Abstract
Cystic fibrosis (CF) is a lethal genetic disease characterized by progressive lung damage and airway obstruction. The majority of patients demonstrate airway hyperresponsiveness (AHR), which is associated with more rapid lung function decline. Recent studies in the neonatal CF pig demonstrated airway smooth muscle (ASM) dysfunction. These findings, combined with observed CF transmembrane conductance regulator (CFTR) expression in ASM, suggest that a fundamental defect in ASM function contributes to lung function decline in CF. One established driver of AHR and ASM dysfunction is transforming growth factor (TGF) β1, a genetic modifier of CF lung disease. Prior studies demonstrated that TGFβ exposure in CF mice drives features of CF lung disease, including goblet cell hyperplasia and abnormal lung mechanics. CF mice displayed aberrant responses to pulmonary TGFβ, with elevated PI3K signaling and greater increases in lung resistance compared with controls. Here, we show that TGFβ drives abnormalities in CF ASM structure and function through PI3K signaling that is enhanced in CFTR-deficient lungs. CF and non-CF mice were exposed intratracheally to an adenoviral vector containing the TGFβ1 cDNA, empty vector, or PBS only. We assessed methacholine-induced AHR, bronchodilator response, and ASM area in control and CF mice. Notably, CF mice demonstrated enhanced AHR and bronchodilator response with greater ASM area increases compared with non-CF mice. Furthermore, therapeutic inhibition of PI3K signaling mitigated the TGFβ-induced AHR and goblet cell hyperplasia in CF mice. These results highlight a latent AHR phenotype in CFTR deficiency that is enhanced through TGFβ-induced PI3K signaling.
Keywords: cystic fibrosis, CFTR, transforming growth factor β, airway smooth muscle, airway hyperresponsiveness
Clinical Relevance
Airway hyperresponsiveness (AHR) is found in the majority of people with cystic fibrosis (CF) and is associated with worse outcomes, but there is little understanding of the mechanism driving this abnormality. This study demonstrates that transforming growth factor β, a well-known modulator of CF disease, causes AHR in a mouse model of CF. This may allow for development of therapeutics targeting AHR and improved symptom management and lung function for people with CF.
Cystic fibrosis (CF) is a lethal genetic disorder characterized by progressive lung damage secondary to altered ion transport, mucus obstruction, infection, and inflammation (1). In addition to defects typically associated with epithelial CF transmembrane conductance regulator (CFTR) dysfunction, evidence is building regarding a fundamental defect in airway smooth muscle (ASM) structure and function in CF. Airway hyperresponsiveness (AHR) is found in over half of patients with CF and is associated with more rapid pulmonary function decline (2). Children with CF have increased ASM quantified on endobronchial biopsies (3). Although increased ASM burden and AHR are also found in individuals with asthma, the diagnosis of asthma in a given patient with CF is difficult, and the best therapies to address AHR in CF are unclear (4). Bronchospasm related to AHR may also lead to intolerance of efficacious CF therapies, such as hypertonic saline and lumacaftor/ivacaftor (5, 6).
For years, it has remained unclear if the alterations in ASM found in CF are primary defects or secondary to the cumulative impact of recurrent pulmonary inflammation and infection. However, recent studies in the CF pig have identified abnormalities in ASM tone and contractility before the onset of inflammation/infection (7, 8). Cook and colleagues (7) reported in 2016 that uninflamed, uninfected newborn CF pigs have increased baseline smooth muscle tone, increased airflow obstruction, and delayed reuptake of calcium in the ASM sarcoplasmic reticulum (SR) after cholinergic stimulation. CFTR was identified in the SR of wild-type pigs, leading the authors to hypothesize that the loss of CFTR-dependent chloride transport in the SR delays calcium reuptake due to disturbed electrochemical gradients within the myocytes. Further analysis of the transcriptome of isolated CF pig ASM identified abnormal increases in mitogen-activated protein kinase (MAPK) and PI3K signaling that were associated with CFTR deficiency (8).
Taken together, these findings in patients with CF and animal models implicate alterations in ASM function as a clinically relevant source of variability in the CF lung phenotype. CF lung disease severity cannot be predicted simply by CFTR genotype; over 50% of this variability is attributable to non-CFTR genetic influences (9). These observations have led to a search for genetic modifiers of CF lung disease, identifying transforming growth factor (TGF) β1 as a potent disease modifier in genome-wide association studies (10, 11). TGFβ is a cytokine that drives diverse and context-dependent cellular activities in the lung, including remodeling, inflammation, and fibrosis; it has been proposed as a master regulator of lung health and disease (12). Higher-producing TGFβ polymorphisms (at the −509 promoter region and in codon 10) predispose patients with CF to more severe lung disease, and higher levels of TGFβ1 in the BAL fluid (BALF) are linked to worse outcomes (10, 13, 14). Downstream of TGFβ, two general cellular pathways are activated: canonical Smad signaling pathways and noncanonical pathways, including the MAPK and PI3K signaling cascades. These pathways have been implicated in driving CF-relevant lung pathology, including goblet cell hyperplasia, pulmonary fibrosis, aberrant inflammation, and CFTR downregulation (12, 15).
Despite these findings, we lack a clear mechanistic understanding of how TGFβ modifies CF lung disease (16). One possible mechanism is direct downregulation of CFTR in CF-affected epithelia. Several groups have demonstrated that exposure to TGFβ downregulates expression and function of CFTR in airway epithelial cells (15, 17, 18). The relevance of this is unclear however, as CFTR function is already very low/absent in patients with CF at baseline (before CFTR modulation). TGFβ also reverses F508 del CFTR rescue with the CFTR corrector VX-809 (15). In addition to these direct effects on CFTR, another potential mechanism through which TGFβ may impact CF lung disease is through exacerbation of ASM abnormalities.
We hypothesized that chronic, low-level TGFβ exposure would drive increased AHR and altered ASM function in CF. Traditional transgenic mouse models of TGFβ overexpression do not accurately replicate what happens in the lungs of patients with CF, where TGFβ exposure is low and protracted. Conditional overexpression in the airway epithelium of adult mice causes a devastating, obliterative lung disease characterized by overwhelming fibrosis (19). To achieve physiologically relevant pulmonary TGFβ1 exposure in mice, we use a nonreplicating adenoviral vector carrying a constitutively active TGFβ1 transgene, adenoviral (Ad)-TGFβ (20). We have previously published a study of pulmonary Ad-TGFβ exposure in CF and non-CF mice, focusing on the effects on pulmonary remodeling and function (21). CF mice exposed to Ad-TGFβ display more pronounced increases in PI3K signaling and worse lung mechanics abnormalities compared with Ad-TGFβ–exposed non-CF mice (21). This novel model therefore allowed us to investigate the impact of isolated exposure to physiologic levels of TGFβ in the CF lung in vivo. Our results elucidate fundamental abnormalities in CF pulmonary responses, including altered ASM structure and function, triggered by TGFβ exposure. Some of the results of these studies have been previously reported in the form of an abstract (22).
Methods
Institutional Approval
This study was approved by the Institutional Animal Care and Use Committee at Cincinnati Children’s Hospital Research Foundation.
Adenoviral Vector
Ad-TGFβ is a previously described, nonreplicating adenoviral vector containing a TGFβ1 transgene (20). Empty vector (Ad-dl70-3) was used to control for vector effects (23). At our dose of 5 × 107 plaque-forming unit (pfu), Empty vector does not produce appreciable lung pathology and Ad-TGFβ produces a nonfibrotic lung disease at Day 7 (20, 21).
Mouse Studies
F508 del CFTR homozygous C57BL/6J mice with gut correction (CFTRtm1kth, “CF mice”) were obtained from Case Western Reserve University (24). Heterozygous littermates (“non-CF mice”) were controls. Adult male and female mice were anesthetized with ketamine-xylazine and intratracheally administered Ad-TGFβ (5 × 107 pfu), empty vector (5 × 107 pfu), or vehicle (PBS) on Day 0. Mice were killed at Day 7. A subgroup was intraperitoneally treated with pan-PI3K inhibitor, LY294002 (25 mg/kg; Calbiochem) or vehicle (DMSO) at Days −1 and 2 and killed at Day 1 or Day 3.
Lung Mechanics
Mice were anesthetized with ketamine-xylazine solution. After cannulation, lung mechanics were measured on a flexiVent system (SCIREQ). To determine AHR, PBS or the bronchoconstrictor methacholine (acetyl-β-methylcholine chloride; Sigma) was administered via nebulizer (Aeroneb Lab Nebulizer, Aerogen). Resistance was calculated from 12 forced oscillation perturbations (single frequency) obtained approximately every 12 seconds. Albuterol sulfate (5 mg/ml; Akorn) was nebulized in a subset of animals before methacholine, as previously described (25).
BAL
BALF was collected via tracheal cannula by flushing of 1 ml sterile PBS. Total cell count was determined. A total of 200 cells per mouse was counted to determine differential cell counts after Kwik-Diff Stain (Thermofisher Scientific).
Lung Histology
Lungs were inflated with 10% formalin, 25 cm H2O, and embedded in paraffin blocks. Staining for ACTA2 (α-smooth muscle actin [αSMA], A2547; Sigma) was performed to stain ASM. All transversely cut airways with diameters of 100–400 μM were analyzed. Morphometric analysis of αSMA staining was performed as previously described (26) using MetaMorph software (Molecular Devices). Goblet cells were identified by periodic acid–Schiff (PAS) stain (Poly Scientific R&D). Airway goblet cell percentage was calculated as described previously (21).
Western Blot Analysis
Whole lungs and BAL cell pellets were homogenized and prepared for Western blot analysis. Primary antibodies used were: phosphorylated-S6 (CS4858; Cell Signaling), S6 (CS2217; Cell Signaling), phosphorylated extracellular signal-regulated protein kinases (ERKs) 1/2 (CS4370; Cell Signaling), ERK1/2 (CS9102; Cell Signaling), phosphorylated Smad2 (Ab3849; Millipore), and Smad2 (CS5339; Cell Signaling).
TGFβ ELISA
A TGFβ ELISA (R&D Systems) was used to quantify TGFβ levels in mouse BALF. Total TGFβ levels were measured after acidification to activate TGFβ; active TGFβ levels were measured without acidification.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (GraphPad). Student’s two-tailed t test was used for two-group comparison, whereas one-way or two-way ANOVA where appropriate, were used to compare three or more groups. Data are expressed as mean (±SD).
Results
TGFβ Induces AHR in CF, but Not Non-CF Mice
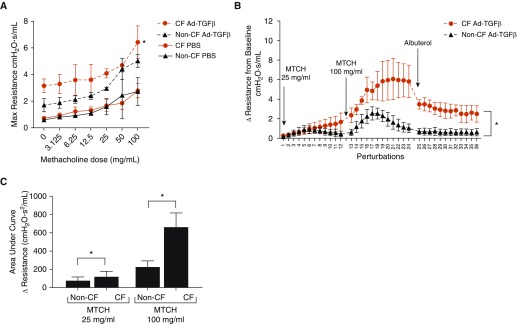
Previously, we have shown that Ad-TGFβ administered intratracheally at 5 × 107 pfu produces a nonfibrotic lung disease characterized by goblet cell hyperplasia, reduced CFTR expression, and heightened resistance and elastance (21). Despite the lack of intrinsic lung disease in CF mice, this physiologically relevant, subacute TGFβ exposure caused greater increases in resistance as well as PI3K signaling in CF mice compared with non-CF littermates (21). Notably, empty vector adenoviral administration did not induce discernable lung disease at this dosage. To determine if pulmonary Ad-TGFβ exposure produced alterations in AHR, CF and non-CF mice were treated with PBS, empty vector, or Ad-TGFβ and exposed to increasing doses of the bronchoconstrictor, methacholine (Figure 1A). Consistent with previous results, Ad-TGFβ induced greater baseline increases in maximal lung resistances in CF mice. This heightened resistance was seen at all doses of methacholine in CF mice, where non-CF mice treated with TGFβ did not have significantly different methacholine responses compared with PBS-treated non-CF control mice. Empty vector treatment did not induce altered methacholine responses in either CF or non-CF mice (see Figure E1A in the data supplement).
Figure 1.
(A) Adenoviral (Ad)-transforming growth factor (TGF) β–exposed cystic fibrosis (CF) mice have greater increases in lung resistance at methacholine doses up to 100 mg/ml compared with PBS-exposed CF mice. *P < 0.05 versus CF mice treated with PBS by two-way ANOVA with Tukey’s post hoc test. (B) Ad-TGFβ–exposed CF mice demonstrate a delayed methacholine (MTCH)-induced peak change in resistance and delayed resolution of resistance alterations compared with non-CF mice. Resistance was measured during each of 12 single-frequency forced oscillation technique perturbations, taken approximately every 12 seconds after each nebulized treatment. *P < 0.05 by two-way ANOVA. (C) The areas under the curve of the resistance changes induced by 25 and 100 mg/ml of methacholine are higher in CF than non-CF TGFβ-exposed mice. *P < 0.05 by two-tailed t test. Data are presented as mean ± SD.
Altered Bronchoconstriction Dynamics in TGFβ-treated CF Mice
Previous studies in the CF pig implicated delayed Ca2+ reuptake, and thus delayed relaxation, as a fundamental defect in CF ASM (7). To investigate the kinetics of AHR in our model, the time course of acute lung resistance changes after 25 and 100 mg/ml methacholine were determined in CF and non-CF mice. TGFβ-treated CF mice demonstrated delayed peak methacholine response and delayed resolution of methacholine-induced heightened resistance (Figure 1B). The area under the curve of the methacholine-induced change in resistance from baseline was significantly higher at both 25 and 100 mg/ml methacholine in CF mice compared with non-CF mice (Figure 1C).
β-Agonist Treatment Does Not Alter Baseline Resistance, but Reverses AHR in TGFβ-treated CF Mice
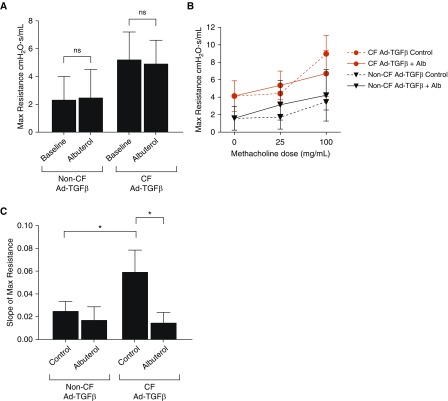
To determine if enhanced ASM contraction was responsible for the heightened baseline resistance in CF mice, Ad-TGFβ–transduced CF and non-CF mice were treated with nebulized albuterol and lung resistance was measured. This treatment did not significantly alter resistance in either group, indicating that increases in baseline resistance secondary to Ad-TGFβ treatment were not primarily due to reversible increases in ASM tone (Figure 2A).
Figure 2.
(A) Albuterol treatment does not reduce the heightened TGFβ-dependent baseline resistance in either CF or non-CF mice (ns = not significant by two-tailed t test, P > 0.05). (B) Nebulized albuterol (Alb) treatment immediately before methacholine challenge blunted the methacholine-induced resistance increases in Ad-TGFβ–exposed CF mice. Control mice were treated with PBS before methacholine challenge. (C) Albuterol pretreatment significantly reduced the slope of methacholine-induced resistance in CF mice, but not in non-CF mice. *P < 0.05 by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean ± SD.
In contrast to the failure of albuterol to reverse the increase in baseline resistance produced by Ad-TGFβ in both CF and non-CF mice, albuterol pretreatment reduced the TGFβ-dependent AHR phenotype specifically in CF mice. Pretreatment with albuterol before each methacholine dose blunted the resistance increases in CF mice (Figure 2B), with a concomitant reduction in the methacholine response slope in CF mice only (Figure 2C).
ASM Area Is Increased in TGFβ-exposed CF Mice
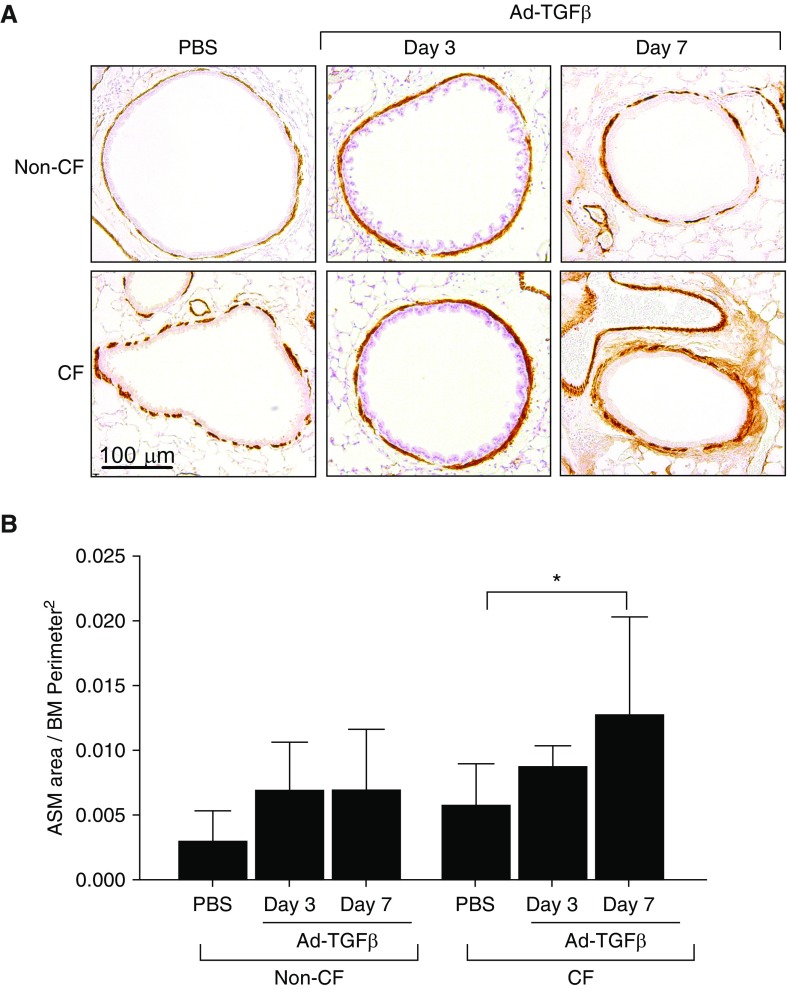
To determine the ASM burden and time course of ASM remodeling, immunohistochemistry for αSMA was performed at Days 3 and 7 after Ad-TGFβ administration (Figure 3A). Quantification of αSMA staining around airways was performed and corrected to basement membrane perimeter squared. CF mice examined at 7 days after Ad-TGFβ treatment demonstrated a significant increase in ASM area (Figure 3B). Non-CF mice trended toward increased ASM area at Day 7, but this did not reach significance (P = 0.296). At Day 3, both groups demonstrated an intermediate phenotype with a slight, nonsignificant increase in ASM area. Empty vector–treated controls did not demonstrate alterations in ASM burden (Figure E1B).
Figure 3.
(A) Immunohistochemistry for ACTA2-smooth muscle actin (αSMA) demonstrated airway smooth muscle (ASM) (brown) around airways in PBS control and Ad-TGFβ–treated CF and non-CF mice. Scale bar: 100 μm. (B) Quantification of αSMA area, corrected to basement membrane (BM) perimeter squared, demonstrated that TGFβ exposure significantly increased ASM area at Day 7 in CF mice only. *P < 0.05 by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean ± SD.
PI3K Inhibition Decreases AHR and Goblet Cell Hyperplasia in CF Mice
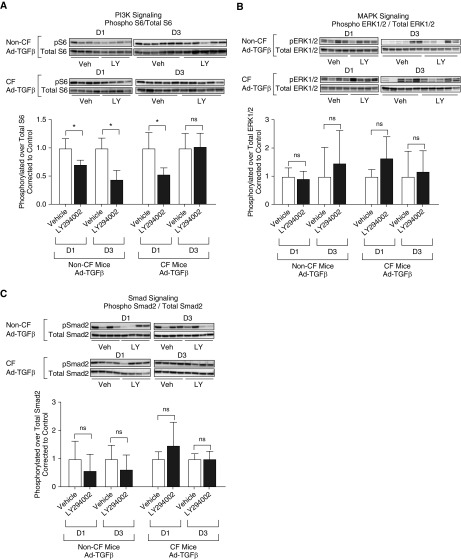
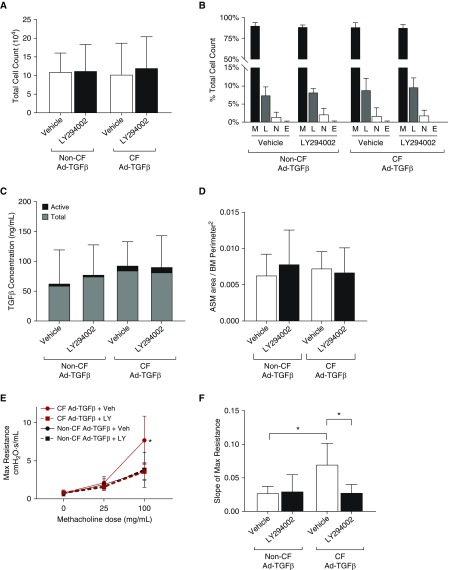
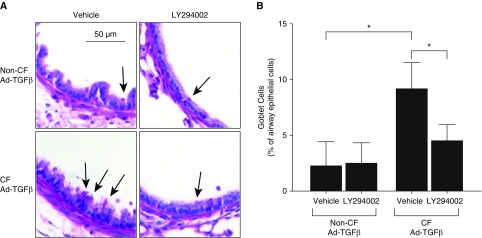
The studies above demonstrated minimal ASM remodeling at Day 3 after Ad-TGFβ transduction, providing a logical time point to further investigate the mechanism driving AHR (vs. ASM increase) in CF mice produced by TGFβ. Based on our previous findings identifying heightened PI3K signaling in CF mice after Ad-TGFβ transduction (21) and heightened PI3K signaling as a potential driver of ASM abnormalities in CF pigs (8), CF and non-CF mice transduced with intratracheal Ad-TGFβ were pretreated with intraperitoneal LY294002, a pan-PI3K inhibitor, or vehicle. LY294002 pretreatment reduced PI3K signaling compared with vehicle in both CF and non-CF mice 1 day after Ad-TGFβ exposure (Figure 4A). Interestingly, PI3K signaling inhibition was overcome in CF mice 3 days after Ad-TGFβ exposure, whereas inhibition continued in non-CF mice (Figure 4A). Neither MAPK (ERK1/2) nor canonical Smad signaling was measurably impacted by LY294002 treatment (Figures 4B and 4C). In the predominantly inflammatory cells recovered from BAL, PI3K signaling was not altered by LY294002 pretreatment (Figure E2). Similar to previously published findings at Day 7 after Ad-TGFβ treatment (21), CF and non-CF mice at Day 3 after Ad-TGFβ treatment demonstrated similar total cell counts and cell type distribution in BALF, and this was not altered by LY294002 treatment (Figures 5A and 5B). Active and latent TGFβ levels in the BAL were also similar in CF and non-CF controls and unchanged by vehicle or LY294002 treatment (Figure 5C). ASM area was likewise unaffected by PI3K inhibition in TGFβ-exposed CF and non-CF mice (Figure 5D). Despite similar levels of pulmonary inflammation, pulmonary TGFβ concentration, and ASM burden, PI3K inhibition significantly reduced methacholine-induced AHR in CF mice, but not in non-CF controls (Figures 5E and 5F). The percent of goblet cells per airway was also significantly reduced by LY294002 treatment in CF mice only (Figures 6A and 6B).
Figure 4.
After treatment with the pan-PI3K inhibitor LY294002 (LY), PI3K signaling, but not mitogen-activated protein kinase (MAPK) or canonical Smad signaling, is inhibited in the lungs of mice treated with intratracheal Ad-TGFβ. Western blot analysis was performed on whole-lung homogenates from mice treated with intraperitoneal LY before Ad-TGFβ exposure. (A) PI3K signaling, as measured by phosphorylation of S6, is inhibited in both CF and non-CF mouse lungs at Day 1 after Ad-TGFβ treatment as compared with vehicle (veh)-treated mice. At 3 days after TGFβ exposure, CF mice, but not non-CF mice, have overcome PI3K inhibition. (B and C) Neither Ad-TGFβ–induced MAPK (as measured by phosphorylated extracellular signal-regulated protein kinase [ERK] 1/2) nor canonical Smad (as measured by phosphorylated Smad2) signaling is affected by LY treatment as compared with vehicle treatment. Values are corrected to vehicle-treated mice of the same genotype and time point. *P < 0.05 by two-tailed t test. ns indicates P > 0.05 by two-tailed t test. Data are presented as mean ± SD.
Figure 5.
(A and B) Treatment with a PI3K inhibitor, LY, did not reduce total cell count (A) or alter differential cell count (B) in the BAL of Ad-TGFβ–treated CF or non-CF mice. E = eosinophils; L = lymphocytes; M = macrophages; N = neutrophils. (C) Similarly, LY treatment did not alter TGFβ levels, either total or active, in BAL of treated mice. (D) PI3K inhibition with LY did not reduce ASM area in TGFβ-treated CF or non-CF mice. (E) Despite similar levels of pulmonary inflammation, TGFβ, and ASM burden, LY treatment significantly reduced airway hyperresponsiveness (AHR) in Ad-TGFβ–exposed CF mice, but not Ad-TGFβ–exposed non-CF mice. *P < 0.05 versus CF LY-treated mice by two-way ANOVA with Sidak’s post hoc analysis. (F) Slope of max resistances was significantly decreased by LY in CF mice, indicating reduced AHR after PI3K inhibition in the setting of CF transmembrane conductance regulator deficiency only. *P < 0.05 by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean ± SD.
Figure 6.
PI3K inhibition with LY decreased goblet cell hyperplasia in Ad-TGFβ–exposed CF, but not non-CF, mice. (A) Periodic acid–Schiff–stained, mucin-containing goblet cells (arrows) were more numerous in Ad-TGFβ–exposed CF mice treated with vehicle. Scale bar: 50 μm. (B) The percentage of goblet cells around airways was significantly increased in vehicle-treated CF mice after Ad-TGFβ exposure, but this goblet cell hyperplasia was decreased by LY treatment. Percent goblet cells was not changed by PI3K inhibition in Ad-TGFβ–exposed non-CF mice. *P < 0.05 by one-way ANOVA with Tukey’s post hoc analysis. Data are presented as mean ± SD.
Discussion
Several published reports have linked increased TGFβ with abnormalities in ASM structure and function. TGFβ drives ASM contraction, proliferation, and hyperresponsiveness in vitro and in mouse models (27, 28), and inducible TGFβ overexpression in the mouse lung leads to ASM enlargement (29). Mice deficient in Smad3, which is critical for Smad-dependent canonical TGFβ signaling, fail to develop ASM thickening in an allergic mouse model of asthma compared with wild-type mice (30). Noncanonical pathways activated downstream of TGFβ, such as PI3K, have also been implicated in driving AHR in experimental mouse models (31). TGFβ has been implicated as a genetic modifier and biomarker in asthma, and TGFβ levels are increased in BALF of children with severe asthma compared with control subjects (32). Finally, overproducing TGFβ1 polymorphisms in patients are linked to worsening asthma severity, indicating a potential link between TGFβ signaling and ASM dysfunction in humans (33).
In our studies reported here, we have demonstrated that CF mice have greater alterations in ASM structure and function after TGFβ exposure, despite lack of ASM abnormalities or AHR at baseline. CF mice treated with intrapulmonary Ad-TGFβ have increased AHR that is rescued with β-adrenergic agonist treatment and greater ASM burden compared with littermate non-CF mice. These data correlate with clinical studies in patients with CF that show heightened ASM burden on biopsies and enhanced AHR, even in the absence of a clear asthma diagnosis (2, 3). Combined with recent studies in the CF pig demonstrating a hypercontractile ASM phenotype observed at birth and contributing to airway obstruction (7, 8), our study supports the notion that loss of CFTR function in ASM results in alterations in ASM structure and function. In the CF mouse model, TGFβ exposure reveals a latent ASM phenotype contributing to enhanced lung pathology and airway obstruction.
A recent study of ASM strips isolated from patients with CF and those without CF revealed IL-13–induced hypercontractility in CF ASM, supporting our findings that CFTR-deficient ASM have altered responses to cytokine stimuli (34). This study also demonstrated decreased response of CF ASM strips to the β-agonist isoproterenol after IL-13 exposure. This contrasts with our finding of increased bronchodilator response during methacholine challenge in TGFβ-exposed CF mice. We speculate that IL-13 and TGFβ may produce differential responses in CF ASM, or that chronic β-agonist use in patients with CF contributes to tolerance to bronchodilator effects, as has been reported in patients with asthma (35). Of note, over 90% of patients with CF use short-acting β-agonists (such as albuterol) or long-acting β-agonists daily (36).
Although CF and asthma are distinct clinical entities, there are clear shared pathologies and disease drivers: sustained inflammation; goblet cell hyperplasia; and ASM hypercontractility and proliferation (4). Heterozygosity for CFTR mutations has been associated with both asthma and AHR (37). However, few studies have investigated the use of typical asthma therapies in CF, although AHR in CF is associated with more rapid pulmonary decline (2). CF pulmonary guidelines from the Cystic Fibrosis Foundation recommend against routine use of inhaled corticosteroids, as there is little evidence of efficacy, and chronic use may increase the risk of airway infection and impair growth (38). Ivacaftor, a CFTR modulator that rapidly improves CFTR function in CFTR mutations causing gating defects, relieves pulmonary obstruction within 48 hours, indicating acute improvements in ASM function after restoration of CFTR activity (39). These results suggest that AHR and associated ASM abnormalities are a potential mechanism for airway obstruction and TGFβ disease modification in CF. Given its complex regulation of injury and repair response, TGFβ itself may be a difficult therapeutic target in CF. However, a better understanding of the effects of TGFβ in CF might lead to more focused therapies targeting the downstream drivers of pathology.
LY294002 is a widely used pan-PI3K inhibitor that has been studied extensively in animal models, including mouse models of asthma (31, 40). However, there are concerns about the nonspecificity of LY294002 inhibitory effects (41). Both canonical Smad signaling and MAPK signaling have been reported to be inhibited by LY294002 in certain model systems (42, 43). Other investigators have reported that LY294002 does not affect MAPK or Smad signaling in their studies (44, 45). These non–PI3K-specific effects of LY294002 are likely dependent on concentration, timing, mode of administration, and model system. In this study, we observed LY294002-induced pulmonary PI3K inhibition, and not alterations in MAPK or Smad signaling. However, the possibility remains that off-target effects of LY294002 are influencing our results. Furthermore, we cannot exclude effects on canonical and/or noncanonical TGFβ signaling pathways in different cell types within tissue compartments. In future studies, isoform-specific PI3K inhibitors and alternative molecular approaches will be needed to confirm the specificity of our results. Furthermore, as LY294002 was administered intraperitoneally, we expect broad PI3K inhibition in extrapulmonary tissues. As noted above, we demonstrated PI3K inhibition in whole-lung homogenates, but not inflammatory BAL cells after LY294002 treatment. These results implicate pulmonary PI3K signaling as a driver of our findings. Comparing modes of PI3K inhibitor delivery, such as systemic compared with inhalation, may clarify where inhibition of PI3K within the lung impacts TGFβ-dependent responses (e.g., epithelia, smooth muscle, or the pulmonary parenchyma).
In this study, PI3K inhibition reduced AHR in TGFβ-transduced CF mice; we have previously shown greatly heightened whole-lung activation of PI3K signaling after TGFβ exposure in CF mice only (21). The PI3K pathway has also been implicated in driving AHR in animal models of asthma, potentially through a reduction in pulmonary eosinophilic inflammation (31, 40). We did not observe eosinophilic inflammation in our model, however, and PI3K inhibition with LY294002 did not produce changes in total BALF cell count, differential cell count, or PI3K signaling in BAL cells. These observations suggest that, in the current studies, the reduction of AHR produced by PI3K inhibition is not primarily through altered cellular inflammation. PI3K inhibition did mitigate TGFβ-induced goblet cell hyperplasia, a major pathologic feature of CF, in CF mice, but not in non-CF mice, after 3 days of TGFβ exposure. Interestingly, although goblet cell hyperplasia was similar in CF and non-CF mice after 7 days of TGFβ exposure, only CF mice developed AHR at this time point. This suggests that goblet cell hyperplasia alone does not produce the AHR phenotype uniquely seen in CF mice. Thus, we propose that the reduction of AHR produced by PI3K inhibition is driven, at least in part, through ASM responses. Further studies of isolated ASM cells will be necessary to further define the mechanism of TGFβ-induced abnormalities in ASM structure and function in the setting of CFTR deficiency.
PI3K signaling has been previously implicated in CF pathology. In an analysis of CF human nasal mucosal tissue, increased PI3K signaling was associated with worse lung disease (46). Furthermore, inhibition of PI3K in F508 del CF bronchial epithelial cells has been shown to enhance CFTR stability and expression (47). These findings support the notion that PI3K may represent a viable therapeutic target with the potential to improve both CFTR function and ASM function in CF.
This study has several limitations that should be considered when interpreting the results. Ad-TGFβ intratracheal administration creates heterogenous transduction primarily in the bronchiolar–alveolar epithelium, which may not accurately replicate TGFβ expression patterns in patients (20). CF mice fail to develop significant spontaneous lung disease, a clear contrast with the effect of CFTR loss in humans. Despite this phenotype, CF mice have been a valuable model to study and understand CF lung disease, including hyperinflammatory response, delayed clearance of Pseudomonas, susceptibility to lung fibrosis, and TGFβ-dependent AHR as described here (48, 49). Thus, the CF mouse provides a valuable model to investigate abnormalities in cellular responses and lung remodeling in a setting of functional CFTR deficiency. Pseudomonas aeruginosa infection may also impact TGFβ-induced lung pathology in CF, as Pseudomonas infection has been found to be associated with increased TGFβ levels in patients with CF (14). Examining the role of infection is beyond the scope of this paper, but is an important direction for future work. Finally, heightened TGFβ exposure in patients with CF likely takes place over months to years, whereas, here, we are studying 3–7 days of increased TGFβ expression. Despite these limitations, our studies provide clear evidence linking TGFβ to lung pathology and ASM dysfunction specific to CF, including a novel role for TGFβ-dependent AHR mediated by PI3K signaling.
In conclusion, our data suggest a fundamental defect in CF ASM, leading to altered structure and function. Modest elevations in pulmonary TGFβ levels, due to either high-producing TGFβ polymorphisms or as a result of environmental stimuli, may prime the CF airway to develop enhanced AHR, greater ASM burden, and progressive airway obstruction. Although it is unclear what impact current CFTR modulators will have on this phenotype, our results support the examination of AHR in future trials of CFTR therapies. Furthermore, TGFβ may contribute to ASM dysfunction in other airway disease processes with altered CFTR function (37, 50), supporting a broader significance of our observations. Targeting of pathways that result in AHR and ASM-dependent airway obstruction in CF has the potential to impact the trajectory of lung disease progression.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Drs. William Hardie and John Brewington, Cincinnati Children’s Hospital, Cincinnati, OH, for their support of this work. The Ad-TGFβ vector was kindly provided by Dr. Martin Kolb, McMaster University (ON, Canada).
Footnotes
Supported in part by National Institutes of Health grant KL2-TR-001429 (E.L.K.), a New Horizons grant from Cystic Fibrosis Research, Inc. (E.L.K.), and the Cincinnati Children’s Hospital Procter Scholar Award (E.L.K.).
Author Contributions: Conceived and designed the study—E.L.K. and J.P.C.; performed experiments—E.L.K. and C.D.; analyzed and interpreted the data—E.L.K., S.K.M., K.M.H., C.D., and J.P.C.; wrote the manuscript—E.L.K. and J.P.C.; edited the manuscript—E.L.K., S.K.M., K.M.H., C.D., and J.P.C.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0158OC on January 10, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Eggleston PA, Rosenstein BJ, Stackhouse CM, Alexander MF. Airway hyperreactivity in cystic fibrosis: clinical correlates and possible effects on the course of the disease. Chest. 1988;94:360–365. doi: 10.1378/chest.94.2.360. [DOI] [PubMed] [Google Scholar]
- 3.Regamey N, Ochs M, Hilliard TN, Mühlfeld C, Cornish N, Fleming L, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–843. doi: 10.1164/rccm.200707-977OC. [DOI] [PubMed] [Google Scholar]
- 4.Kent BD, Lane SJ, van Beek EJ, Dodd JD, Costello RW, Tiddens HA. Asthma and cystic fibrosis: a tangled web. Pediatr Pulmonol. 2014;49:205–213. doi: 10.1002/ppul.22934. [DOI] [PubMed] [Google Scholar]
- 5.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 6.Wainwright CE, Elborn JS, Ramsey BW. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for phe508del cftr. N Engl J Med. 2015;373:1783–1784. doi: 10.1056/NEJMc1510466. [DOI] [PubMed] [Google Scholar]
- 7.Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR, et al. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle: implications for airway contractility. Am J Respir Crit Care Med. 2016;193:417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cook DP, Adam RJ, Zarei K, Deonovic B, Stroik MR, Gansemer ND, et al. CF airway smooth muscle transcriptome reveals a role for PYK2. JCI Insight. 2017;2:95332. doi: 10.1172/jci.insight.95332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanscoy LL, Blackman SM, Collaco JM, Bowers A, Lai T, Naughton K, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:1036–1043. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, et al. Gene Modifier Study Group. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 11.Arkwright PD, Laurie S, Super M, Pravica V, Schwarz MJ, Webb AK, et al. TGF-beta(1) genotype and accelerated decline in lung function of patients with cystic fibrosis. Thorax. 2000;55:459–462. doi: 10.1136/thorax.55.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54:647–655. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris WT, Muhlebach MS, Oster RA, Knowles MR, Noah TL. Transforming growth factor-beta(1) in bronchoalveolar lavage fluid from children with cystic fibrosis. Pediatr Pulmonol. 2009;44:1057–1064. doi: 10.1002/ppul.21079. [DOI] [PubMed] [Google Scholar]
- 14.Harris WT, Muhlebach MS, Oster RA, Knowles MR, Clancy JP, Noah TL. Plasma TGF-β1 in pediatric cystic fibrosis: potential biomarker of lung disease and response to therapy. Pediatr Pulmonol. 2011;46:688–695. doi: 10.1002/ppul.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun H, Harris WT, Kortyka S, Kotha K, Ostmann AJ, Rezayat A, et al. Tgf-beta downregulation of distinct chloride channels in cystic fibrosis–affected epithelia. PLoS One. 2014;9:e106842. doi: 10.1371/journal.pone.0106842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer EL, Clancy JP. TGFβ as a therapeutic target in cystic fibrosis. Expert Opin Ther Targets. 2018;22:177–189. doi: 10.1080/14728222.2018.1406922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snodgrass SM, Cihil KM, Cornuet PK, Myerburg MM, Swiatecka-Urban A. Tgf-β1 inhibits Cftr biogenesis and prevents functional rescue of ΔF508-Cftr in primary differentiated human bronchial epithelial cells. PLoS One. 2013;8:e63167. doi: 10.1371/journal.pone.0063167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe KL, Wang A, Hunter MM, Stanton BA, McKay DM. TGFbeta down-regulation of the CFTR: a means to limit epithelial chloride secretion. Exp Cell Res. 2004;298:473–484. doi: 10.1016/j.yexcr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Lee CG, Kang HR, Homer RJ, Chupp G, Elias JA. Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc. 2006;3:418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warshamana GS, Pociask DA, Fisher KJ, Liu JY, Sime PJ, Brody AR. Titration of non-replicating adenovirus as a vector for transducing active TGF-beta1 gene expression causing inflammation and fibrogenesis in the lungs of C57BL/6 mice. Int J Exp Pathol. 2002;83:183–201. doi: 10.1046/j.1365-2613.2002.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer EL, Hardie WD, Madala SK, Davidson C, Clancy JP. Subacute TGFβ expression drives inflammation, goblet cell hyperplasia, and pulmonary function abnormalities in mice with effects dependent on CFTR function. Am J Physiol Lung Cell Mol Physiol. 2018;315:L456–L465. doi: 10.1152/ajplung.00530.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer E, Madala S, Hardie W, Hudock KM, Davidson C, Ostmann A, et al. Tgf beta, early airway obstruction and smooth muscle dysfunction in cf. Pediatr Pulmonol. 2018;53:183–184. [Google Scholar]
- 23.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA. 1994;91:8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darrah RJ, Bederman IR, Mitchell AL, Hodges CA, Campanaro CK, Drumm ML, et al. Ventilatory pattern and energy expenditure are altered in cystic fibrosis mice. J Cyst Fibros. 2013;12:345–351. doi: 10.1016/j.jcf.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Addison KJ, Morse J, Robichaud A, Daines MO, Ledford JG. A novel in vivo system to test bronchodilators. J Infect Pulm Dis. 2017;3:1–5. doi: 10.16966/2470-3176.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant PJ, North ML, Ward A, Ward M, Khanna N, Correa J, et al. Hypertrophic airway smooth muscle mass correlates with increased airway responsiveness in a murine model of asthma. Am J Respir Cell Mol Biol. 2012;46:532–540. doi: 10.1165/rcmb.2011-0293OC. [DOI] [PubMed] [Google Scholar]
- 27.Ojiaku CA, Cao G, Zhu W, Yoo EJ, Shumyatcher M, Himes BE, et al. TGF-β1 evokes human airway smooth muscle cell shortening and hyperresponsiveness via Smad3. Am J Respir Cell Mol Biol. 2018;58:575–584. doi: 10.1165/rcmb.2017-0247OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossé Y, Stankova J, Rola-Pleszczynski M. Transforming growth factor-beta1 in asthmatic airway smooth muscle enlargement: is fibroblast growth factor-2 required? Clin Exp Allergy. 2010;40:710–724. doi: 10.1111/j.1365-2222.2010.03497.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200:377–389. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in Smad 3–deficient mice. J Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 31.Medina-Tato DA, Ward SG, Watson ML. Phosphoinositide 3-kinase signalling in lung disease: leucocytes and beyond. Immunology. 2007;121:448–461. doi: 10.1111/j.1365-2567.2007.02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown SD, Baxter KM, Stephenson ST, Esper AM, Brown LA, Fitzpatrick AM. Airway TGF-β1 and oxidant stress in children with severe asthma: association with airflow limitation. J Allergy Clin Immunol. 2012;129:388–396, 396.e1–8. doi: 10.1016/j.jaci.2011.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFbeta1 allele association with asthma severity. Hum Genet. 2001;109:623–627. doi: 10.1007/s00439-001-0617-y. [DOI] [PubMed] [Google Scholar]
- 34.Matusovsky OS, Kachmar L, Ijpma G, Panariti A, Benedetti A, Martin JG, et al. Contractile properties of intrapulmonary airway smooth muscle in cystic fibrosis. Am J Respir Cell Mol Biol. 2019;60:434–444. doi: 10.1165/rcmb.2018-0005OC. [DOI] [PubMed] [Google Scholar]
- 35.Salpeter SR, Ormiston TM, Salpeter EE. Meta-analysis: respiratory tolerance to regular beta2-agonist use in patients with asthma. Ann Intern Med. 2004;140:802–813. doi: 10.7326/0003-4819-140-10-200405180-00010. [DOI] [PubMed] [Google Scholar]
- 36.North American Cystic Fibrosis Foundation. Patient registry: annual data report. 2016 Aug [accessed 2019 Apr 23]. Available from: https://www.cff.org/our-research/cf-patient-registry/2015-patient-registry-annual-data-report.pdf.
- 37.Nielsen AO, Qayum S, Bouchelouche PN, Laursen LC, Dahl R, Dahl M. Risk of asthma in heterozygous carriers for cystic fibrosis: a meta-analysis. J Cyst Fibros. 2016;15:563–567. doi: 10.1016/j.jcf.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Balfour-Lynn IM, Welch K. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev. 2016;(8):CD001915. doi: 10.1002/14651858.CD001915.pub5. [DOI] [PubMed] [Google Scholar]
- 39.Adam RJ, Hisert KB, Dodd JD, Grogan B, Launspach JL, Barnes JK, et al. Acute administration of ivacaftor to people with cystic fibrosis and a G551D-CFTR mutation reveals smooth muscle abnormalities. JCI Insight. 2016;1:e86183. doi: 10.1172/jci.insight.86183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol. 2005;5:495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, Hancox T, Saghir N, Timms JF, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007;404:15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacob A, Cooney D, Pradhan M, Coggeshall KM. Convergence of signaling pathways on the activation of ERK in B cells. J Biol Chem. 2002;277:23420–23426. doi: 10.1074/jbc.M202485200. [DOI] [PubMed] [Google Scholar]
- 43.Qiao J, Kang J, Ko TC, Evers BM, Chung DH. Inhibition of transforming growth factor-beta/Smad signaling by phosphatidylinositol 3-kinase pathway. Cancer Lett. 2006;242:207–214. doi: 10.1016/j.canlet.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Kuramitsu Y, Baron B, Kitagawa T, Tokuda K, Akada J, et al. PI3K inhibitor LY294002, as opposed to wortmannin, enhances AKT phosphorylation in gemcitabine-resistant pancreatic cancer cells. Int J Oncol. 2017;50:606–612. doi: 10.3892/ijo.2016.3804. [DOI] [PubMed] [Google Scholar]
- 45.Wilkes MC, Mitchell H, Penheiter SG, Doré JJ, Suzuki K, Edens M, et al. Transforming growth factor-beta activation of phosphatidylinositol 3-kinase is independent of Smad2 and Smad3 and regulates fibroblast responses via p21-activated kinase-2. Cancer Res. 2005;65:10431–10440. doi: 10.1158/0008-5472.CAN-05-1522. [DOI] [PubMed] [Google Scholar]
- 46.Polineni D, Dang H, Gallins PJ, Jones LC, Pace RG, Stonebraker JR, et al. Airway mucosal host defense is key to genomic regulation of cystic fibrosis lung disease severity. Am J Respir Crit Care Med. 2018;197:79–93. doi: 10.1164/rccm.201701-0134OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reilly R, Mroz MS, Dempsey E, Wynne K, Keely SJ, McKone EF, et al. Targeting the PI3K/Akt/mTOR signalling pathway in cystic fibrosis. Sci Rep. 2017;7:7642. doi: 10.1038/s41598-017-06588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schroeder TH, Reiniger N, Meluleni G, Grout M, Coleman FT, Pier GB. Transgenic cystic fibrosis mice exhibit reduced early clearance of Pseudomonas aeruginosa from the respiratory tract. J Immunol. 2001;166:7410–7418. doi: 10.4049/jimmunol.166.12.7410. [DOI] [PubMed] [Google Scholar]
- 49.Huaux F, Noel S, Dhooghe B, Panin N, Lo Re S, Lison D, et al. Dysregulated proinflammatory and fibrogenic phenotype of fibroblasts in cystic fibrosis. PLoS One. 2013;8:e64341. doi: 10.1371/journal.pone.0064341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.