Abstract
Activation of microglia and astrocytes secondary to inflammatory processes contributes to the development and perpetuation of pain with a neuropathic phenotype. This pain state presents as a chronic debilitating condition and affects a large population of patients with conditions like rheumatoid arthritis and diabetes, or after surgery, trauma, or chemotherapy. Here, we review the regulation of lipid rafts in glial cells and the role they play as a key component of neuroinflammatory sensitization of central pain signaling pathways. In this context, we introduce the concept of an inflammaraft (i-raft), enlarged lipid rafts harboring activated receptors and adaptor molecules and serving as an organizing platform to initiate inflammatory signaling and the cellular response. Characteristics of the inflammaraft include increased relative abundance of lipid rafts in inflammatory cells, increased content of cholesterol per raft, and increased levels of inflammatory receptors, such as toll-like receptor (TLR)4, adaptor molecules, ion channels, and enzymes in lipid rafts. This inflammaraft motif serves an important role in the membrane assembly of protein complexes, for example, TLR4 dimerization. Operating within this framework, we demonstrate the involvement of inflammatory receptors, redox molecules, and ion channels in the inflammaraft formation and the regulation of cholesterol and sphingolipid metabolism in the inflammaraft maintenance and disruption. Strategies for targeting inflammarafts, without affecting the integrity of lipid rafts in noninflammatory cells, may lead to developing novel therapies for neuropathic pain states and other neuroinflammatory conditions.
Keywords: cholesterol, sphingolipids, neuropathic pain, microglia, astrocytes
Tissue injury and injury to the peripheral nerve often lead to highly disruptive persistent pain states in animals and humans. In this review, we draw attention to the role played in the development of these persistent pain states by signaling organized through lipid rafts in microglia and astrocytes. The concept of lipid rafts was originally proposed based on the self-associative properties of sphingolipid and cholesterol that facilitate lateral segregation of lipids and proteins in the plasma membrane (1). An umbrella effect of the sphingomyelin headgroup stabilizes cholesterol near the sphingomyelin molecules by concealing the hydrophobic cholesterol core from interfacial water (2). These and other structural characteristics of lipid rafts and the biophysical models of microdomains in the plasma membrane have been described in detail in many excellent reviews (3–5). The purpose of this article is different, it focuses on the biology and changes in glial cell lipid rafts underlying neuroinflammation and nociceptive signaling. The nidus around which this review on lipid rafts, glia, and pain processing is organized results from three convergent observations. They focus on toll-like receptor (TLR)4 signaling but have much broader implications.
First, we and others observed that spinal TLR4 signaling has little effect upon the acute pain state (as after a brief application of a high-intensity thermal stimulus or a mechanical compression), but appears to mediate development of the persistent pain phenotype secondary to local inflammation and to peripheral nerve injury (6–13). This transition of pain from an acute stimulus-linked condition to a chronic state has been shown to be a surprisingly common event in both animals and humans (14). For example, in rheumatoid arthritis, joint pain can persist despite resolution of swelling (15, 16). Following surgeries, up to 30% of the population report pain that lasts greater than 3 months (17, 18). Current work emphasizes that these persistent changes may result from neuroinflammatory processes in the spinal cord resembling those arising from physical or chemical injury to the peripheral nerve, with peripheral and central (spinal cord and dorsal root ganglion) immune and glial cells being activated, resulting in a facilitated input/output function of the dorsal horn secondary to neuronal sensitization (10, 19).
Next came the realization that these effects of Tlr4 knockout or TLR4 inhibition on pain processing are mediated, at least in part, by an action upon spinal glial cells. Intrathecal delivery of agents that are thought to maintain microglia in a quiescent state, such as miR-124 (20) or minocycline (21, 22), have been reported to attenuate hyperalgesia (abnormally increased sensitivity to high intensity “painful” stimuli) in models of tissue and nerve injury, albeit the specificity of microglial “inhibitors” is not absolute (23). Spinal microglia express TLR4 in abundance and when activated, it mediates a robust secretion of cytokines, chemokines, and lipids (10, 24–26). These products acting upon neuraxial receptors and targets (dorsal horn neurons) can lead to a pronounced facilitation of neuraxial afferent processing, yielding an enhanced pain signal driven by an otherwise innocuous or moderately noxious stimulus, i.e., allodynia or hyperalgesia. We note here that while there is a consensus that spinal glial cells are activated and induce sensitization of nociceptive neuronal pathways, disagreement exists regarding the specific cell types that play a major role in various pain conditions. As an example, a pivotal role of microglial activation is suggested for the pain state generated by injuries to nerve trunks, i.e., mononeuropathies (6). Conversely, activation of astrocytes is seen in pain states associated with polyneuropathies induced by chemotherapeutics. Treatment with several agents (oxaliplatin, paclitaxel, or bortezomib) induces increases in spinal astrocytes (27, 28). Other investigators show changes in spinal microglia associated with chemotherapy-induced peripheral neuropathy (CIPN) (29, 30).
Finally, the pivot from TLR4 activation in glial cells to lipid rafts comes from the recognition that the first step in TLR4 signaling, receptor dimerization, requires the ordered membrane microenvironment of lipid rafts (31, 32). The requirement for lipid raft localization is important to activation not only of TLR4 but also of a large number of other inflammatory receptors, enzymes, and ion channels, as will be considered in this review. In this context, the TLR4 dimerization, which can be easily assessed with a flow cytometry method, reflects not only a ligand-induced TLR4 receptor activation event but also the permissive membrane microenvironment that supports receptor dimerization. Thus, depletion of cholesterol inhibits lipopolysaccharide (LPS)-induced TLR4 dimerization (33, 34). apoA-I binding protein (AIBP), which augments cholesterol efflux and reduces lipid rafts, also inhibits TLR4 dimerization and alleviates neuropathic pain in mouse models (33). Conversely, increased cholesterol and lipid raft content in the plasma membrane, secondary to knockout of the cholesterol transporters ABCA1 and ABCG1, augments TLR4 dimerization (35, 36).
Based on the three sets of observations outlined above and current literature, we concluded that cholesterol-rich membrane rafts in glial cells serve as local organizing matrices for membrane receptors and channels involved in neuroinflammation and pain processing in the spinal cord. In this article, we summarize the current knowledge in support of this hypothesis. We also suggest a new framework that we believe can be useful for operating in the space of lipid rafts and neuroinflammation, the inflammaraft or, for brevity, the i-raft.
THE INFLAMMARAFT
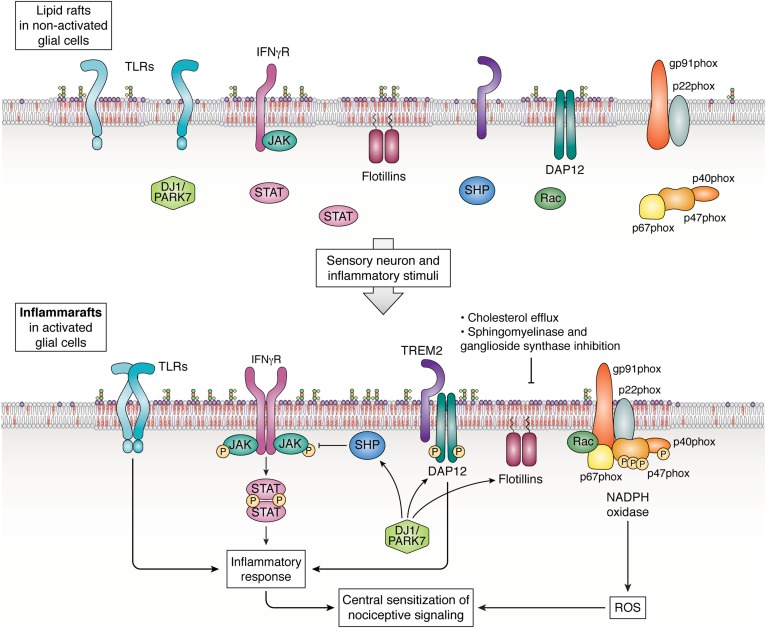
In the course of many conversations on the topic of lipid rafts and their role in inflammatory processes, particularly in spinal microglia, the authors of this article often employed an “inflammasome of the lipid raft” phraseology. It conveyed the connotation of the role played by membrane lipid rafts as a focal point for the organization of a cascade defining the cellular inflammatory response. The notion, while useful, was conversationally cumbersome and not entirely correct. The term “NLRP3 inflammasome” has been reserved for an intracellular molecular complex, which triggers the release of the cytokines interleukin (IL)-1β and IL-18 (37). Thus, a new term was coined, “inflammaraft,” defined, for the purposes of this article, as enlarged lipid rafts harboring activated receptors and adaptor molecules and serving as a scaffold to organize the cellular inflammatory response (Fig. 1).
Fig. 1.
The inflammaraft: Enlarged lipid rafts serving as an organizing platform to initiate inflammatory signaling in glial cells. The diagram illustrates the involvement of lipid raft dynamics in inflammatory activation of microglia and astrocytes. Peripheral-nerve injury elicits ATP release from spinal interneurons and upregulation of P2X and P2Y receptors in spinal microglia (not shown). The P2X and P2Y receptors, localized to lipid rafts, together with a variety of inflammatory cytokines and pathogen- and damage-associated molecular patterns that activate innate immune receptors, contribute to inflammatory activation of microglia (68, 136). This results in the formation of an inflammaraft, which consolidates scattered components of receptor/adaptor molecule complexes and serves as a platform for initiation of inflammatory signaling, as well as for negative feedback regulation. Inflammaraft-associated processes in glial cells include TLR4 homodimerization (33), TLR4/TLR2 and TLR4/TLR6 heterodimerization (137–139); IFNγR-induced JAK-STAT signaling (65), which is also induced by gangliosides (128); TREM2/DAP12 activation (61); and activation of NADPH oxidase activity and increased ROS production (140). DJ1/PARK regulates expression and localization of TREM2 (141) and lipid raft-resident flotillins (95), as well as SHP1 and SHP2 (65, 97, 128). SHP1 and SHP2, upon recruitment to lipid rafts, dephosphorylate their targets and help prevent steady-state inflammatory activation. Reversal of inflammarafts back into lipid rafts of a nonactivated cell can be achieved by augmented cholesterol efflux regulated by the LXR-ABCA1 pathway (102–104, 110), β-cyclodextrins, and AIBP (33); inhibition of ganglioside synthases (125–128); and delicate control of sphingomyelinases, which hydrolyze sphingomyelin but produce inflammatory and raft-residing ceramides (129, 132). For illustrative purposes, all protein components are depicted as residing in one large, shared inflammaraft. Certainly, this is one hypothetical configuration and there may be others. Not shown on the diagram but reviewed in the article are inflammaraft-associated P2X, P2Y, and NK1 receptors and EV budding.
In our own work, we observed that in response to inflammatory stimuli there was increased cellular binding of cholera toxin B to a raft-localized ganglioside, monosialotetrahexosyl ganglioside (GM)1, indicating enlarged lipid rafts, increased cholesterol levels in isolated lipid rafts, increased TLR4 occupancy in lipid rafts, and most important in our view, increased TLR4 dimerization (33). Thus, we suggest that characteristics of the inflammaraft should include (but are not be limited to) the following: i) increased relative abundance of lipid rafts in inflammatory cells; ii) increased content of cholesterol per raft; iii) increased levels of inflammatory receptors, adaptor molecules, ion channels, and enzymes in lipid rafts; together with iv) the evidence of protein complex assemblies in the plasma membrane. The data supporting this characterization of the inflammaraft are ample but scattered throughout literature. Other characteristics may emerge as equally important from work in other laboratories.
The purpose of this article is to review the current evidence emphasizing the functionality of inflammarafts, specifically in glial cells, providing their relevance to systems functioning in nociceptive processing. It is well appreciated that the function of many ion channels and synaptic transmission require lipid rafts (38, 39), and an extensive literature exists to highlight roles of lipid rafts in the regulation of neuronal activity, including in pain processing (40–43). Yet, as we discussed above, glial cells are considered to play an important role as a component of neuroinflammatory sensitization of neuraxial signaling pathways. We will demonstrate that the concept of inflammaraft helps to formalize the lipid raft-centric view of neuroinflammation in the context of neuropathic pain and its control by cholesterol and sphingolipid metabolism, as well as processing of pro- and anti-inflammatory signals at the cell surface.
REGULATION OF INFLAMMARAFTS: PROTEINS OR LIPIDS IN THE LEAD?
The self-assembly property of cholesterol and sphingolipids is in the core of lipid raft formation (1). Because cholesterol concentrates in lipid rafts, proteins that have specific cholesterol binding motifs tend to localize to the rafts: examples of such protein assemblies being TLRs, several gated ion channels, and G protein-coupled receptors (44–46). Binding to gangliosides guides amyloid proteins to lipid rafts (47). In addition, post-translational modification of proteins by saturated lipids, e.g., glycosylphosphatidylinositol anchors, N-terminal myristic acid tails, cysteine acylation, isoprenylation, and C-terminal sterol moieties, recruits them to rafts (48). These data suggest that in addition to being a structural backbone of lipid rafts responsible for forming ordered membrane domains, cholesterol and sphingolipids are often required for targeting specific proteins to lipid rafts.
Further, inflammarafts form as a result of inflammatory signal-dependent oligomerization of resident raft proteins, which then pulls together isolated lipid rafts to form larger assemblies, with a longer lifetime and higher occupancy of raft-associated lipids and proteins, many of which are recruited from non-raft regions of the membrane or from the cytosol (4). Thus, protein-dependent signals constitute the front end of inflammaraft formation, driving lipid raft clustering and enlargement. Conversely, because the integrity of lipid rafts depends on their lipid composition, depletion of cholesterol and/or sphingolipids disrupts lipid rafts and serves to inhibit inflammaraft-associated cellular processes.
In the next two sections, we will summarize current knowledge of protein-driven inflammaraft formation and lipid-dependent mechanisms of its disassembly, specifically related to activation of microglia and astrocytes in neuropathic pain.
ACTIVATION OF INFLAMMARAFT-ASSOCIATED PROTEINS IN PAIN PROCESSING
TLR4
It is well documented that TLR4 is activated in lipid rafts (31–34, 36, 49–53). In a model of spinal L5 nerve transection, tactile and thermal hypersensitivity are significantly attenuated in Tlr4 knockout and point-mutant mice, as well as in rats administered intrathecally with Tlr4 antisense oligonucleotides or siRNA. In these models, TLR4 deficiency is associated with decreased expression of spinal microglial markers and pro-inflammatory cytokines as compared with respective control groups (6, 54). The TLR4 coreceptor, cluster of differentiation (CD)14, which is localized to lipid rafts via its glycosylphosphatidylinositol anchor, is also involved in the pathogenesis of neuropathic pain. CD14 expression is increased in the ipsilateral lumbar spinal cord following L5 nerve transection, together with increases in microglial size and granularity. CD14 knockout mice display significantly decreased mechanical allodynia and thermal hyperalgesia (55).
Additional evidence of the involvement of spinal TLR4 in the genesis of nociceptive processing comes from experiments in which mice received intrathecal injections of the specific TLR4 agonist LPS: wild-type but not Tlr4 knockout mice or mice lacking adaptor signaling proteins develop severe tactile allodynia (56, 57). In these experiments, the intrathecal LPS models the onset of delayed spinal neuroinflammation induced by peripheral injury as discussed below. Similarly, intrathecal TLR2 and TLR3 ligands induce allodynia in wild-type but not respective knockout mice, the effect that is in part dependent on TNFα secretion (56, 57). Interestingly, inhibition of 12/15-lipoxygenase (12/15-LO), which is highly expressed in microglia, but not of cyclooxygenase, completely prevents intrathecal LPS-induced tactile allodynia, suggesting that TLR4-induced production of the bioactive lipids by 12/15-LO, including hepoxilin, mediates, at least in part, neuronal sensitization (25), although the contribution of 12/15-LO expressed in other cell types cannot be excluded.
TLR4 signaling plays a critical role in the transition from acute to chronic postinflammatory mechanical hypersensitivity. Several examples of this have been identified. In the K/BxN passive serum transfer model of arthritis, mice develop a long-lasting inflammation, which resolves in 2–3 weeks. Assessment of the sensitivity to light touch reveals that significant allodynia is observed coincident with the onset of inflammation; but of note, the allodynia persists for many weeks after the inflammation has resolved. It has been demonstrated that a Tlr4 knockout or intrathecal delivery of the TLR4 antagonist, LPS from Rhodobacter sphaeroides (LPS-RS), during the inflammatory phase has no effect upon the early inflammation or allodynia, but prevents the postinflammation allodynia (8). In another example, following the injection of formalin into a hind paw, the animals display a robust time-limited flinching behavior. After 5–7 days, the animals develop a robust tactile allodynia. Again, in Tlr4 knockout mice or after intrathecal delivery of a TLR4 antagonist (TAK-242), there is no effect upon the initial flinching, but Tlr4 knockout or intrathecal antagonism prevent the late phase allodynia (9). Both of these models emphasize that spinal TLR4 regulates the transition from an acute to a chronic pain state, at the stage when peripheral injury triggers spinal neuroinflammation.
In CIPN, which remains an intractable health problem, the prevalence of a pain phenotype is 68% 1 month after the end of chemotherapy, 60% at 3 months, and 30% at 6 months or more (58); TLR4 is attracting attention as a potential therapeutic target. Preclinical models show attenuated hyperalgesia and allodynia in cisplatin-treated TLR4-deficient mice (13, 59). Disruption of all downstream TLR signaling by mutations in the Myd88 and Ticam1 adaptor genes has completely prevented the onset of cisplatin-initiated allodynia at any time (13, 59). Intrathecal treatment with the TLR4 antagonist, LPS-RS, transiently reverses paclitaxel-induced mechanical hypersensitivity (60).
TREM2
Following spinal nerve injury, expression of the triggering receptor expressed on myeloid cells (TREM)2 and its adaptor protein, DNAX activation protein (DAP)12, is increased in microglia of the dorsal horn. Intrathecal administration of TREM2 agonistic antibody induces pro-inflammatory cytokine expression and neuropathic pain; this effect is abrogated in DAP12-deficient mice (61). Conversely, in a cisplatin-induced CIPN mouse model, allodynia is significantly diminished with administration of a neutralizing antibody against TREM2 (30). TREM2 colocalizes in lipid rafts with DAP12 (62) and also with membrane-spanning 4-domains subfamily A (MS4A), which modulates the production of soluble TREM2 (63).
IFNγR
Stimulation with IFNγ leads to lipid raft-dependent activation of IFNγR and downstream Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathways (64, 65). Spinal microglia express high levels of IFNγR, and IFNγ induces microglial activation and long-lasting tactile allodynia, in part via upregulation of the tyrosine protein kinase, Lck/Yes-related novel protein tyrosine kinase (LYN), and the purinergic P2X purinoreceptor cation channel family (P2X)4 receptor (66). The lipid raft-associated P2X4 (67) is a microglia receptor for the ATP released from neurons post peripheral nerve injury, which mediates release of brain-derived neurotrophic factor (BDNF) and other factors involved in central sensitization (68, 69).
P2X and P2Y purinergic receptors
P2X4 receptor-positive microglia play a pivotal role in the mechanism of neuropathic pain (69, 70). CCL2 promotes P2X4 receptor trafficking to the cell surface in microglia (71). A mechanism for pain hypersensitivity after peripheral nerve injury through the P2X4 receptor involves an increase in Ca2+ and activation of p38 MAPK leading to the synthesis and exocytotic release of BDNF from microglia, which is blocked by inhibiting soluble NSF attachment protein receptor (SNARE)-mediated exocytosis (72). Studies in other cell types propose that the P2X4 and P2X7 receptors’ membrane localization is regulated by the association with lipid rafts, or through vesicle exocytosis (71, 73, 74) and that cholesterol regulates P2X receptor activity (75). Contributing to sexual dimorphism in pain, the P2X4 receptor pathway is specifically engaged in male but not female rodents (76).
The P2Y purinergic G protein-coupled receptor family (P2Y)12 receptor is expressed only in microglia in the CNS and its surface expression is increased following peripheral nerve injury. P2Y12 antagonism reduces pain by reducing microglial activation and p38 MAPK phosphorylation in a spinal nerve ligation neuropathic pain model (77). Similarly, the expression of the P2Y12 receptor in peripheral satellite glia is also related to pain and its antagonism leads to reduced cytokine production and p38 MAPK phosphorylation and higher withdrawal thresholds (78). It has been demonstrated that in platelets, activation of the P2Y12 receptor occurs in lipid rafts (79, 80). However, whether lipid rafts are required for P2Y12 activation in microglia remains to be demonstrated.
NK1 receptor
Human neurons, microglia, and astrocytes express neurokinin 1 receptor (NK1R), which augments inflammatory signaling pathways upon substance P release from nociceptive primary afferents (81). Administration of opioids, which block the release of substance P from small primary afferents (82), or selective NK1R antagonism decreases glial inflammation and attenuates paradoxical pain sensitization upon opioid withdrawal (83). The NK1R localizes to lipid rafts and caveolae; cholesterol extraction and replenishment demonstrate that activation of NK1R is dependent on lipid raft integrity (84).
Redox inflammarafts
Reactive oxygen species (ROS) can induce several proteins, including TLR4, to translocate to lipid rafts (50). NADPH oxidase (NOX)2-derived ROS in spinal microglia contribute to peripheral nerve injury-induced neuropathic pain. NOX2-deficient mice show reduced spinal nerve transection-induced ROS generation, microglial activation, and pro-inflammatory cytokine expression in the spinal cord. Spinal nerve transection-induced mechanical allodynia and thermal hyperalgesia are similarly attenuated in NOX2-deficient mice (85). NOX2 induction in pain is mediated by TLR4 and TLR2 in spinal microglia (86, 87). TLR4 induces the recruitment of cytosolic NOX2 adaptor proteins to the membrane by phosphorylation of Ser345 in p47phox (87). Activated NOX2 localizes to lipid rafts together with p22phox (88). Similarly, the activation of NOX1 and NOX3 by NOXO1 is preceded by the interaction of the PX domain with phosphatidylinositol (PtdIns)(4,5)P2, important in lipid raft biogenesis (89). NOX5 directly interacts, via its N-terminal region, with PtdIns(4,5)P2, promoting localization to cholesterol- and caveolin-rich lipid rafts (90).
Extracellular vesicles
The budding of extracellular vesicles (EVs) from inflammarafts is facilitated by externalization of acid sphingomyelinase, which, by locally increasing ceramide levels in the inner leaflet of the plasma membrane, modifies the membrane curvature and facilitates EV biogenesis (91). The release of EVs propagates inflammatory signals from activated glial cells. Thus, EVs shed from microglia activated by a P2X7-p38 MAPK pathway and carrying IL-1β are involved in the development of neuropathic pain induced by spinal nerve ligation in rats (92). EV-transmitted signals induce increased expression of costimulatory molecule CD86 and pro-inflammatory genes IL-1β, IL-6, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)2 in recipient microglia (93). Astrocyte-secreted EVs released in response to ethanol carry inflammatory-related proteins [TLR4, NFκB-p65, IL-1R, caspase-1, and NACHT, LRR, and PYD domains-containing protein 3 (NLRP3)] and microRNAs (mir-146a, mir-182, and mir-200b), but no such changes have been observed in EVs from ethanol-treated Tlr4 knockout astrocytes (94). These results suggest that EVs originating from inflammarafts could act as cellular transmitters of inflammation signaling by spreading the membrane and cytosolic cargo and amplifying neuroinflammation.
Negative feedback in inflammarafts
Protein deglycase DJ-1 (DJ-1), also known as Parkinson disease protein 7 (PARK7), a protein associated with familial Parkinson’s disease, plays a major role in supporting lipid raft integrity and negative feedback mechanisms to repress inflammatory activation. Deficiency in DJ-1 decreases the stability and half-life of flotillin-1 in primary astrocytes. DJ-1 regulates flotillin-1 stability by direct interaction, and thus, dysregulation of flotillin-1 in DJ-1-deficient astrocytes causes an alteration in the cellular cholesterol levels, membrane fluidity, and lipid raft-dependent endocytosis (95). Defective endocytosis of raft-associated TLR4 may explain augmented response to LPS in DJ-1-deficient astrocytes (96). DJ-1 may also function as a scaffold protein that facilitates the phosphatase Src homology region 2 domain-containing phosphatase (SHP)1 interaction with p-STAT1, thereby preventing extensive and prolonged activation of the STAT1 pathway by IFNγ (65). Similarly, ROS-induced phosphorylation of SHP2 and its translocation into lipid rafts to dephosphorylate STAT3 prevents steady-state STAT3 activation in astrocytes (97).
The DJ-1 deficiency also impairs glutamate uptake, a major function of astrocytes in the maintenance of CNS excitatory homeostasis (95). The loss of astrocyte glutamate uptake function is likely due to translocation of the glutamate transporter, excitatory amino acid transporter (EAAT)2, away from lipid rafts (98). Astrocyte production of macrophage inflammatory protein (MIP)-2γ in response to LPS or TNFα reduces expression of the glutamate transporter (GLT)-1, a mouse analog of EAAT2, and induces its redistribution away from lipid rafts, resulting in reduced glutamate uptake (99). Glutamate homeostasis can also be affected by release of EVs with increased content of Na+/K+ ATPase and EAAT1 and EAAT2 glutamate transporters (100). Despite the important role of DJ-1 in the regulation of the inflammaraft in glial cells, we are unaware of studies that have investigated DJ-1 in facilitated pain states.
Research reports summarized in this section support the notion that inflammaraft-associated signaling via TLR4 and other TLRs, TREM2, IFNγR, P2X, and P2Y purinergic receptors and NK1 receptor, redox regulation, and EV-propagated neuroinflammation significantly contribute to the transition from acute to chronic pain and to the perpetuation of neuropathic pain. Thus, targeting inflammarafts may provide a method for reducing many of these inflammatory events, breaking the self-reinforcing cycle of neuroinflammation and ultimately alleviating neuropathic pain.
LIPID-ASSOCIATED MECHANISMS OF INFLAMMARAFT REGULATION
Cholesterol efflux
Cholesterol efflux from astrocytes, in conjunction with apoE expression, contributes to formation of CNS lipoproteins, serving in adult brain to deliver cholesterol and other lipids to neurons (101). The function of the ABCA1 cholesterol transporter in astrocytes is essential for lipidation of CNS lipoproteins (102, 103), while the roles of ABCG1 and ABCG4 are less well understood, and they appear to have overlapping functions in promoting sterol efflux from astrocytes (101). The lipid efflux process in astrocytes is regulated by LXR (104) and BDNF (105).
In addition to metabolic support of neurons, cholesterol efflux regulates inflammarafts in microglia and astrocytes. The LXR-ABCA1/ABCG1-cholesterol efflux pathway is a major conduit of anti-inflammatory responses (106). Intrathecal injections of specific LXR agonists prevent the development of mechanical allodynia and alleviate the established allodynia in rats and mice subjected to spinal nerve injury or intrathecal LPS (33, 107). The effect of LXR agonists is abolished in LXRα-deficient mice, which also show significantly increased glial activation in response to spinal nerve injury (107). Microglia from mice deficient in ABCA1 exhibit augmented LPS-induced secretion of TNFα and decreased phagocytic activity (108). Anti-inflammatory effects of taraxasterol, a pentacyclic-triterpene isolated from dandelion (Taraxacum officinale), in LPS-stimulated BV2 microglia cells are mediated by activation of the LXRα-ABCA1 signaling pathway, which induces cholesterol efflux and disruption of lipid rafts (109). Similarly, platycodin D, a triterpenesaponin extracted from Chinese bellflower (Platycodon grandiflorum) and also known to have anti-inflammatory effects, inhibits LPS-induced ROS, TNF-α, IL-6, and IL-1β production in primary rat microglial cells. The mechanism involves reduction of lipid raft abundance and inhibition of LPS-induced TLR4 translocation into lipid rafts. The inhibition of inflammatory responses by platycodin D is reversed by knockdown of LXRα, supporting the cholesterol efflux mechanism (110).
Exposure to LPS inhibits cholesterol efflux to gain lipid raft support to inflammatory signaling, including in microglia (33, 111, 112). Intrathecal apoA-I, serving as a cholesterol efflux acceptor, transiently reduces intrathecal LPS-induced tactile allodynia in mice (33). AIBP, via its binding to TLR4, facilitates cholesterol efflux from LPS-activated microglia but not from unstimulated cells (33), suggesting selective regulation of cholesterol efflux under inflammatory conditions. As a result, AIBP reverses LPS-induced changes in the microglial plasma membrane, characteristic of an inflammaraft: increased lipid raft abundance, cholesterol and TLR4 content in lipid rafts, and TLR4 dimerization. In mice, intrathecal administration of AIBP prevents LPS-induced TLR4 dimerization and IBA1 and glial fibrillary acidic protein (GFAP) expression in the spinal cord and reduces levels of inflammatory cytokines in the cerebrospinal fluid (33). These features of disrupted spinal inflammarafts in mice that received intrathecal AIBP parallel the reversal of mechanical allodynia in a CIPN model and of delayed allodynia in an arthritis model. Importantly, intrathecal AIBP does not result in any adverse effects on sensory or motor function, suggesting targeted regulation of the inflammaraft in activated microglia (and possibly other spinal cells) by AIBP, without affecting normal lipid raft-associated functions in non-inflammatory cells (33).
As an alternative to manipulating endogenous pathways regulating cholesterol efflux, β-cyclodextrins are widely used to deplete the plasma membrane of cholesterol and disrupt lipid rafts in laboratory settings (113). Cyclodextrins are also widely used in drug formulations for complexation and delivery of many therapeutics, including nonsteroidal anti-inflammatory drugs and local anesthetics (114, 115). Direct therapeutic effects have been described for intrathecal 2-hydroxypropyl-β-cyclodextrin in treatment of Niemann-Pick disease, type C1 (116, 117). In a mouse model of atherosclerosis, administration of 2-hydroxypropyl-β-cyclodextrin reduces plaque size, in part via increased oxysterol production and activation of the LXR-dependent cholesterol efflux pathway (118). However, to the best of our knowledge, no studies have been conducted to test the analgesic effects of a cyclodextrin as a stand-alone therapy.
Cholesterol biosynthesis
Statins, a class of potent inhibitors of cholesterol biosynthesis, have well-documented anti-inflammatory effects, some of them independent of statin’s inhibition of cholesterol biosynthesis (119). For example, a recent article provides evidence that lovastatin directly binds to myeloid differentiation (MD)-2, the TLR4 coreceptor, and thereby inhibits TLR4 signaling and attenuates neuropathic pain (120). Thus, the reported effects of statins on reducing neuroinflammation and neuropathic pain, referring to the cholesterol synthesis mechanism, should be interpreted with caution. Atorvastatin attenuates neuropathic pain in a rat neuropathy model by downregulating oxidative damage at peripheral, spinal, and supraspinal levels (121). Systemic administration of simvastatin or rosuvastatin completely prevents the development of mechanical allodynia and thermal hyperalgesia in a nerve ligation model. When administered postinjury, statins dose-dependently reduce established hypersensitivity and are able to abolish IL-1β expression and significantly reduce spinal nerve ligation-triggered spinal microglia and astrocyte activation (122). In cell culture experiments, exposure of human and murine microglia to simvastatin reduces cell surface expression of the chemokine receptors, C-C motif chemokine receptor (CCR)5 and C-X-C motif chemokine receptor (CXCR)3, and abolishes chemokine-induced microglial cell motility (123). The mechanism likely involves simvastatin-induced disruption of lipid rafts, resulting in loss of the cell surface expression of CCR5 (124).
Sphingolipid metabolism
Upregulation of the lipid raft component ganglioside GD3 in astrocytes results in increased recruitment of platelet-derived growth factor receptor (PDGFR)α to lipid rafts and the promotion of malignant phenotypes such as cell proliferation and invasion in gliomas (125). Conversely, knockout of GD3 and GM2/GD2 synthases results in dispersion of caveolin-1 and flotillin-1 from lipid raft fractions and increased expression of inflammatory cytokines IL-1β and TNFα (126). The deficiency in lysosomal β-hexosaminidase, the cause of Sandhoff disease, results in excessive accumulation of GM2 ganglioside, a lipid raft component, and progressive neurodegeneration. Microglia from the mouse model of Sandhoff disease are characterized by increased dimerization of the P2Y6 receptor, which mediates extracellular nucleotide-induced secretion of macrophage inflammatory protein (MIP)-1α and neuroinflammation (127). In cultured rat brain microglia, gangliosides induce rapid but transient activation of the JAK-STAT pathway; the transient effect is due to subsequent activation of the phosphatase SHP2. The latter depends on ganglioside-induced rapid SHP2 recruitment to membrane lipid rafts, where it binds and dephosphorylates JAK2 (128).
The product of hydrolysis of lipid raft-localized sphingomyelin is ceramide, which remains in lipid rafts and further promotes receptor dimerization (129). Tibial nerve transection significantly increases levels of products of sphingomyelin-ceramide metabolism in the dorsal horn of rats, and the increased levels of the endogenous ceramide metabolite N,N-dimethylsphingosine induce mechanical hypersensitivity in vivo (130). The mechanism likely includes an increase in intracellular Ca2+ concentration and the inhibition of glutamate uptake by astrocytes (131). Intrathecal inhibition of neutral sphingomyelinase significantly attenuates partial sciatic nerve ligation-induced activation of microglia and tactile allodynia (132).
This section outlined the lipid regulation pathways that can be exploited to disrupt inflammarafts as a new target for treatment of neuropathic pain, which will result in cumulative inhibition of inflammatory receptors localized to lipid rafts. Certainly, an inflammaraft therapy is a thin line to walk, because normal cellular functions that occur in lipid rafts of non-inflammatory cells must be preserved.
OTHER INTERVENING VARIABLES: SEXUAL DIMORPHISM AND AGING
An additional level of potential complexity to the inflammaraft story is the role of sexual dimorphism in neuraxial signaling systems. Two examples can be cited. One is the involvement of microglia in neuropathic pain processing where, according to a recent study, microglia play a primary role only in male mice (133). This difference is despite a similar degree of pain hypersensitivity in male and female mice; however, in females, adaptive immune responses likely associated with T cell release of proalgesic mediators enhance neuronal activity leading to mechanical allodynia (133). The other example is work with nerve injury and persistent inflammation, where TLR4 signaling has been reported to play a pivotal role in males in mediating the persistent pain state after nerve injury (7) and inflammation (8). With regard to aging, recent work has revealed that cortical lipid rafts are modified by aging in a sex-dependent fashion, being more pronounced in women than in men (134). The effect of such sex-dependent changes in nociceptive processing remain to be assessed.
CONCLUSIONS
In the context of neuroinflammation as a central factor in the transition from acute to chronic pain and maintenance of the facilitated pain state arising from tissue and nerve injury, the inflammaraft is a lipid-protein assembly in cells including glia, which organizes inflammatory signaling cascades originating from the cell surface. Although discussing exact lipid-protein composition and biophysical properties of an inflammaraft is outside the scope of this article, we summarize here the current knowledge of inflammatory receptors and adaptor molecules localized to lipid rafts in activated glia, as well as regulation of cholesterol and sphingolipid levels, which make or break the functional integrity of the inflammaraft. It is important to note that cholesterol and sphingolipids may regulate receptor function not only through altering lipid rafts, but also via direct interactions with the proteins. Yet, the effect of cholesterol or sphingolipid depletion would be similar for both mechanisms.
Many unresolved questions remain. Although LPS stimulation of TLR4 signaling appears to be a major tool for laboratory studies of inflammarafts, factors that modulate lipid rafts in general and in spinal glial cells in particular and the role this regulation plays in in vivo processes are poorly understood. The notion that ligand-dependent activation of cellular receptors leads to inflammaraft formation, but that lipid depletion serves to mediate inflammaraft destruction, is almost certainly oversimplified. Future studies will reveal whether cholesterol- and sphingolipid-related mechanisms (outside of genetic deficiency) are also involved in inflammaraft formation. Other questions include, what is the exact architecture of receptor-adaptor molecule assemblies in inflammarafts? How extensive is the cross-talk between different receptors in the same inflammaraft, and what regulates such cross-talk? What is the involvement of cytoskeleton and cell-cell contacts in regulation of an inflammaraft?
From the therapeutic point of view, consideration of inflammarafts raises the question of whether there is a viable option of modulating inflammarafts without affecting the normal function of proteins localized to lipid rafts in nonactivated cells. The use of AIBP, which binds to cells expressing high levels of TLR4 concentrated in inflammarafts and which promotes cholesterol efflux, offers one possibility. Importantly, the selective actions of AIBP are to be distinguished from those of promiscuous cholesterol removal agents, such as the cyclodextrins. This approach might still allow TLR4 activation to defend against host pathogens while reducing protracted large raft aggregation sustaining neuropathic pain signaling. Future studies will explore other innate protective mechanisms targeting inflammarafts that can be developed into therapeutic strategies to treat neuroinflammation and neuropathic pain.
In closing, the authors would like to recognize that it has been 10 years since the passing of Robert Galambos. Professor Galambos in a prescient review article in 1951 (135) emphasized the role of glia in regulating neuronal networks. It is noteworthy that current work has elaborated on his thinking, and today we recognize that astrocytes and microglia play a pivotal role as components of the tripartite synapse in processes regulating neuronal excitability and neuraxial function, truly a perceptive insight!
Acknowledgments
The authors thank Dr. Luciana Giono for her creative artistic work to illustrate this article.
Footnotes
Abbreviations:
- 12/15-LO
- 12/15-lipoxygenase
- AIBP
- apoA-I binding protein
- BDNF
- brain-derived neurotrophic factor
- CD
- cluster of differentiation
- CIPN
- chemotherapy-induced peripheral neuropathy
- DAP
- DNAX activation protein
- DJ-1/PARK7
- protein deglycase DJ-1 (also known as Parkinson disease protein 7)
- EAAT
- excitatory amino acid transporter
- EV
- extracellular vesicle
- GM
- monosialotetrahexosyl ganglioside
- IL
- interleukin
- iNOS
- inducible nitric oxide synthase
- JAK
- Janus kinase
- LPS
- lipopolysaccharide
- LPS-RS
- lipopolysaccharide from Rhodobacter sphaeroides
- NK1R
- neurokinin 1 receptor
- NLRP3
- NACHT, LRR, and PYD domains-containing protein 3
- NOX
- NADPH oxidase
- P2X
- P2X purinoreceptor cation channel family
- P2Y
- P2Y purinergic G protein-coupled receptor family
- ROS
- reactive oxygen species
- SHP
- Src homology region 2 domain-containing phosphatase
- STAT
- signal transducer and activator of transcription
- TLR
- toll-like receptor
- TREM
- triggering receptor expressed on myeloid cells
This work was supported by National Institutes of Health Grants NS102432, HL135737, HL136275, NS104769, NS099338, and T32AR064194. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Y.I.M. and T.L.Y. are inventors listed in patent applications related to the topic of this article and are scientific founders of Raft Pharmaceuticals LLC.
REFERENCES
- 1.Simons K., and Ikonen E.. 1997. Functional rafts in cell membranes. Nature. 387: 569–572. [DOI] [PubMed] [Google Scholar]
- 2.Hanashima S., Murakami K., Yura M., Yano Y., Umegawa Y., Tsuchikawa H., Matsumori N., Seo S., Shinoda W., and Murata M.. 2019. Cholesterol-induced conformational change in the sphingomyelin headgroup. Biophys. J. 117: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lingwood D., and Simons K.. 2010. Lipid rafts as a membrane-organizing principle. Science. 327: 46–50. [DOI] [PubMed] [Google Scholar]
- 4.Sezgin E., Levental I., Mayor S., and Eggeling C.. 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson C. V., Rohacs T., and Hansen S. B.. 2019. Tools for understanding nanoscale lipid regulation of ion channels. Trends Biochem. Sci. 44: 795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanga F. Y., Nutile-McMenemy N., and DeLeo J. A.. 2005. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. USA. 102: 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorge R. E., LaCroix-Fralish M. L., Tuttle A. H., Sotocinal S. G., Austin J. S., Ritchie J., Chanda M. L., Graham A. C., Topham L., Beggs S., et al. 2011. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 31: 15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christianson C. A., Dumlao D. S., Stokes J. A., Dennis E. A., Svensson C. I., Corr M., and Yaksh T. L.. 2011. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain. 152: 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woller S. A., Ravula S. B., Tucci F. C., Beaton G., Corr M., Isseroff R. R., Soulika A. M., Chigbrow M., Eddinger K. A., and Yaksh T. L.. 2016. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav. Immun. 56: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q., and Yaksh T. L.. 2011. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 24: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woller S. A., Ocheltree C., Wong S. Y., Bui A., Fujita Y., Goncalves Dos Santos G., Yaksh T. L., and Corr M.. 2019. Neuraxial TNF and IFN-beta co-modulate persistent allodynia in arthritic mice. Brain Behav. Immun. 76: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran R., Wang Z., Saavedra C., DiNardo A., Corr M., Powell S. B., and Yaksh T. L.. 2019. Role of Toll-like receptor 4 signaling in mast cell-mediated migraine pain pathway. Mol. Pain. 15: 1744806919867842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woller S. A., Corr M., and Yaksh T. L.. 2015. Differences in cisplatin-induced mechanical allodynia in male and female mice. Eur. J. Pain. 19: 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woller S. A., Eddinger K. A., Corr M., and Yaksh T. L.. 2017. An overview of pathways encoding nociception. Clin. Exp. Rheumatol. 35 (Suppl. 107): 40–46. [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe F., and Michaud K.. 2007. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J. Rheumatol. 34: 1674–1683. [PubMed] [Google Scholar]
- 16.Taylor P., Manger B., Alvaro-Gracia J., Johnstone R., Gomez-Reino J., Eberhardt E., Wolfe F., Schwartzman S., Furfaro N., and Kavanaugh A.. 2010. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J. Int. Med. Res. 38: 1213–1224. [DOI] [PubMed] [Google Scholar]
- 17.Katz J., and Seltzer Z.. 2009. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev. Neurother. 9: 723–744. [DOI] [PubMed] [Google Scholar]
- 18.Kehlet H., and Rathmell J. P.. 2010. Persistent postsurgical pain: the path forward through better design of clinical studies. Anesthesiology. 112: 514–515. [DOI] [PubMed] [Google Scholar]
- 19.Yaksh T. L., Woller S. A., Ramachandran R., and Sorkin L. S.. 2015. The search for novel analgesics: targets and mechanisms. F1000Prime Rep. 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willemen H. L., Huo X. J., Mao-Ying Q. L., Zijlstra J., Heijnen C. J., and Kavelaars A.. 2012. MicroRNA-124 as a novel treatment for persistent hyperalgesia. J. Neuroinflammation. 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mei X. P., Xu H., Xie C., Ren J., Zhou Y., Zhang H., and Xu L. X.. 2011. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neurosci. Res. 70: 305–312. [DOI] [PubMed] [Google Scholar]
- 22.Hua X. Y., Svensson C. I., Matsui T., Fitzsimmons B., Yaksh T. L., and Webb M.. 2005. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 22: 2431–2440. [DOI] [PubMed] [Google Scholar]
- 23.Möller T., Bard F., Bhattacharya A., Biber K., Campbell B., Dale E., Eder C., Gan L., Garden G. A., Hughes Z. A., et al. 2016. Critical data-based re-evaluation of minocycline as a putative specific microglia inhibitor. Glia. 64: 1788–1794. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H., Alam A., Chen Q., Eusman M. A., Pal A., Eguchi S., Wu L., and Ma D.. 2017. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br. J. Anaesth. 118: 504–516. [DOI] [PubMed] [Google Scholar]
- 25.Gregus A. M., Buczynski M. W., Dumlao D. S., Norris P. C., Rai G., Simeonov A., Maloney D. J., Jadhav A., Xu Q., Wei S. C., et al. 2018. Inhibition of spinal 15-LOX-1 attenuates TLR4-dependent, nonsteroidal anti-inflammatory drug-unresponsive hyperalgesia in male rats. Pain. 159: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno K., Woller S. A., Miller Y. I., Yaksh T. L., Wallace M., Beaton G., and Chakravarthy K.. 2018. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain. 159: 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson C. R., Zhang H., and Dougherty P. M.. 2014. Astrocytes, but not microglia, are activated in oxaliplatin and bortezomib-induced peripheral neuropathy in the rat. Neuroscience. 274: 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makker P. G., Duffy S. S., Lees J. G., Perera C. J., Tonkin R. S., Butovsky O., Park S. B., Goldstein D., and Moalem-Taylor G.. 2017. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One. 12: e0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beh S. T., Kuo Y. M., Chang W. W., Wilder-Smith E., Tsao C. H., Tsai C. H., Chen L. T., and Liao L. D.. 2019. Preventive hypothermia as a neuroprotective strategy for paclitaxel-induced peripheral neuropathy. Pain. 160: 1505–1521. [DOI] [PubMed] [Google Scholar]
- 30.Hu L. Y., Zhou Y., Cui W. Q., Hu X. M., Du L. X., Mi W. L., Chu Y. X., Wu G. C., Wang Y. Q., and Mao-Ying Q. L.. 2018. Triggering receptor expressed on myeloid cells 2 (TREM2) dependent microglial activation promotes cisplatin-induced peripheral neuropathy in mice. Brain Behav. Immun. 68: 132–145. [DOI] [PubMed] [Google Scholar]
- 31.Fessler M. B., and Parks J. S.. 2011. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 187: 1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tall A. R., and Yvan-Charvet L.. 2015. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woller S. A., Choi S. H., An E. J., Low H., Schneider D. A., Ramachandran R., Kim J., Bae Y. S., Sviridov D., Corr M., et al. 2018. Inhibition of neuroinflammation by AIBP: spinal effects upon facilitated pain states. Cell Reports. 23: 2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers K. A., Szaszi K., Khadaroo R. G., Tawadros P. S., Marshall J. C., Kapus A., and Rotstein O. D.. 2006. Oxidative stress generated by hemorrhagic shock recruits Toll-like receptor 4 to the plasma membrane in macrophages. J. Exp. Med. 203: 1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yvan-Charvet L., Welch C., Pagler T. A., Ranalletta M., Lamkanfi M., Han S., Ishibashi M., Li R., Wang N., and Tall A. R.. 2008. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 118: 1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X., Owen J. S., Wilson M. D., Li H., Griffiths G. L., Thomas M. J., Hiltbold E. M., Fessler M. B., and Parks J. S.. 2010. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J. Lipid Res. 51: 3196–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F., Burns K., and Tschopp J.. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell. 10: 417–426. [DOI] [PubMed] [Google Scholar]
- 38.Aureli M., Grassi S., Prioni S., Sonnino S., and Prinetti A.. 2015. Lipid membrane domains in the brain. Biochim. Biophys. Acta. 1851: 1006–1016. [DOI] [PubMed] [Google Scholar]
- 39.Zaidi A., Adewale M., McLean L., and Ramlow P.. 2018. The plasma membrane calcium pumps-the old and the new. Neurosci. Lett. 663: 12–17. [DOI] [PubMed] [Google Scholar]
- 40.Sághy E., Szoke E., Payrits M., Helyes Z., Borzsei R., Erostyak J., Janosi T. Z., Setalo G. Jr., and Szolcsanyi J.. 2015. Evidence for the role of lipid rafts and sphingomyelin in Ca2+-gating of transient receptor potential channels in trigeminal sensory neurons and peripheral nerve terminals. Pharmacol. Res. 100: 101–116. [DOI] [PubMed] [Google Scholar]
- 41.Schrattenholz A., and Soskic V.. 2006. NMDA receptors are not alone: dynamic regulation of NMDA receptor structure and function by neuregulins and transient cholesterol-rich membrane domains leads to disease-specific nuances of glutamate-signalling. Curr. Top. Med. Chem. 6: 663–686. [DOI] [PubMed] [Google Scholar]
- 42.Gnanasekaran A., Sundukova M., van den Maagdenberg A. M., Fabbretti E., and Nistri A.. 2011. Lipid rafts control P2X3 receptor distribution and function in trigeminal sensory neurons of a transgenic migraine mouse model. Mol. Pain. 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Startek J. B., Boonen B., Lopez-Requena A., Talavera A., Alpizar Y. A., Ghosh D., Van Ranst N., Nilius B., Voets T., and Talavera K.. 2019. Mouse TRPA1 function and membrane localization are modulated by direct interactions with cholesterol. eLife. 8: e46084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruysschaert J. M., and Lonez C.. 2015. Role of lipid microdomains in TLR-mediated signalling. Biochim. Biophys. Acta. 1848: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 45.Katritch V., Cherezov V., and Stevens R. C.. 2013. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 53: 531–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fantini J., Epand R. M., and Barrantes F. J.. 2019. Cholesterol-recognition motifs in membrane proteins. Adv. Exp. Med. Biol. 1135: 3–25. [DOI] [PubMed] [Google Scholar]
- 47.Yahi N., and Fantini J.. 2014. Deciphering the glycolipid code of Alzheimer’s and Parkinson’s amyloid proteins allowed the creation of a universal ganglioside-binding peptide. PLoS One. 9: e104751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levental I., Grzybek M., and Simons K.. 2010. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 49: 6305–6316. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Lizarbe S., Pascual M., Gascon M. S., Blanco A., and Guerri C.. 2008. Lipid rafts regulate ethanol-induced activation of TLR4 signaling in murine macrophages. Mol. Immunol. 45: 2007–2016. [DOI] [PubMed] [Google Scholar]
- 50.Wong S. W., Kwon M. J., Choi A. M. K., Kim H. P., Nakahira K., and Hwang D. H.. 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284: 27384–27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shridas P., Bailey W. M., Talbott K. R., Oslund R. C., Gelb M. H., and Webb N. R.. 2011. Group X secretory phospholipase A2 enhances TLR4 signaling in macrophages. J. Immunol. 187: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng A. M., Handa P., Tateya S., Schwartz J., Tang C., Mitra P., Oram J. F., Chait A., and Kim F.. 2012. Apolipoprotein A-I attenuates palmitate-mediated NF-kappaB activation by reducing Toll-like receptor-4 recruitment into lipid rafts. PLoS One. 7: e33917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M., Zhao G. J., Yin K., Xia X. D., Gong D., Zhao Z. W., Chen L. Y., Zheng X. L., Tang X. E., and Tang C. K.. 2018. Apolipoprotein A-1 binding protein inhibits inflammatory signaling pathways by binding to apolipoprotein A-1 in THP-1 macrophages. Circ. J. 82: 1396–1404. [DOI] [PubMed] [Google Scholar]
- 54.Wu F. X., Bian J. J., Miao X. R., Huang S. D., Xu X. W., Gong D. J., Sun Y. M., Lu Z. J., and Yu W. F.. 2010. Intrathecal siRNA against Toll-like receptor 4 reduces nociception in a rat model of neuropathic pain. Int. J. Med. Sci. 7: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao L., Tanga F. Y., and Deleo J. A.. 2009. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 158: 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stokes J. A., Corr M., and Yaksh T. L.. 2013. Spinal toll-like receptor signaling and nociceptive processing: regulatory balance between TIRAP and TRIF cascades mediated by TNF and IFNbeta. Pain. 154: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stokes J. A., Cheung J., Eddinger K., Corr M., and Yaksh T. L.. 2013. Toll-like receptor signaling adapter proteins govern spread of neuropathic pain and recovery following nerve injury in male mice. J. Neuroinflammation. 10: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seretny M., Currie G. L., Sena E. S., Ramnarine S., Grant R., MacLeod M. R., Colvin L. A., and Fallon M.. 2014. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 155: 2461–2470. [DOI] [PubMed] [Google Scholar]
- 59.Park H. J., Stokes J. A., Corr M., and Yaksh T. L.. 2014. Toll-like receptor signaling regulates cisplatin-induced mechanical allodynia in mice. Cancer Chemother. Pharmacol. 73: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y., Zhang H., Zhang H., Kosturakis A. K., Jawad A. B., and Dougherty P. M.. 2014. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J. Pain. 15: 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi M., Konishi H., Sayo A., Takai T., and Kiyama H.. 2016. TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J. Neurosci. 36: 11138–11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poliani P. L., Wang Y., Fontana E., Robinette M. L., Yamanishi Y., Gilfillan S., and Colonna M.. 2015. TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125: 2161–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deming Y., Filipello F., Cignarella F., Cantoni C., Hsu S., Mikesell R., Li Z., Del-Aguila J. L., Dube U., Farias F. G., et al. 2019. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 11: eaau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sehgal P. B., Guo G. G., Shah M., Kumar V., and Patel K.. 2002. Cytokine signaling: STATS in plasma membrane rafts. J. Biol. Chem. 277: 12067–12074. [DOI] [PubMed] [Google Scholar]
- 65.Kim J. H., Choi D. J., Jeong H. K., Kim J., Kim D. W., Choi S. Y., Park S. M., Suh Y. H., Jou I., and Joe E. H.. 2013. DJ-1 facilitates the interaction between STAT1 and its phosphatase, SHP-1, in brain microglia and astrocytes: a novel anti-inflammatory function of DJ-1. Neurobiol. Dis. 60: 1–10. [DOI] [PubMed] [Google Scholar]
- 66.Tsuda M., Masuda T., Kitano J., Shimoyama H., Tozaki-Saitoh H., and Inoue K.. 2009. IFN-γ receptor signaling mediates spinal microglia activation driving neuropathic pain. Proc. Natl. Acad. Sci. USA. 106: 8032–8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allsopp R. C., Lalo U., and Evans R. J.. 2010. Lipid raft association and cholesterol sensitivity of P2X1-4 receptors for ATP: chimeras and point mutants identify intracellular amino-terminal residues involved in lipid regulation of P2X1 receptors. J. Biol. Chem. 285: 32770–32777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salter M. W., and Stevens B.. 2017. Microglia emerge as central players in brain disease. Nat. Med. 23: 1018–1027. [DOI] [PubMed] [Google Scholar]
- 69.Beggs S., Trang T., and Salter M. W.. 2012. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 15: 1068–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsuda M., Shigemoto-Mogami Y., Koizumi S., Mizokoshi A., Kohsaka S., Salter M. W., and Inoue K.. 2003. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 424: 778–783. [DOI] [PubMed] [Google Scholar]
- 71.Toyomitsu E., Tsuda M., Yamashita T., Tozaki-Saitoh H., Tanaka Y., and Inoue K.. 2012. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 8: 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Trang T., Beggs S., Wan X., and Salter M. W.. 2009. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 29: 3518–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonnord P., Delarasse C., Auger R., Benihoud K., Prigent M., Cuif M. H., Lamaze C., and Kanellopoulos J. M.. 2009. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 23: 795–805. [DOI] [PubMed] [Google Scholar]
- 74.Weinhold K., Krause-Buchholz U., Rodel G., Kasper M., and Barth K.. 2010. Interaction and interrelation of P2X7 and P2X4 receptor complexes in mouse lung epithelial cells. Cell. Mol. Life Sci. 67: 2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murrell-Lagnado R. D. 2017. Regulation of P2X purinergic receptor signaling by cholesterol. Curr. Top. Membr. 80: 211–232. [DOI] [PubMed] [Google Scholar]
- 76.Mapplebeck J. C. S., Dalgarno R., Tu Y., Moriarty O., Beggs S., Kwok C. H. T., Halievski K., Assi S., Mogil J. S., Trang T., et al. 2018. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain. 159: 1752–1763. [DOI] [PubMed] [Google Scholar]
- 77.Yu T., Zhang X., Shi H., Tian J., Sun L., Hu X., Cui W., and Du D.. 2019. P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis. 10: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi Z., Xie L., Zhou C., Yuan H., Ouyang S., Fang Z., Zhao S., Jia T., Zou L., Wang S., et al. 2018. P2Y12 receptor upregulation in satellite glial cells is involved in neuropathic pain induced by HIV glycoprotein 120 and 2′,3′-dideoxycytidine. Purinergic Signal. 14: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savi P., Zachayus J-L., Delesque-Touchard N., Labouret C., Hervé C., Uzabiaga M-F., and Herbert J-M.. 2006. The active metabolite of clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc. Natl. Acad. Sci. USA. 103: 11069–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rabani V., Montange D., Meneveau N., and Davani S.. 2018. Impact of ticagrelor on P2Y1 and P2Y12 localization and on cholesterol levels in platelet plasma membrane. Platelets. 29: 709–715. [DOI] [PubMed] [Google Scholar]
- 81.Burmeister A. R., Johnson M. B., Chauhan V. S., Moerdyk-Schauwecker M. J., Young A. D., Cooley I. D., Martinez A. N., Ramesh G., Philipp M. T., and Marriott I.. 2017. Human microglia and astrocytes constitutively express the neurokinin-1 receptor and functionally respond to substance P. J. Neuroinflammation. 14: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kondo I., Marvizon J. C., Song B., Salgado F., Codeluppi S., Hua X. Y., and Yaksh T. L.. 2005. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J. Neurosci. 25: 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tumati S., Largent-Milnes T. M., Keresztes A. I., Yamamoto T., Vanderah T. W., Roeske W. R., Hruby V. J., and Varga E. V.. 2012. Tachykinin NK(1) receptor antagonist co-administration attenuates opioid withdrawal-mediated spinal microglia and astrocyte activation. Eur. J. Pharmacol. 684: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monastyrskaya K., Hostettler A., Buergi S., and Draeger A.. 2005. The NK1 receptor localizes to the plasma membrane microdomains, and its activation is dependent on lipid raft integrity. J. Biol. Chem. 280: 7135–7146. [DOI] [PubMed] [Google Scholar]
- 85.Kim D., You B., Jo E. K., Han S. K., Simon M. I., and Lee S. J.. 2010. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA. 107: 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim H., Kim D., and Lee S. J.. 2013. Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J. Biol. Chem. 288: 7572–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dang P. M., Stensballe A., Boussetta T., Raad H., Dewas C., Kroviarski Y., Hayem G., Jensen O. N., Gougerot-Pocidalo M. A., and El-Benna J.. 2006. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Invest. 116: 2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vilhardt F., and van Deurs B.. 2004. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 23: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng G., and Lambeth J. D.. 2004. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J. Biol. Chem. 279: 4737–4742. [DOI] [PubMed] [Google Scholar]
- 90.Kawahara T., and Lambeth J. D.. 2008. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol. Biol. Cell. 19: 4020–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., Saglietti L., Schuchman E. H., Furlan R., Clementi E., et al. 2009. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J., Li X., Jiang X., Yang M., Yang R., Burnstock G., Xiang Z., and Yuan H.. 2017. Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal. 13: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verderio C., Muzio L., Turola E., Bergami A., Novellino L., Ruffini F., Riganti L., Corradini I., Francolini M., Garzetti L., et al. 2012. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 72: 610–624. [DOI] [PubMed] [Google Scholar]
- 94.Ibáñez F., Montesinos J., Ureña-Peralta J. R., Guerri C., and Pascual M.. 2019. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J. Neuroinflammation. 16: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim J. M., Cha S. H., Choi Y. R., Jou I., Joe E. H., and Park S. M.. 2016. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci. Rep. 6: 28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim K. S., Kim J. S., Park J-Y., Suh Y. H., Jou I., Joe E-H., and Park S. M.. 2013. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum. Mol. Genet. 22: 4805–4817. [DOI] [PubMed] [Google Scholar]
- 97.Park S. J., Kim H. Y., Kim H., Park S. M., Joe E. H., Jou I., and Choi Y. H.. 2009. Oxidative stress induces lipid-raft-mediated activation of Src homology 2 domain-containing protein-tyrosine phosphatase 2 in astrocytes. Free Radic. Biol. Med. 46: 1694–1702. [DOI] [PubMed] [Google Scholar]
- 98.Tian G., Kong Q., Lai L., Ray-Chaudhury A., and Lin C-G.. 2010. Increased expression of cholesterol 24S-hydroxylase results in disruption of glial glutamate transporter EAAT2 association with lipid rafts: a potential role in Alzheimer’s disease. J. Neurochem. 113: 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang J., Han D., Hong J., Tan Q., and Tian Y.. 2012. The chemokine, macrophage inflammatory protein-2gamma, reduces the expression of glutamate transporter-1 on astrocytes and increases neuronal sensitivity to glutamate excitotoxicity. J. Neuroinflammation. 9: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gosselin R. D., Meylan P., and Decosterd I.. 2013. Extracellular microvesicles from astrocytes contain functional glutamate transporters: regulation by protein kinase C and cell activation. Front. Cell. Neurosci. 7: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vance J. E., and Hayashi H.. 2010. Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta. 1801: 806–818. [DOI] [PubMed] [Google Scholar]
- 102.Hirsch-Reinshagen V., Zhou S., Burgess B. L., Bernier L., McIsaac S. A., Chan J. Y., Tansley G. H., Cohn J. S., Hayden M. R., and Wellington C. L.. 2004. Deficiency of ABCA1 impairs apolipoprotein E metabolism in brain. J. Biol. Chem. 279: 41197–41207. [DOI] [PubMed] [Google Scholar]
- 103.Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., and Holtzman D. M.. 2004. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 279: 40987–40993. [DOI] [PubMed] [Google Scholar]
- 104.Abildayeva K., Jansen P. J., Hirsch-Reinshagen V., Bloks V. W., Bakker A. H., Ramaekers F. C., de Vente J., Groen A. K., Wellington C. L., Kuipers F., et al. 2006. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281: 12799–12808. [DOI] [PubMed] [Google Scholar]
- 105.Spagnuolo M. S., Donizetti A., Iannotta L., Aliperti V., Cupidi C., Bruni A. C., and Cigliano L.. 2018. Brain-derived neurotrophic factor modulates cholesterol homeostasis and apolipoprotein E synthesis in human cell models of astrocytes and neurons. J. Cell. Physiol. 233: 6925–6943. [DOI] [PubMed] [Google Scholar]
- 106.Zelcer N., and Tontonoz P.. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu J., Feng Y-W., Liu L., Wang W., Zhong X-X., Wei X-H., and Liu X-G.. 2017. Liver X receptor α is involved in counteracting mechanical allodynia by inhibiting neuroinflammation in the spinal dorsal horn. Anesthesiology. 127: 534–547. [DOI] [PubMed] [Google Scholar]
- 108.Karasinska J. M., de Haan W., Franciosi S., Ruddle P., Fan J., Kruit J. K., Stukas S., Lutjohann D., Gutmann D. H., Wellington C. L., et al. 2013. ABCA1 influences neuroinflammation and neuronal death. Neurobiol. Dis. 54: 445–455. [DOI] [PubMed] [Google Scholar]
- 109.Liu B., He Z., Wang J., Xin Z., Wang J., Li F., and Fu Y.. 2018. Taraxasterol inhibits LPS-induced inflammatory response in BV2 microglia cells by activating LXRalpha. Front. Pharmacol. 9: 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu Y., Xin Z., Liu B., Wang J., Wang J., Zhang X., Wang Y., and Li F.. 2018. Platycodin D inhibits inflammatory response in LPS-stimulated primary rat microglia cells through activating LXRalpha-ABCA1 signaling pathway. Front. Immunol. 8: 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Baranova I., Vishnyakova T., Bocharov A., Chen Z., Remaley A. T., Stonik J., Eggerman T. L., and Patterson A. P.. 2002. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun. 70: 2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin K., Liao D. F., and Tang C. K.. 2010. ATP-binding membrane cassette transporter A1 (ABCA1): a possible link between inflammation and reverse cholesterol transport. Mol. Med. 16: 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zidovetzki R., and Levitan I.. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768: 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carneiro S. B., Costa Duarte F. I., Heimfarth L., Siqueira Quintans J. S., Quintans L. J. Jr., Veiga V. F. D. Jr., and Neves de Lima A. A.. 2019. Cyclodextrin-drug inclusion complexes: in vivo and in vitro approaches. Int. J. Mol. Sci. 20: E642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Oliveira S. B., Araya E. I., Gambeta E., Ferreira L. E. N., Franz-Montan M., Claudino R. F., and Chichorro J. G.. 2019. Comparison of antinociceptive effects of plain lidocaine versus lidocaine complexed with hydroxypropyl-beta-cyclodextrin in animal models of acute and persistent orofacial pain. Naunyn Schmiedebergs Arch. Pharmacol. 392: 573–583. [DOI] [PubMed] [Google Scholar]
- 116.Ory D. S., Ottinger E. A., Farhat N. Y., King K. A., Jiang X., Weissfeld L., Berry-Kravis E., Davidson C. D., Bianconi S., Keener L. A., et al. 2017. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet. 390: 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davidson J., Molitor E., Moores S., Gale S. E., Subramanian K., Jiang X., Sidhu R., Kell P., Zhang J., Fujiwara H., et al. 2019. 2-Hydroxypropyl-beta-cyclodextrin is the active component in a triple combination formulation for treatment of Niemann-Pick C1 disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 1864: 1545–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zimmer S., Grebe A., Bakke S. S., Bode N., Halvorsen B., Ulas T., Skjelland M., De Nardo D., Labzin L. I., Kerksiek A., et al. 2016. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8: 333ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Antonopoulos A. S., Margaritis M., Lee R., Channon K., and Antoniades C.. 2012. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr. Pharm. Des. 18: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng Y., Zhang X., Zhang T., Grace P. M., Li H., Wang Y., Li H., Chen H., Watkins L. R., Hutchinson M. R., et al. 2019. Lovastatin inhibits Toll-like receptor 4 signaling in microglia by targeting its co-receptor myeloid differentiation protein 2 and attenuates neuropathic pain. Brain Behav. Immun. 82: 432–444. [DOI] [PubMed] [Google Scholar]
- 121.Pathak N. N., Balaganur V., Lingaraju M. C., Kant V., Latief N., More A. S., Kumar D., Kumar D., and Tandan S. K.. 2014. Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochem. Int. 68: 1–9. [DOI] [PubMed] [Google Scholar]
- 122.Shi X. Q., Lim T. K., Lee S., Zhao Y. Q., and Zhang J.. 2011. Statins alleviate experimental nerve injury-induced neuropathic pain. Pain. 152: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 123.Kuipers H. F., Rappert A. A., Mommaas A. M., van Haastert E. S., van der Valk P., Boddeke H. W., Biber K. P., and van den Elsen P. J.. 2006. Simvastatin affects cell motility and actin cytoskeleton distribution of microglia. Glia. 53: 115–123. [DOI] [PubMed] [Google Scholar]
- 124.Kuipers H. F., Biesta P. J., Groothuis T. A., Neefjes J. J., Mommaas A. M., and van den Elsen P. J.. 2005. Statins affect cell-surface expression of major histocompatibility complex class II molecules by disrupting cholesterol-containing microdomains. Hum. Immunol. 66: 653–665. [DOI] [PubMed] [Google Scholar]
- 125.Ohkawa Y., Momota H., Kato A., Hashimoto N., Tsuda Y., Kotani N., Honke K., Suzumura A., Furukawa K., Ohmi Y., et al. 2015. Ganglioside GD3 enhances invasiveness of gliomas by forming a complex with platelet-derived growth factor receptor alpha and yes kinase. J. Biol. Chem. 290: 16043–16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ohmi Y., Tajima O., Ohkawa Y., Yamauchi Y., Sugiura Y., Furukawa K., and Furukawa K.. 2011. Gangliosides are essential in the protection of inflammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice. J. Neurochem. 116: 926–935. [DOI] [PubMed] [Google Scholar]
- 127.Kawashita E., Tsuji D., Kanno Y., Tsuchida K., and Itoh K.. 2016. Enhancement by uridine diphosphate of macrophage inflammatory protein-1 alpha production in microglia derived from Sandhoff disease model mice. JIMD Rep. 28: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim H. Y., Park S. J., Joe E. H., and Jou I.. 2006. Raft-mediated Src homology 2 domain-containing proteintyrosine phosphatase 2 (SHP-2) regulation in microglia. J. Biol. Chem. 281: 11872–11878. [DOI] [PubMed] [Google Scholar]
- 129.Beckmann N., Sharma D., Gulbins E., Becker K. A., and Edelmann B.. 2014. Inhibition of acid sphingomyelinase by tricyclic antidepressants and analogons. Front. Physiol. 5: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Patti G. J., Yanes O., Shriver L. P., Courade J. P., Tautenhahn R., Manchester M., and Siuzdak G.. 2012. Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat. Chem. Biol. 8: 232–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee Y. K., Kim H. L., Kim Y. L., and Im D. S.. 2007. Multiple actions of dimethylsphingosine in 1321N1 astrocytes. Mol. Cells. 23: 11–16. [PubMed] [Google Scholar]
- 132.Kobayashi Y., Kiguchi N., Maeda T., Ozaki M., and Kishioka S.. 2012. The critical role of spinal ceramide in the development of partial sciatic nerve ligation-induced neuropathic pain in mice. Biochem. Biophys. Res. Commun. 421: 318–322. [DOI] [PubMed] [Google Scholar]
- 133.Sorge R. E., Mapplebeck J. C., Rosen S., Beggs S., Taves S., Alexander J. K., Martin L. J., Austin J. S., Sotocinal S. G., Chen D., et al. 2015. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Díaz M., Fabelo N., Ferrer I., and Marín R.. 2018. “Lipid raft aging” in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer’s disease. Neurobiol. Aging. 67: 42–52. [DOI] [PubMed] [Google Scholar]
- 135.Galambos R. 1961. A glia-neural theory of brain function. Proc. Natl. Acad. Sci. USA. 47: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Baral P., Udit S., and Chiu I. M.. 2019. Pain and immunity: implications for host defence. Nat. Rev. Immunol. 19: 433–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez-Lizarbe S., Montesinos J., and Guerri C.. 2013. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J. Neurochem. 126: 261–273. [DOI] [PubMed] [Google Scholar]
- 138.Wang Y. C., Zhou Y., Fang H., Lin S., Wang P. F., Xiong R. P., Chen J., Xiong X. Y., Lv F. L., Liang Q. L., et al. 2014. Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann. Neurol. 75: 876–889. [DOI] [PubMed] [Google Scholar]
- 139.Shmuel-Galia L., Klug Y., Porat Z., Charni M., Zarmi B., and Shai Y.. 2017. Intramembrane attenuation of the TLR4-TLR6 dimer impairs receptor assembly and reduces microglia-mediated neurodegeneration. J. Biol. Chem. 292: 13415–13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schilling T., and Eder C.. 2010. Importance of lipid rafts for lysophosphatidylcholine-induced caspase-1 activation and reactive oxygen species generation. Cell. Immunol. 265: 87–90. [DOI] [PubMed] [Google Scholar]
- 141.Trudler D., Weinreb O., Mandel S. A., Youdim M. B., and Frenkel D.. 2014. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J. Neurochem. 129: 434–447. [DOI] [PubMed] [Google Scholar]