatively regulates S100A9-mediated lung damage during respiratory syncytial virus exacerbations. Mucosal Immunol. 2016;9:1317–1329. doi: 10.1038/mi.2015.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty DF, Nath S, Poon J, Foronjy RF, Ohlmeyer M, Dabo AJ, et al. Protein phosphatase 2A reduces cigarette smoke–induced cathepsin S and loss of lung function. Am J Respir Crit Care Med. 2019;200:51–62. doi: 10.1164/rccm.201808-1518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribas V, García-Ruiz C, Fernández-Checa JC. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ursini F, Maiorino M, Brigelius-Flohé R, Aumann KD, Roveri A, Schomburg D, et al. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15:435–445. doi: 10.1016/0891-5849(93)90043-t. [DOI] [PubMed] [Google Scholar]
- 34.Thu VT, Kim HK, Ha SH, Yoo JY, Park WS, Kim N, et al. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 35.de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, et al. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohé R, et al. Glutathione peroxidase 2, the major cigarette smoke–inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Zhou L, Zhou K, Zhang J, Shang F, Zhang X. Celastrol protects RPE cells from oxidative stress–induced cell death via activation of Nrf2 signaling pathway. Curr Mol Med. 2019;19:172–182. doi: 10.2174/1566524019666190424131704. [DOI] [PubMed] [Google Scholar]
- 40.Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10:2023–2033. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao XK, Che P, Cheng ML, Zhang Q, Mu M, Li H, et al. Tristetraprolin down-regulation contributes to persistent TNF-alpha expression induced by cigarette smoke extract through a post-transcriptional mechanism. PLoS One. 2016;11:e0167451. doi: 10.1371/journal.pone.0167451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross EA, Naylor AJ, O’Neil JD, Crowley T, Ridley ML, Crowe J, et al. Treatment of inflammatory arthritis via targeting of tristetraprolin, a master regulator of pro-inflammatory gene expression. Ann Rheum Dis. 2017;76:612–619. doi: 10.1136/annrheumdis-2016-209424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman MM, Rumzhum NN, Hansbro PM, Morris JC, Clark AR, Verrills NM, et al. Activating protein phosphatase 2A (PP2A) enhances tristetraprolin (TTP) anti-inflammatory function in A549 lung epithelial cells. Cell Signal. 2016;28:325–334. doi: 10.1016/j.cellsig.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Matoušková P, Hanousková B, Skálová L. MicroRNAs as potential regulators of glutathione peroxidases expression and their role in obesity and related pathologies. Int J Mol Sci. 2018;19:E1199. doi: 10.3390/ijms19041199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Y, Schoenberg DR. c-Src activates endonuclease-mediated mRNA decay. Mol Cell. 2007;25:779–787. doi: 10.1016/j.molcel.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince MR, Frisoli JK. Beta-carotene accumulation in serum and skin. Am J Clin Nutr. 1993;57:175–181. doi: 10.1093/ajcn/57.2.175. [DOI] [PubMed] [Google Scholar]
- 47.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 48.Hong J, Lambert JD, Lee SH, Sinko PJ, Yang CS. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem Biophys Res Commun. 2003;310:222–227. doi: 10.1016/j.bbrc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Ueda Y, Igishi T, Hashimoto K, Suyama H, Araki K, Sumikawa T, et al. Synergistic cell growth inhibition by the combination of amrubicin and Akt-suppressing tyrosine kinase inhibitors in small cell lung cancer cells: implication of c-Src and its inhibitor. Int J Oncol. 2009;34:689–696. doi: 10.3892/ijo_00000195. [DOI] [PubMed] [Google Scholar]
- 50.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.