Abstract
Cellular membranes are not homogenous mixtures of proteins; rather, they are segregated into microdomains on the basis of preferential association between specific lipids and proteins. These microdomains, called lipid rafts, are well known for their role in receptor signaling on the plasma membrane (PM) and are essential to such cellular functions as signal transduction and spatial organization of the PM. A number of disease states, including atherosclerosis and other cardiovascular disorders, may be caused by dysfunctional maintenance of lipid rafts. Lipid rafts do not occur only in the PM but also have been found in intracellular membranes and extracellular vesicles (EVs). Here, we focus on discussing newly discovered functions of lipid rafts and microdomains in intracellular membranes, including lipid and protein trafficking from the ER, Golgi bodies, and endosomes to the PM, and we examine lipid raft involvement in the production and composition of EVs. Because lipid rafts are small and transient, visualization remains challenging. Future work with advanced techniques will continue to expand our knowledge about the roles of lipid rafts in cellular functioning.
Keywords: microdomains, cholesterol trafficking, extracellular vesicles, exosomes
MICRODOMAINS AND CELLULAR CHOLESTEROL HOMEOSTASIS
Cellular membranes are not homogenous mixtures of lipids and proteins, rather, they are composed of lipids and proteins, some of which segregate into lipid microdomains called lipid rafts, which are enriched in free cholesterol (FC) and glycosphingolipids, like SM, and are resistant to detergent extraction. In this review, domains enriched in FC and SM will be designated as “lipid rafts,” while other lipid domains that are not as well characterized, particularly those in mitochondria, will be referred to as lipid microdomains. The segregation of these structures regulates cellular polarity and vesicular traffic as well as cell signaling pathways affecting a plethora of biological processes (1). However, over two decades since the functional lipid raft model of the plasma membrane (PM) was first published by Simons and Ikonen (2), many of the fundamental questions about the biogenesis and structure of lipid rafts still remain unanswered. Although less so today than in the past, experimental obstacles in visualizing lipid rafts have hampered progress in understanding raft size, when and where they are formed, and how they are turned over or removed from the cell. However, new techniques have been developed that provide a clearer vision of how lipid rafts form and how they function. Since our previous review of lipid rafts in inflammatory receptor signaling and atherosclerosis (1), a great deal of information has emerged providing new insights into how lipid rafts affect a number of disease states. In this current review, we will discuss newly discovered functions of lipid rafts as well as the contribution of lipid rafts to the composition of extracellular vesicles (EVs).
Two common types of lipid rafts have been described: planar lipid rafts and invaginated lipid rafts, which curve inward and are termed caveolae. Caveolae are specialized lipid rafts found in very high numbers in adipocytes, which associate with the protein caveolin-1 (CAV1) to form concave regions on the cell membrane (3). Formation of lipid rafts is due to phase separation of lipids within the membrane that is thermodynamically driven and depends on the amounts and types of membrane lipids and the amounts of FC and SM, both essential components of planar lipid rafts and caveolae. Regions of the PM that are rich in FC and SM are more ordered and often referred to as the liquid-ordered phase as compared with the rest of the lipid membrane, which is called the liquid-disordered phase. The size of lipid rafts has been estimated to average around 500 nm2, and they have lifetimes on the PM that range from seconds to minutes (4). Because lipid rafts are mobile and “float” like an iceberg in the liquid-disordered lipid phase, they can also associate to form larger raft-domains, when proteins that associate with liquid-ordered domains facilitate oligomerization of protein complexes. Studies of both rabbit erythrocytes and Chinese hamster ovary cells suggest that PMs accommodate lipid rafts having different lipid packing and sizes (5, 6).
Changes in the FC composition, which modulates lipid raft content, can affect cell mobility. For example, increasing the FC concentration in THP1 cells leads to increased chemotactic response to monocyte chemoattractant protein-1 (MCP-1) due to alterations in C-C motif chemokine receptor 2 (CCR2) levels in lipid rafts. Modulating human monocyte FC affected rolling of these cells on E-selectin-coated surfaces by changing E-selectin counterreceptor CD44 distribution on the lipid rafts, suggesting a mechanism through which FC concentration modulates monocyte adhesion by regulation of receptor mobility (7, 8).
All mammalian cells have an outer protective barrier made up of a phospholipid bilayer that associates with a variety of proteins, aiding in cellular integrity and communication. The PM is highly organized and displays asymmetry with respect to the types of phospholipids found in the inner and outer bilayer leaflets and with respect to FC distribution. For example, phosphatidylcholine (PC) predominates in the bilayer with a higher percentage of PC and SM in the outer leaflet, while phosphatidylserine (PS) and phosphatidylethanolamine (PE) are preferentially found in the inner leaflet (9, 10). Bilayers associated with intracellular organelles have somewhat different phospholipid to FC ratios, and FC is reported to be asymmetrically distributed between the inner and outer leaflets (11–13). Recent studies suggest that long chain C24 sphingolipids modulate this membrane bilayer asymmetry (14).
Despite years of research, membrane lipid rafts remain controversial. Because of their dynamic nature the proportions of raft to non-raft membrane lipids are difficult to quantify and image in real time. The most common method to study lipid raft composition is to isolate detergent-resistant membrane (DRM) from cells (15). DRMs from model membranes and cell membranes contain a subset of lipid-anchored and integral PM proteins. Intact membrane-associated regions can be visualized using fluorescently labeled antibodies that recognize proteins or fluorescent ganglioside analogs (16) sequestered in the raft regions. Newer, non-detergent methods for isolating bulk lipid rafts from different cell types have been developed permitting analysis of their lipid content and associated proteins (17, 18). More detailed investigations have recently employed Foster resonance energy transfer (FRET), atomic force microscopy, stimulated emission depletion microscopy (STED), and other super-resolution techniques. An overall consensus is that lipid rafts range in diameter estimated from 5 to 80 nm, with relatively short lifetimes, rapidly responding to cellular needs. With the refinement of the super-resolution techniques, we are now poised to discover new information on factors leading to how these elusive and highly dynamic structures condense and dissolve.
CONTRIBUTION OF CHOLESTEROL SYNTHESIS
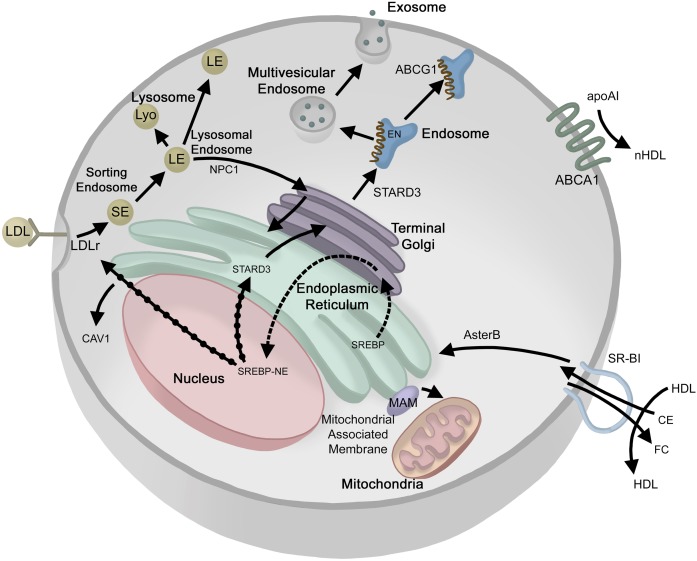
The FC found in lipid rafts can be derived endogenously by de novo FC synthesis in the ER or exogenously from lipoproteins or vesicles. Newly synthesized FC rapidly leaves the ER, mostly through nonvesicular mechanisms bypassing ER-Golgi membrane transport (19, 20), maintaining low ER membrane sterol content, as illustrated in Fig. 1. The newly produced FC can be transported to the PM, thereby becoming available to extracellular acceptors (21), or it can be transported to cholesterol pools in other intracellular compartments, e.g., endosomes (22). Interestingly, a membrane-FC-gradient exists in cells with the FC levels lowest in the ER, and increasing as it approaches the PM. Because excess FC is cytotoxic and cannot be effectively removed by catabolism, cells devote considerable effort to sensing intracellular FC levels and adjusting synthesis and receptor-mediated lipoprotein uptake as well as FC efflux to balance the gradient and maintain proper cellular cholesterol levels. When a cholesterol-rich diet is consumed, the excess FC perturbs cellular cholesterol balance, thus impacting lipid raft homeostasis (23). Excess FC is either stored as cholesteryl ester (CE) or actively exported through efflux pathways. Cholesterol exported from peripheral tissues is then transported back to the liver by HDLs for recycling, conversion to bile acids, or excretion in feces, accounting for about 1.1 g of FC per day (24). Cellular FC balance is mediated in a complex manner by two main nuclear receptor systems, SREBPs and LXRs. Activated SREBP undergoes assisted migration to the Golgi where it is further cleaved releasing the SREBP-nuclear element, which migrates to the nucleus causing increased transcription of gene products leading to an increase in cellular FC from de novo synthesis or uptake of cholesterol from circulating lipoproteins (25). LXR activation by oxysterols promotes FC efflux (26). Key processes in the maintenance of the FC pool take place at several different subcellular localizations; therefore, FC transport between these sites is important for the overall regulation of intracellular FC concentration. FC can be transferred between subcellular membranes by vesicular transport and by nonvesicular mechanisms [reviewed in (27)]. Figure 1 illustrates our current knowledge on the movement of FC within the cell and at the cell surface.
Fig. 1.
Ins and outs of cellular cholesterol transport. EN, endosomes; EX, exosome; LE, late endosome; LY, lysosome; MVE, multivesicular endosome; NUC; nucleus; SE, sorting endosome; TG, trans-Golgi; SREBP-NE, SREBP-nuclear element. Cholesterol movement (solid arrows); activation (solid arrows with black dots); SREBP movement (dashed arrow).
Modulation of cellular FC levels may contribute to certain disease conditions. For example, metastatic ovarian tumor cells reportedly promote FC efflux from tumor-associated macrophages, thereby depleting FC in macrophage lipid rafts, which in turn reduces interferon-γ gene expression and increases interleukin-4-mediated reprogramming. This series of events explains how these macrophages are forced to adopt a tumor-promoting immunosuppressive response rather than an intrinsic tumoricidal activity, which would normally suppress tumor development (28).
CONTRIBUTION OF LIPOPROTEIN CHOLESTEROL
An alternative to FC acquisition via de novo synthesis is through receptor-mediated uptake of FC contained in plasma lipoproteins. apoB- or apoE-containing particles, such as chylomicron remnants, VLDLs, and LDLs, bind to the LDL receptor (LDLr) bound to the PM by a transmembrane domain, but not associated with a lipid raft. After binding the LDLr, the LDL-LDLr complex is endocytosed through clathrin-coated vesicles into a sorting endosome (Fig. 1) where LDL and LDLr dissociate at lower pH. LDLr is then recycled to the cell surface, while the LDL is transported to acidic lysosomal compartments where CEs are hydrolyzed to FC. Interestingly, although multivesicular bodies harbor most of the FC in the endocytic pathway (29), lysosomal membranes are normally FC poor (30). Multivesicular late endosomes contain two lysosomal proteins, Niemann-Pick type C (NPC)1 and NPC2, which are essential for moving FC out of the endosomal system. Genetic and phenotypic evidence in mutant mice suggest that the NPC proteins participate in different steps of the same pathway and that neither can compensate for the other (31). Deficiency of either protein leads to the accumulation of LDL-derived FC in late endocytic organelles (32). Recent advances have revealed new insights into the architecture of NPC1, including luminal interaction with NPC2 (33, 34), a membrane-embedded sterol-sensing domain (35) and a C-terminal luminal domain (36). Current hypotheses suggest that NPC2 binds FC in the lysosome and then hands it off to NPC1, which has membrane-spanning domains in the lysosomal/endosomal membrane. Transfer of FC by NPC1 to the membrane or the cytoplasm is still under active investigation. NPC2, but not NPC1, was suggested to be essential for ABCA1-mediated flux of FC from the (endo)lysosomal compartment toward the PM (37).
ENDOSOMAL CHOLESTEROL TRAFFICKING AND EXOSOMES
The endosomal compartment is at the crossroads of vesicular flow between the Golgi, lysosomes, ER, PM, and other cellular compartments. Sorting (early) endosomes give rise to recycling endosomes, which are in constant exchange with the PM. They are also the source of multi-vesicular endosomes, which mature into late endosomes that are in exchange with either the trans-Golgi network (TGN) or lysosomes, or fuse with the PM to release exosomes. After release from the endolysosomal system, FC is delivered to other membranes, such as the PM, ER, recycling endosomes, and, in steroid hormone-producing cells, the mitochondria. When the PM concentration of FC exceeds ∼30 mol% of total PM lipid, an accessible pool of mobile FC forms, some of which moves to the ER to signal a FC excess and to moderate activation of SREBP (38, 39). Exactly which membrane(s) serve as the initial acceptor of endosomal FC has not yet been determined; however, recent findings argue that the bulk of LDL-cholesterol is transported to the PM (38, 39). Because SREBP-mediated FC sensing takes place in the ER, lipoprotein-derived cholesterol also needs to be delivered to the ER. However, the mechanisms of post-NPC1 endosome to ER trafficking are still largely unknown (Fig. 1), despite the presence of FC-binding and ER-interacting domains in late endosomal membrane proteins (40). Oxysterol-binding protein-related protein 1L (ORP1L) and StAR-related lipid transfer protein 3 (STARD3) can bind vesicle-associated membrane protein (VAP) in the ER, bridging between late endosomes and the ER. Interestingly, it was recently reported that both ORP1L and STARD3 also transfer FC from the ER to endosomes, employing VAP as the ER partner (41, 42).
ROLE OF NON-ENDOSOMAL CHOLESTEROL MOVEMENT
Scavenger receptor BI (SR-BI) is a PM receptor that mediates the selective uptake of CE from lipoproteins, and is known as the HDL receptor. The selective HDL-cholesterol uptake pathway is distinct from the LDLr pathway, as it does not require clathrin-dependent receptor-mediated whole particle uptake and lysosomal degradation (Fig. 1). SR-BI removes and transports CE from HDL into the cells, which are then hydrolyzed by a neutral CE hydrolase releasing FC (43). The identity of the hydrolase depends on the cell type and carboxyesterase 1 has been suggested as the neutral hydrolase in liver tissue (44). Recently three ER-resident cholesterol transport proteins (Aster-A, -B, and -C) were described that bind FC and facilitate its removal from the PM (45). Moreover, Aster-B was shown to move FC from the PM to the ER downstream of SR-BI and, in that way, enable the ER to sense fluctuations in the pool of PM FC and to link this with regulation of the sterol-sensing SREBP-2 pathway. When PM FC levels rise above homeostatic levels, Asters form bridges between the PM and the ER, and the sterol-binding ASTER domain extracts FC from the PM and moves it down the concentration gradient to the ER (45) (Fig. 1).
ROLE OF CHOLESTEROL EFFLUX
Efflux or removal of excess cholesterol from cells is an ATP-dependent process carried out by ABCA1, which mediates FC movement across the PM to an acceptor such as apoA-I for generating new HDL by the liver or for peripheral tissues to send FC back to the liver in a process called reverse cholesterol transport (46). apoA-I binds with ABCA1, which then packages FC, SM, and phospholipids into newly formed or nascent HDL (nHDL) particles (18). These FC-rich particles are then remodeled by LCAT, also called PC-sterol acyltransferase, into particles having a core of CE. Alternatively, ABCA1 will also efflux FC to other proteins, e.g., apoA-II, apoA-IV, apoC-I, apoC-II, apoC-III, and apoE (47). ABCA1 is present on the PM, not in lipid rafts, but generates nHDL that has a lipid composition typical of the lipid raft. ABCG1 participates in intracellular sterol movement (48) but has not been shown to directly transfer cholesterol to apolipoproteins or lipoproteins at the surface of the cells (48, 49) as originally proposed (50, 51). Given the ubiquitous cellular presence of ABCA1, FC efflux to apoA-I appears to be a continuous housekeeping function of all cells. However, most cells, with the exception of hepatocytes and enterocytes, do not produce apoA-I (52). They must utilize apoE, which is synthesized by many peripheral tissues (53), or they must generate lipid-poor apoA-I from mature HDL following CE removal by SR-BI, although there is evidence that partially lipidated apoA-I is a poor substrate for ABCAI (54). SR-BI has also been implicated as a cholesterol efflux transporter, as it induces FC flux down an FC-concentration gradient (55, 56). Efficient SR-BI-mediated CE uptake and possibly FC efflux require the presence of an extracellular matrix protein, procollagen C-endopeptidase enhancer protein 2 (PCPE2) (57, 58). However, the mechanism by which PCPE2 influences SR-BI function remains to be fully elucidated.
Lipid rafts provide a platform for organizing the signaling of many receptors and proteins. Because many of these processes involve inflammation-related pathways, lipid raft composition is carefully regulated. Available FC is either utilized for cellular membrane maintenance, stored, or moved to a substrate pool for export via ABCA1 (59). ABCA1, whose expression is modulated by LXR, is a uniquely sensitive master controller of membrane FC that is essential for maintaining lipid raft composition and function (60–66) (Fig. 1). In accordance with this notion, the lipid composition of nHDL was found to be identical to that typically found in lipid rafts isolated from cells (18, 67). Synthesis of nHDL containing raft FC from three molecules of lipid-poor apoA-I yields an ∼9–11 nm diameter particle that contains ∼240 molecules of lipid, 100 of which are FC (18). Therefore, ABCA1 exports FC from peripheral tissues, which helps modulate the FC available to form lipid rafts.
ROLE OF INTRACELLULAR BILAYER MEMBRANES
The formation of lipid rafts is not merely confined to the PM. Prior to proposing the raft as an essential component of the PM, lipid rafts were operationally defined as a lipid-regulated sorting mechanism for transport of cholesterol from the TGN to the PM. In 1993, Fiedler et al. (68) found that both the Golgi and the PM formed protein-sphingolipid microdomains. Indeed, both ER and mitochondria reportedly have lipid raft-like microdomains (69, 70). Several studies have shown that these organelles differ both quantitatively and qualitatively in their lipid content [reviewed in (9)].
PMs are enriched in sphingolipids and FC (typically 20–25%), which are packed at a higher density than glycerophospholipids and are more resistant to mechanical stress. FC is also abundant in the endocytic recycling compartment and part of the Golgi complex, with an enrichment toward the TGN. In contrast, ER membranes are, despite the organelle being the main site of FC synthesis, extremely low in sterol and complex sphingolipid content. Mitochondrial membranes are also relatively low in sterols, except for mitochondria in sterol-producing cells, which import and utilize FC for sterol production. A substantial amount of cellular lipid metabolism and synthesis occurs in the mitochondria. Lipids present in the mitochondrial membrane include lysophosphatidic acid, phosphatidic acid, phosphatidylglycerol, and cardiolipin (CL), a lipid that is unique to the mitochondrial membrane. The presence of phosphatidylglycerol and high CL content in the inner membrane in addition to a high PE/PC ratio are reminiscent of the bacterial origin of this membrane and are probably required to support oxidative phosphorylation.
ROLE OF GOLGI AND TGN
The classical secretory route for many proteins is from the ER to the Golgi and from the TGN to the PM, following the FC and SM gradient, which increases from ER to Golgi to PM (71). FC content is critical for Golgi-related vesicle transport, as Golgi morphology and intra-Golgi transport are disrupted by reducing FC content by 10% (72). Both raft and non-raft domains in the Golgi/TGN have been shown to be important for vesicle transport within or from the Golgi (73, 74). Non-raft coated vesicles are instrumental for trafficking proteins to the basolateral PM or within the Golgi-ER compartment. Specific raft-associated proteins are important for sorting and transport from the TGN to the PM (75, 76). Whether lipids or proteins initiate and mediate the raft formation or sorting process is still under debate.
However, there are nonvesicular transport mechanisms that include proteins in the glycolipid transport protein superfamily (77–79), oxysterol binding proteins (80), and ceramide transport protein (81, 82). Moreover, specific lipid signals at the TGN, the phosphatidylinositol phosphates PI4P and PI4,5P2, can be recognized by lipid transport proteins (83). Phosphatidylinositol phosphates can be localized in rafts and in that way contribute to transport and metabolism of other raft lipids in a process that has yet to be elucidated (83). Therefore, it is likely that Golgi-related vesicle trafficking and protein sorting require the proper lipid environment, which includes lipid rafts.
MITOCHONDRIAL INTERACTIONS WITH THE ER
The interaction of the ER with mitochondria occurs via certain subdomains of the ER, named mitochondria-associated membranes (MAMs), which allow membrane scrambling between these organelles and contribute to the complex series of ER functions (84) (Fig. 1). In mammalian cells, MAMs play a critical role in the early steps in the formation of autophagosomes (85, 86). MAMs are enriched in CAV1 (87), lipid synthesis enzymes (88, 89), and FC (90), with more cholesterol present in MAMs than in either the ER or the mitochondria (91). The latter suggests that they act as sites of nonvesicular lipid transfer between the ER and mitochondria. Moreover, the ganglioside GM1, which contains one sialic acid residue, can accumulate at the ER membranes and can promote the juxtaposition of ER and mitochondria at the MAMs.
Calnexin (CANX), a prototypical calcium-binding ER palmitoylated chaperone protein enriched in the MAMs, was recruited to the detergent TX-100-insoluble fractions corresponding to lipid microdomains during autophagy-stimulating conditions (92). Disialoganglioside having three glycosyl groups (GD3) was detectable in the MAMs of cells under autophagic stimulation (92) and enhanced the association of GD3 with autophagy and beclin 1 regulator 1 (AMBRA1) and increased the association of AMBRA1 with WD repeat domain phosphoinositide-interacting protein 1. siRNA-mediated inhibition of GD3 synthesis in stimulated conditions reduced autophagy and CANX-AMBRA1 association, suggesting that the protein composition of MAMs was modulated by the concentration of GD3, leading to impaired starvation-induced association of core complex molecules at the MAMs (92). Together, these data suggest that lipid microdomains may participate in autophagy and organelle recycling.
MITOCHONDRIAL DYNAMICS AND APOPTOSIS
Functional studies suggest that mitochondrial lipid microdomains participate in the fusion, fission, and apoptosis of the mitochondrial network during remodeling (93). Mitochondrial fusion induced by mitochondrial division inhibitor 1 (Mdivi-1) required GD3 to co-associate with mitofusin (Mfn)2 to participate in the fusion process (94). The mitochondrial network constantly remodels its shape through fusion and fission to regulate their number and function. In human cells, mitochondrial fusion is regulated by the mitofusins Mfn1 and Mfn2, which are embedded in the mitochondrial outer membrane, and by the optic atrophy 1 protein, located in the mitochondrial inner membrane (95, 96). Mitochondrial fission is directed by recruitment of the soluble dynamin-related GTPase called dynamin-like protein 1 (DLP1) from the cytosol to mitochondria (97, 98). This GTPase localization marks sites of future fission. Human mitochondrial fission 1 protein (hFis1), an integral outer mitochondrial membrane protein, has an essential role in completing fission (99).
Mitochondrial fission is required during cell proliferation and apoptosis (100). Glycosphingolipid microdomains were proposed to mediate multiple steps in the apoptosis cascade including recruitment of Fas cell surface death receptor and TNF-α receptors (101–103) and recruitment of the proapoptotic Bcl-2 family proteins, including truncated Bid, t-Bid, and Bax, following Fas cell surface death receptor triggering (69). The activation of Fas cell surface death receptor and TNF-α receptor may also induce an intracellular movement of lipid raft components, such as GD3, toward mitochondria (69, 104, 105). During apoptosis, CL may be present in lipid microdomains, where it acts as an activation platform for caspase-8 translocation on mitochondria, at contact sites between inner and outer membranes, facilitating self-activation (69, 106). Proapoptotic members of the Bcl-2 family were suggested to associate with mitochondrial fission sites and mitochondrial fission proteins during apoptosis (107). hFis1 is constitutively associated with mitochondrial lipid microdomains, while DLP1 is recruited to lipid microdomains only on apoptotic Fas cell surface death receptor triggering (93). Disruption of lipid rafts leads to an impairment of DLP1 recruitment, a reduction of mitochondrial fission, and a significant reduction of apoptosis (93, 107). Mitochondria depolarization and cytochrome c release may also be dependent on lipid microdomain integrity, because disruption of lipid microdomains prevented depolarization, cytochrome c release, and apoptosis upon apoptotic triggering (69). These studies suggest that mitochondrial lipid microdomains act as activating platforms where key reactions take place that determine cell fate.
When protein content of mitochondrial DRM was investigated using stable isotope labeling by amino acids in cell culture, quantitative mass spectrometric proteomic analysis identified F1/F0 ATPase subunits, voltage-dependent anion selective channel proteins, and several other mitochondrial proteins. However, the amounts of these proteins were not sensitive to FC disruption and they were not enriched in DRMs compared with whole cell membranes (108) suggesting that these proteins at best are partially copurifying contaminants. Proteins that participate in mitochondrial fusion or fission were not detected in the mass spectrometric analysis suggesting that these proteins do not reside in lipid raft-like domains or that their concentration was below the limit of detection.
EVS: EXOMERES, EXOSOMES, MICROPARTICLES, AND LIPOPROTEINS
Communication between cells and tissues is essential for maintaining biochemical integrity. The role of secreted proteins, peptides, and eicosanoid lipids in intercellular communication has been recognized for many years; however, communication between cells by EVs is receiving more attention. Non-lipoprotein EVs or microparticle vesicles are being investigated as a means for transferring information among cells in both animals (109) and plants (110). This is an ancient system that has been reported in several species of bacteria and fungi (111). These particles are reported to carry proteins, lipids, and RNA.
A large number and variety of EVs are released by mammalian tissues and have been recently reviewed (112). These vesicles are reported to transport a variety of proteins, RNAs, and lipids among cells. Another class of particles that are often co-isolated with EVs include the following lipoproteins: HDL, LDL, IDL, VLDL, and chylomicrons. These particles are reported to carry many proteins, microRNAs, and lipids among cells. In particular, lipoproteins are primarily responsible for cholesterol exchange among cells via lymph and plasma. Lipoproteins consist of an exterior monolayer of phospholipids that interacts with apolipoprotein(s) on the surface of the particle. The interior consists of a mixture of hydrophobic lipids, like FC, CE, and triglycerides (TGs). HDL and LDL have well-defined roles in nutrient transfer, while HDL may have additional anti-inflammatory properties. Proteomic analyses suggest that these particles associate with a variety of proteins (113, 114), but the principal proteins for each particle are apoB100 for LDL, IDL, and VLDL, and apoA-I for HDL and chylomicrons. Other characteristic apolipoproteins carried are apoE on IDL, VLDL, chylomicrons, and HDL, and apoA-II and apoM on HDL. Chylomicrons carry a truncated form of apoB, apoB48, which has 48% of the apoB100 sequence. VLDL is secreted by the liver with preliminary assembly of apoB100 and TG taking place in the ER with final assembly in the Golgi (115). Secretory granules from the Golgi merge with the PM to release VLDL. IDL and LDL are generated in the plasma by remodeling of VLDL, while HDL is generated outside of the cell by lipidation of apoA-I by ABCA1 with the primary site of lipidation at the liver and intestine. Lipids carried on the surface of the lipoproteins are predominantly the amphipathic lipids PC and SM, while the interior or core carries hydrophobic lipids like TG, FC, and CE. VLDL and chylomicrons are particularly rich in TGs, while HDL and LDL carry predominantly CE with lesser amounts of FC and TGs.
A thorough discussion of criteria for defining non-lipoprotein EVs is given in a consensus paper by Thery et al. (116). EVs are often divided into three classes based on diameter: exomeres, 30–50 nm; exosomes, 50–150 nm; and microparticles, 100–1,000 nm. These size ranges overlap with diameters of lipoproteins like HDL and LDL (5–35 nm) and VLDL and chylomicrons (20–1,200 nm) and may be isolated with EVs (117). In addition to having diameters similar to those of EVs, the densities of lipoproteins overlap with the density ranges that characterize exosomes and microparticles (118). Thus, the relative contributions of EVs and lipoproteins will require increased attention to the details of particle isolation.
Exosomes and microparticles are generated by different cellular processes. Exosomes are secreted through multivesicular bodies and are enriched in tetraspanins (119), while microparticles are formed by budding of the PM (120), which requires modulation of ATP-dependent transporters, e.g., flippase and floppase, and scramblase activity. The primary cellular mechanism for assembling multivesicular bodies uses the endosomal sorting complex required for transport (ESCRT) of intraluminal vesicles into the endosome (121, 122). An ESCRT-independent pathway has been identified that may be ceramide dependent (123) and may have significant effect on the secretion of selected proteins (124). Most cells shed EVs into the tissue environment and these products are taken up by other cells by endocytosis, receptor mediated uptake of EV-bound ligands, and fusion of EV lipid with cell membranes.
EVs have been shown to carry proteins, lipids, and RNAs from an origin cell to a receptive cell (109, 125). The cargo is most often antigens, cell surface proteins, cytoplasmic contents, and various nuclear components. Under healthy conditions, in vivo levels of EVs are generally relatively low, but these levels often increase in disease states or during muscle repair (126), and it has been suggested that their concentration and composition might be used for diagnosis and prognosis (125, 127). EVs from platelets participate in normal procoagulant activity and thrombin production, and their production is crucial for physiologic coagulation (128). The absence of these platelet-derived EVs leads to the development of a bleeding disorder called the Scott syndrome.
In other cases, EVs facilitate movement of proteins from cells expressing these proteins to cells that do not express these proteins, e.g., the transfer of chemokine CCR5 (129), which enables retrograde signaling through EV-mediated transport of synaptotagmin 4 (130), or the transfer of oncogenic receptor epidermal growth factor receptor variant III (EGFRvIII) from EGFRvIII expressing cells to RGFRvIII-negative cells (131). A recent study of adipocyte-specific knockout of CAV1 showed that the essential adipocyte protein CAV1 could be transferred by EVs from endothelial cells normally resident in fat tissue to adipocytes (132). Adipocytes are also reported to release lipid-filled vesicles that are a source of lipid for local macrophages (133). Health-promoting functional roles for EVs have been established for many cell types; however, in some cases exosomes promote disease states. CD63+/CD66b+ exosomes from neutrophils carrying α1-antitrypsin-insensitive neutral elastase cause emphysema in mice by binding and degrading extracellular matrix. CD66b+/NE+ PMN exosomes isolated from COPD patients were found to induce a COPD phenotype in mice (134). EVs have been connected with information exchange within the tumor microenvironment (118, 131) and implicated as participants in diabetes, myocardial infarction, and metabolic syndrome (125, 135, 136).
LIPID RAFTS AND EV LIPIDS
Exosomes and microparticles are characterized by having a lipid bilayer that contains intraparticle proteins and metabolites, which support transmembrane and surface bound proteins. In contrast, exomers generally carry lesser amounts of lipids (137). Some groups have described exosomes as having membranes with lipid compositions that are lipid raft-like (138–140), suggesting that they might be directly formed from lipid rafts. Table 1 summarizes lipid compositions for exosomes with diameters of around 50–100 nm. For comparison, the composition of both nHDL, the first-formed HDL particles generated by ABCA1, and one defined mature HDL particle, HDL2b, are included. Both exosomes and nHDL show an enrichment in both FC and SM, which is usually associated with lipid rafts (2, 18, 141, 142), an enrichment not found in mature HDL, suggesting that exosomes and nHDL have origins associated with lipid rafts. EVs in the first four columns of Table 1 show high PS levels (138, 143). Higher concentrations of PS on the outer leaflet of the lipid bilayer would promote uptake by other cells, like macrophages (144, 145). Exosomes from adipocytes have little PS. Increased levels of FC and SM strongly suggest that the lipid profile of exosomes is more like lipid rafts, but it is unclear at this time if this lipid composition has functional significance or is simply related to the site of vesicle generation where the predominant composition is that of a lipid raft.
TABLE 1.
Cellular exosome lipid composition compared with HDL
| Lipid Class | PC-3 Cells (139) | DiFi Cells (137) | Oli-neu Cells (123) | Platelets (146) | Adipocytes (147) | nHDL (18) (Diameter = 12 nm) | HDL(2b) (148, 149) (Diameter = 10.4 nm) |
| FC | 43.5 | 60.3 | 43 | 42.5 | 64 | 45.7 | 8.2 |
| CE | 0.1 | 6.7 | — | 4.1 | — | 3 | 39.9 |
| SM | 16.3 | 6.5 | 8.2 | 12.5 | 8 | 10.3 | 10.4 |
| PC | 15.3 | 16.8 | 26.7 | 15.9 | 22 | 39.5 | 38.4 |
| PE | 5.8 | 3 | 10.9 | 3.1 | 3 | 0.6 | 0.83 |
| PS | 11.7 | 6.5 | 14.9 | 10.5 | 1 | 0.9 | 0.02 |
| PI | 0.13 | — | — | 5.2 | 2 | 1.6 | 0.83 |
Mole percent lipid composition of exosomes from several cellular sources compared with nHDL and mature plasma HDL. For some of these reports the mole percentages were estimated from mass data. PI, phosphatidylinositol.
CONCLUDING REMARKS
Lipid rafts are essential for maintaining cellular functions, including spatial PM organization, signal transduction, and receptor activation, as well as newer functions involving intracellular lipid and protein trafficking from the ER, Golgi, and endosomes to the PM. Lipid microdomains have also been shown to be involved in mitochondrial function by facilitating interaction between the ER and mitochondrial membranes for the exchange of lipid and proteins. Most recently, cellular EVs are now recognized as important elements in the transfer of information and/or biomolecules among cells. Exosomes, representing one EV size class, are enriched in FC and SM, similar to that found in lipid rafts. These findings suggest that EVs may be derived from endosomes that originated from lipid rafts, thereby reinforcing the concept that the formation, stability, and turnover of lipid rafts is critically dependent on the maintenance of a cellular FC gradient, where FC content increases markedly proceeding from intracellular organelle membranes to the PM. The PM FC concentration gradient is maintained through tight feedback control of FC synthesis, uptake, and efflux.
Because of their transient nature and small size, lipid rafts appear to lack structural boundaries, making their visualization challenging. Advances in our technical ability to study and define these lipid microdomains will be needed in order to expand our knowledge of their roles in cellular functioning. These advances will no doubt show that lipid microdomains participate and regulate even more cellular processes than we can currently know.
Acknowledgments
The authors acknowledge Dr. Theresa Camille Maatman for her valuable assistance in preparing the artwork for Fig. 1.
Footnotes
Abbreviations:
- AMBRA1
- autophagy and beclin 1 regulator 1
- Aster
- ER-resident cholesterol transport protein
- CAV1
- caveolin-1
- CL
- cardiolipin
- CE
- cholesteryl ester
- DLP1
- dynamin-like protein 1
- DRM
- detergent-resistant membrane
- EV
- extracellular vesicle
- FC
- free cholesterol
- GD3
- disialoganglioside with three glycosyl groups
- LDLr
- LDL receptor
- MAM
- mitochondria-associated membrane
- Mfn
- mitofusin
- nHDL
- nascent HDL
- NPC
- Niemann-Pick type C
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PM
- plasma membrane
- PS
- phosphatidylserine
- SR-BI
- scavenger receptor BI
- TG
- triglyceride
- TGN
- trans-Golgi network
This work was supported by National Institutes of Health Grants RO1 HL127649 and HL138907 (to M.G.S-T.) and American Heart Association Grant 19TPA34890023 (M.J.T.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- 1.Sorci-Thomas M. G., and Thomas M. J.. 2016. Microdomains, inflammation, and atherosclerosis. Circ. Res. 118: 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons K., and Ikonen E.. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 3.Ariotti N., Fernandez-Rojo M. A., Zhou Y., Hill M. M., Rodkey T. L., Inder K. L., L. B. Tanner, M. R. Wenk, J. F. Hancock, and R. G. Parton. 2014. Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. J. Cell Biol. 204: 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pralle A., Keller P., Florin E. L., Simons K., and Horber J. K.. 2000. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148: 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez S. A., Tricerri M. A., and Gratton E.. 2012. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl. Acad. Sci. USA. 109: 7314–7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levental I., Grzybek M., and Simons K.. 2011. Raft domains of variable properties and compositions in plasma membrane vesicles. Proc. Natl. Acad. Sci. USA. 108: 11411–11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha A. K., Mousavi M., Dallo S. F., Evani S. J., and Ramasubramanian A. K.. 2018. Influence of membrane cholesterol on monocyte chemotaxis. Cell. Immunol. 324: 74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saha A. K., Osmulski P., Dallo S. F., Gaczynska M., Huang T. H., and Ramasubramanian A. K.. 2017. Cholesterol regulates monocyte rolling through CD44 distribution. Biophys. J. 112: 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Meer G., Voelker D. R., and Feigenson G. W.. 2008. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivel T., Ramseyer C., and Yesylevskyy S.. 2019. The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin. Sci. Rep. 9: 5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S. L., Sheng R., Jung J. H., Wang L., Stec E., O’Connor M. J., S. Song, R. K. Bikkavilli, R. A. Winn, D. Lee, et al. 2017. Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol. Nat. Chem. Biol. 13: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogasawara F., Kano F., Murata M., Kimura Y., Kioka N., and Ueda K.. 2019. Changes in the asymmetric distribution of cholesterol in the plasma membrane influence streptolysin O pore formation. Sci. Rep. 9: 4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steck T. L., and Lange Y.. 2018. Transverse distribution of plasma membrane bilayer cholesterol: picking sides. Traffic. 19: 750–760. [DOI] [PubMed] [Google Scholar]
- 14.Courtney K. C., Pezeshkian W., Raghupathy R., Zhang C., Darbyson A., Ipsen J. H., D. A. Ford, H. Khandelia, J. F. Presley, and X. Zha. 2018. C24 sphingolipids govern the transbilayer asymmetry of cholesterol and lateral organization of model and live-cell plasma membranes. Cell Reports. 24: 1037–1049. [DOI] [PubMed] [Google Scholar]
- 15.Raggi C., Diociaiuti M., Caracciolo G., Fratini F., Fantozzi L., Piccaro G., K. Fecchi, E. Pizzi, G. Marano, F. Ciaffoni, et al. 2019. Caveolin-1 endows order in cholesterol-rich detergent resistant membranes. Biomolecules. 9: E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki K. G. N., Ando H., Komura N., Konishi M., Imamura A., Ishida H., M. Kiso, T. K. Fujiwara, and A. Kusumi. 2018. Revealing the raft domain organization in the plasma membrane by single-molecule imaging of fluorescent ganglioside analogs. Methods Enzymol. 598: 267–282. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald J. L., and Pike L. J.. 2005. A simplified method for the preparation of detergent-free lipid rafts. J. Lipid Res. 46: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 18.Sorci-Thomas M. G., Owen J. S., Fulp B., Bhat S., Zhu X., Parks J. S., D. Shah, W. G. Jerome, M. Gerelus, M. Zabalawi, et al. 2012. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three ApoA-I monomers. J. Lipid Res. 53: 1890–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumann N. A., Sullivan D. P., Ohvo-Rekila H., Simonot C., Pottekat A., Klaassen Z., C. T. Beh, and A. K. Menon. 2005. Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry. 44: 5816–5826. [DOI] [PubMed] [Google Scholar]
- 20.Heino S., Lusa S., Somerharju P., Ehnholm C., Olkkonen V. M., and Ikonen E.. 2000. Dissecting the role of the Golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc. Natl. Acad. Sci. USA. 97: 8375–8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lusa S., Heino S., and Ikonen E.. 2003. Differential mobilization of newly synthesized cholesterol and biosynthetic sterol precursors from cells. J. Biol. Chem. 278: 19844–19851. [DOI] [PubMed] [Google Scholar]
- 22.Cruz J. C., and Chang T. Y.. 2000. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J. Biol. Chem. 275: 41309–41316. [DOI] [PubMed] [Google Scholar]
- 23.Hermetet F., Buffiere A., Aznague A., Pais de Barros J. P., Bastie J. N., Delva L. and Quéré R.. 2019. High-fat diet disturbs lipid raft/TGF-beta signaling-mediated maintenance of hematopoietic stem cells in mouse bone marrow. Nat. Commun. 10: 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Millar J. S., and Cuchel M.. 2018. Cholesterol metabolism in humans: a review of methods and comparison of results. Curr. Opin. Lipidol. 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein J. L., DeBose-Boyd R. A., and Brown M. S.. 2006. Protein sensors for membrane sterols. Cell. 124: 35–46. [DOI] [PubMed] [Google Scholar]
- 26.Tontonoz P., and Mangelsdorf D. J.. 2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17: 985–993. [DOI] [PubMed] [Google Scholar]
- 27.Maxfield F. R., and Tabas I.. 2005. Role of cholesterol and lipid organization in disease. Nature. 438: 612–621. [DOI] [PubMed] [Google Scholar]
- 28.Goossens P., Rodriguez-Vita J., Etzerodt A., Masse M., Rastoin O., Gouirand V., T. Ulas, O. Papantonopoulou, M. Van Eck, N. Auphan-Anezin, et al. 2019. Membrane cholesterol efflux drives tumor-associated macrophage reprogramming and tumor progression. Cell Metab. 29: 1376–1389.e4. [DOI] [PubMed] [Google Scholar]
- 29.Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., and H. J. Geuze. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 4: 222–231. [DOI] [PubMed] [Google Scholar]
- 30.Friedland N., Liou H. L., Lobel P., and Stock A. M.. 2003. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc. Natl. Acad. Sci. USA. 100: 2512–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sleat D. E., Wiseman J. A., El-Banna M., Price S. M., Verot L., Shen M. M., G. S. Tint, M. T. Vanier, S. U. Walkley, and P. Lobel. 2004. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA. 101: 5886–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturley S. L., Patterson M. C., Balch W., and Liscum L.. 2004. The pathophysiology and mechanisms of NP-C disease. Biochim. Biophys. Acta. 1685: 83–87. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y., Ren J., Harlos K., and Stuart D. I.. 2016. Structure of glycosylated NPC1 luminal domain C reveals insights into NPC2 and Ebola virus interactions. FEBS Lett. 590: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Saha P., Li J., Blobel G., and Pfeffer S. R.. 2016. Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc. Natl. Acad. Sci. USA. 113: 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Wang J., Coutavas E., Shi H., Hao Q., and Blobel G.. 2016. Structure of human Niemann-Pick C1 protein. Proc. Natl. Acad. Sci. USA. 113: 8212–8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Lu F., Trinh M. N., Schmiege P., Seemann J., Wang J., and G. Blobel. 2017. 3.3 Å structure of Niemann-Pick C1 protein reveals insights into the function of the C-terminal luminal domain in cholesterol transport. Proc. Natl. Acad. Sci. USA. 114: 9116–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boadu E., Nelson R. C., and Francis G. A.. 2012. ABCA1-dependent mobilization of lysosomal cholesterol requires functional Niemann-Pick C2 but not Niemann-Pick C1 protein. Biochim. Biophys. Acta. 1821: 396–404. [DOI] [PubMed] [Google Scholar]
- 38.Das A., Brown M. S., Anderson D. D., Goldstein J. L., and Radhakrishnan A.. 2014. Three pools of plasma membrane cholesterol and their relation to cholesterol homeostasis. eLife. 3: doi:10.7554/eLife.02882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Infante R. E., and Radhakrishnan A.. 2017. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife. 6: doi:10.7554/eLife.25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo J., Jiang L., Yang H., and Song B-L.. 2017. Routes and mechanisms of post-endosomal cholesterol trafficking: a story that never ends. Traffic. 18: 209–217. [DOI] [PubMed] [Google Scholar]
- 41.Eden E. R., Sanchez-Heras E., Tsapara A., Sobota A., Levine T. P., and Futter C. E.. 2016. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev. Cell. 37: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilhelm L. P., Wendling C., Védie B., Kobayashi T., Chenard M-P., Tomasetto C., G. Drin, and F. Alpy. 2017. STARD3 mediates endoplasmic reticulum-to-endosome cholesterol transport at membrane contact sites. EMBO J. 36: 1412–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connelly M. A., Kellner-Weibel G., Rothblat G. H., and Williams D. L.. 2003. SR-BI-directed HDL-cholesteryl ester hydrolysis. J. Lipid Res. 44: 331–341. [DOI] [PubMed] [Google Scholar]
- 44.Yuan Q., Bie J., Wang J., Ghosh S. S., and Ghosh S.. 2013. Cooperation between hepatic cholesteryl ester hydrolase and scavenger receptor BI for hydrolysis of HDL-CE. J. Lipid Res. 54: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandhu J., Li S., Fairall L., Pfisterer S. G., Gurnett J. E., Xiao X., T. A. Weston, D. Vashi, A. Ferrari, J. L. Orozco, et al. 2018. Aster proteins facilitate nonvesicular plasma membrane to ER cholesterol transport in mammalian cells. Cell. 175: 514–529.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang N., Silver D. L., Costet P., and Tall A. R.. 2000. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J. Biol. Chem. 275: 33053–33058. [DOI] [PubMed] [Google Scholar]
- 47.Remaley A. T., Stonik J. A., Demosky S. J., Neufeld E. B., Bocharov A. V., Vishnyakova T. G., T. L. Eggerman, A. P. Patterson, N. J. Duverger, S. Santamarina-Fojo, et al. 2001. Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem. Biophys. Res. Commun. 280: 818–823. [DOI] [PubMed] [Google Scholar]
- 48.Tarling E. J., and Edwards P. A.. 2011. ATP binding cassette transporter G1 (ABCG1) is an intracellular sterol transporter. Proc. Natl. Acad. Sci. USA. 108: 19719–19724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pagler T. A., Wang M., Mondal M., Murphy A. J., Westerterp M., Moore K. J., F. R. Maxfield, and A. R. Tall. 2011. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ. Res. 108: 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy M. A., Barrera G. C., Nakamura K., Baldan A., Tarr P., Fishbein M. C., J. Frank, O. L. Francone, and P. A. Edwards. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131. [DOI] [PubMed] [Google Scholar]
- 51.Wang N., Lan D., Chen W., Matsuura F., and Tall A. R.. 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA. 101: 9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtiss L. K., Valenta D. T., Hime N. J., and Rye K. A.. 2006. What is so special about apolipoprotein AI in reverse cholesterol transport? Arterioscler. Thromb. Vasc. Biol. 26: 12–19. [DOI] [PubMed] [Google Scholar]
- 53.Williams D. L., Dawson P. A., Newman T. C., and Rudel L. L.. 1985. Synthesis of apolipoprotein E by peripheral tissues. Potential functions in reverse cholesterol transport and cellular cholesterol metabolism. Ann. N. Y. Acad. Sci. 454: 222–229. [DOI] [PubMed] [Google Scholar]
- 54.Mulya A., Lee J. Y., Gebre A. K., Thomas M. J., Colvin P. L., and Parks J. S.. 2007. Minimal lipidation of pre-beta HDL by ABCA1 results in reduced ability to interact with ABCA1. Arterioscler. Thromb. Vasc. Biol. 27: 1828–1836. [DOI] [PubMed] [Google Scholar]
- 55.Ji Y., Jian B., Wang N., Sun Y., Moya M. L., Phillips M. C., G. H. Rothblat, J. B. Swaney, and A. R. Tall. 1997. Scavenger receptor BI promotes high density lipoprotein-mediated cellular cholesterol efflux. J. Biol. Chem. 272: 20982–20985. [DOI] [PubMed] [Google Scholar]
- 56.Jian B., de la Llera-Moya M., Ji Y., Wang N., Phillips M. C., Swaney J. B., A. R. Tall, and G. H. Rothblat. 1998. Scavenger receptor class B type I as a mediator of cellular cholesterol efflux to lipoproteins and phospholipid acceptors. J. Biol. Chem. 273: 5599–5606. [DOI] [PubMed] [Google Scholar]
- 57.Sorci-Thomas M. G., Pollard R. D., and Thomas M. J.. 2015. What does procollagen C-endopeptidase enhancer protein 2 have to do with HDL-cholesteryl ester uptake? Or how I learned to stop worrying and love reverse cholesterol transport? Curr. Opin. Lipidol. 26: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollard R. D., Blesso C. N., Zabalawi M., Fulp B., Gerelus M., Zhu X., E. W. Lyons, N. Nuradin, O. L. Francone, X. A. Li, et al. 2015. Procollagen C-endopeptidase enhancer protein 2 (PCPE2) reduces atherosclerosis in mice by enhancing SR-BI mediated HDL-cholesteryl ester uptake. J. Biol. Chem. 290: 15496–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neufeld E. B., O’Brien K., Walts A. D., Stonik J. A., Malide D., Combs C. A., and A. T. Remaley. 2014. The human ABCG1 transporter mobilizes plasma membrane and late endosomal non-sphingomyelin-associated-cholesterol for efflux and esterification. Biology (Basel). 3: 866–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma L., Dong F., Zaid M., Kumar A., and Zha X.. 2012. ABCA1 protein enhances Toll-like receptor 4 (TLR4)-stimulated interleukin-10 (IL-10) secretion through protein kinase A (PKA) activation. J. Biol. Chem. 287: 40502–40512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., and Tang C.. 2012. Regulation of ABCA1 functions by signaling pathways. Biochim. Biophys. Acta. 1821: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin K., Chen W. J., Zhou Z. G., Zhao G. J., Lv Y. C., Ouyang X. P., X. H. Yu, Y. Fu, Z. S. Jiang, and C. K. Tang. 2012. Apolipoprotein A-I inhibits CD40 proinflammatory signaling via ATP-binding cassette transporter A1-mediated modulation of lipid raft in macrophages. J. Atheroscler. Thromb. 19: 823–836. [DOI] [PubMed] [Google Scholar]
- 63.Dong F., Mo Z., Eid W., Courtney K. C., and Zha X.. 2014. Akt inhibition promotes ABCA1-mediated cholesterol efflux to ApoA-I through suppressing mTORC1. PLoS One. 9: e113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reboulleau A., Robert V., Vedie B., Doublet A., Grynberg A., Paul J. L., and N. Fournier. 2012. Involvement of cholesterol efflux pathway in the control of cardiomyocytes cholesterol homeostasis. J. Mol. Cell. Cardiol. 53: 196–205. [DOI] [PubMed] [Google Scholar]
- 65.Nofer J. R. 2015. Signal transduction by HDL: agonists, receptors, and signaling cascades. Handb. Exp. Pharmacol. 224: 229–256. [DOI] [PubMed] [Google Scholar]
- 66.Bocchetta S., Maillard P., Yamamoto M., Gondeau C., Douam F., Lebreton S., S. Lagaye, S. Pol, F. Helle, W. Plengpanich, et al. 2014. Up-regulation of the ATP-binding cassette transporter A1 inhibits hepatitis C virus infection. PLoS One. 9: e92140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sorci-Thomas M. G., and Thomas M. J.. 2012. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler. Thromb. Vasc. Biol. 32: 2561–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiedler K., Kobayashi T., Kurzchalia T. V., and Simons K.. 1993. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 32: 6365–6373. [DOI] [PubMed] [Google Scholar]
- 69.Garofalo T., Giammarioli A. M., Misasi R., Tinari A., Manganelli V., Gambardella L., A. Pavan, W. Malorni, and M. Sorice. 2005. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 12: 1378–1389. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi T., and Su T. P.. 2003. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 306: 718–725. [DOI] [PubMed] [Google Scholar]
- 71.Brügger B., Sandhoff R., Wegehingel S., Gorgas K., Malsam J., Helms J. B., W. D. Lehmann, W. Nickel, and F. T. Wieland. 2000. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J. Cell Biol. 151: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stüven E., Porat A., Shimron F., Fass E., Kaloyanova D., Brügger B., F. T. Wieland, Z. Elazar, and J. B. Helms. 2003. Intra-Golgi protein transport depends on a cholesterol balance in the lipid membrane. J. Biol. Chem. 278: 53112–53122. [DOI] [PubMed] [Google Scholar]
- 73.Dodonova S. O., Diestelkoetter-Bachert P., von Appen A., Hagen W. J., Beck R., Beck M., F. Wieland, and J. A. Briggs. 2015. Vesicular transport. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 349: 195–198. [DOI] [PubMed] [Google Scholar]
- 74.Manneville J. B., Casella J. F., Ambroggio E., Gounon P., Bertherat J., Bassereau P., J. Cartaud, B. Antonny, and B. Goud. 2008. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc. Natl. Acad. Sci. USA. 105: 16946–16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerriero C. J., Lai Y., and Weisz O. A.. 2008. Differential sorting and Golgi export requirements for raft-associated and raft-independent apical proteins along the biosynthetic pathway. J. Biol. Chem. 283: 18040–18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surma M. A., Klose C., and Simons K.. 2012. Lipid-dependent protein sorting at the trans-Golgi network. Biochim. Biophys. Acta. 1821: 1059–1067. [DOI] [PubMed] [Google Scholar]
- 77.D’Angelo G., Polishchuk E., Di Tullio G., Santoro M., Di Campli A., Godi A., G. West, J. Bielawski, C. C. Chuang, A. C. van der Spoel, et al. 2007. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 449: 62–67. [DOI] [PubMed] [Google Scholar]
- 78.Mattjus P. 2016. Specificity of the mammalian glycolipid transfer proteins. Chem. Phys. Lipids. 194: 72–78. [DOI] [PubMed] [Google Scholar]
- 79.Yamaji T., Kumagai K., Tomishige N., and Hanada K.. 2008. Two sphingolipid transfer proteins, CERT and FAPP2: their roles in sphingolipid metabolism. IUBMB Life. 60: 511–518. [DOI] [PubMed] [Google Scholar]
- 80.Ridgway N. D. 2010. Oxysterol-binding proteins. Subcell. Biochem. 51: 159–182. [DOI] [PubMed] [Google Scholar]
- 81.Hanada K., Kumagai K., Yasuda S., Miura Y., Kawano M., Fukasawa M., and M. Nishijima. 2003. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 426: 803–809. [DOI] [PubMed] [Google Scholar]
- 82.Kumagai K., Yasuda S., Okemoto K., Nishijima M., Kobayashi S., and Hanada K.. 2005. CERT mediates intermembrane transfer of various molecular species of ceramides. J. Biol. Chem. 280: 6488–6495. [DOI] [PubMed] [Google Scholar]
- 83.Martin T. F. 2001. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13: 493–499. [DOI] [PubMed] [Google Scholar]
- 84.Raturi A., and Simmen T.. 2013. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim. Biophys. Acta. 1833: 213–224. [DOI] [PubMed] [Google Scholar]
- 85.Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., H. Oomori, T. Noda, T. Haraguchi, Y. Hiraoka, et al. 2013. Autophagosomes form at ER-mitochondria contact sites. Nature. 495: 389–393. [DOI] [PubMed] [Google Scholar]
- 86.Matarrese P., Garofalo T., Manganelli V., Gambardella L., Marconi M., Grasso M., A. Tinari, R. Misasi, W. Malorni, and M. Sorice. 2014. Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy. 10: 750–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sala-Vila A., Navarro-Lerida I., Sanchez-Alvarez M., Bosch M., Calvo C., Lopez J. A., E. Calvo, C. Ferguson, M. Giacomello, A. Serafini, et al. 2016. Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci. Rep. 6: 27351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vance J. E., Stone S. J., and Faust J. R.. 1997. Abnormalities in mitochondria-associated membranes and phospholipid biosynthetic enzymes in the mnd/mnd mouse model of neuronal ceroid lipofuscinosis. Biochim. Biophys. Acta. 1344: 286–299. [DOI] [PubMed] [Google Scholar]
- 89.Vance J. E. 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265: 7248–7256. [PubMed] [Google Scholar]
- 90.Fujimoto M., Hayashi T., and Su T. P.. 2012. The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochem. Biophys. Res. Commun. 417: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Area-Gomez E., Del Carmen Lara Castillo M., Tambini M. D., Guardia-Laguarta C., de Groof A. J., Madra M., J. Ikenouchi, M. Umeda, T. D. Bird, S. L. Sturley, et al. 2012. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 31: 4106–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garofalo T., Matarrese P., Manganelli V., Marconi M., Tinari A., Gambardella L., A. Faggioni, R. Misasi, M. Sorice, and W Malorni. 2016. Evidence for the involvement of lipid rafts localized at the ER-mitochondria associated membranes in autophagosome formation. Autophagy. 12: 917–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciarlo L., Manganelli V., Garofalo T., Matarrese P., Tinari A., Misasi R., W. Malorni, and M. Sorice. 2010. Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ. 17: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 94.Ciarlo L., Vona R., Manganelli V., Gambardella L., Raggi C., Marconi M., W. Malorni, M. Sorice, T. Garofalo, and P. Matarrese. 2018. Recruitment of mitofusin 2 into “lipid rafts” drives mitochondria fusion induced by Mdivi-1. Oncotarget. 9: 18869–18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen H., Detmer S. A., Ewald A. J., Griffin E. E., Fraser S. E., and Chan D. C.. 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 160: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olichon A., Emorine L. J., Descoins E., Pelloquin L., Brichese L., Gas N., E. Guillou, C. Delettre, A. Valette, C. P. Hamel, et al. 2002. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 523: 171–176. [DOI] [PubMed] [Google Scholar]
- 97.Yoon Y., Pitts K. R., Dahan S., and McNiven M. A.. 1998. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J. Cell Biol. 140: 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitts K. R., Yoon Y., Krueger E. W., and McNiven M. A.. 1999. The dynamin-like protein DLP1 is essential for normal distribution and morphology of the endoplasmic reticulum and mitochondria in mammalian cells. Mol. Biol. Cell. 10: 4403–4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon Y., Krueger E. W., Oswald B. J., and McNiven M. A.. 2003. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol. Cell. Biol. 23: 5409–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., C. L. Smith, and R. J. Youle. 2001. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 1: 515–525. [DOI] [PubMed] [Google Scholar]
- 101.Garofalo T., Misasi R., Mattei V., Giammarioli A. M., Malorni W., Pontieri G. M., A. Pavan, and M. Sorice. 2003. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8-ganglioside interaction in T cells. J. Biol. Chem. 278: 8309–8315. [DOI] [PubMed] [Google Scholar]
- 102.Legler D. F., Micheau O., Doucey M. A., Tschopp J., and Bron C.. 2003. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 18: 655–664. [DOI] [PubMed] [Google Scholar]
- 103.Scheel-Toellner D., Wang K., Singh R., Majeed S., Raza K., Curnow S. J., M. Salmon, and J. M. Lord. 2002. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem. Biophys. Res. Commun. 297: 876–879. [DOI] [PubMed] [Google Scholar]
- 104.García-Ruiz C., Colell A., Morales A., Calvo M., Enrich C., and Fernandez-Checa J. C.. 2002. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. J. Biol. Chem. 277: 36443–36448. [DOI] [PubMed] [Google Scholar]
- 105.De Maria R., Lenti L., Malisan F., d’Agostino F., Tomassini B., Zeuner A., M. R. Rippo, and R. Testi. 1997. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 277: 1652–1655. [DOI] [PubMed] [Google Scholar]
- 106.Sorice M., Manganelli V., Matarrese P., Tinari A., Misasi R., Malorni W., and T. Garofalo. 2009. Cardiolipin-enriched raft-like microdomains are essential activating platforms for apoptotic signals on mitochondria. FEBS Lett. 583: 2447–2450. [DOI] [PubMed] [Google Scholar]
- 107.Brooks C., Wei Q., Feng L., Dong G., Tao Y., Mei L., Z. J. Xie, and Z. Dong. 2007. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc. Natl. Acad. Sci. USA. 104: 11649–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng Y. Z., Berg K. B., and Foster L. J.. 2009. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 50: 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mause S. F., and Weber C.. 2010. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 107: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 110.Rybak K., and Robatzek S.. 2019. Functions of extracellular vesicles in immunity and virulence. Plant Physiol. 179: 1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gill S., Catchpole R., and Forterre P.. 2019. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 43: 273–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathieu M., Martin-Jaular L., Lavieu G., and Thery C.. 2019. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21: 9–17. [DOI] [PubMed] [Google Scholar]
- 113.Lee S. E., Schulze K., Stewart C. P., Cole R. N., Wu L. S., Eroglu A., J. D. Yager, J. Groopman, P. Christian, and K. P. West, Jr. 2019. Plasma proteome correlates of lipid and lipoprotein: biomarkers of metabolic diversity and inflammation in children of rural Nepal. J. Lipid Res. 60: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.von Zychlinski A., Williams M., McCormick S., and Kleffmann T.. 2014. Absolute quantification of apolipoproteins and associated proteins on human plasma lipoproteins. J. Proteomics. 106: 181–190. [DOI] [PubMed] [Google Scholar]
- 115.Tiwari S., and Siddiqi S. A.. 2012. Intracellular trafficking and secretion of VLDL. Arterioscler. Thromb. Vasc. Biol. 32: 1079–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Théry C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., A. Antoniou, T. Arab, F. Archer, G. K. Atkin-Smith, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 7: 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simonsen J. B. 2017. What are we looking at? Extracellular vesicles, lipoproteins, or both? Circ. Res. 121: 920–922. [DOI] [PubMed] [Google Scholar]
- 118.Menard J. A., Cerezo-Magana M., and Belting M.. 2018. Functional role of extracellular vesicles and lipoproteins in the tumour microenvironment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373: doi:10.1098/rstb.2016.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Escola J. M., Kleijmeer M. J., Stoorvogel W., Griffith J. M., Yoshie O., and Geuze H. J.. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273: 20121–20127. [DOI] [PubMed] [Google Scholar]
- 120.Record M., Subra C., Silvente-Poirot S., and Poirot M.. 2011. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 121.Juan T., and Furthauer M.. 2018. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin. Cell Dev. Biol. 74: 66–77. [DOI] [PubMed] [Google Scholar]
- 122.Stuffers S., Sem Wegner C., Stenmark H., and Brech A.. 2009. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 10: 925–937. [DOI] [PubMed] [Google Scholar]
- 123.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., and Simons M.. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 124.Baietti M. F, Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A., Y. Ivarsson, F. Depoortere, C. Coomans, E. Vermeiren, et al. 2012. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14: 677–685. [DOI] [PubMed] [Google Scholar]
- 125.Herring J. M., McMichael M. A., and Smith S. A.. 2013. Microparticles in health and disease. J. Vet. Intern. Med. 27: 1020–1033. [DOI] [PubMed] [Google Scholar]
- 126.Romero M., Keyel M., Shi G., Bhattacharjee P., Roth R., Heuser J. E., and P. A. Keyel. 2017. Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ. 24: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Badimon L., Suades R., Arderiu G., Pena E., Chiva-Blanch G., and Padro T.. 2017. Microvesicles in atherosclerosis and angiogenesis: from bench to bedside and reverse. Front. Cardiovasc. Med. 4: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macey M. G., Wolf S. I., and Lawson C.. 2010. Microparticle formation after exposure of blood to activated endothelium under flow. Cytometry A. 77: 761–768. [DOI] [PubMed] [Google Scholar]
- 129.Mack M., Kleinschmidt A., Bruhl H., Klier C., Nelson P. J., Cihak J., Plachý J., Stangassinger M., Erfle V., and Schlöndorff D.. 2000. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat. Med. 6: 769–775. [DOI] [PubMed] [Google Scholar]
- 130.Korkut C., Li Y., Koles K., Brewer C., Ashley J., Yoshihara M., and V. Budnik. 2013. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron. 77: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Al-Nedawi K., Meehan B., Micallef J., Lhotak V., May L., Guha A., and J. Rak. 2008. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10: 619–624. [DOI] [PubMed] [Google Scholar]
- 132.Crewe C., Joffin N., Rutkowski J. M., Kim M., Zhang F., Towler D. A., R. Gordillo, and P. E. Scherer. 2018. An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell. 175: 695–708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Flaherty S. E. 3rd, Grijalva A., Xu X., Ables E., Nomani A., and Ferrante A. W. Jr.. 2019. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 363: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Genschmer K. R., Russell D. W., Lal C., Szul T., Bratcher P. E., Noerager B. D., M. Abdul Roda, X. Xu, G. Rezonzew, L. Viera, et al. 2019. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell. 176: 113–126.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Agouni A., Lagrue-Lak-Hal A. H., Ducluzeau P. H., Mostefai H. A., Draunet-Busson C., Leftheriotis G., C. Heymes, M. C. Martinez, and R. Andriantsitohaina. 2008. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am. J. Pathol. 173: 1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Loyer X., Zlatanova I., Devue C., Yin M., Howangyin K. Y., Klaihmon P., C. L. Guerin, M. Kheloufi, J. Vilar, K. Zannis, et al. 2018. Intra-cardiac release of extracellular vesicles shapes inflammation following myocardial infarction. Circ. Res. 123: 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Q., Higginbotham J. N., Jeppesen D. K., Yang Y. P., Li W., McKinley E. T., R. Graves-Deal, J. Ping, C. M. Britain, K. A. Dorsett, et al. 2019. Transfer of functional cargo in exomeres. Cell Rep. 27: 940–954.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., T. Kobayashi, J. P. Salles, B. Perret, C. Bonnerot, et al. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Llorente A., Skotland T., Sylvanne T., Kauhanen D., Rog T., Orlowski A., I. Vattulainen, K. Ekroos, and K. Sandvig. 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 1831: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 140.Rappa G., Mercapide J., Anzanello F., Pope R. M., and Lorico A.. 2013. Biochemical and biological characterization of exosomes containing prominin-1/CD133. Mol. Cancer. 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Skotland T., Sandvig K., and Llorente A.. 2017. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66: 30–41. [DOI] [PubMed] [Google Scholar]
- 142.Fielding C. J., and Fielding P. E.. 2004. Membrane cholesterol and the regulation of signal transduction. Biochem. Soc. Trans. 32: 65–69. [DOI] [PubMed] [Google Scholar]
- 143.Chapuy-Regaud S., Dubois M., Plisson-Chastang C., Bonnefois T., Lhomme S., Bertrand-Michel J., B. You, S. Simoneau, P. E. Gleizes, B. Flan, et al. 2017. Characterization of the lipid envelope of exosome encapsulated HEV particles protected from the immune response. Biochimie. 141: 70–79. [DOI] [PubMed] [Google Scholar]
- 144.Matsumoto A., Takahashi Y., Nishikawa M., Sano K., Morishita M., Charoenviriyakul C., H. Saji, and Y. Takakura. 2017. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J. Pharm. Sci. 106: 168–175. [DOI] [PubMed] [Google Scholar]
- 145.Fadok V. A., de Cathelineau A., Daleke D. L., Henson P. M., and Bratton D. L.. 2001. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 146.Pienimaeki-Roemer A., Kuhlmann K., Bottcher A., Konovalova T., Black A., Orso E., G. Liebisch, M. Ahrens, M. Eisenacher, H. E. Meyer, et al. 2015. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion. 55: 507–521. [DOI] [PubMed] [Google Scholar]
- 147.Durcin M., Fleury A., Taillebois E., Hilairet G., Krupova Z., Henry C., S. Truchet, M. Trötzmüller, H. Köfeler, G. Mabilleau, et al. 2017. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles. 6: 1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Darabi M., and Kontush A.. 2016. Can phosphatidylserine enhance atheroprotective activities of high-density lipoprotein? Biochimie. 120: 81–86. [DOI] [PubMed] [Google Scholar]
- 149.Kontush A., Therond P., Zerrad A., Couturier M., Negre-Salvayre A., de Souza J. A., S. Chantepie, and M. J. Chapman. 2007. Preferential sphingosine-1-phosphate enrichment and sphingomyelin depletion are key features of small dense HDL3 particles: relevance to antiapoptotic and antioxidative activities. Arterioscler. Thromb. Vasc. Biol. 27: 1843–1849. [DOI] [PubMed] [Google Scholar]