Idiopathic pulmonary fibrosis (IPF) is the most common form of idiopathic interstitial pneumonia and the most aggressive form of diffuse parenchymal lung disease, characterized by progressive scarring of the alveolar network that eventually leads to respiratory failure. Although the precise pathophysiology of IPF is not yet fully understood, the prevailing knowledge suggests that recurrent injury to alveolar epithelial cells (AECs) represents a key trigger for the accumulation of myofibroblasts that excessively deposit extracellular matrix proteins such as collagen and fibronectin (1). Bleomycin injury is commonly used to model IPF in experimental mice. Although this injury model does not fully recapitulate the human condition, and despite the fact that bleomycin-induced pulmonary fibrosis is reversible and human IPF is irreversible, bleomycin instillation is still the most commonly used injury model because of the wealth of knowledge regarding its different phases (inflammation, fibrosis, and repair), dosage, susceptibility of various mouse strains, and so forth.
FGFs (fibroblast growth factors) are critical molecules for normal development/organogenesis, homeostasis, and repair after injury. Most FGFs exert their biological functions by binding to and activating tyrosine kinase FGF receptors (FGFRs), namely FGFR1–4. FGFR1/2/3 have two alternative splice variants or isoforms: IIIb (mostly epithelial) and IIIc (mostly mesenchymal) (2). Prototypic FGFs (FGF1 and FGF2) were originally discovered and purified from bovine brain and pituitary, and they were found to promote proliferation of fibroblasts such as the 3T3-L1 cell line. However, many FGFs target epithelial cells and are important for wound healing and repair after injury in many organs, including the lung (3). In fact, accumulating evidence suggests that FGF/FGFR signaling in the lung epithelium is essentially antifibrotic. For instance, exogenous or genetically overexpressed FGF1, FGF7, or FGF10 protects against experimental pulmonary fibrosis by enhancing AEC proliferation and inhibiting TGF-β1 (transforming growth factor-β1) signaling (4–6). Furthermore, Fgf2-knockout mice have increased mortality and weight loss in response to bleomycin injury (7), whereas overexpression of Fgf2 attenuates bleomycin-induced pulmonary fibrosis (8). Interestingly, FGF1, FGF7, and FGF10 have been shown to be upregulated in IPF lungs (9–11), and nintedanib, which is a small-molecule multi–tyrosine kinase inhibitor that also targets FGFR, is one of the two currently approved medications for the treatment of IPF (together with pirfenidone). Despite the upregulation of these ligands in the lungs of patients with IPF, the epithelial isoforms (IIIb) of FGFR1/2 are downregulated, whereas the mesenchymal isoforms (IIIc) of FGFR1/2/3 are upregulated, in IPF lungs compared with control donors (9). In addition, it is important to mention that FGF1 binds to and activates all FGFR splice variants (epithelial and mesenchymal), whereas FGF2 mainly interacts with the mesenchymal isoforms (IIIc) of FGFR1/2 and FGF7/FGF10 mainly interacts with the epithelial isoform (IIIb) of FGFR2 (FGF10 also interacts with FGFR1-IIIb) (2). Thus, epithelial FGFR signaling, particularly in AECs, seems to be protective and important for repair after lung injury and fibrosis.
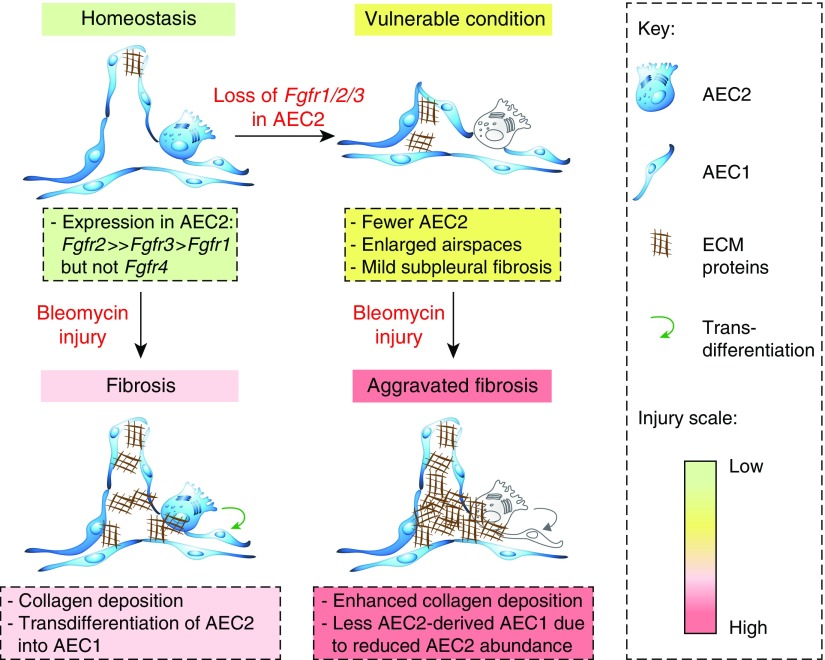
In this issue of the Journal (pp. 608–621), Dorry and colleagues (12) demonstrate that AEC2-specific FGFR signaling is required for AEC2 homeostasis and protection after bleomycin-induced lung injury. To achieve this, they generated triple conditional knockouts for Fgfr1/2/3 in AEC2s (SftpcCre-ERT2;Fgfr1flox/flox;Fgfr2flox/flox;Fgfr3flox/flox or SPC-TCKO), double conditional knockouts for Fgfr2/3 (SftpcCre-ERT2;Fgfr2flox/flox;Fgfr3flox/flox or SPC-R2/R3), and single conditional knockouts for Fgfr2 and Fgfr3 (SPC-R2 and SPC-R3, respectively). FGFR1 alone was not investigated on the basis of the authors’ data that it is expressed at much lower levels in AEC2s than Fgfr2 and Fgfr3, which is in agreement with a previous study (9). After intraperitoneal tamoxifen administration, SPC-TCKO mice showed decreased numbers of lineage-labeled cells and total AEC2s (likely due to decreased proliferation and increased apoptosis of preexisting AEC2s), enlarged alveolar diameter, and mild subpleural fibrosis (Figure 1). This phenomenon was observed also in doxycycline-inducible Sftpc-rtTA;TetO-Cre;Fgfr1flox/flox;Fgfr2flox/flox;Fgfr3flox/flox mice. Interestingly, the study also shows that the proportion of lineage-negative, SFTPC (surfactant protein C)-positive (GFP-negative SPC-positive) cells out of total SPC-positive cells increases in SPC-TCKO mice compared with control animals. The authors hinted at the possibility that a compensatory mechanism replenishes lost AEC2s from SPC-negative precursors or from unlabeled AEC2s. SPC-TCKO mice show not only increased susceptibility to lung injury but also a 100% mortality rate by 14 days after bleomycin administration (0.5 U/kg). Therefore, the authors titrated the dose of bleomycin to 0.02 U/kg to achieve enough survival that allows study of further fibrosis development in SPC-TCKO mice. Analysis at 21 days after bleomycin administration showed that SPC-TCKO mice develop more severe fibrosis than control animals and display less AEC2-derived AEC1s out of total AEC1s, likely due to reduced AEC2 abundance (Figure 1).
Figure 1.
Role of FGFR2 in type 2 alveolar epithelial cell (AEC2) homeostasis and response to injury. AEC2s mainly express Fgfr2 and Fgfr3. Bleomycin instillation leads to fibrosis development coupled to AEC2-to-AEC1 transdifferentiation. Loss of Fgfr1/2/3 in AEC2s (SPC-TCKO triple–conditional knockout mice) leads to a vulnerable state with fewer AEC2s, enlarged airspaces, and mild subpleural fibrosis. Bleomycin injury to SPC-TCKO or SPC-R2 (SftpcCre-ERT2;Fgfr2flox/flox) mice leads to aggravated lung fibrosis with fewer AEC2s, enhanced collagen deposition, and a decreased proportion of AEC2-derived AEC1s out of total AEC1s due to reduced AEC2 abundance. ECM = extracellular matrix; Fgfr = fibroblast growth factor receptor.
To identify which FGFR is responsible for exerting protective effects against bleomycin injury, the authors used SPC-R2 and SPC-R3 mice. Eventually, SPC-R2, but not SPC-R3, mice recapitulated the phenotype of SPC-TCKO mice in terms of reduced numbers of lineage-labeled cells and increased lung injury (12). Even during homeostasis, SPC-R2 mice showed fewer lineage-labeled cells than control animals 6 weeks after tamoxifen administration. These findings demonstrate a critical role for FGFR2 in AEC2 not only for protection against lung injury but also for homeostatic maintenance (Figure 1).
AEC2s are regarded as stem cells in the alveolar compartment of the lung. These cells can self-renew and give rise to AEC1s during repair after injury. One likely explanation for the increased susceptibility of SPC-TCKO and SPC-R2 mice to bleomycin injury is reduced AEC2 abundance. The authors show that after bleomycin administration, mutant lungs express lower levels of AEC2 and AEC1 marker genes, indicating loss of alveolar epithelial integrity. Pulmonary surfactant secreted by AEC2s lowers alveolar surface tension, thereby preventing alveolar collapse. Mutations in the SFTPC gene have been reported in familial forms of IPF, and a recent study has shown that expression of mutated Sftpc in vivo causes spontaneous lung fibrosis in mice (13). Furthermore, SFTPC mutations can cause protein misfolding, endoplasmic reticulum stress, and ultimately AEC2 apoptosis (14, 15). SHP2 (protein tyrosine phosphatase nonreceptor type 11) is one of the essential mediators for FGF/FGFR signal transduction. Knocking out Shp2 in AECs induces a marked reduction in surfactant protein production, disorganized lamellar bodies, increased AEC apoptosis, and spontaneous development of pulmonary fibrosis (16). Therefore, loss of FGFR2 signaling in AEC2 likely initiates a pathological cascade that leads to enhanced vulnerability to injury and fibrosis development (Figure 1).
It is also important to identify which ligand(s) are responsible for the antifibrotic effect of FGFR2 (presumably FGFR2-IIIb) signaling in AEC2s. In the adult mouse lung, the highest-expressed FGF ligands are FGF1, FGF2, FGF7, FGF9, FGF10, FGF18, and FGF23 (3). Among these ligands, FGF1, FGF7, and FGF10 are known for their high affinity to FGFR2-IIIb (2). Fgf10 expression has been shown to mark a subpopulation of lipofibroblasts in the embryonic and postnatal mouse lung (17), and autocrine/paracrine FGF10 signaling through FGFR2-IIIb is also important for lipofibroblast formation (18). Lipofibroblasts are regarded as niche cells for AEC2 stem cells in the lung, and apart from providing triglycerides to adjacent AEC2s to assist in the process of surfactant production, they presumably play a role in AEC2 maintenance during homeostasis and repair after injury. Reciprocal signaling between AEC2s and lipofibroblasts is believed to ensure the integrity of the alveolar stem cell niche. In this context, lipofibroblasts transdifferentiate into myofibroblasts during fibrosis development in vivo (10). It is therefore highly plausible that lipofibroblast-derived FGF10 activates FGFR2-IIIb in adjacent AEC2s and ensures their maintenance and protection against bleomycin injury.
A previous study reported that FGFR2-IIIb ligand inhibition does not impact weight loss, mortality, lung injury, or fibrosis compared with controls in bleomycin-induced lung fibrosis (19). One of the reasons behind the discrepancy with the study by Dorry and colleagues might be that the dominant-negative soluble decoy receptor approach might trap multiple ligands that also signal through mesenchymal receptor isoforms of not only FGFR2 but also other FGFRs. In other words, such an approach might neutralize not only antifibrotic but also profibrotic FGFs, thereby neutralizing the net outcome in terms of fibrosis development. Disruption of FGFR signaling in AEC2s might also activate FGF signaling in other cell types because of increased bioavailability of FGF ligands. In addition, the experimental design of the study by Dorry and colleagues leads to deleting both the IIIb and IIIc splice variants of FGFR2 in AEC2s (12). Interestingly, FGFR2-IIIb can be alternatively spliced into FGFR2-IIIc on the basis of the surrounding environment in prostate cancer, and this class switching contributes to epithelial-to-mesenchymal transition (20). Although further studies are needed to detect and dissect the roles of potential FGFR2 splice variants in AEC2s, the study by Dorry and colleagues emphasizes the importance of FGFR2 signaling in AEC2s during homeostasis and in response to injury.
IPF is typically diagnosed at the end-stage phase in aged males. Most studies, including the present one, investigate the preventive, rather than the therapeutic, effect of candidate signaling pathways, and bench-to-bedside success stories have been rare for IPF. In the future, more comprehensive studies with cell-type–specific deletions of ligands and receptors coupled to single-cell analysis, functional assays, and validation in human-derived material might help improve understanding of the involvement of FGF signaling as well as other developmental programs in lung disease and regeneration and pave the path for targeted therapy and personalized medicine.
Supplementary Material
Footnotes
Supported by the German Research Foundation (KFO 309 P7 and SFB CRC 1213), Institute for Lung Health, Cardio-Pulmonary Institute (EXC 2026; project ID 390649896), University Hospital Giessen and Marburg, and the German Center for Lung Research (E.E.A.).
Originally Published in Press as DOI: 10.1165/rcmb.2020-0013ED on January 15, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Selman M, Pardo A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell Signal. 2020;66:109482. doi: 10.1016/j.cellsig.2019.109482. [DOI] [PubMed] [Google Scholar]
- 2. Ornitz DM, Itoh N. The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Agha E, Seeger W, Bellusci S. Therapeutic and pathological roles of fibroblast growth factors in pulmonary diseases. Dev Dyn. 2017;246:235–244. doi: 10.1002/dvdy.24468. [DOI] [PubMed] [Google Scholar]
- 4. Shimbori C, Bellaye PS, Xia J, Gauldie J, Ask K, Ramos C, et al. Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J Pathol. 2016;240:197–210. doi: 10.1002/path.4768. [DOI] [PubMed] [Google Scholar]
- 5. Sugahara K, Iyama K, Kuroda MJ, Sano K. Double intratracheal instillation of keratinocyte growth factor prevents bleomycin-induced lung fibrosis in rats. J Pathol. 1998;186:90–98. doi: 10.1002/(SICI)1096-9896(199809)186:1<90::AID-PATH137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6. Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, et al. Overexpression of fibroblast growth factor-10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2009;180:424–436. doi: 10.1164/rccm.200811-1794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guzy RD, Stoilov I, Elton TJ, Mecham RP, Ornitz DM. Fibroblast growth factor 2 is required for epithelial recovery, but not for pulmonary fibrosis, in response to bleomycin. Am J Respir Cell Mol Biol. 2015;52:116–128. doi: 10.1165/rcmb.2014-0184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koo HY, El-Baz LMF, House S, Cilvik SN, Dorry SJ, Shoukry NM, et al. Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation. J Pathol. 2018;246:54–66. doi: 10.1002/path.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res. 2015;16:83. doi: 10.1186/s12931-015-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20:261–273.e3. doi: 10.1016/j.stem.2016.10.004. [Published erratum appears in Cell Stem Cell 20:571.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Agha E, Schwind F, Ruppert C, Günther A, Bellusci S, Schermuly RT, et al. Is the fibroblast growth factor signaling pathway a victim of receptor tyrosine kinase inhibition in pulmonary parenchymal and vascular remodeling? Am J Physiol Lung Cell Mol Physiol. 2018;315:L248–L252. doi: 10.1152/ajplung.00140.2018. [DOI] [PubMed] [Google Scholar]
- 12. Dorry SJ, Ansbro BO, Ornitz DM, Mutlu GM, Guzy RD. FGFR2 is required for AEC2 homeostasis and survival after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2020;62:608–621. doi: 10.1165/rcmb.2019-0079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128:4008–4024. doi: 10.1172/JCI99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mulugeta S, Nguyen V, Russo SJ, Muniswamy M, Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang X, Zhang Y, Tao B, Teng L, Li Y, Cao R, et al. Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB J. 2012;26:2338–2350. doi: 10.1096/fj.11-200139. [DOI] [PubMed] [Google Scholar]
- 17. El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Alam D, El Agha E, Sakurai R, Kheirollahi V, Moiseenko A, Danopoulos S, et al. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development. 2015;142:4139–4150. doi: 10.1242/dev.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. MacKenzie B, Henneke I, Hezel S, Al Alam D, El Agha E, Chao CM, et al. Attenuating endogenous Fgfr2b ligands during bleomycin-induced lung fibrosis does not compromise murine lung repair. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1014–L1024. doi: 10.1152/ajplung.00291.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katoh Y, Katoh M. FGFR2-related pathogenesis and FGFR2-targeted therapeutics (review) Int J Mol Med. 2009;23:307–311. doi: 10.3892/ijmm_00000132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.