Long-chain fatty acid (LCFA) transport is fundamental to human pathophysiology, and its impairment has been implicated in cardiovascular disease, cancer, and obesity-linked diabetes (1–4). Physiologically, LCFAs are an energy source, precursors to regulatory molecules, and components of complex lipids such as triacylglycerols (TAGs), phospholipids, and cholesteryl esters, which occur in plasma lipoproteins and living cells. Most physiological LCFAs contain 16 or 18 carbons with up to three double bonds (5, 6) and associate with lipid surfaces at diffusion-controlled rates with kon ~109 M−1sec−1. They spontaneously transfer as monomers between lipid surfaces and albumin at rates that increase with decreasing chain length with a t1/2 of ~5–200 msec (7). Lipoprotein turnover occurs with t1/2 ~3 to 5 days so that LCFA transfer is comparatively rapid.
Given that the destinations of plasma LCFAs are cells in plasma-perfused tissues, mechanisms by which the LCFAs enter cells are relevant to both normal human physiology and pathophysiology. The plasma membrane comprises a polarity gradient that is hydrocarbon-like at the midmost and polar near leaflet edges (Fig. 1). How LCFAs transfer between lipid surfaces separated by an aqueous phase is largely uncontroversial; LCFAs enter cells by diffusion to and insertion into the outer leaflet, translocation to the inner leaflet, and desorption into the cytoplasm. LCFA desorption occurs by the longitudinal movement of the carboxylate group, followed by the acyl chain. According to the principle of microscopic reversibility, the mechanisms for insertion (kon) and desorption (koff) are the same (8), so that the reverse process (kon), occurs by LCFA insertion into a membrane leaflet acyl chain first, followed by desorption of the anionic, charged form (9). However, the debate over the middle step—LCFA translocation from the outer to the inner leaflet (ktr)—continues.
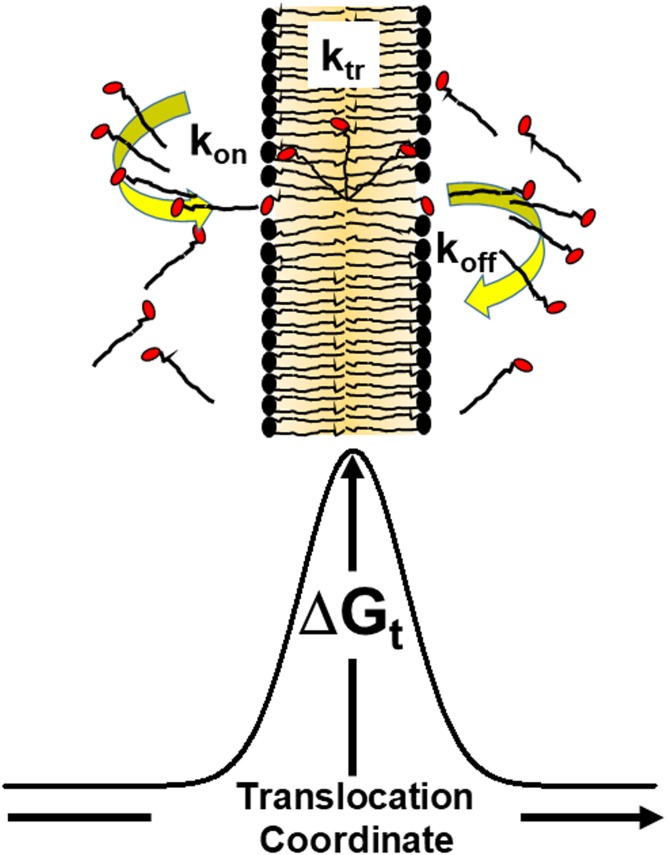
Fig. 1.
Model for LCFA transfer across phospholipid bilayer membranes. The plasma membrane has nonpolar hydrocarbons in the middle and is more polar near the leaflet edges. LCFAs (FA acyl chains are shown as black lines and carboxylate groups in red) diffuse to and insert into the outer leaflet and translocate to the inner leaflet from where they enter the cytoplasm.
The ongoing search for the LCFA translocation mechanisms was influenced by a precedent: glucose transport! Because it has many hydroxyl groups, glucose is insoluble in hydrocarbons and does not spontaneously translocate across the plasma membrane. Rather, its transporter, glucose transporter type 4 (GLUT4), carries glucose into cells, an important process in both adipose tissue and muscle (10). This analogy and the assumption that LCFAs are anionic (pKa ~5) have provoked a search for LCFA translocators. One of these, FA transport protein (FATP) was discovered by expression cloning, which identified proteins associated with the uptake and retention of a fluorescent FA (11). Hypothetically, this approach reveals all proteins with activities that convert LCFA to a form that is retained by the cell. Ultimately, FATP was identified as an acyl-CoA synthetase (12) that converts LCFA to its CoA analog, which does not pass through the plasma membrane because of the high polarity (13).
CD36, also known as FAT or scavenger receptor class B member 3 (SCARB3), has also been reported to be an LCFA translocator. CD36 is localized to the plasma membrane outer leaflet, and the mechanism by which it transfers LCFA to the inner leaflet adjacent to the cytoplasm is unknown. Several carefully conducted studies have reported that CD36 enhances cellular LCFA uptake (14–16), without providing a molecular mechanism. Subsequent studies have compared LCFA uptake by control HEK cells (17), in which LCFA metabolism is slower than LCFA translocation, with that of CD36-transfected HEK cells, and found identical rates of LCFA binding and translocation. However, they also revealed diversion of LCFA to TAG synthesis in CD36-expressing cells. This process supports a cellular LCFA concentration gradient of low intracellular and high extracellular LCFA concentration. In a broader context, this gradient could also be maintained by LCFA activation, incorporation into complex lipids, or β-oxidation; in other words, metabolism.
In this issue of the Journal of Lipid Research, Jay et al. tested three well-rationalized two-step models of cellular LCFA uptake. Two of these involved protein-based LCFA translocators; in the third, translocation occured solely via a transverse diffusion mechanism sometimes called flip-flop. The authors followed the movement of a natural LCFA (oleic acid) according to its binding to the cell membrane outer leaflet and its translocation to the inner leaflet. The authors monitored these processes by using dual fluorescence probes—acrylodan-labeled intestinal fatty acid-binding protein (ADIFAB) and 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), a pH sensor—that respond to each of these steps, respectively. Each of the aforementioned three models has a predicted distinctive profile for ADIFAB and BCECF kinetics. Only the diffusion model gave near-parallel kinetics in adipocytes, which contain putative LCFA transporters, and in protein-free phospholipid vesicles.
In control adipocytes, a decline in ADIFAB fluorescence paralleled the growth of BCECF fluorescence, indicating the concurrent disappearance of LCFA in the outer leaflet and external media and its appearance on the inner leaflet, respectively. According to the same fluorescence probes, LCFA uptake differed only slightly at 4°C and 37°C, whereas recovery of the intracellular pH was slower at the lower temperature, a finding consistent with the predicted slowing of intracellular LCFA metabolism at a reduced temperature. The authors complemented these analyses with tests of numerous inhibitors of cellular LCFA transport, and a similar but variable effect was observed with putative inhibitors of LCFA translocation, suggesting, but not proving, that reduced LCFA entry into the cell is due to inhibited metabolism. The LCFA uptake by protein-free vesicles was not affected, suggesting that the metabolic machinery of cells, and not uptake itself, is impaired by the inhibitors.
The authors’ other experiments focused on the chemistry of several inhibitors of LCFA translocation, notably sulfosuccinimidyl oleate (SSO), reported to highly specifically and competitively inhibit CD36-mediated LCFA transport (18). Previous studies with this molecule led to the original postulate that CD36 translocates LCFA. The experiments by Jay and colleagues revealed that SSO modified CD36 as expected; unexpectedly, however, SSO also modified numerous other proteins. The authors speculate that this occurs via SSO reaction with the ϵ-amino groups of lysine residues. Two other observations favor the hypothesis that the inhibitors suppress intracellular metabolism. First, all putative LCFA translocation inhibitors reduce the formation of intracellular TAG, the most likely product of LCFA-loaded adipocytes. Second, SSO reacts with many other cellular proteins in addition to CD36. Given the observed nonspecificity of SSO, some of these are likely involved in glycerolipid synthesis. Importantly, overexpression of either glycerol phosphate acyltransferase or lysoglycerol phosphate acyltransferase, enzymes that catalyze key steps in the synthesis of glycerolipids, including TAG, potentiates cellular LCFA uptake (19, 20).
At phospholipid interfaces, the pKa of LCFA is ~7 (21), meaning that half of it is in the protonated and uncharged form. Given that the major physicochemical barrier to LCFA translocation is the hydrocarbon interior of the lipid bilayer, it is intuitively satisfying that the diffusion model behaves according to the solubility of protonated LCFA in hydrocarbons (22, 23) (22, 23). In studies of cellular LCFA transport, there has been an ongoing debate between the “diffusionists” and the “translocationists” (14, 24–27) because mechanisms guide therapeutic strategies. Physiologically, uncontrolled LCFA movement into and out of cells would be expected to impair the cellular response to changing energy demands. Whereas a regulated translocator could be the needed controller, an alternative mechanism, which is supported by the Jay et al. paper, is that this can also be achieved by regulating the balance between intracellular TAG synthesis versus hydrolysis, which transfer LCFAs into or liberate LCFAs from fat droplets, respectively. The findings of this article do not prove that there are no FA translocators, but rather provide compelling support for a model of metabolism-driven translocation by diffusion.
Impaired FA uptake by adipose tissue and the resulting excess of plasma LCFA are mechanistically linked to impaired glucose disposal, a hallmark of type 2 diabetes (28). Proposals for therapeutic approaches that address this impairment differ between translocationists and diffusionists—translocationists would target the putative LCFA transporters. In the context of this paper, however, the diffusionists would target intracellular metabolism that consumes LCFA, notably, by oxidation, TAG synthesis (19, 20), and possibly the regulatory role of CD36. The paper by Jay and colleagues provides a compelling context for the development of these much-needed therapeutic approaches favored by the diffusionists.
Footnotes
The author declares no conflicts of interest with the contents of the article.
REFERENCES
- 1.Kris-Etherton P. M. 1999. AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation. 100: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 2.Simopoulos A. P. 1999. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 70: 560S–569S. [DOI] [PubMed] [Google Scholar]
- 3.Wilk J. B., Tsai M. Y., Hanson N. Q., Gaziano J. M., Djousse L.. 2012. Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am. J. Clin. Nutr. 96: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palomer X., Pizarro-Delgado J., Barroso E., Vazquez-Carrera M.. 2018. Palmitic and oleic acid: the yin and yang of fatty acids in type 2 diabetes mellitus. Trends Endocrinol. Metab. 29: 178–190. [DOI] [PubMed] [Google Scholar]
- 5.Abdelmagid S. A., Clarke S. E., Nielsen D. E., Badawi A., El-Sohemy A., Mutch D. M., Ma D. W.. 2015. Comprehensive profiling of plasma fatty acid concentrations in young healthy Canadian adults. PLoS One. 10: e0116195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pownall H. J., Reynaud A. S., Harper E., Choi S., Rohrbach K., Pao Q., Reeves R. S., and Gotto A. M.. Effects of 12 weeks of dietary fish oil, polyunsaturated fat, monounsaturated fat, and saturated fat on plasma lipoprotein structure and composition. Omega-3 Fatty Acids in Nutrition, Vascular Biology, and Medicine. 1994:263. American Heart Association, Dallas, TX.
- 7.Massey J. B., Bick D. H., Pownall H. J.. 1997. Spontaneous transfer of monoacyl amphiphiles between lipid and protein surfaces. Biophys. J. 72: 1732–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis G. N. 1925. A new principle of equilibrium. Proc. Natl. Acad. Sci. USA. 11: 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamp F., Hamilton J. A.. 1992. pH gradients across phospholipid membranes caused by fast flip-flop of un-ionized fatty acids. Proc. Natl. Acad. Sci. USA. 89: 11367–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James D. E., Brown R., Navarro J., Pilch P. F.. 1988. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 333: 183–185. [DOI] [PubMed] [Google Scholar]
- 11.Schaffer J. E., Lodish H. F.. 1994. Expression cloning and characterization of a novel adipocyte long fatty acid transport protein. Cell. 79: 427–436. [DOI] [PubMed] [Google Scholar]
- 12.Coe N. R., Smith A. J., Frohnert B. I., Watkins P. A., Bernlohr D. A.. 1999. The fatty acid transport protein (FATP1) is a very long chain acyl-CoA synthetase. J. Biol. Chem. 274: 36300–36304. [DOI] [PubMed] [Google Scholar]
- 13.Wolkowicz P. E., Pownall H. J., McMillin-Wood J. B.. 1982. (I-pyrenebutyryl)carnitine and 1-pyrenebutyryl coenzyme A: fluorescent probes for lipid metabolite studies in artificial and natural membranes. Biochemistry. 21: 2990–2996. [DOI] [PubMed] [Google Scholar]
- 14.Abumrad N. A., el-Maghrabi M. R., Amri E. Z., Lopez E., Grimaldi P. A.. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268: 17665–17668. [PubMed] [Google Scholar]
- 15.Luiken J. J., Glatz J. F., Bonen A.. 2000. Fatty acid transport proteins facilitate fatty acid uptake in skeletal muscle. Can. J. Appl. Physiol. 25: 333–352. [PubMed] [Google Scholar]
- 16.Glatz J. F., Vork M. M., Cistola D. P., van der Vusse G. J.. 1993. Cytoplasmic fatty acid binding protein: significance for intracellular transport of fatty acids and putative role on signal transduction pathways. Prostaglandins Leukot. Essent. Fatty Acids. 48: 33–41. [DOI] [PubMed] [Google Scholar]
- 17.Xu S., Jay A., Brunaldi K., Huang N., Hamilton J. A.. 2013. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry. 52: 7254–7261. [DOI] [PubMed] [Google Scholar]
- 18.Harmon C. M., Abumrad N. A.. 1993. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J. Membr. Biol. 133: 43–49. [DOI] [PubMed] [Google Scholar]
- 19.Ruan H., Pownall H. J.. 2001. Overexpression of 1-acyl-glycerol-3-phosphate acyltransferase-alpha enhances lipid storage in cellular models of adipose tissue and skeletal muscle. Diabetes. 50: 233–240. [DOI] [PubMed] [Google Scholar]
- 20.Igal R. A., Wang S., Gonzalez-Baro M., Coleman R. A.. 2001. Mitochondrial glycerol phosphate acyltransferase directs the incorporation of exogenous fatty acids into triacylglycerol. J. Biol. Chem. 276: 42205–42212. [DOI] [PubMed] [Google Scholar]
- 21.Doody M. C., Pownall H. J., Kao Y. J., Smith L. C.. 1980. Mechanism and kinetics of transfer of a fluorescent fatty acid between single-walled phosphatidylcholine vesicles. Biochemistry. 19: 108–116. [DOI] [PubMed] [Google Scholar]
- 22.Calvo B. C., Collado I., Cepeda E. A.. 2009. Solubilities of palmitic acid in pure solvents and its mixtures. J. Chem. Eng. Data. 54: 64–68. [Google Scholar]
- 23.Smith R., Tanford C.. 1973. Hydrophobicity of long chain n-alkyl carboxylic acids, as measured by their distribution between heptane and aqueous solutions. Proc. Natl. Acad. Sci. USA. 70: 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pownall H., Moore K.. 2014. Commentary on fatty acid wars: the diffusionists versus the translocatists. Arterioscler. Thromb. Vasc. Biol. 34: e8–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonen A., Chabowski A., Luiken J. J., Glatz J. F.. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda). 22: 15–29. [DOI] [PubMed] [Google Scholar]
- 26.Glatz J. F. C., Luiken J.. 2018. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J. Lipid Res. 59: 1084–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton J. A. 2007. New insights into the roles of proteins and lipids in membrane transport of fatty acids. Prostaglandins Leukot. Essent. Fatty Acids. 77: 355–361. [DOI] [PubMed] [Google Scholar]
- 28.McGarry J. D. 1992. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 258: 766–770. [DOI] [PubMed] [Google Scholar]