Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal age-associated disease with no cure. Although IPF is widely regarded as a disease of aging, the cellular mechanisms that contribute to this age-associated predilection remain elusive. In this study, we sought to evaluate the consequences of senescence on myofibroblast cell fate and fibrotic responses to lung injury in the context of aging. We demonstrated that nonsenescent lung myofibroblasts maintained the capacity for dedifferentiation, whereas senescent/IPF myofibroblasts exhibited an impaired capacity for dedifferentiation. We previously demonstrated that the transcription factor MyoD acts as a critical switch in the differentiation and dedifferentiation of myofibroblasts. Here, we demonstrate that decreased levels of MyoD preceded myofibroblast dedifferentiation and apoptosis susceptibility in nonsenescent cells, whereas MyoD expression remained elevated in senescent/IPF myofibroblasts, which failed to undergo dedifferentiation and demonstrated resistance to apoptosis. Genetic strategies to silence MyoD restored the susceptibility of IPF myofibroblasts to undergo apoptosis and led to a partial reversal of age-associated persistent fibrosis in vivo. The capacity for myofibroblast dedifferentiation and subsequent apoptosis may be critical for normal physiologic responses to tissue injury, whereas restricted dedifferentiation and apoptosis resistance in senescent cells may underlie the progressive nature of age-associated human fibrotic disorders. These studies support the concept that senescence may promote profibrotic effects via impaired myofibroblast dedifferentiation and apoptosis resistance, which contributes to myofibroblast accumulation and ultimately persistent fibrosis in aging.
Keywords: MyoD, senescence, apoptosis resistance, pulmonary fibrosis, myofibroblast plasticity
Clinical Relevance
Attempts to develop antifibrotic treatment strategies have largely focused on targeting fibrosis initiation events (fibroblast proliferation, fibroblast-to-myofibroblast differentiation, and extracellular matrix generation). One potential explanation for the relative limited efficacy of current antifibrotic approaches is that for most patients, fibrosis is well established at the time of diagnosis. The current study suggests that targeting age-associated pathological dysfunction to promote fibrosis resolution (myofibroblast dedifferentiation or apoptotic clearance of senescent cells) may be more effective than strategies that block fibrosis development.
Fibrotic disease affects most organ systems, including the heart, blood vessels, kidney, liver, skin, and lung. Fibrotic disorders account for increasing morbidity and mortality worldwide. It has been estimated that ∼45% of all deaths in the United States are attributed to organ fibrosis (1, 2). Idiopathic pulmonary fibrosis (IPF) is the most severe form of lung fibrosis, which is a uniformly fatal disorder. IPF is characterized by progressive scar tissue formation that ultimately leads to respiratory failure. IPF is widely regarded as a disease of aging (3–7), as it disproportionately affects the elderly population. The incidence and prevalence of IPF increase with advancing age, with a mean age of 66 years at the time of diagnosis (8), yet few studies have investigated the mechanisms associated with this age-associated predilection.
The myofibroblast is a key effector cell type and a central mediator of fibrotic diseases, including IPF. In response to injury, fibroblasts migrate to the site of damage and differentiate into myofibroblasts. These “muscle-like” cells are responsible for synthesizing extracellular matrix (ECM) components via a TGF-β–dependent mechanism (9). As wound healing culminates, myofibroblasts undergo apoptosis and re-epithelialization occurs (10–12). Conversely, myofibroblast accumulation and impaired re-epithelialization are the pathologic hallmarks of fibrotic disease (13–15). The inability to terminate the host reparative response, with persistent myofibroblast activation/accumulation in injured tissues, may underlie the progressive nature of fibrotic diseases such as IPF (16, 17).
The myofibroblast has long been believed to represent a terminally differentiated cell type (18). Therefore, several attempts have been made to target myofibroblast activation in the development of antifibrotic treatment strategies, including inhibiting proliferation, differentiation, or ECM generation. More recently, our group was the first to demonstrate that myofibroblasts are not a terminally differentiated cell type, as they retain the capacity for dedifferentiation and subsequent proliferation (19). This finding has since been confirmed by several groups (20–23). MyoD is a basic helix-loop-helix transcription factor that acts as a critical switch in the differentiation and dedifferentiation of myofibroblasts. Induction of MyoD is necessary for myofibroblast differentiation, whereas mitogenic stimuli promote downregulation of MyoD and dedifferentiation (19). Although various mitogenic stimuli have been shown to induce myofibroblast dedifferentiation (20–23), it is not known whether this capacity for dedifferentiation is altered in the context of aging.
Cellular senescence is defined as irreversible cell cycle arrest (even in the presence of mitogenic signals). Senescent cells are typically characterized by a large flattened morphology, a senescence-associated secretory phenotype, and the presence of several senescence-associated markers, including SA-β-gal (senescence-associated β-galactosidase), p16, and p21 (among others) (24). It is well established that senescent cells accumulate with advancing age (25), and several recent studies have demonstrated that senescent myofibroblasts accumulate in the lungs of patients with IPF (26–29). In the current study, we sought to evaluate the impact of senescence on myofibroblast dedifferentiation capacity and subsequent apoptotic fate. Our studies suggest that nonsenescent cells that undergo dedifferentiation are susceptible to apoptosis, whereas senescent/IPF lung myofibroblasts exhibit an impaired capacity for dedifferentiation and apoptosis resistance. Furthermore, we demonstrate that MyoD is persistently expressed in senescent/IPF myofibroblasts that fail to undergo dedifferentiation, and in the lungs of both aged mice with persistent fibrosis and human patients with IPF. Genetic targeting of MyoD restored IPF myofibroblast susceptibility to apoptosis in vitro and led to a partial reversal of age-dependent persistent fibrosis in vivo. The capacity for myofibroblast dedifferentiation and the subsequent apoptotic fate of these cells may be critical to physiologic versus pathologic responses to tissue injury. These studies support the concept that senescence may promote profibrotic effects via impaired myofibroblast dedifferentiation and resistance to apoptosis.
Methods
Cell Culture
Human lung fibroblasts (IMR-90) were purchased from Coriell Cell Repositories. Fibroblasts were isolated from the lungs of patients with IPF using collagenase digestion as previously described (30) under protocols approved by the institutional review boards of the University of Alabama at Birmingham (#N140903002) and the University of Arizona (#1200000347). IPF lung fibroblasts were also purchased from Lonza. Lung fibroblasts from age-matched healthy individuals (48–67 yr old) were purchased from ATCC and Lonza. Primary murine fibroblasts were isolated from the lungs of young (2 mo old) C57BL/6 mice as previously described (26). All cells were cultured in Dulbecco’s modified Eagle medium (Life Technologies, Inc.) supplemented with 10% FBS (Hyclone Laboratories), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1.25 μg/ml amphotericin B, at 37°C in 5% CO2, 95% air (26).
In Vitro Replicative Senescence Model
Human lung fibroblasts (IMR-90) were passaged in culture until they reached exhaustion of proliferative potential (data not shown). Cellular senescence was confirmed by microscopic evaluation, quantitative fluorescence assay for SA-β-gal activity, SA-β-gal staining, and expression of senescence markers (increased p16, p21, and decreased phosphorylated retinoblastoma protein) as compared with “nonsenescent” cells (young cells from the same population that were not subjected to repeated passaging in culture). These methods are consistent with a previously established protocol, which utilized lung fibroblasts as a model of replicative senescence (31).
Fibroblast-to-Myofibroblast Differentiation/Dedifferentiation
Cells were plated for 24 hours, followed by serum starvation for 16 hours. The cells were then treated with TGF-β (2 ng/ml) for 48 hours to induce myofibroblast differentiation. “Day 0” in the schematic diagrams (Figure 1G; see Figure 3A) represents differentiated myofibroblasts induced by TGF-β. Myofibroblasts were then treated with media supplemented with 20% FBS (a mitogenic stimulus that was previously shown to induce myofibroblast dedifferentiation) (19) or 0% FBS, which was replaced daily for 5 days. The cells were then evaluated for their capacity to dedifferentiate (Figure 1G) and/or for their susceptibility to apoptosis (see Figure 3A).
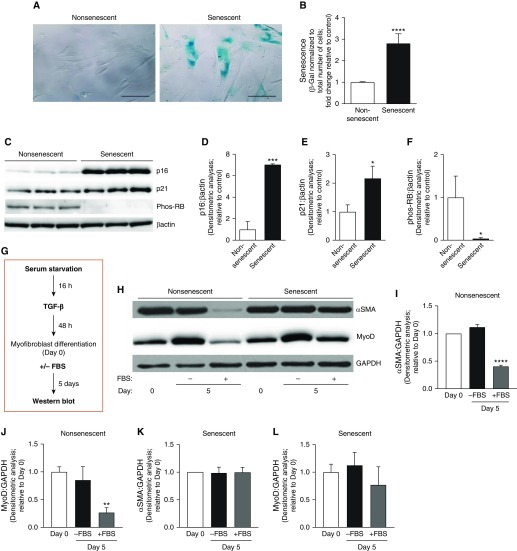
Figure 1.
Senescent myofibroblasts have a diminished capacity for dedifferentiation. Lung fibroblasts at low and high population doublings (nonsenescent and senescent, respectively) were used and cultured ex vivo. (A–F) Senescence was evaluated by qualitative (A) and quantitative (B) measurements of senescence-associated β-galactosidase (β-gal) activity, expression of the senescence markers by Western blotting (C), and densitometric analyses (D–F). Scale bars: 100 μm. *P < 0.05, ***P < 0.001, and ****P < 0.0001 as compared with nonsenescent, using Student’s two-tailed t-test. (G–L) Cells were serum starved for 16 hours and treated with TGF-β (transforming growth factor β; 2 ng/ml) for 48 hours. At 48 hours after TGF-β treatment (Day 0), the media was replaced with TGF-β–free media with 0% or 20% FBS and cells were incubated for 5 days. (G) Schematic diagram illustrating the treatment protocol. (H–L) Protein expression of α-SMA (α-smooth muscle actin), MyoD (myogenic differentiation), and GAPDH was assessed by Western blotting (H) and quantified by densitometric analyses (I–L). **P < 0.01 and ****P < 0.0001 as compared with Day 0, using Student’s two-tailed t-test. All values represent means ± SEM; n = 3–5 biological replicates, 3–5 independent experiments. Phos-RB = phosphorylated retinoblastoma protein.
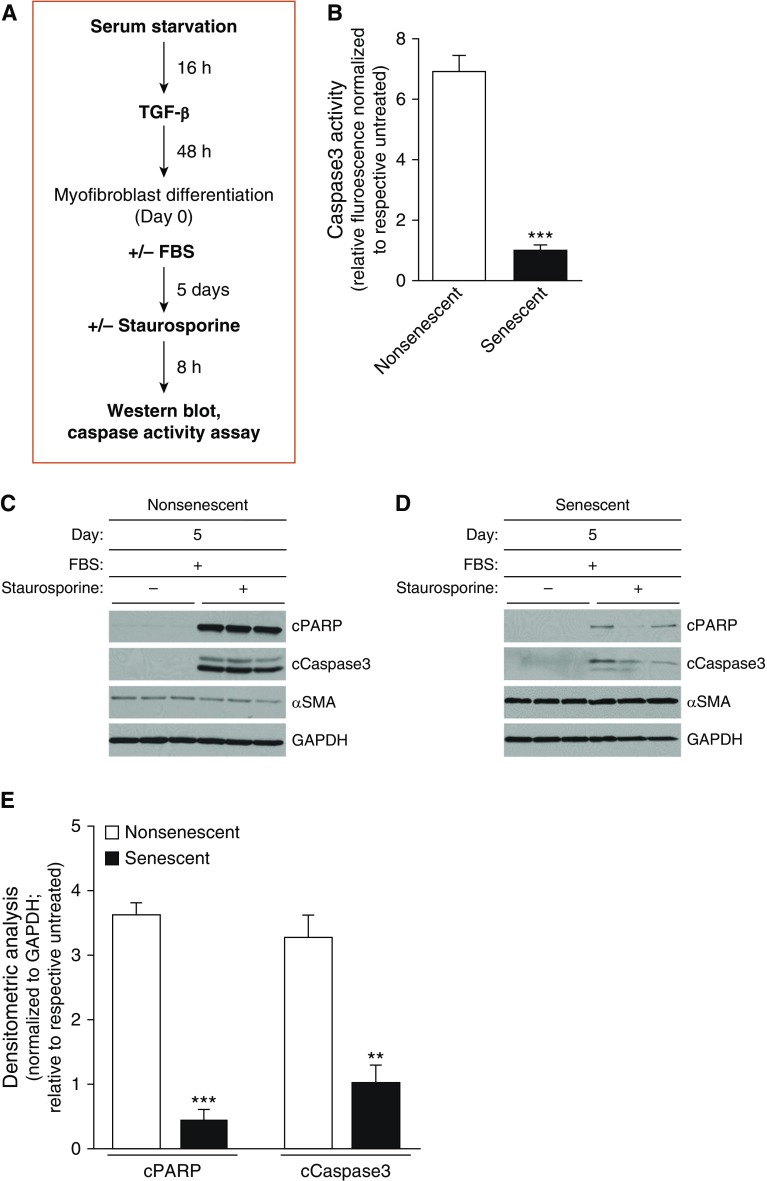
Figure 3.
Impaired capacity for dedifferentiation in senescent myofibroblasts is associated with apoptosis resistance. Nonsenescent and senescent lung fibroblasts were serum starved for 16 hours, treated with TGF-β (2 ng/ml) for 48 hours (Day 0), and then treated with 0% or 20% FBS for 5 days. Cells were treated with vehicle or staurosporine (300 nM), an apoptosis-inducing agent, for 8 hours. (A) Schematic diagram illustrating the treatment protocol and endpoints assessed. (B) Caspase 3 activity was assessed. (C–E) Expression of cPARP, cCaspase 3, α-SMA, and GAPDH was assessed by Western blotting (C and D) and by densitometric analyses (E). All values represent means ± SEM; n = 3 biological replicates from 2 independent experiments; **P < 0.01 and ***P < 0.001 as compared with nonsenescent, using Student’s two-tailed t-test. cPARP = cleaved poly-ADP-ribose polymerases.
Murine Model of Bleomycin-induced Lung Injury
Young (2 mo old) and aged (18 mo old) female C57BL/6 mice (The Jackson Laboratory or the National Institute on Aging) were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Bleomycin (1.25 U/kg; 50 μl) was administered intratracheally to induce lung fibrosis as previously described (26). The mice were killed by CO2 inhalation. All procedures were approved by the institutional animal care and use committees at the University of Alabama at Birmingham or the University of Arizona.
RNA Interference
For in vivo RNA interference studies, MyoD siRNA (CAG CAG ACG ACU UCU AUG) or nontargeting siRNA (UAA GGC UAU GAA GAG AUA C) (Dharmacon) was reconstituted in PBS and administered to the lungs of aged mice by intranasal delivery (50 μg in 30 μl). Treatments were administered every other day during Weeks 3–6 after injury for a total of 10 treatments, as previously described (26). For in vitro studies, MyoD siRNA was transfected into fibroblasts using Lipofectamine 2000 (Life Technologies) according to the manufacturer’s instructions.
Statistical Analysis
Graphs were generated and statistical analyses were performed with GraphPad Prism (GraphPad Software). Data are expressed as means ± SEM. Differences among groups were assessed with one-way ANOVA multiple comparisons with Tukey’s post test, and between pairs with Student’s two-tailed t test. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001).
Additional details regarding the materials and methods used for hydroxyproline assay, Western blot, Caspase activity assay, and lung histology are provided in the data supplement.
Results
Senescent and IPF Myofibroblasts Demonstrate an Impaired Capacity for Dedifferentiation
The myofibroblast has long been characterized as a terminally differentiated cell type, as fibroblast-to-myofibroblast differentiation and fibrosis were considered to be irreversible processes (18). Our group was the first to demonstrate that myofibroblasts retain the capacity to undergo dedifferentiation, which is accompanied by the dissolution of α-SMA (α-smooth muscle actin) stress fibers and restored proliferative capacity (19). We found that myofibroblast differentiation and dedifferentiation/proliferation were mediated through reciprocal signaling between TGF-β1/ALK5/MyoD and mitogen(s)/ERK-MAPK/CDKs, respectively (19). Since that initial discovery, several other reports have confirmed that fibroblast-to-myofibroblast differentiation can in fact be reversed (19–23, 32). However, the capacity for myofibroblast dedifferentiation has not been evaluated in the context of aging/senescence. To evaluate the impact of cellular senescence on myofibroblast dedifferentiation capacity in vitro, we used a cellular model of replicative senescence with human lung fibroblasts. Compared with “nonsenescent” (young) cells, “senescent” fibroblasts exhibited cellular hypertrophy, increased SA-β-gal activity, elevated expression of p16 and p21, and decreased phosphorylation of retinoblastoma protein (Figures 1A–1F). We previously demonstrated that MyoD is required for myofibroblast differentiation, which leads to the induction of α-SMA (a defining feature of the differentiated myofibroblast) (19). We therefore treated nonsenescent and senescent fibroblasts with TGF-β to induce myofibroblast differentiation. The cells were then subjected to a previously established dedifferentiation protocol (Figure 1G; schematic diagram illustrating the myofibroblast dedifferentiation protocol) (19), and their capacity for dedifferentiation was assessed. In response to mitogenic stimuli (high-FBS treatment), nonsenescent myofibroblasts underwent dedifferentiation, characterized by a marked downregulation of α-SMA (Figures 1H and 1I) and MyoD (Figures 1H and 1J) protein levels, whereas α-SMA and MyoD were persistently expressed in senescent myofibroblasts (Figures 1H, 1K, and 1L). These data demonstrate that the capacity for dedifferentiation is impaired in senescent myofibroblasts.
Previous reports by our group and others have demonstrated that IPF lung myofibroblasts exhibit a senescent phenotype (27, 29), and that senescent myofibroblasts accumulate in fibroblastic foci of IPF lungs (26–29). Consistent with these reports, we confirmed that IPF lung myofibroblasts exhibited increased levels of senescence markers, including SA-β-gal staining and activity, and increased expression of p16, as compared with “adult healthy” myofibroblasts (isolated from the lungs of age-matched healthy subjects) (Figures 2A–2D). IPF and adult healthy myofibroblasts were evaluated for their capacity to undergo dedifferentiation using the same treatment protocol described above (Figure 1G). In response to FBS treatment, adult healthy myofibroblasts demonstrated the ability to undergo dedifferentiation, as indicated by significantly decreased expression of α-SMA and MyoD (Figures 2E–2G). In contrast, the expression of α-SMA and MyoD remained persistently elevated in senescent IPF myofibroblasts (Figures 2H–2J), indicating that the capacity for dedifferentiation was impaired in these cells.
Figure 2.
Idiopathic pulmonary fibrosis (IPF) lung myofibroblasts demonstrate impaired dedifferentiation capacity. (A–D) Fibroblasts isolated from the lungs of healthy adults and patients with biopsy-proven IPF were evaluated for cellular senescence by qualitative (A) and quantitative (B) measurements of senescence-associated β-gal activity, expression of the senescence markers by Western blotting (C), and densitometric analyses (D). Scale bars: 100 μm. *P < 0.05 and ****P < 0.0001 as compared with adult healthy lungs, using Student’s two-tailed t-test. (E–J) Cells were serum starved for 16 hours, treated with TGF-β (2 ng/ml) for 48 hours (Day 0), and then treated with 0% or 20% FBS for 5 days. Expression of α-SMA, MyoD, and GAPDH was assessed by Western blotting in fibroblasts isolated from adult healthy lungs (E) and from IPF lungs (H). (F–J) α-SMA and MyoD were quantified by densitometric analyses for adult healthy fibroblasts (F and G) and for IPF fibroblasts (I and J). **P < 0.01 and ****P < 0.0001 as compared with Day 0, using Student’s two-tailed t-test. All values represent means ± SEM; n = 3 biological replicates from 3 independent experiments.
Impaired Dedifferentiation in Senescent and IPF Myofibroblasts Is Associated with Apoptosis Resistance
Studies in our laboratory and others support the acquisition of an apoptosis-resistant phenotype with myofibroblast senescence (26, 33, 34). We therefore sought to evaluate whether the capacity for myofibroblast dedifferentiation affects their ultimate apoptotic fate. Nonsenescent and senescent myofibroblasts were subjected to the same dedifferentiation protocol, and then susceptibility to apoptosis was evaluated in response to staurosporine, a well-known inducer of apoptosis (35) (Figure 3A). Nonsenescent cells, which had undergone dedifferentiation (associated with decreased levels of MyoD and α-SMA), were highly susceptible to apoptosis (Figures 3B, 3C, and 3E). In contrast, senescent cells, which failed to undergo dedifferentiation and exhibited persistently elevated MyoD and α-SMA levels, demonstrated resistance to apoptosis (Figures 3B, 3D, and 3E).
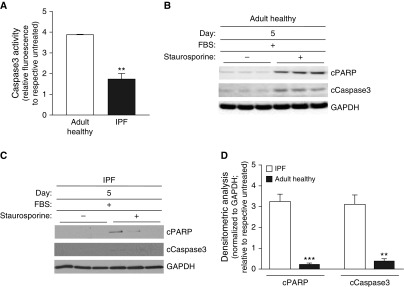
We next evaluated IPF and adult healthy lung myofibroblasts, using the same treatment protocol, to assess their susceptibility to apoptosis (Figure 3A). Similar to the above findings, adult healthy myofibroblasts, which retained the capacity to undergo dedifferentiation accompanied by reductions in MyoD and α-SMA, demonstrated a susceptibility to apoptosis (Figures 4A, 4B, and 4D), whereas senescent IPF myofibroblasts, which exhibited an impaired capacity for dedifferentiation associated with persistently elevated levels of MyoD and α-SMA, were resistant to apoptosis (Figures 4A, 4C, and 4D). Overall, these studies indicate that senescent myofibroblasts that fail to undergo dedifferentiation, associated with persistently elevated levels of MyoD/α-SMA, acquire an apoptosis-resistant phenotype.
Figure 4.
Impaired dedifferentiation in IPF fibroblasts is associated with apoptosis resistance. Adult healthy and IPF lung fibroblasts were serum starved for 16 hours, treated with TGF-β (2 ng/ml) for 48 hours (Day 0), and then treated with 0% or 20% FBS for 5 days. Cells were treated with vehicle or staurosporine (300 nM) for 8 hours, and Caspase 3 activity was assessed (A). (B–D) Expression of cPARP, cCaspase 3, and GAPDH was assessed by Western blotting (B and C) and densitometric analyses (D). Values represent means ± SEM; n = 3 biological replicates; **P < 0.01 and ***P < 0.001 using Student’s two-tailed t test.
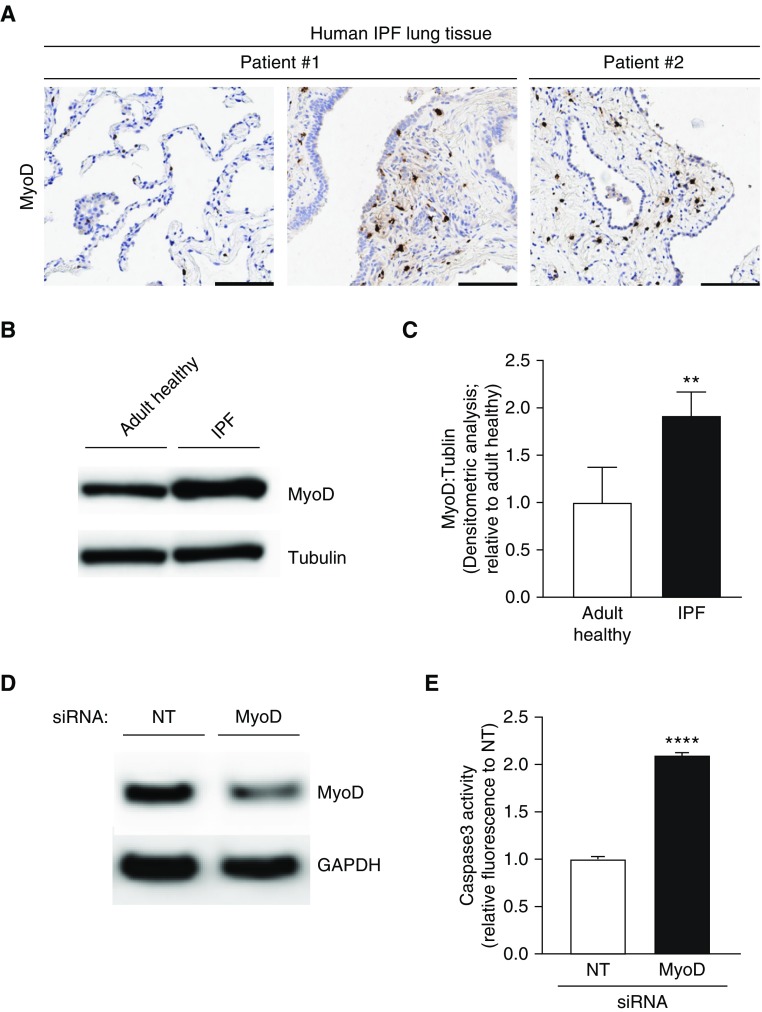
MyoD Is Persistently Elevated in IPF Lung Myofibroblasts, and Genetic Knockdown Restores Susceptibility to Apoptosis
To our knowledge, the relative expression of MyoD in the IPF lung has not been previously explored. Therefore, we evaluated the expression of MyoD in lung tissue from patients with IPF. We detected high levels of MyoD expression in fibrotic regions of two different patients with confirmed IPF (Figure 5A). However, MyoD expression was not observed in normal lung parenchymal (nonfibrotic) regions from the same patient (Figure 5A, left panel). To confirm that the elevated expression of MyoD in IPF lung tissue was specific to myofibroblast cells, we evaluated MyoD expression levels in myofibroblasts isolated from IPF lungs. Indeed, MyoD expression was significantly elevated in IPF myofibroblasts as compared with adult healthy myofibroblasts (Figures 5B and 5C). We next sought to determine whether persistently elevated MyoD expression contributes to the apoptosis-resistant phenotype of these cells. IPF myofibroblasts were transfected with MyoD siRNA or a nontargeting control siRNA, and knockdown was confirmed by Western blotting (Figure 5D). Transfected cells were then evaluated for their susceptibility to apoptosis. MyoD knockdown led to increased susceptibility to apoptosis in IPF myofibroblasts (Figure 5E). Taken together, these data indicate that persistently elevated levels of MyoD may contribute to the apoptosis-resistant pathologic phenotype of IPF lung myofibroblasts.
Figure 5.
MyoD is upregulated in IPF lung myofibroblasts, and genetic targeting restores apoptosis susceptibility. (A) IPF lung tissue sections were analyzed by immunohistochemistry for expression of MyoD. Scale bars: 100 μm. (B and C) Adult healthy and IPF lung fibroblasts were evaluated for protein expression of MyoD by Western blotting (B) and densitometric analyses (C). (D and E) IPF fibroblasts were transfected with MyoD siRNA. Downregulation of MyoD was confirmed by Western blotting (D) and caspase activity of transfected cells was assessed (E). Values represent means ± SEM; n = 3 biological replicates; **P < 0.01 and ****P < 0.0001 using Student’s two-tailed t test. NT = nontargeting siRNA.
MyoD Expression Is Elevated in Age-associated Persistent Fibrosis
MyoD is required for myofibroblast differentiation, and diminished MyoD expression is associated with myofibroblast dedifferentiation in vitro (19). However, the role of MyoD in the induction and maintenance of fibrogenic responses to lung injury in vivo has not been previously evaluated. We used the bleomycin model to induce lung fibrosis, which peaks 2–3 weeks after injury, followed by fibrosis resolution in young mice (26, 36). In contrast, aged mice exhibit an impaired capacity for fibrosis resolution, with persistent fibrosis up to 4 months after injury (26). Here, we evaluated the expression of MyoD in the context of resolving versus nonresolving lung fibrosis, using young (2 mo old) and aged (18 mo old) mice, respectively. After bleomycin-induced lung injury, both young and aged mice showed elevated expression of MyoD at 3 weeks after injury (Figure 6A, immunohistochemistry; Figure E1 in the data supplement, Western blotting); at this time point, the severity of fibrosis is similar in both young and aged mice (26). However, at 2 months after injury, when young mice are actively resolving fibrosis and aged mice demonstrate persistent fibrosis (26), expression of MyoD remained elevated only in the lungs of aged mice (Figures 6A, 6B, and E1). These results support the concept that downregulation of MyoD in young mice may promote dedifferentiation of myofibroblasts and ultimately resolution of fibrosis, whereas persistent MyoD expression in aged mice may impair dedifferentiation of senescent myofibroblasts, which contributes to persistent fibrosis.
Figure 6.
MyoD is upregulated in the lungs of aged mice with persistent fibrosis. Young (2 mo old) and aged (18 mo old) C57BL/6 mice were subjected to lung injury by airway instillation of intratracheal bleomycin (1.25 U/kg). Lung tissue was harvested at 0 (uninjured), 3 weeks, and 2 months after injury. (A) Immunohistochemistry analysis of MyoD. Scale bars: 100 μm. (B) Densitometric analyses of MyoD protein expression in whole-lung tissues at 2 months after injury. Values represent means ± SEM; n = 4–5 biological replicates; *P < 0.05 compared with young mice using Student’s two-tailed t-test.
Therapeutic Targeting of MyoD in Aged Mice with Persistent Fibrosis Restores the Capacity for Fibrosis Resolution
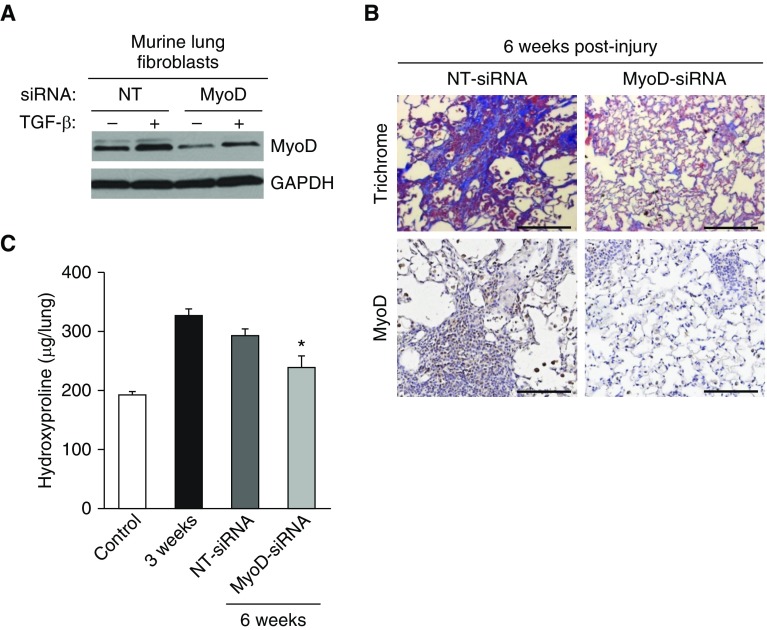
We next evaluated whether therapeutic targeting of MyoD in age-associated established fibrosis could restore the capacity for fibrosis resolution. To validate the knockdown efficiency of siRNA targeting MyoD in murine lung fibroblasts, cells were isolated from the lungs of young (2 mo old) mice and cultured ex vivo. Fibroblasts were transfected with MyoD siRNA or a nontargeting siRNA (control), and then treated with TGF-β to induce MyoD expression. MyoD knockdown was confirmed by Western blotting (Figure 7A). For in vivo targeting, MyoD siRNA or nontargeting control siRNA was administered to the lungs of aged (18 mo old) mice by intranasal delivery after bleomycin injury. Specifically, siRNA was administered during the period of established/persistent fibrosis (Weeks 3–6 after injury; siRNA was given every other day for a total of 10 treatments). Aged mice that received MyoD siRNA treatment showed markedly reduced MyoD expression in the lungs, as demonstrated by immunohistochemical staining for MyoD (Figure 7B, bottom panels). Importantly, MyoD knockdown led to a partial resolution of age-associated persistent fibrosis, as determined by Masson’s trichrome blue staining for collagen (Figure 7B, upper panels) and total lung hydroxyproline (Figure 7C). Overall, these studies indicate that MyoD plays a role in mediating fibrogenic responses to lung injury.
Figure 7.
Therapeutic targeting of MyoD in the lungs of aged mice with established fibrosis leads to fibrosis resolution. (A) Isolated lung fibroblasts from uninjured young mice were transfected with MyoD-targeting or -nontargeting siRNA. Transfected cells were serum starved overnight and treated with/without TGF-β (2 ng/ml) for 48 hours. MyoD was assessed by Western blotting. (B and C) Aged (18 mo old) C57BL/6 mice were subjected to lung injury by airway instillation of intratracheal bleomycin (1.25 U/kg). The mice were then treated with intranasal instillation of MyoD or vehicle siRNA every other day from Week 3 to Week 6. Lung tissue was harvested at 0 week (uninjured), 3 weeks, and 6 weeks after injury/treatment. Fibrosis was assessed by Masson’s trichrome blue staining for collagen (top panels) and MyoD was assessed by immunohistochemical staining for MyoD (bottom panels) (B), and whole-lung homogenates were analyzed by quantitative hydroxyproline assay (C). Scale bars: 100 μm. Data are expressed as total micrograms of hydroxyproline per whole lung. Differences among groups were assessed with one-way ANOVA multiple comparisons with Tukey’s post test. Values represent means ± SEM; n = 6–11 biological replicates; *P < 0.05 as compared with NT-siRNA using one-way ANOVA multiple comparisons with Tukey’s post test.
Discussion
In response to tissue injury, the local activation and proliferation of fibroblasts, which differentiate to myofibroblasts, are largely responsible for ECM deposition, a defining feature of fibrosis. Consequently, attempts to develop antifibrotic treatment strategies have largely focused on targeting fibrosis initiation events (e.g., fibroblast proliferation, fibroblast-to-myofibroblast differentiation, and ECM generation) (37, 38). In theory, inhibition of these processes would halt the progression of the disease. Potent inducers of myofibroblast differentiation, including TGF-β, endothelin, and connective tissue growth factor, have been targeted as potential therapies (39, 40), with limited success. The two U.S. Food and Drug Administration (FDA)-approved drugs for IPF, pirfenidone (Genentech) and nintedanib (Boehringer Ingelheim), mediate antifibrotic effects by targeting fibroblast activation. Pirfenidone downregulates the production of growth factors and procollagens I and II (41). Nintedanib is a tyrosine-kinase inhibitor that targets proliferative pathways, including vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor, and type II TGF-β receptor (42). However, despite their FDA approval, these therapies only moderately slow the progression of lung decline, and no available data indicate that these drugs promote reversal of established fibrosis. Importantly, these FDA-approved therapies are associated with a number of significant and intolerable side effects, and they do not improve survival or quality of life for patients with IPF (43–45). One potential explanation for the relative limited efficacy of current antifibrotic approaches is that for most patients, fibrosis is well established at the time of diagnosis. From this standpoint, it is not likely that targeting pathways/phenotypes involved in initiation of the fibrotic process would promote resolution and/or cure fibrotic disease. In contrast, the current study suggests that targeting phenotypes that promote fibrosis resolution (e.g., myofibroblast dedifferentiation and apoptotic clearance of senescent cells) may be more effective than strategies that block fibrosis development.
Cellular senescence is pleiotropic, as it may confer protective or detrimental effects in tissue repair. For example, in response to stress-induced oncogene activation, induction of cellular senescence serves as a critical barrier against tumor development (46), which prevents proliferation and ultimately the transmission of deleterious mutations (24). However, the functional contribution of senescence to noncancer human pathologies, such as fibrosis, is less understood. Several studies using murine models have demonstrated that senescence acts as an antifibrotic mechanism (26, 47–51). Previous work from our lab indicates that in response to lung injury, transient senescence is associated with the resolution of lung fibrosis (26). Furthermore, eliminating senescent cells in vivo has been shown to exacerbate fibrotic responses in murine models of lung (47), cardiac (48), kidney (49), liver (50), and skin (51) fibrosis. However, it is important to note that the animal studies that demonstrated antifibrotic effects of senescence (described above) (47–51) were all performed in young mice, when senescence appears to be a transient process. In contrast, we have demonstrated that aged mice exhibit persistent senescence and nonresolving fibrosis in response to lung injury, suggesting that senescence is a profibrotic mechanism in the context of aging (26). Thus, it is feasible that the fate of senescent cells during fibrotic responses to injury could be highly divergent depending on age. In support of this concept, a growing body of evidence suggests that senescence drives age-associated pathologies in late life (24). Numerous studies have demonstrated the accumulation of senescent cells in pathologic tissue regions of human patients with chronic fibrotic diseases, including IPF (26–29, 52), liver fibrosis (53, 54), kidney fibrosis (55), and cardiac fibrosis (56)—all of which predominantly afflict the elderly population. The current study sheds light on potential mechanisms by which myofibroblast senescence could exert profibrotic effects in the context of aging.
The precise mechanisms regulating the fate of senescent cells during injury-repair responses remain poorly understood. In young animals, senescent cells have been shown to identify themselves to the immune system, enabling their efficient clearance to promote fibrosis resolution (50, 57). In contrast, senescent lung myofibroblasts in aged mice acquire an apoptosis-resistant phenotype, which contributes to their accumulation in nonresolving lung fibrosis (26–28). Similarly, lung myofibroblasts from patients with IPF have been found to exhibit apoptosis resistance (26–29). The current study further validates that observation, as we also demonstrate that senescent/IPF myofibroblasts exhibit resistance to apoptosis. Impaired immune surveillance has been shown to accelerate the accumulation of senescent cells in aged lung tissues (58). Thus, it is likely that the accumulation of senescent cells in fibrotic disease is related (at least in part) to the established decline of immune-system function with age. Furthermore, in response to chronic damage, the production of senescent cells may outpace their clearance. Such a state, although initially beneficial, may eventually trigger an aberrant repair-response leading to disease pathogenesis. Our studies raise the possibility that the loss of plasticity (e.g., the inability to dedifferentiate) observed in senescent myofibroblasts is a key mechanism that may explain their apoptosis-resistant fate in aging. It is conceivable that a multitude of age-associated biological and immunological alterations may compound an aberrant repair-response, resulting in persistent/progressive fibrotic disease.
Our previous studies demonstrated a critical role for the myogenic transcription factor MyoD in the unexpected plasticity of myofibroblasts (19). The current study demonstrates that senescent/IPF myofibroblasts that fail to undergo dedifferentiation exhibit persistently elevated levels of MyoD and α-SMA. Because senescence is an irreversible and terminal cell phenotype, it is not surprising that these cells lose their ability to dedifferentiate into their proliferative progenitors. Our studies provide proof-of-concept that targeting MyoD can restore the apoptosis susceptibility of senescent IPF myofibroblasts in vitro, and can partially reverse age-dependent established fibrosis in vivo. However, the precise mechanisms responsible for the persistent upregulation of MyoD in senescent myofibroblasts, and how this contributes to apoptosis resistance, require further investigation. Given that IPF fibroblasts develop a senescence-associated secretory phenotype, which includes increased levels of secreted TGF-β (27–29), it is feasible that autocrine TGF-β signaling may contribute to the persistent upregulation of MyoD. The relationship between MyoD and α-SMA protein levels is not stoichiometric, which could be attributed to their relative protein stabilities (the protein half-life of α-SMA is ∼100 times greater than that of MyoD) (59, 60). This, along with other factors described above (including immune senescence), may explain the partial resolution of fibrosis (vs. complete reversal) observed after MyoD knockdown in aged mice. Overall, these studies support the concept that dedifferentiation and/or apoptosis of myofibroblasts are critical to facilitate fibrosis resolution. Furthermore, our studies implicate MyoD as a novel target that (at least in part) regulates these processes.
Although our findings indicate that IPF myofibroblasts exhibit a significantly impaired dedifferentiation capacity, a previous report demonstrated that IPF myofibroblasts retain the capacity for dedifferentiation (61). Several potential factors may account for the discrepancy in these observations, including patient age, accumulation of senescence cells, disease state, and/or genetic variation. Cells isolated from older patients and/or patients with advanced disease would likely exhibit a greater accumulation of senescent cells than those obtained from younger patients or patients with early-stage disease. In the current study, we confirmed that IPF fibroblasts (obtained from various sources) exhibited greater senescence than cells isolated from age-matched cohorts, whereas senescence of IPF fibroblasts was not evaluated in the previous study (61). These factors may also explain the variable rates of disease progression observed in individual patients with IPF. Furthermore, recent studies have also suggested that alveolar epithelial cell senescence contributes to IPF pathogenesis (27, 52). Additional studies are needed to fully understand how senescence of specific cell populations within IPF lungs contributes to disease progression.
Although aging is among the greatest risk factors for developing IPF, therapeutic strategies have yet to exploit the targeting of age-associated pathological mechanisms. These studies provide proof-of-concept that therapeutic strategies aimed at altering the apoptotic fate of senescent myofibroblasts may be a more tenable approach for patients with age-associated established fibrosis than targeting events involved in the development of the fibrotic process. Recent studies suggest that senolytics, an emerging class of drugs that selectively kill senescent cells, may be a promising therapeutic approach for IPF. One study demonstrated that the senolytic agent quercetin restored apoptosis susceptibility in IPF lung myofibroblasts, and inhibited fibrotic responses to lung injury in aged mice (62). Furthermore, the first-in-human pilot study using a combination of senolytic drugs (dasatinib and quercetin) in patients with IPF provided evidence that senolytics may alleviate physical dysfunction (63). The identification of novel senolytic agents that selectively target senescent myofibroblasts (without affecting other cell types) could be of paramount importance for developing improved therapies for age-dependent fibrotic diseases. Overall, shifting the focus of therapeutic strategies beyond cellular phenotypes involved in initiation of the fibrotic process holds great promise for the development of treatments that could promote the resolution of established fibrosis.
Supplementary Material
Footnotes
Supported by the Office of the Assistance Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under award no. W81XWH-17-1-0443 (L.H.); Veterans Administration Health System grant 1 I01 BX003919-01A1 (L.H.); National Institutes of Health grants 1R21AG054766-01 (L.H.), P01 HL114470 (V.J.T.), and R01 AG046210 (V.J.T.); and VA Merit Award I01BX003056 (V.J.T.).
Author Contributions: V.J.T. and L.H. conceived the project and provided funding. L.H. supervised all studies. K.K. and L.H. wrote the manuscript. K.K., N.J.L., Y.-J.S., S.P., and L.H. designed and conducted experiments, and/or analyzed results. D.K. contributed to animal studies. K.A. and K.S.K. assisted with institutional review board protocols. K.A. performed idiopathic pulmonary fibrosis cell/tissue isolation. All authors contributed intellectual input to the overall project, analyses of data, and figure and manuscript preparation.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0092OC on January 21, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hecker L, Thannickal VJ. Nonresolving fibrotic disorders: idiopathic pulmonary fibrosis as a paradigm of impaired tissue regeneration. Am J Med Sci. 2011;341:431–434. doi: 10.1097/MAJ.0b013e31821a9d66. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR. The age of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:771–772. doi: 10.1164/rccm.201001-0049ED. [DOI] [PubMed] [Google Scholar]
- 6.Hecker L. Mechanisms and consequences of oxidative stress in lung disease: therapeutic implications for an aging populace. Am J Physiol Lung Cell Mol Physiol. 2018;314:L642–L653. doi: 10.1152/ajplung.00275.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016;13:S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 9.Duan FF, Barron G, Meliton A, Mutlu GM, Dulin NO, Schuger L. P311 promotes lung fibrosis via stimulation of transforming growth factor-β1, -β2, and -β3 translation. Am J Respir Cell Mol Biol. 2019;60:221–231. doi: 10.1165/rcmb.2018-0028OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 11.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmoulière A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 13.Bai L, Bernard K, Tang X, Hu M, Horowitz JC, Thannickal VJ, et al. Glutaminolysis epigenetically regulates antiapoptotic gene expression in idiopathic pulmonary fibrosis fibroblasts. Am J Respir Cell Mol Biol. 2019;60:49–57. doi: 10.1165/rcmb.2018-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scruggs AM, Koh HB, Tripathi P, Leeper NJ, White ES, Huang SK. Loss of CDKN2B promotes fibrosis via increased fibroblast differentiation rather than proliferation. Am J Respir Cell Mol Biol. 2018;59:200–214. doi: 10.1165/rcmb.2017-0298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munguía-Reyes A, Balderas-Martínez YI, Becerril C, Checa M, Ramírez R, Ortiz B, et al. R-Spondin-2 is upregulated in idiopathic pulmonary fibrosis and affects fibroblast behavior. Am J Respir Cell Mol Biol. 2018;59:65–76. doi: 10.1165/rcmb.2017-0115OC. [DOI] [PubMed] [Google Scholar]
- 16.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 17.Misharin AV, Budinger GRS. Targeting the myofibroblast in pulmonary fibrosis. Am J Respir Crit Care Med. 2018;198:834–835. doi: 10.1164/rccm.201806-1037ED. [DOI] [PubMed] [Google Scholar]
- 18.Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation—the role of Smad proteins. Exp Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- 19.Hecker L, Jagirdar R, Jin T, Thannickal VJ. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–1921. doi: 10.1016/j.yexcr.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 21.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261–273.e3. doi: 10.1016/j.stem.2016.10.004. [Published erratum appears in Cell Stem Cell 20:571.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, et al. Reversal of myofibroblast differentiation by prostaglandin E(2) Am J Respir Cell Mol Biol. 2013;48:550–558. doi: 10.1165/rcmb.2012-0262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driesen RB, Nagaraju CK, Abi-Char J, Coenen T, Lijnen PJ, Fagard RH, et al. Reversible and irreversible differentiation of cardiac fibroblasts. Cardiovasc Res. 2014;101:411–422. doi: 10.1093/cvr/cvt338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 25.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters DW, Blokland KEC, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, et al. Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2018;315:L162–L172. doi: 10.1152/ajplung.00037.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Álvarez D, Cárdenes N, Sellarés J, Bueno M, Corey C, Hanumanthu VS, et al. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1164–L1173. doi: 10.1152/ajplung.00220.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beauséjour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- 33.Sanders YYLH, Liu H, Zhang X, Hecker L, Bernard K, Desai L, et al. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol. 2013;1:8–16. doi: 10.1016/j.redox.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Khalaf HH, Aboussekhra A. Survivin expression increases during aging and enhances the resistance of aged human fibroblasts to genotoxic stress. Age (Dordr) 2013;35:549–562. doi: 10.1007/s11357-011-9378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayorga M, Bahi N, Ballester M, Comella JX, Sanchis D. Bcl-2 is a key factor for cardiac fibroblast resistance to programmed cell death. J Biol Chem. 2004;279:34882–34889. doi: 10.1074/jbc.M404616200. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins RG, Moore BB, Chambers RC, Eickelberg O, Königshoff M, Kolb M, et al. ATS Assembly on Respiratory Cell and Molecular Biology. An official American Thoracic Society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017;56:667–679. doi: 10.1165/rcmb.2017-0096ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Chen B, Liu T, Chen X. Reversal of myofibroblast differentiation: a review. Eur J Pharmacol. 2014;734:83–90. doi: 10.1016/j.ejphar.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulasekaran P, Scavone CA, Rogers DS, Arenberg DA, Thannickal VJ, Horowitz JC. Endothelin-1 and transforming growth factor-beta1 independently induce fibroblast resistance to apoptosis via AKT activation. Am J Respir Cell Mol Biol. 2009;41:484–493. doi: 10.1165/rcmb.2008-0447OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 41.Rangarajan S, Locy ML, Luckhardt TR, Thannickal VJ. Targeted therapy for idiopathic pulmonary fibrosis: where to now? Drugs. 2016;76:291–300. doi: 10.1007/s40265-015-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangarajan S, Kurundkar A, Kurundkar D, Bernard K, Sanders YY, Ding Q, et al. Novel mechanisms for the antifibrotic action of nintedanib. Am J Respir Cell Mol Biol. 2016;54:51–59. doi: 10.1165/rcmb.2014-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karimi-Shah BA, Chowdhury BA. Forced vital capacity in idiopathic pulmonary fibrosis: FDA review of pirfenidone and nintedanib. N Engl J Med. 2015;372:1189–1191. doi: 10.1056/NEJMp1500526. [DOI] [PubMed] [Google Scholar]
- 44.Raghu G, Selman M. Nintedanib and pirfenidone: new antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191:252–254. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, et al. INBUILD Trial Investigators. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 46.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. doi: 10.1038/nature10599. [DOI] [PubMed] [Google Scholar]
- 47.Cui H, Ge J, Xie N, Banerjee S, Zhou Y, Antony VB, et al. miR-34a inhibits lung fibrosis by inducing lung fibroblast senescence. Am J Respir Cell Mol Biol. 2017;56:168–178. doi: 10.1165/rcmb.2016-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu F, Li Y, Zhang J, Piao C, Liu T, Li HH, et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8:e74535. doi: 10.1371/journal.pone.0074535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2010;299:F1486–F1495. doi: 10.1152/ajprenal.00378.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 54.Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 55.Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295:F1563–F1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 56.Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol. 2016;67:2018–2028. doi: 10.1016/j.jacc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 57.Wallach-Dayan SB, Elkayam L, Golan-Gerstl R, Konikov J, Zisman P, Dayan MR, et al. Cutting edge: FasL(+) immune cells promote resolution of fibrosis. J Autoimmun. 2015;59:67–76. doi: 10.1016/j.jaut.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 58.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barja F, Coughlin C, Belin D, Gabbiani G. Actin isoform synthesis and mRNA levels in quiescent and proliferating rat aortic smooth muscle cells in vivo and in vitro. Lab Invest. 1986;55:226–233. [PubMed] [Google Scholar]
- 60.Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- 61.Reddy AT, Lakshmi SP, Zhang Y, Reddy RC. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J. 2014;28:5299–5310. doi: 10.1096/fj.14-256263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hohmann MS, Habiel DM, Coelho AL, Verri WA, Jr, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60:28–40. doi: 10.1165/rcmb.2017-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.