Cystic fibrosis (CF), the most common inherited recessive genetic disease in the white population, is lethal and characterized by defects in chloride and bicarbonate transport, mucus secretion, lung bacterial infection, and inflammation (1, 2). Other clinical features of CF include deterioration of lung architecture and function, pancreatic insufficiency, male infertility, and meconium ileus in newborns (3). Mutations in the CFTR (CF conductance regulator) gene result in deficient and/or defective CFTR protein, and over 2,000 variant mutations have been identified in the CFTR gene. Deletion of phenylalanine 508 (∆F508) is the most common one in white individuals that impairs CFTR folding and biosynthesis, as well as endocytic processing and chloride channel function (4–6). CFTR is expressed in the submucosal glands of the airway, in multiciliated surface epithelial cells, and in the recently identified airway epithelium-rich pulmonary ionocytes (7). Depletion or defects in CFTR of the airway epithelial cells have been implicated in mucociliary clearance impairment and airway remodeling (8). Although chronic pulmonary infection is a hallmark of CF lung disease, the majority of patients with CF demonstrate airway hyperresponsiveness (AHR) suggestive of airway smooth muscle (ASM) dysfunction. Recent studies showed CFTR expression in the sarcoplasmic reticulum in ASM, and loss of CFTR was found to alter the ASM transcriptome (9) and to increase basal tone and bronchodilator response and disrupt calcium handling in neonatal CF pigs (10), suggesting a causal link between CFTR and ASM cell function.
Multiple intra- and extracellular signaling pathways, such as HIF-1α (hypoxia-inducible factor-1α), NF-κB, PI3K, protein kinase B (or AKT), mTOR (mammalian target of rapamycin), PDK1 (3-phosphoinositide–dependent protein kinase), SGK1 (serum/glucocorticoid regulated kinase-1), PYK2 (proline-rich tyrosine kinase-2), and MAPKs (mitogen-activated protein kinases), have been implicated in CF lung pathogenesis (11–13). Many of these pathways are activated by TGF-β (transforming growth factor-β) signaling via TGF-βR1 (TGF-β receptor R1) and TGF-βR2, which drive CF lung disease (14). TGF-β levels are elevated in plasma and BAL fluid of patients with CF, and have been shown to be associated with pulmonary exacerbations and the severity of CF lung pathogenesis (15). Therefore, TGF-β is recognized as a therapeutic target in CF; however, the role and mechanism(s) of TGF-β in AHR and ASM dysfunction have not been well investigated.
In a study presented in this issue of the Journal, using a homozygous mouse model of F508 del CFTR with gut correction, Kramer and colleagues (pp. 657–667) found that subacute TGF-β expression by an adenoviral TGF-β vector in CFTRtm1kth mice (“CF mice”) enhanced a latent AHR phenotype and ASM dysfunction (16). This is the first in vivo study to clearly demonstrate that expression of TGF-β in CF mice drives abnormalities in AHR and ASM dysfunction compared with non-CF control mice. Altered bronchoconstriction dynamics and increased ASM area were observed in TGF-β–exposed CF mice. Furthermore, albuterol (β-agonist) pretreatment had no effect on baseline resistance but reversed AHR in TGF-β–expressing CF mice. Building on an earlier observation of enhanced PI3K signaling in CF mice after Ad-TGF-β transduction (14), Kramer and colleagues demonstrated that blocking PI3K activity in vivo with a pan-PI3K inhibitor, LY294002, decreased methacholine-induced AHR and goblet cell hyperplasia in CF mice. However, inhibition of PI3K had no effect on Smad or MAPK (ERK1/2) signaling in CF mice, or on ASM area in both CF and non-CF mice. These studies highlight the significance of the TGF-β/PI3K signaling axis in AHR and ASM dysfunction in the ∆F508-deleted CF mouse model, and suggest PI3K as a potential therapeutic target by itself or in combination with TGF-β in CF lung disease (Figure 1).
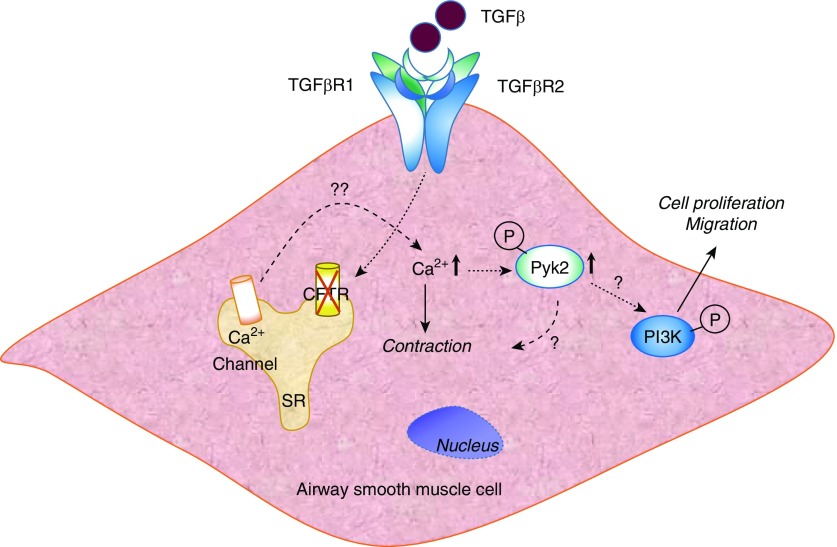
Figure 1.
Schema depicting TGF-β (transforming growth factor-β)–mediated PI3K signaling and airway remodeling in a CFTR (cystic fibrosis transmembrane conductance regulator)-deficient airway smooth muscle cell. Loss of CFTR in the airway smooth muscle cell potentiates TGF-β–induced release of intracellular Ca2+ from the sarcoplasmic reticulum (SR) via TGF-βR1 (TGF-β receptor R1) or TGF-βR2, which can lead to activation and autophosphorylation of PYK (proline-rich tyrosine kinase) (9). Phosphorylated (P) PYK2 can promote PI3K activation and stimulate cell proliferation and migration. Increased Ca2+ levels either independently or in conjunction with phosphorylated PYK2 can induce cell contraction. The upstream and downstream targets of Ca2+, PYK2, and PI3K in modulating cell contraction, proliferation, and migration in normal and CFTR-deficient airway smooth muscle cells from humans, mice, and pigs warrant future investigations. Furthermore, additional canonical and noncanonical pathways stimulated by TGF-β in normal and CFTR-deficient airway smooth muscle cells can contribute to contractile and proliferative pathways.
There are several limitations to the study. LY294002, a pan-PI3K inhibitor that was used to block PI3K activity in vivo, may exhibit off-target effects. Other approaches, such as instillation of PI3K shRNA or siRNA directly into the airway to block PI3K, will be necessary to corroborate the inhibitor results. Furthermore, PI3K is divided into four classes (classes I–IV) based on primary structure, regulation, and in vitro substrate specificity. It is unclear which class of PI3K is activated by TGF-β in ASM cells to induce AHR, cell proliferation, and ASM dysfunction. PI3K is a lipid kinase that uses phosphatidylinositol and other polyphosphoinositides as substrates to generate corresponding phosphorylated phosphoinositides via binding to plekstrin homology domain of AKT or PDK1, resulting in translocation to the plasma membrane and partial activation. However, the downstream target(s) of PI3K has not been identified in ASM cells, which makes it difficult to define the mechanism(s) of AHR and ASM dysfunction in CF. In vitro studies with isolated ASM cells from non-CF and F508 del CFTR homozygous mice or CF pigs will provide mechanistic insights into the canonical and noncanonical signaling pathways mediated by TGF-β in AHR, cell proliferation, and ASM dysfunction in CF lung disease.
Supplementary Material
Acknowledgments
Acknowledgment
The author thanks Dr. Ramaswamy Ramchandran for helpful discussion and for drawing the schema.
Footnotes
Supported by U.S. National Institutes of Health grants HLBI P01 077806, HLBI P01 126609, and HLBI R01 127342 (V.N.).
Originally Published in Press as DOI: 10.1165/rcmb.2020-0029ED on February 3, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2. Wine JJ, Hansson GC, König P, Joo NS, Ermund A, Pieper M. Progress in understanding mucus abnormalities in cystic fibrosis airways. J Cyst Fibros. 2018;17:S35–S39. doi: 10.1016/j.jcf.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 3. Castellani C, Assael BM. Cystic fibrosis: a clinical view. Cell Mol Life Sci. 2017;74:129–140. doi: 10.1007/s00018-016-2393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83:121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 5. Riordan JR. Cystic fibrosis as a disease of misprocessing of the cystic fibrosis transmembrane conductance regulator glycoprotein. Am J Hum Genet. 1999;64:1499–1504. doi: 10.1086/302429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 7. Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adam D, Roux-Delrieu J, Luczka E, Bonnomet A, Lesage J, Mérol JC, et al. Cystic fibrosis airway epithelium remodelling: involvement of inflammation. J Pathol. 2015;235:408–419. doi: 10.1002/path.4471. [DOI] [PubMed] [Google Scholar]
- 9. Cook DP, Adam RJ, Zarei K, Deonovic B, Stroik MR, Gansemer ND, et al. CF airway smooth muscle transcriptome reveals a role for PYK2. JCI Insight. 2017;2:95332. doi: 10.1172/jci.insight.95332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook DP, Rector MV, Bouzek DC, Michalski AS, Gansemer ND, Reznikov LR, et al. Cystic fibrosis transmembrane conductance regulator in sarcoplasmic reticulum of airway smooth muscle: implications for airway contractility. Am J Respir Crit Care Med. 2016;193:417–426. doi: 10.1164/rccm.201508-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bodas M, Vij N. The NF-κB signaling in cystic fibrosis lung disease: pathophysiology and therapeutic potential. Discov Med. 2010;9:346–356. [PMC free article] [PubMed] [Google Scholar]
- 12. Caohuy H, Yang Q, Eudy Y, Ha T-A, Xu AE, Glover M, et al. Activation of 3-phosphoinositide-dependent kinase 1 (PDK1) and serum- and glucocorticoid-induced protein kinase 1 (SGK1) by short-chain sphingolipid C4-ceramide rescues the trafficking defect of ΔF508-cystic fibrosis transmembrane conductance regulator (ΔF508-CFTR) J Biol Chem. 2014;289:35953–35968. doi: 10.1074/jbc.M114.598649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reilly R, Mroz MS, Dempsey E, Wynne K, Keely SJ, McKone EF, et al. Targeting the PI3K/Akt/mTOR signalling pathway in cystic fibrosis. Sci Rep. 2017;7:7642. doi: 10.1038/s41598-017-06588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kramer EL, Hardie WD, Madala SK, Davidson C, Clancy JP. Subacute TGFβ expression drives inflammation, goblet cell hyperplasia, and pulmonary function abnormalities in mice with effects dependent on CFTR function. Am J Physiol Lung Cell Mol Physiol. 2018;315:L456–L465. doi: 10.1152/ajplung.00530.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kramer EL, Clancy JP. TGFβ as a therapeutic target in cystic fibrosis. Expert Opin Ther Targets. 2018;22:177–189. doi: 10.1080/14728222.2018.1406922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kramer EL, Madala SK, Hudock KM, Davidson C, Clancy JP. Subacute TGFβ exposure drives airway hyperresponsiveness in cystic fibrosis mice through the PI3K pathway. Am J Respir Cell Mol Biol. 2020;62:657–667. doi: 10.1165/rcmb.2019-0158OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.