Abstract
In this retrospective case series, chest CT scans of 21 symptomatic patients from China infected with the 2019 novel coronavirus (2019-nCoV) were reviewed, with emphasis on identifying and characterizing the most common findings. Typical CT findings included bilateral pulmonary parenchymal ground-glass and consolidative pulmonary opacities, sometimes with a rounded morphology and a peripheral lung distribution. Notably, lung cavitation, discrete pulmonary nodules, pleural effusions, and lymphadenopathy were absent. Follow-up imaging in a subset of patients during the study time window often demonstrated mild or moderate progression of disease, as manifested by increasing extent and density of lung opacities.
© RSNA, 2020
Summary
The 2019 novel coronavirus manifests with characteristic chest CT imaging features, which are helpful to the radiologist for the early detection and diagnosis of this emerging global health emergency.
Key Results
■ Of 21 patients with the 2019 novel coronavirus, 15 (71%) had involvement of more than two lobes at chest CT, 12 (57%) had ground-glass opacities, seven (33%) had opacities with a rounded morphology, seven (33%) had a peripheral distribution of disease, six (29%) had consolidation with ground-glass opacities, and four (19%) had crazy-paving pattern.
■ Lung cavitation, discrete pulmonary nodules, pleural effusions, and lymphadenopathy were absent.
■ Fourteen percent of patients (three of 21) presented with a normal CT scan.
Introduction
On December 31, 2019, the World Health Organization (WHO) was alerted to several cases of a respiratory illness of unknown origin emerging from Wuhan City, Hubei Province of China, with clinical presentations resembling those of viral pneumonia and manifesting as fever, cough, and dyspnea. As of January 30, 2020, the WHO has designated this outbreak as a global health emergency. Patients often had pulmonary parenchymal opacities at chest radiography. By January 3, 2020, 44 patients with this unexplained illness were reported to the WHO, with preliminary epidemiologic investigation demonstrating that most patients worked at or were frequent visitors to the Huanan Seafood Wholesale Market (1). Analysis of bronchoalveolar lavage fluid samples and electron microscopy revealed the culprit to be a coronavirus, a virus with a characteristic crown morphology at scanning electron microscopy, which is due to the presence of viral spike peplomers emanating from the viral envelope.
This newly discovered virus has been temporarily named the 2019 novel coronavirus (2019-nCoV). Coronaviruses belong to the family Coronaviridae and the order Nidovarales, a family that includes viruses that cause diseases ranging from the common cold to severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS) (2). The SARS outbreak started in southern China and was recognized as a global public health threat in March 2003, resulting in 774 deaths out of 8098 individuals who were infected from November 2002 through July 2003 (3). MERS was first reported in 2012 in Saudi Arabia and resulted in 858 deaths among 2494 infected individuals to date (4).
As of January 28, 2020, 4593 confirmed 2019-nCoV cases have been reported globally. Of these, 4537 were from China and 56 were from 14 other countries. Furthermore, an additional 6973 cases are suspected in China. As of January 28, 2020, there have been 106 deaths—all in China (5). On January 30, 2020, the China coronavirus was declared as a global health emergency by the WHO. An initial prospective analysis of the clinical features of 41 of the initially admitted patients in Wuhan with laboratory-confirmed 2019-nCoV demonstrated that 2019-nCoV caused a severe illness similar clinically to SARS and sometimes resulted in admission to the intensive care unit (13 of 41 patients [32%]) and death (six of 41 patients [15%]). All 41 patients with pneumonia in that study had abnormal findings on chest CT scans, with preliminary reports indicating bilateral abnormal lung opacities in each patient (6).
Herein we describe and characterize the key CT findings in a group of 21 patients infected with 2019-nCoV in China, with the goal of familiarizing radiologists and clinical teams with the imaging manifestations of this new outbreak. Early disease recognition can speed up treatment and prompt early patient isolation. This will allow for early implementation of public health surveillance, containment, and response to this communicable disease.
Materials and Methods
Patients and Chest CT
Our institutional review board waived the requirement to obtain written informed consent for this retrospective case series, which evaluated de-identified data and involved no potential risk to patients. To avert any potential breach of confidentiality, no link between the patients and the researchers was made available.
From January 18, 2020, until January 27, 2020, 21 patients admitted to three hospitals in three provinces in China with confirmed 2019-nCoV underwent chest CT. Ten patients were from Zhuhai (Guangdong Province) and were imaged with 1-mm-thick slices with a UCT 760 scanner (United Imaging, Shanghai, China). Nine patients were from Nanchang (Jiangxi Province) and were imaged with 8-mm-thick slices with an Emotion 16 scanner (Siemens Healthineers, Erlangen, Germany). Two patients were from Qingdao (Shandong Province) and were imaged with 5-mm-thick slices, one with a BrightSpeed scanner (GE Medical Systems, Milwaukee, Wis) and one with an Aquilion ONE scanner (Toshiba Medical Systems, Tokyo, Japan). All scans were obtained with the patient in the supine position during end-inspiration without intravenous contrast material. All patients were positive for 2019-nCov at laboratory testing of respiratory secretions obtained by means of bronchoalveolar lavage, endotracheal aspirate, nasopharyngeal swab, or oropharyngeal swab.
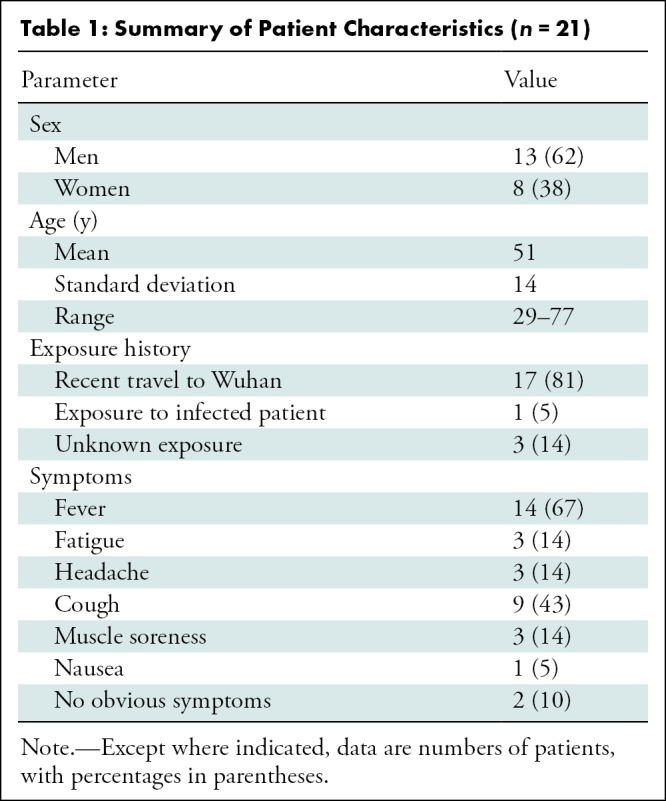
Patient selection for this study was consecutive in each of the three institutions, and no exclusion criteria were applied (Table 1). In addition to age and sex, clinical information collected included severity and time course of symptoms as well as travel and exposure history.
Table 1:
Summary of Patient Characteristics (n = 21)
CT Image Review
All CT images were reviewed by two fellowship-trained cardiothoracic radiologists with approximately 5 years of experience each (M.C. and A.B.) by using a viewing console. Images were reviewed independently, and final decisions were reached by consensus. For disagreement between the two primary radiologist interpretations, a third fellowship-trained cardiothoracic radiologist with 10 years of experience (A.J.) adjudicated a final decision. No negative control cases were examined, and no blinding occurred.
For each of the 21 patients, the initial CT scans were evaluated for the following characteristics: (a) presence of ground-glass opacities, (b) presence of consolidation, (c) number of lobes affected by ground-glass or consolidative opacities, (d) degree of lobe involvement in addition to overall lung “total severity score,” (e) presence of nodules, (f) presence of a pleural effusion, (g) presence of thoracic lymphadenopathy (defined as lymph node size of ≥10 mm in short-axis dimension), and (h) presence of underlying lung disease such as emphysema or fibrosis. Other abnormalities (eg, cavitation, reticulation, interlobular septal thickening, calcification, and bronchiectasis) were noted. Ground-glass opacification was defined as hazy increased lung attenuation with preservation of bronchial and vascular margins, and consolidation was defined as opacification with obscuration of margins of vessels and airway walls (7). Each of the five lung lobes was assessed for degree of involvement and classified as none (0%), minimal (1%–25%), mild (26%–50%), moderate (51%–75%), or severe (76%–100%). No involvement corresponded to a lobe score of 0, minimal involvement to a lobe score of 1, mild involvement to a lobe score of 2, moderate involvement to a lobe score of 3, and severe involvement to a lobe score of 4. An overall lung “total severity score” was reached by summing the five lobe scores (range of possible scores, 0–20). Eight patients underwent follow-up chest CT during the study time window. These scans were also evaluated to assess for change or progression over time, which was evaluated qualitatively with a consensus approach by two of the radiologists (M.C. and A.B.).
Results
There were 13 men and eight women studied (age range, 29–77 years; mean age ± standard deviation, 51 years ± 14).
Ground-Glass Opacities and Consolidation
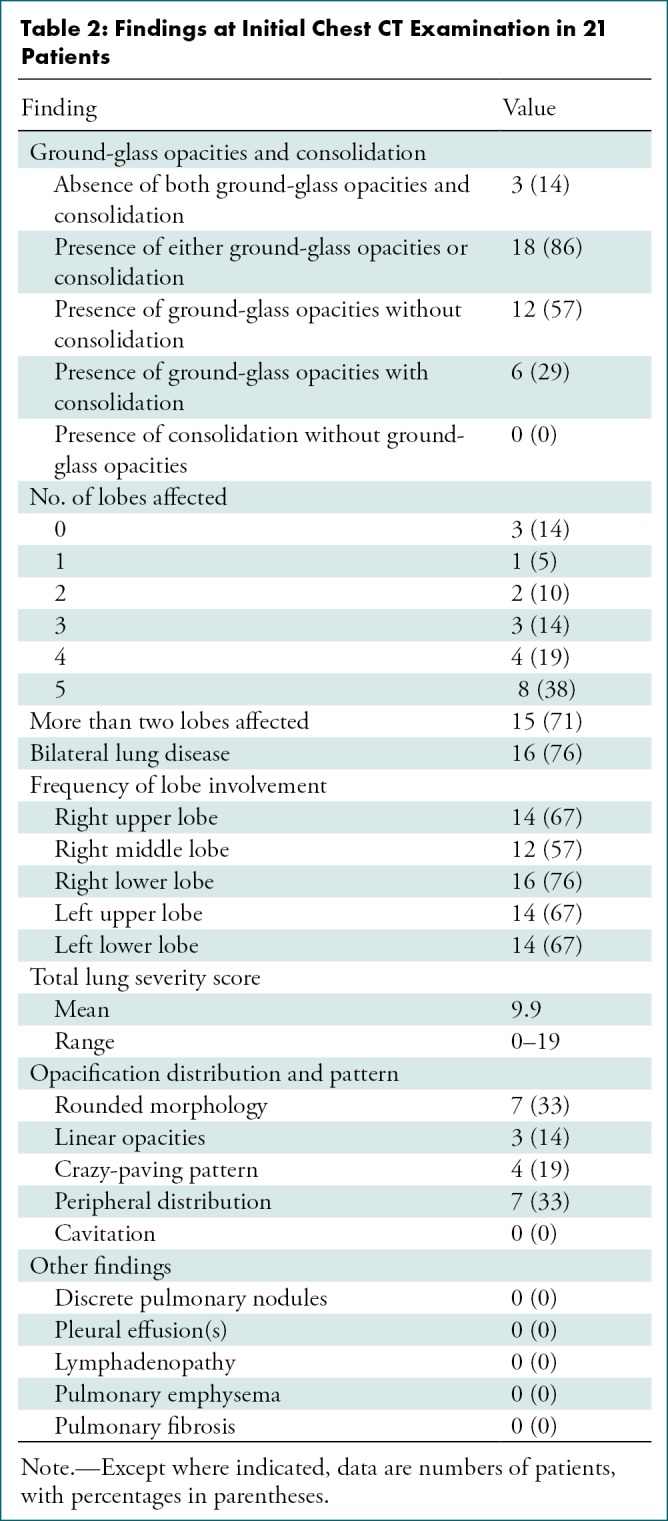
Of the 21 initial chest CT scans, three (14%) showed no ground-glass opacities or consolidation; in fact, these three patients had entirely normal chest CT examinations at presentation (Table 2). Of the 18 patients with ground-glass opacities, consolidation, or both, 12 had only ground-glass opacities (with no consolidation); no patient demonstrated consolidation without ground-glass opacification.
Table 2:
Findings at Initial Chest CT Examination in 21 Patients
Opacification Distribution and Patterns
Excluding the three patients with a normal initial chest CT examination, the remaining 18 of 21 patients (86%) by definition had disease affecting at least one lobe. Of the 21 patients, one patient (5%) had one affected lobe, two patients (10%) had two affected lobes, three patients (14%) had three affected lobes, four patients (19%) had four affected lobes, and eight patients (38%) had disease affecting all five lobes.
The right upper lobe was involved in 14 of the 21 patients at initial CT (67%), the right middle lobe was involved in 12 (57%), the right lower lobe was involved in 16 (76%), the left upper lobe was involved in 14 (67%), and the left lower lobe was involved in 14 (67%). Of the 18 patients with lung opacities, 16 had bilateral disease and two had unilateral disease (both had right lung involvement only).
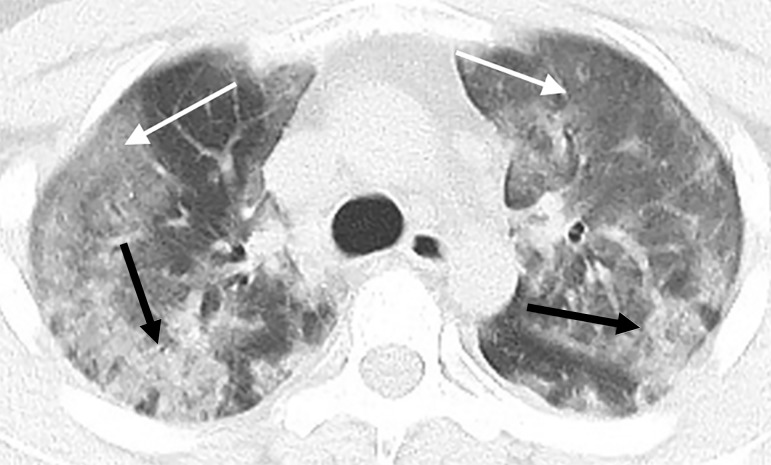
The total lung severity score ranged from 0 (in the three normal CT examinations) to a maximum of 19, with a mean score of 9.9. The patient with the highest lung severity score was admitted to the intensive care unit (Fig 1).
Figure 1a:
Images in a 29-year-old man with unknown exposure history who presented with fever and cough ultimately requiring admission to intensive care unit. (a) Axial thin-section unenhanced CT scan shows diffuse bilateral confluent and patchy ground-glass (white arrows) and consolidative (black arrows) pulmonary opacities. (b) Axial unenhanced image shows that the disease in the right middle and lower lobes has a striking peripheral distribution (arrows).
Figure 1b:

Images in a 29-year-old man with unknown exposure history who presented with fever and cough ultimately requiring admission to intensive care unit. (a) Axial thin-section unenhanced CT scan shows diffuse bilateral confluent and patchy ground-glass (white arrows) and consolidative (black arrows) pulmonary opacities. (b) Axial unenhanced image shows that the disease in the right middle and lower lobes has a striking peripheral distribution (arrows).
Seven of the initial 21 patients (33%) demonstrated ground-glass and/or consolidative opacities with a rounded morphology (Fig 2). Three of the 21 patients (14%) demonstrated a predominantly linear abnormality and four (19%) demonstrated a crazy-paving pattern (Fig 3). Seven patients (21%) had a peripheral distribution of disease (Fig 4). No patients had cavitation in the lung, discrete pulmonary nodules, pleural effusion(s), lymphadenopathy, underlying pulmonary emphysema, or fibrosis.
Figure 2:
Image in a 36-year-old man with history of recent travel to Wuhan who presented with fever, fatigue, and myalgias. Coronal thin-section unenhanced CT image shows ground-glass opacities with a rounded morphology in both upper lobes (arrows).
Figure 3:
Image in a 66-year-old woman with history of recent travel to Wuhan who presented with fever and productive cough. Axial thin-section collimated unenhanced CT image shows a crazy-paving pattern, as manifested by right lower lobe ground-glass opacification and interlobular septal thickening (arrow) with intralobular lines.
Figure 4:
Image in a 69-year-old man with history of recent travel to Wuhan who presented with fever. Axial thin-section unenhanced CT scan shows ground-glass opacities in the lower lobes with a pronounced peripheral distribution (arrows).
Follow-up Chest CT
Eight patients underwent follow-up chest CT during the study date range, one of whom underwent two follow-up CT examinations (Table 3). The mean time between initial chest CT and follow-up was 2.5 days (range, 1–4 days). One of the eight patients (13%) had normal initial and follow-up chest CT examinations, with no interval change. No patients demonstrated interval improvement. Five of the eight patients (63%) demonstrated mild progression and two (25%) demonstrated moderate progression. No patients demonstrated severe progression. One patient with mild progression at the first follow-up CT examination performed 1 day after the initial CT examination underwent a second CT examination 3 days later (4 days after the initial CT examination). The second CT examination showed marked progression, demonstrating increasing number of opacities and density of consolidation. Finally, one patient had a normal chest CT scan at initial presentation, followed by a CT scan obtained 3 days later showing a new solitary, rounded peripheral ground-glass lesion (Fig 5).
Table 3:
Qualitative Change at Follow-up Chest CT Examination in Eight Patients
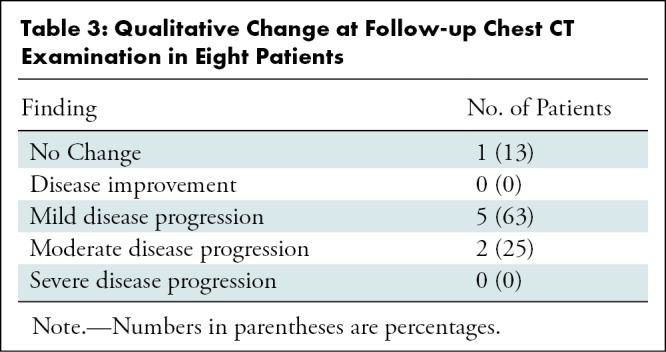
Figure 5a:
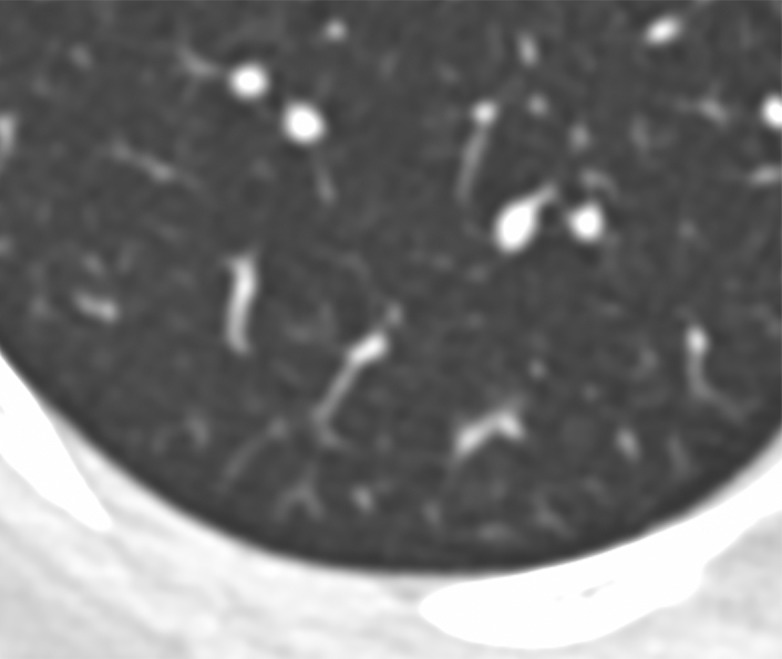
Images in a 43-year-old woman with a history of travel to Wuhan who presented with fever. (a) Axial thin-section unenhanced CT image obtained January 18, 2020, shows normal lung. (b) Follow-up CT image obtained January 21, 2020, shows a new solitary, rounded, peripheral ground-glass lesion in the right lower lobe (arrow).
Figure 5b:

Images in a 43-year-old woman with a history of travel to Wuhan who presented with fever. (a) Axial thin-section unenhanced CT image obtained January 18, 2020, shows normal lung. (b) Follow-up CT image obtained January 21, 2020, shows a new solitary, rounded, peripheral ground-glass lesion in the right lower lobe (arrow).
Discussion
The 2019 novel coronavirus (2019-nCoV) is a new disease outbreak with potentially far-reaching public health ramifications. Chest CT is a key component of the diagnostic work-up for patients with suspected infection, and our investigation has shown some imaging findings frequently encountered in affected patients. Among 21 patients studied, ground-glass opacities were observed in 12 patients (57%) and consolidation was observed in six (29%). There was a high likelihood of this disease affecting more than two lobes (15 of 21 patients, 71%) with bilateral involvement (16 of 21 patients, 76%).
The secondary findings included opacities with a rounded morphology (seven of 21 patients, 33%), reticulation (three of 21 patients, 14%), and crazy paving (four of 21 patients, 19%). A peripheral location of the opacities was also reasonably common (seven of 21 patients, 33%). Pertinent negative findings included the absence of discrete pulmonary nodules, cavitation, pleural effusions, and lymphadenopathy.
Viruses are a common cause of respiratory infection. Imaging findings of viral pneumonias are varied, overlapping with other infectious and inflammatory lung diseases. Viruses in the same viral family share a similar pathogenesis; therefore, CT may help identify distinguishing patterns and features in immunocompetent patients (8). These preliminary data suggest that the CT findings for 2019-nCoV have many similar features to the other coronaviruses: SARS and MERS.
SARS and MERS outbreaks were also due to a coronavirus; therefore, experience from those epidemics may be helpful in managing the current outbreak. Correlating imaging findings in patients with SARS and MERS to those in patients with 2019-nCoV may prove valuable. The disease processes are similar insofar as ground-glass opacities and consolidation are the primary findings on CT scans. Lung involvement with a peripheral predominance was also seen in SARS and MERS. Likewise, previous coronavirus pneumonias were also associated with a crazy-paving pattern (defined as thickened interlobular septa and intralobular lines with superimposed ground-glass opacification), which was also seen in some of our patients. The absence of pulmonary cavitation, pleural effusions, and lymphadenopathy noted in our data was also characteristic of previous SARS studies (9).
Multifocal involvement of 2019-nCoV was more common in our study than in earlier coronavirus reports (eg, SARS studies with typically unifocal lung disease) (10). Moreover, prior work evaluating CT patterns in patients with MERS demonstrated a tendency for lung disease to have a basilar and subpleural distribution (11). However, our patient sample showed no definite lobar or craniocaudal distribution. After recovery from MERS pneumonia, airspace disease showed marked improvement but with often residual fibrotic changes. Although it is too early to have imaging descriptions of 2019-nCoV in the more subacute, chronic, or treated patient population, one might hypothesize that the lungs may respond and heal in a similar fashion (12).
Our patient sample is unique from those of other published series on the 2019-nCoV in that three of our patients had normal initial chest CT scans. One of these patients progressed 3 days later and developed a solitary rounded ground-glass lesion in the right lower lobe, indicating that this pattern may represent the very first radiographically visible manifestation of disease in some patients infected with 2019-nCoV. Another of these three patients underwent follow-up chest CT 4 days after the initial CT examination that remained entirely normal. Ramifications of negative imaging in known infected patients suggests that chest CT lacks complete sensitivity and cannot alone reliably fully exclude this disease, particularly early in the infection. This may be related to the fact that 2019-nCoV has an incubation period of several days, and there may be a prodromal phase where viral infection manifests with symptoms before the emergence of imaging manifestations. The Centers for Disease Control and Prevention has noted that symptoms of 2019-nCoV may appear in as few as 2 days or as long as 2 weeks after exposure, which is similar to the incubation period of MERS (13).
Our study had several limitations. We had a relatively small number of patients, including only eight of 21 patients with follow-up CT imaging. We did not review chest radiographs. Instead, we limited our study to chest CT as CT is likely more sensitive to early and/or mild disease, as was the case with previous coronavirus outbreaks (14). However, chest radiography may have some utility, with the potential to serve as a screening tool on the front lines in medical settings with high disease prevalence but limited resources. Finally, 11 of the patients had CT scans that were 5 mm in slice thickness or more, in which case subtle findings such as early and/or minimal emphysema and tiny pulmonary nodules could be occult or overlooked. However, the thicker-slice images provided for review serve as an accurate representation of a radiologist’s daily practice in some areas of the world that are being affected by the outbreak.
In summary, this work represents an early investigation of chest CT findings in the 2019 novel coronavirus (2019-nCoV), with the intention of creating familiarity with common imaging manifestations of the disease. The radiologist plays a crucial role in the rapid identification and early diagnosis of new cases, which can be of great benefit not only to the patient but to the larger public health surveillance and response systems. There is value in recognizing that the CT appearance of 2019-nCoV shares some similarities with that of other diseases that cause viral pneumonia, particularly those within the same viral family (SARS and MERS). Presently, worldwide public health measures are updating and evolving on a daily basis to manage this current outbreak. As new cases are identified, other unique pulmonary CT imaging manifestations may emerge as potential points for discernment in this patient population. Future studies will be instrumental in determining how patients with parenchymal lung disease evolve after treatment.
Disclosures of Conflicts of Interest: M.C. disclosed no relevant relationships. A.B. disclosed no relevant relationships. X.M. disclosed no relevant relationships. N.Z. disclosed no relevant relationships. M.H. disclosed no relevant relationships. X.Z. disclosed no relevant relationships. J.C. disclosed no relevant relationships. W.X. disclosed no relevant relationships. Y.Y. disclosed no relevant relationships. Z.A.F. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: is a paid consultant for Trained Therapeutics Discovery; institution has stock/stock options in Trained Therapeutics Discovery. Other relationships: institution has a patent licensed to Trained Therapeutics Discovery. A.J. disclosed no relevant relationships. K.L. disclosed no relevant relationships. S.L. disclosed no relevant relationships. H.S. disclosed no relevant relationships.
Abbreviations:
- MERS
- Middle East respiratory syndrome
- SARS
- severe acute respiratory syndrome
- 2019-nCov
- 2019 novel coronavirus
- WHO
- World Health Organization
References
- 1.Novel Coronavirus – China. World Health Organization. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Published January 12, 2020.
- 2.Novel Coronavirus (2019-nCoV). World Health Organization. https://www.who.int/emergencies/diseases/novel-Coronavirus-2019. Published January 7, 2020.
- 3.Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. World Health Organization. https://www.who.int/csr/sars/country/table2004_04_21/en/. Published April 21, 2004.
- 4.Middle East respiratory syndrome Coronavirus (MERS-CoV). World Health Organization. https://www.who.int/emergencies/mers-cov/en/.
- 5.Situation report - 8. World Health Organization. https://www.who.int/docs/default-source/Coronaviruse/situation-reports/20200128-sitrep-8-ncov-cleared.pdf?sfvrsn=8b671ce5_2. Published January 28, 2020.
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020 Jan 24 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246(3):697–722. [DOI] [PubMed] [Google Scholar]
- 8.Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT Features of Viral Pneumonia. RadioGraphics 2018;38(3):719–739. [DOI] [PubMed] [Google Scholar]
- 9.Ooi GC, Khong PL, Müller NL, et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology 2004;230(3):836–844. [DOI] [PubMed] [Google Scholar]
- 10.Wong KT, Antonio GE, Hui DS, et al. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology 2003;228(2):401–406. [DOI] [PubMed] [Google Scholar]
- 11.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol 2014;203(4):782–787. [DOI] [PubMed] [Google Scholar]
- 12.Choi WJ, Lee KN, Kang EJ, Lee H. Middle East respiratory syndrome-Coronavirus infection: a case report of serial computed tomographic findings in a young male patient. Korean J Radiol 2016;17(1):166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.About 2019 Novel Coronavirus (2019-nCoV). Centers for Disease Control and Prevention. https://www.cdc.gov/Coronavirus/2019-ncov/about/symptoms.html. Updated January 31, 2020.
- 14.Paul NS, Roberts H, Butany J, et al. Radiologic pattern of disease in patients with severe acute respiratory syndrome: the Toronto experience. RadioGraphics 2004;24(2):553–563. [DOI] [PubMed] [Google Scholar]