Abstract
Tomato mosaic virus (ToMV) and tobacco mosaic virus (TMV) are critical pathogens causing severe crop production losses of solanaceous plants. The present study was undertaken to evaluate the antiviral effects of extracts of Alpinia plants on ToMV and TMV infection in Nicotiana benthamiana. The aqueous extracts of Alpinia zerumbet (Pers.) B.L. Burtt and R.M. Smith and Alpinia kumatake, which grow widely in subtropical and tropical regions including East Asia, were effective in reducing ToMV infection when plants were treated prior to virus inoculation. We also found that the extracts of A. zerumbet isolated from Okinawa (Japan), locally referred to as shima-gettou, strongly inhibited ToMV and TMV infection. To obtain an active fraction, the aqueous extract of A. zerumbet isolate OG1 was separated by ethyl acetate, and the antiviral active compound was found to be present in the water layer. Based on our results, the extract of Alpinia plants has potential as an antiviral reagent for practical application in solanaceous crop production.
Keywords: antiviral activity, Alpinia zerumbet, tobacco mosaic virus, tomato mosaic virus
Plant viruses cause many economically significant plant diseases, and infected plants show a range of symptoms, including mosaic, crinkle, yellowing, and stunting. Tobacco mosaic virus (TMV) and tomato mosaic virus (ToMV) both belong to the genus Tobamovirus in the family Virgaviridae, and they have a wide host range, including Solanaceous species such as tomato (Solanum lycopersicum L.), pepper (Capsicum annuum L.), and Nicotiana benthamiana Domin. TMV was the first virus discovered in the late 19th century, and it is one of the most thoroughly studied plant viruses (Zaitlin 1998). ToMV is one of the most critical pathogen of tomato. These viruses cause significant losses in both quantity and quality of crops on a global scale. To control the spread of pathogens, agricultural chemicals are typically used.
However, plant virus infection cannot be controlled directly by the use of chemicals. Therefore, traditional cultural practices to control plant viruses depend on chemical or biological control of the insect vector of a plant virus, growing genetically resistant cultivars, and planting virus-free material. In this study, we strove to develop an antiviral agent using natural plant materials. Although several antiviral compounds are used to treat animal and human infections that have been made from plant compounds (Hudson 1990), it is unclear whether these compounds also inhibit plant viruses and treat infections, and their agricultural use has yet to be evaluated.
Alpinia zerumbet (Pers.) B.L. Burtt and R.M. Smith, syn. Alpinia speciosa, also referred to as shell ginger, is a member of the Alpinia genus in the Zingiberaceae family (Teschke and Xuan 2018) (Figure 1). Alpinia zerumbet plants grow widely in subtropical and tropical regions in East Asia including Japan with the northern edge of the natural range in Cape Sata in Kagoshima Prefecture. In Japan, A. zerumbet is known as gettou or shima-gettou, and in Okinawa and the Amami islands, it is also referred to as san-nin and sanen, respectively. Alpinia zerumbet is a perennial herb with rhizomes, grows 2.5 to 3 m tall, and produces two-ranked leaves, which are aromatic. In Okinawa and the Amami islands, their leaves are used for traditional herbal tea, to flavor noodles, and to wrap mochi rice cakes called muchi. It also has been utilized as a folk medicine for its anti-oxidant, bacteriostatic, and fungistatic properties, as well as in cosmetics (Elzaawely et al. 2007a, 2007b; Tu and Tawata 2015; Yonaha et al. 2013; Zoghbi et al. 1999). These uses and properties make A. zerumbet an attractive candidate for use as a novel agricultural resource. However, its bioactive phytochemicals have not been sufficiently utilized or evaluated as agricultural materials.
Figure 1. Alpinia plants grown in Okinawa (A) Alpinia zerumbet and (B) Alpinia kumatake.
In this study, we examined the antiviral activity of extract from the stem and leaves of three greenhouse grown varieties of Alpinia plants: two A. zerumbet isolates (OG1 and AG1) were collected from Nakijinn-son, Okinawa, and the Amami island, respectively, and an isolate of Alpinia kumatake (KB1) (Sharma and Hashinaga 2004), known as kumatakerann in Japan, was collected from Uruma-shi, Okinawa.
Aqueous extracts were obtained by squeezing leaves and stems of Alpinia plants with a sugar cane squeezer (YBK-2, Yabiku, Japan). The extracts were centrifuged at 3,260×g for 10 min, and the supernatant was first filtered with Whatman no. 1 filter paper and then with a membrane filter (0.22 µm, Bottle Top Filter, TPP). To evaluate the inhibitory effect of the Alpinia extracts, OG1, AG1, and KB1, the extracts were applied to N. benthamiana plants (the third true leaf stage) as foliar sprays, and 3 days after the treatment the plants were inoculated with a plant virus as described below.
To prepare inoculum of ToMV, pTL-derived plasmids (pTLBN.G3), which contain a full length ToMV cDNA as well as a gene encoding green fluorescent protein (GFP) (Kubota et al. 2003), were linearized by restriction with MluI and used as a template for in vitro transcription. The AmpliCap-Max T7 High Yield Message Maker Kit (CELLSCRIPT, USA) was used for in vitro transcription according to the manufacturer’s instructions at 37°C for 40 min. The transcription mixture was then diluted by 40-fold in water. The diluted mixture mixed with abrasive carborundum (600 mesh; Nacalai Tesque, Japan) was mechanically applied to the third true leaves of N. benthamiana that had been treated with the Alpinia extract. Two other antiviral agents, i.e., L-ascorbic acid (Fujiwara et al. 2013) and Lentemin (NSK Co., Ltd., Japan), were also used as controls to evaluate antiviral activity. Control plants underwent the inoculation with only water.
Green fluorescent protein foci were used to detect virus infection, and they were observed under blue-light irradiation 3 and 11 days post-infection (dpi). The antiviral activity was assessed based on the number of GFP foci formed on the control and treated N. benthamiana leaves. The number of GFP foci formed on the inoculated leaves was calculated at 3 dpi.
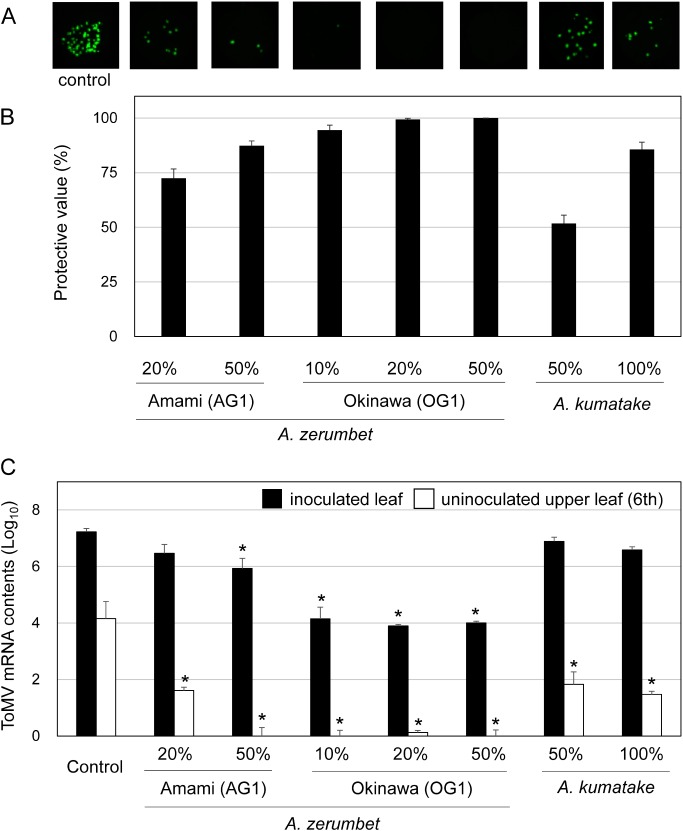
Our results showed that treatments with the extract of A. zerumbet isolates OG1 and AG1, as well as A. kumatake isolate KB1 effectively protected N. benthamiana leaves against ToMV-GFP infection, when compared with the control (Figures 2 and 3). In addition, the application of extract from isolate OG1 showed greater antiviral activity than the application of extracts from isolates AG1 and KB1. Furthermore, we used quantitative real-time polymerase chain reaction (qPCR) analysis to confirm these findings at the RNA level. Equal amounts of total RNA were subjected to cDNA synthesis and then specific ToMV sequences were amplified. The results obtained from qPCR (Figure 3C) were not significantly different from those obtained from assays based on the number of GFP foci (Figure 3A, B). These results indicate that the number of GFP foci is a highly correlated indicator for ToMV infection.
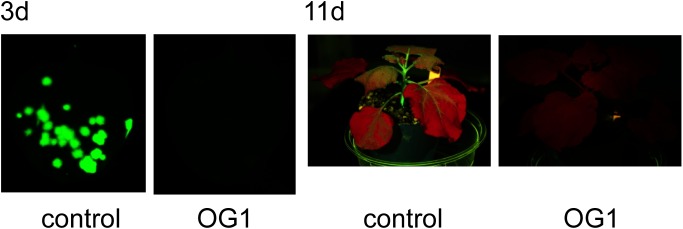
Figure 2. Accumulation of GFP in a Nicotiana benthamiana plant infected with GFP-tagged ToMV. Plants were grown for 21 days at 24°C with 16 h-light/8 h-dark cycles. The plants were treated with water and the extracts of A. zerumbet isolates OG1. The third true leaf was mechanically inoculated with GFP-tagged ToMV 3 days after treatment. Viral infection sites are seen as GFP-fluorescent spots. (A) GFP foci formed on N. benthamiana at 3 dpi. Pictures were taken under blue-light irradiation with a ChemiDoc™ MP Imaging System (Bio-rad, USA). (B) GFP fluorescence pattern on mosaic leaves at 11 dpi. Pictures were taken under blue-light irradiation with LEDGFP/L-HNDY (Optocode Corp.).
Figure 3. Effects of foliar application of the extracts of Alpinia plants against GFP-tagged ToMV virions. Nicotiana benthamiana plants were treated with water and the extracts of Alpinia plants (A. zerumbet isolates OG1 and AG1, and Alpinia kumatake isolate KB1), then inoculated with GFP-tagged ToMV 3 days after treatment. (A) GFP foci formed on N. benthamiana at 3 dpi. Pictures were taken under blue-light irradiation with ChemiDoc™ MP Imaging System (Bio-rad, USA). (B) The number of GFP spots formed on the inoculated leaves was calculated at 3 dpi. Protective value=(1−number of GFP spots formed on treated plants/number of GFP spots formed on untreated plants)×100. (C) Levels of ToMV mRNA accumulation in inoculated and uninoculated upper leaves collected at 3 dpi. Bars indicate the standard error (SE). The experiment was independently performed twice (n>3 per experiment). Asterisks indicate significant differences between the control and treated plants at each leaf position by one-way ANOVA with Dunnett’s multiple comparisons test (p<0.01).
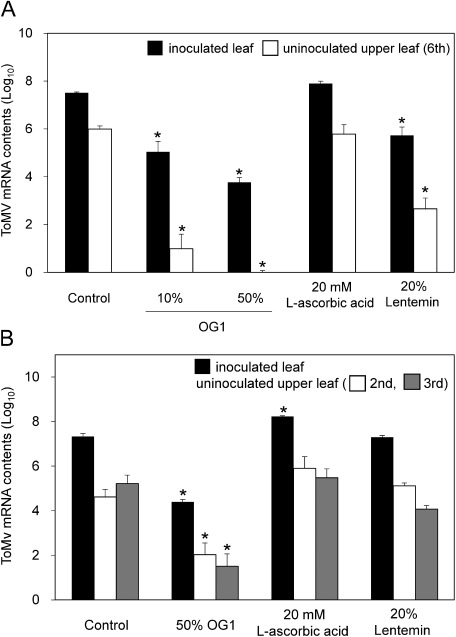
In both N. benthamiana and tomato hosts, the extracts of A. zerumbet isolate OG1 protected both inoculated and uninoculated upper leaves against ToMV as compared with water-treated plants (Figure 4). Furthermore, A. zerumbet extract application on N. benthamiana and tomato was more effective than the application of L-ascorbic acid or Lentemin in reducing viral infection. These results indicate that the Alpinia extracts play positive role in plant protection to ToMV.
Figure 4. Comparison of antiviral effects of the extracts of A. zerumbet and antiviral reagents in (A) N. benthamiana and (B) tomato plants after inoculation with GFP-tagged ToMV. The plants were treated with water, the extracts of A. zerumbet isolate OG1, L-ascorbic acid, and Lentemin. The third true leaf of N. benthamiana and the first true leaf of tomato were inoculated with the GFP-tagged ToMV inoculum 3 days after treatment. Levels of ToMV mRNA accumulation in inoculated and uninoculated upper leaves collected at 3 dpi (N. benthamiana) and 6 dpi (tomato) are shown. Bars indicate the standard error (SE). The experiment was independently performed twice (n>3 per experiment). Asterisks indicate significant differences between the control and treated plants at each leaf position by one-way ANOVA with Dunnett’s multiple comparisons test (p<0.01).
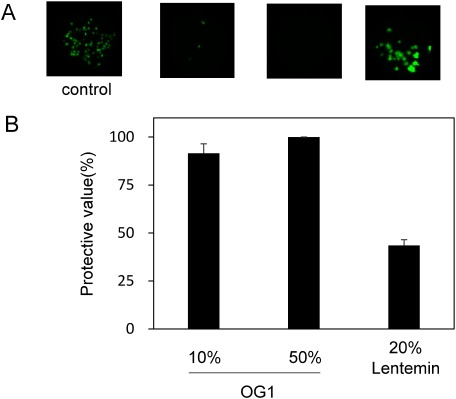
Given the effectiveness of A. zerumbet OG1 extract, we also tested N. benthamiana plants treated with it against TMV tagged with GFP. To prepare inoculum of TMV, plasmids of pTMV-30B:GFP (Shivprasad et al. 1999) were linearized by restriction with KpnI, and used as a template for in vitro transcription using the AmpliCap-Max T7 High Yield Message Maker Kit according to the manufacturer’s instructions at 37°C for 40 min. The transcription mixture was then diluted 6-fold in water. Inoculation was performed using the same method used in the ToMV-inoculation. The results showed that the extract protected treated N. benthamiana plants against TMV infection as well (Figure 5).
Figure 5. Effects of foliar application of the extracts from A. zerumbet plants against GFP-tagged TMV virions. The plants were treated with water, the extracts from A. zerumbet isolate OG1, and Lentemin, and then inoculated with GFP-tagged TMV 3 days after treatment. (A) GFP foci formed on N. benthamiana at 3 dpi. Pictures were taken under blue-light irradiation with ChemiDoc™ MP Imaging System (Bio-rad, USA). (B) The number of GFP spots formed on the inoculated leaves was calculated at 3 dpi. Bars indicate the standard error (SE). The experiment was independently performed twice (n>3 per experiment).
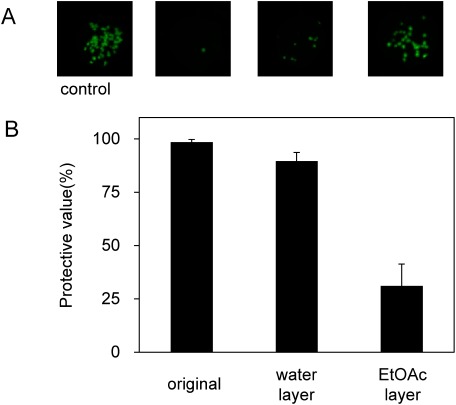
Producing enough extract for evaluation was challenging, and if the extract were to be used in a commercial application, it would need to be produced in larger quantities. To increase the amount of active fraction obtained, the aqueous extract of A. zerumbet isolate OG1 was separated by ethyl acetate (EtOAc). The EtOAc layer was dried using a rotary evaporator and stored at −80°C until use. The water layer was also freeze-dried and stored at −80°C until use. The concentration of these fractions was adjusted to be the concentration of the original solution. The application of water layer of the extraction was also able to protect N. benthamiana plants against ToMV infection (Figure 6).
Figure 6. Effects of foliar application of the extract of A. zerumbet isolate OG1 separated by ethyl acetate as plant protection against GFP-tagged ToMV virions. The plants were treated with ethyl acetate and the extract present in the water layers, and then inoculated with GFP-tagged ToMV 3 days after treatment. (A) GFP foci formed on N. benthamiana at 3 dpi. Pictures were taken under blue-light irradiation with ChemiDoc™ MP Imaging System (Bio-rad, USA). (B) The number of GFP spots formed on the inoculated leaves was calculated at 3 dpi. Bars indicate the standard error (SE). The experiment was independently performed twice (n>3 per experiment).
Since the different Alpinia isolate extracts had varying levels of antiviral activity, we determined how closely related the isolates were by using sequences of nrDNA internal transcribed spacer (ITS), which is the spacer DNA situated between the small-subunit ribosomal RNA (rRNA) and large-subunit rRNA genes as molecular markers to distinguish the Alpinia plants. Sequences of ITS regions for all of the species examined were identical, indicating that the species and isolates are closely related to each other.
In this study, we demonstrated that the extract of Alpinia plants, especially isolate OG1, had high antiviral activity on two different viruses, ToMV and TMV, when used to treat tomato and N. benthamiana. Moreover, in comparing GFP foci and qPCR analysis, measuring GFP foci appears to be a useful indicator for measuring antiviral activity. Based on our results, the extract of Alpinia plants has potential as an antiviral reagent for practical application in crop production. A limitation on the use may be producing sufficient extract quantities; however, our results also showed that extract production protocols can be improved. Future work on identifying the active compound within the extracts with antiviral activity and characterizing the antiviral mechanism will provide further insight into the use of antiviral agents in crops.
Acknowledgments
We would like to thank Dr. Masayuki Ishikawa (National Institute of Agrobiological Sciences) for kindly providing the pTLBN.G3. We would also like to thank Hiroshi Higa and Tsutomu Agena (Bios no Oka) and Ayumi Nakaima (Southeast Botanical Gardens Co., Ltd.) for kindly providing plant materials. We would like to thank Aya Okada, Shoko Nieda, Masami Miyamoto, and Yuriko Imai of RIBS as well for their excellent technical assistance. This work was supported by grants from the Project of the NARO Bio-oriented Technology Research Advancement Institution (Integration research for agriculture and interdisciplinary fields to Y.Y. and Y.N. and Research program on development of innovative technology to Y.Y., T.H. and Y.N.), Science and technology research promotion program for agriculture, forestry, fisheries and food industry to Y.Y., T.H. and Y.N. and by Grants-in-Aid for Scientific Research (KAKENHI) to M.N. (17KT0152).
Abbreviations
- TMV
tobacco mosaic virus
- ToMV
tomato mosaic virus
References
- Elzaawely AA, Xuan TD, Koyama H, Tawata S (2007a) Antioxidant activity and contents of essential oil and phenolic compounds in flowers and seeds of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. Food Chem 104: 1648–1653 [Google Scholar]
- Elzaawely AA, Xuan TD, Tawata S (2007b) Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chem 103: 486–494 [Google Scholar]
- Fujiwara A, Shimura H, Masuta C, Sano S, Inukai T (2013) Exogenous ascorbic acid derivatives and dehydroascorbic acid are effective antiviral agents against Turnip mosaic virus in Brassica rapa. J Gen Plant Pathol 79: 198–204 [Google Scholar]
- Hudson JB (1990) Antiviral compounds from Plants. Boca Raton, CRC Press, Florida, p 200
- Kubota K, Tsuda S, Tamai A, Meshi T (2003) Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J Virol 77: 11016–11026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma KD, Hashinaga F (2004) Alpinia leaf extract: A prospective natural food preservative. J Sci Ind Res (India) 63: 689–693 [Google Scholar]
- Shivprasad S, Pogue GP, Lewandowski DJ, Hidalgo J, Donson J, Grill LK, Dawson WO (1999) Heterologous sequences greatly affect foreign gene expression in tobacco mosaic virus-based vectors. Virology 255: 312–323 [DOI] [PubMed] [Google Scholar]
- Teschke R, Xuan TD (2018) Viewpoint: A contributory role of shell ginger (Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm) for human longevity in Okinawa, Japan? Nutrients 10: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PT, Tawata S (2015) Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 20: 16723–16740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonaha M, Aoki Y, Iwabuchi M, Nagahata T, Fujiwara Y (2013) The Inhibitory effects of the hot-water extract of fermented shell ginger (Alpinia speciosa K. Schum.) on melanogenesis in B16 mouse melanoma cells. J Home Economics Japan 64: 215–224 [Google Scholar]
- Zaitlin M (1998) The discovery of the causal agent of the tobacco mosaic disease (PDF). In: Kung SD, Yang SF (eds) Discoveries in Plant Biology. World Publishing Co., Hong Kong, pp 105–110
- Zoghbi MGB, Andrade EHA, Maia JGS (1999) Volatile constituents from leaves and flowers of Alpinia speciosa K. Schum. and A. purpurata (Viell.) Schum. Flavour Fragrance J 14: 411–414 [Google Scholar]