Abstract
Adoptive cellular therapy with chimeric antigen receptor T cells (car-ts) has recently received approval from Health Canada and the U.S. Food and Drug Administration after remarkable and durable remissions were seen in children with recurrent or refractory leukemia and adults with non-Hodgkin lymphoma—responses that were so impressive that a shift in the paradigm of care has now occurred for children with acute lymphoblastic leukemia.
The concept behind car-t immunotherapy is that modification of a patient’s own T cells to facilitate their localization to the cancer cell, with subsequent activation of the T cell effector mechanism and proliferation, will result in targeted killing of cancer cells. The car-ts are a novel drug in that the starting material for the manufacture of the car-t product comes from the patient, whose viable T cells are then genetically modified. Thus, collaboration is needed between the pharmaceutical companies, which must meet good manufacturing standards for each patient’s unique product, and the treating sites. For regulators and health authorities, this new class of drugs requires new paradigms for assessment and approval. Treatments with car-ts require that institutions address unique logistics requirements and management of novel toxicities.
The Hospital for Sick Children has had early experience with both the licensing of clinical trials and the introduction of the first commercial product. Here, we provide an overview of basic concepts and treatment, with caveats drawn from what we have learned thus far in bringing this new therapy to the clinical front line.
Keywords: Chimeric antigen receptor T cells, pediatrics, leukemia, lymphoma, cytokine release syndrome
INTRODUCTION
The treatment of cancer is being disrupted with the introduction of treatments focusing not on cytotoxic therapies, but rather on immunologic approaches. Allogeneic hematopoietic stem (or “progenitor”)–cell therapy (hsct) was the first successful application of replacement of the immunologic system as an anti-leukemia or -lymphoma strategy. Chimeric antigen receptor T cell (car-t) therapy, first conceptualized at the end of the 1980s, predicted that the body’s own immune cells could be repurposed to target cancer cells—if the correct target could be identified, and if the T cells would be stimulated on contact to mobilize effector, expansion, and survival mechanisms. Early clinical trials were disappointing, until a handful of patients with chronic lymphocytic leukemia and acute lymphoblastic leukemia (all) experienced phenomenal responses: complete and durable remissions in what were hopeless situations1. Success was based on high expression of CD19 on most leukemia or lymphoma cells and otherwise limited expression of CD19 in the body (basically on B cells, whose main output of immune globulins could be compensated by supplementation, as is common practice in patients with primary B cell immunodeficiencies).
The early clinical experience has been systematically reviewed by many groups2,3. The goal of the present clinically focused review is to provide an overview of the treatment process and the early experience with car-t treatment, with a particular focus on pediatric all, providing caveats about treatment management in the car-t process and the likely evolution of car-t therapy over the next few years. Our experience is with CD19-targeting car-t therapy for all, and hence that experience is the focus of the discussion.
REVIEW
General Principles: Not All CAR T Cells Are the Same
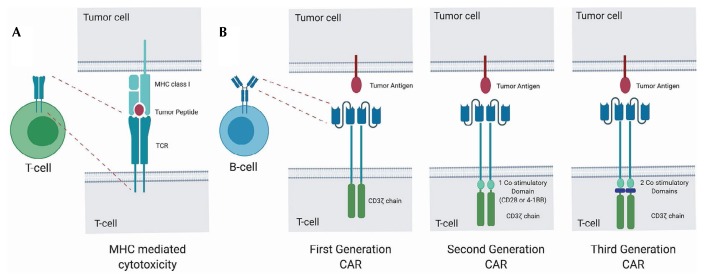
Conventionally, T cells act by targeting specific peptides in the context of major histocompatibility complex restriction [Figure 1(A)]. Using an engineered car to modify the binding region, the major histocompatibility complex can be bypassed and cell surface antigens can be targeted in an independent fashion [Figure 1(B)]4, thus directing the patient’s own T cells to become activated on contact with the target cells—in our case, cells that express CD19 on their surface. A single-chain variable fragment derived from an antibody is typically used to target the antigen.
FIGURE 1.
Overview of chimeric antigen receptor (CAR) T cell therapy. (A) Cytotoxicity mediated by T cell receptor (TCR) restriction of the major histocompatibility complex (MHC) of a cell-surface antigen. (B) Cytotoxicity through direct targeting of a cell-surface antigen by a CAR T cell.
The concept of using car T cells to target tumour-surface antigens was described in the late 1980s5, but first-generation car-ts, which included only the receptor component CD3z as an intracellular domain, showed limited efficacy. Significant responses were observed only after researchers went back to the gene construct and added a single costimulatory domain derived from either CD28 or 4-1BB—the so-called second-generation car-ts6. A viral vector is used to deliver the genetic material—which includes the targeting antibody–based variable region, a transmembrane domain, a costimulatory domain, and the CD3z signalling domain—into the patient’s T cells. Third-generation car-ts contain additional costimulatory domains, with the aim to improve proliferation, cytokine secretion, and in vivo persistence; compared with second-generation car-ts, they have shown improved effector functions and in vivo persistence.
Currently, the U.S. Food and Drug Administration and Health Canada have approved two constructs for car-t production: tisagenlecleucel, a 4-1BB–based construct, for relapsed or refractory (r/r) all in children and r/r B cell lymphoma in adults; and axicabtagene ciloleucel, which uses the CD28 costimulatory construct, for the treatment of r/r B cell lymphoma in adults. Toxicities for the two constructs differ, and although 4-1BB–containing car-ts can persist for years, CD28-based car-ts generally persist for only months2. The length of time that is needed for the persistence of car-ts is not known, but the shorter-lived car-t products are usually followed by allogeneic transplantation.
The CAR-T Therapy Process
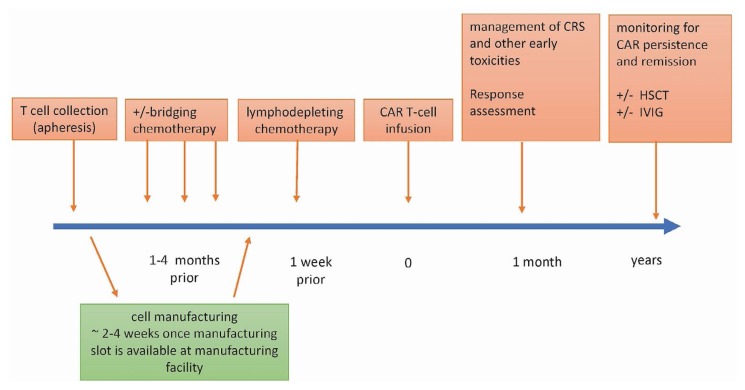
The subsections that follow describe the activities that constitute the car-t therapy process (Figure 2).
FIGURE 2.
Treating patients with chimeric antigen receptor (CAR) T cell therapy—basic concepts. CRS = cytokine release syndrome; HSCT = hematopoietic stem-cell transplantation; IVIG = intravenous immunoglobulin.
Collection of Mononuclear Cells (Apheresis) and Manufacturing of CAR-Ts
The starting material for car-t manufacturing comes from the patient. Protocol requirements can vary, but a circulating CD3 count of at least 150/mm3 is generally needed to reliably collect a number of T cells sufficient for manufacturing7,8. Some products are manufactured with freshly collected cells; others start with frozen product. Timing and logistics can be challenging in patients with relapsed disease. Given the rapid clinical introduction of car-t therapy, obtaining a manufacturing slot can be challenging. Washout periods for chemotherapy and immunotherapy before the collection are important considerations to ensure a sufficient number of functional T cells.
In adolescents and adults, the required apheresis might feasibly be performed using peripheral intravenous lines. In children, central venous access is generally required for blood flow to be sufficient for collection. If the patient lacks a central line at the time of relapse, we recommend insertion of an apheresis-compatible line that can be used both for the collection (and potentially re-collection in case of manufacturing failure) and for the subsequent treatment.
Any apheresis platform can be used for cell collection. In Canada, the most commonly used platform is the Spectra Optia system (Terumo BCT, Lakewood, CO, U.S.A.). The continuous and intermittent cell collection approaches have both worked well in our hands (unpublished data); the choice should be based on institutional experience and expertise.
Although the manufacturing process involves a T cell selection step, we have found that, in patients with high peripheral blast counts, T cell yields are low, risking manufacturing failure. The total white blood cell count, CD3+ T cell count, and blast percentage should all be checked before the collection procedure. We avoid collections in patients with a white blood cell count greater than 20×109/L if most of the cells are blasts; however, achieving that degree of disease control at the time of collection and at the same time fulfilling the chemotherapy washout requirements can be challenging. Even when sufficient T cells are collected, there is a risk of manufacturing failure, as occurred in 7%–8% of patients in the eliana and juliet trials9,10. In the zuma-1 study, a manufacturing failure occurred for only 1% of patients11. At time of collection, low lymphocyte counts, low T cell counts, a high blast percentage in the peripheral blood, and age less than 3 years have been associated with manufacturing failure12,13. Manufacturing failure has been attributed to inherent T cell defects that might be patient-specific or related to the amount and intensity of prior treatment. In a patient cohort with chronic lymphocytic leukemia, Fraietta et al.14 showed that sustained remissions were associated with T cell collections that were enriched for a proliferative CD27+ CD45RO–CD8+ T cell subset. Expression of exhaustion markers such as PD-1 and tim-3 on the manufactured car-t product was also negatively correlated with anti-leukemic responses.
Patients who relapse after allogeneic hsct deserve special consideration. In such a situation, the T cells collected and the T cells that are subsequently manufactured will be derived mostly from the engrafted donor T cells. Donor cell–driven graft-versus-host disease (gvhd) is a risk in the presence of alloreactive T cell expansion. A phase i study with activated and expanded donor lymphocytes was associated with grades i–ii gvhd in 5 of 18 patients and grade iii gvhd in 2 of 18 patients15; and at least 2 patients were reported to have developed acute and chronic gvhd after car-t therapy16,17. To avoid collection of alloreactive T cells, it is recommended that, for at least 2 weeks before cell collection, patients stop immune suppression without any signs of significant gvhd18. Studies that used donor graft–derived CD19 car-t products reported higher rates of gvhd, and such products are therefore not routinely used19.
With an appreciation of the challenges of collection in patients with advanced disease and of the possibility that the T cell phenotype might be exhausted, there is a trend to collect T cells earlier in the patient’s trajectory. That approach does not work if allogeneic transplantation before the car-t therapy is anticipated (given that donor-derived T cells would be the cells needed for manufacturing) or if the product has to be shipped fresh for manufacturing.
Bridging Chemotherapy
Patients who are currently eligible for car-t treatment for all often present with active r/r disease and require treatment to safely bridge the time of cell manufacturing (from T cell collection to car-t infusion). In the eliana trial, 65 of 75 patients received bridging chemotherapy. Notably, 10 of 92 patients who were enrolled in the study were not able to undergo car-t infusion because of disease progression, toxicity, or infection-related complications, illustrating the challenges of treating patients during the manufacturing period10.
The optimal chemotherapy regimen for bridging depends on the patient’s treatment history and prior toxicities. Unlike conventional all chemotherapy treatment, whose goal is to achieve complete remission, the goal for patients awaiting car-t treatment is to temporize the disease until the car-ts are manufactured, avoiding toxicities that might prevent the patient from receiving the car-t product. In our institutional experience, most patients can be bridged successfully with low-intensity regimens that can generally be administered on an outpatient basis and that are not associated with toxicities such as mucositis or infections (unpublished data). The drugs commonly used for bridging regimens include steroids, vincristine, mercaptopurine, methotrexate (weekly dosing), low-dose cytosine arabinoside, cyclophosphamide, etoposide, and asparaginase18,20. It is important that enough time be allowed between drug dosing and car-t infusion—the washout time—so as to not impair car-t function. The optimal interval could be specific to a clinical research protocol or to a specific car-t construct. Generally, recommended washout times are 2 weeks for systemic chemotherapy, 4 weeks for pegylated l-asparaginase, and 72 hours for steroids18,20.
Other considerations in the bridging regimen strategy include availability of a target for car-t expansion after infusion. A high disease burden before car-t infusion has been associated with greater expansion of car-ts, which correlates with severity of cytokine release syndrome (crs)21,22. The effect of disease burden on outcome might differ depending on the car-t construct used. Park et al.21 showed that a higher disease burden before infusion with a CD28-based CD19 car-t construct correlated with inferior outcomes. Treating physicians therefore have to carefully consider the intensity of the bridging regimen. Normal B cells express CD19 and will serve as additional targets that can cause expansion of the car-ts. Thus, therapies such as inotuzumab and blinatumomab, which deplete the B cell pool, should be used with caution during the bridging phase. For example, Gardner et al.17 showed that low numbers of CD19-positive cells in the bone marrow before infusion of a 4-1BB–based CD19 car was associated with decreased persistence of car-ts, which has itself been associated with worse outcomes. Blinatumomab, the bispecific antibody that targets CD19, is generally avoided because of concern that it might select for CD19-negative clones18,23. Of course, the relevant decisions are ultimately driven by the aggressiveness of the leukemia or lymphoma, which might require more-intensive regimens to control the disease during manufacturing. In our experience, the latter situation is the exception rather than the rule.
Lymphodepleting Chemotherapy
To promote expansion and persistence of the car-ts after infusion, lymphodepleting chemotherapy is generally recommended in the week before the car-t infusion10,24. Lymphodepletion has been shown to enhance the effectiveness of adoptively transferred T cells25, and it can eliminate regulatory T cells as well as other competing elements of the immune system that act as “cytokine sinks,” enhancing the availability of cytokines essential for T cell proliferation26. Fludarabine and cyclophosphamide are most commonly used in a range of combinations24,27. The eliana trial regimen consisted of intravenous cyclophosphamide (500 mg/m2 daily for 2 days) and fludarabine (30 mg/m2 daily for 4 days)10,20,28. Alternative regimens have been used, but the combination of fludarabine and cyclophosphamide has been shown to be the more favourable regimen—in some clinical settings, at least24,27.
For patients with low cell or lymphocyte counts, car-ts could be infused without prior lymphodepleting chemotherapy. The data for this particular subgroup of patients are limited, but patients have been successfully so treated29.
Presentation and Management of Acute Side Effects
Therapy with car-ts has unique acute toxicities that require coordinated management by multiple teams. Major acute toxicities include crs, neurologic toxicity, tumour lysis syndrome, and cytopenias. Given the potential for severe and rapidly evolving complications, patients must be followed closely after infusion. Hospitalization of patients receiving car-ts varies with the centre. If the patient is clinically well, has a limited tumour burden, and lives in close proximity to the hospital, our centre has moved to outpatient management for patients receiving tisagenlecleucel. With other constructs, especially CD28-based cars, which can have more accelerated kinetics, hospitalization for at least 7 days after the infusion is still mandated30.
Cytokine release syndrome is a systemic inflammatory response resulting from the release of cytokines from activated car-ts and bystander immune cells. It is typically characterized by fever, hypoxia, tachycardia, hypotension, and multi-organ dysfunction31, and it is not unique to car-t therapies. Cytokine release syndrome can occur after antibody therapies (such as blinatumomab) and checkpoint inhibitor therapy, but the intensity tends to be more severe after car-t therapy.
The timing and severity of symptoms in patients receiving car-t products vary depending on the construct used, the type of cancer, disease burden, and the patient’s age and comorbidities31,32. For tisagenlecleucel and axicabtagene ciloleucel, the incidence of crs is 77% and 93%10,11. Various grading systems have been developed32–34. The consensus guidelines recently published by the American Society for Transplantation and Cellular Therapy have defined 4 grades of crs (depending on the presence of fever, the degree of hypotension, and hypoxia), which will both standardize and simplify reporting of crs35.
Cytokine release syndrome is a diagnosis of exclusion, and with the development of fever, an empiric broad-spectrum antibiotic is recommended. Guidelines for crs management focus on symptom management (cardiovascular support, dialysis, antibiotics, fever management). High cytokine levels, including interleukin 6, are hallmarks of crs and correlate with symptom severity36,37. Tocilizumab, an antibody targeting interleukin 6, has been effectively used for the treatment of crs and is considered the first-line treatment for progressing crs10,28,35,38. For patients who do not respond to a first dose, an additional dose of tocilizumab can be given; if crs cannot be controlled with 1 or 2 doses of tocilizumab, second-line treatment options include other immunosuppressive agents such as steroids or siltuximab39,40. Steroids are avoided after car-t infusion, but if crs symptoms are severe, judicious and sparing use of steroids can help in dampening the crs and minimizing severe toxicity without jeopardizing response.
Neurotoxicity is commonly reported in car-t trials and can occur at the same time as crs, be delayed, or even occur in the absence of crs9–11,28. Several mechanisms seem to be responsible, including trafficking of cytokines into the central nervous system and penetration of car-ts through the blood–brain barrier22,28. Symptoms include headaches, seizures, delirium, tremor, aphasia, and impaired writing, which can be used as a daily monitoring test. Consensus guidelines from the American Society for Transplantation and Cellular Therapy advise use of the scores on the immune effector cell–associated encephalopathy assessment and the Cornell Assessment of Pediatric Delirium to grade immune effector cell–associated neurotoxicity syndromes (4 grades). Treatment is mainly supportive and similar to that for crs, and exclusion of other causes is essential35,40. Cerebral edema has been described in rare cases32. To avoid severe neurotoxicity, patients with active central nervous system disease should be treated before car-t infusion.
Cytopenias are common after car-t infusion and can last for months: 37%–78% of patients show cytopenia 1 month after infusion with tisagenlecleucel and axicabtagene ciloleucel10,11. The cause of the cytopenias is often not clear. Early cytopenias might be attributable to the lymphodepleting chemotherapy; prolonged cytopenias, lasting months after infusion, are likely attributable to myelosuppression mediated by car-t persistence. The latter concept is supported by the fact that patients who have not received lymphodepleting chemotherapy can develop significant cytopenias41,42. In the setting of leukemia, relapse is always a concern.
Post-Therapy Management
As long as the car-ts are functional, the patient will be B cell–penic. Regular assessment of circulating B cells can be beneficial in all, especially in the first year after transplantation (discussed in more detail a little later in this article). We check monthly for the first year. In non-Hodgkin lymphoma, persistence of the car-t product has not been associated with better long-term lymphoma control. Recommendations for substitution differ for adults and children, but monthly immune globulin replacement (intravenous or subcutaneous) is generally recommended for the pediatric population as long as B cell aplasia is evident. Replacement therapy in adults is based on both immunoglobulin and infectious history43,44.
Retroviral or lentiviral gene transfer carries the theoretical risk of replication-competent viruses and insertional mutagenesis. The data from clinical trials to date show no evidence of the realization of those concerns, but long-term follow-up data are needed45,46. For that reason, Health Canada requires 15-year follow-up for patients receiving car-t products, and patients should be regularly screened for secondary malignancies.
Prolonged cytopenia might require transfusions or treatment with granulocyte colony–stimulating factor. Graft-versus-host disease and autoimmune diseases are potential concerns and should be part of the regular screening schedule. For patients who undergo hsct after car-t therapy, follow-up consists mainly of screening for late effects of the transplantation and underlying disease because the car-t product will be ablated with the preparative regimen before transplantation.
Mechanisms of Treatment Failure
Despite the success with which car-ts target B cell antigens, treatment failure remains a major challenge. Leukemia relapses after CD19 car-t therapy can be broadly divided into CD19-positive and CD19-negative relapses. In a series examining relapse after car-t therapy, 12 of 17 patients (71%) were found to have CD19-negative relapses. Further genetic analysis showed that all 12 patients carried mutations in exons 2–5, preventing anchorage of the CD19 protein to the cell surface, thereby eliminating the target antigen. Acquired loss of heterozygosity was described in 8 of the 12 patients47. Alternative splicing of CD19 has been shown to be a mechanism of escape48. No mutations were found in other potential target antigens such as CD20 or CD22, leaving the possibility of targeting other antigens or using bispecific car-ts. Other mechanisms of target loss and tumour escape, such as disrupted membrane trafficking of CD19, have been described for blinatumomab49. In refractory large B cell lymphoma, CD19-directed car-t therapy showed lower rates of complete remission, but only 14% of patients experienced relapses, with 27% of the relapses being attributed to antigen loss11.
Sustained remissions after tisagenlecleucel were associated with the persistence of car-ts and with B cell aplasia (a surrogate marker for car-t persistence) that continued beyond 2–3 months, suggesting continued effector function. The CD19-positive relapses that occurred during that time were largely attributable to early car-t loss. Early B cell recovery, within 6 months of tisagenlecleucel infusion, occurred in approximately 22% of patients, reflecting poor tisagenlecleucel persistence, and was associated with higher risk of relapse50.
How best to address early car-t loss remains unclear. In the eliana trial, 8 of 75 infused patients (11%) underwent allogeneic hsct while in remission, with 2 experiencing B cell recovery within 6 months after infusion10. During the manufacturing process, additional doses of product might be manufactured and be available for re-infusion. The efficacy of car-t re-infusion for early B cell recovery remains the subject of current trials. In a phase i study, Maude et al.51 reported on re-treatment or re-infusion with humanized tisagenlecleucel in 16 children and young adults with r/r B cell all or lymphoblastic lymphoma who had either prior exposure to another car-t product for CD19-positive relapse (n = 10), no response (n = 1), or B cell recovery (n = 5). Of the 16 patients, 9 (56%) achieved a complete remission, defined as morphologic remission with B cell aplasia. Other strategies include collecting T cells earlier in the disease course (before development of an exhausted phenotype) or promoting persistence through the use of checkpoint inhibitors after car-t infusion. Pembrolizumab, a PD-1 blocker, has been associated with some encouraging results in case reports and early clinical studies, prolonging car-t persistence and car-t efficacy52,53.
Relapse with lineage switch is a rare but well-described process that occurs during or after chemotherapy. It occurs more often with specific genetic subtypes such as MLL rearrangements and can occur either by selection of a previously undetected clone or by reprogramming from an immature stem-cell clone54,55. Gardner et al.56 reported about 7 patients with MLL-rearranged B cell all who received a 4-1BB–based CD19 car construct. All patients achieved remission on day 21, but 1 adult and 1 child with infant all relapsed within 6 weeks. One showed a clonally-related acute myeloid leukemia phenotype, and one tested negative for the previous rearrangement, suggesting relapse from an immature stem-cell clone. Interleukin-6 has been implicated as a factor for myeloid differentiation that might play in role in that process, especially during crs57.
Escape mechanisms can vary with the cellular target used. Fry et al.58 published data from a phase i study using a 4-1BB CD22 car construct. Of 21 patients treated, 73% achieved a complete remission. Subsequently, 8 patients relapsed, and it was noticed that most still expressed CD22 on the blast surface, but that the expression level had decreased. In vitro and in vivo follow-up experiments in mice with cell lines expressing CD22 at various levels suggest that target downregulation might play an important role in tumour escape in that particular setting.
There was also a case in which leukemic blasts were transfected with the CD19 car-t vector during manufacturing. Expression of the car vector on leukemic blasts led to binding—and thereby masking—of the CD19 epitope, enabling tumour escape. The authors then retrospectively looked at 116 apheresis products, none of which contained transfected blast cells59.
Role of Allogeneic HSCT After CAR-T Therapy
The need for consolidation after car-t therapy remains an open question. Factors that inform the decision to use hsct include prior transplantation, co-stimulatory domain of the car-t construct, donor availability, and patient and physician preference. Patients whose treatment is CD28-based or less than 3 months in the past might benefit from hsct36. In contrast, Park et al. reported on 32 patients with all and negative measurable residual disease after treatment with a CD28-based car, finding no significant difference in overall survival between patients who underwent allogenic hsct and those who did not21.
Patients who receive a 4-1BB construct with the potential of long-term persistence remain at risk for either CD19-positive or CD19-negative relapse. Given that car-t persistence has been shown to correlate with the risk of CD19-positive relapse, physicians can choose to monitor for B cell persistence and to proceed to hsct only if (early) car-t loss occurs23,60. Relapse 9–12 months after infusion is less common; if patients lose their car-ts after 9 months, waiting and monitoring is an option. In the eliana trial, 8 of 61 patients who achieved a remission underwent hsct, including 2 patients with bone marrow positive for measurable residual disease and 2 patients with early B cell recovery. No relapses were reported in that patient group10.
Implementation in the Canadian Health Care System
Implementation of car-t therapy has required tremendous collaboration across Canada and between manufacturing companies, regulators, and provincial providers. The carts for infusion are regulated as drugs, and uniquely, the patient provides the raw material—their own T cells—for manufacturing the car-t product. Thus, the clinical site is both a source establishment for the manufacturing material and an end user of the manufactured product. Each product is patient-specific; hence, each car-t product is a batch and requires comprehensive testing to meet pharmaceutical standards.
Therapy with car-ts is being assessed by the Health Technology Assessment program of the Canadian Agency for Drugs and Technologies in Health, which assesses medical devices and clinical interventions. That approach was implemented at the request of Canada’s federal, provincial, and territorial ministries of health and the Canadian Association of Provincial Cancer Agencies and is consistent with the work of several other health technology assessment bodies, including the Institut national d’excellence en santé et services sociaux in Quebec. This type of review was new for the Canadian Agency for Drugs and Technologies in Health and was performed with intensive consultation, the first report having recommended equitable access across Canada, with well-defined indications for therapy and collection of standardized data to assess effectiveness, safety, and cost-effectiveness over time61. The federal, provincial, and territorial ministries of health are working through interprovincial agreements, and clinical collaborative groups across Canada (the C17 Research Network, Cell Therapy Transplant Canada, the National Cancer Institute of Canada) have established sharing platforms for best practices and patient navigation.
Cost is a major issue. The anticipated cost of the manufactured product falls into the CA$300,000—CA$450,000 range and constitutes only part of the total cost of care. The additional costs are related to apheresis, venous access, bridging and lymphodepleting chemotherapy, and management of toxicities, which can include intensive care support (currently in about 25% of patients). The required long-term patient outcome monitoring and reporting is also costly. Those costs can easily equal the cost of the drug. As the application of car-t therapy expands to more patients, challenges abound. Cost-effectiveness will depend on whether car-t replaces more-expensive therapies, how effective it is, and whether its costs can come down62,63. BioCanRx is a Canadian immunotherapy network supported by the Canadian Institutes of Health Research that is working toward manufacturing car-t products for clinical trials by using specialized core facilities for the production of viral vectors compliant with Good Manufacturing Practices, immune monitoring compliant with Good Laboratory Practice, and car-t manufacturing.
Implementation at the Institutional Level
Initially at least, centres administering car-t therapy will be centres with hematopoietic stem-cell transplantation experience and accreditation from the Foundation for the Accreditation of Cellular Therapy. However, it is not sufficient to be a transplant centre: centres are having to develop new standard operating procedures and having to modify existing ones to address needs relating to the collection of optimal starting material (the apheresis product), management of the patient until manufacturing is complete, and care of the patient after car-t therapy. The care team has to include the emergency room and critical care units. Close collaboration between the leukemia/lymphoma team and the cellular therapy team during the bridging stage is vital. Training and maintenance of competency are complex and time-consuming. Tools such as the consensus grading for crs and neurologic toxicity secondary to immune effector cells from the American Society for Transplantation and Cellular Therapy35 and patient information packages are helping to standardize communication. Pediatric centres must develop age-appropriate tools to identify neurocognitive toxicity. We have had to develop individually adapted play tools supplemented with a simple handwritten sentence (if the child is able). Given that patients will be repatriating back to home institutions, the long-term follow-up requirements will be challenging and have to be anticipated.
Institutions have to contract as a supplier of manufacturing material (patient T cells used to make the car-t product). That means documenting pharmaceutical-grade process control in the collection, testing, storage, and shipping of that material. Contracting between the hospital, the manufacturing site, and provincial funders is complex and nuanced. At this point, multilateral contracting with each pharmaceutical company and product-specific modifications are required.
As a result, organizational commitment is required not only in areas in which clinical need will increase (laboratories, including the cellular therapy laboratory; apheresis; clinical teams), but also for resourcing of case management and patient navigation through the car-t journey. Urgency often attends the collection of leucocytes before intensive chemotherapy and the challenge of maintaining the patient through bridging chemotherapy. Health care resources are also used in monitoring and reporting, at the least, the outcomes required by the Center for International Blood and Marrow Transplant Research (immune effector cell reporting), plus costing and resource utilization. Those added patient care and reporting costs have to be included when developing program costing.
Future Directions
The first success stories should be seen as a start, heralding the as-yet-unknown potential of car-t therapy. Currently, research subjects in this therapeutic area include further improvements to current strategies; moving the treatment earlier into the therapy journey to avoid the morbidity and mortality of prolonged use of some combination of chemotherapy, radiation therapy, and hsct; and expanding the treatment approach to other malignancies.
Manufacturing a personalized car-t product takes time and is not always successful. To address that issue, the design of off-the-shelf car-t strategies is being pursued by multiple groups. A pilot (phase i) study using cells from universal donors that have been genetically engineered to express a CD19 car and a disrupted T cell receptor alpha chain (to prevent gvhd) showed rates of complete remission or complete remission with incomplete hematologic recovery of 88% in 20 pediatric and adult patients treated64,65. Other strategies to improve current car-t constructs use multiple targeted approaches such as combined CD19 and CD22 cars—similar to the combinatorial approach of chemotherapy66. Early clinical experience has shown the feasibility of that approach, but further studies are needed67.
The use of hsct is associated with significant acute and long-term morbidity and mortality. With the success of current car-t therapies in the r/r setting, studies to test whether car-t therapy can replace hsct in certain situations are on the way. For example, the Children’s Oncology Group, in collaboration with Novartis, has a study for children with high-risk all and persistent measurable residual disease after consolidation. Outcomes for this patient group are poor with conventional therapies, and such patients are generally offered hsct. The trial will address whether, in high-risk patients, those with high minimal residual disease at end of all induction or consolidation will benefit from earlier car-t therapy (see NCT03876769 at https://ClinicalTrials.gov/).
Expansion of the concept of antigen targeting with car-ts to other malignancies and solid tumours has thus far proven difficult. Off-target toxicities, the immunosuppressive environment of solid tumours, the difficulty of optimal target selection, antigen heterogeneity with tumour escape, and T cell exhaustion demand novel approaches to achieve a sustainable response with an acceptable toxicity profile68,69.
SUMMARY
The early experience with car-t therapy in pediatric all and adult recurrent non-Hodgkin lymphoma has already transformed the approach to the management of primary refractory disease or relapse after allogeneic transplantation. Caution is warranted, in that this therapy is not yet “one and done” (Table I). For many patients, manufacturing of the car-t product fails, comorbidities are too great for therapy toleration, or relapse occurs despite successful car-t targeting of the relevant tumour antigen. We are very much in the learning stages. Many questions remain to be answered before we know where this immunomodulatory approach fits into the cancer treatment tool chest.
TABLE I.
Caveats for care of the patient on the chimeric antigen receptor (CAR) T cell therapy journey
| Anticipate need: collect early in the disease course. |
| Be aware of chemotherapy and immunotherapy washout periods. Steroids affect normal T lymphocyte number and function, which are the starting product for manufacturing. |
| After allogeneic transplant, the new T cell population will be the starting product for manufacturing. |
| Avoid collecting T cells in the presence of high circulating blast counts. |
| During bridging chemotherapy, the goal is not to achieve remission, but rather to temporize. |
| Cytokine release syndrome can come on swiftly and later; careful monitoring by trained care-team members, who include members of the emergency room and critical care teams, is essential. |
| Have established care plans for management of severe cytokine release syndrome and neurologic toxicity. |
| Avoid steroids after CAR-T infusion unless cytokine release syndrome is established. |
In the meantime, the first products are moving into the clinic. We have outlined both the steps needed to bring a patient through treatment and the steps needed to develop the supportive clinical and laboratory programs. It is taking a massive effort from the pharmaceutical industry, regulatory bodies, and health care funders to make the therapy happen, and many quiet champions are assisting in the process.
It is likely that advances will be made in clinical approaches (timing of treatment, patient or disease selection, dosing strategies), car-t constructs (target antigens, costimulatory elements), the starting T cell material, manufacturing, and management of toxicities that will refine the therapy—improving safety and efficacy, and decreasing toxicity. It is critical to bring the costs for the treatment down if car-t is to be feasible in the long term.
Finally, the early successes in the treatment of childhood leukemia and adult non-Hodgkin lymphoma are just that. The concept of “teaching” a patient’s T cells to recognize cancer cells is one that has the potential of being applicable to any tumour that has unique antigen targets. Moreover, “teaching” T cells to recognize autoantibody-producing cells or infectious agents are further applications that are being explored70. It is not yet known how far this car-t care journey will take us.
ACKNOWLEDGMENTS
We thank the extended SickKids care team that, in addition to our frontline care teams, has been involved in the development of the cellular therapy program, including our many colleagues in administration, legal, regulatory, and laboratory. The true heroes are the patients and their families, who braved the unknown and accepted the challenge of this new therapy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: DAW has consulted for Novartis for an unrelated drug and is an institutional investigator on Novartis and Kite/Gilead trials; JK is the institutional principal investigator for Novartis-sponsored trials with tisagenlecleucel and for Kite/Gilead–sponsored trials with KTE-X19; JK has also consulted for Novartis, but has not received any honoraria.
REFERENCES
- 1.Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2016;30:157–67. doi: 10.1016/j.blre.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Frey NV, Grupp SA, Maude SL. car T cell therapy in acute lymphoblastic leukemia and potential for chronic lymphocytic leukemia. Curr Treat Options Oncol. 2016;17:28. doi: 10.1007/s11864-016-0406-4. [DOI] [PubMed] [Google Scholar]
- 3.Pettitt D, Arshad Z, Smith J, Stanic T, Holländer G, Brindley D. car-t cells: a systematic review and mixed methods analysis of the clinical trial landscape. Mol Ther. 2018;26:342–53. doi: 10.1016/j.ymthe.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–81. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin–T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: cars take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuirk J, Waller EK, Qayed M, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19:1015–24. doi: 10.1016/j.jcyt.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Even-Or E, Mola MD, Ali M, et al. Optimizing autologous mononuclear cell collections for cellular therapy using the novel Optia apheresis device in pediatric patients with high risk leukemia [abstract 168] Biol Blood Marrow Transplant. 2017;23(suppl):S161–2. doi: 10.1016/j.bbmt.2016.12.273. [DOI] [Google Scholar]
- 9.Schuster SJ, Bishop MR, Tam CS, et al. on behalf of the juliet investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 10.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel car T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen ES, Stroncek DF, Ren J, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion. 2017;57:1133–41. doi: 10.1111/trf.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MM, Fesnak A, Leskowitz RM, et al. Predictors of manufacturing (mfg) success for chimeric antigen receptor (car) T cells in non-Hodgkin lymphoma (nhl) [abstract 180] Cytotherapy. 2017;19(suppl):S118–19. doi: 10.1016/j.jcyt.2017.02.190. [DOI] [Google Scholar]
- 14.Fraietta JA, Lacey SF, Orlando EJ, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (car) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24:563–71. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–31. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. CD19 car-t cells of defined CD4+:CD8+ composition in adult B cell all patients. J Clin Invest. 2016;126:2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 car T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–31. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansagra AJ, Frey NV, Bar M, et al. Clinical utilization of chimeric antigen receptor T cells in B cell acute lymphoblastic leukemia: an expert opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019;25:e76–85. doi: 10.1016/j.bbmt.2018.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood. 2018;131:1045–52. doi: 10.1182/blood-2017-08-752121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novartis Pharmaceuticals Canada Inc. Kymriah: Tisagenlecleucel [product monograph] Dorval, QC: Novartis Pharmaceuticals Canada Inc.; 2018. [Google Scholar]
- 21.Park JH, Rivière I, Gönen M, et al. Long-term follow-up of CD19 car therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller KT, Maude SL, Porter DL, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–25. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grupp SA, Maude SL, Shaw PA, et al. Durable remissions in children with relapsed/refractory all treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019) Blood. 2015;126:681. doi: 10.1182/blood.V126.23.681.681. [DOI] [Google Scholar]
- 24.Turtle CJ, Berger C, Sommermeyer D, et al. Anti-CD19 chimeric antigen receptor–modified T cell therapy for B cell non-Hodgkin lymphoma and chronic lymphocytic leukemia: fludarabine and cyclophosphamide lymphodepletion improves in vivo expansion and persistence of car-t cells and clinical outcomes [abstract 184] Blood. 2015;126 [Google Scholar]
- 25.Dummer W, Niethammer AG, Baccala R, et al. T Cell homeostatic proliferation elicits effective antitumour autoimmunity. J Clin Invest. 2002;110:185–92. doi: 10.1172/JCI0215175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell–mediated tumor immunotherapy. Trends Immunol. 2005;26:111–17. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee DW, III, Stetler-Stevenson M, Yuan CM, et al. Long-term outcomes following CD19 car t cell therapy for b-all are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-car hematopoietic stem cell transplantation. Blood. 2016;128:218. doi: 10.1182/blood.V128.22.218.218. [DOI] [Google Scholar]
- 28.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–21. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kite Pharma Inc. Yescarta: Axicabtagene Ciloleucel [product monograph] Santa Monica, CA: Kite Pharma Inc.; 2019. [Google Scholar]
- 31.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the car t cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [Erratum in: Blood 2015;126:1048; dosage error in article text: Blood 2016;128:1533] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DW, Santomasso BD, Locke FL, et al. asbmt consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–38. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T Cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davila ML, Rivière I, Wang X, et al. Efficacy and toxicity management of 19–28z car t cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen F, Teachey DT, Pequignot E, et al. Measuring il-6 and sil-6R in serum from patients treated with tocilizumab and/ or siltuximab following car t cell therapy. J Immunol Methods. 2016;434:1–8. doi: 10.1016/j.jim.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. on behalf of the Pediatric Acute Lung Injury and Sepsis Investigators network. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. 2019;16:45–63. doi: 10.1038/s41571-018-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochenderfer JN, Dudley ME, Carpenter RO, et al. Donorderived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–39. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [Erratum in: N Engl J Med 2016;374:998] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doan A, Pulsipher MA. Hypogammaglobulinemia due to car T-cell therapy. Pediatr Blood Cancer. 2018;65:e26914. doi: 10.1002/pbc.26914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1–46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Cornetta K, Duffy L, Turtle CJ, et al. Absence of replication-competent lentivirus in the clinic: analysis of infused T cell products. Mol Ther. 2018;26:280–8. doi: 10.1016/j.ymthe.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–41. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24:1504–6. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 48.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to cart-19 immunotherapy. Cancer Discov. 2015;5:1282–95. doi: 10.1158/2159-8290.CD-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braig F, Brandt A, Goebeler M, et al. Resistance to anti–CD19/CD3 bite in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–4. doi: 10.1182/blood-2016-05-718395. [DOI] [PubMed] [Google Scholar]
- 50.Maude SL, Barrett DM, Rheingold SR, et al. Efficacy of humanized CD19-targeted chimeric antigen receptor (car)–modified T cells in children and young adults with relapsed/refractory acute lymphoblastic leukemia. Blood. 2016;128:217. doi: 10.1182/blood.V128.22.217.217. [DOI] [Google Scholar]
- 51.Maude SL, Hucks GE, Callahan C, et al. Durable remissions with humanized CD19-targeted chimeric antigen receptor (car)–modified T cells in car-naive and car-exposed children and young adults with relapsed/refractory acute lymphoblastic leukemia [abstract 1319] Blood. 2017;130(suppl 1) doi: 10.1182/blood-2017-10-808592. [DOI] [Google Scholar]
- 52.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (car)–modified T cells: refueling the car. Blood. 2017;129:1039–41. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li AM, Hucks GE, Dinofia AM, et al. Checkpoint inhibitors augment CD19-directed chimeric antigen receptor (car) T cell therapy in relapsed B-cell acute lymphoblastic leukemia. Blood. 2018;132(suppl 1):556. doi: 10.1182/blood-2018-99-112572. [DOI] [Google Scholar]
- 54.Rossi JG, Bernasconi AR, Alonso CN, et al. Lineage switch in childhood acute leukemia: an unusual event with poor outcome. Am J Hematol. 2012;87:890–7. doi: 10.1002/ajh.23266. [DOI] [PubMed] [Google Scholar]
- 55.Jacoby E, Nguyen SM, Fountaine TJ, et al. CD19 car immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. doi: 10.1038/ncomms12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardner R, Wu D, Cherian S, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged b-all from CD19 car-t–cell therapy. Blood. 2016;127:2406–10. doi: 10.1182/blood-2015-08-665547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen A, Petsche D, Grunberger T, Freedman MH. Interleukin 6 induces myeloid differentiation of a human biphenotypic leukemic cell line. Leuk Res. 1992;16:751–60. doi: 10.1016/0145-2126(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 58.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted car T cells induce remission in b-all that is naive or resistant to CD19-targeted car immunotherapy. Nat Med. 2018;24:20–8. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruella M, Xu J, Barrett DM, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24:1499–503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulsipher MA. Are car T cells better than antibody or hct therapy in b-all? Hematology Am Soc Hematol Educ Program. 2018;2018:16–24. doi: 10.1182/asheducation-2018.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Canadian Agency for Drugs and Technologies in Health (cadth) Tisagenlecleucel for Acute Lymphoblastic Leukemia and Diffuse Large B-Cell Lymphoma: Recommendations. Ottawa, ON: CADTH; 2019. [Google Scholar]
- 62.Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36:3192–202. doi: 10.1200/JCO.2018.79.0642. [DOI] [PubMed] [Google Scholar]
- 63.Fesnak A, Lin C, Siegel DL, Maus MV. car-t cell therapies from the transfusion medicine perspective. Transfus Med Rev. 2016;30:139–45. doi: 10.1016/j.tmrv.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant b-all after infusion of universal talen gene-edited car t cells. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aaj2013. pii:eaaj2013. [Erratum in: Sci Transl Med 2017;9:pii:aam9292] [DOI] [PubMed] [Google Scholar]
- 65.Benjamin R, Graham C, Yallop D, et al. Preliminary data on safety, cellular kinetics and anti-leukemic activity of UCART19, an allogeneic anti-CD19 car T-cell product, in a pool of adult and pediatric patients with high-risk CD19+ relapsed/refractory B-cell acute lymphoblastic leukemia [abstract 896] Blood. 2018;132(suppl 1) doi: 10.1182/blood-2018-99-111356. [DOI] [Google Scholar]
- 66.Zah E, Lin MY, Silva-Benedict A, Jensen MC, Chen YY. T Cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gardner R, Annesley C, Finney O, et al. Early clinical experience of CD19×CD22 dual specific car T cells for enhanced anti-leukemic targeting of acute lymphoblastic leukemia [abstract 278] Blood. 2018;132(suppl 1) [Google Scholar]
- 68.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell. 2017;31:476–85. doi: 10.1016/j.ccell.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Long AH, Haso WM, Shern JF, et al. 4-1BB Costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellebrecht CT, Bhoj VG, Nace A, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–84. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]