Abstract
Rationale: Antenatal factors, such as chorioamnionitis, preeclampsia, and postnatal injury, are associated with an increased risk for bronchopulmonary dysplasia (BPD) and pulmonary hypertension (PH) after preterm birth. IGF-1 (insulin-like growth factor-1) is markedly decreased in normal preterm infants, but whether IGF-1 treatment can prevent BPD or PH is unknown.
Objectives: To evaluate whether postnatal treatment with rhIGF-1 (recombinant human IGF-1)/BP3 (binding peptide 3) improves lung growth and prevents PH in two antenatal models of BPD induced by intraamniotic exposure to endotoxin (ETX) or sFlt-1 (soluble fms-like tyrosine kinase 1), and in a postnatal model due to prolonged hyperoxia.
Methods: ETX or sFlt-1 were administered into the amniotic sac of pregnant rats at Embryonic Day 20 to simulate antenatal models of chorioamnionitis and preeclampsia, respectively. Pups were delivered by cesarean section at Embryonic Day 22 and treated with rhIGF-1/BP3 (0.02–20 mg/kg/d intraperitoneal) or buffer for 2 weeks. Study endpoints included radial alveolar counts (RACs), vessel density, and right ventricular hypertrophy (RVH). Direct effects of rhIGF-1/BP3 (250 ng/ml) on fetal lung endothelial cell proliferation and tube formation and alveolar type 2 cell proliferation were studied by standard methods in vitro.
Measurements and Main Results: Antenatal ETX and antenatal sFlt-1 reduced RAC and decreased RVH in infant rats. In both models, postnatal rhIGF-1/BP3 treatment restored RAC and RVH to normal values when compared with placebo injections. rhIGF-1/BP3 treatment also preserved lung structure and prevented RVH after postnatal hyperoxia. In vitro studies showed that rhIGF-1/BP3 treatment increased lung endothelial cell and alveolar type 2 cell proliferation.
Conclusions: Postnatal rhIGF-1/BP3 treatment preserved lung structure and prevented RVH in antenatal and postnatal BPD models. rhIGF-1/BP3 treatment may provide a novel strategy for the prevention of BPD in preterm infants.
Keywords: insulin-like growth factor-1, angiogenesis, lung development, prematurity, pulmonary hypertension
At a Glance Commentary
Scientific Knowledge on the Subject
Antenatal determinants, including chorioamnionitis, preeclampsia, and postnatal hyperoxia, contribute to the development of bronchopulmonary dysplasia (BPD) and pulmonary hypertension (PH) after preterm birth, but therapies that prevent BPD and PH are lacking.
What This Study Adds to the Field
We found that early treatment with IGF-1 (recombinant human insulin-like growth factor-1)/BP3 preserves lung growth and maintains lung function in infant rats after exposure to antenatal stresses, such as endotoxin and sFlt-1 (soluble fms-like tyrosine kinase 1), as well as postnatal hyperopia. These findings support the speculation that IGF-1 treatment may prevent BPD and PH in human infants.
Despite improvements in care, preterm children remain at high risk for mortality and respiratory morbidities due to the development of bronchopulmonary dysplasia (BPD) (1). BPD is the chronic lung disease of prematurity that develops in infants who require respiratory support at birth because of immaturity of the preterm lung. BPD occurs in over 40% of infants born below 29 weeks gestation, leading to roughly 15,000 new cases in the United States each year (1–4). BPD is strongly associated with significant complications, including the prolonged need for mechanical ventilation, respiratory support and oxygen therapy, longer duration of hospitalization, and higher rates of late respiratory disease and nonrespiratory comorbidities (5, 6). Sustained abnormalities of lung function, poor exercise tolerance, and the need for chronic respiratory medications throughout childhood and adolescence are also increased in former preterm infants (7–11).
Advances in postnatal care have increased survival of extremely preterm infants, but the incidence of BPD has not changed over the past few decades (4), which may contribute to greater numbers of extremely preterm infants who are at highest risk for severe BPD (2, 5). Although postnatal factors, such as hyperoxia, mechanical ventilation, and others, contribute to the risk for BPD, epidemiologic studies have further identified important roles for antenatal factors in disease pathogenesis (9, 11–21). Recent findings emphasize antenatal factors that are independent of postnatal injury as major determinants of risk for BPD and late respiratory outcomes (20, 21). An NHLBI-sponsored workshop discussed the importance of prenatal influences on lung growth and development and subsequent respiratory function after preterm birth that can persist throughout childhood (11).
Antenatal factors, such as chorioamnionitis (CA), preeclampsia (PE), and others, are strongly associated with an increased risk for BPD, especially when associated with intrauterine growth restriction (IUGR) as a biomarker for severe placental dysfunction and fetal stress (15, 22–29). Early changes in circulating angiogenic peptides, including decreased proangiogenic factors, increased antiangiogenic factors (including sFlt-1 [soluble fms-like tyrosine kinase 1], an endogenous vascular endothelial growth factor inhibitor that is markedly increased in the blood and amniotic fluid of women with PE), and decreased endothelial progenitor cells, are associated with both abnormal placental vascular disease and high risk for BPD and pulmonary hypertension (PH) (27–32). These data support the hypotheses that antenatal mechanisms that promote an antiangiogenic fetal environment contribute to high risk for BPD and PH in preterm infants and suggest novel targets for disease prevention.
Although mechanisms linking placental dysfunction with BPD risk are incompletely understood, early disruption of critical signaling pathways, including IGF-1 (insulin-like growth factor-1), which has strong angiogenic activity, have been implicated in the etiology of BPD (32). IGF-1 plays a key role in normal lung development in utero and impaired IGF-1 signaling contributes to disease pathobiology in experimental models of acute lung injury and PH (33–36). Importantly, low IGF-1 levels are also strongly associated with IUGR and PE (31) and persist after preterm birth (37), but whether treatment with IGF-1 can improve respiratory outcomes after preterm birth and prevent BPD and PH is unknown.
Thus, the need for preventive strategies remains a major challenge to better improve long-term respiratory outcomes after preterm birth. Based on the important role of IGF-1 in lung development and reports that low IGF levels shortly after birth are associated with the subsequent development of BPD, we propose the hypothesis that postnatal treatment with rhIGF-1 (recombinant human IGF-1)/BP3 (binding peptide 3) would enhance lung alveolar and vascular growth and lung function and prevent the development of PH in experimental BPD as induced by antenatal stress.
To test this hypothesis, we performed a series of studies using two models of BPD: one model that is associated with CA as induced by intraamniotic exposure to endotoxin (ETX) (38) and the second model that mimics the effects of PE, which is induced by intraamniotic injection of sFlt-1 (39, 40). We then tested the hypothesis that IGF-1 therapy could preserve lung growth and function in a traditional postnatal model of BPD due to hyperoxia exposure. We report that postnatal therapy with rhIGF-1/BP3 improves lung structure and function and prevents PH in each of these animal models, suggesting that IGF-1 may provide a novel strategy for the prevention of BPD.
Methods
General Approach
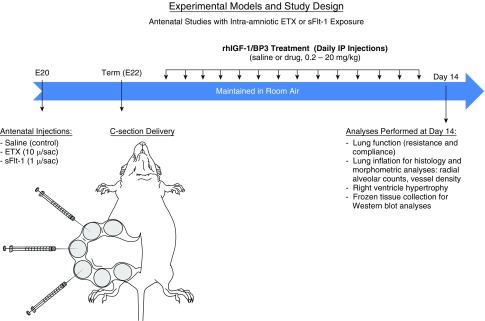
Based on strong epidemiologic and clinical data demonstrating the critical role of antenatal determinants in the pathogenesis of BPD in preterm infants (20, 21), we studied the impact of rhIGF-1/BP3 therapy on lung alveolar and vascular growth and lung function. In this study, we used rodent models of CA as induced by intraamniotic ETX injection and PE as mimicked by sFlt-1 injection, as previously described (38–40) (Figure 1). To further determine whether rhIGF-1/BP3 therapy could improve lung structure and function induced by postnatal hyperoxia exposure, we also studied the effects of rhIGF-1/BP3 in a long-standing model of the “old BPD,” which reflects the adverse effects of postnatal interventions, such as hyperoxia (1, 41), without consideration of the important roles of antenatal determinants in BPD pathobiology of the modern era (2–4, 20, 21, 42, 43). All procedures and protocols were approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center. See the online supplement for more details regarding methods used in this study.
Figure 1.
Schematic illustration of experimental models and study design for antenatal stress induced by intraamniotic injections of ETX and sFlt-1 (soluble fms-like tyrosine kinase 1) to simulate chorioamnionitis and preeclampsia. BP3 = binding peptide 3; C-section = cesarean section; E20 = Embyronic Day 20; E22 = Embryonic Day 22; ETX = endotoxin; rhIGF = recombinant human insulin-like growth factor.
Study Design
Experimental PE due to intraamniotic sFlt-1 administration
Details regarding methods used to develop the approach to establish this animal model of PE have been previously published (38, 39). As illustrated in Figure 1, pregnant rats were prepared for receiving intraamniotic injections at 20 days gestation (term: 22 d). Pregnant rats were randomly assigned to saline (control rats) or sFlt-1 treatment. The control group received 50 μl of normal saline per amniotic sac and the sFlt-1 group received 1 μg of recombinant human sFlt-1-Fc diluted to 50 μl with normal saline per sac. Under aseptic conditions, a midline abdominal incision of 3 to 4 cm in length was made to expose the amniotic sacs for intraamniotic injections.
Cesarean section delivery and postnatal treatment.
Two days after intraamniotic injections, cesarean section was performed under general anesthesia, as previously described (38–41). Newborn rats were randomized to daily treatment with rhIGF-1/BP3 at one of four doses (0.02, 0.2, 2, or 20 mg/kg) or saline (control rats) by intraperitoneal injection. Dose selection was based on preliminary studies that showed efficacy without any signs of toxicity even at the highest dose. At 14 days of age, rat lungs were harvested for physiologic and histological assessments, which included assessment of lung mechanics, inflation of lungs for morphometric analysis, collecting and freezing lung tissue for Western blot analyses, and heart collections for later assessment of right ventricular hypertrophy (RVH).
Experimental CA due to intraamniotic ETX administration
Similarly, pups received either ETX (10 μg/sac) or saline (control rats) and were delivered by cesarean section on Embryonic Day 22. Pups were randomized to daily treatment with rhIGF-1/BP3 or saline and were killed at 14 days for study endpoints. To determine whether brief rhIGF-1/BP3 treatment would be as effective as more prolonged therapy, we randomized 12 pups for rhIGF-1/BP3 treatment at a dose of 20 mg/kg daily for only 3 days, which were then killed at 14 days of age for studies of lung structure.
Experimental model of BPD induced by postnatal hyperoxia exposure
To determine the effects of rhIGF-1/BP3 in a postnatal model of BPD, we studied the effects of IGF-1/BP3 therapy in rat pups exposed to prolonged hyperoxia, as previously described (44). Neonatal rat pups were exposed to high oxygen tension (FiO2, 0.90) from Day 1 to 14 of postnatal life. Animals received daily intraperitoneal injections of rhIGF-1/BP3 (2 mg/kg daily) or saline (control rats) for 14 days. We further compared the effects of short (3 d) versus prolonged (14 d) therapy on lung structure at 2 weeks of age, while maintaining hyperoxia conditions throughout the entire 2-week study period. Studies on the effects of brief rhIGF-1/BP3 treatment were performed to explore whether early intervention to accelerate postnatal adaptation of the lung during hyperoxia would be sufficient to provide sustained improvement in lung growth.
Study Measurements
Lung function
Lung function was determined in 14-day old pups with the flexiVent system (SCIREQ), as previously described (40).
Tissue for histological analysis.
Lungs were inflated with 4% paraformaldehyde and maintained at 20 cm H2O pressure for overnight fixation (38–40). A 2-mm-thick transverse section was taken from the midplanes of the right lower and left lobes of the fixed lungs per animal to process and embed in paraffin wax.
Immunohistochemistry
Slides with 5-μm paraffin sections were stained with hematoxylin and eosin for assessment of alveolar structure, and with von Willebrand factor, an endothelial cell-specific marker, for assessment of vascular density.
Morphometric analysis.
Radial alveolar counts (RACs) and pulmonary vessel density were determined by standard morphometric techniques (38–41, 45, 46).
Indices of RVH.
The right ventricle (RV) and left ventricle plus septum were dissected and weighed. The ratios of RV to left ventricle plus septum weights and RV/body weights were determined to evaluate RVH.
Western blot analysis.
Proteins were obtained from rapidly frozen lung samples for Western blot analysis of IGF-1 and endothelial nitric oxide synthase (eNOS) per standard methods (see online supplement).
RNA isolation and microarray analysis.
The whole lungs of offspring from intraamniotic ETX exposed and control pups were harvested on Day 0 of life (n = 4 for each group). Total RNA was extracted using the miRNeasy Mini Kit (Qiagen) per manufacturer’s instructions. Following isolation, total RNA was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). RNA from these samples were analyzed by standard procedures using Affymetric microarrays (Clariom D Rat Transcriptome Array 1.0). Additional details are provided in the online supplement.
Isolated Neonatal Lung Endothelial Cells and Alveolar Type 2 Cells
Lung endothelial cells (LECs) and alveolar type 2 cells (AT2Cs) were collected from neonatal lungs and studied in vitro by standard methods. We also studied the effects of antenatal ETX exposure on LEC and AT2C growth, and the in vitro response to IGF-1 treatment.
Statistical Analyses
Statistical analysis was performed with the GraphPad Prism 8.0 software package (GraphPad Software). Statistical comparisons were made between groups using the t test or ANOVA with Kruskal-Wallis and Dunn’s post hoc analysis for significance. P < 0.05 was considered significant. Data are presented as interquartile range with minimum–maximum data points.
Results
Effects of Postnatal rhIGF-1/BP3 Treatment after Antenatal sFlt-1 Exposure
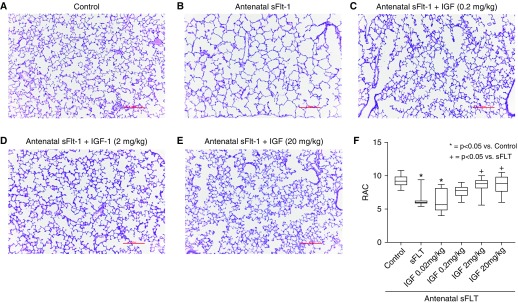
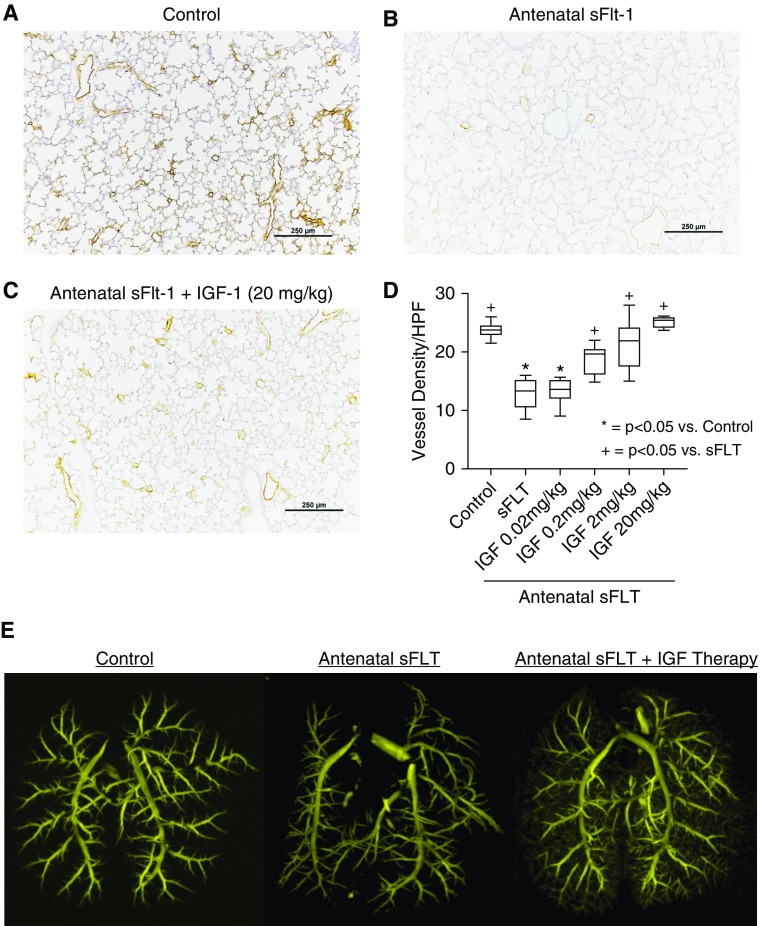
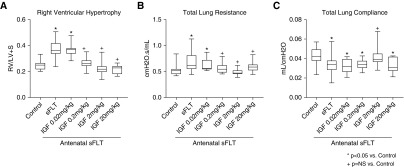
Antenatal injection of sFlt-1 caused distal lung simplification in infant rats, as evident from increased distal airspace size and reduced RAC (Figure 2). Birth weights were not different between sFlt-1–treated and control pups. In comparison with saline control rats, prenatal exposure to sFlt-1 reduced RAC by 32% ± 1 (P < 0.0001). rhIGF-1/BP3 treatment caused dose-related improvement in lung histology, as quantified by increases in infant lung RAC to values that were not different from normal control rats (see Figure 2F). Lung histology and vessel counts show that after antenatal sFlt-1 exposure vascular density in the distal lung was reduced by 42% ± 3 (P < 0.0038) when compared with untreated control rats (Figure 3). Postnatal rhIGF-1/BP3 treatment caused dose-related increases in vessel density after antenatal sFlt-1 treatment, restoring vascular growth to levels observed in control 14-day-old infant rats. Angiograms showed enhanced filling of distal vascular branches in infant rats after rhIGF-1/BP3 treatment (see Figure 3E). In addition to structural improvement in distal lung airspace and vascular growth, we found that rhIGF-1/BP3 treatment reduced RVH and improved lung function in infant rats at Day 14 that had been exposed to antenatal sFlt-1 (Figure 4). Additional analysis of these data found no differences in the severity of impairment of lung structure or function, severity of RVH in response to antenatal sFlt-1, or differences in responsiveness to rhIGF-1/BP3 treatment according to sex.
Figure 2.
Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on distal lung structure in infant rats after antenatal intraamniotic injection of sFlt-1 (soluble fms-like tyrosine kinase 1) in experimental preeclampsia. (A and B) In comparison with saline control rats (A), prenatal exposure to sFlt-1 causes lung simplification (B). (C–F) As shown, postnatal IGF-1 treatment causes dose-related improvement in lung histology (C–E), as quantified by changes in RAC (F). Micrographs are representative and were obtained at the same magnification (scale bars, 100 μm; box plots represent median, interquartile range, and minimum–maximum data points). Animal numbers per study group: control rats = 20; sFlt-1 = 18; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 20; 2 mg/kg = 20; and 20 mg/kg = 10. RAC = radial alveolar count.
Figure 3.
Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on lung vascular density in infant rats after antenatal intraamniotic injection of sFlt-1 (soluble fms-like tyrosine kinase 1) in experimental preeclampsia. (A–D) Lung histology and vessel counts after immunostaining of endothelial cells with von Willebrand factor show that sFlt-1–exposed rats have decreased vascular density when compared with control rats (A and B) and that IGF-1 treatment restores vessel density at 14 days (C and D) (scale bars, 250 μm). (E) Angiograms after barium vascular injection illustrates improved distal branching in infant rats after IGF-1 treatment. Box plots represent median, interquartile range, and minimum–maximum data points. Animal numbers per study group: control rats = 10; sFlt-1 = 10; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 10; 2 mg/kg = 10; and 20 mg/kg = 10. HPF = high-powered field.
Figure 4.
(A–C) Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on RV hypertrophy (A) and lung function (B and C) in infant rats after antenatal intraamniotic injection of sFlt-1 (soluble fms-like tyrosine kinase 1) in experimental preeclampsia. (B and C) As shown, IGF-1 treatment causes dose-related improvement in RV hypertrophy, total respiratory system resistance (B), and compliance (C), with the exception of lung compliance as measured in the IGF-1 20 mg/kg group. Box plots represent median, interquartile range, and minimum–maximum data points. Animal numbers per study group: control rats = 19; sFlt-1 = 18; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 20; 2 mg/kg = 20; and 20 mg/kg = 10. LV+S = left ventricle plus septum; NS = not significant; RV = right ventricle.
Effects of Postnatal rhIGF-1/BP3 Treatment after Antenatal ETX Exposure
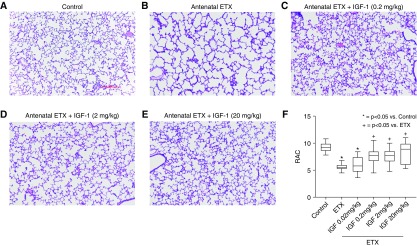
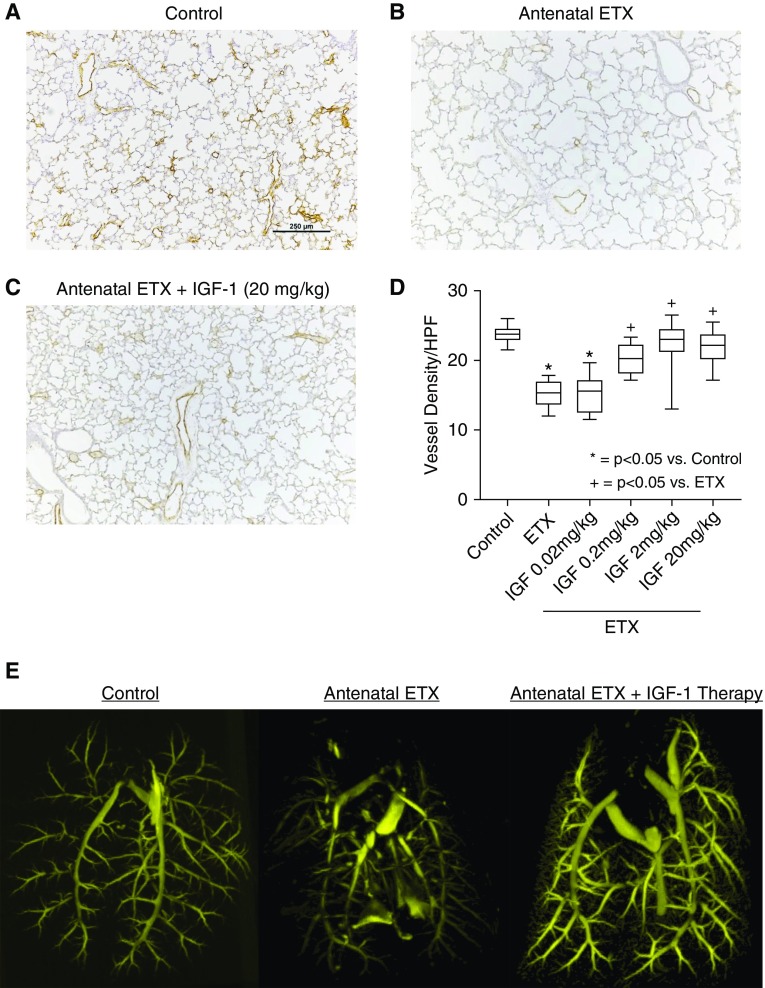
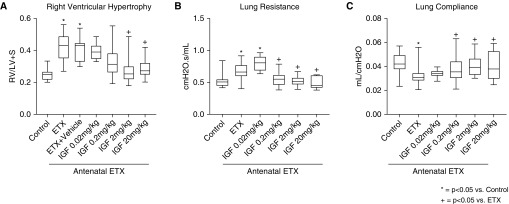
As observed after antenatal sFlt-1, intraamniotic ETX injection to induce an inflammatory model of CA decreased distal lung growth, as evident from the reduction in RAC by 39 ± 1% at 2 weeks of age when compared with vehicle-treated saline control rats (P < 0.0001) (Figure 5). Birth weights were not different between ETX-treated and control pups. Postnatal treatment with rhIGF-1/BP3 causes dose-related improvements in lung structure, resulting in similar RAC as observed in unexposed control rats. Vessel density at 2 weeks was reduced by 36% in pups that had been exposed to antenatal ETX, which was restored to normal values by postnatal rhIGF-1/BP3 treatment (Figure 6). rhIGF-1/BP3 treatment improved lung vascular density in 2-week-old infant rats after antenatal ETX injection in experimental CA (see Figure 6). Lung histology and vessel counts after immunostaining of endothelial cells show that ETX-exposed rats had decreased vascular density when compared with control rats and that rhIGF-1/BP3 treatment restored vessel density to normal values. rhIGF-1/BP3 treatment prevented RVH and improved total respiratory system compliance and resistance in infant rats after antenatal ETX injection (Figure 7). Additional analysis of these data found no differences in the severity of impairment of lung structure or function, severity of RVH in response to antenatal ETX, or differences in responsiveness to rhIGF-1/BP3 treatment according to sex.
Figure 5.
Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on distal lung structure in infant rats after antenatal intraamniotic injection of endotoxin (ETX) in experimental chorioamnionitis. (A and B) Prenatal exposure to ETX decreases septation when compared with saline control rats. (C–F) Postnatal treatment with IGF-1 causes dose-related improvements in lung structure (C–E) and RAC (F). Micrographs are representative and were obtained at the same magnification (scale bars, 100 μm; box plots represent median, interquartile range, and minimum–maximum data points). Animal numbers per study group: control rats = 20; ETX = 19; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 18; 2 mg/kg = 19; and 20 mg/kg = 8. RAC = radial alveolar count.
Figure 6.
Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on lung vascular density in 2-week-old infant rats after antenatal endotoxin (ETX) injection in experimental chorioamnionitis. (A–D) Lung histology and vessel counts after immunostaining of endothelial cells with von Willebrand factor show that ETX-exposed rats have decreased vascular density when compared with control rats (A and B) and that rhIGF-1/BP3 treatment restores vessel density (C and D). (E) Angiograms after barium vascular injection illustrates improved distal branching in infant rats after rhIGF-1/BP3 treatment. Box plots represent median, interquartile range, and minimum–maximum data points. Scale bar, 250 μm. Animal numbers per study group: control rats = 10; ETX = 10; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 10; 2 mg/kg = 10; and 20 mg/kg = 10. HPF = high-powered field.
Figure 7.
(A–C) Effects of IGF-1 (insulin-like growth factor-1) treatment on RV hypertrophy (A) and lung function (B and C) in infant rats after antenatal endotoxin (ETX) injection in experimental chorioamnionitis rhIGF-1 (recombinant human IGF-1)/BP3 (binding peptide 3) treatment causes dose-related improvement in RV hypertrophy, total respiratory system resistance (B), and compliance (C). Box plots represent median, interquartile range, and minimum–maximum data points. Animal numbers per study group: control rats = 20; ETX = 19; rhIGF-1/BP3 0.02 mg/kg = 10; 0.2 mg/kg = 18; 2 mg/kg = 19; and 20 mg/kg = 8. LV+S = left ventricle plus septum; RV = right ventricle.
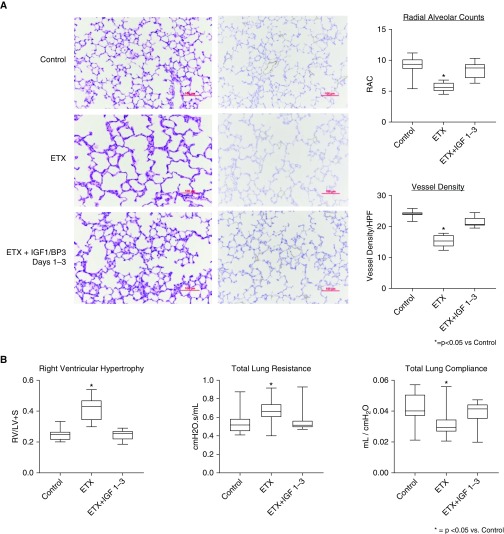
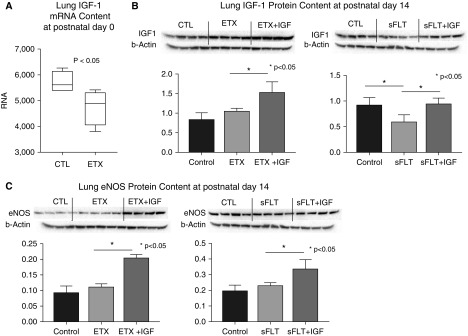
We sought to determine whether shorter periods of therapy would be sufficient to improve distal lung structure and vessel density, and we treated rat pups with rhIGF-1/BP3 (20 mg/kg) during the first 3 days of life and assessed lung structure at 14 days (Figure 8). As shown, 3 days of treatment improved RAC and vessel density in infant lungs, suggesting that shorter duration of treatment may be sufficient in this model. To further explore mechanisms related to the effects of rhIGF-1/BP3 treatment on lung structure in these models, we studied the effects of rhIGF-1/BP3 treatment on lung IGF-1 (Figure 9) and eNOS protein contents at 2 weeks of age after intraamniotic ETX or sFlt-1 exposure. In comparison with untreated rats, rhIGF-1/BP3 infusions increased lung IGF and eNOS content at Day 14.
Figure 8.
Effects of short duration of postnatal rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment after exposure to antenatal endotoxin (ETX) on lung structure in infant rat lungs at 14 days of life. (A and B) As shown, 3 days of treatment improved RAC and vessel density in infant lungs (A) and rhIGF-1/BP3 treatment for 3 days improved right ventricular hypertrophy and lung mechanics at Day 14 in comparison with control rats who received antenatal ETX only (B). Box plots represent median, interquartile range, and minimum–maximum data points. Scale bars, 100 μm. Animal numbers per study group: control rats = 10; ETX = 10; and rhIGF-BP3 Days 1 to 3, 2 mg/kg = 10. HPF = high-powered field; LV+S = left ventricle plus septum; RAC = radial alveolar count; RV = right ventricle.
Figure 9.
Effects of IGF-1 (insulin-like growth factor-1) treatment on lung IGF-1 mRNA (A) and protein (B) and lung eNOS (endothelial nitric oxide synthase) protein (C) contents at 2 weeks of age after intraamniotic ETX or sFlt-1 (soluble fms-like tyrosine kinase 1) exposure. In comparison with untreated rats, rhIGF-1 (recombinant human IGF-1)/BP3 (binding peptide 3) infusions increased lung IGF and eNOS content in treated animals. Representative blots are shown to represent each study group, with 4 animals per group. Box plots represent median, interquartile range, and minimum–maximum data points. Bars represent mean ± SEM. CTL = control; ETX = endotoxin.
Effects of Postnatal rhIGF-1/BP3 Treatment after Prolonged Hyperoxia Exposure
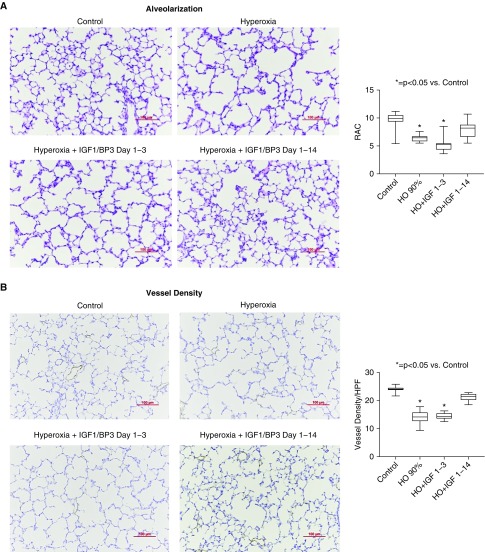
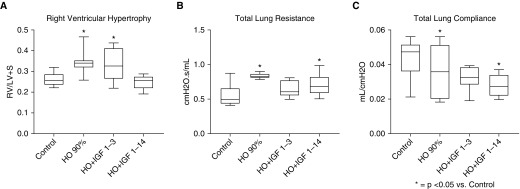
As noted, rhIGF-1/BP3 treatment preserved lung structure and function and prevented RVH in two distinct models of antenatal stress that mimic CA and PE. To determine whether rhIGF-1/BP3 treatment would also be effective in a more traditional BPD model induced by postnatal hyperoxia, we treated rats with rhIGF-1/BP3 during exposure to 90% FiO2 for 2 weeks and assessed lung structure and function for comparisons (Figure 10). rhIGF-1/BP3 treatment prevented the adverse effects of hyperoxia when administered throughout the study period; however, the beneficial effects of rhIGF-1/BP3 treatment were not observed if treatment was halted after only 3 days (see Figure 10 and Figure 11). There were no differences in the severity of impairment of lung structure or function, or severity of RVH, in response to hyperoxia, or differences in responsiveness to rhIGF-1/BP3 treatment as based on sex.
Figure 10.
Effects of short (1–3 d) or long (1–14 d) rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on distal lung structure in infant rats at 14 days after concurrent exposure to hyperoxia (HO). (A) As shown, hyperoxia decreases lung septation when compared with saline control rats. (A and B) Postnatal treatment with IGF-1 for the entire 14-day exposure but not for 3 days improves lung structure, as reflected by increased RAC (A) and vessel density (B). Micrographs are representative and were obtained at the same magnification (scale bars, 100 μm; box plots represent median, interquartile range, and minimum–maximum data points). Animal numbers per study group: control rats = 15; HO = 10; rhIGF-BP3 Days 1 to 3, 2 mg/kg = 10; and rhIGF-BP3 Days 1 to 14 2 mg/kg = 15. HPF = high-powered field; RAC = radial alveolar count.
Figure 11.
(A–C) Effects of short (1–3 d) or long (1–14 d) rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on distal right ventricular function (A) and lung function (B and C) in infant rats at 14 days exposure to hyperoxia (HO). Box plots represent median, interquartile range, and minimum–maximum data points. Animal numbers per study group: control rats = 15; HO = 10; rhIGF-BP3 Days 1 to 3, 2 mg/kg = 10; and rhIGF-BP3 Days 1 to 14, 2 mg/kg = 15. LV+S = left ventricle plus septum; RV = right ventricle.
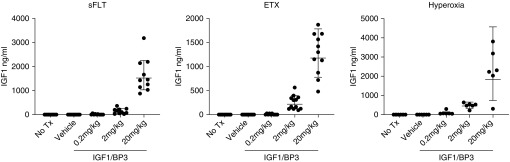
We also examined dose-related changes in serum IGF-1 levels in rats 60 minutes after rhIGF-1/BP3 infusion in pups from each of the models, including those exposed to sFlt-1, ETX, or postnatal hyperoxia. In each study protocol, rhIGF-1/BP3 infusion increased serum IGF-1 levels in dose-related fashion (Figure 12).
Figure 12.
Dose-related changes in serum IGF-1 (insulin-like growth factor-1) levels in rats after rhIGF-1 (recombinant human IGF-1)/BP3 (binding peptide 3) infusion in rats exposed to (left) sFlt-1 (soluble fms-like tyrosine kinase 1), (middle) ETX, or (right) postnatal hyperoxia. Levels were measured from samples drawn at 60 minutes after injections of rhIGF-1/BP3 at Day 14 of life (dose range: 0.2–20 mg/kg). All data points are shown, as well as median and interquartile range. ETX = endotoxin; Tx = therapy.
Effects of rhIGF-1/BP3 Treatment on Fetal LECs and AT2Cs In Vitro
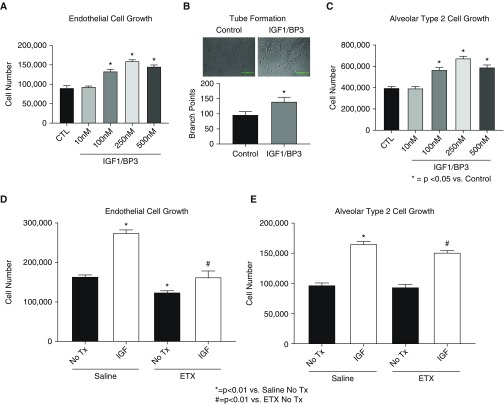
To explore potential mechanisms underlying the effects of rhIGF-1/BP3 treatment in vivo, we studied the direct effects of rhIGF-1/BP3 on isolated LEC and AT2C growth in vitro. (Figure 13) As shown, rhIGF-1/BP3 enhanced LEC growth and tube formation in dose-related fashion and directly stimulated neonatal rat AT2C growth (see Figure 13C). LECs and AT2Cs that were harvested after antenatal ETX exposure had impaired growth in comparison with control rats but were also responsive to exogenous rhIGF-1/BP3 exposure.
Figure 13.
(A and B) Effects of rhIGF-1 (recombinant human insulin-like growth factor-1)/BP3 (binding peptide 3) treatment on neonatal rat pulmonary artery endothelial cell (LEC) growth (A) and tube formation (B) in vitro. As shown, rhIGF-1/BP3 enhanced LEC growth and tube formation in a dose-related fashion. (C) IGF-1 treatment also stimulates neonatal rat alveolar type 2 cell growth. (D and E) In comparison with control rats, LECs (D) and alveolar type 2 cells (E) harvested after antenatal ETX exposure had decreased basal growth, which was increased with IGF-1 treatment. Bars represent mean ± SEM. CTL = control; ETX = endotoxin; Tx = therapy.
Discussion
Since its original description over 50 years ago, there has been a growing awareness of the impact of antenatal determinants as key etiologic mechanisms of impaired lung growth that contribute to the persistently high incidence of BPD (9, 11, 15–23). In this study, we show that rhIGF-1/BP3 therapy preserves lung structure and function and prevents RVH in infant rat pups after exposure to two experimental models of antenatal stress that impair lung development, which include intraamniotic injections of ETX or sFlt-1 to mimic key pathogenetic features of CA and PE, respectively. We further found that in the standard postnatal model of hyperoxia-induced BPD, rhIGF-1/BP3 therapy also enhances lung growth and function and prevents RVH in infant rats. We show that rhIGF-1/BP3 treatment upregulates eNOS and directly stimulates neonatal LEC proliferation and tube formation, reflecting its proangiogenic properties. rhIGF-1/BP3 treatment also enhances AT2C growth, suggesting at least two mechanisms that may contribute to the effectiveness of rhIGF-1/BP3 in each of these animal models. Thus, in both antenatal models and in the postnatal hyperoxia model of BPD, rhIGF-1/BP3 therapy has demonstrable beneficial effects on enhancing lung growth, normalizing lung function, and preventing RVH in infant rats.
Although the pathogenesis of BPD is multifactorial and disease severity is modulated by the adverse effects of postnatal exposures, such as hyperoxia, mechanical ventilation, and other factors, strong experimental and clinical data show that antenatal events are key determinants of high risk for the “new BPD” independent of postnatal injury alone (20, 21, 26, 42). These clinical findings are supported by preclinical studies in which prenatal insults, even in the absence of postnatal hyperoxia or other injuries, are sufficient to cause sustained impairment of lung structure and function and RVH (38–40). As a result, strategies to reduce the incidence and severity of BPD will likely require early identification of at-risk infants with the application of early therapeutic interventions shortly after birth. The animal models used in this study address the need to examine pathogenetic mechanisms through which antenatal stress contribute to disease pathogenesis underlying the new BPD. (42, 43) The new BPD has been characterized by greater recognition of the impact of adverse fetal events in the pathobiology of BPD and late respiratory outcomes, as reflected by recent prospective studies (20, 21).
Several studies have shown a greater risk for BPD in preterm infants with IUGR, a key marker for adverse antenatal conditions strongly linked with placental dysfunction. A recent study reported a roughly twofold increase in early (28 d) and late (36 wk postmenstrual age) mortality, and the risk for BPD in IUGR infants born at or below 32 weeks gestation (24). Additionally, preterm infants who were born with IUGR remain at high risk for late respiratory morbidities and lung function at school age (22–25). When studied at a mean age of 11 years, children who were born prematurely with IUGR had lower lung function, including FEV1 and diffusion capacity, suggesting abnormal airway function and decreased lung surface area, in comparison with non-IUGR prematurely born children (24). Although IUGR itself does not sufficiently provide mechanistic understanding of BPD risk per se, IUGR serves as a useful biomarker that strongly supports the hypothesis that antenatal events play critical roles in BPD etiology and long-term respiratory outcomes (25). Based on these changes in the features and persistent challenges of BPD, more studies that incorporate antenatal models that lead to impaired lung development will provide insights into disease pathobiology and develop potential preventive strategies.
The presence of placental vascular abnormalities with preterm birth are strongly associated with the subsequent risk for BPD and BPD with PH (27–29). Specific mechanisms through which placental dysfunction disrupts lung development and contributes to BPD and PH are uncertain. Preclinical studies suggest that disruption of angiogenesis due to adverse antenatal factors, such as CA, PE, maternal smoking, and postnatal injury after preterm birth, can cause pulmonary vascular disease that not only leads to PH but can also impairs distal lung growth. Laboratory studies have shown that the developing endothelial cell plays a key role in regulation and coordination of epithelial growth and distal airspace structure through the production of critical “angiocrines,” such as nitric oxide, hepatocyte growth factor, vitamin A, IGF-1, and others (47–49).
Because angiogenesis is necessary for normal alveolarization (49–51), it has been suggested that protecting the developing pulmonary vasculature from early injury may not only lower PH and improve gas exchange but may enhance distal lung growth and improve long-term respiratory outcomes (42). Clinical studies have shown that early echocardiographic findings of pulmonary vascular disease after preterm birth are strongly associated with the development and severity of BPD and PH at 36 weeks corrected age (52–54). Interestingly, these findings were also associated with a worse respiratory course during the initial hospitalization but also late respiratory outcomes, including respiratory exacerbations, hospitalizations, and the need for asthma medications (53). Therapeutic strategies that target enhanced endothelial survival, function, and growth may provide novel approaches toward the prevention of BPD after early diagnosis of high risk within preterm populations.
Our findings of beneficial effects of exogenous IGF-1 treatment in preserving lung structure and function in experimental models of BPD further support previous works that have identified a critical role for IGF-1 in mediating normal lung development. IGF-1 regulates fetal growth and organogenesis by stimulating cell proliferation, maturation, and differentiation throughout fetal life and during the early postnatal period (55–62). Lung IGF-I mRNA expression is high during fetal life and decreases after birth (61, 62). Studies using genetic mouse models found that IGF-1 and the IGF receptor-1 play key roles during lung development in utero because fetal lungs in mice with downregulation of IGF-1 or IGFR-1 are small and have abnormal interstitial and distal lung structure (60, 61). In addition, impaired growth and angiogenesis has been demonstrated in fetal LECs in an experimental IUGR model due to heat chamber–exposed pregnant ewes (62). We report decreased lung IGF-1 mRNA expression in neonatal lungs of rat pups exposed to intraamniotic ETX, further supporting the concept that early (antenatal) disruption of IGF-1 signaling may contribute to the pathobiology of BPD.
Clinically, circulating IGF-1 levels rise during the third trimester in utero but remain low in neonates who are born premature (38, 63). Importantly, low circulating IGF-1 levels were found in cord blood samples from newborns delivered in pregnancies complicated by PE (63), and low IGF-1 levels have been further associated with the subsequent development of BPD (37, 64). Recently, published data from a multicenter randomized trial of rhIGF-1/BP3 for the prevention of severe retinopathy of prematurity failed to show significant differences as related to the primary retinopathy of prematurity endpoint (65). However, this small pilot trial had the striking finding that preterm infants that received early and prolonged rhIGF-1/BP3 infusions successfully achieved higher circulating IGF-1 levels and had a marked reduction in the risk for severe BPD by 53% in the full analysis set and by 89% in the evaluable set when compared with control rats (65). Although not the primary study endpoint, this striking reduction in BPD severity supports the concept that early restoration of circulating IGF-1 levels may improve respiratory outcomes in preterm infants.
Conclusions
We report that rhIGF-1/BP3 treatment promoted lung angiogenesis, preserved lung airspace growth and structure, improved lung function, and prevented the development of RVH in three diverse experimental models of BPD, including two antenatal models that better reflect the new BPD. Overall, these findings support concepts that early disruption of IGF-1 signaling after premature birth may play an important role in the subsequent development of BPD and PH, and that IGF-1 treatment may provide a novel treatment strategy for improving cardiorespiratory outcomes in preterm infants.
Supplementary Material
Footnotes
This work was supported by NIH grants HL068702 and HL145679 and a grant from Takeda Pharmaceuticals.
Author Contributions: Contributions to the conception and design of the work: G.S., C.K., B.W., E.W.M., D.S., and S.H.A. Major role in data acquisition, analysis, or interpretation of data for the work; drafting the manuscript; final approval of the version submitted for publication; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-1975OC on February 26, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt B, Roberts RS, Davis PG, Doyle LW, Asztalos EV, Opie G, et al. Caffeine for Apnea of Prematurity (CAP) Trial Investigators; Caffeine for Apnea of Prematurity CAP Trial Investigators. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr. 2015;167:982–986, e2. doi: 10.1016/j.jpeds.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Abman SH. Bronchopulmonary dysplasia. New York, NY: Informa; 2010. [Google Scholar]
- 7.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–156. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunville CF, Sontag MK, Stratton KA, Ranade DJ, Abman SH, Mourani PM. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr. 2010;157:209–214.e1. doi: 10.1016/j.jpeds.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thoracic Soc. 2014;11(Suppl 3):S146–S153. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Prematurity and Respiratory Outcomes Program. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the Prematurity and Respiratory Outcomes Program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manuck TA, Levy PT, Gyamfi-Bannerman C, Jobe AH, Blaisdell CJ. Prenatal and perinatal determinants of lung health and disease in early life: A National Heart, Lung, and Blood Institute Workshop Report. JAMA Pediatr. 2016;170:e154577. doi: 10.1001/jamapediatrics.2015.4577. [DOI] [PubMed] [Google Scholar]
- 12.Farstad T, Bratlid D, Medbø S, Markestad T Norwegian Extreme Prematurity Study Group. Bronchopulmonary dysplasia: prevalence, severity and predictive factors in a national cohort of extremely premature infants. Acta Paediatr. 2011;100:53–58. doi: 10.1111/j.1651-2227.2010.01959.x. [DOI] [PubMed] [Google Scholar]
- 13.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wai KC, Kohn MA, Ballard RA, Truog WE, Black DM, Asselin JM, et al. Trial of Late Surfactant (TOLSURF) Study Group. Early cumulative supplemental oxygen predicts bronchopulmonary dysplasia in high risk extremely low gestational age newborns. J Pediatr. 2016;177:97–102, e2. doi: 10.1016/j.jpeds.2016.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, et al. Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- 17.Van Marter LJ, Dammann O, Allred EN, Leviton A, Pagano M, Moore M, et al. Developmental Epidemiology Network Investigators. Chorioamnionitis, mechanical ventilation, and postnatal sepsis as modulators of chronic lung disease in preterm infants. J Pediatr. 2002;140:171–176. doi: 10.1067/mpd.2002.121381. [DOI] [PubMed] [Google Scholar]
- 18.Hansen AR, Barnés CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010;156:532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Erikson L, Haglund B, Odlind V, Altman M, Ewald U, Keiler H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015;104:259–263. doi: 10.1111/apa.12888. [DOI] [PubMed] [Google Scholar]
- 20.Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196:364–374. doi: 10.1164/rccm.201612-2414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller RL, Feng R, DeMauro SB, Ferkol T, Hardie W, Rogers EE, et al. Prematurity and Respiratory Outcomes Program. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. 2017;187:89–97.e3. doi: 10.1016/j.jpeds.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jobe AH. Effects of chorioamnionitis on the fetal lung. Clin Perinatol. 2012;39:441–457. doi: 10.1016/j.clp.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal MK, Manktelow BN, Draper ES, Field DJ Population-based study. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics. 2003;111:483–487. doi: 10.1542/peds.111.3.483. [DOI] [PubMed] [Google Scholar]
- 24.Ronkainen E, Dunder T, Kaukola T, Marttila R, Hallman M. Intrauterine growth restriction predicts lower lung function at school age in children born very preterm. Arch Dis Child Fetal Neonatal Ed. 2016;101:F412–F417. doi: 10.1136/archdischild-2015-308922. [DOI] [PubMed] [Google Scholar]
- 25.Greenough A, Yuksel B, Cheeseman P. Effect of in utero growth retardation on lung function at follow-up of prematurely born infants. Eur Respir J. 2004;24:731–733. doi: 10.1183/09031936.04.00060304. [DOI] [PubMed] [Google Scholar]
- 26.Taglauer E, Abman SH, Keller RL. Recent advances in antenatal factors predisposing to bronchopulmonary dysplasia. Semin Perinatol. 2018;42:413–424. doi: 10.1053/j.semperi.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, et al. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–574. doi: 10.1016/j.placenta.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Check J, Gotteiner N, Liu X, Su E, Porta N, Steinhorn R, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013;33:553–557. doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voller SB, Chock S, Ernst LM, Su E, Liu X, Farrow KN, et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum Dev. 2014;90:195–200. doi: 10.1016/j.earlhumdev.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–1522. doi: 10.1183/09031936.00017312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olmos A, Díaz L, Vila E, Barrera D, López-Marure R, Biruete B, et al. Associations between insulin-like growth factor I, vascular endothelial growth factor and its soluble receptor 1 in umbilical serum and endothelial cells obtained from normotensive and preeclamptic pregnancies. Growth Factors. 2013;31:123–129. doi: 10.3109/08977194.2013.802692. [DOI] [PubMed] [Google Scholar]
- 32.Capoluongo E, Ameglio F, Zuppi C. Insulin-like growth factor-I and complications of prematurity: a focus on bronchopulmonary dysplasia. Clin Chem Lab Med. 2008;46:1061–1066. doi: 10.1515/CCLM.2008.211. [DOI] [PubMed] [Google Scholar]
- 33.Perkett EA, Badesch DB, Roessler MD, Stenmark KR, Meyrick B, et al. Insulin-like growth factor I and pulmonary hypertension induced by continuous air embolization in sheep. Am J Respir Cell Mol Biol. 1992;6:82–87. doi: 10.1165/ajrcmb/6.1.82. [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Ramchandran R, Chen J, Yang Q, Raj JU. Smooth muscle insulin-like growth factor-1 mediates hypoxia-induced pulmonary hypertension in neonatal mice. Am J Respir Cell Mol Biol. 2016;55:779–791. doi: 10.1165/rcmb.2015-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi JE, Lee SS, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, et al. Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Respir Crit Care Med. 2009;179:212–219. doi: 10.1164/rccm.200802-228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ionescu L, Byrne RN, van Haaften T, Vadivel A, Alphonse RS, Rey-Parra GJ, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Löfqvist C, Hellgren G, Niklasson A, Engström E, Ley D, Hansen-Pupp I WINROP Consortium. Low postnatal serum IGF-I levels are associated with bronchopulmonary dysplasia (BPD) Acta Paediatr. 2012;101:1211–1216. doi: 10.1111/j.1651-2227.2012.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, et al. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol. 2010;299:L735–L748. doi: 10.1152/ajplung.00153.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L36–L46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wallace B, Peisl A, Seedorf G, Nowlin T, Kim C, Bosco J, et al. Anti-sFlt-1 therapy preserves lung alveolar and vascular growth in antenatal models of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2018;197:776–787. doi: 10.1164/rccm.201707-1371OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonikos DS, Bensch KG, Ludwin SK, Northway WH., Jr Oxygen toxicity in the newborn: the effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest. 1975;32:619–635. [PubMed] [Google Scholar]
- 42.Abman SH, Bancalari E, Jobe A. The evolution of bronchopulmonary dysplasia after 50 years. Am J Respir Crit Care Med. 2017;195:421–424. doi: 10.1164/rccm.201611-2386ED. [DOI] [PubMed] [Google Scholar]
- 43.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–172. doi: 10.1097/MOP.0b013e3283423e6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, et al. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res. 2005;58:22–29. doi: 10.1203/01.PDR.0000163378.94837.3E. [DOI] [PubMed] [Google Scholar]
- 45.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1. Postnatal lung growth. Thorax. 1982;37:572–579. doi: 10.1136/thx.37.8.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35:544–547. doi: 10.1136/adc.35.184.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun EJ, Lorizio W, Seedorf G, Abman SH, Vu TH. VEGF and endothelium-derived retinoic acid regulate lung vascular and alveolar development. Am J Physiol Lung Cell Mol Physiol. 2016;310:L287–L298. doi: 10.1152/ajplung.00229.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seedorf G, Metoxen AJ, Rock R, Markham N, Ryan S, Vu T, et al. Hepatocyte growth factor as a downstream mediator of vascular endothelial growth factor-dependent preservation of growth in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2016;310:L1098–L1110. doi: 10.1152/ajplung.00423.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 50.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 51.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol. 2002;283:L555–L562. doi: 10.1152/ajplung.00408.2001. [DOI] [PubMed] [Google Scholar]
- 52.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mourani PM, Mandell EW, Meier M, Younoszai A, Brinton JT, Wagner BD, et al. Early pulmonary vascular disease in preterm infants is associated with late respiratory outcomes in childhood. Am J Respir Crit Care Med. 2019;199:1020–1027. doi: 10.1164/rccm.201803-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A.Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia J Pediatr 2014165909–914, e1.[Published erratum appears in J Pediatr 166:782.] [DOI] [PubMed] [Google Scholar]
- 55.Epaud R, Aubey F, Xu J, Chaker Z, Clemessy M, Dautin A, et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One. 2012;7:e48071. doi: 10.1371/journal.pone.0048071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bach LA. Endothelial cells and the IGF system. J Mol Endocrinol. 2015;54:R1–R13. doi: 10.1530/JME-14-0215. [DOI] [PubMed] [Google Scholar]
- 57.Jacobo SMP, Kazlauskas A. Insulin-like growth factor 1 (IGF-1) stabilizes nascent blood vessels. J Biol Chem. 2015;290:6349–6360. doi: 10.1074/jbc.M114.634154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hellstrom A, Perruzzi C, Ju M, Engstrom E, Hard AL, Liu JL, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA. 2001;98:5804–5808. doi: 10.1073/pnas.101113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Masood A, Yi M, Lau M, Belcastro R, Ivanovska J, et al. The IGF-I/IGF-R1 pathway regulates postnatal lung growth and is a nonspecific regulator of alveologenesis in the neonatal rat. Am J Physiol Lung Cell Mol Physiol. 2013;304:L626–L637. doi: 10.1152/ajplung.00198.2012. [DOI] [PubMed] [Google Scholar]
- 61.Han RN, Post M, Tanswell AK, Lye SJ. Insulin-like growth factor-I receptor-mediated vasculogenesis/angiogenesis in human lung development. Am J Respir Cell Mol Biol. 2003;28:159–169. doi: 10.1165/rcmb.4764. [DOI] [PubMed] [Google Scholar]
- 62.Rozance PJ, Seedorf GJ, Brown A, Roe G, O’Meara MC, Gien J, et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L860–L871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conti E, Zezza L, Ralli E, Caserta D, Musumeci MB, Moscarini M, et al. Growth factors in preeclampsia: a vascular disease model. A failed vasodilation and angiogenic challenge from pregnancy onwards? Cytokine Growth Factor Rev. 2013;24:411–425. doi: 10.1016/j.cytogfr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Hellström A, Ley D, Hansen-Pupp I, Hallberg B, Löfqvist C, van Marter L, et al. Insulin-like growth factor 1 has multisystem effects on foetal and preterm infant development. Acta Paediatr. 2016;105:576–586. doi: 10.1111/apa.13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ley D, Hallberg B, Hansen-Pupp I, Dani C, Ramenghi LA, Marlow N, et al. study team. rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J Pediatr. 2019;206:56–65, e8. doi: 10.1016/j.jpeds.2018.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.