Abstract
Kenaf, Hibiscus cannabinus, is a fiber-enriched plant belonging to Malvaceae and is an important fiber crop. The features of kenaf of being fast-growing and fiber-enriched suggest the potential for the use of kenaf in biomass and materials. Here, we modified procedures for regeneration from kenaf explants in order to establish efficient genetic modification for improving the property of kenaf as a material. We tested several tissues of kenaf seedlings for callus induction and subsequent shoot regeneration by supplying several combinations of plant growth factors. We show the cotyledons to be the best tissue for efficient callus induction, and the rate for callus induction reached ∼98% on an improved callus inducing medium. We also show the efficient regeneration of kenaf from cotyledon-derived calli achieved by shoot induction on a shoot-inducing medium. We also achieved seed setting of the regenerated kanaf plants under a regulated growth condition in a chamber. Our efficient regeneration method and seed setting condition will enable the production of stably transformed kenaf that can improve the properties of kenaf as a material.
Keywords: kenaf, Malvaceae, seed setting, tissue culture
Introduction
Kenaf, Hibiscus cannabinus, is an annual plant belonging to Malvaceae, which includes cotton and okra. Kenaf grows quickly and has a fiber-enriched woody base; thus, it is regarded as an important fiber crop. The properties of kenaf enable it to be applied to biocomposite materials as a filler, especially in automotive parts (Holbery and Houston 2006). Conventional breeding can improve the property of kenaf, however, establishment of complete genetic transformation procedure is essential to dramatically improve the physical properties of kenaf as a structural material in short term. Among the several ways of genetic transformation established in plants, agrobacterium-mediated transformation is the most popular, and, in general, it utilizes the regeneration of plants from the callus that can be induced by the combination of the plant growth regulators auxins and cytokinins. There are several reports on regeneration of shoots from kenaf using various explants (Herath et al. 2004; McLean et al. 1992; Samanthi et al. 2013; Srivatanakul et al. 2000; Zapata et al. 1999). Using the kenaf shoot apex or shoot, shoots are efficiently regenerated without the callus state or adding plant growth regulators, but the addition of N6-benzyladenine (BA) and thidiazuron, cytokinin-like plant growth regulators, can increase the number of regenerating shoots (Herath et al. 2004; Srivatanakul et al. 2000; Zapata et al. 1999). McLean et al. (1992) showed efficient induction of the callus from kenaf internodal stems by supplementing 1-naphthaleneacetic acid (NAA)/BA or 2,4-dichlorophenoxyacetic acid (2,4-D)/kinetin as the auxin/cytokinin combination, but the regeneration efficiency was lower than 15%. Samanthi et al. (2013) showed the induction of the kenaf callus from an intact cotyledon with a maximum of 80% efficiency by adding indole-3-butyric acid (IBA) as the auxin and BA, and the callus regenerated shoots with 68.7% efficiency. Since the callus induction and shoot regeneration has not been achieved 100% efficiency yet, there are still possibilities to improve the regeneration efficiency. Moreover, information regarding the flowering and seed setting is lacking in kenaf. Here, we report the complete regeneration of kenaf including improved callus induction and shoot regeneration, flowering, and seed setting methods.
Materials and methods
Plant material and seed germination
Kenaf Hibiscus cannabinus seeds were purchased from Fujita seed corporation (Hyogo, Japan). Kenaf seeds were sterilized with 70% (v/v) ethanol for 20 min and then with 1% (w/v) sodium hypochlorite for 30 min, followed by rinsing with sterilized water. The seeds were placed on Murashige-Skoog (MS) (Murashige and Skoog 1962) medium (Sigma-Aldrich Co., USA) containing 3% (w/v) sucrose (Wako Pure Chemical Corporation, Japan) with or without growth regulators and were cultivated at 22°C under a 16 h photoperiod in a growth chamber (LH-100S, NK System, Japan).
Condition for callus induction
For callus induction from seeds, sterilized seeds were placed on MS medium containing 3% (w/v) sucrose, 0.3 mg l−1 of 2,4-D (Wako Pure Chemical Corporation, Japan), 0.3 mg l−1 of Kinetin (Sigma-Aldrich Co., USA) and 0.3% (w/v) of phytagel (Sigma-Aldrich Co., USA), and then were cultivated for one week at 22°C under a 16-h daily photoperiod. Regarding hypocotyls, those from aseptically grown 10-day-old seedlings were cut into a 5-mm length or chopped into smaller fragments, and then were placed on MS medium containing 3% (w/v) sucrose, 2.0 mg l−1 of 2,4-D, 2.0 mg l−1 of Kinetin and 0.3% (w/v) phytagel. They were cultivated for one to four weeks at 22°C under a 16-h daily photoperiod. Regarding cotyledons, those from aseptically grown 10-day-old seedlings were cut into 5-mm pieces and were placed on MS, 1/2 MS, or Gamborg B5 medium (Sigma-Aldrich Co., USA) containing 2% (w/v) sucrose, supplemented with 1.5 mg l−1 of BA (Sigma-Aldrich Co., USA) and 0–0.05 mg l−1 of IBA (Sigma-Aldrich Co., USA) and 0.3% (w/v) phytagel or 0.8% (w/v) agar. Calli were induced for three weeks at 22°C under dark conditions.
Conditions for shoot induction
Calli induced from seeds were transferred to MS medium containing 2% (w/v) sucrose, 1.0 mg l−1 of BA and 0.3% (w/v) phytagel, and then were cultivated for four weeks at 30°C under continuous light. Calli induced from hypocotyls were transferred to MS medium containing 2% (w/v) sucrose, 0.1–5.0 mg l−1 of BA, 0.1–5.0 mg l−1 of forchlorfenuron (Tokyo Chemical Industry Co., Ltd., Japan) or 1.0 mg l−1 of trans-Zeatin (Sigma-Aldrich Co., USA), 0–0.3 mg l−1 of Indole-3-acetic acid (IAA) (Sigma-Aldrich Co., USA), and 0.3% (w/v) phytagel, and then were cultivated for four weeks at 30°C under continuous light. Calli induced from cotyledons were transferred to MS medium containing 2% (w/v) sucrose, 0.5–2.0 mg l−1 of BA, 0–0.05 mg l−1 of IBA, 0.3 mg l−1 of GA (Sigma-Aldrich Co., USA), and 0.3% (w/v) phytagel, and then were cultivated for four weeks at 30°C under continuous light.
Flowering and seed maturation
Shoots regenerated from the callus were cultivated on MS medium containing 2% (w/v) sucrose and 0.3% (w/v) phytagel at 30°C under continuous light. The plants were then transferred to a 2 : 1 mixture of soil and vermiculite and were cultivated at 22°C or 30°C under continuous light. The plants were then cultivated at 22°C or 30°C under an 8 h daily photoperiod in a growth chamber LH-100S.
Results and discussion
Callus induction from kenaf tissues
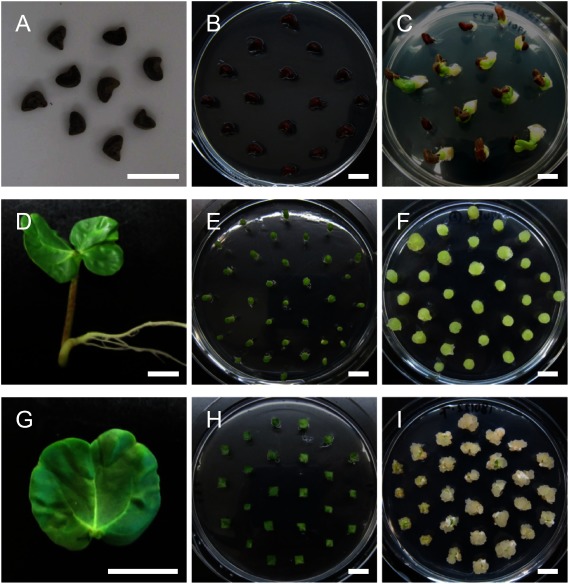
The callus induction efficiency depends on the tissues type. We tested the seeds, hypocotyls, and cotyledons of kenaf seedlings for their capability to induce callus by cultivating them on a callus-inducing medium (CIM), a widely used auxin-rich medium to induce the plant callus. By using some combinations of auxins and cytokinins reported to induce callus from kenaf explants (McLean et al. 1992; Samanthi et al. 2013), all the tested tissues induced the callus efficiently (Figure 1). However, the calli from the tissues showed some differences in properties as follows.
Figure 1. Callus induction from kenaf seed, hypocotyl, and cotyledon. (A–C) Callus induction from kenaf seeds. Kenaf seeds (A) were sterilized and then were placed on CIM (B). Calli were induced from the seeds by cultivating on CIM for one week (C). (D–F) Callus induction from the kenaf hypocotyl. Hypocotyls from 10-day-old seedlings (D) were cut and placed vertically on CIM (E). Calli were induced from the fragmented hypocotyls by cultivating on CIM for one week (F). (G–I) Callus induction from the kenaf cotyledon. Cotyledons from 10-day-old seedlings (G) were cut into small pieces and then were cultivated on CIM (H). Calli were induced from cotyledon pieces by cultivating on CIM for three weeks (I). Bars=10 mm.
In the case of seeds, after root development in germination, calli were induced from the roots, but subsequent cultivation of the calli on a shoot-inducing medium (SIM), a cytokinin-rich medium for the induction of shoot regeneration, induced the regeneration of only roots but not shoots (Supplementary Figure S1, Supplementary Table S1). This suggests that the calli induced from roots were not suitable for shoot regeneration, inconsistent with the related species Hibiscus sabdariffa (Konar et al. 2018). Hypocotyls were fragmented and then were placed vertically or horizontally, or placed horizontally after longitudinal split, or were chopped since wounding generally promotes callus formation (Ikeuchi et al. 2013), and then they were cultivated on CIM. These sectioning and cultivating procedures, except for chopping, succeeded in inducing the callus, and the vertically cultivated hypocotyls seemed to show the highest efficiency for induction (Figure 1D–F, Supplementary Figure S2, Supplementary Table S1). Too much fragmentation may disturb callus induction from hypocotyls. The calli from the vertically cultivated hypocotyls were then tested for the induction of shoot regeneration on SIM with several combinations of plant growth regulators. However, no shoots were regenerated from these hypocotyl-derived calli (Supplementary Figure S3, Supplementary Table S1), suggesting the calli derived from hypocotyls were not suitable for shoot regeneration, as well as the calli from roots. Cotyledons showed efficient callus induction on CIM supplemented with indole-3-butyric acid (IBA) in combination with BA when they were cut into 5-mm pieces, and subsequent cultivation on SIM induced greening of the calli as will hereinafter be described in detail, which should lead to shoot development. These results show that kenaf cotyledons, but not seeds or hypocotyls, efficiently induce calli that potentially regenerate fertile shoots.
Optimization of conditions for callus induction from cotyledons
To increase the efficiency of callus induction from cotyledons, we carried out optimization of plant growth regulator concentrations, basal salts, and solidification reagents for callus induction. First, we analyzed the effect of several concentrations of IBA and BA as supplements to MS medium. The results showed that 1.5 mg l−1 of BA +0.01 mg l−1 of IBA and 1.5 mg l−1 of BA only yielded the highest and lowest callus induction from the kenaf cotyledon in terms of efficiency and homogeneity of the calli with low induction of root formation, respectively (Table 1, Supplementary Figure S4). This shows that IBA is important for the efficient induction of callus from the cotyledon, on the contrary, an excess amount of IBA inhibits callus formation. The root formation was induced by a low cytokinin/auxin ratio in agreement with a report by Skoog and Miller (1957). It is noted that the callus-induction efficiency reached 97.0–98.8% in the medium supplemented with 1.5 mg l−1 of BA +0.005–0.01 mg l−1 of IBA (Table 1), which shows an almost complete induction of callus from cotyledon pieces, and the efficiency was higher than that obtained by Samanthi et al. (2013).
Table 1. Callus induction and root formation efficiency from kenaf cotyledons.
| IBA concentration (mg l−1) combination with 1.5 mg l−1 of BA | ||||
|---|---|---|---|---|
| 0 | 0.005 | 0.01 | 0.05 | |
| Rate of callus formation (callus/explant) | 61.1% (214/350) | 98.8% (395/400) | 98.0% (392/400) | 97.0% (388/400) |
| Rate of root formation (%) (with root/callus) | 0% (0/214) | 10.1% (40/395) | 4.8% (19/392) | 38.1% (148/388) |
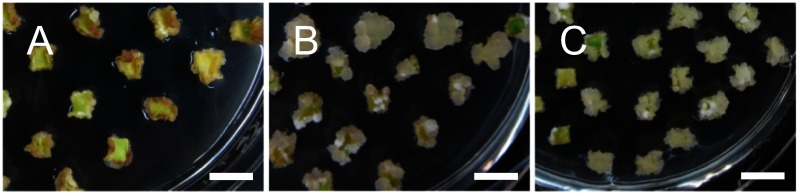
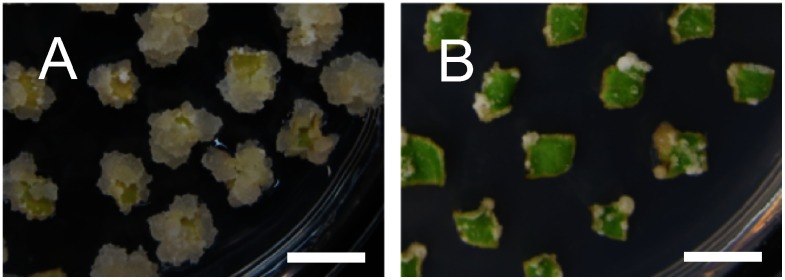
Next, we tested the effect of inorganic basal salts, Gamborg B5 (Gamborg et al. 1968), MS, or 1/2 MS, supplemented with 1.5 mg l−1 of BA +0.01 mg l−1 of IBA for the induction of callus from cotyledon pieces. In contrast to the callus hardly induced in the B5 medium, the callus was efficiently induced in both MS medium, especially in the MS medium (Figure 2, Table 2), suggesting that MS is the more appropriate basal salts than Gamborg B5 and that callus induction from the cotyledon requires a high concentration of nutrients. Using the MS medium supplemented with 1.5 mg l−1 of BA +0.01 mg l−1 of IBA, our analysis also clarified that the use of agar instead of phytagel (gellan gum) as a solidification reagent significantly disturbed callus induction from the kenaf cotyledon (Figure 3, Table 2), suggesting that solidification reagents are important for kenaf callus induction as well as the effect of plant growth regulators.
Figure 2. Effect of inorganic basal salts for callus induction from cotyledons. Calli were induced from cotyledon pieces on CIM composed of Gamborg B5 (A), 1/2 MS (B), or MS (C) supplemented with 1.5 mg l−1 of BA and 0.01 mg l−1 of IBA by cultivating for three weeks. Bars=10 mm.
Table 2. Callus induction efficiency from kenaf cotyledons.
| Basal salt | B5 | MS | 1/2MS | MS | |
|---|---|---|---|---|---|
| Solidification reagent | phytagel | phytagel | agar | ||
| Rate of callus formation (callus/explant) | 88.4% (221/250) | 93.6% (234/250) | 86.0% (215/250) | 97.5% (195/200) | 71.5% (143/200) |
Figure 3. Effect of solidification reagents on callus induction from cotyledons. Calli were induced from cotyledon pieces on MS medium containing 1.5 mg l−1 of BA and 0.01 mg l−1 of IBA, and 0.3% phytagel (A) or 0.8% agar (B) by cultivating for three weeks. Bars=10 mm.
Shoot regeneration from the callus
Using the induced kenaf calli, we next tested the regeneration of shoots with several patterns of plant growth regulator concentrations. Calli formed on CIM were transferred to SIM supplemented with 0.3 mg l−1 of gibberellin (GA) in which the concentration of BA and IBA were modified to 0.5–2 mg l−1 and 0–0.05 mg l−1, respectively. As a result of cultivation on the SIM, greening of the calli was induced in the SIMs containing both 1–2 mg l−1 of BA and 0.05 mg l−1 of IBA, but not in other SIMs (Supplementary Figure S5, Table 3). This suggests a lower concentration of BA (<1 mg l−1) or IBA (<0.05 mg l−1) affects the greening of calli. Subsequent cultivation of these greened calli on the SIM supplemented with 1.5 mg l−1 of BA +0.05 mg l−1 of IBA +0.3 mg l−1 of GA led to the regeneration of multiple shoots from a single callus (Figure 4C). The efficiency of shoot regeneration from the greened calli was 63.4% (118 of 186), and the efficiency of shoot regeneration in our experiment was lower than that obtained by Samanthi et al. (2013) in spite of same hormone composition used. To induce the development of the regenerated shoot with roots, they were transferred to an MS medium without any plant growth regulators. Cultivation on the MS led to the induction of shoot elongation and root development (Figure 4D, E) that could grow and mature in the soil (Figure 4F). Thus, our data showed that SIM supplemented with 1.5 mg l−1 of BA +0.05 mg l−1 of IBA +0.3 mg l−1 of GA is sufficient for the greening and shoot development of the kenaf callus.
Table 3. The efficiency for greening of callus on SIM.
| BA (mg l−1) | 0.5 | 1 | 1.5 | 2 | 1.5 | |
| IBA (mg l−1) | 0.05 | 0.01 | 0 | |||
| Greening/callus | 25% | 35% | 43% | 41% | 14% | 0% |
Figure 4. Regeneration of kenaf from cotyledons via calli. (A) Calli induced from cotyledon pieces by cultivating on CIM for 21 days. (B) Calli cultivated on SIM for four weeks. (C) Greening of calli after 14 weeks cultivation. (D) Multiple shoots regenerated from the calli. (E) The shoots cultivated on growth regulator-free MS medium. (F) Plants cultivated on soil. Bars=10 mm.
Flowering and seed maturation of kenaf
Producing the next generation is essential to obtain stable homozygous transgenic plants. However, the regulated condition for flowering and seed maturation of kenaf, especially the regenerated one, is not well defined. We cultivated regenerated kenaf plants on soil at 22°C or 30°C, lowest or highest temperature for optimum growing of kenaf (Rowell and Stout 2006), respectively, under an 8 h photoperiod in a chamber. Cultivation of kenaf at 22°C succeeded in the development of flower buds, while cultivation at 30°C failed, suggesting a high temperature affected the development of the flower bud (Figure 5B). The kenaf plants bearing flower buds were then cultivated at 22°C or 30°C because, in general, the accumulated temperature after flowering influences the seed maturation; cultivating at a higher temperature is expected to accelerate seed maturation. Both kenaf plants cultivated at 22°C and 30°C induced flowering normally (Figure 5C), and subsequent cultivation at 22°C led to the development of fruits from the flowers and maturation of seeds in the fruits (Figure 5D), although the initial few fruits were dropped without seed maturation. Contrastingly, cultivation at 30°C after flowering failed to set fruit because of the development of an abscission layer at the base of the fruit in the initial stage of fruit development. These results collectively suggest that high temperature inhibits the development of flower and fruit buds but not the growth of the flower. Finally, the individual kenaf plants developed three to four matured fruits at 22°C, and the fruits contained 8 to 14 seeds (Figure 5E). The seeds from the regenerated kenaf were evaluated by sawing them on MS medium. Forty-seven seeds from four fruits of the regenerated kenaf were germinated at a rate of 62%, suggesting a sufficient germination rate of the seeds from the regenerated kenaf. In conclusion, cultivation at 22°C under an 8-h daily photoperiod fulfills the conditions for both flowering and seed development of kenaf.
Figure 5. Flowering and seed maturation of regenerated kenaf. (A) Regenerated kenaf grown on soil in a growth chamber. (B–E) Representative images of flower buds (B), a flower (C), an immature seed pod, and matured seeds (E). Bars=10 cm in A and 1 cm in B–E.
Conclusion
In this report, we discussed the successful complete regeneration of kenaf, Hibiscus sabdariffa, via the callus state induced from cotyledon explants. Multiple shoot regeneration from the greened callus, flowering, and seed maturation of the regenerated plant in a regulated condition enabled production of the next generation. In addition to the agrobacterium infection method that is still incomplete for the production of the kenaf T2 generation (Banks et al. 1993; Herath et al. 2005; Kojima et al. 2004), a new gene transformation method with functional peptide has recently been developed in plants (Chuah and Numata 2018; Lakshmanan et al. 2013; Miyamoto et al. 2018). In combination with the transformation methods, our complete regeneration method would produce stable genetically transformed kenaf. Since kenaf can be used as a filler of composite materials (Holbery and Houston 2006; Rozman et al. 2011; Sanadi et al. 1995), modification of the physical property of kenaf by stable transformation is expected to be applied to the development of improved materials.
Acknowledgments
This work was supported by Grants-in-Aid from the Japan Science and Technology Agency Exploratory Research for Advanced Technology (JST-ERATO; Grant No., JPMJER1602).
Supplementary Data
References
- Banks SW, Gossett DR, Lucas C, Millhollon EP, LaCelle MG (1993) Agrobacterium-mediated transformation of kenaf (Hibiscus cannabinus L.) with the β-glucuronidase (GUS) gene. Plant Mol Biol Report 11: 101–104 [Google Scholar]
- Chuah JA, Numata K (2018) Stimulus-responsive peptide for effective delivery and release of DNA in plants. Biomacromolecules 19: 1154–1163 [DOI] [PubMed] [Google Scholar]
- Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50: 151–158 [DOI] [PubMed] [Google Scholar]
- Herath SP, Suzuki T, Hattori K (2004) Multiple shoot regeneration from young shoots of kenaf (Hibiscus cannabinus). Plant Cell Tissue Organ Cult 77: 49–53 [Google Scholar]
- Herath SP, Suzuki T, Hattori K (2005) Factors influencing Agrobacterium mediated genetic transformation of kenaf. Plant Cell Tissue Organ Cult 82: 201–206 [Google Scholar]
- Holbery J, Houston D (2006) Natural-fiber-reinforced polymer composites applications in automotive. Jom-Us 58: 80–86 [Google Scholar]
- Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: Mechanisms of induction and repression. Plant Cell 25: 3159–3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Shioiri H, Nogawa M, Nozue M, Matsumoto D, Wada A, Saiki Y, Kiguchi K (2004) In planta transformation of kenaf plants (Hibiscus cannabinus var. aokawa No. 3) by Agrobacterium tumefaciens. J Biosci Bioeng 98: 136–139 [DOI] [PubMed] [Google Scholar]
- Konar S, Karmakar J, Ray A, Adhikari S, Bandyopadhyay TK (2018) Regeneration of plantlets through somatic embryogenesis from root derived calli of Hibiscus sabdariffa L. (Roselle) and assessment of genetic stability by flow cytometry and ISSR analysis. PLoS One 13: e0202324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan M, Kodama Y, Yoshizumi T, Sudesh K, Numata K (2013) Rapid and efficient gene delivery into plant cells using designed peptide carriers. Biomacromolecules 14: 10–16 [DOI] [PubMed] [Google Scholar]
- McLean KS, Lawrence GW, Reichert NA (1992) Callus induction and adventitious organogenesis of kenaf (Hibiscus cannabinus L.). Plant Cell Rep 11: 532–534 [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Tsuchiya K, Numata K (2019) Block copolymer/plasmid dna micelles postmodified with functional peptides via thiol-maleimide conjugation for efficient gene delivery into plants. Biomacromolecules 20: 653–661 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Rowell RM, Stout HP (2006) Jute and kenaf. In: Lewin M (ed) Handbook of Fiber Chemistry, Third Edition. CRC Press, pp405–449
- Rozman H, Musa L, Azniwati A, Rozyanty A (2011) Tensile properties of kenaf/unsaturated polyester composites filled with a montmorillonite filler. J Appl Polym Sci 119: 2549–2553 [Google Scholar]
- Samanthi PW, Puad AM, Suhaimi N, Kumar SM, Aini ASN (2013) In vitro shoot regeneration from leaf explants of kenaf (Hibiscus cannabinus L.). Sains Malays 42: 1505–1510 [Google Scholar]
- Sanadi AR, Caulfield DF, Jacobson RE, Rowell RM (1995) Renewable agricultural fibers as reinforcing fillers in plastics: Mechanical properties of kenaf fiber-polypropylene composites. Ind Eng Chem Res 34: 1889–1896 [Google Scholar]
- Skoog F, Miller CO (1957) Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11: 118–130 [PubMed] [Google Scholar]
- Srivatanakul M, Park S, Sanders J, Salas M, Smith R (2000) Multiple shoot regeneration of kenaf (Hibiscus cannabinus L.) from a shoot apex culture system. Plant Cell Rep 19: 1165–1170 [DOI] [PubMed] [Google Scholar]
- Zapata C, Srivatanakul M, Park S-H, Lee B-M, Salas MG, Smith RH (1999) Improvements in shoot apex regeneration of two fiber crops: Cotton and kenaf. Plant Cell Tissue Organ Cult 56: 185–191 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.