Abstract
Lithospermum erythrorhizon, a medicinal plant growing in Asian countries, produces shikonin derivatives that are lipophilic secondary metabolites. These red naphthoquinone pigments are traditionally used as a natural drug and a dye in East Asia. In intact L. erythrorhizon plants, shikonin derivatives are produced in the root epidermal cells and secreted into extracellular spaces. The biosynthetic pathway for shikonin derivatives remains incompletely understood and the secretion mechanisms are largely unknown. Understanding the molecular mechanisms underlying shikonin biosynthesis and transport in L. erythrorhizon cells requires functional analysis of candidate genes using transgenic plants. To date, however, standard transformation methods have not yet been established. This study describes an efficient method for L. erythrorhizon transformation using hairy roots by Rhizobium rhizogenes strain A13, present domestically in Japan. Hairy roots of L. erythrorhizon were generated from explants of the axenic shoots that were infected with R. rhizogenes strain A13. Integration into the genome was assessed by PCR amplifying a transgene encoding green fluorescent protein (GFP) and by monitoring GFP expression. This method enhanced transformation efficiency 50–70%. Although methods for the systematic stable transformation of L. erythrorhizon plants have not yet been reported, the method described in this study resulted in highly efficient stable transformation using hairy roots. This method enables the functional analysis of L. erythrorhizon genes.
Keywords: hairy root, Lithospermum erythrorhizon, meropenem, Rhizobium rhizogenes A13, shikonin, stable transformation
Introduction
Lithospermum erythrorhizon is a herbal medicinal plant native to Japan, Korea, and China. Because of their many bioactivities, including their anti-inflammatory and anti-bacterial properties, dried roots of L. erythrorhizon, called “shikon” in Japanese, have been traditionally used as a crude drug (Yazaki 2017). The main medicinal compounds of this crude drug are shikonin derivatives, consisting lipophilic red naphthoquinone pigments.
The biosynthesis of this red pigment has been intensively studied. The key regulatory reaction in its biosynthetic pathway is the conjugation of a geranyl moiety to p-hydroxybenzoic acid, yielding m-geranyl-p-hydroxybenzoic acid, the first lipophilic intermediate in this pathway. This reaction is catalyzed by a membrane-bound prenyltransferase, LePGT1 (Ohara et al. 2009). The substrates of LePGT1, geranyl diphosphate and p-hydroxybenzoic acid, are derived from the mevalonate and phenylpropanoid pathways, respectively. After this reaction, at least five more enzymes are involved in synthesizing the shikonin molecule (Wong 2019). To date, however, only one cytochrome P450 enzyme, CYP76B74, has been found to be involved in the biosynthesis of shikonin in Arnebia euchroma (Wang et al. 2019).
Plant metabolites include many lipophilic compounds, which are often secreted into extracellular spaces. In Laminaceae plants, monoterpenes are produced by secretory cells and secreted into the subcuticular cavity inside the glandular trichome (Lange and Croteau 1999). Furanocoumarins are biosynthesized in the flavedo of citrus species and accumulate specifically in oil cavities, which are apoplastic spaces surrounded by epithelial cells (Voo et al. 2012). The molecular mechanisms by which these compounds are secreted from cells that produce them, however, are still largely unknown. Shikonin derivatives are produced in the root epidermal cells of L. erythrorhizon and secreted from these cells into their extracellular spaces. Under dark conditions, as in soil, L. erythrorhizon plants produce large quantities of shikonin derivatives. Among the benefits of using L. erythrorhizon to study lipid secretion are the red color of shikonin derivatives, making them visible by bright field microscopy, and their auto-fluorescence, allowing their subcellular movement and accumulation pattern to be monitored by confocal microscopy (Tatsumi et al. 2016). Another advantage is that shikonin production is reversibly regulated by other factors, with skinonin production negatively regulated by illumination and ammonium ion and positively regulated by methyl jasmonate and copper ion. Illumination is the strongest inhibitor of shikonin biosynthesis in L. erythrorhizon plants and dominantly shuts off its production under all conditions (Yazaki 2017). These properties enhance the ability to study shikonin derivative transport mechanisms in L. erythrorhizon as a model system, and may lead to greater understanding of the intracellular trafficking of lipophilic metabolites.
Comparative transcriptome and proteome analyses of L. erythrorhizon identified several candidate genes that may be involved in shikonin biosynthesis and/or transport (Takanashi et al. 2019). Evaluating the function and involvement of these genes in shikonin production and transport requires their introduction into L. erythrorhizon. These genes may induce the overproduction or suppression of shikonin derivatives, providing insight into the molecular mechanisms that control the production and transport of shikonin derivatives.
Hairy root is an attractive material for studying the molecular mechanism involved in the biosynthesis and transport of shikonin derivatives, because these compounds are specifically produced in root tissues, especially by epidermal cells. Hairy roots of L. erythrorhizon have been generated with the soil-borne bacterium Rhizobium rhizogenes strain ATC C15834 as a gene carrier (Fang et al. 2016; Yazaki et al. 1998), with these transfected genes reported to function in the cultured hairy roots of L. erythrorhizon. However, a detailed standardized protocol for the generation of L. erythrorhizon hairy roots has not yet been established. Rather, concerns were raised about the reproducibility of current protocols, including the low efficiency of transgene integration and the low survival rate on selection medium. Another serious limitation was usage of R. rhizogenes strain ATC C15834, which originated in the United States, is strictly controlled by the Plant Protection Station. Due to this limitation, hairy roots harboring transgenes of interest cannot be shared with collaborators outside the licensed laboratory, even if they have permission. There is, therefore, a strong need to identify a usable R. rhizogenes strain, which generates hairy roots at a rate similar to that of ATC C15834. This new strain may facilitate more multilateral, collaborative studies of shikonin production. The present study reports the establishment of a highly efficient method for transforming L. erythrorhizon using a Japanese strain of R. rhizogenes, termed A13, and describes a detailed experimental protocol for the stable transformation of L. erythrorhizon hairy roots.
Materials and methods
Plant materials and growth condition
Axenic cultures of L. erythrorhizon shoots were grown on 1/2 MS agar medium at pH 5.7 under 16 h-light/8 h-dark photoperiod for two weeks.
RNA extraction and cDNA synthesis
Total RNA was extracted from shikonin-producing cultured cells of L. erythrorhizon using RNeasy Plant Mini Kit (QIAGEN, Hirden, Germany) and treated with DNase using DNA-free Kit (Ambion, Carlsbad, CA). The resulting DNA-free RNA samples were reverse transcribed to cDNA using SuperScript Reverse Transcriptase (Invitrogen, Carlsbad, CA).
Vector construction
The gene encoding monomeric green fluorescent protein (mGFP) contained in the vector pGWB405m (Nakagawa et al. 2007b; Segami et al. 2014) was amplified using PrimeSTAR Max DNA Polymerase (TaKaRa, Kusatsu, Japan), and the amplified fragment was inserted into the vector pENTR/D-TOPO by TOPO reaction (Invitrogen). The mGFP gene was inserted into the destination vector pGWB502 (Nakagawa et al. 2007b) by LR recombination reaction (Invitrogen), yielding the plasmid pGWB502_mGFP.
The open reading frame (ORF) of LePGT1 in L. erythrorhizon cDNA was amplified with KOD Plus Neo DNA polymerase (Toyobo, Osaka, Japan) and cloned into the pENTR/D-TOPO by In-Fusion reaction (Clontech, Mountain View, CA). The ORF of LeDI2 in the vector pDR196_LeDI2 (Yazaki et al. 2001) was amplified by KOD Plus Neo and cloned into pENTR/D-TOPO by TOPO reaction. The LePGT1 and LeDI2 fragments were inserted into the destination vectors pGWB405m and pGWB505 (Nakagawa et al. 2007b), respectively, by LR recombination, yielding the plasmids pGWB405m_LePGT1 and pGWB505_LeDI2, respectively.
LeDI2-GFP in pGWB505_LeDI2 was amplified with KOD Plus Neo and inserted into two vectors, pBin19 derivative (Gatz et al. 1992) digested with XbaI and SacI and pRI201AN (Takara) digested with SalI and SacI, using T4 ligase (Promega, San Louis Obispo, CA), yielding the plasmids pBin19_LeDI2-GFP and pRI201AN_LeDI2-GFP, respectively. To amplify LeDI2-GFP for insertion to pBin19, overlap PCR was performed to mutagenize the XbaI restriction site. Primers used for plasmid construction are listed in Supplementary Table S1.
Transformation of R. rhizogenes strain A13
R. rhizogenes strain A13 (MAFF 02-10266) was kindly provided by Dr. M. Mii of Chiba University (Daimon et al. 1990), and vector constructs were introduced into this strain using the modified freeze–thaw transformation method. Briefly, strain A13 was mixed with 200–1,000 ng plasmid, frozen in liquid nitrogen for two minutes and heated at 37°C for five minutes. This freeze–thaw treatment was repeated three times. After incubation at 28°C for three hours, the A13 mixture was incubated on YEB medium containing antibiotics at 28°C for two to three days, and bacterial transformation was confirmed by colony PCR.
Rhizobium-mediated transformation of L. erythrorhizon
L. erythrorhizon hairy roots were generated by Rhizobium-mediated transformation in a manner similar to that used to transform tomato plants (Sun et al. 2006). Detailed procedure is described in Results and discussion section. Callus induction medium which are used to induce dedifferentiated tissues consisted of 1/2 MS medium containing 1% sucrose, 1 µM potassium indoleacetate (Nacalai Tesque, Kyoto, Japan), and 10 µM kinetin (Sigma, St. Louis, MO). Inoculation buffer to suspend R. rhizogenes consisted of 1/2 MS liquid medium containing 1% sucrose and 100 µM acetosyringone (Sigma). Co-cultivation medium was 1/2 MS medium without KH2PO4, CaCl2·2H2O, NH4NO3, and KNO3, but containing 1% sucrose and 100 µM acetosyringone to improve hairy root induction (Valimehr et al. 2014). Root induction medium consisted of 1/2 MS medium containing 1% sucrose, and 20 mg l−1 meropenem trihydrate (Wako) to eliminate R. rhizogenes. Generated hairy roots were excised from the explants and cultured in root induction medium. All procedures were performed under axenic conditions. Details of the compositions of each medium are described in Supplementary Table S2.
Genotyping
Genomic DNA was extracted from each hairy root line before selection on the plate with antibiotics by use of DNA extraction buffer (200 mM Tris–HCl [pH 8.0], 250 mM NaCl, 25 mM EDTA [pH 8.0], and 0.5% SDS). GFPh and Actin genes were amplified by KOD FX Neo DNA polymerase (Toyobo). Primers used for genotyping are listed in Supplementary Table S1.
Fluorescent microscopic analysis
GFP fluorescence of hairy roots was detected using a stereoscopic M165 FC fluorescent microscope (Leica Microsystems, Wetzlar, Germany) equipped with a CCD camera (VB-7010, Keyence, Osaka, Japan). The bright field and GFP fluorescent images were taken at the same field of view.
Sensitivity of hairy roots to antibiotics
The established lines of pBI121_GFPh-transformed hairy roots, which had been emerged and excised from stem explants, were grown on 1/2 MS medium containing different concentrations (0–100 mg l−1) of hygromycin B (Wako, Osaka, Japan) or kanamycin sulfate (Wako), in the presence of 1% sucrose and 20 mg l−1 meropenem, for 20 days. Elongation of root tissue was measured using ImageJ software (http://rsb.info.nih.gov/ij).
Accession numbers
Nucleotide sequence data used in this study can be found in the GenBank/EMBL data libraries under accession numbers AB055078.1 (LePGT1) and D45901.1 (LeDI2).
Results and discussion
Application of R. rhizogenes strain A13 to L. erythrorhizon
Because L. erythrorhizon roots produce shikonin derivatives, functional analysis of hairy roots, to which target genes are introduced for knock out/down or overexpression, can facilitate the molecular biological analysis of the mechanisms underlying the biosynthesis and transport of shikonin derivatives. The present study, therefore, involved the use of domestic R. rhizogenes strain A13 to stably transform the hairy roots of L. erythrorhizon in a high efficient and reproducible manner.
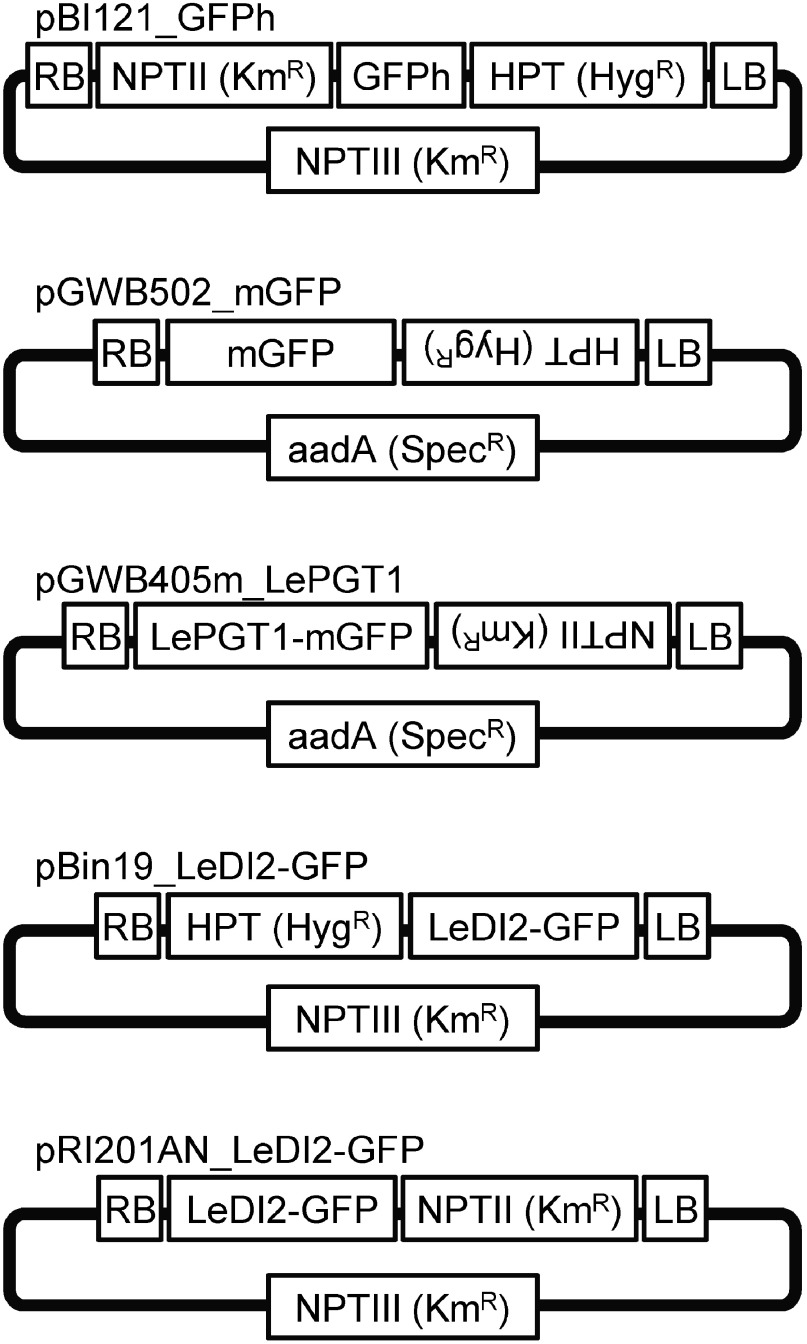
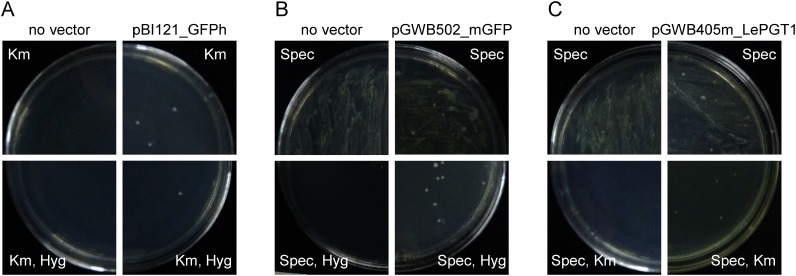
Initially, plasmid vectors were introduced into R. rhizogenes strain A13 using a freeze–thaw method. Strain A13 was transformed with pBI121_GFPh, which was generated by the insertion of an endoplasmic reticulum marker and a hygromycin resistance gene into a pBI121 vector (Ueda et al. 2010) (Figure 1). In this vector, three gene expression cassettes are located in the T-DNA region; an endoplasmic reticulum marker and a hygromycin resistance gene are driven by 35S promoter, and a kanamycin resistance gene is driven by nopaline synthase promoter. The transformed A13 was selected by growth on both plates containing only kanamycin and containing kanamycin with hygromycin (Figure 2A). In contrast, introduction into A13 of the plasmids pGWB502_mGFP and pGWB405m_LePGT1, both of which encode spectinomycin resistance genes for bacterial selection (Figure 1), resulted in complete coverage of the plates containing spectinomycin (Figure 2B, C). In parallel, control A13, which was subjected to the freeze–thaw protocol in the absence of plasmids, also completely covered the plates. Therefore, to isolate transformed A13 as a colony, we used antibiotics for plant selection in addition to spectinomycin, because the resistant gene of plant selection marker that is located in T-DNA region of plant binary vector is sometimes used for bacterial selection (Akama et al. 1992; Nakagawa et al. 2007a). A13 transformants grew on agar media containing antibiotics for plant selection, hygromycin (pGWB502_mGFP) or kanamycin (pGWB405m_LePGT1), whose expression was driven by nopaline synthase promoter, whereas A13 bacteria without plasmids did not grow on these media (Figure 2B, C). These results showed that spectinomycin resistance gene was not able to be used as a selection marker in R. rhizogenes. On the other hand, A13 did not show the resistance for kanamycin and hygromycin, thus these resistance genes in T-DNA region were effective on the selection of the transformed strains.
Figure 1. Structure of the plasmids vectors used in this study. LB, left border; RB, right border; NPT, neomycin phosphotransferase; HPT, hygromycin phosphotransferase; aad, aminoglycoside adenylyltransferase; Km, kanamycin; Hyg, hygromycin; Spec, spectinomycin.
Figure 2. Screening of transformed R. rhizogenes strain A13. A13 clones transformed with pBI121_GFPh (A), pGWB502_mGFP (B), and pGWB405m_LePGT1 (C) were culture on media containing antibiotics, for bacterial selection alone (upper panel) or for both bacterial and plant selections (lower panel). Each left panel shows A13 transformation without vector and each right panel shows A13 transformed with each vector plasmid.
Procedure to generate hairy roots of L. erythrorhizon harboring target genes
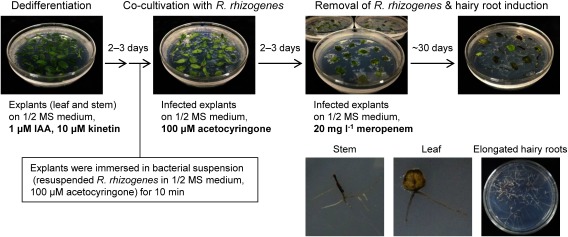
A multi-step protocol was developed to generate hairy roots of L. erythrorhizon harboring target genes of interest (Figure 3).
Figure 3. Schematic showing the method of L. erythrorhizon transformation using R. rhizogenes strain A13. Upper panels represent transformation flow and lower panels show emerged hairy roots from stem and leaf explants and elongated hairy roots.
Explants of leaves and stems cut from axenic shoot cultures of L. erythrorhizon were dedifferentiated on callus induction medium for 2–3 days under illumination.
Colonies of transformed R. rhizogenes A13 were checked by colony PCR (Supplementary Figure S1) and cultured overnight in YEB liquid medium at 28°C.
After reaching an OD600 0.6–0.8, the cultured A13 preparations were centrifuged at 1,500×g at 4°C for 10 min.
The pellet was resuspended in inoculation buffer and diluted to OD600 0.6.
The lightly dedifferentiated L. erythrorhizon explants were immersed in the bacterial suspension for 10 min.
These explants were placed onto sterilized paper towels to eliminate excess bacterial suspension.
The infected explants were transferred to co-cultivation medium with the leaf adaxial side downward and incubated in the dark for 2–3 days at 25°C.
These co-cultivated explants were subsequently transferred to root induction medium with the leaf adaxial side upward and incubated in the dark, with transfer to fresh medium every 10–30 days.
Hairy roots usually emerged from explants about 30 days after the explants were transferred to root induction medium.
These hairy roots were analyzed to determine their transgenicity by PCR-based genotyping.
Detection of transgenes in hairy roots
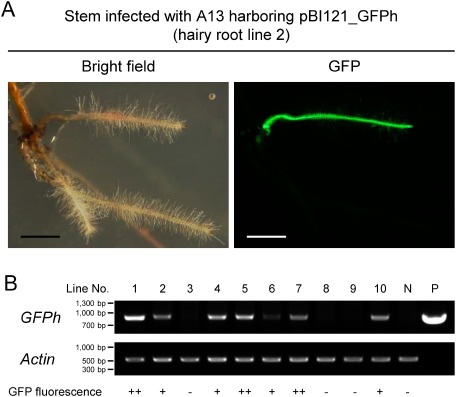
Fluorescent microscopic examination of pBI121_GFPh-transformed hairy roots showed that, although multiple hairy roots often emerged from each infection site of stem explants, some were positive for GFP fluorescence and the others were not (Figure 4A). To determine whether transgenes had integrated into these hairy roots, genomic DNA was extracted from each and the insertion of transgenes was assessed by PCR. Amplified GFPh was found in transferred T-DNA regions of hairy roots positive for GFP fluorescence, but not in the hairy roots negative for GFP fluorescence (Figure 4B). Additionally, some hairy roots harboring GFPh showed strong GFP fluorescence whereas the others showed weak GFP fluorescence (Figure 4B), indicating that the expression of transgene largely varied depending on each hairy root clone.
Figure 4. GFP fluorescence and genotyping of hairy roots. Analysis of hairy roots generated from stem explants, which had been infected with A13 harboring pBI121_GFPh. (A) Generated hairy root (line 2) showing GFP fluorescence. Bright field (left) and GFP fluorescence (right) images were taken at the same field of view. Scale bars, 2 mm. (B) Confirmation of transgene integration by genotyping PCR. Genomic DNA extracted from hairy roots was subjected to PCR amplification of the transgene GFPh and the endogenous gene Actin. Controls consisted of genomic DNA extracted from hairy roots generated with wild-type A13 (N, no plasmid) and the plasmid pBI121_GFPh (P). ++, very strong fluorescence; +, strong fluorescence; −, no fluorescence.
To compare the transformation efficiency for two points, difference of inserted sequences and vector backbone, the efficiency was investigated using three distinct types of vectors: pBI121_GFPh, pBin19_LeDI2-GFP, and pRI201AN_LeDI2-GFP (Table 1). pBin19_LeDI2-GFP and pRI201AN_LeDI2-GFP were constructed by insertion into pBin19 and pRI201AN, respectively, of a GFP-fused LeDI2 gene that encodes a shikonin production-related protein (Yazaki et al. 2001) (Figure 1). Eighty-three stem explants were infected with the A13 strain harboring pBI121_GFPh. After incubation on root induction medium for 30 days, 63 hairy roots emerged from 42 of these stems. The 51 roots that showed elongation on root induction medium were subjected to PCR-based genotyping to determine whether transgene was inserted. Of these 51 roots, 33 were found to possess the transgene, GFP. In contrast, infection of 66 leaf explants resulted in the emergence of 11 hairy roots from seven of these explants, suggesting that the rate of root emergence from stem explants (51%) was much higher than that from leaf explants (11%). Six lines of transgenic hairy roots were obtained from the 11 elongated roots of leaf explants, indicating that the transformation efficiency was slightly higher when stems (65%) than leaves (55%) were used as infected explants.
Table 1. Transformation efficiency of hairy roots.
| Vector backbone | Inserted gene | Type of infected explants | Infected explants [a] | Explants with emerged hairy roots [b] | Rate of root emergence [b/a] | Elongated hairy roots [c] | Transgenic hairy roots [d] | Transformation efficiency [d/c] |
|---|---|---|---|---|---|---|---|---|
| pBI121 | GFPh | Stem | 83 | 42 | 51% | 51 (35) | 33 (28) | 65% |
| Leaf | 66 | 7 | 11% | 11 (7) | 6 (5) | 55% | ||
| pBin19 | LeDI2-GFP | Stem | 34 | 5 | 15% | 4 (3) | 2 (2) | 50% |
| Leaf | 42 | 5 | 12% | 6 (5) | 2 (2) | 33% | ||
| pRI201AN | LeDI2-GFP | Stem | 58 | 15 | 26% | 24 (15) | 2 (2) | 8% |
| Leaf | 39 | 9 | 23% | 7 (7) | 5 (5) | 71% |
The rate of root emergence (%) was calculated by dividing the number of explants with emerged hairy roots (b) by the number of infected explants (a). Transformation efficiency (%) was calculated by dividing the number of transgenic hairy roots (d) by the number of elongated hairy roots (c). Total numbers of hairy roots are listed in the columns “Elongated hairy roots” and “Transgenic hairy roots”. Numbers of explants with elongated hairy roots and those with elongated transgenic hairy roots are listed in parentheses of (c) and (d), respectively. The number of replication in this experiment is one time.
Use of the two other plasmids, pBin19_LeDI2-GFP and pRI201AN_LeDI2-GFP, to generate transgenic hairy roots showed that the rates of root emergence from the stem and leaf explants were similar (Table 1). That is the transformation efficiencies of pBin19_LeDI2-GFP were 50% for stems and 33% for leaves as infected explants, whereas the transformation efficiencies of pRI201AN_LeDI2-GFP were 8.3% for stems and 71% for leaves. These results indicate that strain A13 can yield transgenic hairy roots of L. erythrorhizon. Despite the same inserted sequences, the transformation efficiencies of pBin19_LeDI2-GFP and pRI201AN_LeDI2-GFP were different, which suggested the transformation efficiency depended on the vector backbone. In addition, while both pBI121_GFPh and pBin19_LeDI2-GFP were originated from same vector backbone, these transformation efficiencies were different, indicating that inserted sequences also affect transformation efficiency.
Selection of hairy roots harboring transgenes
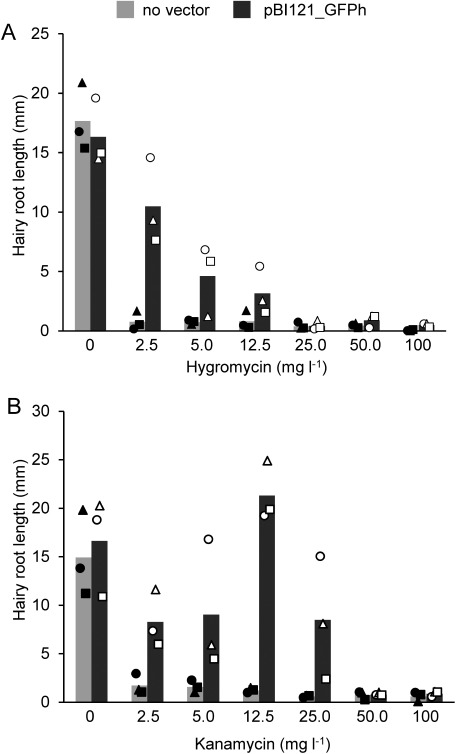
The selection of transgene-positive cells is a critical step in the establishment of transgenic lines. Both non-transgenic and transgenic cells can proliferate during cultivation of generated hairy roots, as both cell types usually exist as chimeras in the transgenic hairy roots. Antibiotics, including kanamycin, hygromycin, and bialaphos, are often used to select and isolate transgenic lines. However, suitable antibiotics and their effective concentrations differ greatly, depending on plant species and plant tissues. To determine the optimal antibiotic concentrations for L. erythrorhizon hairy roots, pBI121_GFPh-transformed hairy roots, which were emerged from stem explants and the transgene integration was confirmed by genotyping PCR before giving the selection pressure, were cultured with various concentrations of kanamycin and hygromycin (0–100 mg l−1) for 20 days, and root elongation was evaluated. Growth of hairy roots generated by wild-type A13 was arrested on 2.5 mg l−1 hygromycin, whereas roots transformed with pBI121_GFPh were able to grow on media containing up to 12.5 mg l−1 hygromycin (Figure 5A). As hygromycin concentration increased, root elongation decreased, indicating that 2.5 mg l−1 hygromycin was sufficient to select hairy root clones harboring the marker gene, hygromycin phosphotransferase (HPT). Similarly, growth of control roots was arrested on plates containing 2.5 mg l−1 kanamycin (Figure 5B) as well as hygromycin. Hairy roots harboring pBI121_GFPh elongated on media containing 25 mg l−1 kanamycin, with resistance to kanamycin being prominent at 12.5 mg l−1 (Figure 5B). These results suggested that 12.5 mg l−1 kanamycin was appropriate to select transformed hairy roots. A comparison of these antibiotics indicated that the hairy roots of L. erythrorhizon were more sensitive to hygromycin than to kanamycin.
Figure 5. Resistance of hairy root lines to different concentrations of antibiotics. Hairy roots transformed with pBI121_GFPh were grown on medium containing the plant selection antibiotics hygromycin (A) or kanamycin (B), at concentrations of 0–100 mg l−1, for 20 days, and the extended root lengths were measured. Hairy roots generated with non-transformed A13 were used as a control (no vector). Bars represent the mean of three independent lines. These lines are genetically independent hairy root lines emerged from distinct explant tissues. Different shaped plots depict different independent lines and each plot represents the mean of six roots from each line.
The present study describes a transformation method using R. rhizogenes A13, a domestic strain in Japan, to obtain transgenic L. erythrorhizon with high transformation efficiency. A13 generated hairy roots from L. erythrorhizon in a comparable efficiency as ATC C15834, as reported in Cannabis sativa (Berahmand et al. 2016). Several experimental conditions, such as the plant tissue used for infection and the type and concentration of antibiotics were optimized, from the transformation of R. rhizogenes to the screening of generated transgenic hairy roots. In this study, we found the highest transformation efficiency was 71% when leaf was infected with pRI201AN_LeDI2-GFP vector. The combination of stem/leaf explants with pBI121_GFPh vector and that of stem with pBin19_LeDI2-GFP vector also showed more than 50% of transformation efficiency. These efficiencies of present method using domestic strain A13 were much higher than that of previous report (20%) using ATC C15834 (Yazaki et al. 1998). Further, selection using optimal concentration of antibiotics enables only transgenic cells to grow in the hairy root. The time required from infection to obtaining L. erythrorhizon hairy roots containing the gene of interest was about three months. In conclusion, our transgenic protocol will allow in-planta functional analysis of candidate genes from L. erythrorhizon, and may contribute to understanding the molecular mechanisms underlying shikonin biosynthesis and secretion.
Acknowledgments
The authors thank Dr. Masahiro Mii and Dr. Tomoko Igawa (Chiba University) for providing R. rhizogenes strain A13 and Dr. Hirobumi Yamamoto (Toyo University) for providing cultured L. erythrorhizon cells. The authors also thank Dr. Takahiro Hamada (Okayama University of Science), Dr. Haruko Ueda, and Dr. Ikuko Hara-Nishimura (Konan University) for providing the pBI121_GFPh vector; Dr. Shoji Segami and Dr. Masayoshi Maeshima (Nagoya University) for providing the pGWB405m vector; and Dr. Tsuyoshi Nakagawa (Shimane University) for providing the pGWB502 and pGWB505 vectors. The authors are also grateful to Ms. Kaori Kanazawa and Mr. Kohei Nakanishi (Kyoto University) for technical support.
Abbreviations
- PGT
p-hydroxybenzoic acid geranyltransferase
- LeDI2
L. erythrorhizon dark-inducible gene 2
- GFP
green fluorescent protein
Funding
This work was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI to K.Y. (JP19H05638), a JSPS Research Fellowship for Young Scientists DC2 to K.T. (201811502), and the New Energy and Industrial Technology Development Organization (NEDO) to K.Y. (16100890-0).
Supplementary Data
References
- Akama K, Shiraishi H, Ohta S, Nakamura K, Okada K, Shimura Y (1992) Efficient transformation of Arabidopsis thaliana: Comparison of the efficiencies with various organs, plant ecotypes and Agrobacterium strains. Plant Cell Rep 12: 7–11 [DOI] [PubMed] [Google Scholar]
- Berahmand F, Beizaee N, Nayyeri MD, Sharafi A, Manjili HK, Danafar H, Sohi HH (2016) Cannabis sativa L. genetically transformed root based culture via Agrobacterium rhizogenes. Pharm Biomed Res 2: 13–18 [Google Scholar]
- Daimon H, Fukami M, Mii M (1990) Hairy root formation in peanut by the wild type strains of Agrobacterium rhizogenes. Plant Tissue Cult Lett 7: 31–34 (in Japanese) [Google Scholar]
- Fang R, Wu F, Zou A, Zhu Y, Zhao H, Zhao H, Liao Y, Tang RJ, Yang T, Pang Y, et al. (2016) Transgenic analysis reveals LeACS-1 as a positive regulator of ethylene-induced shikonin biosynthesis in Lithospermum erythrorhizon hairy roots. Plant Mol Biol 90: 345–358 [DOI] [PubMed] [Google Scholar]
- Gatz C, Frohberg C, Wendenburg R (1992) Stringent repression and homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants. Plant J 2: 397–404 [DOI] [PubMed] [Google Scholar]
- Lange BM, Croteau R (1999) Genetic engineering of essential oil product in mint. Curr Opin Plant Biol 2: 139–144 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T (2007a) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007b) Improved Gateway binary vectors: High-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Ohara K, Muroya A, Fukushima N, Yazaki K (2009) Functional characterization of LePGT1, a membrane-bound prenyltransferase involved in the geranylation of p-hydroxybenzoic acid. Biochem J 421: 231–241 [DOI] [PubMed] [Google Scholar]
- Segami S, Makino S, Miyake A, Asaoka M, Maeshima M (2014) Dynamics of vacuoles and H+-pyrophosphatase visualized by monomeric green fluorescent protein in Arabidopsis: Artifactual bulbs and native intravacuolar spherical structures. Plant Cell 26: 3416–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HJ, Uchii S, Watanabe S, Ezura H (2006) A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol 47: 426–431 [DOI] [PubMed] [Google Scholar]
- Tatsumi K, Yano M, Kaminade K, Sugiyama A, Sato M, Toyooka K, Aoyama T, Sato F, Yazaki K (2016) Characterization of shikonin derivative secretion in Lithospermum erythrorhizon hairy roots as a model of lipid-soluble metabolite secretion from plants. Front Plant Sci 7: 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi K, Nakagawa Y, Aburaya S, Kaminade K, Aoki W, Saida-Munakata Y, Sugiyama A, Ueda M, Yazaki K (2019) Comparative proteomic analysis of Lithospermum erythrorhizon reveals regulation of a variety of metabolic enzymes leading to comprehensive understanding of the shikonin biosynthetic pathway. Plant Cell Physiol 60: 19–28 [DOI] [PubMed] [Google Scholar]
- Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I (2010) Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci USA 107: 6894–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valimehr S, Sanjarian F, Sohi HH, Sharafi A, Sabouni F (2014) A reliable and efficient protocol for inducing genetically transformed roots in medicinal plant Nepeta pogonosperma. Physiol Mol Biol Plants 20: 351–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voo SS, Grimes HD, Lange BM (2012) Assessing the biosynthetic capabilities of secretory glands in Citrus peel. Plant Physiol 159: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang R, Liu T, Lv C, Liang J, Kang C, Zhou L, Guo J, Cui G, Zhang Y, et al. (2019) CYP76B74 catalyzes the 3″-hydroxylation of geranylhydroquinone in shikonin biosynthesis. Plant Physiol 179: 402–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DCJ (2019) Harnessing integrated omics approaches for plant specialized metabolism research: New insights into shikonin biosynthesis. Plant Cell Physiol 60: 4–6 [DOI] [PubMed] [Google Scholar]
- Yazaki K (2017) Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol 34: 131–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K, Matsuoka H, Shimomura K, Bechthold A, Sato F (2001) A novel dark-inducible protein, LeDI-2, and its involvement in root-specific secondary metabolism in Lithospermum erythrorhizon. Plant Physiol 125: 1831–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki K, Tanaka S, Matsuoka H, Sato F (1998) Stable transformation of Lithospermum erythrorhizon by Agrobacterium rhizogenes and shikonin production of the transformants. Plant Cell Rep 18: 214–219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.