Abstract
Accumulating evidence suggests that fibrosis is a multicellular process with contributions from alveolar epithelial cells (AECs), recruited monocytes/macrophages, and fibroblasts. We have previously shown that AEC injury is sufficient to induce fibrosis, but the precise mechanism remains unclear. Several cell types, including AECs, can produce CCL2 and CCL12, which can promote fibrosis through CCR2 activation. CCR2 signaling is critical for the initiation and progression of pulmonary fibrosis, in part through recruitment of profibrotic bone marrow–derived monocytes. Attempts at inhibiting CCL2 in patients with fibrosis demonstrated a marked upregulation of CCL2 production and no therapeutic response. To better understand the mechanisms involved in CCL2/CCR2 signaling, we generated mice with conditional deletion of CCL12, a murine homolog of human CCL2. Surprisingly, we found that mice with complete deletion of CCL12 had markedly increased concentrations of other CCR2 ligands and were not protected from fibrosis after bleomycin injury. In contrast, mice with lung epithelial cell–specific deletion of CCL12 were protected from bleomycin-induced fibrosis and had expression of CCL2 and CCL7 similar to that of control mice treated with bleomycin. Deletion of CCL12 within AECs led to decreased recruitment of exudate macrophages. Finally, injury to murine and human primary AECs resulted in increased production of CCL2 and CCL12, in part through activation of the mTOR pathway. In conclusion, these data suggest that targeting CCL2 may be a viable antifibrotic strategy once the pathways involved in the production and function of CCL2 and other CCR2 ligands are better defined.
Keywords: fibrosis, CCL2, CCL12, alveolar epithelial cell
Clinical Relevance
Our findings demonstrate that CCL2/12 derived from injured alveolar epithelial cells promotes lung fibrosis. We identify a novel mechanism by which injury promotes CCL2/12 expression by alveolar epithelial cells. We demonstrate the importance of alveolar epithelial cell–derived CCL2/12 in recruiting profibrotic macrophages to the lung.
Pulmonary fibrosis is a devastating condition resulting from diverse injuries to the lung or from primary conditions such as idiopathic pulmonary fibrosis (IPF). Clinically, IPF results in a rapid and progressive decline in lung function leading to a poor quality of life and a median survival of 3–5 years from the time of diagnosis (1, 2). Despite intense research into the pathogenesis of IPF and other fibrotic disorders, therapeutic options remain limited. Although a number of profibrotic pathways have been identified, clinical trials aimed at targeting these pathways have mostly led to negative results (3–6). These negative clinical trial results may be due to incomplete understanding of the profibrotic mechanisms and the cells involved that lead to insufficient pathway inhibition, opposing effects on pro- and antifibrotic functions in diverse cell types, or the upregulation of compensatory profibrotic signaling factors (7). A greater understanding of specific pathways and the overall mechanism of lung fibrosis may allow a refined and improved therapeutic approach (8–10).
Fibrosis is a multicellular process that involves contributions from different cell types (7, 11). In addition to the accumulation of activated fibroblasts, an important role for alveolar epithelial cell (AEC) injury/death and profibrotic macrophage recruitment into the lung (12–16) during fibrosis have been implicated in numerous studies. Although likely overly simplistic, a unifying model holds that AEC injury leads to production of cytokines that function to recruit and activate bone marrow–derived cells to the lung, which promotes fibroblast activation and subsequent fibrosis (14, 17–19).
Extensive evidence derived from animal models, as well as human data, demonstrates that CCL2 is involved in the pathogenesis of pulmonary fibrosis (20). CCL2 is a chemoattractant involved in the recruitment of fibrocytes and profibrotic macrophages (12, 21–24). Human CCL2 has two murine homologs: CCL2 and CCL12. Despite the nomenclature, human CCL2 has greater homology to murine CCL12 than it does to murine CCL2 (22). CCL2-null mice have been studied extensively in many different disease models; however, to our knowledge, mice with complete or cell-selective deletion of CCL12 have not previously been generated or characterized, indicating that the understanding of human CCL2 biology remains inadequately studied in the context of in vivo murine models. CCL2 and CCL12 both function through binding to their receptor, CCR2. Other CCR2 ligands, most notably CCL7, have also been implicated in fibrosis (25). A fibrogenic role for CCR2 signaling in fibrosis was first demonstrated in the FITC model of lung fibrosis (21). Importantly, the profibrotic properties of CCR2 were subsequently confirmed in many other complementary models of pulmonary fibrosis (12, 24). In one study, neutralizing antibodies to CCL12, but not to CCL2, prevented fibrosis, suggesting that CCL12 is the more critical profibrotic homolog of human CCL2 (22, 26). CCL2 and CCL12 are produced by multiple cell types, including epithelial cells and a number of distinct bone marrow–derived cells, and although macrophages may account for the highest expression of CCL2/12 on a per-cell basis, the functionally important cellular sources of CCL2/12 during pulmonary fibrosis remain unclear (26–30). CCL12 expression can be induced in cultured AECs in vitro after injury (22, 26); however, the pathways that regulate CCL2/12 expression remain poorly defined.
The strong evidence in support of a profibrotic role for CCL2/CCR2 signaling in pulmonary fibrosis led to a phase 2 clinical trial of a CCL2-neutralizing antibody in patients with IPF (31). In this trial, the CCL2-neutralizing antibody did not alter the progression of lung function decline compared with placebo. Remarkably, the investigators noted that the total serum CCL2 concentrations increased approximately 10–100-fold from baseline in patients treated with the CCL2-neutralizing antibody. Perhaps more striking, the concentration of free CCL2, unbound to the neutralizing antibody, at 24 and 52 weeks was actually about twofold higher than the baseline free CCL2 concentration in the patients treated with CCL2-neutralizing antibody (31). Thus, the primary conclusions of this clinical trial were that the neutralizing antibody was not effective in inhibiting CCL2 activity and that the clinical impact of effective CCL2 inhibition remains untested. These data suggest that although inhibition of CCL2 itself may be problematic because of the compensatory increase in CCL2 production, inhibition of CCL2 activity and the CCL2/CCR2 pathway has not been optimally tested in humans and might still be a viable therapeutic target.
In the present study, we generated mice with complete and cell-selective conditional deletion of CCL12. We found that complete loss of CCL12 led to a dramatic compensatory increase in expression of other CCR2 ligands and had no overall effect on fibrosis. Conversely, AEC-specific loss of CCL12 led to a muted compensatory response, reduced accumulation of profibrotic macrophages, and attenuated fibrosis. We also found that diverse injuries to AECs can induce expression of CCL12 through the mTOR signaling pathway, suggesting that the pathways which lead to AEC production of CCR2 ligands might be a viable therapeutic target.
Methods
Mice
Floxed CCL12 mice were generated by Ingenious Targeting Laboratory using a targeting vector purchased from the KOMP Repository. The flippase recognition target (FRT)-flanked region of the resulting mice was removed by crossing with cytomegalovirus (CMV)/β-actin promoter-FlpO mice purchased from The Jackson Laboratory. The resulting floxed CCL12 mice were then backcrossed for at least 10 generations onto a C57BL/6 background.
To generate CCL12-null mice, floxed CCL12 mice were crossed with mice expressing Cre by the CMV/β-actin promoter, resulting in recombination and deletion of the floxed CCL12 gene in all cells, including germ cells. The mice carrying the CCL12-null allele were subsequently crossed to breed out the CMV/β-actin promoter Cre transgene and to generate CCL12-null homozygous mice. The extent of floxed CCL12 gene recombination was verified by qPCR using primers flanking the floxed region, 5′-CGATACCACGATATCAACAAG-3′ (forward) and 5′-CTCAGACCTAACTTCGTATAATG-3′ (reverse), and normalized using primers outside of the floxed region, 5′-GGCTCTTACCTCATCCTT-3′ (forward) and 5′-CGAAATGAAAGCTAGAGGAG-3′ (reverse). AEC-specific loss of CCL12 was generated by crossing floxed CCL12 mice with surfactant protein-C promoter-reverse tetracycline transactivator (SPC-rtTA)/CMV promoter tet operator Cre (tetO-Cre) transgenic mice (32), and the mice were maintained on doxycycline-containing chow during breeding to specifically and permanently delete ccl12 in lung epithelial cells and their derivatives. Mice were used at 6–8 weeks old. All mice were housed under specific-pathogen–free conditions. The institutional animal care and use committee at the University of Michigan approved all the experiments.
Mice were injured with bleomycin as described before (15, 33). Briefly, mice were administered 40 μl of saline or bleomycin (3 U/kg) dissolved in saline via oropharyngeal aspiration (34). At the indicated time points after injection, mice were killed for collection of BAL, cells, and lung tissue.
Antibodies and Other Reagents
Antibodies against CCL12 were obtained from R&D Systems. ELISA kits for human CCL2, murine CCL2, and murine CCL12 were purchased from R&D Systems. ELISA kits for murine CCL7 were obtained from Abcam. Antibodies against mouse CD45, CD16/32, Ly6C, and CD11b were ordered from BD Biosciences. Antibodies against murine CD11c, CD45, and CD16/32 were purchased from BioLegend. Anti-Scr antibody was obtained from EMD Millipore. Antibody for raptor (regulatory associated protein of mTOR) was purchased from Cell Signaling Technology. Purified TGF-β1 (transforming growth factor-β1) was obtained from R&D Systems. Tunicamycin was purchased from Sigma-Aldrich. Other reagents for type II AEC isolation and culture were described previously (18). Antibodies used for flow cytometry are listed in Table E1 in the data supplement and were described before (12).
Hematoxylin and Eosin Staining and Masson’s Trichrome Assay
Lung sections were analyzed as previously described (35). Briefly, mice were killed with CO2. Their lungs were inflated with formaldehyde to a pressure of 25 cm H2O and fixed overnight. Tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin or Masson’s trichrome by McClinchey Histology Labs.
Hydroxyproline Assay
A hydroxyproline assay was performed as described before (36, 37). Briefly, either entire lung lobes or right lung lobes were isolated and homogenized at 3 weeks after bleomycin administration. Tissue was baked in 12N HCl overnight at 120°C. Aliquots of tissue samples were neutralized with citrate buffer and incubated with chloramine T solution at room temperature for 20 minutes. The samples were further mixed with Erlich’s solution at 65°C for 15 minutes. Absorption at 540 nm was measured. Total hydroxyproline content was quantified by comparison against a standard curve.
Isolation and Culture of Type II AECs
Primary human type II AECs were purchased from Cell Biologics. Primary murine type II AECs were harvested as described previously (11, 14, 32). Briefly, lungs were perfused and lavaged after mice were killed with CO2. Lungs were filled with dispase for incubation at room temperature for 45 minutes. Lungs were teased apart and then incubated with DNase for 10 minutes. The digested samples were serially passed through 70-μm, 40-μm, and 20-μm filters. The cell suspension was incubated with CD16/32 and CD45 antibodies at 37°C for 30 minutes. A negative selection was applied through a magnetic separator. The remaining cells were further negatively selected by plating on a plastic Petri dish for 1–2 hours. AECs were cultured in a 37°C, 5% CO2 incubator. Some AECs were treated with TGF-β (4 ng/ml), tunicamycin (500 ng/ml), H2O2 (500 μM), or appropriate vehicle control. In some experiments, AECs were pretreated with Torin (100 nM) or vehicle control for 1 hour before treatment with TGF-β or tunicamycin.
In some experiments, cells were first treated with lentivirus encoding shRNA to raptor versus scrambled shRNA as a control as previously described. Briefly, the shRNA vector was purchased from Open Biosystems, and lentivirus was generated by the University of Michigan Vector Core. Lentivirus (5 pfu/cell) was used on Days 2 and 3 after AEC isolation to inhibit expression of raptor (vs. lentivirus encoding scrambled shRNA). After an additional 24 hours, cells were analyzed for expression of raptor by immunoblotting or treated with tunicamycin or TGF-β as before (36).
Gene Expression Analysis
Gene expression was analyzed through qRT-PCR as described before (18, 36). Briefly, RNA was isolated from either cells or lung tissues with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized with the SuperScript III First-Strand Synthesis Kit (Invitrogen). The POWER SYBR Green PCR Master Mix Kit (Applied Biosystems) was applied for RT-PCR on the Applied Biosystems 7000 sequence detection system. The fold changes in gene expression were normalized to the housekeeping genes β-actin and GAPDH. Primer sequences used were as follows: arginase, 5′-CAGAAGAATGGAAGAGTCAG (forward) and 5′-CAGATATGCAGGGAGTCACC (reverse); CCL2, 5′-TTCTGGGCCTGCTGTTCACAG (forward) and 5′-CTACTCATTGGGATCATCTTGC (reverse); CCL7, 5′-TGCTTTCAGCATCCAAGTGTG (forward) and 5′-GGACACCGACTACTGGTGATC (reverse); CCL12, 5′-TAGCTACCACCATCAGTCCTC (forward) and 5′-GGGACACTGGCTGCTTGTGAT (reverse); CCR2, 5′-CCTTGGGAATGAGTAACTGTGTGA (forward) and 5′- GACAGGATTAATGCAGCAGTGTGT (reverse); and Scr, 5′-CATCGTTGTGTATGACTACCA (forward) and 5′-CCTGGAAGTTCTGGAGTTTTCT (reverse).
ELISA
CCL2, CCL7, and CCL12 were measured in AEC supernatants or lung homogenates by using ELISA kits from R&D Systems and Abcam according to the manufacturers’ protocols.
Immunoblot Assay
Immunoblot analysis was performed using lung tissues or cells lysed in radioimmunoprecipitation assay buffer as described before (32, 38). Scanned immunoblots are representative of at least three independent experiments.
Single-Cell Lung Leukocyte Flow Cytometry
Lungs from killed mice were minced and digested with proteolytic enzymes to obtain a single-cell suspension of leukocytes as previously described (39). The flow cytometric analysis and gating strategy used to identify lung leukocyte subsets were described in detail before (12), and fluorochrome-conjugated antibodies used for cell staining are listed in Table E1. Briefly, cells were first stained with a fixable viability dye (Zombie aqua; BioLegend) following the manufacturer’s protocol. After Fc receptors were blocked using anti-CD16/32 antibody (clone 93; BioLegend), cells were stained for cell surface markers and then fixed with 2% formaldehyde (Thermo Fisher Scientific) in PBS. Data were acquired using an LSR II or LSRFortessa flow cytometer (both from BD Biosciences) and analyzed using FlowJo software (FlowJo, LLC). At least 150,000 events in the CD45+ gate were acquired per lung sample. Thereafter, the gating strategy detailed in Figure E5 was used to identify Ly6Chigh monocytes as CD45+CD3−CD19−Ly6G−SSClowSiglec F−CD11c−CD11b+Ly6Chigh. Alveolar macrophages were identified as CD45+CD3−CD19−Ly6G−FSCmod/highSiglec F+CD11c+CD11b−, and exudate macrophages were identified as CD45+CD3−CD19−Ly6G−FSCmod/highSiglec F−autofluorescent+CD11c+CD11b+. To determine the number of cells in each population of interest in each sample, the corresponding percentage was multiplied by the total number of viable CD45+ cells in that sample. The latter value was calculated for each sample as the product of the percentage of viable CD45+ cells and the original hemocytometer count of total viable cells identified within that sample. Cell sorting for interstitial macrophages (defined as CD11b+CD64+) was performed using a FACSAria III cell sorter (BD Biosciences) within the University of Michigan Biomedical Research Core Facilities.
Statistical Analysis
Data are expressed as box-and-whisker plots in which the middle line represents the median, the box indicates the 25th and 75th percentiles, and the whiskers represent the minimal and maximal values. For evaluation of group differences, a two-tailed Student’s t test was used, assuming equal variance. P < 0.05 was accepted as significant.
Results
AEC Injury Leads to Increased Expression of CCL2 and CCL12
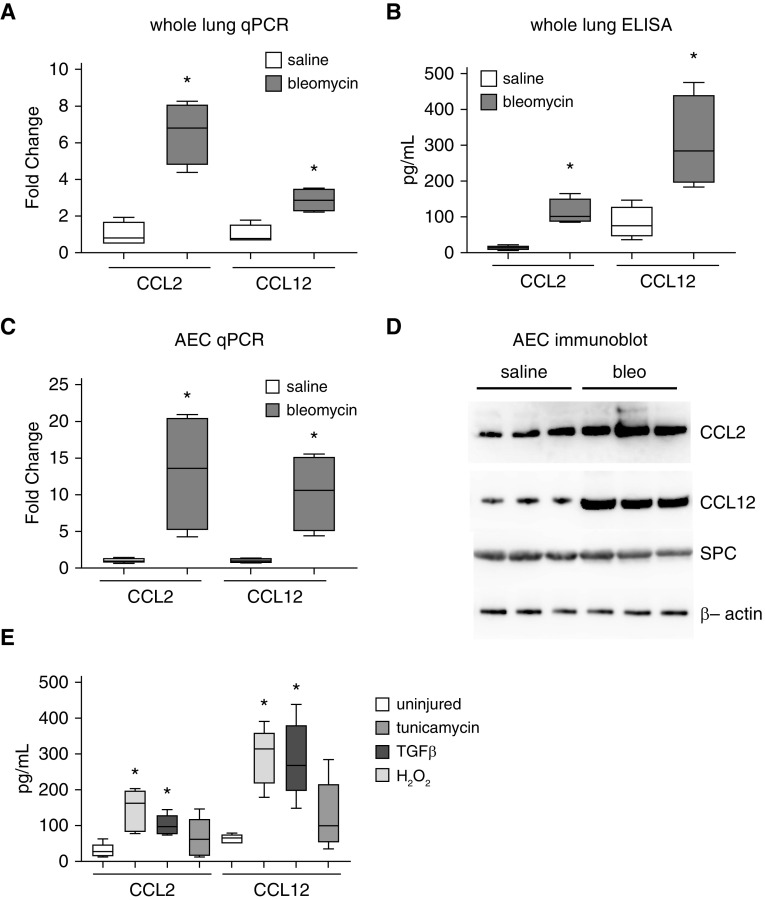
Although increased concentrations of CCL2 and CCL12 have been observed in a number of models of pulmonary fibrosis, whether these chemokines are produced by AECs in vivo has not been well demonstrated. We first injured wild-type (WT) mice with bleomycin (or saline control). In a time-course experiment, we found that CCL2 and CCL12 expression peaked at 10 days after bleomycin, and this time point was chosen for subsequent experiments (Figure E1). Ten days after bleomycin, we found increased expression of CCL2 and CCL12 mRNA and protein in bleomycin-treated mice, consistent with prior reports (Figures 1A and 1B) (23). To further demonstrate upregulation of CCL2/12 in AECs in vivo, 10 days after bleomycin, AECs were isolated, and they were also found to have increased CCL2 and CCL12 mRNA by qPCR and protein by immunoblot analysis (Figures 1C and 1D). Purity of AECs isolated from saline- and bleomycin-treated mice was verified by confirming expression of Scr (Figures 1D and E2), and isolated AECs were not contaminated with CD45-expressing leukocytes (Figure E3). AEC injury is known to occur in the bleomycin model through a number of pathways relevant to human disease, including endoplasmic reticulum stress, oxidative stress, and TGF-β (3, 15, 32). Murine primary AECs were cultured and treated with tunicamycin (an inducer of endoplasmic reticulum stress), TGF-β (an inducer of AEC injury/apoptosis), and H2O2 (an inducer of oxidative stress). After 24 hours, conditioned media from injured AECs were found to have increased concentrations of CCL2 and CCL12 after induction of endoplasmic reticulum stress and TGF-β exposure (Figure 1E). Oxidative stress resulted in a trend toward increased concentrations of CCL2 and CCL12, but the increased expression did not reach statistical significance. Collectively, these studies demonstrate that various AEC insults, both in vitro and in vivo, lead to increased production and release of CCL2 and CCL12.
Figure 1.
Injury promotes alveolar epithelial cell (AEC) production of CCL2 and CCL12. (A and B) Ten days after intrapulmonary saline or bleomycin administration to wild-type (WT) mice, lungs were analyzed for expression of CCL2 and CCL12 by qPCR (A) and ELISA (B). n = 5–8. *P < 0.05 compared with saline-treated mice. (C and D) Ten days after intrapulmonary saline or bleomycin administration to WT mice, AECs were harvested and analyzed for expression of CCL2 and CCL12 by qPCR (C) and immunoblotting (D). n = 5–8. *P < 0.05 compared with saline-treated. Concentrations of Scr (SPC) were similar. (E) Conditioned media from primary AECs injured with TGF-β (transforming growth factor-β), tunicamycin, or H2O2 were analyzed for CCL2 and CCL12 by ELISA. n = 6. *P < 0.05. bleo = bleomycin; qPCR = quantitative PCR.
Generation of CCL12-Null Mice and Mice with AEC-Specific Deletion of CCL12
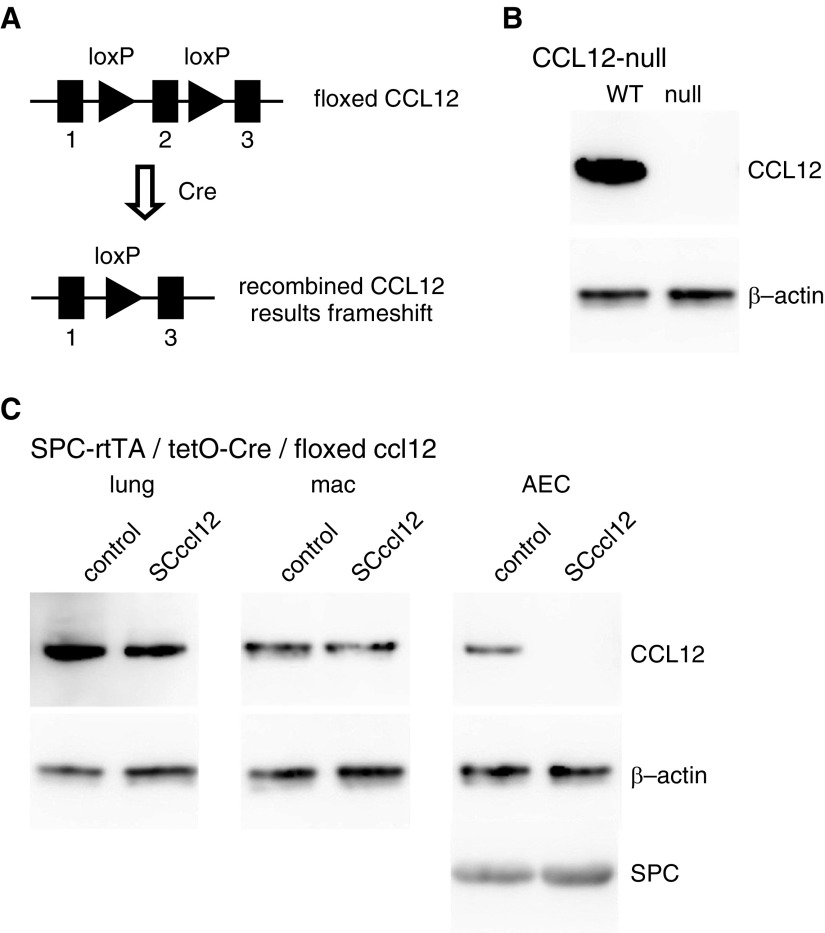
To determine the importance of AEC-specific CCL12, we generated floxed CCL12 mice using a targeting vector from the KOMP Repository. The FRT-flanked neomycin selection cassette was removed from the resulting mice by crossing with a transgenic mouse constitutively expressing FlpO. The FlpO transgene was bred out, and the resulting floxed CCL12 mice (Figure 2A) were backcrossed for at least 10 generations onto a C57BL/6 background. Floxed CCL12 mice were crossed with a CMV-Cre transgenic mouse, resulting in recombination of the floxed CCL12 allele in all cells including the germline. These mice were crossed to breed out the Cre transgene and to yield homozygous mice with complete deletion of CCL12. Deletion of CCL12 was confirmed by immunoblotting from lung lysate of CCL12-null mice (Figure 2B) as well as other tissue (not shown). AEC-specific deletion of CCL12 was achieved by crossing floxed CCL12 mice with transgenic mice in which expression of the reverse tetracycline transactivator is regulated by the SPC promoter (SPC-rtTA) with expression of Cre regulated by a CMV-tetO promoter (tetO-Cre). Breeding pairs were maintained on doxycycline chow to remove CCL12 expression in lung epithelial cells of developing SPC-rtTA/tetO-Cre/floxed CCL12 (SCccl12) mice. AEC-specific deletion of CCL12 was confirmed by immunoblot analysis (Figure 2C) demonstrating preserved concentrations of CCL12 in lung lysate and alveolar macrophages, as well as near-complete loss of CCL12 in AECs. Furthermore, CD64+CD11b+ interstitial macrophages from CCL12-null mice demonstrated complete recombination of the floxed CCL12 gene, whereas interstitial macrophages from SCccl12 mice demonstrated no recombination of the floxed CCL12 by qPCR (Figure E4).
Figure 2.
Generation and validation of mice with complete and AEC-specific deletion of CCL12. (A) Schematic of the floxed CCL12 allele. Cells expressing Cre recombinase will have removal of exon 2, leading to a frameshift and lack of expression thereafter. (B) Floxed CCL12 mice were crossed with mice expressing a constitutive Cre recombinase resulting in a CCL12-null allele. Homozygous CCL12-null mice have complete loss of CCL12, demonstrated by immunoblot from whole lung. (C) Floxed CCL12 mice were crossed with mice carrying the Scr reverse tetracycline transactivator (SPC-rtTA) and tetO-cytomegalovirus-Cre (tetO-Cre) alleles. SPC-rtTA/tet-O Cre/floxed CCL12 (SCccl12) mice have reduced expression of CCL12 in the whole lung, normal expression of CCL12 in macrophages isolated from the lungs, and loss of CCL12 from isolated AECs. loxP = locus of X-over P1; mac = macrophages.
CCL12-Null Mice Are Not Protected from Fibrosis
We first injured CCL12-null and WT control mice with bleomycin. After 3 weeks, fibrosis was assessed by examining trichrome-stained lung sections and by hydroxyproline assay. Surprisingly, CCL12-null mice were not protected from fibrosis. To determine if there was compensatory upregulation of other CCR2 ligands, we measured concentrations of CCL2 and CCL7, which are both CCR2 ligands that have been implicated in fibrosis (25). CCL12-null and WT mice were injured with bleomycin as before. After 10 days, lungs were analyzed for CCL2, CCL7, and CCL12 expression by qPCR and ELISA. As expected, lungs from WT mice exhibited a marked increase in CCL2, CCL7, and CCL12 expression after bleomycin injury. Lungs from CCL12-null mice, however, exhibited a much more robust increase in CCL2 and CCL7 expression after bleomycin than those from WT mice (Figure 3).
Figure 3.
CCL12-null mice are not protected from bleo-induced fibrosis. (A–D) Trichrome staining of lung sections of WT (A and C) or CCL12-null (B and D) mice 21 days after saline (sal) (A and B) or bleo (C and D). Scale bars: 200 μm. (E) Hydroxyproline assay of WT control and CCL12-null mice 21 days after sal or bleo. n = 5–8 per group. (F) Lung expression of CCL2, CCL7, and CCL12 by qPCR of WT control (ctl) or CCL12-null mice 10 days after sal or bleo injury. n = 4–6 per group. *P < 0.01 compared with ctl mice treated with bleomycin. (G) Concentrations of CCL2, CCL7, and CCL12 measured by ELISA from lung lysates of ctl or CCL12-null mice 10 days after saline or bleomycin. n = 4–8 per group. *P < 0.01 compared with WT mice treated with bleo.
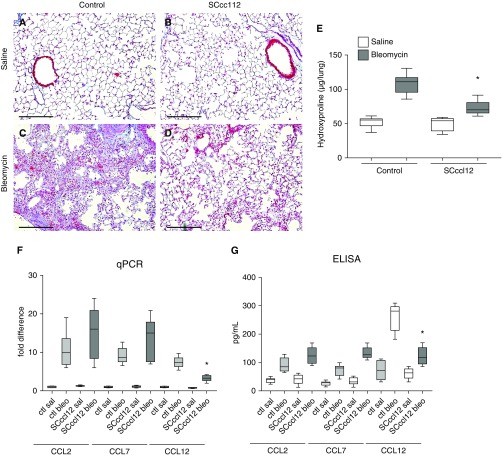
Mice with AEC-Specific Deletion of CCL12 Are Protected from Fibrosis
Next, SCccl12 mice (or littermate control animals lacking one of the transgenes) were injured with saline or bleomycin. After 3 weeks, fibrosis was assessed as before. SCccl12 mice with AEC-specific deletion of CCL12 had an attenuated fibrotic response to bleomycin (Figure 4). As with the CCL12-null mice, SCccl12 mice were analyzed for expression of CCL2 and CCL7. As expected, control mice had a marked increase in expression of CCL2, CCL7, and CCL12 after bleomycin injury. SCccl12 mice had an attenuated increase in CCL12 expression. Concentrations of CCL2 and CCL7 in SCccl12 mice injured with bleomycin trended higher than those in control mice injured with bleomycin, but the differences were not statistically significant. Collectively, these results indicated that SCccl12 mice injured with bleomycin exhibit a muted compensatory upregulation of CCL2 and CCL7 lung expression compared with control mice.
Figure 4.
Mice with AEC-specific deletion of CCL12 are protected from bleo-induced lung fibrosis. (A–D) Trichrome staining of lung sections of SCccl12 mice (B and D) or littermate control mice lacking at least one of the transgenes (A and C) 21 days after sal (A and B) or bleo (C and D). Scale bars: 200 μm. (E) Hydroxyproline assay of littermate control and SCccl12 mice 21 days after sal or bleo. n = 5–8 per group. *P < 0.05 compared with control mice treated with bleo. (F) Lung expression of CCL2, CCL7, and CCL12 by qPCR of control (ctl) or SCccl12 mice 10 days after sal or bleo injury. n = 4–6 per group. *P < 0.01 compared with mice treated with bleo. (G) Concentrations of CCL2, CCL7, and CCL12 measured by ELISA from lung lysate of ctl or SCccl12 mice 10 days after sal or bleo. n = 4–6 per group. *P < 0.01 compared with ctl mice treated with bleo.
AEC-derived CCL12 Is Involved in Recruitment of Exudate Macrophages
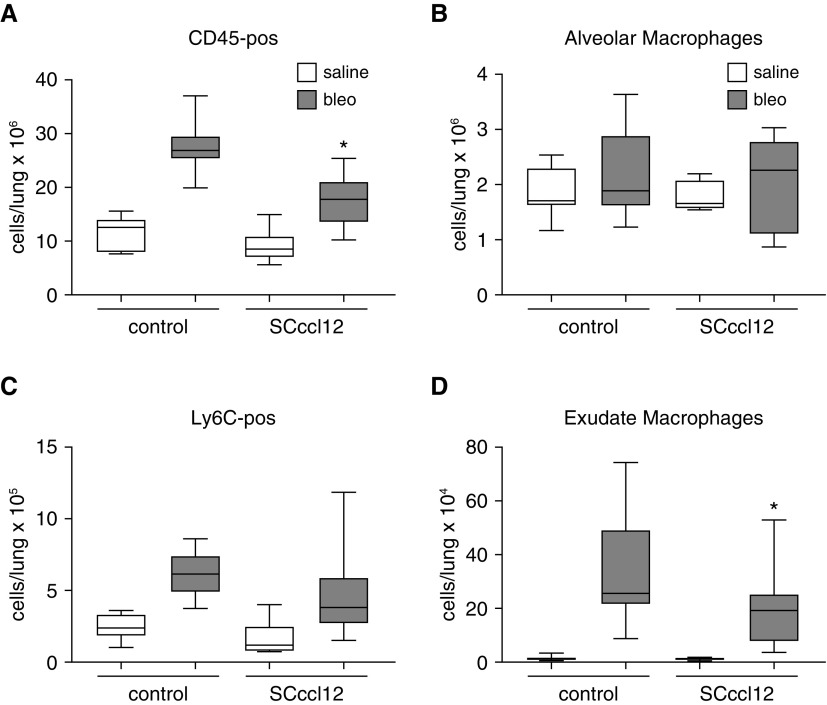
We and others have shown that fibrosis is associated with recruitment of bone marrow–derived cells into the lung and that many of these cells are monocytes which subsequently differentiate into monocyte-derived “exudate” macrophages that display a profibrotic phenotype (12, 16, 24, 40–42). CCR2 signaling is critical for recruitment of bone marrow–derived cells, such as exudate macrophages, during fibrosis (12). However, the important ligand or the cellular source of the ligand remains unknown. To determine if CCL12 specifically released from AECs influenced accumulation of bone marrow–derived cells into the lung, SCccl12 and littermate control mice were injured with bleomycin (or saline). Ten days after bleomycin treatment, lung single-cell leukocyte suspensions from WT and SCccl12 mice were analyzed by flow cytometry as previously described (Figure E5) (12, 43). As expected, WT mice injured with bleomycin had a significant increase in both Ly6C-positive monocytes and exudate macrophages, which have previously been associated with lung fibrosis (12). SCccl12 mice demonstrated a significantly attenuated increase in the numbers of exudate macrophages and a trend toward decreased concentrations of Ly6Chigh monocytes after bleomycin compared with control mice treated with bleomycin (Figure 5).
Figure 5.
Mice with AEC-specific deletion of CCL12 have less recruitment of immune cells to the lung at Day 10 after bleo treatment. (A–D) Lung single-cell suspension from WT and SCccl12−/− mice were analyzed by flow cytometry at Day 10 after bleo or sal treatment. Numbers of lung CD45-positive cells (A), alveolar macrophages (B), Ly6C-positve monocytes (C), and exudate macrophages (D) were quantified. n = 7–13. *P < 0.05 compared with control mice treated with bleomycin.
CCL2 and CCL12 Production after Injury Is Mediated through the mTOR Pathway
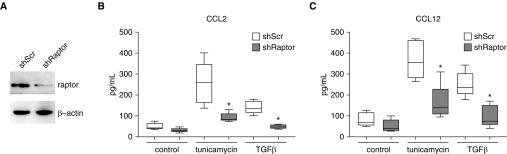
A recent report suggests that epithelial cell production of CCL2 can be regulated through a noncanonical mTOR pathway (44). The mTOR pathway has been implicated in fibrosis, but the precise mechanism remains unclear (45). To explore the possibility that mTOR signaling is involved in expression of CCL2 and CCL12 by AECs, primary murine AECs were cultured and injured with TGF-β or tunicamycin as before. Some wells were pretreated with the mTOR inhibitor Torin (100 nM) or vehicle control for 1 hour before treatment with TGF-β or tunicamycin. As before, we observed increased concentrations of CCL2 and CCL12 in the conditioned media of AECs treated with TGF-β and tunicamycin. AECs pretreated with Torin had a marked reduction in CCL2 and CCL12 production (Figure 6), supporting a role for the mTOR pathway in CCL2 and CCL12 expression. To confirm these results in human AECs, primary human AECs were cultured and treated with tunicamycin, TGF-β, and Torin. Similar to the murine AECs, primary human AECs demonstrated a marked increase in CCL2 production that was blocked by mTOR inhibition. Finally, to substantiate a role for the mTOR pathway, we used lentivirus-mediated shRNA against raptor, which is part of the mTORC1 complex. Primary AECs treated with shRaptor had decreased concentrations of raptor verified by immunoblotting (Figure 7A). As with AECs treated with Torin, AECs with suppressed expression of raptor had an attenuated upregulation of CCL2 and CCL12 in response to TGF-β and tunicamycin (Figures 7B and 7C).
Figure 6.
mTOR pathway regulates AEC expression of CCL2 and CCL12. (A and B) Amount of CCL2 (A) and CCL12 (B) quantified by ELISA of conditioned media from primary murine AECs treated with tunicamycin or TGF-β with or without the mTOR inhibitor Torin. (C) Amount of CCL2 quantified by ELISA of conditioned media from primary human AECs treated with tunicamycin or TGF-β with or without the mTOR inhibitor Torin. n = 4 per group. *P < 0.01 compared with AECs not treated with Torin. h = human; m = mouse/rat.
Figure 7.
Raptor regulates AEC expression of CCL2 and CCL12. (A–C) Primary AECs were treated with lentivirus encoding shRNA for raptor (shRaptor) or scramble control (shScr), and reduced concentrations of raptor were confirmed by immunoblot analysis (A). Amount of CCL2 (B) and CCL12 (C) quantified by ELISA of conditioned media from primary murine AECs treated with shScr or shRaptor and tunicamycin or TGF-β. n = 4 per group. *P < 0.01 compared with AECs treated with shScr.
Discussion
Numerous animal model and human sample studies have suggested that CCL2/CCL12 signaling through CCR2 is a prominent profibrotic pathway (21, 23, 31, 39, 46). However, the cellular source, the pathways involved in CCR2 ligand production, and the profibrotic function of CCR2 activation remain unclear. We found that mice with complete deletion of CCL12 are not protected from fibrosis, whereas mice with lung epithelial cell–specific deletion of CCL12 are protected from fibrosis. CCL12-null mice exhibit a much more robust compensatory increased expression of CCL2 as well as CCL7, which is also a ligand for CCR2 and other C-C chemokine receptors (47), than do SCccl12 mice or control mice. Both of our transgenic mouse models displayed different degrees of compensatory cytokine expression. Although the degree of compensation of CCR2 ligand expression was less in the SCccl12 mice, these mice still expressed increased concentrations of CCL2 and CCL7 (compared with control mice), which may have contributed to the incomplete inhibition of fibrosis. Notably, CCL7 has been implicated as a profibrotic cytokine, and increased concentrations of CCL7 have been observed in IPF lung tissue (25). It is unclear if a monocyte/macrophage autocrine negative feedback loop regulates expression of CCR2 ligands leading to the marked overexpression of CCL2 and CCL7 in mice with total deletion of CCL12 compared with mice with AEC-specific deletion of CCL12 or if there is a threshold effect below which there is a more prominent response in expression of CCR2 ligands. In contrast to complete deletion of CCL12 in this study, inhibition of CCL12 with a neutralizing antibody was effective at attenuating fibrosis in a mouse model (22), which could support a threshold effect, and there may be a narrow therapeutic window within which CCL12 signaling is inhibited without a resulting upregulation of other CCR2 ligands. Potentially, this therapeutic window may narrow further as upregulation of CCR2 ligand expression continues over time. This notion is consistent with a recent phase 2 clinical trial of carlumab, a CCL2-neutralizing antibody that did not reduce the progressive decline in lung function that is characteristic of patients with IPF (31). Similar to results of other clinical trials targeting CCL2, patients with IPF treated with a CCL2-neutralizing antibody demonstrated a marked compensatory increase in serum concentrations of total and free CCL2, suggesting that direct inhibition of CCL2 as therapy is problematic because of loss of negative feedback regulation of CCL2 expression. Further attempts at inhibiting the CCL2/CCR2 pathway for fibrotic therapy could be directed at the chemokine receptors. For example, there is currently interest in dual inhibition of CCR2 and CCR5 as a potential antifibrotic treatment (48).
Other approaches to inhibiting CCR2 signaling might focus on production of CCR2 ligands, which would require a greater understanding of the cellular source of the CCR2 ligands and the signaling pathways involved in their expression. We have previously shown that primary AECs can respond to TGF-β by undergoing apoptosis (11, 15, 32). Others have reported that endoplasmic reticulum stress is another inducer of AEC injury and apoptosis in the context of fibrosis. AEC injury is a common theme in fibrotic diseases and in animal models of fibrosis, but the mechanism by which epithelial cell injury promotes fibrosis remains unknown. We found that, during injury, AECs respond by producing profibrotic CCL2. The in vivo relevance of AEC-derived CCL2 has not previously been adequately studied, owing to lack of an appropriate animal model with targeted deletion of CCL12, a murine homolog of human CCL2. In the present study, we found that mice with AEC-specific deletion of CCL12 were protected from fibrosis and had reduced recruitment of exudate macrophages to the lung. A recent report suggested that mTOR/mTORC1 is a novel pathway which regulates expression of CCL2 in malignant epithelial cells and is involved in recruitment of tumor-associated macrophages (44). Furthermore, mTOR itself has been implicated as a profibrotic pathway. We similarly found that expression of CCL2 by injured primary murine and human AECs is mediated through an mTOR pathway. Interestingly, mTOR inhibition did not influence the expression of CCL2 or CCL12 by macrophages (data not shown), suggesting that AECs and macrophages regulate CCL2 and CCL12 expression by distinct mechanisms. Although several reports have indicated that AECs can upregulate CCL2 and CCL12 after insult, not much is known about the pathways involved in expression of CCL2 or CCL12 in AECs. Future studies could focus on mechanisms involved in CCL2 expression that might offer additional targets for fibrotic therapy. In human epithelial cancer cells, CCL2 transcription was mediated by the transcription factor FOXK1 (forkhead box protein K1) downstream of mTOR/mTORC1 signaling (44). Whether this is also true in AECs after injury or if activation of mTOR/mTORC1 leads to induction of CCL2/CCL12 expression through different transcription factor(s) will be the subject of future studies.
In this study, we found that AECs are a critical source of CCL2/CCL2 during fibrosis. Recent single-cell RNA sequencing analysis from human and murine normal and fibrotic lungs indicated that interstitial macrophages express high concentrations of CCL2 and CCL12, whereas fewer AECs express CCL2 or CCL12 (29, 30). Interestingly, there does appear to be increased expression of CCL2 in AECs isolated from fibrotic IPF lung compared with normal human AECs (29), consistent with the results of this study. The numbers of CCL2/CCL12-expressing AECs may be underrepresented in these reports, because injured AECs or AECs in the early stages of apoptosis may be particularly vulnerable to cell death and loss during the single-cell isolation protocol. Furthermore, recent studies have indicated that single-cell RNA-sequencing analysis has a fairly high lower limit of detection, known as the “dropout effect” (49–51). Therefore, AECs may express lower concentrations of CCL2 and CCL12 below the detection limit of single-cell RNA-sequencing analysis, but they may still account for a significant amount of total CCL2/CCL12, given the abundance of AECs in the lung. Although we cannot exclude the possibility that nonspecificity of the SPC-rtTA/tetO-Cre transgenes are targeting recombination and removal of cell types other than lung epithelial cells, we believe that this is less likely, given the extensive literature describing this approach, the lack of contaminating macrophages in our AEC preparation (Figure E2), and the lack of recombination of floxed CCL12 within alveolar and interstitial macrophages in SCccl12 mice (Figures 2 and E4).
Finally, the effector cells and the mechanisms by which CCR2 activation causes fibrosis remain unclear. Prior reports have suggested that CCR2 activation is required for accumulation of profibrotic hematopoietically derived cells. The signaling mechanisms involved in CCR2-dependent chemotaxis and the function of these cells remain unclear and could be additional targets for fibrosis. We found that exudate macrophages are recruited to the lung by a mechanism dependent on AEC-derived CCL12. This is consistent with our prior report in which AEC-specific injury results in accumulation of exudate macrophages and fibrosis in a CCR2-dependent mechanism (12). Interestingly, in both our prior report and the present study, there were no differences in the numbers of alveolar macrophages, suggesting that recruited exudate macrophages are an important cell type in supporting fibrosis. Recently, numerous studies have suggested an important role for monocyte-derived macrophages in fibrosis (12, 16, 40, 52). Most of these studies have shown an association between fibrosis and accumulation of these bone marrow–derived cells. The function of these cells in the direct contribution of matrix deposition and/or supporting other fibrogenic effector cells through paracrine activities remains controversial (38, 53).
Collectively, our data show that CCL2 and CCL12 are produced by AECs after injury, leading to exudate macrophage recruitment and subsequent lung fibrosis. Our studies suggest that targeting the CCL2/CCR2 pathway may be a viable therapeutic target after gaining a better understanding of the pathways involved in expression of CCL2 and the mechanisms involved in compensatory cytokine production.
Supplementary Material
Footnotes
Supported by National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL108904 (K.K.K.), U.S. Department of Defense grant GW160154 (J.J.O.), NHLBI grant R01 HL131608 (R.L.Z.), and NHLBI grant R01 HL078871 (T.H.S.).
Author Contributions: Conception and design: J.Y., M.A., J.J.O., T.H.S., and K.K.K. Analysis and interpretation: J.Y., M.A., S.L., S.T.-T., R.L.Z., J.J.O., and K.K.K. Drafting of the manuscript: J.Y., M.A., J.J.O., and K.K.K.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0297OC on January 10, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Thoracic Society; European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [Published erratum appears in Am J Respir Crit Care Med 2002;166:426.] [DOI] [PubMed] [Google Scholar]
- 3.Kim KK, Sheppard D, Chapman HA. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10:a022293. doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 5.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KK, Sisson TH, Horowitz JC. Fibroblast growth factors and pulmonary fibrosis: it’s more complex than it sounds. J Pathol. 2017;241:6–9. doi: 10.1002/path.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inchingolo R, Varone F, Sgalla G, Richeldi L. Existing and emerging biomarkers for disease progression in idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2019;13:39–51. doi: 10.1080/17476348.2019.1553620. [DOI] [PubMed] [Google Scholar]
- 9.Kropski JA, Blackwell TS, Loyd JE. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1717–1727. doi: 10.1183/09031936.00163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Noth I, Martinez F. N-acetylcysteine for idiopathic pulmonary fibrosis: the door is still open [letter] Lancet Respir Med. 2017;5:e1–e2. doi: 10.1016/S2213-2600(16)30327-7. [DOI] [PubMed] [Google Scholar]
- 11.Warsinske HC, Wheaton AK, Kim KK, Linderman JJ, Moore BB, Kirschner DE. Computational modeling predicts simultaneous targeting of fibroblasts and epithelial cells is necessary for treatment of pulmonary fibrosis. Front Pharmacol. 2016;7:183. doi: 10.3389/fphar.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, et al. Implicating exudate macrophages and Ly-6Chigh monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol. 2015;185:969–986. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Velikoff M, Canalis E, Horowitz JC, Kim KK. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. Am J Physiol Lung Cell Mol Physiol. 2014;306:L786–L796. doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wheaton AK, Velikoff M, Agarwal M, Loo TT, Horowitz JC, Sisson TH, et al. The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1206–L1217. doi: 10.1152/ajplung.00424.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, et al. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013;183:1559–1570. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KK, Chapman HA. Endothelin-1 as initiator of epithelial-mesenchymal transition: potential new role for endothelin-1 during pulmonary fibrosis [editorial] Am J Respir Cell Mol Biol. 2007;37:1–2. doi: 10.1165/rcmb.2007-0001ED. [DOI] [PubMed] [Google Scholar]
- 20.Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Clinical significance of MCP-1 levels in BALF and serum in patients with interstitial lung diseases. Eur Respir J. 1999;14:376–382. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- 21.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 24.Osterholzer JJ, Christensen PJ, Lama V, Horowitz JC, Hattori N, Subbotina N, et al. PAI-1 promotes the accumulation of exudate macrophages and worsens pulmonary fibrosis following type II alveolar epithelial cell injury. J Pathol. 2012;228:170–180. doi: 10.1002/path.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi ES, Jakubzick C, Carpenter KJ, Kunkel SL, Evanoff H, Martinez FJ, et al. Enhanced monocyte chemoattractant protein-3/CC chemokine ligand-7 in usual interstitial pneumonia. Am J Respir Crit Care Med. 2004;170:508–515. doi: 10.1164/rccm.200401-002OC. [DOI] [PubMed] [Google Scholar]
- 26.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:465–477. doi: 10.1164/rccm.200905-0798OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia GQ, Gonzalo JA, Lloyd C, Kremer L, Lu L, Martinez-A C, et al. Distinct expression and function of the novel mouse chemokine monocyte chemotactic protein-5 in lung allergic inflammation. J Exp Med. 1996;184:1939–1951. doi: 10.1084/jem.184.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie-Pimentel AC, Chiu S, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi N, Watanabe S, Verma R, Jablonski RP, Chen CI, Cheresh P, et al. A spatially restricted fibrotic niche in pulmonary fibrosis is sustained by M-CSF/M-CSFR signaling in monocyte-derived alveolar macrophages. Eur Respir J. 2020;55:1900646. doi: 10.1183/13993003.00646-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghu G, Martinez FJ, Brown KK, Costabel U, Cottin V, Wells AU, et al. CC-chemokine ligand 2 inhibition in idiopathic pulmonary fibrosis: a phase 2 trial of carlumab. Eur Respir J. 2015;46:1740–1750. doi: 10.1183/13993003.01558-2014. [DOI] [PubMed] [Google Scholar]
- 32.Wheaton AK, Agarwal M, Jia S, Kim KK. Lung epithelial cell focal adhesion kinase signaling inhibits lung injury and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2017;312:L722–L730. doi: 10.1152/ajplung.00478.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashley SL, Sisson TH, Wheaton AK, Kim KK, Wilke CA, Ajayi IO, et al. Targeting inhibitor of apoptosis proteins protects from bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol. 2016;54:482–492. doi: 10.1165/rcmb.2015-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, et al. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Exp Lung Res. 2006;32:181–199. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia S, Agarwal M, Yang J, Horowitz JC, White ES, Kim KK. Discoidin domain receptor 2 signaling regulates fibroblast apoptosis through PDK1/Akt. Am J Respir Cell Mol Biol. 2018;59:295–305. doi: 10.1165/rcmb.2017-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Velikoff M, Agarwal M, Disayabutr S, Wolters PJ, Kim KK. Overexpression of inhibitor of DNA-binding 2 attenuates pulmonary fibrosis through regulation of c-Abl and Twist. Am J Pathol. 2015;185:1001–1011. doi: 10.1016/j.ajpath.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cipolla E, Fisher AJ, Gu H, Mickler EA, Agarwal M, Wilke CA, et al. IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J. 2017;31:5543–5556. doi: 10.1096/fj.201700289R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleaveland KR, Moore BB, Kim KK. Paracrine functions of fibrocytes to promote lung fibrosis. Expert Rev Respir Med. 2014;8:163–172. doi: 10.1586/17476348.2014.862154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, et al. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KK, Dotson MR, Agarwal M, Yang J, Bradley PB, Subbotina N, et al. Efferocytosis of apoptotic alveolar epithelial cells is sufficient to initiate lung fibrosis. Cell Death Dis. 2018;9:1056. doi: 10.1038/s41419-018-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCubbrey AL, Barthel L, Mohning MP, Redente EF, Mould KJ, Thomas SM, et al. Deletion of c-FLIP from CD11bhi macrophages prevents development of bleomycin-induced lung fibrosis. Am J Respir Cell Mol Biol. 2018;58:66–78. doi: 10.1165/rcmb.2017-0154OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–8053. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatsumi H, Matsumoto M, Nakayama KI. Noncanonical pathway for regulation of CCL2 expression by an mTORC1-FOXK1 axis promotes recruitment of tumor-associated macrophages. Cell Rep. 2017;21:2471–2486. doi: 10.1016/j.celrep.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 45.Lawrence J, Nho R. The role of the mammalian target of rapamycin (mTOR) in pulmonary fibrosis. Int J Mol Sci. 2018;19:778. doi: 10.3390/ijms19030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurczynski SJ, Procario MC, O’Dwyer DN, Wilke CA, Moore BB. Loss of CCR2 signaling alters leukocyte recruitment and exacerbates γ-herpesvirus-induced pneumonitis and fibrosis following bone marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2016;311:L611–L627. doi: 10.1152/ajplung.00193.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation: therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65:47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- 48.Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One. 2016;11:e0158156. doi: 10.1371/journal.pone.0158156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kharchenko PV, Silberstein L, Scadden DT. Bayesian approach to single-cell differential expression analysis. Nat Methods. 2014;11:740–742. doi: 10.1038/nmeth.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, et al. Massively parallel digital transcriptional profiling of single cells. Nat Commun. 2017;8:14049. doi: 10.1038/ncomms14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riemondy KA, Jansing NL, Jiang P, Redente EF, Gillen AE, Fu R, et al. Single cell RNA sequencing identifies TGFβ as a key regenerative cue following LPS-induced lung injury. JCI Insight. 2019;5:e123637. doi: 10.1172/jci.insight.123637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satoh T, Nakagawa K, Sugihara F, Kuwahara R, Ashihara M, Yamane F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541:96–101. doi: 10.1038/nature20611. [DOI] [PubMed] [Google Scholar]
- 53.Kleaveland KR, Velikoff M, Yang J, Agarwal M, Rippe RA, Moore BB, et al. Fibrocytes are not an essential source of type I collagen during lung fibrosis. J Immunol. 2014;193:5229–5239. doi: 10.4049/jimmunol.1400753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.