To the Editor:
Although encouraging progress continues to be made in the development of novel compounds against tuberculosis (TB), a significant proportion of patients with TB resistant to various first- and second-line drugs are unable to be successfully treated with the current anti-TB armamentarium. Building on evidence of the early bactericidal activity (EBA) of 2 g meropenem (Mero) administered intravenously every 8 hours combined with oral amoxicillin plus clavulanic acid (Amx/Clv) (1), the World Health Organization recently included Mero-Amx/Clv as an additional drug combination for drug-resistant TB (2).
Aiming to simplify carbapenem treatment for TB, we evaluated the 14-day EBA of Mero or ertapenem (Erta), combined with oral Amx/Clv, in patients with rifampicin-susceptible pulmonary TB. We conducted a prospective two-phase randomized trial at a single site in Cape Town, South Africa. Approvals were obtained from the local regulatory authority and ethical review board before study initiation. Participants received either Mero 6 g once daily administered intravenously over 6 hours or a once-daily dose of Erta 1 g intramuscularly, along with two tablets of Amx/Clv 1,000/62.5 mg every 12 hours. A smaller control group received standard-of-care combination therapy with daily isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE). We collected serial sputum samples from each participant before and during study treatment. We used linear mixed-effects modeling to estimate EBA as the daily change in colony-forming units (log10cfu) per milliliter of sputum plated onto 7H11S agar plates. A total of 39 participants were recruited, with 18 and 17 participants enrolled in the Mero and Erta groups, respectively, and 4 in the HRZE group. Overall, 69% of the participants were male, with a median age of 32 years and body mass index of 19.2 kg/m2. All participants were HIV-negative. The mean baseline cfu count was similar between treatment groups.
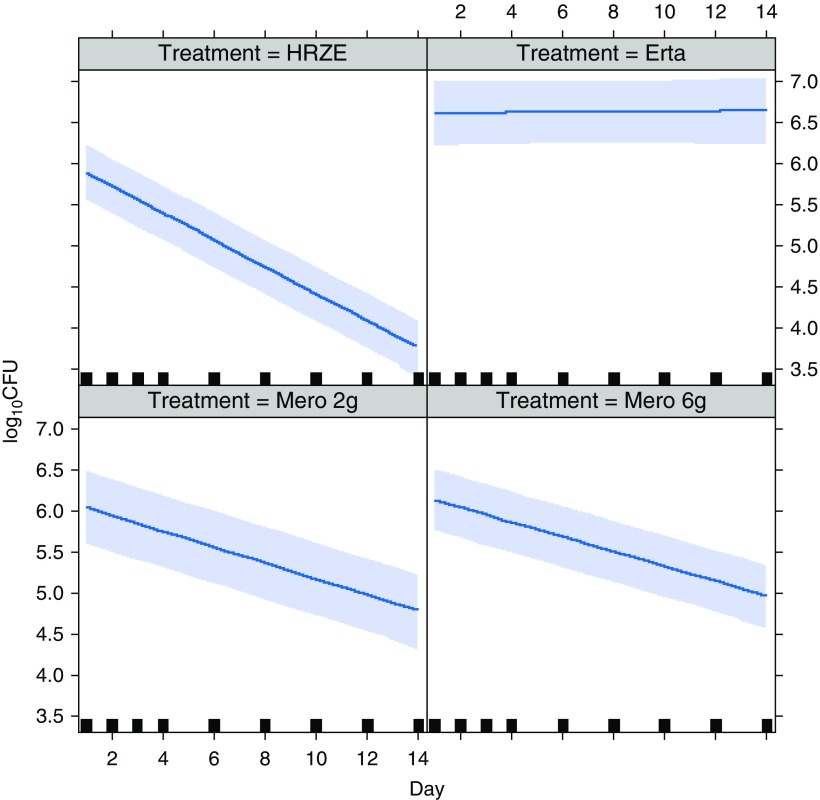
We also evaluated the data from the previous β-lactam study performed by our group in 2015 (1). Finding no statistically significant differences between baseline and control variables for these two studies, we combined the data and directly compared treatment outcomes (Figure 1). Mero-Amx/Clv 6 g once daily showed significant EBA with a daily decrease of 0.094 (95% confidence interval [CI], 0.075 to 0.115; P < 0.0001), which was not different from the Mero-Amx/Clv 2 g 8-hourly EBA decrease of 0.095 (95% CI, 0.073 to 0.118; P < 0.0001). Erta-Amx/Clv showed no EBA (increase of 0.010; 95% CI, −0.010 to 0.031; P = 0.3480). There were no unexpected or severe adverse events.
Figure 1.
Fourteen-day mycobactericidal activity of intravenous meropenem (Mero) 6 g once daily over 6 hours versus 2 g every 8 hours, compared with that of ertapenem (Erta) 1 g once daily intramuscularly and isoniazid, rifampicin, pyrazinamide, and ethambutol (HRZE) dosed once daily according to standard weight bands. All Mero- and Erta-containing groups received amoxicillin plus clavulanic acid orally twice daily. The darker blue lines represent the estimated daily on-treatment change in colony-forming units [log10cfu], adjusted for baseline cfu, age, body mass index, sex, and study. The lighter blue shadows are the associated 95% confidence bands. The solid squares represent the time point of sputum collection. We used a single joint linear mixed-effects model, taking into account the correlation between observations from each participant.
A prolonged once-daily dose of Mero with similar activity to the World Health Organization–recommended every-8-hours dose may prove more practically implementable in low-resource settings where treatment is provided on an outpatient basis. Long-term hospitalization for an every-8-hours overnight administration of intravenous therapy is prohibitive in many areas with a high incidence of TB that is resistant to current orally bioavailable medicines. Based on time above the minimum inhibitory concentration as the driver of carbapenem activity, pharmacokinetic modeling has further predicted that a single 6-g dose of Mero could safely be infused in an even shorter time without critically reducing bactericidal activity (data not shown). Erta administered intravenously and/or at higher doses needs further investigation, as has been suggested by other investigators based on in vitro data and pharmacokinetic studies (3, 4). Meanwhile, the search for an orally bioavailable carbapenem with anti-TB activity must continue.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank David Barros-Aguirre and Joel Lelievre for critical input into this letter.
Footnotes
Supported by the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 733079.
Originally Published in Press as DOI: 10.1164/rccm.201911-2149LE on January 10, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Diacon AH, van der Merwe L, Barnard M, von Groote-Bidlingmaier F, Lange C, García-Basteiro AL, et al. β-Lactams against tuberculosis—new trick for an old dog? N Engl J Med. 2016;375:393–394. doi: 10.1056/NEJMc1513236. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO consolidated guidelines on drug-resistant tuberculosis treatment. 2019. [accessed 2019 Aug]. Available from: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ [PubMed]
- 3.van Rijn SP, Srivastava S, Wessels MA, van Soolingen D, Alffenaar JC, Gumbo T. Sterilizing effect of ertapenem-clavulanate in a hollow-fiber model of tuberculosis and implications on clinical dosing. Antimicrob Agents Chemother. 2017;61:e02039–16. doi: 10.1128/AAC.02039-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuur MA, Ghimire S, Bolhuis MS, Wessels AMA, van Altena R, de Lange WCM, et al. Pharmacokinetics of 2,000 milligram ertapenem in tuberculosis patients. Antimicrob Agents Chemother. 2018;62:e02250-17. doi: 10.1128/AAC.02250-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.