Abstract
Rationale: Excessive daytime sleepiness is a common disabling symptom in obstructive sleep apnea syndrome.
Objectives: To evaluate the efficacy and safety of pitolisant, a selective histamine H3 receptor antagonist with wake-promoting effects, for the treatment of daytime sleepiness in patients with moderate to severe obstructive sleep apnea refusing continuous positive airway pressure treatment.
Methods: In an international, multicenter, double-blind, randomized (3:1), placebo-controlled, parallel-design trial, pitolisant was individually titrated at up to 20 mg/d over 12 weeks. The primary endpoint was the change in the Epworth Sleepiness Scale score. Key secondary endpoints were maintenance of wakefulness assessed on the basis of the Oxford Sleep Resistance test, safety, Clinical Global Impression of severity, patient’s global opinion, EuroQol quality-of-life questionnaire, and Pichot fatigue questionnaire.
Measurements and Main Results: A total of 268 patients with obstructive sleep apnea (75% male; mean age, 52 yr; apnea–hypopnea index, 49/h; baseline sleepiness score, 15.7) were randomized (200 to pitolisant and 68 to placebo) and analyzed on an intention-to-treat basis. The Epworth Sleepiness Scale score was reduced more with pitolisant than with placebo (−2.8; 95% confidence interval, −4.0 to −1.5; P < 0.001). Wake maintenance tests were not improved. The Pichot fatigue score was reduced with pitolisant. The overall impact of pitolisant was confirmed by both physicians’ and patients’ questionnaires. Adverse event incidence, mainly headache, insomnia, nausea, and vertigo, was similar in the pitolisant and placebo groups (29.5% and 25.4%, respectively), with no cardiovascular or other significant safety concerns.
Conclusions: Pitolisant significantly reduced self-reported daytime sleepiness and fatigue and improved patient-reported outcomes and physician disease severity assessment in sleepy patients with obstructive sleep apnea refusing or nonadherent to continuous positive airway pressure.
Clinical trial registered with www.clinicaltrials.gov (NCT01072968) and EU Clinical Trials Register (EudraCT 2009-017251-94).
Keywords: excessive daytime sleepiness, obstructive sleep apnea, continuous positive airway pressure, pitolisant
At a Glance Commentary
Scientific Knowledge on the Subject
Some patients with obstructive sleep apnea who refuse or do not adhere to continuous positive airway pressure treatment have persistent daytime sleepiness.
What This Study Adds to the Field
In this randomized placebo-controlled trial, pitolisant, a selective histamine H3 receptor antagonist with wake-promoting effects, significantly reduced sleepiness and fatigue and improved both global patient-reported outcomes and the physician’s disease severity assessment. Importantly, no detrimental cardiovascular impact was associated with this therapy.
Obstructive sleep apnea (OSA) is a major health concern worldwide that has multiorgan consequences and results in considerable economic, healthcare, and social burdens (1, 2). OSA is often associated with comorbidities such as arterial hypertension, arrhythmia, stroke, coronary heart disease, and metabolic dysfunction. Excessive daytime sleepiness (EDS) and fatigue are among the chief complaints of patients with OSA, and they have disabling consequences: impaired attention and vigilance, cognitive dysfunction, loss of productivity at work, deterioration in quality of life, and increased risk of occupational and motor vehicle accidents (3, 4).
Continuous positive airway pressure (CPAP) is the first-line therapy for symptomatic moderate to severe OSA. When used properly, CPAP normalizes the apnea–hypopnea index (AHI), suppresses nocturnal oxygen desaturations, and decreases sleep fragmentation. As a consequence, there is a general reduction in EDS, and improvements in alertness, cognitive function, and quality of life are seen. CPAP is particularly effective in patients with more pronounced sleepiness and OSA severity (5, 6). However, sleepiness is known to persist in approximately 15% of patients in spite of adequate CPAP therapy, and this residual component may represent a considerable therapeutic drawback (7–9). Wake-promoting agents such as modafinil and armodafinil, as well as, more recently, solriamfetol, in combination with CPAP have been demonstrated to decrease residual sleepiness and improve quality of life in randomized controlled trials (10, 11).
A major issue with CPAP treatment is adherence, with 15% of patients with OSA refusing to try CPAP and 20–30% discontinuing CPAP in the long term (12, 13). Hence, prescribing a wake-promoting agent to selectively treat EDS and not treating the underlying cause is frequently debated, and efficacy data are scarce in this specific context.
Pitolisant is a novel selective histamine H3 receptor antagonist/inverse agonist with strong wake-promoting effects that is well tolerated in patients with narcolepsy (14, 15). This provides a rationale to assess its efficacy and safety in the treatment of EDS in patients with OSA who refuse or do not adhere to CPAP therapy. The objectives of this study were to demonstrate the efficacy and safety of pitolisant administered at 5, 10, or 20 mg once per day versus placebo during 12 weeks for the treatment of EDS in patients with moderate to severe OSA and refusing CPAP therapy.
Methods
Study Design
This phase 3, prospective, double-blind, placebo-controlled, parallel-group, multicenter study evaluated the efficacy and safety of pitolisant over 12 weeks in adult patients with moderate to severe OSA (AHI, ≥15 events/h) experiencing EDS (Epworth Sleepiness Scale [ESS] score, ≥12), refusing CPAP treatment, and without significant cardiovascular disease. The study was conducted in 28 hospital sleep clinics in 10 European countries between October 6, 2011, and May 7, 2014. The study was approved by the appropriate institutional review board or ethics committee of each study center and was performed in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent before participation. The study is registered with www.clinicaltrials.gov (NCT01072968) and the EU Clinical Trials Register (EudraCT 2009-017251-94).
Patients
Patients were adults with OSA diagnosed according to the International Classification of Sleep Disorders 2nd Edition (16) criteria and refusing or not adhering to CPAP therapy. Included patients with OSA were those with an AHI ≥15 events per hour assessed during the previous year and a complaint of EDS, defined as an ESS score ≥12.
Key noninclusion criteria were as follows:
-
•
History of a medical disorder other than OSA associated with EDS (periodic limb movement arousal index, >10 events/h; 13-item Beck Depression Inventory [BDI-13] score, >16 or item G = 0; Mini Mental State Examination score < 28)
-
•
Body mass index (BMI) >40 kg/m2 (owing to the risk of obesity hypoventilation syndrome and because morbid obesity might be a significant cause of sleepiness)
-
•
Surgery for OSA, including uvulopalatopharyngoplasty
-
•
Use of a mandibular advancement device
-
•
Nighttime or variable work shifts
-
•
Current or recent history of drug, alcohol, or other substance abuse or dependence
-
•
Presence of an unstable or clinically significant medical condition
-
•
A behavior or psychiatric disorder or medical history that could affect safety or interfere with study assessments
-
•
Use of any treatment that could affect the evaluation of EDS
Cardiovascular disease, including myocardial infarction, hypertension, angina, arterial hypertension or dysrhythmia, electrocardiogram, Bazett’s corrected QT interval longer than 450 ms on an ECG, left ventricular hypertrophy, or mitral valve prolapse, was not exclusionary, unless the investigator deemed it unstable or recent.
Randomization Procedure
Randomization was centralized and performed via an electronic web randomization server (Arone Projection; https://www.bioprojet-studies.org/) that automatically assigned a patient number at screening and then automatically assigned a study treatment number when the patient was randomized. The randomization list was established on a balanced 3:1 (three active for one placebo) basis. Pitolisant and placebo were contained within sealed capsules, similar in appearance and taste, and containing a one-fourth, one-half, or one full tablet of pitolisant 20 mg or lactose only (placebo). The patients, their sleep and/or respiratory physicians, and staff were blinded to the treatment allocation.
Intervention
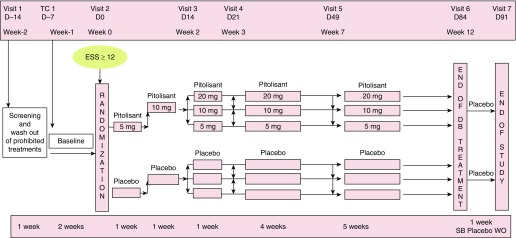
Patients who fulfilled selection criteria were randomized 3:1 to the following treatment groups: pitolisant at 5, 10, or 20 mg once daily or placebo consumed on an empty stomach within 1 hour of waking in the morning. Treatment was initiated at 5 mg by an individual 2-week titration period, escalating the dose on the basis of efficacy and tolerance to the treatment, followed by treatment with the selected dose for a further 10 weeks (Figure 1).
Figure 1.
Study design. D = day; DB = double-blind; ESS = Epworth Sleepiness Scale; SB = single blind; TC = telephone call; WO = wash out.
Outcomes
The primary efficacy endpoint was the change from baseline to Week 12 in the ESS score, a reliable patient self-assessment method to measure EDS. The key secondary endpoint was the change from baseline to Week 12 in the Oxford Sleep Resistance (OSLER) test, a test of behavioral maintenance of wakefulness that objectively measures the ability to maintain wakefulness. The OSLER test consisted of three sessions each of 40-minute sleep resistance challenges performed at 9:00 a.m., 11:00 a.m., and 1:00 p.m. The mean sleep latency (mean of the three tests) and the number of errors (three to six consecutive errors indicating microsleep and seven or more errors indicating sleep onset) were calculated (17, 18). Other secondary endpoints were responders according to the ESS (ESS score, ≤10 or improvement ≥3 points), Pichot fatigue scale, sleep diary (sleepiness and sleep episodes), Trail Making Test parts A and B, Clinical Global Impression (CGI) severity and change scales, patient’s global opinion (PGO), Leeds Sleep Evaluation Questionnaire (LSEQ), and EuroQol quality-of-life questionnaire (EQ-5D). Safety was assessed by evaluating adverse events (particularly treatment-emergent adverse events [TEAEs]), clinical laboratory parameters (hematology, biochemistry, and electrolytes), vital signs, physical examination, ECG data, BDI-13 score, amphetamine-like withdrawal symptoms, and the patient’s overall evaluation of tolerance.
Statistical Analysis
Sample size calculation
Results of exploratory studies on pitolisant provided an estimate of ESS score residual variability with an SD of 6 points. The minimal clinically important difference was arbitrarily fixed by agreement between the investigators at ESS score –3, corresponding to an effect size of 0.5. Recent independent studies (19, 20) have established the minimal clinically important improvement of the ESS score to lie between −2 and −3. The correlation between final and baseline ESS scores was conservatively estimated as r = 0.4. Assuming an analysis of covariance with a 0.95 confidence level as the main confirmatory test, a difference of at least 3 points should be detected with a power of 90% by including at least 60 patients in the placebo group and 180 patients in the pitolisant treatment group.
Description of the different populations analyzed
The intention-to-treat (ITT) population included all randomized patients. The safety population corresponded to all patients who received at least one dose of study medication, regardless of the outcome, and for whom at least one valid postbaseline evaluation (including any adverse event) was available. The per-protocol population included all patients in the ITT population with no major protocol violation regarding inclusion or noninclusion criteria or during the treatment phase and no premature discontinuation in the double-blind phase of the study. The per-protocol population was confirmed by blinded review of the data before database lock.
Demographic data and other baseline characteristics were analyzed for the ITT population. The efficacy analysis of the ITT population was considered as the primary analysis. The safety population was used for safety, concomitant medications, exposure, dosing, and compliance analyses.
The statistical analysis was performed by an independent external statistician. Another third-party statistician independently reviewed the statistical analysis report. Descriptive statistics were used for the quantitative variables, and the frequency distribution was used for the ordinal and nominal variables. Exact 95% confidence intervals (CIs) are given for selected variables.
The final ESS score was compared between the two arms using a linear mixed effects model, considering treatment as a fixed factor, center as a random factor, and ESS score and BMI at baseline as adjustment covariates. It was foreseen that the ESS score might be logarithmically transformed, depending on normality of the residuals. However, it appeared that this was not necessary.
The analysis of safety data was descriptive, except for between-group comparisons of the frequencies of TEAEs by means of logistic regression. Missing data for the primary efficacy variable and for response were allocated using the last observation carried forward (LOCF) method. An additional sensitivity analysis was performed in which missing data were allocated using multiple imputation (see additional analysis and Tables E1 and E2 in the online supplement). A sensitivity analysis for the primary efficacy variable was performed using the baseline ESS value carried forward, adjusting for ESS score and BMI at baseline.
The statistical analysis took into account the possibility of imbalance between centers and treatment by considering a random factor for centers. All statistical tests were two sided at a 5% level of significance.
Results
Patient Flow
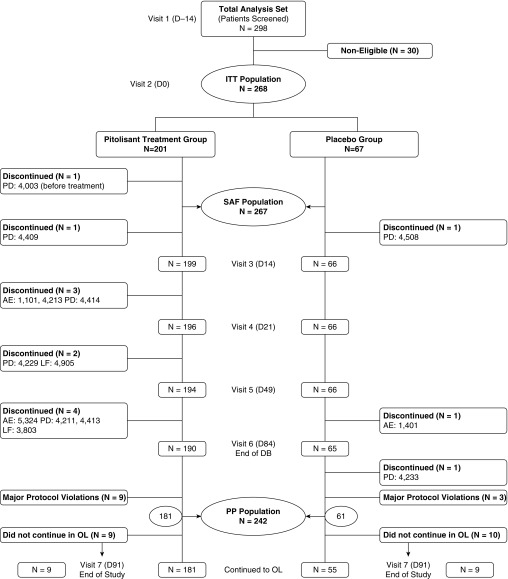
Two hundred ninety-eight patients were screened for inclusion (Figure 2). Of these, 268 patients (89.9%) were eligible for entry into the double-blind phase of the study and were randomized to pitolisant (n = 201) or placebo (n = 67). Among patients in this ITT population, 267 received at least one dose of study medication and had a validated postbaseline assessment, comprising 200 in the pitolisant group and 67 in the placebo group. These patients constituted the safety population. Twelve patients in the ITT population had at least one major protocol deviation (Table E3), and 14 patients discontinued the study prematurely. These 26 patients were excluded from the per-protocol analysis that included 181 in the pitolisant group and 61 in the placebo group.
Figure 2.
Study flowchart. Causes of noneligibility were as follows: 10 due to patient decision and 20 due to exclusion criteria (6 significant cardiovascular disease or abnormality, 3 Epworth Sleepiness Scale score <12, 2 severe insomnia not associated with obstructive sleep apnea, 2 apnea–hypopnea index <15 events/h, 2 positive serological test [HIV], 1 severe depression [Beck Depression Inventory score >16], 1 substance abuse [opioids], 1 significant periodic limb movement disorder and central sleep apnea, 1 age <18 yr, and 1 adhering to continuous positive airway pressure therapy). AE = adverse event; D = day; DB = double-blind; ITT = intention to treat; LF = lost to follow-up; OL = open label; PD = patient decision; PP = per protocol; SAF = safety.
The ITT population was primarily male (75.4%) and obese, and the mean age was 52.0 years. On average, the time since diagnosis of OSA was 11.9 months; AHI at diagnosis was 49.3 events per hour; nocturnal SaO2 was 90.1%; and Mini Mental State Examination score was 29.4. No significant differences in demographic or clinical characteristics were found between the treatment groups (Table 1).
Table 1.
Demography and Characteristics at Baseline
| Parameter | Pitolisant (n = 201) | Placebo (n = 67) | All Patients (N = 268) |
|---|---|---|---|
| Age, yr, mean (SD) (range) | 51.9 (10.6) (25–75) | 52.1 (11.0) (30–76) | 52.0 (10.6) (25–76) |
| Sex, n (%) | |||
| M | 151 (75.1) | 51 (76.1) | 202 (75.4) |
| F | 50 (24.9) | 16 (23.9) | 66 (24.6) |
| Weight at inclusion, kg, mean (SD) | 97.7 (15.7) | 99.8 (16.1) | — |
| BMI, kg/m2, mean (SD) | 32.8 (4.6) | 33.0 (4.3) | — |
| Cardiovascular disease, n (%) | 110 (54.7) | 35 (52.2) | 145 (54.1) |
| AHI at date of diagnosis, events/h, mean (SD) | 50.2 (44.3) | 46.9 (22.8) | 49.3 (40.0) |
| Nocturnal SaO2 at date of diagnosis, %, mean (SD) | 89.8 (9.1) | 90.9 (3.8) | 90.1 (8.2) |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index.
Primary Efficacy Endpoint
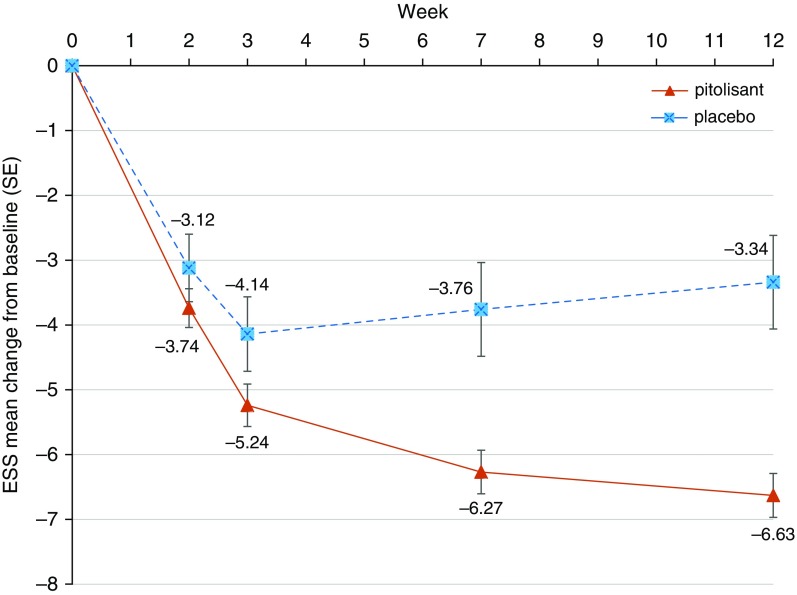
The primary endpoint, change in ESS score from baseline to end of intervention (LOCF for ESS), was −6.3 in the pitolisant group and −3.6 in the placebo group (P < 0.001). For the mean LOCF for the final ESS score, the 95% CI for pitolisant (8.8–10.1) versus placebo (10.7–13.5) did not overlap. The primary analysis showed a significant difference in effect between arms of −2.8 (95% CI, −4.0 to −1.5; P < 0.001) (Figure 3 and Table 2). When missing data were allocated using multiple imputation, the multiple imputation analysis confirmed the LOCF analysis (see Tables E1 and E2). This was due to the small number of missing values in this study, which was only 3.0% of the final ESS dataset. Predefined sensitivity analyses (baseline observation carried forward) adjusted for BMI and ESS at baseline showed the same significant treatment effects.
Figure 3.
Changes in Epworth Sleepiness Scale (ESS) score during treatment.
Table 2.
Efficacy Results for Primary Endpoint: ESS Score
| Parameter | Pitolisant (n = 201) | Placebo (n = 67) | P Value |
|---|---|---|---|
| ESS score at inclusion, mean (SD) | 15.7 (3.1) | 15.7 (3.6) | — |
| ESS score at end of treatment, mean (SD) | 9.1 (4.7) | 12.2 (6.1) | — |
| Final ESS score, DB-LOCF, mean (SD) (95% CI) | 9.4 (4.6) (8.8–10.1) | 12.1 (5.8) (10.7–13.5) | <0.001 |
| ESS score change, DB-LOCF – V2 | −6.3 (4.5) | −3.6 (5.5) | <0.001 |
| R1 response (ESS score ≤10) | |||
| n (%) | 135 (67.2) | 30 (44.8) | <0.001 |
| 95% CI | 60.2–73.6 | 32.6–57.4 | |
| R2 response (ESS score ≤10 or ESS score improvement ≥3) | |||
| n (%) | 162 (80.6) | 36 (53.7) | <0.001 |
| 95% CI | 74.4–85.8 | 41.1–66.0 |
Definition of abbreviations: CI = confidence interval; DB-LOCF = database with last observation carried forward; ESS = Epworth Sleepiness Scale; R1 = first secondary enpoint result; R2 = second secondary endpoint result; V2 = visit 2.
Secondary Efficacy Outcomes
Pitolisant normalized the ESS score (ESS, ≤10) in 67.2% of patients in the study arm versus 44.8% in the placebo group. An “ESS response,” defined as either an ESS score ≤10 or improvement by 3 or more points, was observed in 80.6% in the pitolisant group and 53.7% in the placebo group (P < 0.001) (Table 2).
The baseline mean sleep latencies during OSLER tests were 14.79 ± 10.95 minutes and 15.92 ± 11.04 minutes for the pitolisant and placebo groups, respectively. The percentages of patients exhibiting the maximum of 40 minutes were 5.5% and 6%, whereas those in the 30–40-minute range were 6.5% and 4.5%, in the pitolisant and placebo groups, respectively.
The ratios of increase in mean sleep latency during OSLER tests were 1.65 and 1.39 in the pitolisant and placebo groups, respectively (P = 0.108 using a mixed model). The analysis of the mean difference of the logarithms of sleep latencies between pitolisant and placebo showed an estimate of 0.1 (95% CI, −0.0 to 0.3), not reaching significance. The numbers and types of errors did not differ between the treatment groups (Table 3). Similar results were found in the per-protocol analysis.
Table 3.
Efficacy Results for Secondary Outcomes
| Parameter | Pitolisant (n = 201) | Placebo (n = 67) | P Value |
|---|---|---|---|
| OSLER test | |||
| OSLER test mean sleep latency at inclusion, min, (SD) | 14.79 (10.95) | 15.92 (11.04) | — |
| Number of patients with OSLER test = 40 min at inclusion | 11 (5.5%) | 4 (6%) | — |
| Number of patients with OSLER test ≥30 min and <40 min at inclusion | 13 (6.5%) | 3 (4.5%) | — |
| OSLER test mean sleep latency at end of treatment, min | 21.95 (13.53) | 20.25 (13.42) | — |
| Ratio of OSLER test V6/OSLER test V2, geometric mean | 1.65 | 1.39 | 0.120 |
| Mean difference of pitolisant and placebo logarithms of sleep latency at end of DB treatment (95% CI) | 0.1 (0.0–0.3) |
— | |
| Normal vigilance (number of 3–6 and ≥7 errors = 0 for each of the three tests) | |||
| At baseline (V2) | 2.0% (0.5–5.0%) | 3.0% (0.4–10.4%) | — |
| At the end of DB treatment (V6) | 8.5% (4.9–13.5%) | 6.3% (1.7–15.2%) | 0.487 |
| Pichot fatigue score, mean change (SD) | −3.6 (5.6) | −1.0 (6.3) | 0.005 |
| Sleep diary variables | |||
| Mean change in daily number of sleep/sleepiness episodes (SD) | −1.79 (1.97) | −1.30 (1.86) | 0.056* |
| Mean change in daily duration of sleep/sleepiness episodes (SD) | −47.87 (53.39) | −32.24 (48.82) | 0.066† |
| EQ-5D, mean change in VAS score | 7.3 ± 16.2 | 1.8 ± 16.3 | 0.059 |
| Leeds Sleep Evaluation Questionnaire | |||
| Mean change in modified getting to sleep (SD) | 10.21 (24.99) | 2.42 (23.51) | 0.155 |
| Mean change in quality of sleep (SD) | 17.70 (26.08) | 13.00 (25.56) | 0.108 |
| Mean change in awake after sleep (SD) | 19.19 (26.61) | 14.00 (25.18) | 0.160 |
| Mean change in behavior after awakening (SD) | 21.96 (22.26) | 13.35 (20.89) | 0.018 |
| Mean change in global LSEQ score (SD) | 17.26 (14.80) | 10.69 (14.80) | 0.005 |
| TMT A, mean change in average time (SD) | −8.9 (12.7) | −7.3 (13.7) | 0.389 |
| TMT B, mean change in average time (SD) | −22.5 (40.0) | −16.3 (33.8) | 0.648 |
| CGI | <0.001 | ||
| Very much improved | 21 (11.1%) | 3 (4.7%) | |
| Much improved | 84 (44.2%) | 19 (29.7%) | |
| Minimally improved | 55 (28.9%) | 14 (21.9%) | |
| No change | 30 (15.8%) | 22 (34.4%) | |
| Minimally worse | 0 (0.0%) | 6 (9.4%) | |
| Much worse | 0 (0.0%) | 0 (0.0%) | |
| Very much worse | 0 (0.0%) | 0 (0.0%) | |
| CGI improvement at end of DB treatment (V6) | |||
| n (%) | 160 (84.2%) | 36 (56.3%) | |
| 95% CI | 78.2–89.1% | 43.3–68.6% | |
| Patient’s global opinion | <0.001 | ||
| Improvement at V6, n (%) | 164 (86.3%) | 39 (60.9%) | |
| 95% CI | 80.6–90.9% | 47.9–72.9% | |
Definition of abbreviations: CGI = Clinical Global Impression; CI = confidence interval; DB = double-blind; EQ-5D = EuroQol five-dimension quality of life scale; LSEQ = Leeds Sleep Evaluation Questionnaire; OSLER = Oxford Sleep Resistance test; TMT = Trail Making Test; V2 = visit 2; V6 = visit 6; VAS = visual analogue scale.
P = 0.049 in the per-protocol population.
P = 0.050 in the per-protocol population.
There were trends in improvement in sleep diary variables in the pitolisant group compared with the placebo group (number and duration of sleep and sleepiness episodes; P = 0.056 and P = 0.066, respectively). Significance was achieved for these variables in per-protocol analysis (number and duration of sleep and sleepiness episodes; P = 0.049 and P = 0.05, respectively).
The EQ-5D visual analogue scale showed average increases of 7.3 mm in the pitolisant group and 1.8 mm in the placebo group (P = 0.059). No between-group differences were found regarding the items in the LSEQ, except for behavior after waking with pitolisant (P = 0.018). No changes were found for the mean time to perform Trail Making Test part A or B. At the end of the double-blind phase, 84.2% of patients in the pitolisant group had improved their CGI severity and change scale scores (11.1% very much improved, 44.2% much improved, and 28.9% minimally improved) compared with 56.3% in the placebo group (4.7% very much improved, 29.7% much improved, and 21.9% minimally improved) (P < 0.001). Improvement in the PGO was expressed by 86.3% of patients in the pitolisant group (marked effect, 30.0%; moderate effect, 33.7%; minimal effect, 22.6%) compared with 60.9% in the placebo group (marked effect, 21.9%; moderate effect, 18.8%; minimal effect, 20.3%) (P < 0.001). The mean Pichot fatigue scale scores decreased from 13.0 ± 6.5 at baseline to 9.2 ± 6.6 at 12 weeks in the pitolisant group and from 11.1 ± 5.9 to 10.5 ± 6.1 in the placebo group, with a significant mean change between groups (−3.6 ± 5.6 vs. −1.0 ± 6.3; P = 0.005) (Table 3). During the double-blind phase, the maximum dose prescribed was 20 mg/d for 82.5% of patients in the pitolisant group and for 86.6% of the patients in the placebo group.
Safety
The safety evaluation was based on the incidence of TEAEs. No differences were found for TEAE frequency between the pitolisant (29.5%) and placebo groups (25.4%). The most frequently reported TEAE was headache (8.5% and 11.9% in the pitolisant and placebo groups, respectively). Other frequent TEAEs were insomnia, nausea, and vertigo, reported in 5.5%, 2.5%, and 2.0% with pitolisant, respectively, and in 3.0%, 1.5%, and 2.0% with placebo, respectively (Table 4). Moreover, the frequency of TEAEs that were considered treatment related was similar (24.0% with pitolisant and 19.4% with placebo; P = 0.377).
Table 4.
Safety Parameters
| Parameter | Pitolisant (n = 200) | Placebo (n = 67) | P Value |
|---|---|---|---|
| Any TEAE, n (%) | 59 (29.5) | 17 (25.4) | |
| Any treatment-related TEAE, n (%) | 48 (24.0) | 13 (19.4) | 0.377 |
| Any serious TEAE, n (%) | 2 (1.0) | 0 (0.0) | |
| Any TEAEs leading to study drug withdrawal, n (%) | 3 (1.5) | 2 (3.0) | |
| Systolic blood pressure (SD) | |||
| At baseline (V2) | 128.2 (11.6) | 127.2 (7.2) | |
| Range | 97 to 180 | 110 to 145 | |
| At end of DB treatment (V6) | 127.4 (11.4) | 128.5 (10.1) | |
| Range | 95 to 185 | 110 to 160 | |
| Mean change (SD) | −0.7 (11.6) | 1.3 (9.3) | 0.313 |
| Range | −55 to 30 | −20 to 33 | |
| Diastolic blood pressure (SD) | |||
| At baseline (V2) | 80.1 (6.6) | 80.3 (5.1) | |
| Range | 57 to 112 | 60 to 91 | |
| At end of DB treatment (V6) | 79.8 (6.4) | 80.4 (5.2) | |
| Range | 60 to 108 | 64 to 101 | |
| Mean change (SD) | −0.2 (7.5) | 0.2 (5.9) | 0.622 |
| Range | −42 to 24 | −24 to 33 | |
| Heart rate (SD) | |||
| At baseline (V2) | 74.2 (10.2) | 72.9 (10.2) | |
| Range | 50 to 104 | 57 to 101 | |
| At end of DB treatment (V6) | 73.5 (9.8) | 73.7 (10.8) | |
| Range | 46 to 103 | 48 to 99 | |
| Mean change (SD) | −0.3 (9.7) | 0.3 (8.4) | 0.725 |
| Range | −24 to 26 | −24 to 10 | |
| BDI* total score | |||
| Mean score at baseline (V2) (SD) | 4.7 (3.4) | 4.4 (3.6) | |
| 95% CI | 4.3 to 5.2 | 3.5 to 5.2 | |
| Mean score at end of DB treatment (V6) (SD) | 3.7 (3.3) | 3.5 (3.7) | |
| 95% CI | 3.2 to 4.2 | 2.6 to 4.5 | |
| Mean change between baseline and end of DB treatment | −1.0 (2.7) | −0.9 (3.2) | 0.960 |
| 95% CI | −1.4 to −0.6 | −1.7 to −0.1 |
Definition of abbreviations: BDI = Beck Depression Inventory; CI = confidence interval; DB = double-blind; TEAE = treatment-emergent adverse event; V2 = visit 2; V6 = visit 6.
Thirteen items.
TEAEs leading to study drug withdrawal were reported for three patients (1.5%) in the pitolisant group and two patients (3.0%) in the placebo group. Serious TEAEs were reported for two patients (1.0%; one prolonged QT interval on the ECG and one cardiopulmonary failure leading to death) during pitolisant treatment and considered unlikely to be treatment related and in none of the patients receiving placebo.
The occurrence of amphetamine-like withdrawal syndrome (dysphoria, defined as at least three of the following symptoms: fatigue, vivid and unpleasant dreams, insomnia or hypersomnia, increased appetite, and psychomotor retardation and/or agitation [21]) was assessed in all participants. None of the patients experienced amphetamine-like withdrawal syndrome, and specifically neither hypersomnia nor fatigue rebound, at treatment interruption at the end of the study. All features and symptoms of withdrawal syndrome are reported in Table E4. BDI scores, blood chemistry, and hematological or cardiovascular parameters did not change in either group. During treatment, there were no changes from baseline in systolic and diastolic blood pressure or heart rate for both groups (Table 4). Mean values of the ECG variables were comparable in the two treatment groups. However, in the pitolisant treatment group, three patients (1.5%) had at least one postdose corrected QT interval by Fredericia’s corrected QT interval (QTcF) longer than 450 ms, and four patients (2.0%) had one QTcF elongation greater than or equal to 60 ms, whereas there was one patient with QTcF longer than 450 ms in the placebo group.
Discussion
Pitolisant reduced self-reported EDS as measured by the ESS score together with an overall improvement in both patient-reported outcomes and physician-assessed severity in adult patients with OSA with daytime sleepiness refusing CPAP treatment. The study population corresponded to patients with OSA refusing or not tolerating CPAP treatment. Personalization of sleep apnea treatment is crucial (22) and is a prerequisite for optimizing adherence, which in turn leads to effectiveness. In the case of CPAP refusal or nonadherence, clinicians should attempt alternative treatments to CPAP (23) before offering solely pharmacologic treatment for sleepiness. Evidence supports the use of mandibular advancement devices in mild to moderate OSAS as providing a health benefit equivalent to CPAP (24). Maxillomandibular osteotomy and upper airway stimulation seem to be as efficient as CPAP in selected young patients with OSA without comorbidities. Finally, lifestyle interventions, including weight loss (25), exercise (26), and/or positional therapy (27) can be considered as able to at least reduce OSA severity.
After the dose escalation period, the mean ESS score was significantly reduced compared with placebo. The estimate of the treatment effect based on the change in ESS score between the beginning and end of the double-blind intervention was −2.8 (95% CI, −4.0 to −1.5). Moreover, the normalization (ESS score ≤ 10) and responder rates (ESS score ≤ 10 or improvement ≥3) were greater in the group treated with pitolisant. The magnitude of the change in ESS score was close to that observed in studies of modafinil, armodafinil, and solriamfetol in patients with OSA (10, 11, 28). However, this should be confirmed by head-to-head comparisons between wake-promoting agents. The magnitude of ESS improvement with pitolisant at Week 12 was substantial and clinically relevant (19, 20), albeit slightly weaker than that reported for patients with narcolepsy treated with pitolisant with doses often reaching 40 mg (14, 15).
ESS is a patient-reported assessment of propensity to fall asleep in different situations of everyday life, whereas the OSLER test is an objective measure of ability to maintain wakefulness in a laboratory environment and the related daytime vigilance. We found no significant between-group changes in the OSLER test. However, the pitolisant group had lower mean sleep latency at baseline (14.79) than the placebo group (15.92), and a considerable proportion of patients exhibited OSLER test mean sleep latencies in the normal range at baseline (12% had mean sleep latency ≥30 min in the pitolisant group compared with 10% in the placebo group), and this may have limited the potential for improvement because of a ceiling effect.
For several other secondary outcomes (sleep diaries and EQ-5D), results were close to statistical significance, becoming statistically significant in per-protocol analysis. This suggests that the study might have been underpowered for some secondary outcomes. This may also be due to the inclusion criteria of without CPAP treatment and with baseline ESS scores ≥12. Our population thus differs from the pivotal trials of modafinil, armodafinil, and solriamfetol for treatment of EDS in OSA, in which most of the patients were receiving CPAP therapy and with an ESS score ≥10. Pitolisant was superior to placebo at the end of the double-blind phase, with major improvements compared with placebo in terms of patient-centered outcomes, including the Pichot fatigue scale and a feeling of restorative sleep upon waking (explored by the LSEQ). A clear improvement was also observed via the physician-scored (CGI of change) and patient-scored (PGO) questionnaires.
Adherence to CPAP is a major issue when treating OSA. It is estimated that 10–15% of patients with OSA initially refuse or quickly abandon CPAP in the first weeks of treatment. The long-term rate of CPAP discontinuation is consistent across studies at between 20% and 40% (13). No clear improvement in mean CPAP adherence has been seen over the last 20 years, despite considerable technical advances, behavioral interventions, and multimodal telemonitoring systems (29, 30). This leaves thousands of patients with symptomatic OSA with untreated daytime sleepiness. An original and unique aspect of our study was the targeted OSA population comprising only patients with EDS refusing CPAP. In the two recent solriamfetol studies (11, 28), the study populations were heterogeneous, with approximately one-third of patients having no OSA primary treatment. In the study by Strollo and colleagues (11), a post hoc subanalysis showed that ESS score, maintenance of wakefulness, and CGI change scale score were slightly better in nonadherent patients than in those adhering to CPAP. It remains controversial whether targeting EDS alone without addressing the underlying cause is an acceptable practice, and doing so might lead to misuse. However, from a pragmatic point of view, these patients often remain untreated and have a poor quality of life, loss of productivity, and risk of EDS-related accidents. Another major issue beyond symptom improvement is to ensure that a given wake-promoting agent does not precipitate or exacerbate OSA-related cardiovascular comorbidities, as already reported for psychostimulants used in narcolepsy acting via dopamine or norepinephrine release (31, 32). However, in this pitolisant trial, no significant changes were found in terms of blood pressure or heart rate, which provides a reassurance of safety. In contrast, in a recent meta-analysis, modafinil and armodafinil were associated with a mean increase in systolic blood pressure of 3.0 mm Hg (95% CI, 0.8–5.2 mm Hg) and a mean increase in diastolic blood pressure of 1.9 mm Hg (95% CI, 0.5–3.3 mm Hg) in three of seven trials (33). In a recent study using solriamfetol, the highest dose caused increases in systolic and diastolic blood pressure of 2.5 and 1.5 mm Hg, respectively, and heart rate increased by 2–3 beats per minute at doses greater than 75 mg. Again, head-to-head comparisons of the various clinically available wake-promoting agents, with particular focus on cardiovascular outcomes, are needed.
Our results confirm the favorable safety profile of pitolisant already reported in patients with narcolepsy (14, 15). No key changes were found in physical examination parameters or vital signs, depressive symptoms, and ECG or laboratory test results during the study. The changes reported in QTc (QTcF, >450 ms and elongation >60 ms) did not differ significantly between pitolisant and placebo. The pitolisant to placebo subject ratio was 3:1. Hence, there was no difference in the number of subjects with post-treatment QTcF values greater than 450 ms (3 of 201 patients [1.5%] in the pitolisant treatment group and 1 of 67 patients [1.5%] in the placebo group). These results are similar to those observed in the previous clinical cardiovascular safety study (through QT/QTc) with pitolisant (40 and 120 mg acute), including 58 healthy volunteers (males and females), in which 1 (1.7%) of 58 subjects exhibited a postdose QTcF longer than 450 ms, comparable to the incidence reported in the placebo period. Accordingly, results reported with pitolisant in the previous randomized controlled trials in patients with narcolepsy (14, 15) did not show any significant increase in QTc. A recent 1-year open-label trial in patients with narcolepsy confirmed the absence of significant ECG changes, including in the QTc (409 ± 25 ms at baseline and 416 ± 25 ms after 12 mo) (34). In line with reports of pitolisant use in patients with narcolepsy, there were no withdrawal symptoms after abrupt discontinuation of the drug in sleepy patients with OSA (14, 15). The overall tolerance was good, and the incidence rates of global as well as treatment-related TEAEs or TEAEs leading to study withdrawal were similar in both treatment groups, with no major safety concerns raised during the study. The most frequently reported TEAE was headache at a frequency of 8.5% with pitolisant and 11.9% with placebo, followed by insomnia, nausea, and vertigo.
The main limitation of this study was its 12-week duration. Long-term maintenance of efficacy and safety are being evaluated in an extension of the study. For most patients, the maximum dose of 20 mg was administered (82.5% in the pitolisant group and 86.6% in the placebo group); however, this dose may potentially have been too low to achieve maximum efficiency, as suggested by narcolepsy studies in which the maximum dose could be as high as 40 mg/d (14, 15). Moreover, no dose–response assessment was conducted, because dose assignment was not randomized, and dose uptitration is the standard approach in the clinical use of pitolisant. In conclusion, in this 12-week, phase 3 clinical trial, pitolisant reduced self-reported EDS and improved patient-reported outcomes, confirmed by the physician’s CGI, in patients with OSA refusing or not adhering to CPAP treatment.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Alison Foote (Grenoble Alpes University Hospital, Grenoble, France) for critically reading and editing the manuscript.
HAROSA II Study Group collaborators: Valerie Attali, Sleep Disorder Unit, Hôpital la Pitié-Salpêtrière, Paris, France and Sorbonne University INSERM UMRS 1158, Paris, France; Patrice Bourgin, Hopital Civil, Strasbourg, France; Frederic Gagnadoux, Department of Pneumology, CHU Angers, France; Farhad Baharloo, Hopital de la Citadelle, Liège, Belgium; Soren Berg, Scan Sleep ApS, Sovnlaegecentret, Copenhagen, Denmark; Olli Polo, Unesta Research Center, Helsinki, Finland; Jan Anders Hedner, Sleep and Wake Disorders, Department of Internal Medicine, University of Gothenburg and Sahlgrenska University Hospital, Gothenburg, Sweden; Yeksel Peker, Sleep Medicine Unit, Skaraborg County Hospital, Skövde, Sweden; Alexander Blau, Advanced Sleep Research GmbH, Berlin, Germany; Diego Garcia Borreguero, Sleep Research Institute, Madrid, Spain; Francisco Javier Puertas Cuesta, Sleep Unit, Neurophysiology Department, Ribera Hospital, Valencia, Spain; Georgi Belev, Department of Pneumology, Saint George Hospital, Plovdiv, Bulgaria; Yavor Ivanov, Clinic of Pneumology, Pleven, Bulgaria; Hristo Metev, Department of Pneumology, Rousse, Bulgaria; Diana Petkova, Department of Pneumology, Varna, Bulgaria; Dejan Dokic, Department of Pneumology and Allergy, Mother Teresa Medical University, Skopje, Macedonia; Merita Ismajli Marku, Neuropraxis, Skopje, Macedonia; Biserka Jokovska Kaeva, Department of Pneumology and Allergy, Mother Teresa Medical University, Skopje, Macedonia; Slavko Jankovic, Neurology Clinic, Belgrade, Serbia; Ivan Kopitovic, Institute of Pneumology of Vojvodina, Sremska Kamenica, Serbia; and Miodrag Vukcevic, Department of Pneumology, Hospital Bezanijska, Belgrade, Serbia.
Footnotes
Supported by Bioprojet, Paris, France.
Author Contributions: J.V., M.P., J.H., T.S., O.G., R. Tiholov, R. Tamisier, and C.S.-G. participated in data acquisition, data interpretation, and revision of the paper. I.L. and J.-M.L. participated in the conception, design, and organization of the study and revision of the paper. P.L. participated in the conception, design, international coordination, and revision of the paper. Y.D. and J.-L.P. participated in the conception and design of the study and wrote the paper. J.-C.S. participated in the conception and design of the study and revised the paper. All authors shared in the decision to submit the manuscript for publication, and all authors attest to the accuracy and completeness of the data and compliance with the study protocol.
Data collected for the study, including deidentified individual participant data and a data dictionary defining each field in the set, will be made available to others after the publication of this article, as will additional related documents (study protocol, statistical analysis plan, and informed consent form), for academic purposes (e.g., meta-analyses), upon request to the sponsor, Bioprojet (jm.lecomte@bioprojet.com), and with a signed data access agreement.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201907-1284OC on January 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the HAROSA II Study Group, Valerie Attali, Patrice Bourgin, Frederic Gagnadoux, Farhad Baharloo, Soren Berg, Olli Polo, Jan Anders Hedner, Yeksel Peker, Alexander Blau, Diego Garcia Borreguero, Francisco Javier Puertas Cuesta, Georgi Belev, Yavor Ivanov, Hristo Metev, Diana Petkova, Dejan Dokic, Merita Ismajli Marku, Biserka Jokovska Kaeva, Slavko Jankovic, Ivan Kopitovic, and Miodrag Vukcevic
References
- 1.Lévy P, Kohler M, McNicholas WT, Barbé F, McEvoy RD, Somers VK, et al. Obstructive sleep apnoea syndrome. Nat Rev Dis Primers. 2015;1:15015. doi: 10.1038/nrdp.2015.15. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT, Bassetti CL, Ferini-Strambi L, Pépin JL, Pevernagie D, Verbraecken J, et al. Baveno Working Group. Challenges in obstructive sleep apnoea. Lancet Respir Med. 2018;6:170–172. doi: 10.1016/S2213-2600(18)30059-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3:404–414. doi: 10.1016/S2213-2600(15)00090-9. [DOI] [PubMed] [Google Scholar]
- 4.Bucks RS, Olaithe M, Rosenzweig I, Morrell MJ. Reviewing the relationship between OSA and cognition: where do we go from here? Respirology. 2017;22:1253–1261. doi: 10.1111/resp.13140. [DOI] [PubMed] [Google Scholar]
- 5.Bratton DJ, Gaisl T, Schlatzer C, Kohler M. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869–878. doi: 10.1016/S2213-2600(15)00416-6. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: a systematic review and meta-analysis. Sleep Breath. 2018;22:555–568. doi: 10.1007/s11325-017-1590-6. [DOI] [PubMed] [Google Scholar]
- 7.Pépin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Lévy P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. 2009;33:1062–1067. doi: 10.1183/09031936.00016808. [DOI] [PubMed] [Google Scholar]
- 8.Gasa M, Tamisier R, Launois SH, Sapene M, Martin F, Stach B, et al. Scientific Council of the Sleep Registry of the French Federation of Pneumology-FFP. Residual sleepiness in sleep apnea patients treated by continuous positive airway pressure. J Sleep Res. 2013;22:389–397. doi: 10.1111/jsr.12039. [DOI] [PubMed] [Google Scholar]
- 9.Kapur VK, Donovan LM. Taking care of persistent sleepiness in patients with sleep apnea [editorial] Am J Respir Crit Care Med. 2019;199:1310–1311. doi: 10.1164/rccm.201812-2291ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avellar AB, Carvalho LB, Prado GF, Prado LB. Pharmacotherapy for residual excessive sleepiness and cognition in CPAP-treated patients with obstructive sleep apnea syndrome: a systematic review and meta-analysis. Sleep Med Rev. 2016;30:97–107. doi: 10.1016/j.smrv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Strollo PJ, Jr, Hedner J, Collop N, Lorch DG, Jr, Chen D, Carter LP, et al. Tones 4 Study Investigators. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155:364–374. doi: 10.1016/j.chest.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Bai XX, Williams SC, Hua SC, Kim JW, Marshall NS, et al. Modafinil increases awake EEG activation and improves performance in obstructive sleep apnea during continuous positive airway pressure withdrawal. Sleep (Basel) 2015;38:1297–1303. doi: 10.5665/sleep.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rotenberg BW, Murariu D, Pang KP. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szakacs Z, Dauvilliers Y, Mikhaylov V, Poverennova I, Krylov S, Jankovic S, et al. HARMONY-CTP study group. Safety and efficacy of pitolisant on cataplexy in patients with narcolepsy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:200–207. doi: 10.1016/S1474-4422(16)30333-7. [DOI] [PubMed] [Google Scholar]
- 15.Dauvilliers Y, Bassetti C, Lammers GJ, Arnulf I, Mayer G, Rodenbeck A, et al. HARMONY I study group. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12:1068–1075. doi: 10.1016/S1474-4422(13)70225-4. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine International Classification of Sleep Disorders: diagnostic and coding manual, 2nd ed. (ICSD-2). Winchester: American Academy of Sleep Medicine; 2005.
- 17.Bennett LS, Stradling JR, Davies RJ. A behavioural test to assess daytime sleepiness in obstructive sleep apnoea. J Sleep Res. 1997;6:142–145. doi: 10.1046/j.1365-2869.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 18.Mazza S, Pepin JL, Deschaux C, Naegele B, Levy P. Analysis of error profiles occurring during the OSLER test: a sensitive mean of detecting fluctuations in vigilance in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:474–478. doi: 10.1164/rccm.2107065. [DOI] [PubMed] [Google Scholar]
- 19.Patel S, Kon SSC, Nolan CM, Barker RE, Simonds AK, Morrell MJ, et al. The Epworth Sleepiness Scale: minimum clinically important difference in obstructive sleep apnea [letter] Am J Respir Crit Care Med. 2018;197:961–963. doi: 10.1164/rccm.201704-0672LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crook S, Sievi NA, Bloch KE, Stradling JR, Frei A, Puhan MA, et al. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax. 2019;74:390–396. doi: 10.1136/thoraxjnl-2018-211959. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association Diagnostic and statistical manual of mental disorders, 4th ed. (DSM-IV-TR). Washington, DC: APA; 2000.
- 22.Sutherland K, Kairaitis K, Yee BJ, Cistulli PA. From CPAP to tailored therapy for obstructive sleep apnoea. Multidiscip Respir Med. 2018;13:44. doi: 10.1186/s40248-018-0157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Randerath W, Bassetti CL, Bonsignore MR, Farre R, Ferini-Strambi L, Grote L, et al. Challenges and perspectives in obstructive sleep apnoea: report by an ad hoc working group of the Sleep Disordered Breathing Group of the European Respiratory Society and the European Sleep Research Society. Eur Respir J. 2018;52:1702616. doi: 10.1183/13993003.02616-2017. [DOI] [PubMed] [Google Scholar]
- 24.Chan ASL, Sutherland K, Cistulli PA. Mandibular advancement splints for the treatment of obstructive sleep apnea. Expert Rev Respir Med. 2020;14:81–88. doi: 10.1080/17476348.2020.1686978. [DOI] [PubMed] [Google Scholar]
- 25.Hudgel DW, Patel SR, Ahasic AM, Bartlett SJ, Bessesen DH, Coaker MA, et al. American Thoracic Society Assembly on Sleep and Respiratory Neurobiology. The role of weight management in the treatment of adult obstructive sleep apnea: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e70–e87. doi: 10.1164/rccm.201807-1326ST. [DOI] [PubMed] [Google Scholar]
- 26.Mendelson M, Bailly S, Marillier M, Flore P, Borel JC, Vivodtzev I, et al. Obstructive sleep apnea syndrome, objectively measured physical activity and exercise training interventions: a systematic review and meta-analysis. Front Neurol. 2018;9:73. doi: 10.3389/fneur.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pépin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13:813–824. doi: 10.5664/jcsm.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweitzer PK, Rosenberg R, Zammit GK, Gotfried M, Chen D, Carter LP, et al. TONES 3 Study Investigators. Solriamfetol for excessive sleepiness in obstructive sleep apnea (TONES 3): a randomized controlled trial. Am J Respir Crit Care Med. 2019;199:1421–1431. doi: 10.1164/rccm.201806-1100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pépin JL, Jullian-Desayes I, Sapène M, Treptow E, Joyeux-Faure M, Benmerad M, et al. Multimodal remote monitoring of high cardiovascular risk patients with OSA initiating CPAP: a randomized trial. Chest. 2019;155:730–739. doi: 10.1016/j.chest.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Pépin JL, Tamisier R, Hwang D, Mereddy S, Parthasarathy S. Does remote monitoring change OSA management and CPAP adherence? Respirology. 2017;22:1508–1517. doi: 10.1111/resp.13183. [DOI] [PubMed] [Google Scholar]
- 31.Bosco A, Lopez R, Barateau L, Chenini S, Pesenti C, Pépin JL, et al. Effect of psychostimulants on blood pressure profile and endothelial function in narcolepsy. Neurology. 2018;90:e479–e491. doi: 10.1212/WNL.0000000000004911. [DOI] [PubMed] [Google Scholar]
- 32.Pepin JL, Borel AL, Tamisier R, Baguet JP, Levy P, Dauvilliers Y. Hypertension and sleep: overview of a tight relationship. Sleep Med Rev. 2014;18:509–519. doi: 10.1016/j.smrv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Chapman JL, Vakulin A, Hedner J, Yee BJ, Marshall NS. Modafinil/armodafinil in obstructive sleep apnoea: a systematic review and meta-analysis. Eur Respir J. 2016;47:1420–1428. doi: 10.1183/13993003.01509-2015. [DOI] [PubMed] [Google Scholar]
- 34.Dauvilliers Y, Arnulf I, Szakacs Z, Leu-Semenescu S, Lecomte I, Scart-Gres C, et al. HARMONY III study group. Long-term use of pitolisant to treat patients with narcolepsy: Harmony III Study. Sleep (Basel) 2019;42:zsz174. doi: 10.1093/sleep/zsz174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.