Abstract
Potato (Solanum tuberosum) is one of the important crop plants, and many potato cultivars consist of a tetraploid genome with high heterozygosity. The techniques of transformation and genome editing require plant regeneration. However, no efficient regeneration method has been established except for some specific cultivars, such as ‘Sayaka’. In general, it is necessary to determine the adequate concentrations of auxin and cytokinin for plant regeneration. We established an efficient method using a 24-well microplate that easily enabled determination of the concentrations of these plant growth regulators suitable for shoot regeneration. Using this method, the optimal concentrations of these factors were analyzed for two representative potato cultivars, ‘Sayaka’ and ‘Konafubuki’. This analysis revealed there was a large difference in the optimal concentrations between them. Based on this result, a specialized medium for the efficient regeneration of ‘Konafubuki’ cultivars was proposed. This assay method was also applied for determination of hygromycin sensitivity of these potato cultivars, and it was observed that ‘Konafubuki’ was rather sensitive to hygromycin. These findings suggested that the selection of a ‘Konafubuki’ transformant could be achieved using a medium containing a lower amount of hygromycin than that used for ‘Sayaka’.
Keywords: microplate assay, plant growth regulators, potato, regeneration, sensitivity to antibiotics
Introduction
Potato is one of the important crop plants containing nutrition-rich tubers, and is widely used for potato chips, French fries and other processed products. In addition, potato starch is utilized for additive materials in paper, adhesive agents, and some food products (Kraak 1993). There are hundreds of potato varieties worldwide. Among them, the major species cultivated is Solanum tuberosum, which has a tetraploid genome and commonly shows a vegetative reproduction. Obtaining a new potato cultivar by the traditional crossbreeding method is difficult, and many potato cultivars have high heterozygosity (Muthoni et al. 2015).
Transformation techniques have been developed for potato, enabling the creation of genetically modified potato plants, in which various genetic materials have been introduced with a stable integration into the genome (Barrell et al. 2013). This process also enabled the beginning of a new era for new breeding techniques, such as genome editing of potato plants. Till now, targeted mutagenesis has been achieved using TALEN and CRISPR/Cas9 (Kusano et al. 2018; Sawai et al. 2014; Tuncel et al. 2019).
Skoog and Miller (1957) reported that an adequate amount of two plant growth regulators, auxin and cytokinin, strongly affect the promotion of differentiation and dedifferentiation in plant explants. In Arabidopsis, an auxin-rich medium promotes callus formation, and roots and shoots are efficiently regenerated from the callus cultured in a high-auxin or high-cytokinin medium (Valvekens et al. 1988). However, no efficient potato transformation procedure has been established except for some cultivars, such as ‘Sayaka’ and ‘Sassy’ (Yamamizo et al. 2006; Yasumoto et al. 2019).
It has been shown that there are great differences in the efficiency of regeneration from cultured cells depending on the potato cultivars. This largely influences the efficiency of obtaining regenerated potato (Ishige et al. 1991). Currently, potato breeding technologies including new breeding techniques can be applied to only a few potato cultivars. In this study, to obtain potato plants possessing a desired trait, we established a general method that may allow the introduction of an appropriate gene with efficient regeneration techniques. Here, we describe a novel method for the determination of optimal concentrations of plant growth regulators for regeneration. We also demonstrate the effect of antibiotics commonly used as a selection marker for transformants.
Materials and methods
Plant material and tissue culture condition
Potato cultivars, ‘Sayaka’, ‘Konafubuki’, ‘Sakurafubuki’ and ‘Norin-1’, were used in this study. They were kindly provided by Hokkaido Agricultural Research Center, National Agriculture and Food Research Organization (NARO), Japan. In vitro-grown potato plantlets were grown in long-day conditions with 16 h light and 8 h dark at 23°C in a growth chamber. For plant subculture, we used the medium containing MS basal salt mixture (Fuji Film Wako, Tokyo, Japan) (Murashige and Skoog 1962), 3% sucrose, and 0.3% Gelrite (Fuji Film Wako), with pH adjusted to 6.0. For the plant regeneration, 3C5ZR medium (pH 5.8), which contained MS basal salt mixture, 3% sucrose, 0.01% myo-inositol and 0.3% Gelrite, with 1.5 mg l−1 thiamine-HCl, 0.75 mg l−1 nicotinic acid, 0.75 mg l−1 pyridoxine-HCl, and 0.6 mg l−1 aspartic acid, was used as a basal medium. Plant growth regulators and antibiotics were supplemented to this as required.
Assay for shoot regeneration efficiency
Stems of in vitro-grown potato plantlets were cut into approximately 5–8 mm segments using microscissors (MB-50-15, Natsume Seisakusho, Tokyo, Japan). Two pieces of stem segments were placed onto the 2 ml 3C5ZR medium containing a series of concentrations of 3-Indoleacetic Acid (IAA) (Fuji Film Wako) and trans-zeatin riboside (tZR) (Fuji Film Wako), which was set in a well of a 24-well culture plate (nontreated microplate with lid). Potato tissues were grown at 23°C in long-day condition with 16 h light and 8 h dark in a growth chamber. After 30-day culture, regenerated shoots were detected. When shoot regeneration was observed in one of the two pieces of potato stem segments in a well, it was noted as the occurrence of regeneration.
Regeneration of four potato cultivars on the improved medium
Stem segments of potato cultivars, ‘Sayaka’, ‘Konafubuki’, ‘Sakurafubuki’, and ‘Norin-1’ were placed on Medium-I and Medium-II, which were placed in Petri dishes, and they were cultured for 15 days. They were transplanted onto the same media to replenish the components in the medium and cultured for 15 more days. Medium-I consisted of 3C5ZR supplemented with 0.53 mg l−1 IAA and 1.75 mg l−1 tZR, which was conventionally used for potato transformation (Kusano et al. 2016). Medium-II contained 3C5ZR medium supplemented with 0.27 mg l−1 IAA and 2.63 mg l−1 tZR. After 30-day culture, the number of potato tissues showing shoot regeneration was counted. This experiment was performed more than four times each. The regeneration ratio was calculated for each experiment. The statistical significance was determined using a proportion test between regeneration frequencies in the experiments on Medium-I and Medium-II using R software (https://www.r-project.org).
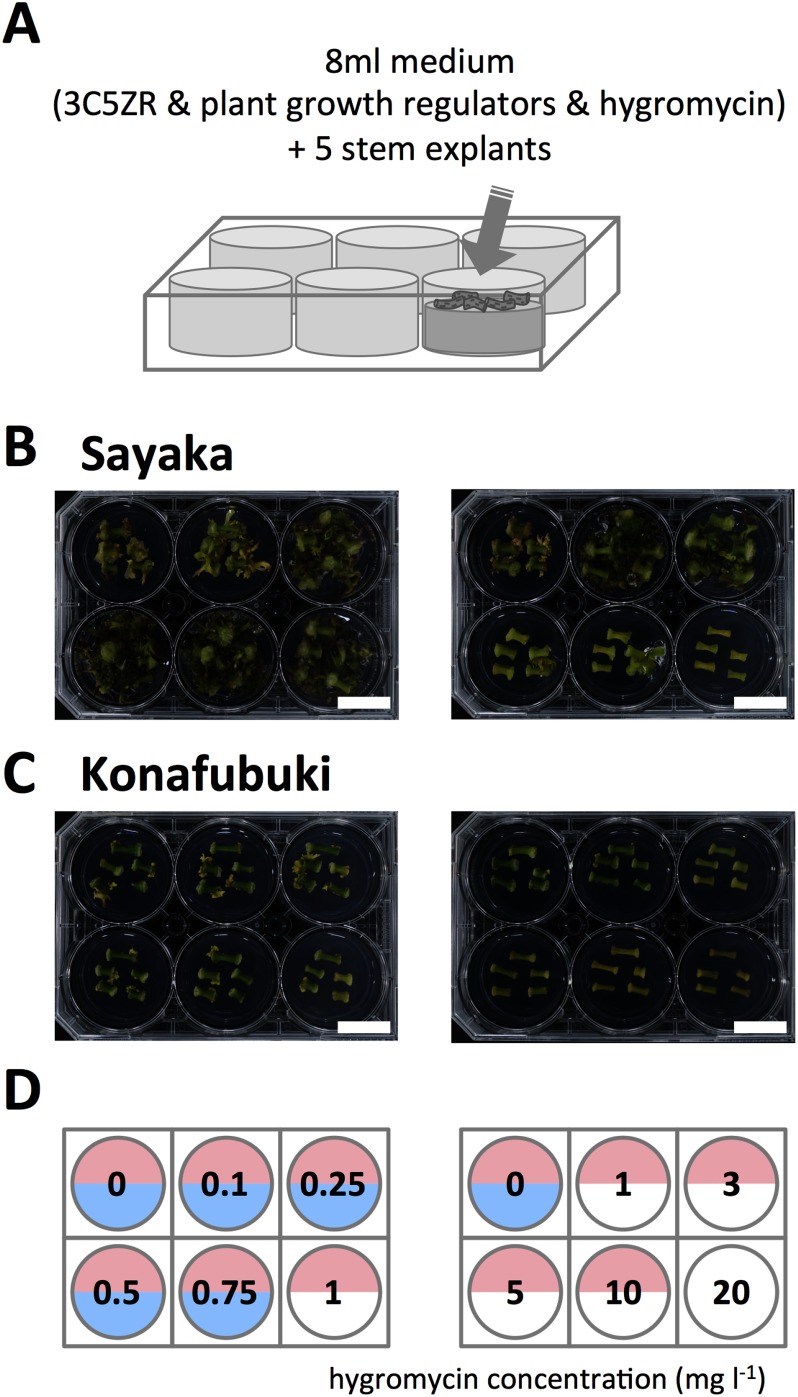
Hygromycin sensitivity test for ‘Sayaka’ and ‘Konafubuki’
Five pieces of stem segments of ‘Sayaka’ and ‘Konafubuki’ were placed in each well of a 6-well plate (untreated microplate with lid) containing 8 ml 3C5ZR medium with a series of concentrations of Hygromycin B (Fuji Film Wako) (Figure 5A). For the cell culture of ‘Sayaka’, the medium containing 0.53 mg l−1 and 1.75 mg l−1 of IAA and tZR, respectively, was used. For the ‘Konafubuki’, the medium containing 0.27 mg l−1 and 1.75 mg l−1 of IAA and tZR, respectively, was used. After a 30-day culture, we analyzed alteration of potato tissue morphology, and evaluated shoot regeneration.
Figure 5. Assay for sensitivity to hygromycin. (A) Schematic representation of the procedure for the determination of sensitivity. Sections of potato stems were placed in a 6-well plate and cultured for 30 days. The wells contained a series of hygromycin concentrations (0 to 20 mg l−1). (B, C) Representative images of cultured cells of ‘Sayaka’ (B) and ‘Konafubuki’ (C). The hygromycin concentrations in the medium is indicated in panel (D). (D) Summary of sensitivity of ‘Sayaka’ and ‘Konafubuki’ to hygromycin. The numbers on the panel indicate the hygromycin concentration in the medium. When shoot regeneration was detected, the corresponding half circles are colored. The results for ‘Sayaka’ and ‘Konafubuki’ are shown in the upper and lower half circles, respectively. Scale bars indicate 2 cm.
Results
Determination of ranges of auxin and cytokinin concentrations for shoot regeneration
Regeneration conditions of potato plants vary depending on the cultivar. To establish a general procedure for potato regeneration, it is necessary to use a culture medium containing the optimal concentrations of plant growth regulators. ‘Sayaka’ is known as a potato cultivar that is efficiently regenerated. Using this cultivar, we examined the effect of various concentrations of auxin and cytokinin in the medium, and found that ‘Sayaka’ was regenerated on the medium containing concentrations of 0 to 1.06 mg l−1 of auxin (as IAA) and concentrations of 0.44 to 14.0 mg l−1 of cytokinin (as tZR). Based on these findings, we developed an assay system to determine the optimal concentration of each plant growth regulator.
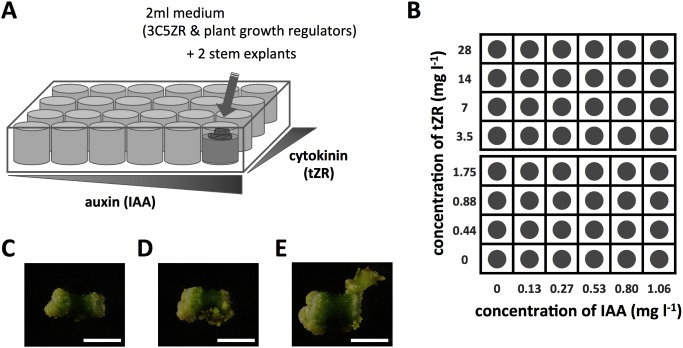
Sections of potato stems of cultivars ‘Sayaka’ and ‘Konafubuki’ were placed onto the medium containing various concentrations of plant growth regulators, cultured for 30 days, and regenerated shoots from the cultured cells were observed. To save space and amount of culture medium, we used 24-well culture plates, each of whose wells contained different concentrations of IAA and tZR (Figure 1).
Figure 1. Outline of the plate assay for regeneration efficiency of potato cultivars. (A) Schematic representation of the regeneration procedure from potato cultured cells using a 24-well culture plate. (B) Layout of the 24-well culture plate. Each well contains a culture medium containing various concentrations of IAA and tZR, ranging 0 to 1.06 mg l−1 and 0 to 28 mg l−1, respectively. (C–E) Photographs of sections of ‘Konafubuki’ stems after 30-day culture. Panels indicate the cells without shoot regeneration (C), and those showing regenerated shoot (D, E). Scale bars indicate 5 mm.
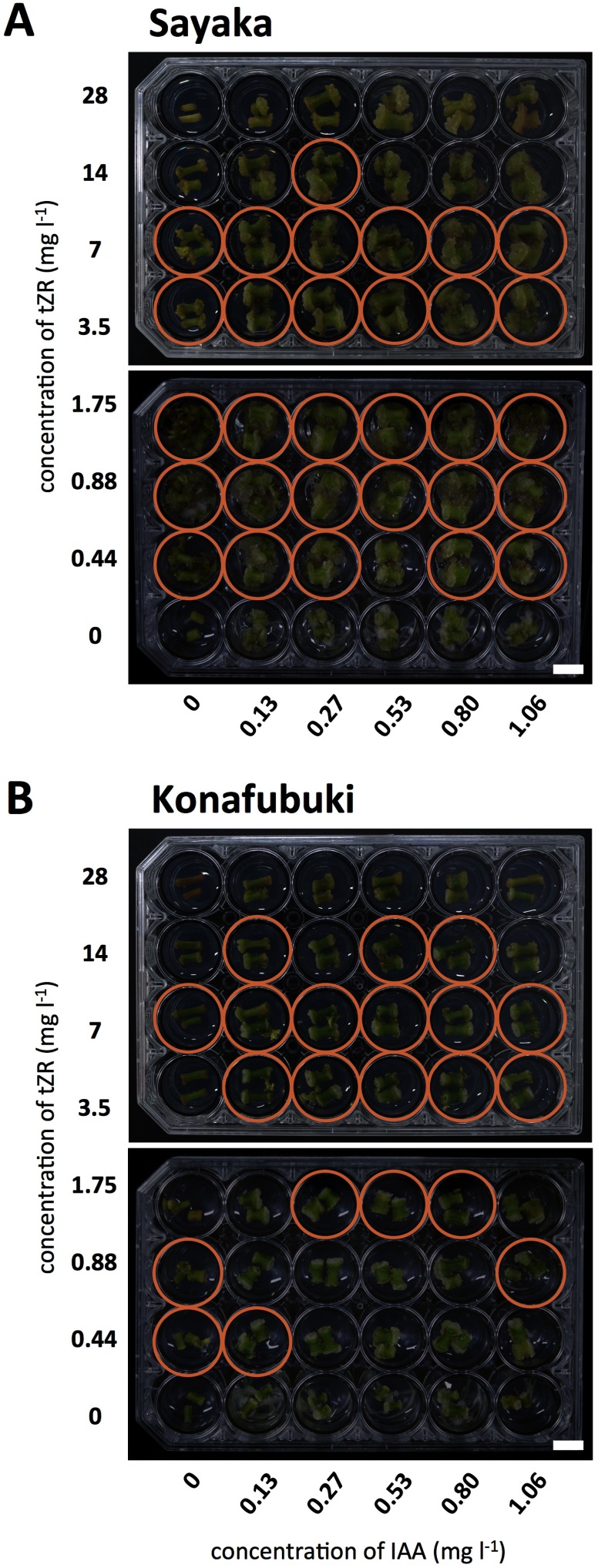
Shoot regeneration was observed in some cultured cells for both ‘Sayaka’ and ‘Konafubuki’. The callus was formed along the cut surface, stem segments were expanded in most wells, and shoot was generated from a part of cultured cells in 30 days after (Figure 1D, E). Regeneration events were observed in many wells with ‘Sayaka’ cells, while ‘Konafubuki’ showed low regeneration frequency (Figure 2). These results indicated that regeneration efficiency was greatly different between these cultivars.
Figure 2. Representative images of the plate assays. ‘Sayaka’ (A) and ‘Konafubuki’ (B) cultured in a 24-well plate for 30 days. The wells contain a series of concentrations of plant growth regulators (0 to 1.06 mg l−1 of IAA and 0 to 28 mg l−1 of tZR). Orange-colored circles indicate the wells in which shoot regeneration was observed. Scale bars indicate 1 cm.
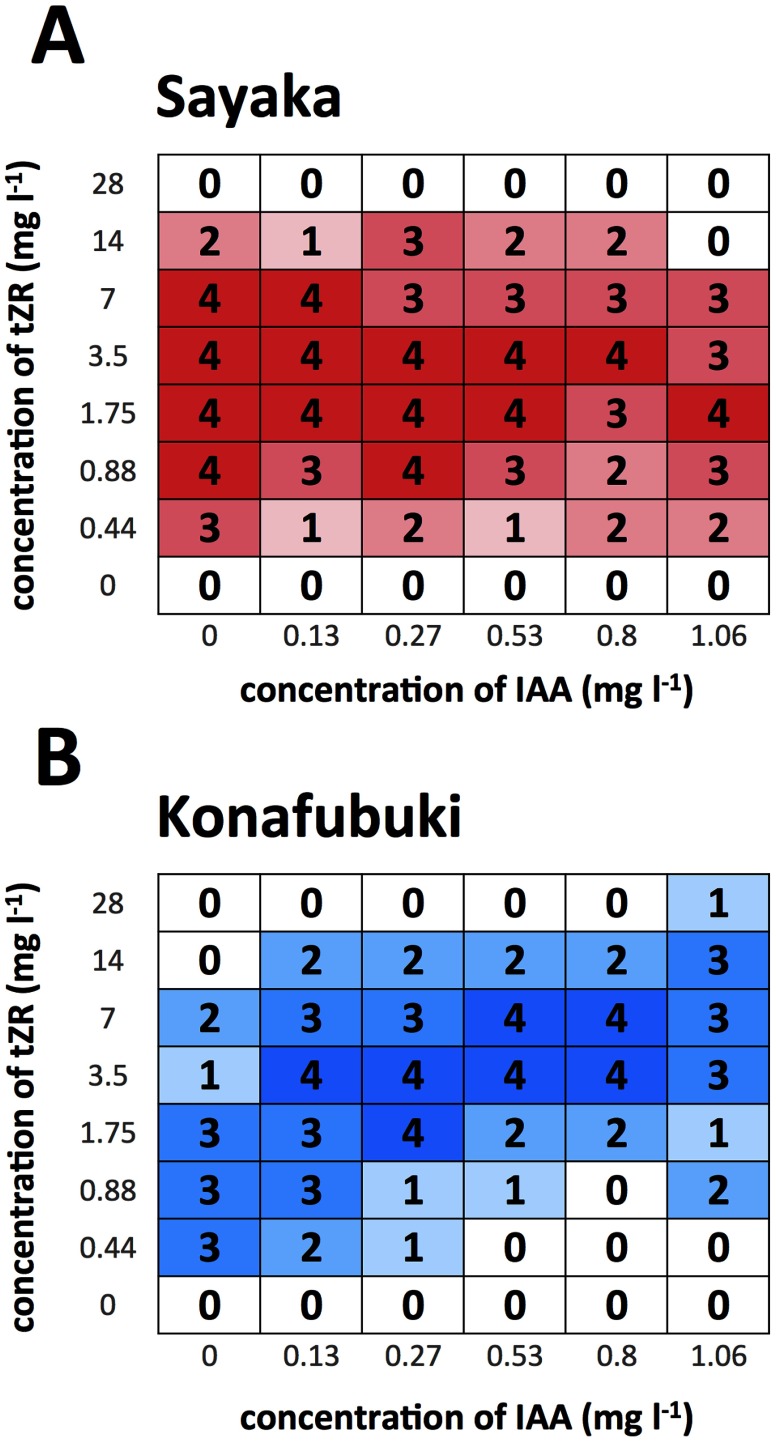
The regeneration efficiency was investigated for ‘Sayaka’ and ‘Konafubuki’ using the abovementioned system. Two pieces of the stem segments of these cultivars were cultured on media containing various concentrations of IAA and tZR, and shoot formation was evaluated. When shoot regeneration was observed in one of the two cultured stem segments in a well, we considered that the regeneration event had occurred on the corresponding culture medium. The number of regeneration events was compared among the different culture media. In the case of ‘Sayaka’, it was found that the optimal plant growth regulator concentration for regeneration was broadly distributed within 0 to 1.06 mg l−1 of IAA, and 0.88 to 7.0 mg l−1 of tZR. In contrast, in the case of ‘Konafubuki’, this was estimated to lie in the narrow range of 0.13 to 0.8 mg l−1 of IAA, and 1.75 to 7.0 mg l−1 of tZR (Figure 3).
Figure 3. Heat map of the shoot formation frequency for the cultured cells of ‘Sayaka’ (A) and ‘Konafubuki’ (B). The occurrence of regeneration was determined using the assay method as shown in the previous figures. When the regenerated shoots were observed on one of the two tissues, it was counted as ‘regenerated’. This assay was performed four times each, and numbers of ‘regenerated’ in total are indicated as the frequency of regeneration. In each box, frequency is shown as the total number as ‘regenerated’ events, with color gradation.
Regeneration ratio for the two media with different plant growth regulator concentrations with four potato cultivars
Based on the results of the estimation for optimal plant growth regulator concentrations, we attempted to improve the culture medium for potato regeneration. In addition to the conventionally used medium, Medium-I, which contained 0.53 mg l−1 IAA and 1.75 mg l−1 tZR (Kusano et al. 2016), we created another medium, Medium-II containing 0.27 mg l−1 IAA and 2.63 mg l−1 tZR. Using these media, regeneration efficiency was determined for the four representative potato cultivars, ‘Sayaka’, ‘Konafubuki’, ‘Sakurafubuki’, and ‘Norin-1’.
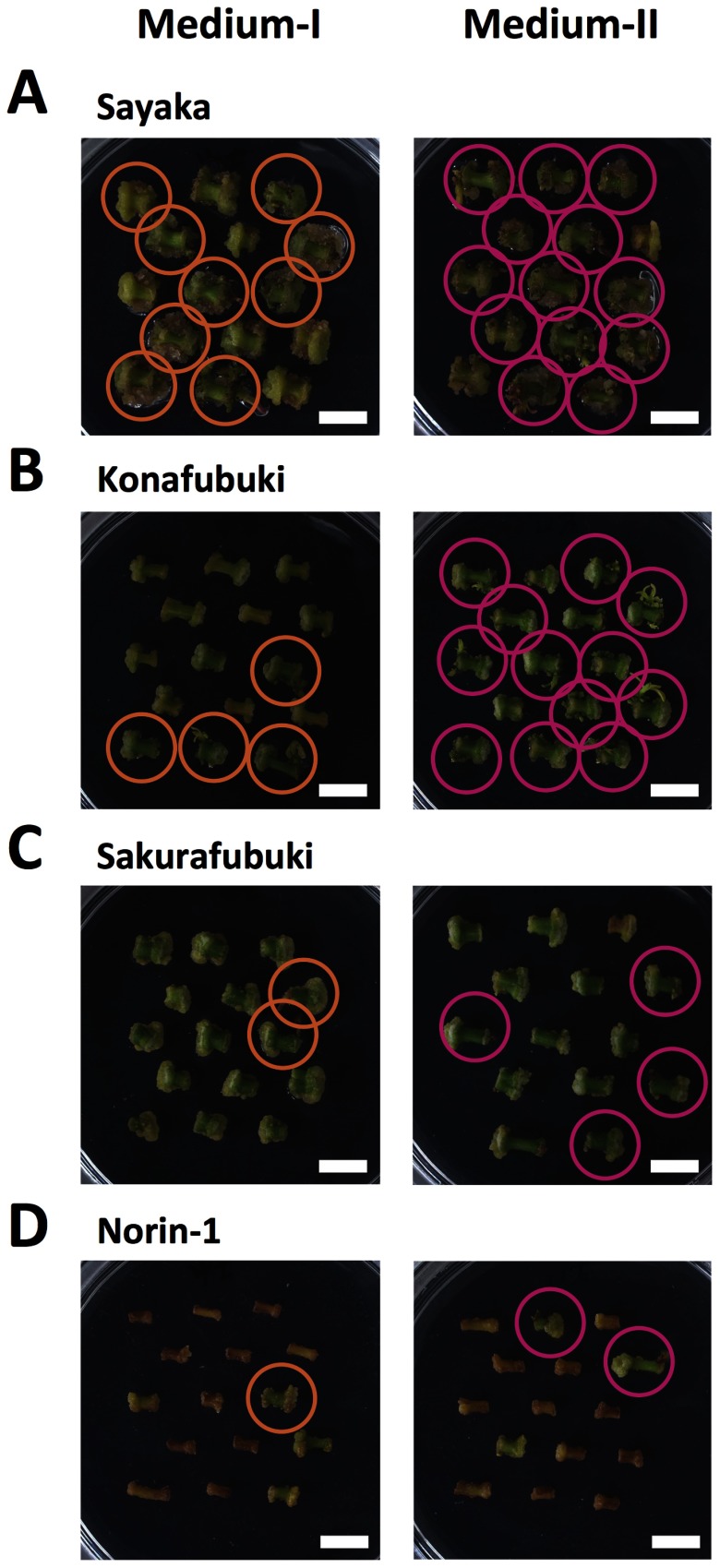
As shown in Figure 4, the regeneration frequency was evidently different between the stem segments cultured on Medium-I and Medium-II; however, ‘Sayaka’ showed a similar efficiency of regeneration in both media. The ratio of occurrence of regeneration in both Medium-I and Medium-II was approximately 70% for the ‘Sayaka’ cultured cells. In contrast, ‘Konafubuki’ and ‘Sakurafubuki’ showed higher values of regeneration efficiency when they were cultured on Medium-II (66.3% and 15.5%, respectively) than on Medium-I (46.7% and 5.2%, respectively). For ‘Norin-1’, the regeneration efficiency was rather low in both media (3.3%) (Table 1). These results suggest that Medium-II containing the optimal concentrations of plant growth regulators is highly effective for increasing the efficiency of potato regeneration in case of ‘Konafubuki’.
Figure 4. Representative images of cell growth and shoot regeneration on Medium-I and Medium-II. Sections of potato stems were cultured for 30 days. Panels indicate ‘Sayaka’ (A), ‘Konafubuki’ (B), ‘Sakurafubuki’ (C), and ‘Norin-1’ (D). Left and right panels show the segments of these potato cultivars on the Medium-I and Medium-II, respectively. Circles indicate the cultured cells that showed shoot regeneration. Scale bars indicate 1 cm.
Table 1. Frequency of shoot regeneration on four potato cultivars.
| Medium-I | Medium-II | |||
|---|---|---|---|---|
| Sayaka | 40/60 | (66.7%) | 42/60 | (70.0%) |
| Konafubuki | 28/60 | (46.7%) | 53/80 | (66.3%)* |
| Sakurafubuki | 3/58 | (5.2%) | 9/58 | (15.5%)* |
| Norin-1 | 2/60 | (3.3%) | 2/60 | (3.3%) |
The sections of potato stems were placed in dishes with Medium-I and Medium-II. Plants were cultured for 30 days, and regenerated shoots were counted. The values indicate the total number of sections of potato stems that regenerated shoots in the stems examined. Values in parentheses show the regeneration ratio. Asterisks indicate the statistical significance between regeneration frequencies in the experiments on Medium-I and Medium-II, which was determined using a proportion test (p<0.1).
Assay for hygromycin sensibility of potato cultivars
Using this assay system, the hygromycin sensitivity of ‘Sayaka’ and ‘Konafubuki’ was analyzed. As shown in Figure 5, ‘Konafubuki’ showed higher sensitivity to hygromycin than ‘Sayaka’. ‘Konafubuki’ failed to regenerate on the medium containing 1 mg l−1 hygromycin, whereas ‘Sayaka’ cells increased normally on the medium containing 3 mg l−1 hygromycin. ‘Sayaka’ survived on the medium containing 10 mg l−1 hygromycin, and sometimes regeneration of the shoot was observed. This suggests that the hygromycin sensibility was rather different depending on the cultivar.
Discussion
For creating a genetically modified plant as well as a genome-edited plant, a technique for plant regeneration is required (Loyola-Vargas and Vázquez-Flota 2006). In the case of potato, it is difficult to obtain a regenerated plant because the conditions of regeneration are greatly different depending on the cultivar. In this study, we developed a new assay system to determine the optimal plant growth regulator concentrations for regeneration from cultured potato cells. Using this assay system, we determined the optimal concentrations of two plant growth regulators, auxin and cytokinin, which are important for the regeneration of potato plants. This assay indicated that the optimal concentrations of these plant growth regulators were different depending on the potato cultivar (Figures 2 and 3).
‘Sayaka’ is commonly used as a cultivar that has a high regeneration ability (Teo et al. 2017; Yamamizo et al. 2006). However, there has been no report on the regeneration of ‘Konafubuki’, which produces a large amount of starch, and has potential as a raw material for industrial starch. If the starch quality is improved by genome editing, new applications of potato starch can be developed and would contribute to increasing its industrial value, resulting in a large ripple (economy) effect. Our assay system indicated that ‘Konafubuki’ was also showed regeneration but the optimal concentration ranges of plant growth regulators for ‘Konafubuki’ were narrow as compared with those of ‘Sayaka’ (Figure 3). Using the improved medium, Medium-II, regenerated ‘Konafubuki’ plants were obtained with high efficiency. However, Medium-II did not lead to a large improvement in the regeneration frequency for ‘Sakurafubuki’ and ‘Norin-1’, although the frequency was slightly higher for ‘Sakurafubuki’ (Table 1). These cultivars could be regenerated on the medium containing the optimal concentrations of plant growth regulators other than Medium-I and Medium-II. In this experiment, we selected three potato cultivars whose genetic background was different from that of ‘Sayaka’. They might have the genes involved in regeneration with a large divergence, and lead to differences in the regeneration frequency.
Hygromycin is commonly used as a selection marker for transgenic potato plants. However, the concentration of hygromycin is critical because cellular growth inhibition often occurs in presence of excess hygromycin. We examined the hygromycin sensitivity of ‘Sayaka’ and ‘Konafubuki’. The findings indicated ‘Konafubuki’ was rather sensitive to hygromycin. In contrast, ‘Sayaka’ grew normally on the medium containing 3 mg l−1 hygromycin and survived on the medium containing 10 mg l−1 (Figure 5). Usually, we used the medium containing 5 mg l−1 hygromycin for selection of potato transformants of ‘Sayaka’ (Kusano et al. 2016). This result suggests that our selection medium contains the amount of hygromycin that is effective in reducing the growth of potato cells without killing them. These findings indicate that the selection of a ‘Konafubuki’ transformant can be achieved using a medium containing a lower amount of hygromycin than that used for ‘Sayaka’. In addition, wild type ‘Sayaka’ survived in the 5 mg l−1 hygromycin condition, and a small number (1 in 5) of plants showed regeneration. We consider these plants were the plants escaping without hygromycin resistance.
Our method may lead to the establishment of culture conditions for various potato cultivars. The favorable culture condition can be easily obtained for each cultivar and applied for genome editing, which would greatly contribute to crop development by improving an appropriate phenotype at a pinpoint.
Acknowledgments
We thank Takahiro Asahi, Daichi Honma, Yukino Okubo, Kosuke Ito, Ami Takeuchi, Takaaki Horie, and Singthongsai Namfa (Tokyo University of Science, Tokyo, Japan) for their kind experimental assistance, and Kenji Asano and Takahiro Noda (Hokkaido Agricultural Research Center, NARO) for providing potato tubers.
Abbreviations
- IAA
3-indoleacetic acid
- tZR
trans-zeatin riboside
References
- Barrell PJ, Meiyalaghan S, Jacobs JME, Conner AJ (2013) Applications of biotechnology and genomics in potato improvement. Plant Biotechnol J 11: 907–920 [DOI] [PubMed] [Google Scholar]
- Ishige T, Ohshima M, Ohashi Y (1991) Transformation of Japanese potato cultivars with the β-glucuronidase gene fused with the promoter of the pathogenesis-related 1a protein gene of tobacco. Plant Sci 73: 167–174 [Google Scholar]
- Kraak A (1993) Industrial applications of potato starch products. Ind Crops Prod 1: 107–112 [Google Scholar]
- Kusano H, Ohnuma M, Mutsuro-Aoki H, Asahi T, Ichinosawa D, Onodera H, Asano K, Noda T, Horie T, Fukumoto K, et al. (2018) Establishment of a modified CRISPR/Cas9 system with increased mutagenesis frequency using the translational enhancer dMac3 and multiple guide RNAs in potato. Sci Rep 8: 13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Onodera H, Kihira M, Aoki H, Matsuzaki H, Shimada H (2016) A simple Gateway-assisted construction system of TALEN genes for plant genome editing. Sci Rep 6: 30234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola-Vargas VM, Vázquez-Flota F (2006) Plant Cell Culture Protocols (2nd edition). Humana Press Inc., New Jersey, pp 3–8
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Muthoni J, Kabira J, Shimelis H, Melis R (2015) Tetrasomic inheritance in cultivated potato and implications in conventional breeding. Aust J Crop Sci 9: 185–190 [Google Scholar]
- Sawai S, Ohyama K, Yasumoto S, Seki H, Sakuma T, Yamamoto T, Takebayashi Y, Kojima M, Sakakibara H, Aoki T, et al. (2014) Sterol Side chain Reductase 2 is a key enzyme in the biosynthesis of cholesterol, the common precursor of toxic steroidal glycoalkaloids in potato. Plant Cell 26: 3763–3774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Teo CJ, Takahashi K, Shimizu K, Shimamoto K, Taoka K (2017) Potato tuber induction is regulated by interactions between components of a tuberigen complex. Plant Cell Physiol 58: 365–374 [DOI] [PubMed] [Google Scholar]
- Tuncel A, Corbin KR, Ahn-Jarvis J, Harris S, Hawkins E, Smedley MA, Harwood W, Warren FJ, Patron NJ, Smith AM (2019) Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes. Plant Biotechnol J 17: 2259–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M (1988) Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA 85: 5536–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizo C, Kuchimura K, Kobayashi A, Katou S, Kawakita K, Jones JDG, Doke N, Yoshioka H (2006) Rewiring mitogen-activated protein kinase cascade by positive feedback confers potato blight resistance. Plant Physiol 140: 681–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S, Umemoto N, Lee HJ, Nakayasu M, Sawai S, Sakuma T, Yamamoto T, Mizutani M, Saito K, Muranaka T (2019) Efficient genome engineering using Platinum TALEN in potato. Plant Biotechnol 36: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]