Abstract
Mesenchymal stem cell extracellular vesicles attenuate pulmonary hypertension, but their ability to reverse established disease in larger animal models and the duration and mechanism(s) of their effect are unknown. We sought to determine the efficacy and mechanism of mesenchymal stem cells’ extracellular vesicles in attenuating pulmonary hypertension in rats with Sugen/hypoxia-induced pulmonary hypertension. Male rats were treated with mesenchymal stem cell extracellular vesicles or an equal volume of saline vehicle by tail vein injection before or after subcutaneous injection of Sugen 5416 and exposure to 3 weeks of hypoxia. Pulmonary hypertension was assessed by right ventricular systolic pressure, right ventricular weight to left ventricle + septum weight, and muscularization of peripheral pulmonary vessels. Immunohistochemistry was used to measure macrophage activation state and recruitment to lung. Mesenchymal stem cell extracellular vesicles injected before or after induction of pulmonary hypertension normalized right ventricular pressure and reduced right ventricular hypertrophy and muscularization of peripheral pulmonary vessels. The effect was consistent over a range of doses and dosing intervals and was associated with lower numbers of lung macrophages, a higher ratio of alternatively to classically activated macrophages (M2/M1 = 2.00 ± 0.14 vs. 1.09 ± 0.11; P < 0.01), and increased numbers of peripheral blood vessels (11.8 ± 0.66 vs. 6.9 ± 0.57 vessels per field; P < 0.001). Mesenchymal stem cell extracellular vesicles are effective at preventing and reversing pulmonary hypertension in Sugen/hypoxia pulmonary hypertension and may offer a new approach for the treatment of pulmonary arterial hypertension.
Keywords: pulmonary vascular remodeling, right ventricular hypertrophy, exosome, macrophage, microRNA
Clinical Relevance
Mesenchymal stem cell extracellular vesicles reversed pulmonary hypertension and improved vascular remodeling in the rat Sugen/hypoxia model. These findings were associated with a reduction in lung macrophages and an increase in the ratio of macrophages activated via the alternative versus the classical pathway (M2/M1). These findings suggest that, on the basis of their immunomodulatory effect on macrophages, mesenchymal stem cell extracellular vesicles may be a new approach for treating pulmonary arterial hypertension.
Pulmonary arterial hypertension (PAH) is a rare disease characterized by loss of peripheral pulmonary vessels and the development of an obliterative vasculopathy in distal pulmonary arterioles (1). These changes lead to progressive increases in pulmonary vascular resistance, elevation of pulmonary arterial pressure, and right heart failure. Current therapies achieve modest improvements in pulmonary hemodynamics, functional capacity, and time to clinical deterioration, but they have little effect on reversing the underlying disease process. As a result, the disease remains incurable, and most patients progress to lung transplant or death within 5 years (2, 3). Hence, there is a pressing need to develop new therapies that target the underlying pathogenesis of this devastating disease.
Mesenchymal stem cells (MSC) have broad antiinflammatory and regenerative properties that have been used in an attempt to improve organ function in a variety of conditions. Several studies have examined their ability to attenuate pulmonary hypertension in preclinical models of the disease (4–9). However, there is little evidence that MSC replace injured cells in the pulmonary circulation. Instead, MSC appear to act in a paracrine fashion to modulate the vascular remodeling effects of other vascular cells. More recently, it has become apparent that many of the antiinflammatory properties of MSC may be mediated by the release of extracellular vesicles (EV). EV are microscopic spheres that consist of a cell membrane encompassing a variety of cytoplasmic proteins, RNA species, and mitochondria (10). EV are secreted by numerous cell types and are increasingly recognized as an important vehicle for cell-to-cell communications (11–13). MSC-EV have broad immunomodulatory effects, especially on macrophages, where they have been shown to shift macrophage activation from the classical inflammatory pathway (M1) to the alternative antiinflammatory pathway (M2) and to decrease secretions of inflammatory cytokines (14–16). Previous studies have shown beneficial effects of MSC-EV in rodent models of pulmonary hypertension and bronchopulmonary dysplasia (17, 18). However, the optimal dose and frequency of administration are not known. Furthermore, the mechanisms responsible for their potential beneficial effects are not well understood. In the present study, we examined the ability of MSC-EV to blunt and reverse pulmonary hypertension in rats treated with Sugen/hypoxia (Su/Hx), a preclinical model of pulmonary hypertension that produces severe elevation of pulmonary artery pressure, right ventricular hypertrophy, and vascular wall thickening (19). We also examined the efficacy of multiple dosing regiments and the effect of MSC-EV on macrophage activation and vascular remodeling.
Methods
Su/Hx Pulmonary Hypertension
Male Sprague Dawley rats (Charles River Laboratories) weighing 180–225 g received injections with the VEGF (vascular endothelial growth factor) receptor 2 antagonist Sugen-5416 (SU5416; Tocris Bioscience) at a dose of 25 mg/kg subcutaneously and placed in hypoxic chambers (10.5% oxygen) for 3 weeks followed by 1, 2, or 3 weeks of normoxic recovery. Normoxic control rats received injections with an equal volume of DMSO vehicle, were kept under normoxic conditions, and received injections of PBS at the same time points that Su/Hx rats received MSC-EV.
Study Protocols
In the disease prevention protocol, rats were administered 100 μg/kg of MSC-EV protein in 500 μl of PBS or 500 μl of PBS alone by tail vein injection once daily for 3 days starting the day rats were administered injections with SU5416. Rats were killed, and pulmonary hypertension was assessed 1 week after removal from hypoxia. In the disease reversal protocols, rats were administered injections with MSC-EV or PBS vehicle once daily for 3 days starting the day after removal from hypoxia. Pulmonary hypertension was assessed 1, 2, or 3 weeks later. In a separate reversal protocol, MSC-EV or PBS vehicle was administered once daily every 5 days starting the day after removal from hypoxia, and pulmonary hypertension was assessed 2 weeks after the first dose. In the dosing protocol, rats were treated with 5, 20, or 100 μg/kg of MSC-EV or vehicle alone for 3 days starting the day after removal from hypoxia, and pulmonary hypertension was assessed 1 week later.
For assessment of pulmonary hypertension, rats were anesthetized with isoflurane, and right ventricular systolic pressure (RVSP) was measured using a Millar catheter (ADInstruments Inc.) inserted into the right ventricle (RV) via the right internal jugular vein. Rats were then killed by exsanguination, and the heart and lungs were removed. RV hypertrophy was assessed by RV to left ventricle (LV) + septum wet weight ratio (RV/LV + S). Lungs were fixed in formalin, embedded in paraffin, and sectioned for hematoxylin and eosin or immunohistochemical staining and analysis. All studies were approved by the Rhode Island Hospital Institutional Animal Care and Use Committee.
To reduce the total number of rats studied, data for the normoxic control and Su/Hx + PBS groups were pooled from the prevention protocol and reversal protocol 1 and from reversal protocols 2 and 4, because rats were killed and assessed for pulmonary hypertension at the same time intervals.
MSC and Lung Fibroblast EV
Human MSC (Lonza) were grown in T-175 flasks as per the manufacturer’s instructions using EV-free Eagle’s minimum essential medium and FBS. Human fibroblasts were cultured in Lonza Fibroblast Growth Basal Medium supplemented with insulin, hFGF (human fibroblast growth factor), and 2% FBS. MSC-EV and fibroblast extracellular vesicles (FL-EV) were isolated at passage 5 as described previously (20, 21). Briefly, the cells were cultured for 3 weeks in T-175 tissue culture flasks and passaged five times. When the desired number of flasks reached 80% confluency, the flasks were rinsed with PBS and fed 20 ml of serum-free RPMI 160 medium. After 24 hours, the media were harvested and centrifuged for 10 minutes at 300 × g to remove cellular debris. The supernatant was ultracentrifuged at 100,000 × g for 1 hour. The pellet was resuspended in PBS containing 1% DMSO. A typical MSC-EV preparation came from 178 flasks with 1 × 106 cells per flask. The EV were collected twice over the course of 4 days. The yield for the two collections would typically be ∼7 × 1011 EV as quantified by nanoparticle tracking analysis. The total protein content would be ∼900 μg as measured by using a Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific) yielding a concentration of ∼8 × 108 EV per microgram of protein. EV were then characterized by EM, Western blotting (Figure E1 in the data supplement), and NanoSight (Malvern Panalytical) analysis (Figure E2), as described by us previously (21).
EM Sample Preparation and Imaging
MSC-EV pellets were resuspended in 5 μl of PBS and deposited on formvar/carbon-coated nickel grids so that EV could adsorb. The grids were rinsed in PBS, fixed with 1% glutaraldehyde, and rinsed in distilled water eight times. Grids were transferred to a uranyl oxalate solution, pH 7, for 5 minutes. The samples were then contrasted with a 4% uranyl acetate/2% methyl cellulose solution for 10 minutes on ice. Grids were then removed from this solution with a stainless steel loop and blotted on Whatman no. 1 filter paper (GE Healthcare Life Sciences) to remove excess fluid. Samples were examined using a Philips 410 Transmission Electron Microscope (Philips) equipped with an Advantage high-resolution charge-coupled device camera from Advanced Microscopy Techniques. Images were acquired using Advanced Microscopy Techniques imaging software.
Histologic Analysis of Lung Sections
Lungs were removed en bloc, and the trachea and main pulmonary artery were cannulated. The airways were infused with 3 ml of 4% paraformaldehyde in PBS, and the pulmonary circulation was flushed with PBS. Both injections were performed at a constant pressure of 20 cm H2O. Lungs were kept in 4% paraformaldehyde until embedded in paraffin; sectioned in 5-μm slices; and stained with antibody against rat ACTA2 (ab5694; Abcam) to assess pulmonary vascular remodeling, antibody against rat von Willebrand factor (vWF) (ab6994; Abcam) to assess distal vessel count, and antibodies against rat Cd206 (ab64693; Abcam) or Cd68 (ED1; Bio-Rad Laboratories) for assessment of macrophage recruitment. Slides were then incubated with the EnVision+ Dual Link System horseradish peroxidase solution (Agilent Technologies) containing antirabbit immunoglobulins conjugated to peroxidase-labeled polymer. After chromogenic development, the slides were counterstained with hematoxylin. Images of the staining were then taken by using a Nikon Eclipse E800 microscope (Nikon Instruments Inc.) equipped with a camera and SPOT Advanced 4.7 software (Diagnostic Instruments Inc.). Muscularization of vessels ≤50 μm was assessed by muscularization index, defined as the total area of the vessel that stained positive for ACTA2 divided by total cross-sectional area of the vessel. The NIH ImageJ program was used to assess vessel areas.
Macrophage In Vitro Polarization
Human primary bone marrow mononuclear cells were purchased from ATCC and cultured in Iscove’s modified Dulbecco’s medium containing 20% FBS supplemented with 50 ng/ml of M-CSF (macrophage colony-stimulating factor) for 7 days. The human primary bone marrow mononuclear cells were first stimulated with 100 nM PMA for 2 days and then polarized with LPS (20 ng/ml) and IFN-γ (20 ng/ml) for M1 macrophages in the presence or absence of MSC-EV (concentrations of 0, 2, 5, and 10 μg/ml) for 2 days. After the treatment, cells were washed and stained with antibodies against human Cd64-phycoerythrin and Cd68-APC–cyanine 7 (both from BioLegend). After flow cytometric analysis on a four-laser LSR-II (BD Biosciences), the percentage of Cd64+Cd68+ M1-polarized macrophages was determined.
RNA was isolated from EV obtained from human MSC or human lung fibroblasts. MicroRNA (miRNA, miR) concentrations were quantitated by TaqMan quantitative PCR directed against card A targeting 380 miRs (Thermo Fisher Scientific). miR expression was normalized relative to the geometric mean.
Statistical Analysis
Data are shown as mean ± SEM. Differences between groups were calculated by unpaired two-tailed Student’s t test or one-way ANOVA when comparing more than two groups using GraphPad Prism version 6.03 software (GraphPad Software, Inc.). For analysis of miR concentrations, significantly expressed miRNAs with average thermocycle values <35 were selected and normalized by geometric mean. Underdetermined thermocycle values were set to 40. Significantly differentially expressed miRNAs were selected by performing a t test. P < 0.05 was considered to be statistically significant.
Results
Effect of MSC-EV on Preventing Pulmonary Hypertension
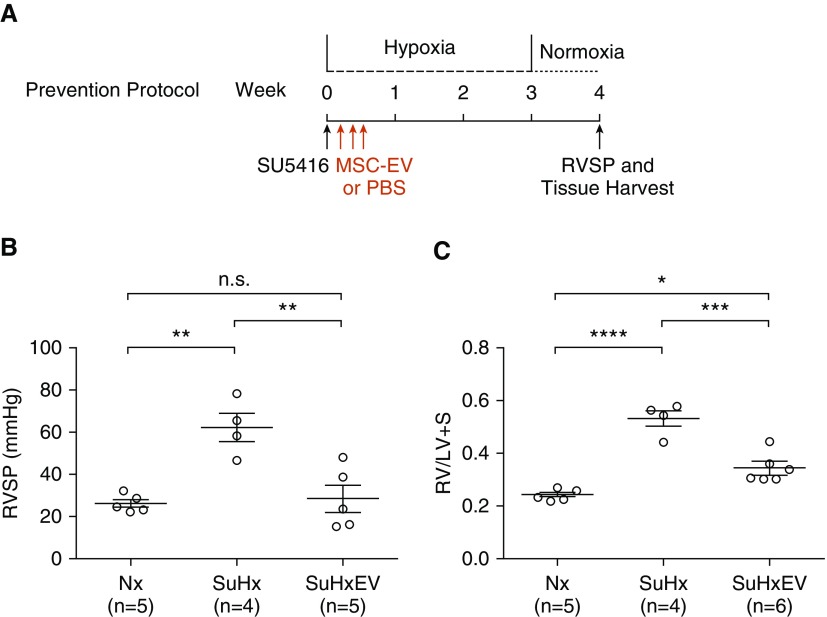
We first established the Su/Hx model of pulmonary hypertension in rats. Compared with control rats, Su/Hx caused severe pulmonary hypertension, as evidenced by marked increase in RVSP (62.2 ± 6.6 vs. 26.5 ± 1.8 mm Hg; P < 0.01), marked increase in RV hypertrophy as indicated by RV/LV + S ratio (0.532 ± 0.030 vs. 0.242 ± 0.009; P < 0.001), and striking vascular remodeling in the lung (Figure E3). In our prevention experiments (Figure 1A), Su/Hx rats treated with MSC-EV had lower RVSP (28.7 ± 6.5 vs. 62.2 ± 6.6 mm Hg; P < 0.01) (Figure 1B) and lower RV/LV + S (0.345 ± 0.022 vs. 0.532 ± 0.030; P < 0.01) (Figure 1C) than Su/Hx rats treated with PBS. RVSP in Su/Hx rats treated with MSC-EV was no different from RVSP in normoxic control rats (28.7 ± 6.5 vs. 26.5 ± 1.8 mm Hg; P = not significant [NS]) (Figure 1B).
Figure 1.
(A) Experimental protocol for prevention of pulmonary hypertension showing timing of administration of mesenchymal stem cell extracellular vesicles (MSC-EV) or PBS vehicle. (B and C) Right ventricular systolic pressure (RVSP) (B) and right ventricle to left ventricle + septum ratio (RV/LV + S) (C) were measured 1 week after removal from 3 weeks of hypoxia. n.s. = not significant; Nx = normoxic control; SuHx = rats treated with Sugen/hypoxia and PBS; SuHxEV = rats treated with Sugen/hypoxia and MSC-EV. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Effect of MSC-EV on Reversing Established Pulmonary Hypertension
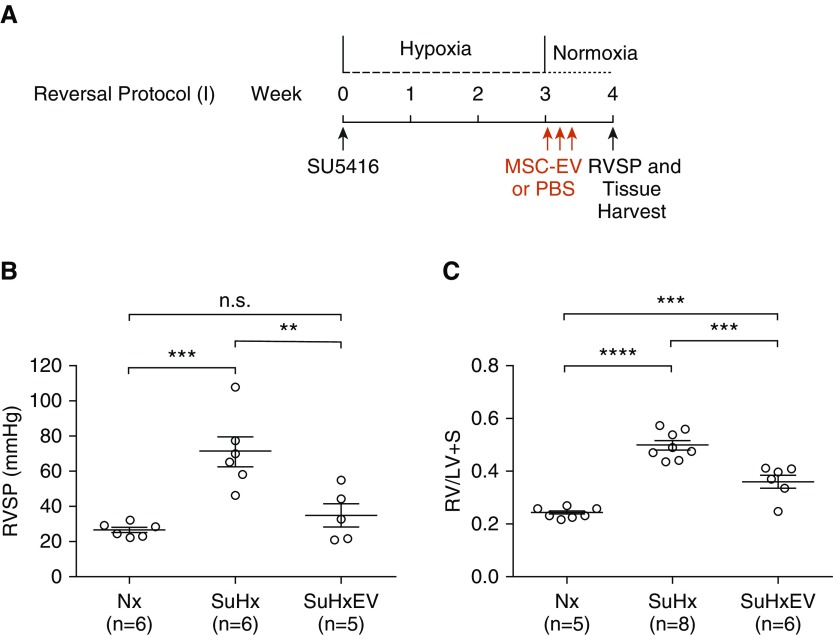
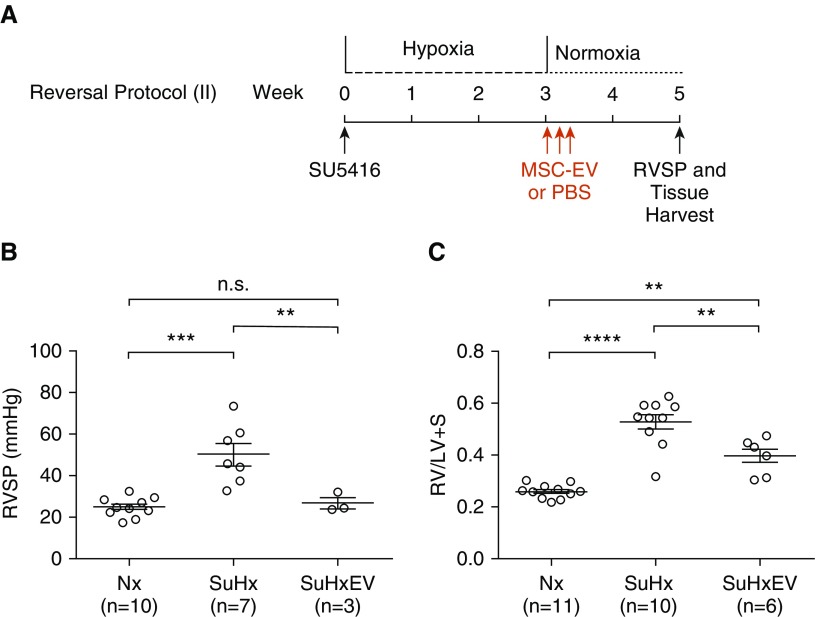
Administration of MSC-EV after 3 weeks of Su/Hx when pulmonary hypertension was already established significantly reduced RV pressure and hypertrophy. One week after removal from hypoxia (reversal protocol 1) (Figure 2A), Su/Hx rats treated with MSC-EV had lower RVSP (35.3 ± 6.6 vs. 71.3 ± 8.6 mm Hg; P < 0.01) (Figure 2B) and RV/LV + S (0.365 ± 0.026 vs. 0.501 ± 0.019; P < 0.001) (Figure 2C) than Su/Hx rats administered PBS alone. In additional experiments, rats were treated with MSC-EV or vehicle alone once daily for 3 days after 3 weeks of Su/Hx as above, but severity of pulmonary hypertension was assessed 2 weeks (reversal protocol 2) and 3 weeks (reversal protocol 3) after treatment. Two weeks after treatment (Figure 3A), RVSP and RV/LV + S were both lower in rats treated with MSC-EV than in rats administered PBS vehicle alone (RVSP, 26.9 ± 2.8 vs. 50.2 ± 5.3 mm Hg; P < 0.01; RV/LV + S, 0.396 ± 0.029 vs. 0.529 ± 0.029; P < 0.01) (Figures 3B and 3C). Three weeks after treatment (Figure 4A), RVSP was significantly reduced in MSC-EV–treated versus PBS-treated rats (30.8 ± 5.8 vs. 67.1 ± 3.5 mm Hg; P < 0.001) (Figure 4B), but the difference in RV/LV + S was no longer statistically significant (0.365 ± 0.029 vs. 0.451 ± 0.045; P = NS) (Figure 4C).
Figure 2.
(A) Experimental protocol for reversal of pulmonary hypertension showing timing of administration of MSC-EV or PBS vehicle. (B and C) RVSP (B) and RV/LV + S (C) were measured 1 week after removal from 3 weeks of hypoxia. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Figure 3.
(A) Experimental protocol for reversal of pulmonary hypertension showing timing of administration of MSC-EV or PBS vehicle. (B and C) RVSP (B) and RV/LV + S (C) were measured 2 weeks after removal from 3 weeks of hypoxia. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Figure 4.
(A) Experimental protocol for reversal of pulmonary hypertension showing timing of administration of MSC-EV or PBS vehicle. (B and C) RVSP (B) and RV/LV + S (C) were measured 3 weeks after removal from 3 weeks of hypoxia. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
In further studies, rats received injections with MSC-EV or PBS vehicle once daily every 5 days starting the day after removal from Su/Hx, and pulmonary hypertension was assessed after 2 weeks in normoxia (reversal protocol 4) (Figure 5A). In these studies, we also examined the effect of MSC-EV on rats without pulmonary hypertension. As in the other studies, RVSP and RV/LV + S were significantly lower in Su/Hx rats treated with MSC-EV than in Su/Hx rats that received vehicle alone (RVSP, 22.5 ± 2.7 vs. 50.2 ± 5.3 mm Hg; P < 0.001; RV/LV + S, 0.413 ± 0.021 vs. 0.529 ± 0.029; P < 0.05) (Figures 5B and 5C). MSC-EV had no effect on RVSP (25.4 ± 4.6 mm Hg) or RV/LV + S (0.274 ± 0.003) in two rats not treated with Su/Hx (Figures 5B and 5C). In all five of the study protocols above, RVSP in rats treated with MSC-EV was not different from RVSP in normoxic control rats.
Figure 5.
(A) Experimental protocol for reversal of pulmonary hypertension showing timing of administration of MSC-EV or PBS vehicle. (B and C) RVSP (B) and RV/LV + S (C) were measured 2 weeks after removal from 3 weeks of hypoxia and treatment with MSC-EV or PBS once daily every 5 days. NxEV = normoxic control rats treated with MSC-EV. *P < 0.05, ***P < 0.001, and ****P < 0.0001.
To determine if the ability of MSC-EV to reverse Su/Hx-induced pulmonary hypertension was unique to EV isolated from MSC, we performed additional experiments using FL-EV. Rats were treated with Su/Hx, exposed to 3 weeks of hypoxia as described above, and then treated with daily injection of MSC-EV, FL-EV, or PBS alone once daily for 3 days and killed after Week 4 (Figure 6A). As in the other studies described above, administration of MSC-EV returned RVSP to baseline levels (Figure 6B) and markedly reduced right ventricular hypertrophy as assessed by RV/LV + S ratio (Figure 6C). However, in rats treated with FL-EV, no differences were seen in RVSP (Figure 6B) or RV/LV + S compared with baseline (Figure 6C). Both RVSP and RV/LV + S were significantly lower in rats treated with MSC-EV than in rats treated with FL-EV or PBS (Figures 6B and 6C).
Figure 6.
(A) Experimental protocol for reversal of pulmonary hypertension showing timing of administration of MSC-EV, fibroblast extracellular vesicles (FL-EV), or PBS vehicle. (B and C) RVSP (B) and RV/LV + S (C) were measured 1 week after removal from 3 weeks of hypoxia. ***P < 0.001 and ****P < 0.0001.
Effect of MSC-EV Dose on Reversing Pulmonary Hypertension
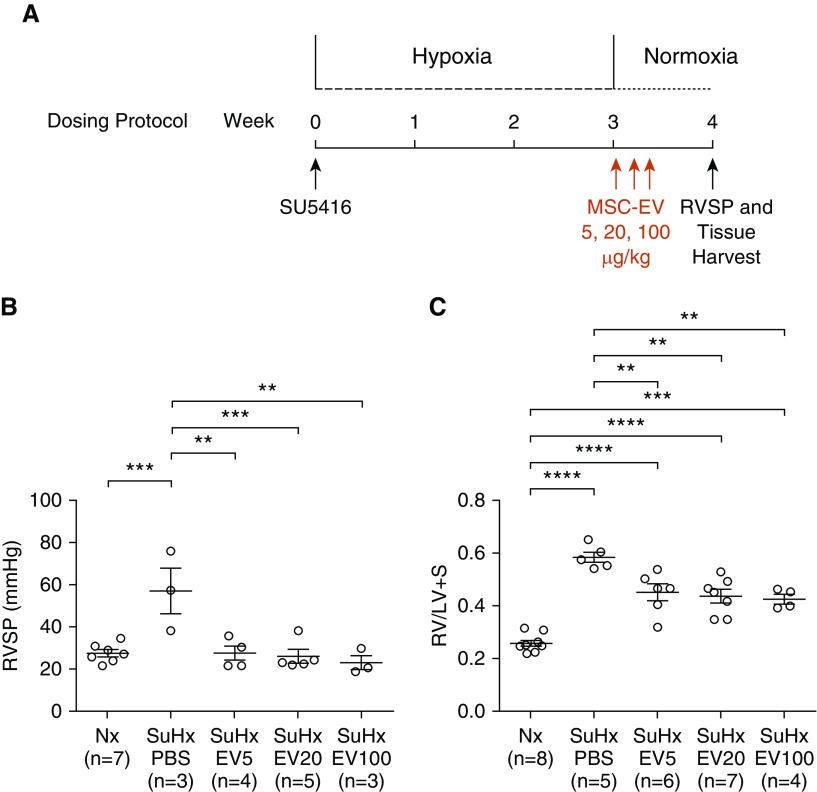
To assess dose response, additional experiments were conducted in which rats were administered MSC-EV for 3 days consecutively starting the day after removal from Su/Hx, and pulmonary hypertension was assessed 1 week later (Figure 7A). As in the other studies, treatment with 100, 20, or 5 μg/kg MSC-EV returned RVSP to baseline (23.0 ± 3.4, 26.0 ± 3.1, and 27.4 ± 3.5 mm Hg, respectively; P < 0.01 for all values; vs. Su/Hx-PBS, 57.2 ± 10.9 mm Hg) and significantly reduced RV/LV + S (0.427 ± 0.18, 0.437 ± 0.026, and 0.453 ± 0.033, respectively; P < 0.01 for all values vs. Su/Hx-PBS, 0.586 ± 0.020) (Figures 7B and 7C).
Figure 7.
(A) Experimental protocol for reversal of pulmonary hypertension with decreasing doses of MSC-EV. (B and C) RVSP (B) and RV/LV + S (C) were measured 1 week after removal from 3 weeks of SuHx. Rats were treated with 5, 20, or 100 μg/kg MSC-EV (EV5, EV20, and EV100, respectively) or an equal volume of PBS vehicle alone once daily for 3 days beginning the day after removal from hypoxia. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Effect of MSC-EV on Reversing Pulmonary Vascular Remodeling
Treatment with Su/Hx caused marked remodeling of peripheral pulmonary vessels, as demonstrated by a near doubling of the muscularization index (0.73 ± 0.03 vs. 0.39 ± 0.07; P < 0.001) (Figures 8A, 8B, and 8E). Treatment with MSC-EV either before or after Su/Hx-induced pulmonary hypertension was established blunted the increase in muscularization index (0.48 ± 0.03 and 0.52 ± 0.03 vs. 0.73 ± 0.01, respectively; P < 0.001 for both) (Figures 8C–8E and E4).
Figure 8.
(A–D) Smooth muscle layer of peripheral pulmonary vessels stained with an antibody against ACTA2 from rats treated with normoxia (A), SuHx plus vehicle (B), SuHx plus MSC-EV in the pulmonary hypertension prevention protocol (C), and SuHx plus MSC-EV in the 1-week reversal protocol (D). Scale bars: 10 μm. (E) Muscularization of vessels ≤50 μm was assessed by muscularization index, defined as the total area of the vessel that stained positive for ACTA2 divided by total cross-sectional area of the vessel. The National Institutes of Health (NIH) ImageJ program was used to assess vessel areas. EV-Prev = rats treated with SuHx and MSC-EV in the prevention protocol; EV-Revs = rats treated with SuHx and MSC-EV in the reversal protocol. *P < 0.05, ***P < 0.001, and ****P < 0.0001.
Effect of MSC-EV on Macrophage Activation and Pulmonary Vascular Angiogenesis
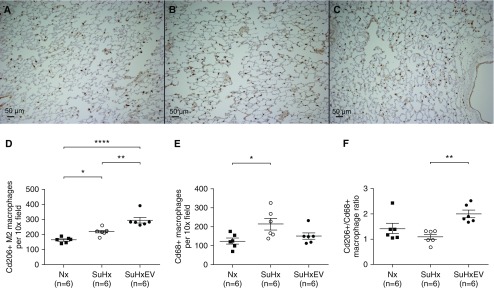
On the one hand, compared with normoxic lungs (Figure 9A), immunohistochemical staining showed that the number of M2 macrophages (Cd206+ cells in brown dots) was increased in Su/Hx rats (Figures 9B and 9D) and was even greater in Su/Hx rats treated with MSC-EV than in those administered PBS (Figures 9C and 9D). On the other hand, compared with normoxic control rats, Su/Hx nearly doubled the number of lung macrophages (Cd68+ cells) in lung sections obtained from rats in the study protocols described above (Figure 9E). However, no increase in lung macrophages was seen in Su/Hx rats treated with MSC-EV (Figure 9E). As a result, the ratio of M2 macrophages to total macrophages (Cd206+/Cd68+ cells) was significantly higher in rats administered MSC-EV, suggesting an increase in the number of macrophages activated via the alternative (M2) pathway (Figure 9F). To confirm our observations on the increase of Cd206+ M2 macrophages, we also used arginase 1 (Arg-1) as another marker to track M2 activation in the lungs. As shown in Figure E5, there was no difference in the number of Arg-1+ cells (brown spots) between normoxic and Su/Hx-treated lungs. However, significantly more Arg-1+ cells could be seen in the lungs of Su/Hx rats treated with MSC-EV than in Nx or Su/Hx rats treated with PBS.
Figure 9.
(A) Immunohistochemical staining of Cd206+ macrophages in lungs of rats treated with normoxia, (B and C) SuHx plus vehicle (B), and SuHx plus MSC-EV (C). Scale bars: 50 μm. (D–F) Average number of Cd206+ per 10× field (D), Cd68+ total rat macrophages per 10× field (E), and ratio of Cd206+/Cd68+ macrophages per 10× lens objective field of view (F) in lung sections from rats treated with Nx, SuHx plus vehicle, or SuHxEV. *P < 0.05, **P < 0.01, and ****P < 0.0001.
Furthermore, we examined the effect of MSC-EV on macrophage activation in vitro. Human bone marrow–derived monocytes were first treated with PMA and then stimulated with LPS and IFN-γ for M1 macrophage polarization in the presence and absence of a series concentrations of MSC-EV. Human monocyte differentiation into macrophages and activation via the classical (M1) pathway were assessed by measuring expression of surface markers Cd64 and Cd68 with flow cytometry. Compared with untreated cells, there was an up to 34% reduction in the number of macrophages that were activated via the M1 pathway (Figure E6). To examine the effect of MSC-EV on pulmonary vascular angiogenesis, lung sections were stained with antibodies against rat vWF, and the number of vessels <50 μm in diameter were counted. No difference in the number of blood vessels was seen between normoxic rats and Su/Hx rats treated with PBS vehicle (Figures 10A, 10B, and 10D). However, rats treated with MSC-EV either before (EV-Prevention; Figure 10C) or after (EV-Reversal) treatment with Su/Hx had a significantly greater number of peripheral pulmonary vessels than normoxic rats or Su/Hx rats treated with PBS alone (Figure 10D). Furthermore, we also counted the number of vWF+ distal vessels in the lungs of normoxic rats treated with MSC-EV. There was no difference in the vessel numbers between normoxic lungs and normoxic lungs treated with MSC-EV (Figure E7).
Figure 10.
(A–C) Immunohistochemical staining for rat von Willebrand factor (vWF) in peripheral pulmonary vessels from rats treated with Nx (A), SuHx plus vehicle (B), and SuHx plus MSC-EV (C). Scale bars: 50 μm. The arrows indicate small pulmonary vessels. (D) Average number of peripheral pulmonary vessels ≤50 μm per 10× lens objective field of view in lungs of rats treated with Nx, SuHx and PBS, EV-Prev, and EV-Revs. **P < 0.01, ***P < 0.001, and ****P < 0.0001.
miRNAs Are Differentially Expressed
The miR content of MSC-EV was quantitated and compared with that of EV isolated from human lung fibroblasts that had no effect on pulmonary hypertension (16). EV were isolated from three separate preparations of MSC and fibroblasts. We found eight miRs that were consistently increased or decreased at least twofold more in MSC-EV than in fibroblast EV (Table 1).
Table 1.
MicroRNA Expression in Extracellular Vesicles Isolated from Human Mesenchymal Stem Cells and Lung Fibroblasts
| miRNA | MSCEV 2207 | MSCEV 2216 | MSCEV 718 | FLEV 001 | FLEV 0853 | FLEV 8197 | P Value by t Test | Fold Increase |
|---|---|---|---|---|---|---|---|---|
| hsa-miR-196b-5p | 0.368 | 1.502 | 0.346 | 11.602 | 12.051 | 8.409 | 0.001 | 988 |
| hsa-miR-615-3p | −0.843 | 0.306 | 3.949 | 11.602 | 6.559 | 8.409 | 0.020 | 210 |
| hsa-miR-452-5p | 1.136 | 1.944 | 2.221 | 5.192 | 4.956 | 8.409 | 0.019 | 21 |
| hsa-miR-224-5p | −2.162 | −1.563 | −0.795 | 0.052 | 2.969 | 1.392 | 0.033 | 7.8 |
| hsa-let-7e-5p | −2.708 | −0.932 | −2.139 | 0.091 | 0.313 | −0.597 | 0.034 | 3.6 |
| hsa-miR-127-5p | −0.150 | −0.499 | 0.009 | 2.100 | 1.345 | 1.414 | 0.003 | 3.6 |
| hsa-miR-23a-3p | −4.576 | −5.383 | −5.156 | −6.130 | −6.183 | −5.849 | 0.018 | 0.50 |
| hsa-miR-744-5p | −1.189 | −0.344 | −0.113 | −1.895 | −1.484 | −2.064 | 0.027 | 0.42 |
| hsa-miR-33b-5p | 8.980 | 8.537 | 9.046 | 5.984 | 5.064 | 1.149 | 0.033 | 0.04 |
Definition of abbreviations: FLEV = lung fibroblast extracellular vesicle; miR = microRNA; MSCEV = mesenchymal stem cell extracellular vesicle.
Normalized number of cycles for select miRs in MSCEV and FLEV.
Discussion
In the present study, MSC-EV were highly effective at both preventing and reversing pulmonary hypertension in the rat Su/Hx model. These findings confirm earlier reports that MSC or their secretome attenuates pulmonary hypertensive responses in multiple animal models of pulmonary hypertension (17, 20, 22), and they extend this observation to the Su/Hx model of pulmonary hypertension in rats. Although the lack of an ideal animal model for PAH continues to impede progress in pulmonary vascular research (23, 24), the rat Su/Hx model has gained popularity for investigating novel therapeutic targets for PAH because it employs a double-hit injury of impaired VEGF signaling plus hypoxia and induces severe increases in pulmonary artery pressure, right ventricular hypertrophy, and pulmonary vascular wall thickness that more closely resemble human disease (19).
In the present study, we show that MSC-EV are able to return RVSP to baseline levels even when administered 3 weeks after exposure to Su/Hx at a time when pulmonary hypertension is already established. Furthermore, we found that MSC-EV were also effective at reversing RV hypertrophy and muscularization of peripheral pulmonary arteries. The effect of MSC-EV persisted for at least 3 weeks after administration and could be achieved with three injections administered on consecutive days at doses of 5, 20, or 100 μg/kg or by once-daily dosing at 100 μg/kg given 5 days apart. These multiple experiments with varying doses and administration schedules demonstrated a consistent and reproducible effect of MSC-EV on attenuating pulmonary hypertension and suggest that MSC-EV may be a promising new approach to treating PAH.
Establishing the minimally effective dose and dosing frequency is crucial for the advancement of MSC-EV as a potential treatment for human PAH. Previously, we found that MSC-EV were effective at reversing monocrotaline-induced pulmonary hypertension in mice at a dose of ∼1,000 μg/kg administered once daily for three consecutive days. In the present study, MSC-EV were equally effective at reversing pulmonary hypertension in a more severe model of pulmonary hypertension at considerably lower doses and were just as effective administered once every 5 days or once daily on three consecutive days. Previous studies of MSC and MSC-EV in other models of pulmonary hypertension have examined a single time point after MSC-EV administration, usually 1–2 weeks after removing animals from the stimulus for inducing pulmonary hypertension. In the present study, we observed no significant attenuation of effect on reducing RVSP to baseline levels between rats studied 1, 2, or 3 weeks after treatment, although the reduction in RV/LV + S at 3 weeks after treatment did not quite reach statistical significance (Figure 4C). Together, the above findings do not suggest a dose-dependent effect of MSC-EV on reversing pulmonary hypertension changes. Rather, a threshold dose of MSC-EV may be necessary to trigger the events responsible for reversing pulmonary hypertension. Further studies aimed at determining the minimally effective dose and maximum duration of effect are currently being conducted, but our present findings suggest that MSC-EV may be effective at reversing pulmonary hypertension at considerably lower doses and for longer periods of time than previously studied.
Despite the growing body of evidence supporting the efficacy of MSC-EV in attenuating pulmonary hypertension, the mechanisms responsible for this effect are unclear. MSC and their EV have broad immunomodulatory effects on multiple inflammatory cells that have been implicated in pulmonary vascular remodeling in pulmonary hypertension, including B cells, T cells, and macrophages (25, 26). In particular, MSC-EV suppress inflammatory responses in macrophages in part by directing macrophage activation toward the alternative, antiinflammatory M2 phenotype (26, 27, 28). Several studies using a variety of lung injuries have demonstrated that MSC-EV can reduce macrophage recruitment to the lung and decrease macrophage expression of proinflammatory cytokines that have been implicated in the pathogenesis of PAH, such as IL-6, MIP-2 (macrophage inflammatory protein 2), and TNF-α (15, 16, 18). This shift from an inflammatory (M1) to a reparative (M2) phenotype has been shown to limit alveolar damage in animal models of acute lung injury and to attenuate pulmonary hypertension in animal models of bronchopulmonary dysplasia (15, 16, 18).
In the present study, Su/Hx increased the number of macrophages in the lung, an effect that was attenuated by treatment with MSC-EV. MSC-EV also increased the number of M2 macrophages in the lung, resulting in an increased ratio of M2 to M1 macrophages compared with Su/Hx control rats. This finding is consistent with an earlier study showing that MSC alone can decrease macrophage recruitment to the lung and increase the number of M2 macrophages in rats with monocrotaline-induced pulmonary hypertension (7). Whether MSC-EV promote M2 activation directly or indirectly by inhibition of M1 activation is unclear, but our in vitro studies suggest the latter. Taken together, these findings suggest that the ability of MSC to blunt macrophage recruitment to the lung and promote alternative activation in pulmonary hypertension is mediated by EV. Although earlier studies have suggested that the M2 phenotype may contribute to the development of PAH (29–31), macrophages demonstrate considerable plasticity in their activation state that is heavily influenced by their microenvironment and includes phenotypes distinct from M1 or M2 that contribute to disease progression (32, 33). Considering the vital role of macrophage-mediated inflammatory responses in the pathogenesis of PAH (34), the immunomodulatory effects of MSC-EV on macrophages are likely to play a prominent role in the ability of MSC-EV to reverse the disease (35).
Some M2 macrophages known as tumor-associated macrophages have also been shown to enhance endothelial cell tube formation in vitro and to support tumor growth by facilitating angiogenesis (36, 37). Interestingly, the increased number of M2 macrophages in the lungs of rats treated with MSC-EV in the present study was associated with an increase in the number of peripheral pulmonary vessels. It is unclear if this finding resulted from stimulation of new vessel growth or inhibition of vessel loss; however, the lack of any effect of MSC-EV on vessel number in normoxic rats suggests the latter. The greater number of peripheral pulmonary vessels in the MSC-EV–treated Su/Hx rats may help explain the marked reduction in RVSP that, which was a consistent observation in all of the study protocols we used. In fact, RVSP in Su/Hx rats treated with MSC-EV was no different from rats that never received Su/Hx in all six of the study protocols. To our knowledge, MSC-EV have not been shown to have a direct vasodilating effect on vascular smooth muscle, although previous studies have demonstrated that EV isolated from rats with hypoxic pulmonary hypertension blunt pulmonary vasoconstriction in isolated pulmonary arterial rings by decreasing pulmonary vascular endothelial nitric oxide synthase expression (38).
The EV preparation used in the present study consisted of a heterogeneous population of vesicles. We omitted the 10,000 × g centrifugation step to include larger EV that contain intact mitochondria (10, 15). Up to 25% of EV isolated from MSC culture media have been found to contain mitochondria (16). These EV have been shown to outsource mitochondria that are taken up by macrophages and result in enhanced oxidative phosphorylation and an antiinflammatory macrophage phenotype with decreased secretion of inflammatory cytokines (15, 16). Other studies by researchers at our laboratory (20) and by others (17, 39) have shown that the exosome fraction of EV, made up of smaller vesicles, is also effective at reversing pulmonary hypertension. Whether both larger and smaller particles are necessary to achieve the optimum effect on reversing pulmonary hypertension in Su/Hx is not known. Additional studies comparing the relative effectiveness of EV versus exosomes may help to elucidate the mechanisms by which MSC-EV mediate their effects.
In our earlier studies, we found that MSC-EV contain a large number of miRs that are differentially expressed compared with EV derived from other cell types that do not attenuate pulmonary hypertension (20). In the present study, we also found that the miR content of MSC-EV differs considerably from that of FL-EV, which had no effect on reversing pulmonary hypertension in Su/Hx rats. For example, the relative concentration of miR-196b was hundreds-fold greater in MSC-EV than in FL-EV. The miR-196 family is a key regulator of the HOXB (homeobox protein B) family of proteins that play important roles in modulating myeloid differentiation of stem cells. In particular, miR-196b has been shown to decrease expression of HOXA5 (homeobox protein Hox-A5) (40), a key transcription factor that promotes granulocyte/monocyte hematopoiesis (41, 42), and may suppress macrophage release of inflammatory cytokines such as IL-6 and TNF-α (43). Whether miR-196b plays a role in mediating the effect of MSC-EV on reversing pulmonary hypertension in Su/Hx is unknown, but it is intriguing that HOXA5 is expressed in the lung and has been shown to be increased in patients with PAH (44). Further studies are needed to identify differences in miR profiles between MSC-EV and other EV that do not reverse pulmonary hypertension and to determine which miRs are most likely to play prominent roles in mediating the beneficial effects of MSC-EV.
In summary, the findings of the present study demonstrate that MSC-EV are effective at preventing and reversing increases in pulmonary artery pressure, right ventricular hypertrophy, and pulmonary vascular remodeling in rats with Su/Hx-induced pulmonary hypertension. Although further studies are needed to better understand the mechanism(s) responsible for these beneficial effects, our findings suggest that MSC-EV decrease macrophage recruitment to the lung, promote the alternative (M2) macrophage activation pathway, and increase vessel formation. The consistent and sustained effects observed in the Su/Hx model of pulmonary hypertension suggest that MSC-EV may be an effective new approach to treating PAH.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Paula Weston for her assistance with EM sample preparation and imaging and Dongfang Yang, Ashlee Sturtevant, and Yan Cheng for immunohistochemical staining and Western blot experiments.
Footnotes
Supported in part by grants from the National Institutes of Health (R01 HL112860, R01 HL123965 [J.R.K.], R01 DK112808, P20 GM119943 [P.J.Q.], P20 GM103652 [P.J.Q.], and T32 HL116249), the American Heart Association (Transformational Project Award 18TPA34110329), and United Therapeutics Corp.
Author Contributions: J.R.K.: Substantial contributions to study conception and design; analysis and interpretation of data; drafting, revision, and final approval of the manuscript; and agreement to be accountable for all aspects of the work. M.P., M.D.T., A.S.B., K.Q.W., M.S.D., T.B., and S.W.: Contributions to study design; acquisition, analysis, and interpretation of data; and revision and final approval of the manuscript. L.R.G., J.M.A., C.E.V., and P.J.Q.: Contributions to interpretation of data and revision and final approval of the manuscript. O.D.L.: Substantial contributions to study conception and design, data acquisition, analysis and interpretation of data, revision and final approval of the manuscript, and agreement to be accountable for all aspects of the work.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0154OC on November 13, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Survival in pulmonary arterial hypertension: a reappraisal of the NIH risk stratification equation. Eur Respir J. 2010;35:1079–1087. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(Suppl):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 5.Aliotta JM, Keaney PJ, Warburton RR, DelTatto M, Dooner MS, Passero MA, et al. Marrow cell infusion attenuates vascular remodeling in a murine model of monocrotaline-induced pulmonary hypertension. Stem Cells Dev. 2009;18:773–782. doi: 10.1089/scd.2008.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CM, Lin W, Huang LT, Chou HC. Human mesenchymal stem cells ameliorate experimental pulmonary hypertension induced by maternal inflammation and neonatal hyperoxia in rats. Oncotarget. 2017;8:82366–82375. doi: 10.18632/oncotarget.19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Mendonça L, Felix NS, Blanco NG, Da Silva JS, Ferreira TP, Abreu SC, et al. Mesenchymal stromal cell therapy reduces lung inflammation and vascular remodeling and improves hemodynamics in experimental pulmonary arterial hypertension. Stem Cell Res Ther. 2017;8:220. doi: 10.1186/s13287-017-0669-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alencar AKN, Pimentel-Coelho PM, Montes GC, da Silva MMC, Mendes LVP, Montagnoli TL, et al. Human mesenchymal stem cell therapy reverses Su5416/hypoxia-induced pulmonary arterial hypertension in mice. Front Pharmacol. 2018;9:1395. doi: 10.3389/fphar.2018.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29:99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 11.Meldolesi J. Exosomes and ectosomes in intercellular communication. Curr Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 12.Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Vorst EPC, de Jong RJ, Donners MMPC. Message in a microbottle: modulation of vascular inflammation and atherosclerosis by extracellular vesicles. Front Cardiovasc Med. 2018;5:2. doi: 10.3389/fcvm.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–1286. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–116. doi: 10.1164/rccm.201705-0925OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 20.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 2016;30:2221–2231. doi: 10.1038/leu.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Z, Liu J, Xiao M, Wang J, Yao F, Zeng W, et al. Mesenchymal stem cell-derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system. J Am Soc Hypertens. 2018;12:470–478. doi: 10.1016/j.jash.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1013–L1032. doi: 10.1152/ajplung.00217.2009. [DOI] [PubMed] [Google Scholar]
- 24.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Favaro E, Carpanetto A, Lamorte S, Fusco A, Caorsi C, Deregibus MC, et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014;57:1664–1673. doi: 10.1007/s00125-014-3262-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831–840. doi: 10.1007/s12026-016-8798-6. [DOI] [PubMed] [Google Scholar]
- 27.Henao Agudelo JS, Braga TT, Amano MT, Cenedeze MA, Cavinato RA, Peixoto-Santos AR, et al. Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front Immunol. 2017;8:881. doi: 10.3389/fimmu.2017.00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, et al. Mesenchymal stem cell-derived extracellular vesicles as mediators of anti-inflammatory effects: endorsement of macrophage polarization. Stem Cells Transl Med. 2017;6:1018–1028. doi: 10.1002/sctm.16-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation. 2011;123:1986–1995. doi: 10.1161/CIRCULATIONAHA.110.978627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amsellem V, Abid S, Poupel L, Parpaleix A, Rodero M, Gary-Bobo G, et al. Roles for the CX3CL1/CX3CR1 and CCL2/CCR2 chemokine systems in hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2017;56:597–608. doi: 10.1165/rcmb.2016-0201OC. [DOI] [PubMed] [Google Scholar]
- 31.Pullamsetti SS, Savai R. Macrophage regulation during vascular remodeling: implications for pulmonary hypertension therapy. Am J Respir Cell Mol Biol. 2017;56:556–558. doi: 10.1165/rcmb.2017-0033ED. [DOI] [PubMed] [Google Scholar]
- 32.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, et al. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006;168:659–669. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis GR, Fernandez-Gonzalez A, Reis M, Mitsialis SA, Kourembanas S. Macrophage immunomodulation: the gatekeeper for mesenchymal stem cell derived-exosomes in pulmonary arterial hypertension? Int J Mol Sci. 2018;19:2534. doi: 10.3390/ijms19092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodelja V, Müller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–493. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 37.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 38.Tual-Chalot S, Guibert C, Muller B, Savineau JP, Andriantsitohaina R, Martinez MC. Circulating microparticles from pulmonary hypertensive rats induce endothelial dysfunction. Am J Respir Crit Care Med. 2010;182:261–268. doi: 10.1164/rccm.200909-1347OC. [DOI] [PubMed] [Google Scholar]
- 39.Hogan SE, Rodriguez Salazar MP, Cheadle J, Glenn R, Medrano C, Petersen TH, et al. Mesenchymal stromal cell-derived exosomes improve mitochondrial health in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2019;316:L723–L737. doi: 10.1152/ajplung.00058.2018. [DOI] [PubMed] [Google Scholar]
- 40.Mo JS, Park YR, Chae SC. MicroRNA 196B regulates HOXA5, HOXB6 and GLTP expression levels in colorectal cancer cells. Pathol Oncol Res. 2019;25:953–959. doi: 10.1007/s12253-018-0399-3. [DOI] [PubMed] [Google Scholar]
- 41.Crooks GM, Fuller J, Petersen D, Izadi P, Malik P, Pattengale PK, et al. Constitutive HOXA5 expression inhibits erythropoiesis and increases myelopoiesis from human hematopoietic progenitors. Blood. 1999;94:519–528. [PubMed] [Google Scholar]
- 42.Fuller JF, McAdara J, Yaron Y, Sakaguchi M, Fraser JK, Gasson JC. Characterization of HOX gene expression during myelopoiesis: role of HOX A5 in lineage commitment and maturation. Blood. 1999;93:3391–3400. [PubMed] [Google Scholar]
- 43.Yuan Y, Lin D, Feng L, Huang M, Yan H, Li Y, et al. Upregulation of miR-196b-5p attenuates BCG uptake via targeting SOCS3 and activating STAT3 in macrophages from patients with long-term cigarette smoking-related active pulmonary tuberculosis. J Transl Med. 2018;16:284. doi: 10.1186/s12967-018-1654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golpon HA, Geraci MW, Moore MD, Miller HL, Miller GJ, Tuder RM, et al. HOX genes in human lung: altered expression in primary pulmonary hypertension and emphysema. Am J Pathol. 2001;158:955–966. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.