When making treatment decisions for children with cancer, many parents and physicians prioritize avoidance of certain late effects over an increased chance of cure.
Abstract
Video Abstract
BACKGROUND:
More than 80% of children with cancer become long-term survivors, yet most survivors experience late effects of treatment. Little is known about how parents and physicians consider late-effects risks against a potential survival benefit when making treatment decisions.
METHODS:
We used a discrete choice experiment to assess the importance of late effects on treatment decision-making and acceptable trade-offs between late-effects risks and survival benefit. We surveyed 95 parents of children with cancer and 41 physicians at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center to assess preferences for 5 late effects of treatment: neurocognitive impairment, infertility, cardiac toxicity, second malignancies, and impaired growth and development.
RESULTS:
Each late effect had a statistically significant association with treatment choice, as did survival benefit (P < .001). Avoidance of severe cognitive impairment was the most important treatment consideration to parents and physicians. Parents also valued cure and decreased risk of second malignancies; physician decision-making was driven by avoidance of second malignancies and infertility. Both parents and physicians accepted a high risk of infertility (parents, a 137% increased risk; physicians, an 80% increased risk) in exchange for a 10% greater chance of cure.
CONCLUSIONS:
Avoidance of severe neurocognitive impairment was the predominant driver of parent and physician treatment preferences, even over an increased chance of cure. This highlights the importance of exploring parental late-effects priorities when discussing treatment options.
What’s Known on This Subject:
Decision-making for pediatric cancer treatment involves numerous considerations, including optimizing cure and minimizing acute and late toxicities of treatment. Questions remain about how parents and physicians weigh late-effects risks and make trade-offs between cure and potential toxicities.
What This Study Adds:
Parents and physicians prioritize avoidance of certain late effects, specifically severe neurocognitive impairment, over an increased chance of cure. Both parents and physicians are willing to accept a high risk of infertility in exchange for an increased chance of cure.
Currently, >80% of children with cancer will become long-term survivors.1 However, nearly all adult survivors of childhood cancer experience a chronic health condition as a result of cancer or its treatment, and almost one-third experience a severe or life-threatening condition.2–4 These late effects have a profound impact on long-term health and quality of life.5
Given the prevalence and impact of late effects, pediatric oncologists employ multiple strategies to minimize the long-term toxicities of treatment, including risk-adapting therapy to reduce treatment of malignancies with excellent chance of cure.6,7 For example, current treatment strategies for Hodgkin’s lymphoma employ radiation sparingly to minimize the long-term risks of second malignancies and cardiac toxicity even if this increases the risk of recurrence.6,8,9 Authors of recent and ongoing studies are investigating the impact of reduction of therapy on the incidence of late effects and risk of relapse in other pediatric cancers.
These trends in research and clinical care suggest that physicians may accept an increased risk of relapse in exchange for a decreased risk of certain late effects. However, we know little about parent perspectives on acceptable trade-offs between late-effects risks and cure. Although many adults facing serious illness consider impaired function and cognition to be of greater concern than mortality,10 parents may feel differently about their children. Furthermore, parents and providers have distinct preferences when considering impairment associated with neonatal care decisions.11,12
In this study, we assessed how parents and physicians weigh late-effects risks versus survival benefits when making cancer treatment decisions. Discrete choice experiment (DCE) is a stated preference method of measuring decision-making preferences and trade-offs through hypothetical scenarios.13 DCEs have been increasingly used to characterize patient preferences in cancer treatment decision-making.14,15 We used a DCE to understand parent and physician preferences and to quantify the relative importance of 5 late effects of pediatric cancer therapy on treatment decision-making. We hypothesized that most parents and physicians would tolerate a high likelihood of late effects in exchange for an increased chance of cure.
Methods
Study Participants
We surveyed parents of children with cancer at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center between November 2016 and June 2018. Parents were eligible to participate if their child was receiving initial cancer-directed therapy and it was within a year of their child's oncologic diagnosis. Additional parent eligibility criteria included a child ≤18 years of age, parent ability to read English, and permission to approach granted by the child’s primary oncologist. Parents of children with no chance of cure as determined by the child’s oncologist were ineligible because this study was focused on long-term outcomes of treatment. Eligible parents were approached in person during clinic visits or inpatient hospital stays and were given a letter describing participation. One parent per child completed the survey. Parent surveys were administered electronically on tablet computers; participating parents received a $20 gift card as a token of appreciation.
Pediatric oncology physicians were surveyed from August 2017 to April 2018. Eligible physicians included fellows and faculty who practice clinical oncology at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center; physicians who participated in pilot testing were ineligible for study participation. Eligible physicians received an e-mail describing the survey with an online link for survey completion. Physicians who did not respond received up to 2 reminder e-mails. Physician participants received a $5 gift card in thanks. The institutional review board of the Dana-Farber Cancer Institute approved this study; documentation of informed consent was waived according to the institutional review board’s recommendation.
DCE
We used a DCE to assess parent and physician treatment preferences. DCEs provide a systematic way of understanding preferences by quantifying the relative importance of each element of a particular choice and can thereby identify acceptable trade-offs between risks and benefits of treatments.13,16 In DCEs, options are characterized by using distinct attributes, each with different levels. Respondents choose their preferred treatment from pairs of hypothetical options that vary across attributes and levels. Data generated from respondents who complete a series of treatment pairings are used to estimate the strength of preferences for each attribute.17
Using best practice guidelines for constructing and analyzing DCEs,13,17,18 we sought to understand how parents and physicians value late-effects risks and likelihood of cure when making treatment decisions. We evaluated 5 specific late effects: neurocognitive impairment, infertility, cardiac toxicity, second malignancies, and impaired growth and development. These late effects were selected on the basis of a literature review and interviews with survivorship experts as common late effects and ones about which parents have the most questions.4,19–22 For infertility, cardiac toxicity, second malignancies, and impaired growth and development, the levels were defined as the numeric risk of the late effect occurring; we evaluated 2 levels for each of these attributes: a low risk and an elevated risk. We assessed 3 levels of neurocognitive impairment (none, mild, and severe) because pretesting revealed that qualitative descriptors were more clinically salient for this late effect and because these levels of disability have more standard definitions.23,24 The levels for each attribute reflect previously described risks associated with standard treatment regimens for common pediatric malignancies (Supplemental Table 5)20,24–29; physicians assessed the relevance of the levels to clinical practice. Each late effect was defined, and its potential impact described, by using language targeted toward an eighth-grade reading level. Chance of cure was used for the measure of benefit to simplify the probability task and because different pediatric malignancies vary considerably in the chance of cure in relapsed disease. A baseline cure rate of 80% was selected to approximate current long-term cure rates across pediatric malignancies.1
The parent instrument was pilot tested in cognitive interviews with 12 parents to assess face and content validity and was iteratively revised. Pilot testing was used to evaluate the clarity and salience of the DCE attribute levels and descriptions, ease of completion, and the number of acceptable tasks. The physician survey was similarly pilot tested with 10 physicians.
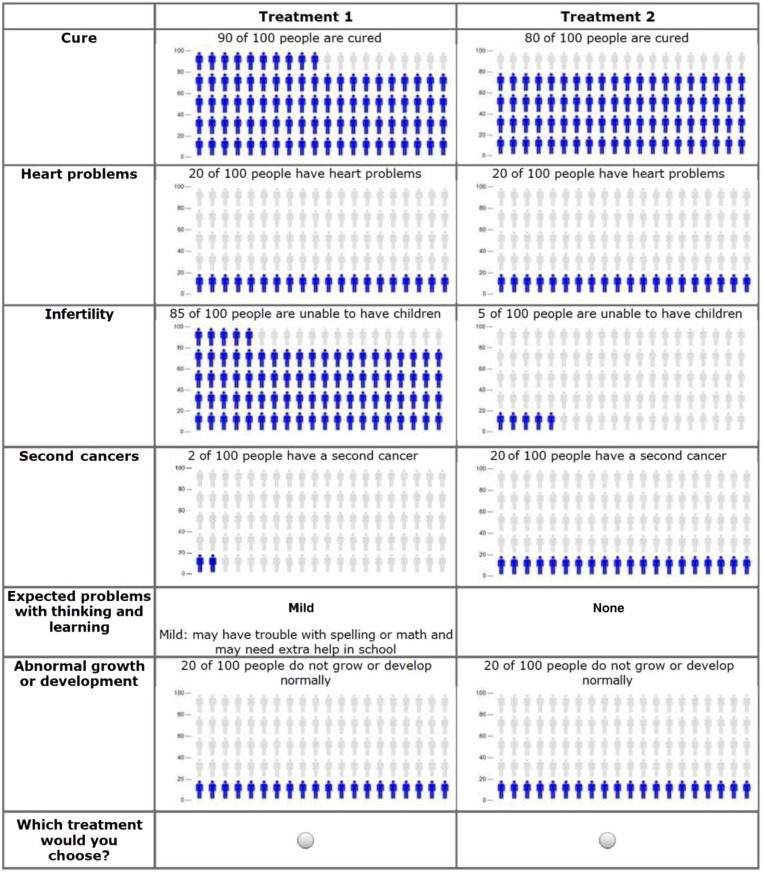
In the final DCE, each parent or physician selected their preferred treatment from 8 choice pairings, each pair varying across 6 attributes (5 late effects plus the chance of cure; Fig 1). Parent and physician DCE tasks were the same aside from introductory language; parents were asked to imagine making a treatment choice for their child, whereas physicians were asked to make a choice for a “newly diagnosed patient.” By employing a balanced-overlap experimental design selected to optimize D-efficiency, balance, and orthogonality,18 there were 20 survey versions, each with 8 tasks. Study versions were randomly assigned to participants by using Lighthouse Studio (Sawtooth Software, Inc, Orem, UT).
FIGURE 1.
Sample parent DCE task.
Survey Instrument
To determine how DCE preferences varied by parent and patient characteristics, parent instruments included 39 standard survey questions in addition to the 8 choice tasks. Parent surveys included previously developed and tested items to assess prognostic understanding, perceived late-effects risks, and information and communication preferences.30–33 Parent attributes, including sex, age, educational level, and race and/or ethnicity, were obtained by a questionnaire. Child characteristics, including age and diagnosis, were obtained by a chart review. The full parent instrument took 20 to 25 minutes to complete. The physician instrument included 11 standard survey items to assess physician characteristics plus the 8 DCE choice tasks and took 8 to 10 minutes to complete.
Statistical Analysis
Parent and physician responses were analyzed separately. Descriptive statistics were used to summarize parent and physician responses to standard survey items. For the DCE, conditional logit models were used to estimate parents’ and physicians’ preference weights of the relative importance of each attribute level on treatment choice. A higher preference weight for a given attribute level indicates a greater likelihood of selecting a treatment that contains that attribute level, assuming other factors being the same. The magnitude of the regression coefficient reflects the relative importance of that factor to treatment choice, with negative numbers indicating less-preferred options. All preference weights were estimated on a uniform scale, allowing direct comparison across attributes. Odds ratios (ORs) indicating the preference for each of the attribute levels relative to the mean effect were calculated by using effects coding.
We conducted post hoc subgroup analyses to determine if prognostic understanding, information preferences, and demographic factors were associated with systematic differences in parent preferences. Hierarchical Bayesian analysis was used to obtain individual utility scores, which were summarized by group by using means and SD. Comparisons of utility scores between groups were performed by using t tests and analysis of variance.
Maximum acceptable risk (MAR) measures the level of risk for a specific late effect that a respondent would be willing to accept in exchange for a treatment benefit. We calculated the MAR for each late effect relative to a 10% increase in chance of cure. MAR was calculated as the average utility of cure divided by the utility per 1% increase in late-effect probability. Variance of the MAR was estimated by using the δ method. Neurocognitive impairment was omitted from the MAR analysis because the numerical risk of neurocognitive impairment was not assessed in the DCE.
Descriptive and comparative analyses were performed by using SAS version 9.4 (SAS Institute, Inc, Cary, NC). DCE models were performed by using Lighthouse Studio (Sawtooth Software, Inc). Two-sided P values <.05 were considered statistically significant.
Results
Eighty-two percent (96 of 117) of eligible parents approached for participation completed the survey. Sixty-five percent (42 of 65) of eligible physicians completed the physician survey. Parents who declined participation (n = 21) had children with similar ages and diagnoses as participating parents; physicians who declined (n = 23) had similar sex and role characteristics as participating physicians. One parent and 1 physician were excluded from the current analysis because they did not complete the DCE; thus, 95 parents and 41 physicians were included in the analytic cohort (Table 1). Participating parents were predominantly female (74%), white (85%), and highly educated (76% with college degrees). Physician participants were mostly attending physicians (80%) with a range of years of experience and varied pediatric oncology practice areas.
TABLE 1.
Characteristics of Participating Parents and Their Children (N = 95) and Physicians (N = 41)
| Characteristics | Results |
|---|---|
| Parent, n (%) | |
| Sex | |
| Female | 70/95 (74) |
| Male | 25/95 (26) |
| Race | |
| White | 80/94 (85) |
| African American | 3/94 (3) |
| Asian American or Pacific Islander | 8/94 (9) |
| Other | 3/94 (3) |
| Ethnicity | |
| Hispanic | 8/94 (9) |
| Education | |
| Less than college graduatea | 23/95 (24) |
| College graduate | 44/95 (46) |
| Graduate or professional school | 28/95 (29) |
| Child | |
| Age at diagnosis, y, n (%) | |
| 0–2 | 17/95 (18) |
| 3–6 | 37/95 (39) |
| 7–12 | 23/95 (24) |
| 13–18 | 18/95 (19) |
| Sex, n (%) | |
| Male | 53/95 (56) |
| Female | 42/95 (44) |
| Diagnosis, n (%) | |
| Hematologic malignancy | 52/95 (55) |
| Extracranial solid tumor | 32/95 (34) |
| Brain tumor | 11/95 (12) |
| Days from diagnosis to survey, median (range) | 140 (29–365) |
| Physician, n (%) | |
| Sex | |
| Female | 24/41 (59) |
| Male | 17/41 (41) |
| Clinical role | |
| Fellow | 8/41 (20) |
| Attending | 33/41 (80) |
| Clinical effort, n (%) | |
| 0%–30% | 23/41 (56) |
| 30%–50% | 9/41 (22) |
| >50% | 9/41 (22) |
| Years in practice (years post medical school graduation), n (%) | |
| <5 | 2/41 (5) |
| 5–10 | 19/41 (46) |
| 11–20 | 13/41 (32) |
| >20 | 7/41 (17) |
| Clinical specialty area (select all that apply), n (%) | |
| Hematologic malignancy | 25/41 (61) |
| Extracranial solid tumor | 21/41 (51) |
| Brain tumor | 13/41 (32) |
| Stem cell transplant | 13/41 (32) |
| Survivorship | 2/41 (5) |
Less than college graduate includes eighth grade or less, high school graduate or equivalent, some college, or technical school.
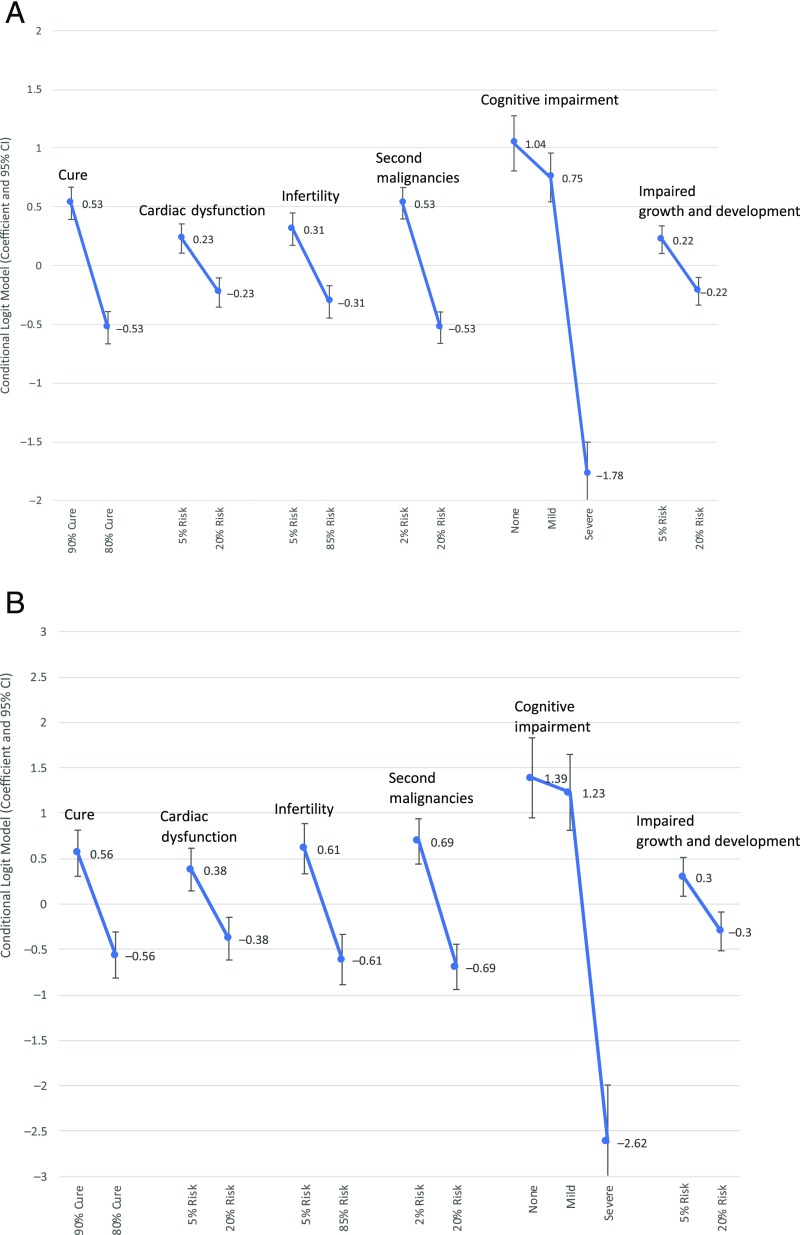
Fig 2 A and B depict the estimated preference weights and associated 95% confidence intervals for parents and physicians, respectively. Parents and physicians preferred the better clinical outcome, increased chance of cure or decreased probability of late effect, across all attributes. Avoidance of severe cognitive impairment was the most important factor to parents (preference weight −1.78 for severe impairment), followed by cure (preference weight −0.53 for 80% chance of cure), and decreased risk of second malignancies (preference weight −0.53 for 20% risk of second malignancy). The most important factors driving physician decision-making were avoidance of severe neurocognitive impairment (preference weight −2.62), avoidance of second malignancies (preference weight −0.69), and risk of infertility (preference weight −0.61 for 85% risk of infertility).
FIGURE 2.
Parent and physician preference weights. Error bars denote the width of the 95% confidence interval (CI). A, Parent preference weights by using the conditional logit model (N = 95). B, Physician preference weights by using the conditional logit model (N = 41).
Each attribute had a statistically significant association with parent and physician treatment choice (Table 2). Parent participants were significantly more likely to choose a treatment with no or mild neurocognitive impairment compared with one that caused severe impairment (OR = 2.83 [P < .001] for none versus severe impairment), as were physicians (OR = 4.01; P < .001).
TABLE 2.
Impact of Late Effects on Parent (N = 95) and Physician (N = 41) Treatment Preferences
| Late Effect | Parents | Physicians | ||||||
|---|---|---|---|---|---|---|---|---|
| Preference Wt | SE | OR (95% CI) | P | Preference Wt | SE | OR (95% CI) | P | |
| Cure | ||||||||
| 80% cure | −0.53 | 0.07 | 0.59 (0.51–0.67) | <.001 | −0.56 | 0.13 | 0.57 (0.44–0.74) | <.001 |
| 90% cure | 0.53 | 0.07 | 1.7 (1.48–1.95) | <.001 | 0.56 | 0.13 | 1.75 (1.36–2.25) | <.001 |
| Cardiac toxicity | ||||||||
| 5% risk | 0.23 | 0.06 | 1.26 (1.11–1.42) | <.001 | 0.38 | 0.12 | 1.47 (1.16–1.86) | <.001 |
| 20% risk | −0.23 | 0.06 | 0.79 (0.7–0.9) | <.001 | −0.38 | 0.12 | 0.68 (0.54–0.86) | <.001 |
| Infertility | ||||||||
| 5% risk | 0.31 | 0.07 | 1.37 (1.19–1.57) | <.001 | 0.61 | 0.14 | 1.84 (1.39–2.43) | <.001 |
| 85% risk | −0.31 | 0.07 | 0.73 (0.64–0.84) | <.001 | −0.61 | 0.14 | 0.54 (0.41–0.72) | <.001 |
| Second malignancy | ||||||||
| 2% risk | 0.53 | 0.07 | 1.7 (1.48–1.94) | <.001 | 0.69 | 0.13 | 2 (1.56–2.56) | <.001 |
| 20% risk | −0.53 | 0.07 | 0.59 (0.52–0.67) | <.001 | −0.69 | 0.13 | 0.5 (0.39–0.64) | <.001 |
| Neurocognitive impairment | ||||||||
| None | 1.04 | 0.12 | 2.83 (2.23–3.58) | <0.001 | 1.39 | 0.23 | 4.01 (2.58–6.24) | <.001 |
| Mild | 0.75 | 0.11 | 2.11 (1.71–2.59) | <.001 | 1.23 | 0.21 | 3.43 (2.26–5.21) | <.001 |
| Severe | −1.78 | 0.14 | 0.17 (0.13–0.22) | <.001 | −2.62 | 0.32 | 0.07 (0.04–0.14) | <.001 |
| Abnormal growth and development | ||||||||
| 5% risk | 0.22 | 0.06 | 1.24 (1.11–1.4) | <.001 | 0.30 | 0.11 | 1.35 (1.09–1.67) | <.001 |
| 20% risk | −0.22 | 0.06 | 0.8 (0.71–0.9) | <.001 | −0.30 | 0.11 | 0.74 (0.6–0.92) | <.001 |
Estimated preference weights, SEs, ORs and corresponding 95% CIs, and P values for each late effect by using the conditional logit model. P values were estimated by assuming a normal distribution. CI, confidence interval.
Post hoc exploratory analyses of differences in parental preferences between groups by using individual utility scores from the hierarchical Bayesian model were conducted to evaluate the strength of preference for an improved chance of cure and avoidance of neurocognitive impairment. DCE findings were similar regardless of parent sex, perceived prognosis, child age, child diagnosis, parent education, time since diagnosis, and race and/or ethnicity (Table 3).
TABLE 3.
Post Hoc Exploratory Analysis for Differences in Parent Preferences (Utility Scores) Between Groups (N = 95)
| Parent Characteristic | n (%) | 90% Cure, Mean Utility Score (SD) | P | No Cognitive Impairment, Mean Utility Score (SD) | P |
|---|---|---|---|---|---|
| Sex | .98 | .89 | |||
| Female | 70 (74) | 47.7 (24.2) | 92.4 (39.4) | ||
| Male | 25 (26) | 47.9 (17) | 91.1 (42) | ||
| Parent perception of prognosis | .87 | .51 | |||
| ≥75% chance of cure | 81 (87) | 48 (23) | 91.1 (42.6) | ||
| <75% chance of cure | 12 (13) | 46.9 (20.9) | 99.4 (17.4) | ||
| Preferred timing of late-effects information | .14 | .75 | |||
| In initial diagnosis and treatment discussions | 68 (72) | 45.6 (22.1) | 92.9 (41.3) | ||
| Not in initial diagnosis and treatment discussions | 27 (28) | 53.2 (22.7) | 90 (36.8) | ||
| Distress associated with late-effects information | .72 | .48 | |||
| Extremely or very upsetting | 62 (67) | 47 (20.9) | 95 (35.5) | ||
| Not extremely or very upsetting | 31 (33) | 48.7 (23.4) | 89.1 (43.3) | ||
| College graduate | .06 | .48 | |||
| Yes | 72 (76) | 50.2 (23.7) | 93.7 (39.9) | ||
| No | 23 (24) | 40.1 (15.9) | 87 (40.4) | ||
| Child age, y | .47 | .68 | |||
| 0–6 | 54 (57) | 46.4 (23.7) | 89.7 (42.9) | ||
| 7–12 | 23 (24) | 52.8 (26) | 98.5 (31.3) | ||
| 13–18 | 18 (19) | 45.5 (10.7) | 90.9 (41.5) | ||
| Diagnosis | .1 | .66 | |||
| Brain tumor | 11 (12) | 38.9 (17.1) | 102 (14.3) | ||
| Extracranial solid tumor | 32 (34) | 43.8 (19.2) | 92.4 (37.6) | ||
| Hematologic malignancy | 52 (55) | 52.1 (24.5) | 89.8 (44.8) | ||
| Race and/or ethnicity | .41 | .25 | |||
| White non-Hispanic | 73 (78) | 46.7 (21.4) | 95.1 (38.6) | ||
| Other race and/or ethnicity | 20 (22) | 51.4 (26.7) | 83.5 (43.3) | ||
| Time from diagnosis to survey completion, d | .6 | .8 | |||
| First 100 | 20 (21) | 44.4 (24.6) | 93.9 (29.8) | ||
| >100 | 75 (79) | 47.6 (23.4) | 91.6 (39.5) | ||
| Recall of discussion of risk of cognitive impairment | .8 | ||||
| Yes | 28/94 (30) | — | 90.7 (33.7) | ||
| No or do not remember | 66/94 (70) | — | 92.3 (39.5) | ||
| Perceived likelihood of cognitive impairment | — | .052 | |||
| Extremely or very | 10/93 (11) | — | 71.8 (49.6) | ||
| Somewhat | 21/93 (23) | — | 85.3 (33.8) | ||
| A little or not at all | 62/93 (67) | — | 98.9 (34.1) |
Mean and SD hierarchical Bayesian utility scores were compared between groups by using the t test or analysis of variance. —, not applicable.
MAR estimates for late effects relative to chance of cure revealed that both parents and physicians were willing to accept a substantially increased risk of infertility (137% increase for parents and 80% increase for physicians) in exchange for a 10% increased chance of cure (Table 4). Parents and physicians were least willing to trade an increased chance of a second malignancy for an improved chance of cure (parents: 18% increased chance of second cancers; physicians: 15% increased chance of second cancers).
TABLE 4.
MAR of Late Effects Parents and Physicians Would Accept in Exchange for a 10% Increased Chance of Cure, With SD
| Average Utilities (Zero-Centered Differences) | Parents’ MAR, % (SD) | Physicians’ MAR, % (SD) |
|---|---|---|
| Cardiac toxicity | 31 (15) | 22 (9) |
| Infertility | 137 (69) | 80 (32) |
| Second malignancy | 18 (9) | 15 (6) |
| Impaired growth and development | 38 (19) | 34 (14) |
Discussion
Although more and more children are cured of their malignancies, that cure comes at a cost to long-term health and quality of life.2–5 As a result, oncologists increasingly design clinical trials with dual goals of optimizing cure while minimizing late effects. In doing so, they make judgments about the relative value of short- and long-term outcomes in patients’ lives. Yet oncologists have largely done so in the absence of information about how parents prioritize avoidance of late effects relative to the chance of cure. In this study, we found that high risks of late effects of pediatric cancer therapy influenced parent and physician treatment preferences. Parents and physicians accepted a decreased chance of cure to avoid severe neurocognitive impairment. Conversely, parents and physicians accepted a high risk of infertility in exchange for an increased chance of cure.
These findings are consistent with those of previous studies that revealed that patients and parents value cognitive and quality-of-life outcomes in decision-making for treatment of serious illness.10,34 Most adults with serious illnesses would not select a life-extending treatment option if it came with severe cognitive or functional impairment.10 Similarly, avoidance of significant neurocognitive disability drives treatment decision-making in pediatric medulloblastoma.23 Although our study included a small number of patients with brain tumors, who face particular risks of neurocognitive impairment from their tumors and treatment, survivors of other childhood cancers, namely acute lymphoblastic leukemia, also face substantial risks of impaired cognition.35 Interestingly, this preference held regardless of patient cancer diagnosis, although there was a trend toward those who perceived their children to be at greatest risk of cognitive impairment placing less value on avoidance of neurocognitive impairment.
There were some notable differences between parent and physician preferences. Whereas parents considered optimizing a chance of cure to be the second most important priority, physicians prioritized cure after risk of neurocognitive impairment, second malignancies, and infertility. It is possible that a 10% increased chance of cure may not be as clinically salient to physicians as to parents, such that physicians would more heavily weigh chance of cure if there were a larger difference between treatment options. However, we selected the 10% difference to approximate real-world treatment choices, and pilot testing revealed it was meaningful to parents and physicians alike. Alternatively, as in previous studies of neonatal outcomes, parents and physicians may have distinct perceptions of the impact of impairment associated with care decisions.11,12 Concern for infertility played a bigger role in physician choices than it did for parents; parental acceptance of potential infertility in exchange for cure aligns with previous research.36
The importance of late-effects risks to parental treatment decision-making has implications for the approach to individual treatment conversations and recommendations. Parents of children who are seriously ill consider making informed medical decisions to be one of the primary components of being a good parent.37 Although we did not evaluate if parent participants felt they faced a treatment choice for their child, previous work has demonstrated that most parents of children with cancer deliberate over a treatment decision.36 Some childhood malignancies have more than one therapeutic option, and others may present a choice between the standard of care and a clinical trial. In these instances, elicitation of parent late-effects priorities and provision of information about late effects can support informed treatment decision-making. For other cancers, oncologists may present a single standard of care. Parents who do not have a choice between treatment options still value involvement in the deliberation phase of decision-making, including receiving and processing information and identifying preferences.38 Therefore, providing high-quality information about potential late effects meets parents’ stated information preferences,36,39 supports parent participation in decision-making, and helps them fulfill their desired parental roles. Although multipage informed consent documents typically list risks of late effects, late effects are generally not discussed at length in informed consent discussions.40 To our knowledge, there are currently no validated tools to solicit parental treatment preferences. Future researchers should explore the best ways to provide late-effects information, with consideration of tools that support information delivery and potentially use DCE to identify individual families’ long-term treatment priorities.
Our study should be interpreted in the context of limitations. DCE is a powerful tool to understand preferences in hypothetical treatment scenarios, but we do not know if our findings would be replicated in actual treatment decisions. We included parents of children facing various pediatric oncology diagnoses with distinct treatment regimens and associated risks of late effects, but the strength of parental preferences may differ when facing particular treatment plans with known risks of late effects. We evaluated preferences for a select number of late effects that were chosen on the basis of their prevalence and importance to families and physicians; additional late effects can be evaluated in future studies. To best elicit preferences and trade-offs, and because decisions to forgo all treatment are rare in pediatric oncology, we did not include a “no treatment” choice in our DCE. Future work to investigate treatment trade-offs in specific pediatric malignancies, particularly those that may include an option for observation only, would be of interest.
We surveyed parents of children currently receiving cancer-directed therapy, which is typically before experiencing late effects; parents of survivors and survivors themselves may have different perceptions on the tolerability of late effects. However, we selected this population to understand the perspectives of parents who recently engaged in treatment decision-making and because parents typically make treatment decisions for their children. Child and adolescent patient and survivor perspectives on treatment decision-making and late-effects tolerability merit further exploration. The generalizability of our results may be somewhat restricted by our single-site sample with limited racial and educational diversity, and we had limited power to conduct subgroup analyses. However, post hoc analyses suggest no differences in parent preferences based on race and/or ethnicity or educational attainment.
Conclusions
As the population of pediatric cancer survivors grows, the quality of survivorship becomes ever more important. Efforts to decrease the long-term toxicities of treatment must be informed by physician perception of intolerable toxicities and by patient and family values. In this study, we found much alignment between parent and physician priorities (particularly around avoidance of neurocognitive impairment and second malignancies) but also important areas of distinction (namely, different acceptable trade-offs for risk of infertility). This nuanced understanding of parental late-effects priorities can help physicians provide the necessary information and support for parent participation in treatment decision-making and may be used to determine priority areas for reductions of late-effects risks in clinical trials. Although cure comes at a cost, some costs may be too excessive to bear.
Acknowledgments
We thank all the parents and physicians who participated in this study for sharing their time and perspectives. We also thank Brett Hauber, PhD, and F. Reed Johnson, PhD, for their help with the MAR analysis.
Glossary
- DCE
discrete choice experiment
- MAR
maximum acceptable risk
- OR
odds ratio
Footnotes
Dr Greenzang obtained funding for this study, conceptualized and designed the study, designed the data collection instruments, coordinated and supervised data collection, conducted the initial analysis, interpreted data, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Al-Sayegh and Ma conducted the analyses and reviewed and revised the manuscript; Drs Najafzadeh and Wittenberg participated in the methodologic approach and design of the survey instrument, contributed to the analyses, and reviewed and revised the manuscript; Dr Mack oversaw the overarching survey design, participated in the methodologic approach and design of the survey instrument, contributed to data interpretation, and critically reviewed the manuscript and suggested revisions; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Funded by the Agency for Healthcare Research and Quality grant K12 HS022986 to Dr Greenzang. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-0498.
References
- 1.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120(16):2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips SM, Padgett LS, Leisenring WM, et al. . Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, et al. . The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. ; Childhood Cancer Survivor Study . Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582 [DOI] [PubMed] [Google Scholar]
- 5.Hudson MM, Mertens AC, Yasui Y, et al. ; Childhood Cancer Survivor Study Investigators . Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592 [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Neglia JP, Woods WG, et al. . Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green DM, Kun LE, Matthay KK, et al. . Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller FG, Castellino SM, Chen L, et al. . Results of the AHOD0431 trial of response adapted therapy and a salvage strategy for limited stage, classical Hodgkin lymphoma: a report from the Children’s Oncology Group. Cancer. 2018;124(15):3210–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh JM, Diller L. Pediatric Hodgkin lymphoma: trade-offs between short- and long-term mortality risks. Blood. 2012;120(11):2195–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061–1066 [DOI] [PubMed] [Google Scholar]
- 11.Saigal S, Stoskopf BL, Feeny D, et al. . Differences in preferences for neonatal outcomes among health care professionals, parents, and adolescents. JAMA. 1999;281(21):1991–1997 [DOI] [PubMed] [Google Scholar]
- 12.Lam HS, Wong SP, Liu FY, Wong HL, Fok TF, Ng PC. Attitudes toward neonatal intensive care treatment of preterm infants with a high risk of developing long-term disabilities. Pediatrics. 2009;123(6):1501–1508 [DOI] [PubMed] [Google Scholar]
- 13.Bridges JF, Hauber AB, Marshall D, et al. . Conjoint analysis applications in health–a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413 [DOI] [PubMed] [Google Scholar]
- 14.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. . Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120(23):3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon PR, Tomlinson G, Pasternak JD, et al. . The role of disease label in patient perceptions and treatment decisions in the setting of low-risk malignant neoplasms. JAMA Oncol. 2019;5(6):817–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320(7248):1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. . Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19(4):300–315 [DOI] [PubMed] [Google Scholar]
- 18.Reed Johnson F, Lancsar E, Marshall D, et al. . Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13 [DOI] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Nathan PC, Kremer LC. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Hematol Oncol Clin North Am. 2010;24(1):129–149 [DOI] [PubMed] [Google Scholar]
- 20.Diller L, Chow EJ, Gurney JG, et al. . Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langeveld NE, Grootenhuis MA, Voûte PA, de Haan RJ, van den Bos C. Quality of life, self-esteem and worries in young adult survivors of childhood cancer. Psychooncology. 2004;13(12):867–881 [DOI] [PubMed] [Google Scholar]
- 22.Lehmann V, Grönqvist H, Engvall G, et al. . Negative and positive consequences of adolescent cancer 10 years after diagnosis: an interview-based longitudinal study in Sweden. Psychooncology. 2014;23(11):1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khakban A, Mohammadi T, Lynd LD, et al. . Societal preferences in the treatment of pediatric medulloblastoma: balancing risk of death and quality of life. Pediatr Blood Cancer. 2017;64(6):e26340. [DOI] [PubMed] [Google Scholar]
- 24.Levine D, Cohen K, Wendler D. Shared medical decision-making: considering what options to present based on an ethical analysis of the treatment of brain tumors in very young children. Pediatr Blood Cancer. 2012;59(2):216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong GT, Oeffinger KC, Chen Y, et al. . Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong GT, Whitton JA, Gajjar A, et al. . Abnormal timing of menarche in survivors of central nervous system tumors: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115(11):2562–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antal Z, Sklar CA. Gonadal function and fertility among survivors of childhood cancer. Endocrinol Metab Clin North Am. 2015;44(4):739–749 [DOI] [PubMed] [Google Scholar]
- 28.Friedman DL, Whitton J, Leisenring W, et al. . Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulder RL, Kremer LC, van Santen HM, et al. . Prevalence and risk factors of radiation-induced growth hormone deficiency in childhood cancer survivors: a systematic review. Cancer Treat Rev. 2009;35(7):616–632 [DOI] [PubMed] [Google Scholar]
- 30.Mack JW, Cook EF, Wolfe J, Grier HE, Cleary PD, Weeks JC. Understanding of prognosis among parents of children with cancer: parental optimism and the parent-physician interaction. J Clin Oncol. 2007;25(11):1357–1362 [DOI] [PubMed] [Google Scholar]
- 31.Mack JW, Fasciano KM, Block SD. Communication about prognosis with adolescent and young adult patients with cancer: information needs, prognostic awareness, and outcomes of disclosure. J Clin Oncol. 2018;36(18):1861–1867 [DOI] [PubMed] [Google Scholar]
- 32.Lindell RB, Koh SJ, Alvarez JM, et al. . Knowledge of diagnosis, treatment history, and risk of late effects among childhood cancer survivors and parents: the impact of a survivorship clinic. Pediatr Blood Cancer. 2015;62(8):1444–1451 [DOI] [PubMed] [Google Scholar]
- 33.Lerman C, Trock B, Rimer BK, Jepson C, Brody D, Boyce A. Psychological side effects of breast cancer screening. Health Psychol. 1991;10(4):259–267 [DOI] [PubMed] [Google Scholar]
- 34.Huang IC, Kenzik KM, Sanjeev TY, et al. . Quality of life information and trust in physicians among families of children with life-limiting conditions. Patient Relat Outcome Meas. 2010;2010(1):141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer NS, Balsamo LM, Bracken MB, Kadan-Lottick NS. Chemotherapy-only treatment effects on long-term neurocognitive functioning in childhood ALL survivors: a review and meta-analysis. Blood. 2015;126(3):346–353 [DOI] [PubMed] [Google Scholar]
- 36.Greenzang KA, Dauti A, Mack JW. Parent perspectives on information about late effects of childhood cancer treatment and their role in initial treatment decision making. Pediatr Blood Cancer. 2018;65(6):e26978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feudtner C, Walter JK, Faerber JA, et al. . Good-parent beliefs of parents of seriously ill children. JAMA Pediatr. 2015;169(1):39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson EG, Wakefield CE, Shaw J, et al. . Decision-making in childhood cancer: parents’ and adolescents’ views and perceptions. Supportive Care Cancer. 2019;27(11):4331–4340 [DOI] [PubMed] [Google Scholar]
- 39.Kessel RM, Roth M, Moody K, Levy A. Day One Talk: parent preferences when learning that their child has cancer. Supportive Care Cancer. 2013;21(11):2977–2982 [DOI] [PubMed] [Google Scholar]
- 40.Ramirez LY, Huestis SE, Yap TY, Zyzanski S, Drotar D, Kodish E. Potential chemotherapy side effects: what do oncologists tell parents? Pediatr Blood Cancer. 2009;52(4):497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]