Abstract
Background
The risks associated with MRI in individuals who have implanted cardiac devices are thought to arise from the interaction between the implanted device and static, gradient, and radiofrequency magnetic fields.
Purpose
To determine the relationship between the peak whole-body averaged specific absorption rate (SAR) and change in magnetic field per unit time (dB/dt), maximum specific energy dose, imaging region, and implanted cardiac device characteristics and their function in patients undergoing MRI.
Materials and Methods
This prospective observational cohort study was conducted from October 16, 2003, to January 22, 2015 (https://ClinicalTrials.gov, NCT01130896). Any individual with an implanted cardiac device who was referred for MRI was included. Clinical MRI protocols without SAR restriction were used. Exclusion criteria were newly implanted leads, abandoned or epicardial leads, and dependence on a pacemaker with an implantable cardioverter defibrillator without asynchronous pacing capability. For each MRI pulse sequence, the calculated whole-body values for SAR, dB/dt, and scan duration were collected. Atrial and ventricular sensing, lead impedance, and capture threshold were evaluated before and immediately after (within 10 minutes) completion of each MRI examination. Generalized estimating equations with Gaussian family, identity link, and an exchangeable working correlation matrix were used for statistical analysis.
Results
A total of 2028 MRI examinations were performed in 1464 study participants with 2755 device leads (mean age, 67 years ± 15 [standard deviation]; 930 men [64%]). There was no evidence of an association between radiofrequency energy deposition, dB/dt, or scan duration and changes in device parameters. Thoracic MRI was associated with decreased battery voltage immediately after MRI (β = −0.008 V, P < .001). Additionally, right ventricular (RV) lead length was associated with decreased RV sensing (β = −0.012 mV, P = .05) and reduced RV capture threshold (β = −0.002 V, P < .01) immediately after MRI.
Conclusion
There was no evidence of an association between MRI parameters that characterize patient exposure to radiofrequency energy and changes in device and lead parameters immediately after MRI. Nevertheless, device interrogation before and after MRI remains mandatory due to the potential for device reset and changes in lead or generator parameters.
© RSNA, 2020
See also the editorial by Shellock in this issue.
Summary
In patients with implanted cardiac devices who were undergoing MRI, we found no evidence of an association between parameters that characterize radiofrequency energy exposure to the patient and changes in device and lead parameters.
Key Results
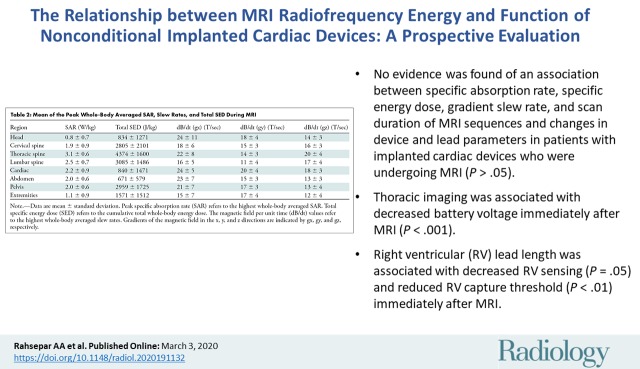
■ We found no evidence of an association between specific absorption rate, specific energy dose, gradient slew rate, and scan duration of MRI sequences and changes in device and lead parameters in patients with implanted cardiac devices who were undergoing MRI (P > .05).
■ Thoracic imaging was associated with decreased battery voltage immediately after MRI (P < .001).
■ Right ventricular (RV) lead length was associated with decreased RV sensing (P = 0.05) and reduced RV capture threshold (P < .01) immediately after MRI.
Introduction
MRI is considered the modality of choice in many clinical situations because of its high spatial resolution, tissue contrast resolution, multiplanar imaging capability, and the absence of ionizing radiation. Despite significant advances and recent changes in guidelines, MRI access is limited for patients with implanted cardiac devices, such as pacemakers, implantable cardioverter defibrillators (ICDs), and cardiac resynchronization therapy systems. Because of the effect of MRI on patient care and worldwide public health, there have been tremendous efforts to develop MRI safety protocols that reduce complications in patients with cardiac devices (1–6).
Risks associated with performing MRI arise from the interaction between the implanted device system and three MRI conditions related to the static and gradient magnetic fields and radiofrequency energy (7). Static magnetic fields necessary for generating magnetization usually cause negligible mechanical forces at 1.5 T (8) but can cause unpredictable reed switch behavior in current pacemaker and ICD systems (9). Gradient fields vary spatially (to encode spatial information), have zero amplitude at the magnet center, and increase toward the edge of the bore. These amplitudes are switched rapidly, and the time-varying magnetic fields (change in magnetic field per unit time [dB/dt], slew rate [measured in Teslas per second]), can induce electrical currents and interfere with cardiac device function (10). Radiofrequency fields used to manipulate magnetization may lead to heating in biologic tissues adjacent to pacing and defibrillation leads (3). In an in vivo study, a temperature increase of up to 20°C was reported during 1.5-T MRI of the heart at a specific absorption rate (SAR) of 3.8 W/kg (11). The radiofrequency-induced thermal injury in these tissues may lead to the subsequent formation of fibrosis and may result in increasing capture thresholds and deterioration of device function (12,13). However, it is noteworthy that alterations in capture threshold commonly occur spontaneously and regardless and independent of exposure to MRI.
Radiofrequency energy exposure during MRI is quantified by the SAR, which is the rate of radiofrequency energy absorbed per unit of mass of an object and which is measured in Watts per kilogram of body weight, and the specific energy dose (SED), which is the amount of radiofrequency energy deposited in an object and which is measured in Joules per kilogram of body weight. As a precaution, an early study (1) limited the whole-body averaged SAR to less than 2.0 W/kg in patients with implanted cardiac devices. Since that time, empirical limitations of SAR values have been considered for patients with cardiac devices who are undergoing MRI (10). However, the importance of limiting energy deposition and time-dependent amplitude variation of gradient magnetic fields during MRI of patients with pacemakers or defibrillators has not been formally assessed. The purpose of this study was to determine the relationship between the peak whole-body averaged SAR and dB/dt, maximum SED, imaging region, and implanted cardiac device characteristics and their function in patients undergoing MRI.
Materials and Methods
Study Design
The Johns Hopkins University institutional review board approved the protocol, and all participants gave written informed consent (https://ClinicalTrials.gov, NCT01130896). Individuals with an implantable cardiac device who were referred for medically necessary MRI were prospectively enrolled consecutively between October 16, 2003, and January 22, 2015. Individuals with newly implanted (<6 weeks) leads or abandoned or epicardial leads and those who were dependent on a pacemaker with an ICD that was not programmable to an asynchronous mode were excluded.
This study cohort includes participants whose safety data have been reported in prior publications (1,2,4). In accordance with the previously described safety protocol (1–3), comparison of device interrogation findings after MRI are published elsewhere (1,2,4), whereas in this study, the association of the maximum whole-body averaged SAR, SED, dB/dt, imaging region, and cardiac device characteristics with device lead parameters are investigated.
Assessment of Devices and Leads Variable Changes
Typical measures to evaluate appropriate device function include atrial and ventricular sensing, lead impedance, and capture threshold. Sensing is defined as the ability of the system to sense an intracardiac intrinsic electrical signal and is vital to inhibit or trigger device function in response to arrhythmia. Lead impedance is defined as the opposition to flow of electrical current through the device circuitry and lead tissue interface. Capture threshold is the minimum pacing energy required to consistently stimulate myocardial contraction. Two experienced investigators (R.H., A.A.R.) evaluated the device and lead variables for each device.
MRI Protocol
MRI was performed at 1.5 T (Avanto or Aera; Siemens Healthcare, Erlangen, Germany). The Avanto system at our institution is equipped with state-of-the art gradient performance (SQ gradient engine), with maximum gradient amplitudes of 45 mT/m per physical direction and a maximum slew rate of 200 T/m/sec at 100% duty cycle rating. Vector performance has a maximum effective amplitude of 72 mT/m and a maximum effective slew rate of 346 T/m/sec. Noninvasive blood pressure was obtained every 3 minutes along with continuous electrocardiographic monitoring. Pulse oximetry was used as a surrogate for rhythm when electrocardiographic monitoring was compromised by MRI-related artifacts. MRI was performed according to standard institutional protocols for the region of interest using standard International Electrotechnical Commission normal-mode SAR and gradient slew rate settings. During the scan, registered nurses with training in cardiac pacing and ICD device management and advanced cardiac life support were present in the MRI suite with immediate back-up from a cardiac electrophysiologist (S.N., H.R.H., R.H.). Scans were repeated as clinically indicated. Whole-body 6-minute time-averaged SAR was reported based on the MRI sequence parameters, applied voltages, transmitter reference adjustment, and patient weight (14). Peak SAR refers to the single pulse sequence with the highest whole-body SAR. Data regarding the gradient slew rate for y-, x-, and z-gradient coils in addition to the scan duration for each sequence were recorded from the scanner.
Device Interrogation and Programming
All devices were interrogated before and immediately (within 10 minutes) after completion of the MRI examination, and long-term device interrogation was provided at routine follow-up in an electrophysiology device clinic (median time to long-term follow-up, 1-year; interquartile range, 0.5–1.7 years). After each interrogation, battery voltage, lead capture thresholds, lead impedances, and sensing signal amplitudes were recorded for all participants. Pacemaker dependence was evaluated immediately before MRI by transient inhibition of pacing with continuous electrocardiographic telemetry and blood pressure monitoring. For participants without a hemodynamically stable escape rhythm, pacing mode was programmed to asynchronous. In participants without hemodynamic pacemaker dependence, either the ventricular- or dual chamber–inhibited pacing mode was used. All other pacing responses and tachyarrhythmia functions were disabled. Immediately after the completion of MRI, device and lead parameters were reinterrogated, and devices were reprogrammed to original settings.
Statistical Analysis
Data were collected before and after the MRI examination and were compared within participants. Continuous variables and discrete variables are summarized as mean ± standard deviation, median and interquartile range, or absolute number and percentage. The association of change in device variables with MRI and device system characteristics was assessed by using generalized estimating equations with Gaussian family, identity link, and an exchangeable working correlation matrix. The models were clustered by patient, which enabled analysis of repeat scans and multiple sequences per patient. All tests were two tailed, and analyses were performed using Stata, version 12 (Stata, College Station, Tex).
Results
Participants and Device Characteristics
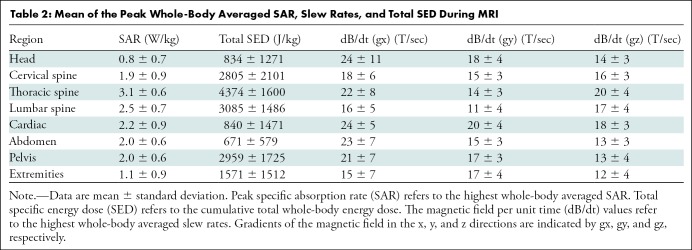
A total of 2028 MRI examinations were performed in the subcohort of 1464 participants with detailed MRI energy data with a total of 2755 leads using routine protocols with standard whole-body SAR settings for the examination (mean age, 67 years ± 15 [standard deviation], 930 male participants [64%]). Of these study participants, 853 (58%) had a permanent pacemaker, 454 (31%) had an ICD, and 157 (11%) had a cardiac resynchronization therapy defibrillator system. Among the study participants, 123 (8%) were device dependent. Lead length, MRI regions, and device manufacturers have been summarized in Table 1. The mean number of separate pulse sequences during each scan was 18 ± 13. The values for peak whole-body averaged SAR, SED, and dB/dt during MRI of different body regions are summarized in Table 2. Interestingly, the calculated average of total SED values was higher for MRI studies performed for the cervical, thoracic, and lumbar spine when compared with other examination types. As previously reported in the overall cohort study (4), one patient with a pacemaker at the estimated replacement interval and less than 1 month of battery life remaining before MRI was performed had the device reset to ventricular-inhibited pacing with end-of-life battery status after MRI. This device could not be reprogrammed and was replaced.
Table 1:
Characteristics of the Study Population Consisting of 1464 Participants Undergoing 2028 MRI Examinations
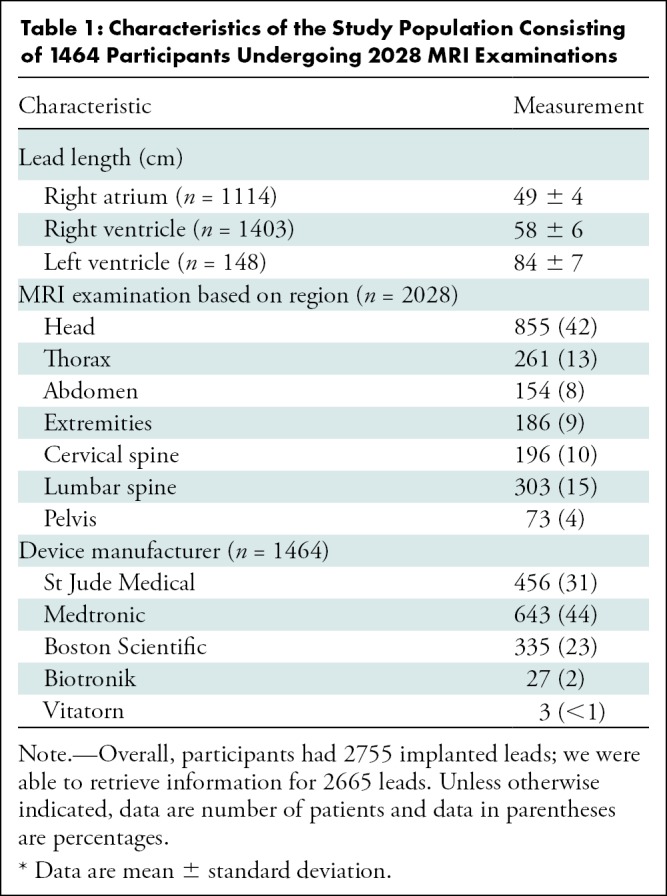
Table 2:
Mean of the Peak Whole-Body Averaged SAR, Slew Rates, and Total SED During MRI
Device Outputs and MRI Parameters
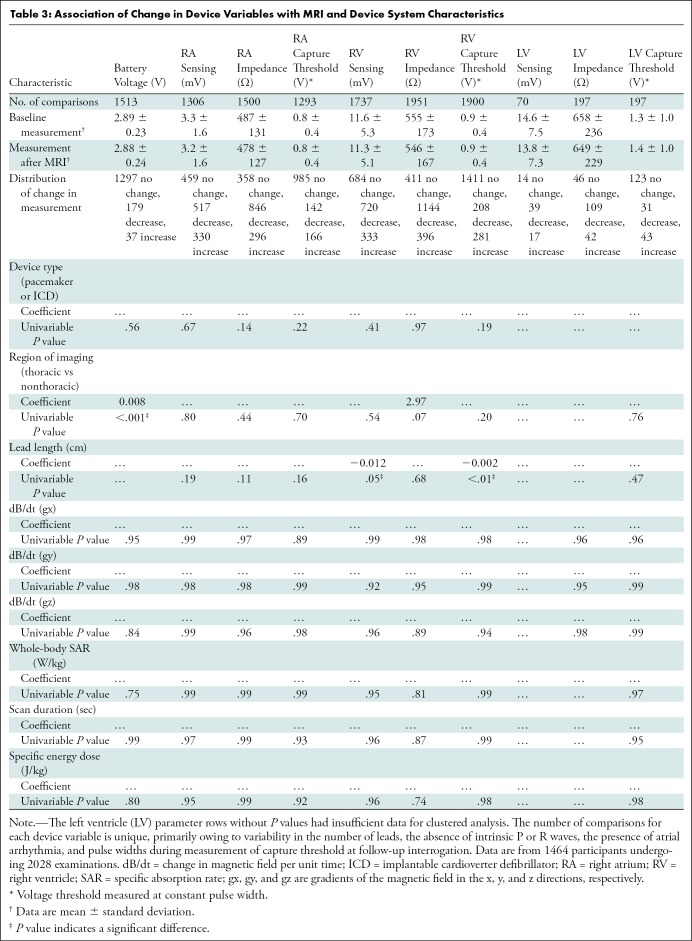
The univariable associations between various device parameters and MRI and device system characteristics have been summarized in Table 3. Thoracic MRI was associated with decreased battery voltage immediately after MRI (mean, 2.89 V ± 0.23 vs 2.89 V ± 0.24, β = −0.008 V, P < .001). Additionally, right ventricle (RV) lead length was associated with decreased RV sensing (mean, 11.6 mV ± 5.3 vs 11.3 mV ± 5.1; β = −0.012 mV; P = 0.05) and reduced RV capture threshold (mean, 0.9 V ± 0.4 vs 0.9 V ± 0.4; β = −0.002 V; P < .01) immediately after MRI. However, we found no association between radiofrequency energy deposition, dB/dt, or scan duration and changes in system parameters.
Table 3:
Association of Change in Device Variables with MRI and Device System Characteristics
Discussion
Many patients are unduly restricted from diagnostic MRI because of safety concerns that arise from the potential interaction between the implanted device and static, gradient, and radiofrequency magnetic fields. To our knowledge, this is the largest prospective study to evaluate the association between MRI field components and changes in cardiac device parameters. The main finding of the current study is that despite a relatively large sample size, we failed to find any association between MRI characteristics, including change in magnetic field per unit time (dB/dt) (P > .05), specific absorption rate (SAR) (P > .05), specific energy dose (SED) (P > .05), and examination duration (P > .05) with adverse device changes. Thoracic MRI was associated with decreased battery voltage immediately after MRI (mean, 2.89 V ± 0.23 vs 2.89 V ± 0.24; β = −0.008 V; P < .001). These findings are particularly relevant for patients with implanted legacy devices that are not considered to be MRI conditional, as the need to scan them will continue for many years. On the basis of our results, the risk of subsequent device malfunction or failure is low if an MRI safety protocol such as the one used here is followed. Additionally, we found no evidence that utilization of the routine clinical protocol using standard International Electrotechnical Commission normal-mode SAR and gradient slew rate settings is associated with adverse outcomes.
In a prior publication from this cohort (4), out of 2103 examinations in 1509 patients, 96% of the MRI examinations were performed without occurrence of a notable change in device settings immediately after MRI (>50% change from baseline) or an event like power-on reset or early termination of the examination. Nevertheless, in 0.4% of the patients, the device reset to a back-up mode, and up to 4% of participants exhibited decreases in P wave amplitude and increases in right atrium (RA), RV, or left ventricle (LV) capture thresholds at immediate or long-term follow up. These results indicate that although most patients did not experience adverse events, in individual patients there is a possibility for device or lead malfunction, and device interrogation must be performed before and particularly after MRI examinations.
The implications of reducing radiofrequency to decrease SAR and SED may lead to diminished image quality and may compromise the diagnostic usefulness of MRI. However, we found no evidence of an association between whole-body SAR and SED and changes in lead impedance, sensing, and threshold. A prior smaller study by Martin et al (12) examined the association of SAR with device parameters in 54 patients with implanted pacemakers who underwent 62 MRI examinations with a 1.5-T MRI system. The investigators found no evidence to suggest that changes in atrial and ventricular capture thresholds were associated with scanned anatomic location and highest whole-body averaged SAR value. A second similar study also failed to find any association between changes in device variables and SAR (15). In another in vitro study, investigators used a 1.5-T MRI scanner with a deep brain stimulation system positioned in a gel saline–filled phantom and measured the increase in temperature over different ranges of SAR. In contrast to the previously mentioned studies and our findings, the authors noted a linear relationship between local and whole-body averaged SAR and electrode heating. They suggested that a 1°C temperature increase at the electrode tips is associated with local and whole-body averaged SARs of 1.2 W/kg and 0.045 W/kg, respectively. The high dB/dt echo-planar imaging sequence had no substantial heating independent of SAR considerations (16).
Poor correlation between heating at different SARs of sequences for different scanners, even those from the same manufacturer, has been reported (14). This suggests that the calculated averaged whole-body SAR is unlikely to serve as a reliable metric with which to estimate the amount of radiofrequency energy irradiation, mainly due to the difference in hardware (different design of transmit radiofrequency body coils) and software (different models and algorithms) between different scanners and manufacturers. Each manufacturer has its own method of calculating SAR, thereby introducing some variability to the metric. SED, or the total accumulated energy absorbed, might be a more relevant metric to monitor for patient safety since this will be directly tied to the temperature increase, but the same problem exists with calculation of SED since it reflects the integration of SAR over time.
Cardiac device leads are generally made of metallic conductive materials. Leads are prone to absorption of radiofrequency energy and localized tissue heating. Increased lead length and conformations like loops may facilitate the transition of energy to the implanted device. Our results showed that RV lead length was associated with changes in RV capture threshold and sensing immediately after MRI examination. It is important to note that some associations reported between device parameters and region of imaging or lead length differ in this study when compared to prior results from this cohort (4). This is primarily due to different statistical methods (generalized estimating equations regression models in the current study vs nonparametric k-sample test on the median or nonparametric test for trend), as well as inclusion of a slightly different subcohort with detailed MRI energy data. In a prior publication from this cohort (4), we found that long-term changes in RV R-wave amplitude were significantly smaller in participants with an RV lead length of 60 cm or less when compared with participants with an RV lead length of more than 60 cm. In addition, long-term changes in atrial capture threshold were significantly larger among participants with an RA lead longer than 50 cm than in those with an RA lead of 50 cm or shorter (4). In consideration of the high dielectric permittivity of surrounding tissues consisting of blood and myocardium (17), a frequency of 64 MHZ (1.5 T) yields a wavelength of 25–28 cm. This is shorter than the average length of ventricular leads (approximately 60 cm) that can result in lead heating and increased capture thresholds. In this setting, we noted an association between RV lead sensing and capture threshold parameters and lead length immediately after MRI. The extent of inductive and radiofrequency energy deposition is likely to be related to the lead length (18), number of lead’s loops (19), implant geometry (20), implant material (21), and design (22), which may be different between RV, RA, and LV leads and may partly explain the association between RV lead length but not RA or LV lead length and device parameter changes immediately after MRI seen in this nested cohort study. In addition, there were fewer participants with RA or LV device leads, resulting in insufficient sample sizes to show an association with lead length in those positions.
In the present study, dB/dt limits of the MRI scanner were monitored for patient peripheral nerve stimulation according to the standards defined by the International Electrotechnical Commission (23) and the guidelines recommended by the Food and Drug Administration (24). Such limits, however, are for patients without any implanted devices. Despite scanning at normal stimulation levels, we found no evidence that that dB/dt was associated with changes in device parameters (Table 3). These results are consistent with an animal study conducted by our group (19). In that animal study, we found that addition of loops can induce high currents, but under conventional implant conditions (without additional lead length coiled into the generator pocket) the magnitude of induced current is less than 0.5 mA (19). In another animal study (25), the pacemaker function was not compromised when gradient fields increased up to 400 mT/sec.
This study was subject to some limitations. First, all the scans were performed using Avanto or Aera (Siemens Healthcare) scanners. As mentioned earlier, the SAR values are reported not to be reproducible between different scanners with different operating systems; therefore, the generalizability of data should be interpreted with caution. Second, the presented data were obtained from one center and may not be generalizable to other MRI facilities. Third, despite the inclusion of many cardiac devices, the numbers of each individual device model were small. Fourth, we were not able to retrieve the local SAR data from the MRI scanners.
We found no evidence of an association between specific absorption rate, specific energy dose, and change in magnetic field per unit time of MRI sequences and changes in device or lead parameters. Nevertheless, device interrogation before and after MRI remains mandatory because of the potential for device reset and changes in lead or generator parameters. These findings suggest the risk of device malfunction or failure is low if precautions are taken.
S.N. supported by the National Institutes of Health (K23HL089333, R01HL116280).
Disclosures of Conflicts of Interest: A.A.R. disclosed no relevant relationships. S.L.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a consultant for Siemens Healthcare. Other relationships: disclosed no relevant relationships. R.H. disclosed no relevant relationships. M.A.G. disclosed no relevant relationships. V.C. disclosed no relevant relationships. D.M. disclosed no relevant relationships. J.E.K. disclosed no relevant relationships. H.R.H. Activities related to the present article: receives royalties from Imricor. Activities not related to the present article: is a consultant for Zoll Medical; institution received a research grant from, has a patent with, and receives royalties from Zoll Medical. Other relationships: disclosed no relevant relationships. S.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a scientific adviser for CardioSolv and Circle Software; institution received grants from Siemens and Imricor. Other relationships: disclosed no relevant relationships.
Abbreviations:
- dB/dt
- change in magnetic field per unit time
- ICD
- implantable cardioverter defibrillator
- LV
- left ventricle
- RA
- right atrium
- RV
- right ventricle
- SAR
- specific absorption rate
- SED
- specific energy dose
References
- 1. Nazarian S, Roguin A, Zviman MM, et al . Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla . Circulation 2006. ; 114 ( 12 ): 1277 – 1284 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nazarian S, Hansford R, Roguin A, et al . A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices . Ann Intern Med 2011. ; 155 ( 7 ): 415 – 424 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roguin A, Zviman MM, Meininger GR, et al . Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T . Circulation 2004. ; 110 ( 5 ): 475 – 482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nazarian S, Hansford R, Rahsepar AA, et al . Safety of Magnetic Resonance Imaging in Patients with Cardiac Devices . N Engl J Med 2017. ; 377 ( 26 ): 2555 – 2564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Russo RJ, Costa HS, Silva PD, et al . Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator . N Engl J Med 2017. ; 376 ( 8 ): 755 – 764 . [DOI] [PubMed] [Google Scholar]
- 6. Indik JH, Gimbel JR, Abe H, et al . 2017. HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices . Heart Rhythm 2017 ; 14 ( 7 ): e97 – e153 . [DOI] [PubMed] [Google Scholar]
- 7. Nazarian S, Halperin HR. . How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices . Heart Rhythm 2009. ; 6 ( 1 ): 138 – 143 . [DOI] [PubMed] [Google Scholar]
- 8. Luechinger R, Duru F, Scheidegger MB, Boesiger P, Candinas R. . Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs . Pacing Clin Electrophysiol 2001. ; 24 ( 2 ): 199 – 205 . [DOI] [PubMed] [Google Scholar]
- 9. Irnich W, Irnich B, Bartsch C, Stertmann WA, Gufler H, Weiler G. . Do we need pacemakers resistant to magnetic resonance imaging? Europace 2005. ; 7 ( 4 ): 353 – 365 . [DOI] [PubMed] [Google Scholar]
- 10. Roguin A, Schwitter J, Vahlhaus C, et al . Magnetic resonance imaging in individuals with cardiovascular implantable electronic devices . Europace 2008. ; 10 ( 3 ): 336 – 346 . [DOI] [PubMed] [Google Scholar]
- 11. Luechinger R, Zeijlemaker VA, Pedersen EM, et al . In vivo heating of pacemaker leads during magnetic resonance imaging . Eur Heart J 2005. ; 26 ( 4 ): 376 – 383 ; discussion 325–327 . [DOI] [PubMed] [Google Scholar]
- 12. Martin ET, Coman JA, Shellock FG, Pulling CC, Fair R, Jenkins K. . Magnetic resonance imaging and cardiac pacemaker safety at 1.5-Tesla . J Am Coll Cardiol 2004. ; 43 ( 7 ): 1315 – 1324 . [DOI] [PubMed] [Google Scholar]
- 13. Sommer T, Naehle CP, Yang A, et al . Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations . Circulation 2006. ; 114 ( 12 ): 1285 – 1292 . [DOI] [PubMed] [Google Scholar]
- 14. Baker KB, Tkach JA, Nyenhuis JA, et al . Evaluation of specific absorption rate as a dosimeter of MRI-related implant heating . J Magn Reson Imaging 2004. ; 20 ( 2 ): 315 – 320 . [DOI] [PubMed] [Google Scholar]
- 15. Mollerus M, Albin G, Lipinski M, Lucca J. . Magnetic resonance imaging of pacemakers and implantable cardioverter-defibrillators without specific absorption rate restrictions . Europace 2010. ; 12 ( 7 ): 947 – 951 . [DOI] [PubMed] [Google Scholar]
- 16. Finelli DA, Rezai AR, Ruggieri PM, et al . MR imaging-related heating of deep brain stimulation electrodes: in vitro study . AJNR Am J Neuroradiol 2002. ; 23 ( 10 ): 1795 – 1802 . [PMC free article] [PubMed] [Google Scholar]
- 17. Gabriel C. . Compilation of the Dielectric Properties of Body Tissues at RF and Microwave Frequencies . 1996. . [Google Scholar]
- 18. Mattei E, Triventi M, Calcagnini G, et al . Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations . Biomed Eng Online 2008. ; 7 ( 1 ): 11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tandri H, Zviman MM, Wedan SR, Lloyd T, Berger RD, Halperin H. . Determinants of gradient field-induced current in a pacemaker lead system in a magnetic resonance imaging environment . Heart Rhythm 2008. ; 5 ( 3 ): 462 – 468 . [DOI] [PubMed] [Google Scholar]
- 20. Calcagnini G, Triventi M, Censi F, et al . In vitro investigation of pacemaker lead heating induced by magnetic resonance imaging: role of implant geometry . J Magn Reson Imaging 2008. ; 28 ( 4 ): 879 – 886 . [DOI] [PubMed] [Google Scholar]
- 21. Serano P, Angelone LM, Katnani H, Eskandar E, Bonmassar G. . A novel brain stimulation technology provides compatibility with MRI . Sci Rep 2015. ; 5 ( 1 ): 9805 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cabot E, Lloyd T, Christ A, et al . Evaluation of the RF heating of a generic deep brain stimulator exposed in 1.5 T magnetic resonance scanners . Bioelectromagnetics 2013. ; 34 ( 2 ): 104 – 113 . [DOI] [PubMed] [Google Scholar]
- 23. IEC 60601-2-33, Ed . 3.0 Medical Electrical Equipment - Part 2-33: Particular Requirements for the Basic Safety and Essential Performance of Magnetic Resonance Equipment for Medical Diagnosis . Geneva, Switzerland: : International Electrotechnical Commission (IEC) , 2010. . [Google Scholar]
- 24. Guidance for Industry and Food and Drug Administration Staff . Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices. U.S. Food and Drug Administration . https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072686.htm. Published 2014. Accessed February 1, 2015 .
- 25. Nahrendorf M, Hiller KH, Hu K, Zeijlemaker V, Griswold M, Bauer WR. . Pacing in high field cardiac magnetic resonance imaging . Pacing Clin Electrophysiol 2004. ; 27 ( 5 ): 671 – 674 . [DOI] [PubMed] [Google Scholar]