Abstract
Immune checkpoint inhibitor–based therapies that target ctla-4, PD-1, or the PD-1 ligand PD-L1 have received approval in Canada and many parts of the world for the treatment of melanoma, renal cell cancer, urothelial cancer, classical Hodgkin lymphoma, and non-small-cell lung cancer. However only a small proportion of patients derive long-term clinical benefit. Here, we describe the biomarkers associated with the complex relationship between tumour-related immune stimulus, T cell–mediated immune response, and immune modulation of the microenvironment that can help to predict improved patient outcomes.
Keywords: Immune checkpoints, PD-1, PD-L1, ctla-4, biomarkers
INTRODUCTION
Since 2009, a paradigm shift has occurred in the understanding of the relationship between the immune system, cancer development, and subsequent disease progression. Loss of immune control is now recognized as a “hallmark” of carcinogenesis1. Thanks to the foundational work of scientists such as James Allison and Tasuku Honjo, immunotherapy is a mainstay of cancer therapy alongside surgery, radiation, chemotherapy, and targeted therapy. In 2012, the humanized anti–ctla-4 antibody ipilimumab was the first immune checkpoint inhibitor approved by the pan-Canadian Oncology Drug Review (pcodr) for the treatment of advanced melanoma. Since 2015, monoclonal antibodies against other immune checkpoint molecules—namely, PD-1 and its cognate ligand PD-L1—have also received pcodr approval for a number of tumour types, including melanoma2, renal cell carcinoma3, urothelial carcinoma4, classical Hodgkin lymphoma5, and non-small-cell lung cancer (nsclc)6–8. In many of those cases, immunotherapy outperforms existing cytotoxic chemotherapy from both a survival and a quality of life perspective.
A consequence of this shift from cytotoxic therapies to immunotherapy is the substantial cost of treating a greater percentage of patients with more expensive therapies. Although phase iii clinical trials show improved survival, they also consistently show modest response rates and, regardless of histology, long-term durable responses to therapy in only approximately 15%–20% of patients. In the current era of “precision oncology,” companion biomarkers that identify patients most likely to benefit from treatment will help to simultaneously improve patient outcomes and reduce health system costs.
REVIEW
Biologic Basis of Immune Checkpoint Inhibition
The multitude of genetic and epigenetic changes seen in cancer cells contribute to the classical “hallmarks of cancer” as described by Douglas Hanahan and Robert Weinberg1. Traditional cancer therapies such as radiation, chemotherapy, and targeted therapy affect a number of those molecular mechanisms and directly exert their cytotoxic or cytostatic effect by interfering directly with cancer cells. As reviewed by Drew Pardoll9, the same factors influencing genetic and epigenetic changes in cancer also provide the opportunity for tumours to express novel antigens (“neoantigens”) that might be unique to cancer cells compared with the noncancerous cells from which they arose. Under “normal” circumstances, the neoantigens would trigger an immune response from T cells, mediated by the T cell receptor on the cell surface, leading to elimination of the cancer cell. To prevent autoimmunity and ongoing damage to healthy tissue when an immune response is triggered, the T cell response is normally tightly regulated by immune checkpoints. Unlike chemotherapy and radiation therapy, immune checkpoint inhibitors do not target the cancer cell directly, but instead work to disrupt the inhibitory immune checkpoint signals that prevent T cells from mounting a response against the evolving tumour. The two main classes of immune checkpoint inhibitors currently in widespread clinical use specifically target the immune checkpoints ctla-4 and PD-1 or PD-L1. Understanding the efficacy of those drugs requires a brief review of the delicate interplay between positive and negative regulatory signals that underpin a normal immune response.
CTLA-4
Upon neoantigen exposure, the initial T cell receptor activation on stimulated T cells leads to co-amplification of the CD28 receptor (mediated by interleukin 2) on their cell surface, which interacts with members of the B7 cell-surface molecule family (CD80, CD86) located on the antigen-presenting cells, increasing T cell proliferation and immune-mediated cell death. The activated cytotoxic T cells also co-amplify the ctla-4 receptor, which recognizes those same B7 proteins and initially helps to recruit T cells to the site of neoantigen exposure10. The greater affinity of ctla-4 compared with CD28 for the B7 proteins prevents overactivation of the T cells and helps to reduce interleukin 2 production and abrogates the immune response9.
PD-1 and PD-L1
The surface protein PD-1 is transcriptionally induced in activated T cells, B cells, and myeloid cells11. Its two ligands, PD-L1 and PD-L212,13, are also members of the B7 family of proteins and share similar sequence homology14. The major roles of PD-1 and its cognate ligands are to limit the activity of T cells in peripheral tissues during an inflammatory response and to limit autoimmunity12,15. A large proportion of tumour-infiltrating lymphocytes (tils) express PD-1 in a variety of tumour histologic types. In addition to tils within the tumour microenvironment expressing PD-1, tumour cells themselves are also recognized to have the tendency to overexpress PD-L1 as a way to avoid immune detection16. Expression of PD-L1 is also induced by a host of pro-inflammatory molecules, with interferon γ being the most potent inducer17,18. In that way, the PD-1/PD-L1 axis is a major contributor to “adaptive immune resistance” within the tumour microenvironment.
Biomarkers of Response to Immune Checkpoint Inhibitors in Cancer
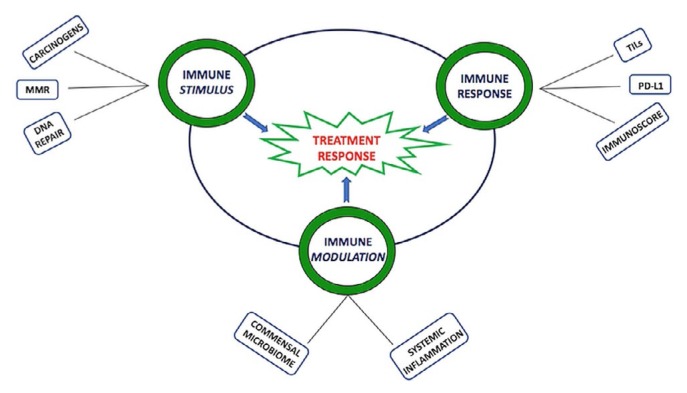
The role of the immune system in the context of cancer is reviewed in detail elsewhere9,19,20. Here, we describe biomarkers of treatment response to ctla-4 and PD-1 or PD-L1 monoclonal antibodies that are approved for cancer treatment in Canada. We explore those biomarkers as they relate to 3 key components of the immunity cycle in cancer (Figure 1): immune stimulus, immune response, and immune modulators. Emerging therapies against other immune checkpoint proteins and self-antigens overexpressed in tumour cells are not discussed.
FIGURE 1.
Multiple factors interact to influence the immune response to the developing tumour as reviewed in the text. MMR = mismatch repair; TILs = tumour-infiltrating lymphocytes.
Immune Stimulus by Increased Neoantigen Production
Tumour Mutational Burden
T Cells are activated by antigenic peptides, typically representing a foreign pathogen, in conjunction with the major histocompatibility complex. In the case of tumour cells, somatic mutations in the genome can result in nonsynonymous single-nucleotide variants that can contribute to the tumour mutational burden (tmb) of the tissue. The elevated tmb can, in turn, generate novel peptides that are then expressed on the surface of the cancer cell as a major histocompatibility complex–associated neoantigen recognized by the immune system as “foreign,” therefore generating a T cell response.
Several observations support the foregoing hypothesis. First, as demonstrated in data from The Cancer Genome Atlas, many of the observed responses to immune checkpoint inhibitors are seen in cancers with a high tmb (such as lung cancer, melanoma, and bladder cancer) and in those known to harbour associations with known carcinogens such as cigarette smoke and ultraviolet light21.
In melanoma, two studies have confirmed the correlation between high tmb, neoantigen load, and benefit from the ctla-4 antibody ipilimumab22,23. In fact, using a discovery cohort of 25 cases and a validation cohort of 39 cases, Alexandra Snyder22 and colleagues were able to demonstrate a significant association between mutational load and long-term clinical benefit, defined as radiographic disease response or stable disease for more than 6 months. In the discovery cohort, a tmb cut-off of more than 100 mutations per sample as determined by whole-exome sequencing (wes) was correlated with increased tumour response and statistically significant overall survival (os), p = 0.04.
In nsclc, Naiyer Rizvi and colleagues24 demonstrated an increased tumour objective response (63% vs. 0%, p = 0.03), duration of clinical benefit, and progression-free survival [pfs: 14.5 months vs. 3.7 months; p = 0.01; hazard ratio (hr): 0.19] with the PD-1 inhibitor pembrolizumab and a tmb greater than 200 per sample (which was the calculated median nonsynonymous mutation rate for the study population) determined by wes. Treatment efficacy also correlated with a molecular smoking signature (higher transversion vs. transition mutations), a higher candidate neoantigen burden, and mutations in dna repair pathway genes. In a retrospective analysis of patients with nsclc treated with the PD-L1 inhibitor atezolizumab in two randomized trials, Gandara and colleagues25 demonstrated that measurements of blood tmb correlated with measurements of tissue tmb. Also, a blood tmb cut-off of 16 or more mutations on their assay could identify patients experiencing better pfs with atezolizumab than with docetaxel chemotherapy.
Similar observations of associations between high tmb and response to immune checkpoint inhibitors have been seen in urothelial cancer (atezolizumab)26, small-cell lung cancer (nivolumab alone or in combination with ipilimumab) 27, and head-and-neck squamous cell cancer negative for the human papilloma virus (PD-1 or PD-L1 inhibitors)28. Subsequently, an analysis of published studies involving 27 tumour types revealed an association between the median number of somatic mutations per megabase and the objective response rate (orr) to PD-1 or PD-L1 inhibitors29. However, that study did reveal outliers, particularly in mismatch repair (mmr)–proficient colorectal cancer, in which, despite a higher tmb per The Cancer Genome Atlas, the response rate to immune checkpoint inhibitors was low. Conversely, the mutational burden in renal cell cancer was relatively low, but the treatment response was higher, suggesting that factors beyond tmb are involved. Those observations were confirmed in an integrative multi-omics approach investigating how cancer-associated mutations might correlate with tumour-associated cytotoxic T cell infiltrate. Only 5 of 19 cancer histology types (melanoma, colorectal cancer, endometrial cancer, lung adenocarcinoma, and endocervical adenocarcinoma) showed a positive correlation of T cell infiltrate with neoantigen levels30. In breast cancer, cytotoxic T cell infiltrates were seen in tumour subsets involving all four expression subtypes, but no associations with neoantigen enrichment were observed. Also, no correlation was seen between T cell infiltrate and high copy number variation, suggesting that that form of genomic instability might not have the same immunogenic potential as mutations associated with single-nucleotide variants.
MMR Deficiency
Healthy or otherwise mmr-proficient cells have carefully regulated processes that identify and correct spontaneous replication-associated dna errors within the genome. Tumours that are mmr-deficient develop many dna mutations that contribute to carcinogenesis and that can encode potential neoantigens. Deficiency in mmr is often seen with an inherited disorder—Lynch syndrome (hereditary non-polyposis colorectal cancer)—caused by mutations in dna repair genes such as MLH1, MSH2, MSH6, and PMS2. Deficiency in mmr can be identified by the presence of microsatellite instability (msi) in tumour tissue.
A phase ii clinical trial explored the efficacy of pembrolizumab monotherapy in 41 patients with known mmr status and treatment-refractory disease31. The mmr-deficient cohort included 11 patients with colorectal cancer, 4 with ampullary cholangiocarcinoma, 2 with endometrial cancer, 2 with small-bowel cancer, and 1 with gastric cancer. The mmr-proficient cohort included 21 patients with colorectal cancer. Also, of the 20 patients with mmr deficiency, all but 1 had non-colorectal clinical Lynch syndrome or a detectable germline mutation. The immune-related orr in evaluable patients was 40% in the mmr-deficient colorectal group and 71% in the mmr-deficient non-colorectal group. No objective responses were seen in 18 evaluable patients with mmr-proficient colorectal cancer. In colorectal cancer, median pfs and os were not reached in mmr-deficient cases and were, respectively, 2.2 months (p < 0.001; hr: 0.1) and 5.0 months (p = 0.05; hr: 0.22) in mmr-proficient cases. As determined by wes, tumours that were mmr-deficient had, on average, 1782 somatic mutations per sample compared with 73 somatic mutations per sample in mmr-proficient tumours (p = 0.007). On 23 May 2017, based on data from 149 patients with 15 different tumour types, the U.S. Food and Drug Administration approved pembrolizumab for the treatment of adult and pediatric patients with previously treated high msi or mmr-deficient solid tumours. That approval marks the first time that the U.S. Food and Drug Administration has approved a cancer treatment for an indication based on a common biomarker rather than on a primary tissue site of origin32. On 18 April 2019, Health Canada issued a Notice of Compliance for the use of pembrolizumab in previously-treated high msi or mmr-deficient colorectal cancer or endometrial cancer. That indication has not yet been approved by the pcodr.
Although mmr deficiency–associated mutations are the most well-explored, other genes have been associated with dna repair pathways that might also cause genomic hypermutation leading to increased neoantigens. Some examples of those dna repair genes include POLE, POLD1, PRKDC, RAD15C, and RAD1724.
Challenges in the Clinic
Because of the higher prevalence of Lynch syndrome in patients with colorectal cancer, clinical practice guidelines recommend routine msi analysis and mmr immunohistochemistry (ihc) after diagnosis. Despite the guidelines, screening is not universally available, and regional variation is significant33. Also, routine screening for mmr deficiency is not yet part of guidelines for non-colorectal-cancer types. Detection of tmb represents an even greater challenge in the clinical setting. The usual definition of tmb is the total number of exonic somatic mutations within the given genomic territory of the assay being used. Most studies correlating the association between tmb and immune checkpoint inhibitor response have relied on wes by next-generation sequencing, which represents a fairly accurate and nonbiased method. The use of next-generation sequencing in clinical laboratories is limited mostly to smaller defined cancer-gene panels because the cost of wes can be prohibitive. Several commercial gene panels correlate with wes-measured tmb25,34, but require prospective validation across multiple cancer types. Finally, with tmb being a continuous variable, appropriate cut-offs can vary with the cancers examined and the methods used. Efforts to harmonize the various processes are required.
Immune Response Through T Cell Recruitment and Activation
TILs
The ultimate goal of immune checkpoint inhibition is to stimulate a T cell response against the “foreign” tumour. Recruitment of the cytotoxic T cells to the tumour is an important part of that process. As early as 1998, Yoshitaka Naito and colleagues35 identified 3 different patterns of cytotoxic CD8-positive T cells when examining a collection of 131 cases of resected colorectal cancer. The patterns included T cells that were infiltrated within cancer-cell nests, distributed in the cancer stroma, and present along the tumour–host interface (invasive margin). In multivariate analysis, only the CD8-positive T cells within the cancer-cell nests had a significant independent association with patient survival (p = 0.016; hr: 0.52). A higher proportion of T cells in cancer-cell nests also had an inverse correlation with Dukes cancer staging: earlier stages were associated with greater T cell infiltration. Similarly, an assessment of 273 lobectomy and segmentectomy samples from patients with stage ia nsclc revealed that high infiltration of tils in the stroma or tumour, compared with lack of such infiltration, was associated with improved 5-year recurrence-free survival (p = 0.011) 36. Limited data also suggest similar positive correlations between the presence of tils and improved patient survival in renal cell carcinoma and urothelial carcinoma37,38. However, in all cancer histologic types, the favourable prognostic role of tils might be oversimplified, as recently reviewed in head-and-neck squamous-cell cancers39; further validation is required.
The density of tils was also found to be predictive of improved outcomes in the setting of immune checkpoint inhibition. One retrospective analysis considered tumour biopsies from 46 patients with metastatic melanoma before and during treatment with pembrolizumab. In treatment responders, compared with patients who progressed on treatment, a higher density of CD8-positive cells was observed in pretreatment samples at the invasive margin, and those cells contained higher levels of phosphorylated stat1 (p = 0.002), a downstream effector of interferon γ binding its receptor40. Upon pembrolizumab exposure, tumours from treatment responders showed increased CD8+ density at the invasive margin and within the tumour. The increase in CD8+ cell density from the pretreatment to the posttreatment sample also correlated with response, and a less diverse repertoire of T cell receptors was observed on the cell surface of those T cells, suggesting a more limited clonal phenotype.
Preliminary data from clinical trials in breast cancer also show similar associations between tils and immune checkpoint inhibitor response. In the phase ib/ii trial of pembrolizumab–trastuzumab in metastatic HER2-positive breast cancer, the presence of tils at baseline was associated with a higher orr and a longer duration of disease control. However, specimens with more tils at baseline were also more likely to be PD-L1–positive41.
PD-L1 Expression
As described earlier, several studies have demonstrated the importance of tils at the invasive margin or within the tumour stroma. However, it is also recognized that not all of those tils can effect their antitumour response even when localized within the proximity of cancer cells. As already mentioned, cancer cells and other immune cells within the microenvironment can express PD-L1 to trigger the adaptive immune response and avoid immune-mediated destruction by the host16. Almost all clinical studies of PD-1/PD-L1 modulating agents in cancer have investigated the possible correlation between tumour or immune cell PD-L1 expression and therapeutic efficacy. Many studies of approved immune checkpoint inhibitors have demonstrated that overexpression of PD-L1 is associated with significantly improved treatment response and os; other studies show no or weaker associations based on histologic subtype, which will be discussed in detail shortly.
In the keynote-001 trial of pembrolizumab monotherapy in melanoma, the orr was 57% in cases in which PD-L1 expression was observed in 33%–66% of tumour cells or tumour-associated immune cells; the orr was 8% in cases without PD-L1 expression42. Conversely, in the Check-Mate 067 study, the combination nivolumab–ipilimumab was more effective than nivolumab monotherapy in patients with low PD-L1 expression, although longer-term outcomes were independent of PD-L1 status43. Although PD-L1 expression is associated with an overall increased response in melanoma, some patients with PD-L1–negative tumours experience a long-term response, and PD-L1 positivity itself does not guarantee a long-term response. Therefore, none of the immune checkpoint inhibitors currently approved for melanoma require knowledge of tumour or immune cell PD-L1 expression status for clinical access (Table I).
TABLE I.
Immune checkpoint inhibitors assessed by the pan-Canadian Oncology Drug Review
| Drug and tumour type | Stage | Indication | Implementation | Biomarker details |
|---|---|---|---|---|
| Atezolizumab | ||||
| NSCLC | IV | Second line | 6 Jul 2018 | — |
| SCLC | Extensive | First line | 14 Feb 2020 | — |
|
| ||||
| Durvalumab | ||||
| NSCLC | III | Consolidative | 21 May 2019 | — |
|
| ||||
| Ipilimumab | ||||
| Melanoma | Advanced | First line | 14 Jan 2015 | — |
| Second line | 2 May 2012 | — | ||
|
| ||||
| Nivolumab | ||||
| Melanoma | Advanced | First line | 18 Apr 2016 | — |
| Node-positive | Adjuvant | 22 Mar 2019 | — | |
| NSCLC | IV | Second line | 20 Jun 2016 | — |
| Renal cell carcinoma | IV | Second line | 19 Sep 2016 | — |
| HNSCC | IV | Second line | 18 Sep 2017 | — |
| Hodgkin lymphoma | — | Failed ASCT | 18 May 2018 | — |
|
| ||||
| Ipilimumab–nivolumab | ||||
| Melanoma | Advanced | First line | 15 Dec 2017 | — |
| Renal cell carcinoma | IV | First line | 16 Nov 2018 | — |
|
| ||||
| Pembrolizumab | ||||
| Melanoma | Advanced | First line | 1 Dec 2015 | — |
| III | Adjuvant | 19 Aug 2019 | — | |
| NSCLC | IV | First line | 8 Sep 2017 | PD-L1 TPS ≥50% |
| First linea | 17 Jun 2019 | — | ||
| First lineb | 20 Jan 2020 | — | ||
| Second line | 18 November 2016 | PD-L1 TPS ≥1% | ||
| Hodgkin lymphoma | — | Relapsed | 22 Jan 2018 | — |
| Urothelial carcinoma | IV | First line | 21 Oct 2019 | PD-L1 CPS ≥10% |
| Second line | 19 Mar 2018 | — | ||
Nonsquamous histology.
Squamous histology.
NSCLC = non-small-cell lung cancer; SCLC = small-cell lung cancer; HNSCC = head and neck squamous-cell carcinoma; ASCT = autologous stem-cell transplantation; TPS = tumour proportion score; CPS = combined positive score.
In nsclc, clinical studies of PD-1 or PD-L1 modulating agents have investigated the possible correlation between tumour PD-L1 expression and therapeutic efficacy. The phase iii CheckMate 017 trial in advanced squamous-cell nsclc and the CheckMate 057 trial in advanced nonsquamous nsclc both demonstrated improved os for nivolumab compared with docetaxel in second-line therapy after platinum doublet chemotherapy6,7. In CheckMate 017, a 20% orr was observed in the nivolumab group, and no associations with PD-L1 expression were observed in subgroup analyses7. CheckMate 057 showed a significant improvement in os with nivolumab (12.2 months vs. 9.4 months; p = 0.002; hr:0.73) and a 19% response rate6. Subgroup analyses demonstrated a significant association between tumour PD-L1 expression (≥1% tumour membrane expression) and both pfs and os in the nivolumab-treated group. However, os in the less than 5% PD-L1 expression group did not differ significantly between the nivolumab and docetaxel arms. Therefore, for all nsclc subtypes, a known PD-L1 expression status is not required to access nivolumab in the second-line setting. The similarly designed phase iii oak trial demonstrated improved survival for atezolizumab compared with docetaxel in combined nsclc subtypes. The atezolizumab group had a 14% orr and a median os duration of 13.8 months44. Patients in the highest PD-L1 tumour or immune cell expression subgroup derived the greatest benefit (median os duration: 20.5 months; hr: 0.41); however, even the subgroup without PD-L1 expression experienced a statistically significantly improved os (median os duration: 12.6 months; hr: 0.75). The phase iii keynote-010 trial compared pembrolizumab monotherapy with docetaxel in nsclc after platinum doublet chemotherapy45. All enrolled patients were required to demonstrate 1% or greater PD-L1 expression on tumour cells (the tumour proportion score, tps). The os and orr were significantly greater for both pembrolizumab groups than they were for the docetaxel group. Access to pembrolizumab for nsclc in previously treated patients in Canada therefore requires mandatory ihc staining for tumour PD-L1.
A meta-analysis of twelve studies in nsclc (3790 patients total) demonstrated an odds ratio for orr of 2.18 (95% confidence interval: 1.45 to 3.29; p = 0.0002) when comparing tumour PD-L1 expression greater than 1% with expression less than 1% in patients treated with PD-1 or PD-L1 inhibitors (atezolizumab, avelumab, durvalumab, nivolumab, and pembrolizumab)46. Furthermore, the odds ratio for orr increased when comparing PD-L1 cut-offs of more than 5% with less than 5%, more than 10% with less than 10%, and more than 50% with less than 50%. Taken together, those data suggest that treatment with PD-1 or PD-L1 inhibitors offers the greatest benefit when tumour PD-L1 expression in nsclc is greater than 1%, and that a possible dose–effect relationship might exist between the intensity of PD-L1 staining and the efficacy of the inhibitor.
In the first-line treatment setting, the phase iii keynote-024 trial compared pembrolizumab monotherapy with standard platinum doublet chemotherapy. Only patients with a PD-L1 tps of 50% or greater with no EGFR or ALK mutations were enrolled8. Based on a preplanned interim analysis, the trial was stopped early because of the superiority of pembrolizumab. Median pfs was significantly higher in the pembrolizumab group than in the platinum-based chemotherapy group (10.3 months vs. 6.0 months; hr: 0.5). Also higher in the pembrolizumab group were the estimated 6-month survival (80.2%) and the response rate (44.8%). The phase iii CheckMate 026 trial also set out to compare the benefit of nivolumab monotherapy with that of platinum doublet chemotherapy in the first-line treatment of nsclc47. Patients were required to have a PD-L1 tps of 5% or greater and no EGFR or ALK mutations. No significant difference in pfs or os was observed between the two study groups. In the nivolumab group, the response rate was 26.1%, and median pfs and os were 4.2 months and 14.4 months respectively. Based on those studies, pembrolizumab is the only immune checkpoint inhibitor monotherapy approved for first-line treatment of advanced nsclc; however, access is limited to tumours with a tps of 50% or greater (Table I). In patients with stage iii nsclc treated with consolidation durvalumab therapy after chemoradiation, an os advantage over placebo was seen independent of PD-L1 expression48.
In renal cell cancer, data about the value of PD-L1 expression and the response to immune checkpoint inhibitors are conflicting. The phase iii CheckMate 025 trial of nivolumab compared with everolimus in previously treated advanced clear-cell renal cell carcinoma demonstrated an improved median os (25.0 months vs. 19.6 months, p = 0.0018; hr: 0.73) in favour of immunotherapy3. Overall, PD-L1 expression was absent in 76% of all assessable pretreatment samples, and an os benefit was observed independent of PD-L1 status. In fact, a numerically improved os was evident for tumours lacking detectable PD-L1 expression compared with those having PD-L1 expression of 1% or more (median: 27.4 months and 21.8 months respectively). Similarly, in the CheckMate 214 study of combination nivolumab–ipilimumab compared with sunitinib for the first-line treatment of metastatic renal cell carcinoma, 71%–74% of all tumours assessed were PD-L1–negative49. Although a positive compared with a negative PD-L1 status was predictive of orr (58% vs. 37% respectively) and pfs (22.8 months vs. 11.0 months respectively), it was not associated with an os benefit. Expression of PD-L1 is therefore not required for the approved use of immunotherapy in renal cell cancer.
The phase iii keynote-045 trial of pembrolizumab compared with single-agent chemotherapy as second-line treatment for advanced urothelial carcinoma progressing after platinum-based chemotherapy showed an improved os with immunotherapy in the overall population (10.3 months vs. 7.4 months, p = 0.002; hr: 0.73)4. However, in patients with PD-L1 expression in tumour and infiltrating immune cells (combined positive score of 10% or greater), the results for os were even more significant in favour of pembrolizumab (8.0 months vs. 5.2 months, p = 0.005; hr: 0.57). Furthermore, the single-arm phase ii keynote-052 trial of pembrolizumab monotherapy as first-line treatment for platinum-ineligible urothelial cancer also showed an association between PD-L1 expression and response50. Of the 374 patients in the study, 24% experienced a response to therapy, and in the 110 patients with a combined positive score of 10% or greater, a 42% orr was observed. Pembrolizumab is currently being evaluated by the pcodr for the first-line treatment of platinum-ineligible patients with a combined positive score of 10% or greater (Table I).
Quantification of Immune Cell Populations
As already described, studies examining tils and PD-L1 expression have typically treated the immune infiltrate as a homogeneous population and have focused predominantly on the location and density of the infiltrate. In reality, the immune infiltrate represents a heterogeneous population of several phenotypically distinct cell populations. Jérôme Galon and colleagues51 recently validated their Immunoscore signature for early-stage colorectal cancer, initially published in 2006, that looks at both cell population and localization within the tumour. The Immunoscore specifically uses ihc for CD3, considering the total lymphocyte population, and ihc for CD8, considering the cytotoxic T cell population at both the tumour centre and the invasive margin. Using a training set of 700 patients and a validation set of 1345 patients, they were able to demonstrate that “inflamed” tumours (high Immunoscore) had a lower risk of 5-year disease recurrence than did “non-inflamed” (low Immunoscore) tumours. The predictive score was independent of TNM stage and msi status. Recently, applying more modern techniques such as single-cell rna sequencing to melanoma has shown that the ratio of CD8-positive T cells with and without expression of the transcription factor tcf7 can be used to identify T cell exhaustion and the likelihood of response to immune checkpoint inhibition52. Further work on immune-cell phenotypes in various cancer types is still required to better understand the role of the various immune cells in each disease.
Challenges in the Clinic
Despite tumour PD-L1 expression being the only approved predictive biomarker of efficacy for PD-1 or PD-L1 inhibitors in Canada, solely relying on that marker is controversial. Several anti–PD-L1 diagnostic antibodies are available, and each antibody is clinically validated only in the context of its companion drug trial: nivolumab (Dako 28-8), pembrolizumab (Dako 22C3), atezolizumab (Ventana SP142), durvalumab (Ventana SP263), and avelumab (Dako 73-10)53. Despite variability in staining intensity and pattern, inter-assay comparability is good, especially for tumour cell staining, but reliability can be quite poor, especially when scoring immune cells. It is impractical for most clinical laboratories to implement processes and training for multiple different PD-L1 assays. Also, with limited correlation between PD-L1 staining and tmb, and with both of those factors being continuous variables, ideal selection of patients for therapy remains a challenge54. The Immunoscore and immune-cell phenotyping represent interesting areas for further exploration; however, as with tmb, widespread access to appropriate gene sequencing technology might be limited. Also, the demonstrated efficacy of chemotherapy–immunotherapy combinations across the entire spectrum of disease might make even the few approved biomarkers—such as PD-L1—potentially obsolete in the future55.
Immune Modulation in the Host and Non-Host Microenvironment
Intuitively, a healthy and active immune system is a prerequisite to mount an immune response against both exogenous infectious pathogens and endogenous threats such as malignant cells. As recently reviewed56, a number of measurable clinical factors that might positively or negatively influence or be associated with inflammatory states could have the ability to predict the likelihood of responding to immune checkpoint inhibitors.
Systemic Inflammation
A complete blood count and serum chemistry are relatively simple to obtain and are regularly performed in most cancer patients at baseline and at regular intervals during the treatment journey. Measurable markers specifically associated with systemic inflammation have been shown to correlate with treatment response. Serum lactate dehydrogenase, associated with a high tumour load and cellular turnover, when greater than the upper limit of normal, was associated with worse survival with immune checkpoint inhibitors in both nsclc and melanoma56,57. Also in melanoma, an increase in lactate dehydrogenase associated with treatment was more prognostic of a poor outcome. Similarly, elevated peripheral lymphocyte counts (total white blood cell count minus absolute neutrophil count) might also reflect the immune system’s ability to mount an antitumour response. Again, in both nsclc and melanoma, a neutrophil-to-lymphocyte ratio of 3 or greater was associated with resistance to immune checkpoint inhibition and worse os.
If peripheral lymphocytes are important to the immune checkpoint inhibitor response, then immunosuppressive agents should impair that effect. A recent study explored outcomes in 90 of 640 patients with nsclc who received 10 mg or more of prednisone at the start of PD-1 or PD-L1 therapy58. Steroid use at baseline was significantly associated with decreased pfs (p = 0.03; hr: 1.3) and os (p= 0.001; hr: 1.7). Although those numbers are small, the routine use of systemic corticosteroids in a patient receiving immune checkpoint inhibitors is generally discouraged.
Commensal Microbiome
In 2013, it was demonstrated for the first time that the composition of the microbiome could influence the efficacy of the immunomodulatory drug cyclophosphamide59. Since then, it has been demonstrated in mouse and human models alike that the composition of the microbiome can also influence the response to immune checkpoint inhibitors. Bertrand Routy and colleagues60 recently examined the influence of the gut microbiome on the efficacy of PD-1–based immunotherapy in epithelial tumours. They demonstrated that concomitant use of broad-spectrum antibiotics compromised the efficacy of PD-1 inhibitors alone or in combination with ctla-4 inhibitors in a mouse xenograft model of melanoma. They next examined 249 patients with tumours of varying histologic type (nsclc, 140; renal cell cancer, 67; urothelial cancer, 42) treated with PD-1 or PD-L1 inhibitors. In 69 patients (28%), antibiotics had been prescribed within 2 months before or 1 month after the start of immunotherapy. In antibiotic-treated patients, compared with their untreated counterparts, pfs and os were both shorter. Shotgun sequencing showed that fecal samples from patients who were classified as having experienced a response to PD-1 blockade (per the Response Evaluation Criteria in Solid Tumors) had a greater abundance of the commensal bacteria Akkermansia muciniphila. Further, in germ-free mice, fecal microbiota transplants from PD-1–responsive patients were demonstrated to be capable of synergizing with PD-1 therapy to mitigate tumour growth. Oral gavage with A. muciniphila was able to restore sensitivity to PD-1 inhibitors in mice that had previously received a fecal microbiota transplant from PD-1–nonresponsive patients or in mice previously sterilized with antibiotics. Those elegant experiments—and the influence of the gut microbiota on immunotherapy and general health—are reviewed elsewhere61.
Challenges in the Clinic
Many of the biomarkers associated with immune modulation still require prospective clinical validation. Also, none of the markers show perfect correlation with treatment response and are insufficient to emphatically deny patients a chance to receive transformative therapy. The association of the microbiome with response represents an exciting direction for future exploration, but limitations in access to technology and expertise again makes widespread clinical access a challenge.
SUMMARY
In the years since 2009, several insights into biomarkers that could potentially help to personalize the use of immunotherapy in cancer and make treatments more cost-effective have been achieved. The application of many biomarkers remains complex, because significant variability remains in the effectiveness of those biomarkers in different cancer types and different clinical stages of the same cancer. The lack of standardization in methods or even a “gold standard” has made implementation of even the more accessible biomarkers challenging. As knowledge of the mechanisms of immune checkpoint inhibition in cancer improves and as the technology advances, improvements can hopefully be made both in the application of existing biomarkers and the identification of new ones to maximize patient outcomes.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: SB is actively participating in immunotherapy clinical trials sponsored by AstraZeneca, Merck, and Roche, and has also received honoraria for advisory board participation from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol–Myers Squibb, GlaxoSmith-Kline, Lilly, Merck, Novartis, Pfizer, Roche, and Takeda. DEM has no conflicts of interest to disclose.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [Erratum in: N Engl J Med 2018;379:2185] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, et al. on behalf of the CheckMate 025 investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellmunt J, de Wit R, Vaughn DJ, et al. on behalf of the keynote-045 investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–26. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–19. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, Rodríguez-Abreu D, Robinson AG, et al. on behalf of the keynote-024 investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knieke K, Hoff H, Maszyna F, et al. CD152 (ctla-4) determines CD4 T cell migration in vitro and in vivo. PLoS One. 2009;4:e5702. doi: 10.1371/journal.pone.0005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–16. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SY, Otsuji M, Gorski K, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 17.Derré L, Rivals JP, Jandus C, et al. btla mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120:157–67. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo A, Yamashita T, Tamura H, et al. Interferon-γ and tumor necrosis factor–α induce an immunoinhibitory molecule, B7-H1, via nuclear factor–κB activation in blasts in myelodysplastic syndromes. Blood. 2010;116:1124–31. doi: 10.1182/blood-2009-12-255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher TN, Kesmir C, van Buuren MM. Biomarkers in cancer immunotherapy. Cancer Cell. 2015;27:12–14. doi: 10.1016/j.ccell.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–18. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to ctla-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to ctla-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. [Errata in: Science 2015;350:aad8366, and Science 2016;352:aaf8264] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med. 2018;24:1441–8. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2019;33:853–61. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanna GJ, Lizotte P, Cavanaugh M, et al. Frameshift events predict anti–PD-1/L1 response in head and neck cancer. JCI Insight. 2018;3:98811. doi: 10.1172/jci.insight.98811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–1. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGrail DJ, Federico L, Li Y, et al. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat Commun. 2018;9:1317. doi: 10.1038/s41467-018-03730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcus L, Lemery SJ, Keegan P, Pazdur R. fda approval summary: pembrolizumab for the treatment of microsatellite instability–high solid tumors. Clin Cancer Res. 2019;25:3753–8. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 33.Jain A, Shafer L, Rothenmund H, et al. Suboptimal adherence in clinical practice to guidelines recommendation to screen for Lynch syndrome. Dig Dis Sci. 2019;64:3489–501. doi: 10.1007/s10620-019-05692-6. [DOI] [PubMed] [Google Scholar]
- 34.Endris V, Buchhalter I, Allgäuer M, et al. Measurement of tumor mutational burden (tmb) in routine molecular diagnostics: in silico and real-life analysis of three larger gene panels. Int J Cancer. 2019;144:2303–12. doi: 10.1002/ijc.32002. [DOI] [PubMed] [Google Scholar]
- 35.Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 36.Horne ZD, Jack R, Gray ZT, et al. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J Surg Res. 2011;171:1–5. doi: 10.1016/j.jss.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 37.Sharma P, Shen Y, Wen S, et al. CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A. 2007;104:3967–72. doi: 10.1073/pnas.0611618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao J, Xi W, Zhu Y, Wang H, Hu X, Guo J. Checkpoint molecule PD-1-assisted CD8+ T lymphocyte count in tumor microenvironment predicts overall survival of patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Cancer Manag Res. 2018;10:3419–31. doi: 10.2147/CMAR.S172039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Meulenaere A, Vermassen T, Aspeslagh S, Vandecasteele K, Rottey S, Ferdinande L. tils in head and neck cancer: ready for clinical implementation and why (not)? Head Neck Pathol. 2017;11:354–63. doi: 10.1007/s12105-016-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loi S, Giobbie-Hurder A, Gombos A, et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (panacea): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019;20:371–82. doi: 10.1016/S1470-2045(18)30812-X. [DOI] [PubMed] [Google Scholar]
- 42.Daud AI, Wolchok JD, Robert C, et al. Programmed death–ligand 1 expression and response to the anti–programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–9. doi: 10.1200/JCO.2016.67.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–56. doi: 10.1056/NEJMoa1709684. [Erratum in: N Engl J Med 2018;379:2185] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1–positive, advanced non-small-cell lung cancer (keynote-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 46.Abdel-Rahman O. Correlation between PD-L1 expression and outcome of nsclc patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematology. 2016;101:75–85. doi: 10.1016/j.critrevonc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2016;34:2980–7. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antonia SJ, Villegas A, Daniel D, et al. on behalf of the pacific investigators. Overall survival with durvalumab after chemoradiotherapy in stage iii nsclc. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 49.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (keynote-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–92. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 51.Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–39. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 52.Sade-Feldman M, Yizhak K, Bjorgaard SL, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013. doi: 10.1016/j.cell.2018.10.038. [Erratum in: Cell 2019;176:404] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of Blueprint phase 2 project. J Thorac Oncol. 2018;13:1302–11. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33:843–52. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. on behalf of the keynote-189 investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 56.Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment—a review from the melanoma perspective and beyond. Front Immunol. 2018;9:1474. doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018;4:351–7. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death–1 and programmed death–ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8. doi: 10.1200/JCO.2018.79.0006. [DOI] [PubMed] [Google Scholar]
- 59.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 61.Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018;15:382–96. doi: 10.1038/s41571-018-0006-2. [DOI] [PubMed] [Google Scholar]