Abstract
Rationale: The use of electronic cigarettes (e-cigarettes) has rapidly increased in the United States, and thousands of e-cigarette flavors are available. However, there remains a dearth of evidence on e-cigarette flavor use patterns among older e-cigarette users.
Objectives: This longitudinal study examined changes in flavor use patterns in long-term e-cigarette users, assessed self-reported adverse reactions, and evaluated users’ anticipated reactions to possible U.S. Food and Drug Administration e-cigarette flavor regulatory scenarios.
Methods: The study population was 383 adult participants who completed two online e-cigarette surveys in 2012–2014 (baseline survey) and in 2017–2019 (follow-up survey). In both surveys, participants were asked, “Thinking about your preferred liquid, what is the name of this liquid flavor?” and to list all flavors used in the past 30 days. Flavor preference was classified using the Penn State Three-Step Flavor Classification method. Participants reported adverse events (open-ended description) with the associated flavor. Regulatory scenarios were presented, and participants selected perceived actions from among a list of 15 options.
Results: Participants’ age averaged 44 ± 12 years; 86% were exclusive e-cigarette users, and 13% reported “poly-use” (i.e., e-cigarette and other tobacco product use). E-cigarette flavor preference migration occurred in all demographic groups: only 36–44% maintained a preference for their original flavor. Preference for tobacco and menthol or mint decreased over time (40% baseline vs. 22% follow-up); preference for fruit remained stable (23% baseline and follow-up), but chocolate/candy or other sweets preference significantly increased (16% baseline vs. 29% follow-up), and other flavors increased slightly. Migration to sweet flavors was more noticeable in younger adults (18–45 yr); exclusive e-cigarette users preferred sweet flavors more commonly than poly-users did (31% vs. 19%). Flavor-associated adverse reactions, mainly respiratory irritations, were reported by 26 (6.9%) participants. Nearly 50% of the participants reported that they would “find a way” to buy their preferred flavor or add flavoring agents themselves if nontobacco flavors were banned.
Conclusions: Flavor migration toward sweet flavors occurred in long-term e-cigarette users, a trend most pronounced in younger and exclusive e-cigarette users. The anticipated maintenance of access to flavors despite regulation suggests an element of e-cigarette–related dependence that requires further evaluation. This information could help clinicians understand the health impacts of e-cigarette flavors, develop appropriate strategies for smoking cessation, and inform the U.S. Food and Drug Administration to plan future regulation of e-cigarette flavors.
Keywords: electronic cigarettes, flavors, Food and Drug Administration regulation
Electronic cigarettes (e-cigarettes) are battery-powered devices that vaporize a nicotine- or non–nicotine-containing liquid into aerosols for inhalation. Beginning in 2006, e-cigarettes were marketed as smoking cessation aids without evidence and were also used as substitutes for combustible cigarettes in smoke-free zones (1). E-cigarettes were not included in the U.S. Food and Drug Administration (FDA) regulations under the 2009 Family Smoking Prevention and Tobacco Control Act (Public Law 111-31), and they rapidly evolved and gained popularity in the United States (2). The National Health Interview Survey indicated that 15.3% of U.S. adults aged 18 years or older had used e-cigarettes at least once in their lifetime and that 3.2% of adults were using e-cigarettes on a daily basis in 2018 (3, 4).
The availability of e-cigarette flavors has also increased rapidly: About 242 new flavors were added to the market each month and more than 7,500 e-cigarettes flavors have become available since 2012 (5). In the PATH (Population Assessment of Tobacco and Health) study, a longitudinal study of tobacco use based on a national representative sample of U.S. population aged 12 years or older, 65% of young adults aged 18–24 years and 50% of adults aged 25 years and older who had ever used e-cigarettes started with flavored e-cigarettes (6). In a 2017 press release, the American Thoracic Society voiced a concern that flavored e-cigarettes were attracting people to initiate e-cigarette use (7, 8). Evidence also suggested that experimentation with flavored e-cigarettes might result in initiating combustible cigarette smoking (9). These results focused on e-cigarettes as an “on-ramp” for nonsmokers.
The rapid increase of flavored e-cigarette use has raised a great public health concern for nicotine addiction, subsequent initiation of cigarette smoking, and unforeseen adverse health effects (10). In 2016, the FDA “deeming rule” extended its authority to regulate e-cigarette products (11), but because e-cigarette products are relatively new in the United States, more evidence is needed to better understand the health impacts of e-cigarette flavors. Previous e-cigarette flavor studies mainly addressed flavor use experience in young people in cross-sectional analysis and showed a popularity of fruit flavors in the majority (9, 12). Nontobacco flavors seemed more appealing to young adults (18–29 yr) (9), but among adults aged 30 years and older or smokers who attempted to reduce or quit smoking, flavor use patterns were inconsistent across studies. In a large online survey of adult frequent e-cigarette users in the United States, use of tobacco flavors (both at e-cigarette initiation and current use) has largely decreased over time, and fruit flavor was the most commonly used flavor (13). The PATH study also indicated the changing pattern of increasing fruit flavor use in all users, but tobacco-associated flavors (tobacco and menthol or mint) were still common in adult users aged 25 years and older (14, 15).
Another group of interest is older adult smokers who become e-cigarette users, either as exclusive e-cigarette users or as poly-users (e-cigarettes and other tobacco products, mainly involving combustion cigarettes). However, flavor use is understudied in this group, and very limited longitudinal studies have been conducted to examine flavor use in older adult users (16, 17). Even in the first wave of the PATH study, only a small sample of exclusive everyday e-cigarette users (N = 156) was identified (18). Also, there is a dearth of evidence on flavor use patterns in relation to long-term e-cigarette use behaviors (13, 17, 19, 20).
From 2012 to 2014, we surveyed a large group of e-cigarette users to examine their e-cigarette use behaviors, and 1,863 participants consented to participate in future research. In 2017, we resurveyed this cohort to assess how their e-cigarette use behaviors had changed over time (21). In this study, we wanted to examine 1) changes in e-cigarette flavor use patterns (both preference and use) over time, 2) flavor preferences among different age groups, and 3) changes in flavor preference by e-cigarette use behaviors. We also asked participants to identify adverse reactions after the use of specific flavors and to anticipate how they would react to potential FDA e-cigarette flavor regulations.
Methods
Study Population and Data Collection
Our 2012–2014 baseline online e-cigarette survey included over 7,000 adult respondents who had used e-cigarettes at least 30 days in their lifetime and were aged 18 years or older (22). We recruited study participants through various online sources (e.g., WebMD [www.webmd.com] or E-Cigarette Forum [www.e-cigarette-forum.com]) and invited them to complete an online survey of e-cigarette use and other tobacco product use. At that time, a total of 1,863 survey respondents provided their contact information and agreed to participate in future research. In January 2017, we recontacted these study participants by sending a unique link to each individual’s e-mail address, inviting them to complete a follow-up survey. We administered the online follow-up survey and collected data using Penn State Research Electronic Data Capture (REDCap), which is a secure, web-based application designed to support data capture for research studies in over 600 U.S. academic institutions (23). We used an implied consent for this follow-up survey and did not offer compensation for participation. This study was approved by the Penn State College of Medicine Institutional Review Board.
Measures
Survey questions
In both the baseline and follow-up surveys, we asked participants about their typical e-cigarette use behaviors and the frequency of their e-cigarette use over the past 30 days and over the past 7 days. In the follow-up survey, we asked participants to recall their flavor use when they first initiated e-cigarette use.
To assess the participants’ preferred e-cigarette flavors, we included an open-ended question in both surveys. The baseline survey asked, “What is your favorite flavor, and what brand of flavored liquid do you prefer?” and the follow-up survey asked, “Thinking about your preferred liquid, what is the name of this liquid flavor?”
We also asked participants to report flavor-associated adverse reactions in the follow-up survey: “Have you had a bad reaction to a particular e-cig liquid flavor, including an allergic reaction?” “If yes, please describe the reaction” (open-ended question), and, “What flavor were you using at that time?” (open-ended question). Regarding FDA regulatory scenarios, we first asked participants if they believed that the FDA should regulate e-cigarettes, and then we let them select their anticipated actions from among a list of 15 options under the scenarios “if your preferred flavor were banned” and “if all nontobacco flavorings, including your preferred flavor, were banned.”
Variable definitions
On the basis of answers to the open-ended questions about preferred e-cigarette flavors at baseline and follow-up, we used the Penn State Three-step Flavor Classification method to determine the preferred flavor for each participant and categorized the flavors into the following groups: tobacco, menthol or mint, clove or spice, fruit, alcoholic drink, chocolate/candy or other sweets, beverage, and all other flavors (24). We divided the study participants into four age groups based on their age at follow-up: 18–30 years, 31–45 years, 46–60 years, and 61 years and older. We defined “current exclusive e-cigarette users” as those who had used e-cigarettes in the last 30 days and used only e-cigarettes in the past 7 days, and we defined “current poly-users” as those who had used both e-cigarettes and any other tobacco products in the past 7 days. The few participants who reported not using e-cigarettes in the past 7 days were excluded from analysis because of a small sample size.
We previously reported e-cigarette–related dependence among adult users, scaling from low to high dependency based on a validated measure, the Penn State Electronic Cigarette Dependence Index (PSECDI) (21, 22). In this study, we reassessed e-cigarette–related dependence using the PSECDI in both surveys. We calculated the PSECDI according to individuals’ responses to 10 questions referring to their typical e-cigarette use behaviors. The PSECDI ranged from 0 to 20 to indicate users’ dependence levels on their e-cigarettes (no dependence = 0–3; low dependence = 4–8; medium dependence = 9–12; and high dependence ≥ 13).
Statistical Analysis
We used the follow-up survey data retrieved in March 2019 and linked each participant’s follow-up survey data with his/her baseline survey data. We calculated means and percentages to describe participants’ sociodemographic characteristics, e-cigarette use behavior, and flavor use at baseline and follow-up. We then compared the overall changes in the preferred flavor between baseline and follow-up and changes within and across age groups. We also compared flavor preference among exclusive e-cigarette users and poly-users at baseline and follow-up. We used a two-sided, paired t test for continuous variables and the McNemar test or chi-square tests for categorical variables and accepted statistical differences at two-sided P values less than 0.05. We also used the Cochran-Armitage trend test two-sided P values to examine the differences in flavor preference across age groups at baseline and follow-up. We performed the statistical analysis using SAS version 9.4 software (SAS Institute).
Results
Characteristics of the Study Population
A total of 742 baseline survey participants answered the follow-up survey. Compared with the nonrespondents, the follow-up survey respondents had higher socioeconomic status and were more likely to report that the most important reason to use an e-cigarette was to quit smoking or avoid relapse (30.6% vs. 24.5%; P = 0.004) (see Table E1 in the online supplement). Regarding e-cigarette use behaviors, the respondents were less likely than nonrespondents to report waking at night to use e-cigarettes (8.2% vs. 16.6%; P < 0.0001) or believing it was hard to quit e-cigarettes (33.4% vs. 38.2%; P = 0.04). There was also a higher proportion of exclusive e-cigarette users among the respondents than among the nonrespondents (76.0% vs. 66.6%; P < 0.0001).
We excluded 359 participants because of missing values in flavor use behaviors, e-cigarette use behaviors, or other tobacco product use and participants with inconsistent responses in age and sex between the baseline and follow-up surveys (Figure E1). The final analytic population was 383 participants with a mean follow-up period of 3.7 years (standard deviation, 0.7; range, 2–6 yr). The majority of the study participants in both surveys were white (94.0%) and male (67.1%) and had similar education levels and employment status (Table 1). The study population at follow-up averaged 44.6 (±12.3) years old (age range, 22–75 yr).
Table 1.
Characteristics at baseline and at follow-up (N = 383)
| Characteristics | Baseline | Follow-Up | P Value* |
|---|---|---|---|
| Sociodemographics | |||
| Age, yr, mean (SD; range) | 40.8 (12.4; 18–71) | 44.6 (12.3; 22–75) | — |
| Sex, M, n (%) | 257 (67.1) | ||
| Race, white, n (%) | 360 (94.0) | ||
| Education level, college or higher, n (%) | 171 (44.7) | 164 (45.2) | 0.31 |
| Full-time employed, n (%) | 256 (66.8) | 238 (65.6) | 0.74 |
| Income >$2,500/mo, n (%) | — | 225 (62.5) | — |
| Current e-cigarette use behaviors | |||
| Exclusive e-cigarette use, n (%) | 300 (83.6) | 308 (85.8) | 0.53 |
| Poly-use (e-cigarettes + any other tobacco products), n (%) | 56 (15.6) | 47 (13.1) | 0.40 |
| Daily e-cigarette use in the past 28 d or 30 d (SD)† | 342 (89.3) | 345 (90.1) | 0.64 |
| Mean e-cigarettes use times per day (SD) | 25.1 (34.7) | 20.7 (23.1) | 0.02 |
| Mean PSECDI (SD) | 8.6 (3.4) | 8.2 (3.9) | 0.03 |
| E-cigarette flavor use behaviors | |||
| Median number of flavors ever used (interquartile range) | NA | 10 (10–50) | — |
| Number of flavors used on a regular basis | NA | ||
| One | — | 4 (1.8) | — |
| Two or three | — | 127 (57.5) | — |
| Four or more | — | 90 (40.7) | — |
| Preferred flavor | |||
| Tobacco | 102 (26.6) | 43 (11.2) | <0.0001 |
| Menthol or mint | 52 (13.6) | 42 (11.0) | 0.09 |
| Fruit | 91 (23.8) | 89 (23.2) | 0.84 |
| Chocolate/candy or other sweets | 63 (16.5) | 113 (29.5) | <0.0001 |
| Beverage | 29 (7.6) | 25 (6.5) | 0.53 |
| Clove or spice | 16 (4.2) | 24 (6.3) | 0.16 |
| Alcoholic drink | 10 (2.6) | 12 (3.1) | 0.65 |
| All other flavors | 20 (5.2) | 35 (9.1) | 0.03 |
| Having had a bad reaction to liquid flavor(s), yes vs. no | NA | 26 (6.9) | — |
| Ever used popcorn-flavored e-cigarette liquid or any e-cigarette flavor containing diacetyl | NA | 90 (23.7) | — |
| Ever used cinnamon-flavored e-cigarette liquid or any e-cigarette flavor containing cinnamaldehyde or 2-methoxicinnamaldehyde | NA | 121 (31.8) | — |
Definition of abbreviations: NA = questions were not comparable in the baseline survey; PSECDI = Penn State Electronic Cigarette Dependence Index; SD = standard deviation.
Boldface indicates statistical significance (P < 0.05).
Paired t test or McNemar test P values to examine changes in characteristics between baseline and follow-up surveys.
In the baseline survey, participants were asked to report the number of e-cigarette use days in the past 28 days; in the follow-up survey, participants were asked to report the number of e-cigarette use days in the past 30 days.
Current E-Cigarette Use Behaviors
In both the baseline and follow-up surveys, study participants reported using e-cigarettes over 95% of the days of the previous month. A majority of the study participants used e-cigarettes exclusively in the past 7 days (85.8% at follow-up), with concomitant use of e-cigarettes and other tobacco products reported by 13.1% of the study population and 1% not using e-cigarettes in the past 7 days (Table 1). The mean number of e-cigarette use times per day decreased (25.1 at baseline vs. 20.7 at follow-up; P = 0.02). The mean PSECDI scores at baseline and follow-up were similar (8.6 vs. 8.2), indicating a low to medium level of e-cigarette–related dependence.
Choices of Flavor Use at E-Cigarette Initiation and during the Past 30 Days
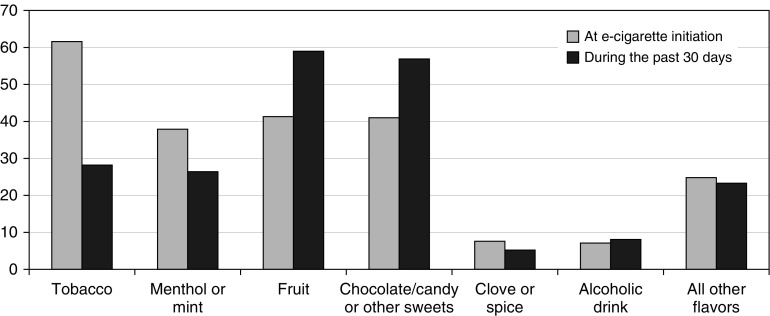
Figure 1 compares flavor uses at e-cigarette initiation and during the past 30 days before the follow-up survey. About 60% recalled using a tobacco flavor when they first started using e-cigarettes, with menthol or mint, fruit, or chocolate/candy or other sweet flavor use reported by around 40% each. The past 30 days flavor use indicated that the dominant flavor use shifted to fruit and chocolate/candy or other sweets in nearly 60% of the participants, whereas the use of tobacco, menthol, or mint dropped to 26–28%. Over 75% of study participants reported having tried at least 10 different flavors (interquartile range, 10–50), and virtually all (98.2%) used 2 or more flavors on a regular basis (Table 1).
Figure 1.
Flavors used at e-cigarette initiation and during the past 30 days. Participants were asked to recall their flavor use at e-cigarette initiation (shown in gray bar) and during the past 30 days at the follow-up survey (shown in black bar) from a list of flavors, including menthol or mint, tobacco, clove or spice, fruit, chocolate, candy or other sweets, alcoholic drink, and other flavor. Participants could report multiple flavor uses, not limited to their preferred flavor.
Preferred Flavor Migration: Baseline versus Follow-Up
At baseline, tobacco was the most preferred flavor (26.6%), followed by fruit (23.8%), chocolate/candy or other sweets (16.5%), and menthol or mint (13.6%) (Table 1). At follow-up, however, chocolate/candy or other sweets became the most preferred e-cigarette flavor group (29.5%; P < 0.0001), and the preference for tobacco flavor decreased to 11.2% (P < 0.0001). Fruit flavor remained one of the top three preferred flavors (23%). There was also a significant increase in the preference for other flavors that were not categorized or self-made (5.2 at baseline vs. 9.1 at follow-up; P = 0.03).
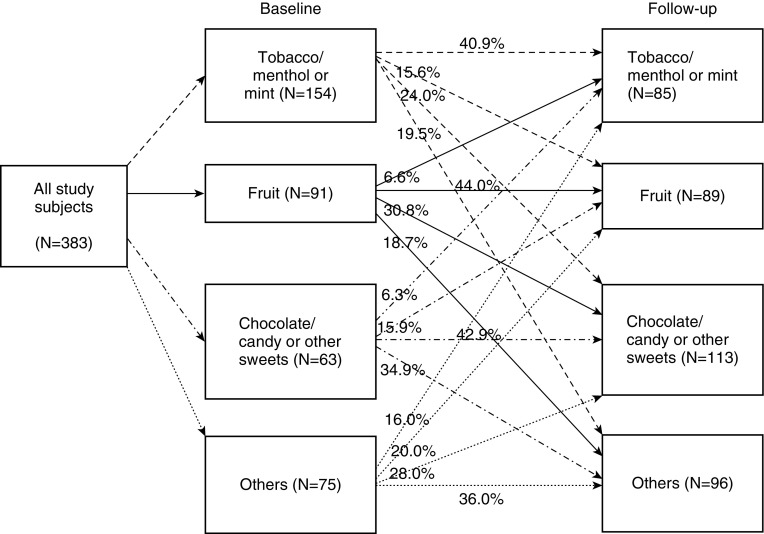
Figure 2 shows details of flavor preference migration. Across all flavor groups, about 40% of participants maintained the same preference at baseline and follow-up. The increase in fruit or chocolate/candy or other sweets preference was reflected in migration from all baseline groups. Switching to the tobacco/menthol or mint flavor was uncommon, ranging from 6% to 16%.
Figure 2.
Changes in flavor preference over time.
Age Patterns in Flavor Preference
Flavor preference was highly varied across adult age groups (Table 2). Among young adults (aged 18–30 yr), tobacco or menthol or mint preference was low at both baseline and follow-up (trend test for the linear variation of tobacco flavor across age groups, P = 0.04 at baseline and P < 0.0001 at follow-up). Young adults had the highest rates of preference for fruit flavor at baseline (trend test across age groups, P = 0.01) and maintained a high preference for fruit flavor at follow-up, but chocolate/candy or other sweets became the top preferred flavor in this group at follow-up (P = 0.04). The migration to chocolate/candy or other sweet flavors was not limited to young adults. For example, there was a striking increase in chocolate/candy or other sweets preference in the 31–45-year-old group, which showed a greater than twofold increase in preference for this flavor group (13.4% at baseline vs. 31.7% at follow-up; P < 0.0001). There was also a tendency to show an increased preference of chocolate/candy or other sweet flavors in the 46–60-year-old groups.
Table 2.
Changes in preferred flavor over time, by age group (N = 383)
| Age Group | Preferred Flavor | Baseline* (n [%]) | Follow-Up* (n [%]) | Change of Flavor Preference (%) | P Value† |
|---|---|---|---|---|---|
| 18–30 (n = 71) | Tobacco | 14 (19.7) | 4 (5.6) | −14.1% | 0.002 |
| Menthol or mint | 7 (9.9) | 8 (11.3) | 1.1% | 0.71 | |
| Fruit | 22 (31.0) | 19 (26.8) | −4.2% | 0.43 | |
| Chocolate/candy or other sweets | 15 (21.1) | 25 (35.2) | 14.1% | 0.04 | |
| 31–45 (n = 142) | Tobacco | 33 (23.2) | 8 (5.6) | −17.6% | <0.0001 |
| Menthol or mint | 23 (16.2) | 15 (10.6) | −5.6% | 0.04 | |
| Fruit | 39 (27.5) | 36 (25.4) | −2.1% | 0.65 | |
| Chocolate/candy or other sweets | 19 (13.4) | 45 (31.7) | 18.3% | <0.0001 | |
| 46–60 (n = 128) | Tobacco | 42 (32.8) | 20 (15.6) | −17.2% | 0.0002 |
| Menthol or mint | 13 (10.2) | 12 (9.4) | −0.8% | 0.84 | |
| Fruit | 24 (18.8) | 27 (21.1) | 2.3% | 0.58 | |
| Chocolate/candy or other sweets | 23 (18.0) | 34 (26.6) | 8.6% | 0.06 | |
| ≥61 (n = 42) | Tobacco | 13 (31.0) | 11 (26.2) | −4.8% | 0.48 |
| Menthol or mint | 9 (21.4) | 7 (16.7) | −4.7% | 0.16 | |
| Fruit | 6 (14.3) | 7 (16.7) | 2.4% | 0.71 | |
| Chocolate/candy or other sweets | 6 (14.3) | 9 (21.4) | 7.1% | 0.41 |
Boldface indicates statistical significance (P < 0.05).
Cochran-Armitage trend test two-sided P values to examine if flavor preference varied linearly across age groups at the baseline survey and at the follow-up survey, respectively:
Tobacco: P = 0.04 and P < 0.0001.
Menthol or mint: P = 0.46 and P = 0.65.
Fruit: P = 0.01 and P = 0.16.
Chocolate/candy or other sweets: P = 0.65 and P = 0.07.
McNemar test two-sided P values to examine changes in flavor preference between the baseline survey and the follow-up survey in each age group.
The preference of tobacco flavor decreased nearly twofold among age groups who were 60 years old or younger. Although tobacco group–associated flavor (tobacco and menthol or mint) was still the top preferred flavor among older adults (>60 yr), the prevalence was reduced by nearly 10% from 52.4% to 42.9%.
Changes in Flavor Preference by E-Cigarette Use Behaviors
We also examined whether flavor preference was related to e-cigarette use behavior (Table 3). Among current exclusive e-cigarette users, tobacco flavor preference decreased between baseline and follow-up (26.3% vs. 10.7%; P < 0.0001), whereas chocolate/candy or other sweet flavor preference increased significantly (16.9% vs. 31.2%; P < 0.0001). The preference for other flavors remained stable. Among poly-users, there was a 10% decrease in the preference of tobacco group–associated flavors (tobacco and menthol or mint) but a slight increase in other flavors, with a smaller sample size limiting statistical power.
Table 3.
Changes in preferred flavor use, by e-cigarette use behaviors at follow-up
| Preferred Flavor | Current Exclusive E-Cigarette Users (n = 308) |
Current Poly-Users (n = 47) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline (n [%]) | Follow-Up (n [%]) | P Value* | Baseline (n [%]) | Follow-Up (n [%]) | P Value* | P Value† | |
| Tobacco | 81 (26.3) | 33 (10.7) | <0.0001 | 12 (25.5) | 7 (14.9) | 0.10 | 0.40 |
| Menthol or mint | 34 (11.0) | 30 (9.7) | 0.41 | 13 (27.7) | 8 (17.0) | 0.06 | 0.13 |
| Fruit | 77 (25.0) | 74 (24.0) | 0.74 | 7 (14.9) | 8 (17.0) | 0.74 | 0.29 |
| Chocolate/candy or other sweets | 52 (16.9) | 96 (31.2) | <0.0001 | 7 (14.9) | 9 (19.2) | 0.53 | 0.09 |
| Beverage | 25 (8.1) | 22 (7.1) | 0.60 | 2 (4.3) | 3 (6.4) | 0.65 | 0.85 |
| Clove or spice | 14 (4.6) | 19 (6.2) | 0.32 | 2 (4.3) | 4 (8.5) | 0.41 | 0.54 |
| Alcoholic drink | 10 (3.3) | 8 (2.6) | 0.62 | 0 (0) | 4 (8.5) | NA | 0.04 |
| Other | 15 (4.9) | 26 (8.4) | 0.06 | 4 (8.5) | 4 (8.5) | 1.00 | 0.99 |
Definition of abbreviation: NA = Not applicable.
Boldface indicates statistical significance (P < 0.05).
McNemar test P values to examine changes in the preferred flavor between the baseline survey and the follow-up survey in current exclusive e-cigarette users or in current poly-users.
Chi-square test P values to examine differences in the preferred flavor between current exclusive e-cigarette users and current poly-users at the follow-up survey.
Possible Adverse Health Effects after E-Cigarette Flavor Use
Twenty-six (6.9%) study participants recalled ever having had a “bad reaction” to e-cigarette flavor use, including coughing/breathing problems/asthma (n = 11), mouth/throat irritation (n = 9), allergic reactions (n = 3), headache or body ache (n = 2), nausea/heartburn (n = 2), and heart problem (n = 1). When asked “What flavor were you using at that time?,” participants identified tobacco or menthol (n = 6), cinnamon (n = 5), fruit (n = 5), beverage (n = 2), other/not sure (n = 3), and high propylene glycol or vegetable glycerin (n = 5). In addition, 23.7% and 31.8% reported having ever used any e-cigarette liquid that contained either 1) popcorn flavor/diacetyl or 2) cinnamon flavor/cinnamaldehyde or 2-methoxicinnamaldehyde, respectively (Table 1). Participants who had ever used these flavors (total n = 154) were more likely than nonusers to report having had a bad reaction (13.0% vs. 2.7%; P < 0.0001).
Anticipated Reactions to Possible FDA E-Cigarette Flavor Regulatory Scenarios
Regarding potential FDA regulations, 156 (42.5%) participants believed that the FDA should regulate e-cigarettes. If their preferred flavor or all the nontobacco flavors were banned, very few participants anticipated that they would stop using e-cigarettes or would use e-cigarettes less, but about 50% reported they would “find a way to buy my preferred flavor” or “add flavoring agents myself” (Table 4). A total of 36 (9.7%) reported that “I would return to smoking traditional tobacco cigarettes” if all nontobacco flavors were banned.
Table 4.
Anticipated reactions to possible U.S. Food and Drug Administration e-cigarette flavor regulatory scenarios
| Anticipated Reactions | If Your Preferred Flavor Were Banned (n [%]) | If All Nontobacco Flavorings Were Banned (n [%]) |
|---|---|---|
| I would miss my old flavor. | 15 (4.0) | 6 (1.6) |
| I would be angry. | 53 (14.2) | 81 (21.8) |
| I would try to stop using e-cigs. | 4 (1.1) | 5 (1.3) |
| I would return to smoking traditional tobacco cigarettes. | 19 (5.1) | 36 (9.7) |
| I don’t really know what I would do. | 24 (6.4) | 10 (2.3) |
| I would use e-cigs less than I do now. | 1 (0.3) | 2 (0.5) |
| I would find a way to buy my preferred flavor. | 76 (20.4) | 71 (19.2) |
| I would continue to use e-cigs about the same as I do now. | 29 (7.8) | 22 (5.9) |
| I might try tobacco cigarettes or smokeless tobacco that comes in my preferred flavor. | 0 (0) | 0 (0) |
| I would not consider using flavored e-cig liquids other than my preferred flavor. | 9 (2.4) | 3 (0.8) |
| I would switch to another flavor. | 26 (7.0) | 13 (3.5) |
| I would be able to stop using e-cigs. | 1 (0.3) | 1 (0.3) |
| I might switch to cigars that are flavored. | 0 (0) | 0 (0) |
| I would add flavoring agents myself. | 114 (30.6) | 122 (32.8) |
| I would use e-cigs more than I do now. | 1 (0.3) | 0 (0) |
Discussion
Our longitudinal study of e-cigarette flavor use patterns conducted over 3.7 years demonstrates a striking increase in the preference for sweet flavors and a decrease in the preference for tobacco flavor in all demographic groups. The majority of the study participants had tried multiple flavors, and only about 40% still preferred the same flavor group that they had used 4 years earlier. Age-specific analysis showed that younger adults (aged 18–30 and 31–45 yr) continued to prefer fruit and chocolate/candy or other sweets, whereas over 40% of the older adults (aged ≥61 yr) still preferred traditional tobacco-related (tobacco and menthol or mint) flavors. The craving and preference for sweet tastes among adolescents and young adults have previously been reported (25); however, our study also showed a preference migration toward chocolate/candy or other sweet flavors in the older age group (46–60 yr) as well.
The majority of the study participants had tried 10 or more flavors since they started using e-cigarettes, and one-fourth of the cohort had tried 50 or more flavors. Although we did not ask whether they had tried new flavors within the previous 7 or 30 days, most people regularly used two or more flavors. As new flavors continue to be added in the marketplace, the changing preference patterns may reflect the current marketing strategies of the e-cigarette manufacturers to promote sweet flavors for the general population (not just limited to adolescents or young adults) or may reflect a user’s own experience that using non–tobacco-related flavors may constitute a flavor that they can happily use and make them less likely to return to cigarette smoking (9). In addition, in our study, there was a significant increase in the preference for chocolate/candy or other sweet flavors between baseline and follow-up among exclusive e-cigarette users, but no significant change in flavor use was found in poly-users, implying that exclusive e-cigarette users may use certain flavors to maintain their e-cigarette use behaviors (13, 19, 20).
Although e-cigarettes contain much lower concentrations of the known harmful toxicants than combustible tobacco products, they are not harmless, and although the current multistate outbreak of vaping-related lung injury in the United States appears to be caused primarily by use of illicit vaping products containing vitamin E acetate or tetrahydrocannabinol, it also indicates the dangers of inhaling unknown chemicals into the lungs (26–28). A recent comprehensive review summarized findings of in vitro studies, animal models, and population studies to describe adverse effects of e-cigarette use on the respiratory system, including the large airways and the alveolar space (29). E-cigarette use in general can cause airway and alveolar inflammation and injury, affect immune function, increase susceptibility to bacterial or viral infections, and worsen existing lung diseases such as asthma or chronic obstructive pulmonary disease, but respiratory effects of e-cigarette flavors are unclear (29, 30). Most e-cigarette flavors, including popular fruit or sweet flavors, contain chemical constituents that can be efficiently aerosolized for inhalation and cause oxidative stress, DNA damage, or pulmonary epithelial toxicity and inflammation (31–35). However, few population studies have examined chronic effects of flavor use, especially in the long term, on exclusive e-cigarette users. About 7% of our study participants reported acute adverse reactions, mainly minor respiratory irritation but also including self-reported asthma attacks, after using certain flavors or high levels of propylene glycol or vegetable glycerin in the e-liquids. About 40% of the study participants had ever used at least one flavor that contained diacetyl or cinnamon flavor/cinnamaldehyde or 2-methoxicinnamaldehyde, and they were also more likely to report having a “bad reaction.” These results suggest flavor-specific adverse health effects and support enhancing the existing adverse experiences reporting system to collect detailed information about e-cigarette flavor use (36). Given the increasing popularity of e-cigarettes, clinicians may need to include e-cigarette use or exposure history (similar to smoking history) in the risk assessment when caring for patients with respiratory symptoms (37). The American Thoracic Society also strongly encourages healthcare professionals and the general public to be educated about e-cigarette–associated respiratory distress (38). Attention to long-term e-cigarette users is warranted because of the potential cumulative toxicities over a longer lifetime of use. In addition, further prospective studies with larger sample sizes are needed to evaluate the unforeseen health effects of e-cigarette flavors. Our data indicating the concurrent use of multiple flavors suggest a complex pattern of flavor use and motivate a need for toxicity testing to assess poly-exposure. In vitro high-throughput methods that look for pathway perturbations may be needed as a toxicity assessment approach.
Our results regarding anticipated reactions to FDA e-cigarette flavor regulation suggest complexities such that the benefits and risks of flavor ban need to be carefully evaluated (19). Restrictions on all nontobacco e-cigarette flavors (or all e-cigarettes) could help prevent nonusers (especially adolescents or youth) from initiating e-cigarette use, and over 40% of our study participants believed in the necessity of implementing regulations. However, a majority anticipated that they would personally attempt to circumvent potential FDA regulations of e-cigarettes by obtaining e-cigarette flavors from various illicit sources (e.g., Internet orders from foreign countries) or even self-making flavors. This maintenance of access to flavors despite regulation suggests an element of e-cigarette–related dependence that requires further evaluation. The use of flavoring agents purchased from unregulated sources could lead to additional unanticipated toxicities. It is also concerning that some established e-cigarette users believed that they would return to cigarette smoking if nontobacco e-cigarette flavors were banned. Thus, for adult e-cigarette users who use certain flavors to facilitate smoking cessation or reduction, banning all nontobacco flavors could precipitate relapse to smoking.
Although our study provides important results to understand migration in flavor use patterns, major limitations need to be discussed when interpreting our results. Our study participants were long-term adult e-cigarette users who volunteered to participate in the online surveys. The study crude retention rate was 39.8%, and we further excluded participants with key missing data. Our study population is therefore not representative of general e-cigarette users, and our results may be biased to reflect the flavor use experience specific to a subset of long-term stable users. Meanwhile, e-cigarette–related adverse respiratory effects could be underestimated if many former or occasional users (who could not tolerate the adverse reactions of e-cigarette use) did not participate in our surveys. Another important limitation for survey research that relies on self-reported data is information bias: E-cigarette users may have changed flavor preference multiple times between the two surveys, or they may not have accurately recalled or known all the flavors they used, especially the flavor used at e-cigarette initiation. We also did not have sufficient data to evaluate if device characteristics would affect flavor use. Despite these limitations, our study included a large number of established exclusive e-cigarette users so that we could examine the flavor use history and distinguish the independent health effects due to e-cigarette use. Our results are consistent with previous research indicating the popularity of non–tobacco-related flavors among e-cigarette users (13, 14). We also provide new evidence to better understand flavor use behaviors in older adults and possible health risks in long-term e-cigarette users.
In conclusion, our study indicates rapidly shifting flavor use patterns in all demographic groups of long-term e-cigarette users. The evidence for regular use of multiple flavors and trial use of many flavors, coupled with evidence of variable adverse respiratory effects from certain e-cigarette flavors, motivates future research to understand the health risks associated with these changing flavor use patterns. The present study can inform clinicians, researchers, and the FDA to address the concern that many long-term users state an intention to seek illicit flavor sources in the event of a flavor ban, which may cause unforeseen health problems.
Supplementary Material
Footnotes
Supported by the National Institute on Drug Abuse of the National Institutes of Health (NIH) and the Center for Tobacco Products of the U.S. Food and Drug Administration (FDA) (P50-DA-036107) for the Penn State Tobacco Center of Regulatory Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Author Contributions: P.D., R.B., and J.F. designed this study. T.F. and A.S. administered the follow-up survey and analyzed the data. J.Y. helped design both surveys and developed the Penn State Flavor Classification method. P.M. provided input on clinical implications. All authors contributed to the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kuehn BM. FDA: electronic cigarettes may be risky. JAMA. 2009;302:937. doi: 10.1001/jama.2009.1245. [DOI] [PubMed] [Google Scholar]
- 2.Hsu G, Sun JY, Zhu SH. Evolution of electronic cigarette brands from 2013–2014 to 2016–2017: analysis of brand websites. J Med Internet Res. 2018;20:e80. doi: 10.2196/jmir.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao W, Xu G, Lu J, Snetselaar LG, Wallace RB. Changes in electronic cigarette use among adults in the United States, 2014–2016 [letter] JAMA. 2018;319:2039–2041. doi: 10.1001/jama.2018.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai H, Leventhal AM.Prevalence of e-cigarette use among adults in the United States, 2014–2018 [letter] JAMA 2019;322:1824–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu SH, Sun JY, Bonnevie E, Cummins SE, Gamst A, Yin L, et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tob Control. 2014;23(Suppl 3):iii3–iii9. doi: 10.1136/tobaccocontrol-2014-051670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanti AC, Johnson AL, Glasser AM, Rose SW, Ambrose BK, Conway KP, et al. Association of flavored tobacco use with tobacco initiation and subsequent use among US youth and adults, 2013–2015. JAMA Netw Open. 2019;2:e1913804. doi: 10.1001/jamanetworkopen.2019.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Thoracic Society. Use big tobacco’s Nov 26 corrective statements to reduce smoking [editorial]. 15 Nov 2017 [accessed 2019 Nov 3]. Available from: https://www.sciencedaily.com/releases/2017/11/171115115019.htm.
- 8.Farber HJ, Neptune ER, Ewart GW. Corrective statements from the tobacco industry: more evidence for why we need effective tobacco control. Ann Am Thorac Soc. 2018;15:127–130. doi: 10.1513/AnnalsATS.201711-845GH. [DOI] [PubMed] [Google Scholar]
- 9.Goldenson NI, Leventhal AM, Simpson KA, Barrington-Trimis JL. A review of the use and appeal of flavored electronic cigarettes. Curr Addict Rep. 2019;6:98–113. doi: 10.1007/s40429-019-00244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Department of Health Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General—executive summary. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2016.
- 11.U.S. Food and Drug Administration, U.S. Department of Health Human Services. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist. 2016;81:28973–29106. [PubMed] [Google Scholar]
- 12.Zare S, Nemati M, Zheng Y. A systematic review of consumer preference for e-cigarette attributes: flavor, nicotine strength, and type. PLoS One. 2018;13:e0194145. doi: 10.1371/journal.pone.0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell C, McKeganey N, Dickson T, Nides M. Changing patterns of first e-cigarette flavor used and current flavors used by 20,836 adult frequent e-cigarette users in the USA. Harm Reduct J. 2018;15:33. doi: 10.1186/s12954-018-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ. Use of flavored e-cigarettes and the type of e-cigarette devices used among adults and youth in the US—results from wave 3 of the Population Assessment of Tobacco and Health Study (2015–2016) Int J Environ Res Public Health. 2019;16:2991. doi: 10.3390/ijerph16162991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soneji SS, Knutzen KE, Villanti AC. Use of flavored e-cigarettes among adolescents, young adults, and older adults: findings from the Population Assessment for Tobacco and Health Study. Public Health Rep. 2019;134:282–292. doi: 10.1177/0033354919830967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JC, Green KM, Arria AM, Borzekowski DLG. Prospective predictors of flavored e-cigarette use: a one-year longitudinal study of young adults in the U.S. Drug Alcohol Depend. 2018;191:279–285. doi: 10.1016/j.drugalcdep.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneller LM, Bansal-Travers M, Goniewicz ML, McIntosh S, Ossip D, O’Connor RJ. Use of flavored electronic cigarette refill liquids among adults and youth in the US—results from wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015) PLoS One. 2018;13:e0202744. doi: 10.1371/journal.pone.0202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Wasserman E, Kong L, Foulds J. A comparison of nicotine dependence among exclusive e-cigarette and cigarette users in the PATH study. Prev Med. 2017;104:86–91. doi: 10.1016/j.ypmed.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JC. Flavored e-cigarette use and cigarette smoking reduction and cessation—a large national study among young adult smokers. Subst Use Misuse. 2018;53:2017–2031. doi: 10.1080/10826084.2018.1455704. [DOI] [PubMed] [Google Scholar]
- 20.Landry RL, Groom AL, Vu TT, Stokes AC, Berry KM, Kesh A, et al. The role of flavors in vaping initiation and satisfaction among U.S. adults. Addict Behav. 2019;99:106077. doi: 10.1016/j.addbeh.2019.106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P, Fan T, Yingst J, Veldheer S, Hrabovsky S, Chen C, et al. Changes in e-cigarette use behaviors and dependence in long-term e-cigarette users. Am J Prev Med. 2019;57:374–383. doi: 10.1016/j.amepre.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res. 2015;17:186–192. doi: 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine Tob Res. 2017;19:1381–1385. doi: 10.1093/ntr/ntw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiol Behav. 1987;39:639–641. doi: 10.1016/0031-9384(87)90166-1. [DOI] [PubMed] [Google Scholar]
- 26.Dinakar C, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375:1372–1381. doi: 10.1056/NEJMra1502466. [DOI] [PubMed] [Google Scholar]
- 27.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems Eaton DL, Kwan LY, Stratton K.Public health consequences of e-cigarettes. Washington, DC: National Academies Press; 2018 [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Atlanta, GA: CDC; 2020 [accessed 2020 Jan 9]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
- 29.Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;366:l5275. doi: 10.1136/bmj.l5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills TA, Pagano I, Williams RJ, Tam EK. E-cigarette use and respiratory 201906472OCdisorder in an adult sample. Drug Alcohol Depend. 2019;194:363–370. doi: 10.1016/j.drugalcdep.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ Health Perspect. 2016;124:733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, et al. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med. 2018;120:72–79. doi: 10.1016/j.freeradbiomed.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur G, Muthumalage T, Rahman I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol Lett. 2018;288:143–155. doi: 10.1016/j.toxlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behar RZ, Luo W, McWhirter KJ, Pankow JF, Talbot P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep. 2018;8:8288. doi: 10.1038/s41598-018-25575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Food and Drug Administration. Tobacco product problem reports. Silver Spring, MD: FDA; 2019 [accessed 2020 Jan 13]. Available from: https://www.fda.gov/tobacco-products/tobacco-science-research/tobacco-product-problem-reports#ends-reports.
- 37.Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, Cates JE, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury — United States, October 2019. MMWR Morb Mortal Wkly Rep. 2019;68:919–927. doi: 10.15585/mmwr.mm6841e3. Lung Injury Response Clinical Working Group; Lung Injury Response Epidemiology/Surveillance Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Thoracic Society. Vaping: the threat to public health and the ATS response. New York, NY: ATS; 2019 [accessed 2019 Nov 3]. Available from: https://www.thoracic.org/professionals/clinical-resources/disease-related-resources/vaping-the-threat-to-public-health-and-the-ats-response.php.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.