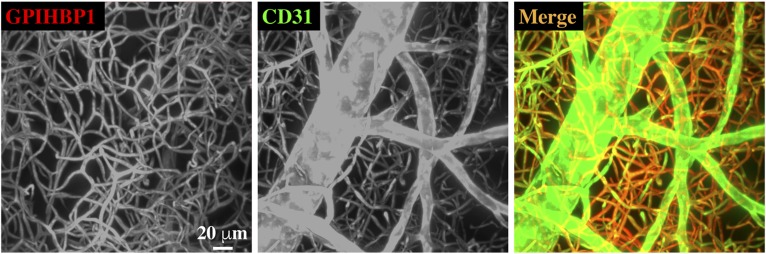
GPIHBP1, a GPI-anchored protein required for lipoprotein lipase (LPL)–mediated processing of triglyceride-rich lipoproteins (TRLs), is expressed by endothelial cells of capillaries but not the endothelial cells of larger blood vessels. Intravascular processing of TRLs provides lipid nutrients for the parenchymal cells of vital tissues (e.g., adipose tissue, heart, skeletal muscle). GPIHBP1 binds LPL with high affinity and stabilizes LPL structure and catalytic activity (1). GPIHBP1-bound LPL is also essential for the margination of TRLs along capillaries, allowing triglyceride hydrolysis to proceed (2). This image of mouse inguinal subcutaneous adipose tissue shows that GPIHBP1 (red) is found exclusively on capillary endothelial cells. A widely used marker of endothelial cells, CD31 (green), is found on endothelial cells of capillaries and larger blood vessels. In this study, a male C57BL/6J mouse was injected intravenously with 100 µg of an Alexa Fluor 568–labeled mouse GPIHBP1–specific monoclonal antibody (11A12) (3) and 50 µg of an Alexa Fluor 647–labeled mouse CD31–specific monoclonal antibody (BioLegend). The mouse was euthanized 30 min later, and the subcutaneous adipose tissue was harvested. The tissue was fixed in 1% paraformaldehyde and 10% sucrose, dehydrated with increasing concentrations of methanol, and then incubated with 2:1 (v/v) dichloromethane/methanol followed by 100% dichloromethane. Adipose tissue blocks were cleared with dibenzyl-ether and scanned by light sheet microscopy at 1 µm intervals with a LaVisionBiotec Ultramicroscope II. Reconstruction of the image stacks was performed with Imaris image analysis software. Projections of the image stacks (x, 258 µm; y, 258 µm; z, 500 µm) were used to create the image shown here. The fact that GPIHBP1 is expressed exclusively by endothelial cells of capillaries makes physiologic sense. The expression of GPIHBP1 in capillary endothelial cells is well suited for capturing the LPL that is secreted by adjacent parenchymal cells and for matching LPL-mediated triglyceride hydrolysis to the metabolic demands of those cells.
EQUIPMENT: Ultramicroscope II (LaVision Biotec)
REAGENTS: Alexa Fluor 568–labeled mouse GPIHBP1–specific monoclonal antibody (11A12) and Alexa Fluor 647–labeled mouse CD31–specific monoclonal antibody (BioLegend)
REFERENCES CITED
- 1.Young S. G., Fong L. G., Beigneux A. P., Allan C. M., He C., Jiang H., Nakajima K., Meiyappan M., Birrane G., and Ploug M.. 2019. GPIHBP1 and lipoprotein lipase, partners in plasma triglyceride metabolism. Cell Metab. 30: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goulbourne C. N., Gin P., Tatar A., Nobumori C., Hoenger A., Jiang H., Grovenor C. R., Adeyo O., Esko J. D., Goldberg I. J., et al. . 2014. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 19: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies B. S. J., Beigneux A. P., Barnes R. H. II, Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I. J., Olivecrona G., et al. . 2010. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]